Combined Theoretical and Experimental Investigations: Design, Synthesis, Characterization, and In Vitro Cytotoxic Activity Assessment of a Complex of a Novel Ureacellobiose Drug Carrier with the Anticancer Drug Carmustine
Abstract
1. Introduction
2. Results
2.1. Conformational Analysis of the Drug Carrier and Carmustine
2.2. The Configurational Search, Structural, and Energetical Parameters of the TN:BCNU Complexation Process
2.3. Cytotoxicity Assay
3. Materials and Methods
Computational Details
4. Experimental Details
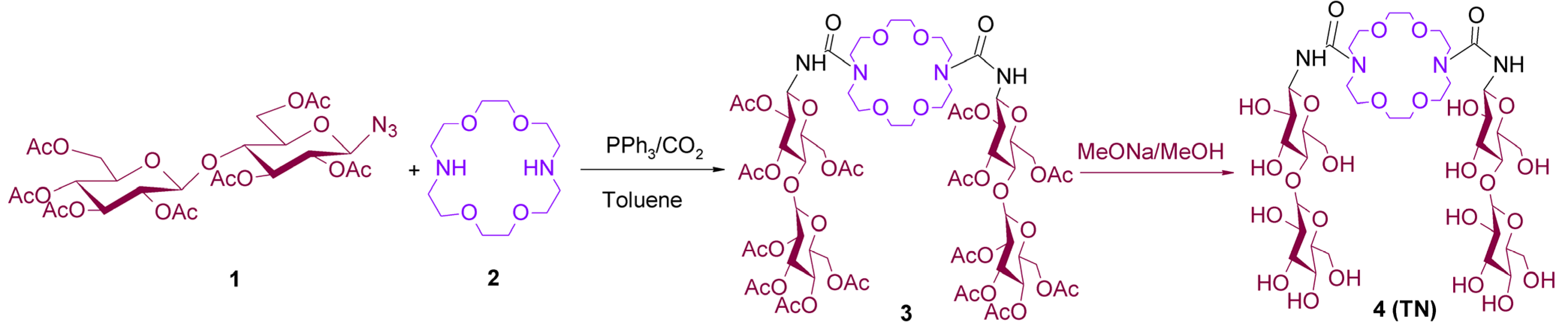
4.1. Synthesis of TN
4.2. Synthesis of the TN:BCNU Complex
4.3. MTT Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeVita, V.T.; Denham, C.; Davidson, J.D.; Oliverio, V.T. The Physiological Disposition of the Carcinostatic 1,3-bis(2-chloroethyU-l-nitrosourea (BCNU) in Man and Animals. Clin. Pharmacol. Ther. 1967, 8, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B.; Issell, B.F. The Nitrosoureas: Carmustine (BCNU) and Lomustine (CCNU). Cancer Treat. Rev. 1982, 9, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Türker, L. Interaction of Carmustine Tautomers with Adenine—DFT Study. Earthline J. Chem. Sci. 2020, 5, 63–76. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The Impact of Lipophilicity on Environmental Processes, Drug Delivery and Bioavailability of Food Components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Jeleń, M.; Martula, E.; Korlacki, R. Study of Lipophilicity and ADME Properties of 1,9-Diazaphenothiazines with Anticancer Action. Int. J. Mol. Sci. 2023, 24, 6970. [Google Scholar] [CrossRef] [PubMed]
- Oriyama, T.; Yamamoto, T.; Nara, K.; Kawano, Y.; Nakajima, K.; Suzuki, H.; Aoyama, T. Prediction of the Permeability of Antineoplastic Agents through Nitrile Medical Gloves by Zone Classification Based on Their Physicochemical Properties. J. Pharm. Health Care Sci. 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; De Bruyn, T.; Wright, M.; Broccatelli, F. Comparing Mechanistic and Preclinical Predictions of Volume of Distribution on a Large Set of Drugs. Pharm. Res. 2018, 35, 87. [Google Scholar] [CrossRef]
- Meulemans, A.; Giroux, B.; Hannoun, P.; Henzel, D.; Bizzari, J.P.; Mohler, J. Permeability of Two Nitrosoureas, Carmustine and Fotemustine in Rat Cortex. Chemotherapy 1989, 35, 313–319. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in Drug Discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.Y.; Yip, A.T.; Lee, B.S.; Kamei, D.T. Modeling Mass Transfer from Carmustine-Loaded Polymeric Implants for Malignant Gliomas. SLAS Technol. 2014, 19, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, F.; Hu, J.; Zhang, X. Nanoparticles for Efficient Drug Delivery and Drug Resistance in Glioma: New Perspectives. CNS Neurosci. Ther. 2024, 30, 14715. [Google Scholar] [CrossRef]
- Bay, J.; Linassier, C.; Biron, P.; Durando, X.; Verrelle, P.; Kwiatkowski, F.; Rosti, G.; Demirer, T. Does High-dose Carmustine Increase Overall Survival in Supratentorial High-grade Malignant Glioma? An EBMT Retrospective Study. Int. J. Cancer 2007, 120, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, C.; Alig, E.; Schmidt, M.U. Crystal Structure of the Anticancer Drug Carmustine Determined by X-ray Powder Diffraction. Powder Diffr. 2021, 36, 148–150. [Google Scholar] [CrossRef]
- Mody, N.; Sharma, R.; Dubey, S.; Vyas, S.P. Combating Cancer with Novel Technologies. Ann. Pharmacol. Pharm. 2017, 2, 1082. [Google Scholar]
- Mali, A.; Bhanwase, A. Brain Targeted Drug Delivery System of Carmustine: Design, Development, Characterization, in Vitro, Ex Vivo Evaluation and in Vivo Pharmacokinetic Study. Acta Chim. Slov. 2024, 71, 26–38. [Google Scholar] [CrossRef]
- van Hoogevest, P.; Liu, X.; Fahr, A. Drug Delivery Strategies for Poorly Water-Soluble Drugs: The Industrial Perspective. Expert Opin. Drug Deliv. 2011, 8, 1481–1500. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Porter, W.; Merdan, T.; Li, L.C. Recent Advances in Intravenous Delivery of Poorly Water-Soluble Compounds. Expert Opin. Drug Deliv. 2009, 6, 1261–1282. [Google Scholar] [CrossRef]
- Ma, D.Q.; Rajewski, R.A.; Vander Velde, D.; Stella, V.J. Comparative Effects of (SBE)7m-β-CD and HP-β-CD on the Stability of Two Anti-neoplastic Agents, Melphalan and Carmustine. J. Pharm. Sci. 2000, 89, 275–287. [Google Scholar] [CrossRef]
- Honmane, S.M.; Charde, M.S.; Choudhari, P.B.; Jadhav, N.R. Development and In Vitro Evaluation of Folate Conjugated Polydopamine Modified Carmustine-Loaded Liposomes for Improved Anticancer Activity. J. Drug Deliv. Sci. Technol. 2023, 90, 105145. [Google Scholar] [CrossRef]
- de Oliveira, V.A.; Negreiros, H.A.; de Sousa, I.G.B.; Farias Mendes, L.K.; Alves Damaceno Do Lago, J.P.; Alves de Sousa, A.; Alves Nobre, T.; Pereira, I.C.; Carneiro da Silva, F.C.; Lopes Magalhães, J.; et al. Application of Nanoformulations as a Strategy to Optimize Chemotherapeutic Treatment of Glioblastoma: A Systematic Review. J. Toxicol. Environ. Health Part B 2024, 27, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Chima, C.M.; Louis, H.; Charlie, D.; Imojara, A.; Benjamin, I.; Uzowuru, E.E.; Adeyinka, A.S. Molecular Simulation of Cu, Ag, and Au-Decorated Molybdenum Doped Graphene Nanoflakes as Biosensor for Carmustine, an Anticancer Drug. Mater. Sci. Semicond. Process 2023, 165, 107669. [Google Scholar] [CrossRef]
- Majumder, R.; Karmakar, S.; Mishra, S.; Mallick, A.B.; Das Mukhopadhyay, C. Functionalized Carbon Nano-Onions as a Smart Drug Delivery System for the Poorly Soluble Drug Carmustine for the Management of Glioblastoma. ACS Appl. Bio Mater. 2024, 7, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Solimannejad, M. In Silico Study of B3O3 Nanosheet as a Disposable Platform for Sensing and Delivery of Carmustine Anticancer Drug. J. Drug Deliv. Sci. Technol. 2023, 87, 104828. [Google Scholar] [CrossRef]
- Bin Iqbal, A.J.; Shahriar, R.; Zubair, A. First-Principles Study of a SiC Nanosheet as an Effective Material for Nitrosourea and Carmustine Anti-Cancer Drug Delivery. Nanoscale Adv. 2024, 6, 2968–2979. [Google Scholar] [CrossRef]
- Li, D.; Ren, T.; Wang, X.; Xiao, Z.; Sun, G.; Zhang, N.; Zhao, L.; Zhong, R. Development and in Vitro Evaluation of Carmustine Delivery Platform: A Hypoxia-Sensitive Anti-Drug Resistant Nanomicelle with BBB Penetrating Ability. Biomed. Pharmacother. 2023, 167, 115631. [Google Scholar] [CrossRef]
- Rani, V.; Venkatesan, J.; Prabhu, A. Carmustine-Loaded Liposomal Delivery Effectively Targets Malignant Glioma Cells and Seizes Endothelial Sprouting In Vitro. J. Clust. Sci. 2024, 35, 1211–1221. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L. Long-Acting Gel Formulations: Advancing Drug Delivery across Diverse Therapeutic Areas. Pharmaceuticals 2024, 17, 493. [Google Scholar] [CrossRef]
- Champeaux-Depond, C.; Jecko, V.; Weller, J.; Constantinou, P.; Tuppin, P.; Metellus, P. Newly Diagnosed High-Grade Glioma Surgery with Carmustine Wafers Implantation. A Long-Term Nationwide Retrospective Study. World Neurosurg. 2023, 173, e778–e786. [Google Scholar] [CrossRef] [PubMed]
- Champeaux-Depond, C.; Jecko, V.; Weller, J.; Constantinou, P.; Tuppin, P.; Metellus, P. Recurrent High Grade Glioma Surgery with Carmustine Wafers Implantation: A Long-Term Nationwide Retrospective Study. J. Neurooncol. 2023, 162, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Kleinberg, L.R. Carmustine Wafers: Localized Delivery of Chemotherapeutic Agents in CNS Malignancies. Expert Rev. Anticancer Ther. 2008, 8, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Aboubakr, O.; Elia, A.; Moiraghi, A.; Benevello, C.; Fathallah, H.; Parraga, E.; Oppenheim, C.; Chretien, F.; Dezamis, E.; et al. Carmustine Wafer Implantation for Supratentorial Glioblastomas, IDH-Wildtype in “Extreme” Neurosurgical Conditions. Neurosurg. Rev. 2023, 46, 140. [Google Scholar] [CrossRef] [PubMed]
- Nozhat, Z.; Heydarzadeh, S.; Shahriari-Khalaji, M.; Wang, S.; Iqbal, M.Z.; Kong, X. Advanced Biomaterials for Human Glioblastoma Multiforme (GBM) Drug Delivery. Biomater. Sci. 2023, 11, 4094–4131. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.-Z.; Wang, Z.-F.; Lan, T.; Huang, W.-H.; Zhao, Y.-H.; Ma, C.; Li, Z.-Q. Carmustine as a Supplementary Therapeutic Option for Glioblastoma: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Zhang, B.; Major, S.; Webb, A. All-trans Retinoic Acid Eluting Poly(Diol Citrate) Wafers for Treatment of Glioblastoma. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 619–628. [Google Scholar] [CrossRef]
- De Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Maira, G.; Mangiola, A. Safety and Efficacy of Gliadel Wafers for Newly Diagnosed and Recurrent Glioblastoma. Acta Neurochir. 2012, 154, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef]
- Rounaghi, G.H.; Mohajeri, M.; Ashrafi, S.; Ghasemi, H.; Sedaghat, S.; Tavakoli, M. Complex Formation of 1,10-Dibenzyl-1,10-Diaza-18-Crown-6 with Ni2+, Cu2+, Ag+ and Cd2+ Metal Cations in Acetonitrile–Dimethylformamide Binary Solutions. J. Incl. Phenom. Macrocycl. Chem. 2007, 58, 1–6. [Google Scholar] [CrossRef]
- Pastuch-Gawołek, G.; Szreder, J.; Domińska, M.; Pielok, M.; Cichy, P.; Grymel, M. A Small Sugar Molecule with Huge Potential in Targeted Cancer Therapy. Pharmaceutics 2023, 15, 913. [Google Scholar] [CrossRef] [PubMed]
- Porwanski, S.; Dumarcay-Charbonnier, F.; Menuel, S.; Joly, J.-P.; Bulach, V.; Marsura, A. Bis-β-Cyclodextrinyl- and Bis-Cellobiosyl-Diazacrowns: Synthesis and Molecular Complexation Behaviors toward Busulfan Anticancer Agent and Two Basic Aminoacids. Tetrahedron 2009, 65, 6196–6203. [Google Scholar] [CrossRef]
- George, A. Jeffrey An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Kamel, M.; Mohammadi, M.; Mohammadifard, K.; Mahmood, E.A.; Poor Heravi, M.R.; Heshmati, J.M.A.; Hossaini, Z. Comprehensive Theoretical Prediction of the Stability and Electronic Properties of Hydroxyurea and Carmustine Drugs on Pristine and Chitosan-Functionalized Graphitic Carbon Nitride in Vacuum and Aqueous Environment. Vacuum 2023, 207, 111565. [Google Scholar] [CrossRef]
- Maranhão, R.C.; Vital, C.G.; Tavoni, T.M.; Graziani, S.R. Clinical Experience with Drug Delivery Systems as Tools to Decrease the Toxicity of Anticancer Chemotherapeutic Agents. Expert Opin. Drug Deliv. 2017, 14, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- HyperChem(TM). HyperChem(TM) Professional 8.0; Hypercube, Inc.: Gainesville, FL, USA, 2008. [Google Scholar]
- Weiner, S.J.; Kollman, P.A.; Case, D.A.; Singh, U.C.; Ghio, C.; Alagona, G.; Profeta, S.; Weiner, P. A New Force Field for Molecular Mechanical Simulation of Nucleic Acids and Proteins. J. Am. Chem. Soc. 1984, 106, 765–784. [Google Scholar] [CrossRef]
- Weiner, S.J.; Kollman, P.A.; Nguyen, D.T.; Case, D.A. An All Atom Force Field for Simulations of Proteins and Nucleic Acids. J. Comput. Chem. 1986, 7, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- James, J.P. Stewart MOPAC2016; Stewart Computational Chemistry: Colorado Springs, CO, USA, 2016; Available online: http://openmopac.net/ (accessed on 14 June 2024).
- Adamiak, M.; Porwański, S.; Ignaczak, A. Conformational Search and Spectroscopic Analysis of Bis -β- d -Glucopyranosyl Azacrown Derivative. Tetrahedron 2018, 74, 2166–2173. [Google Scholar] [CrossRef]
- Adamiak, M.; Ignaczak, A. Quantum Chemical Study of the Complexation Process of Bis-β-d-Glucopyranosyl Diazacrown Derivative with Aspirin and Paracetamol Molecules. Comput. Theor. Chem. 2019, 1167, 112591. [Google Scholar] [CrossRef]
- Adamiak, M.; Ignaczak, A. DFT Studies on the Physicochemical Properties of a New Potential Drug Carrier Containing Cellobiose Units and Its Complex with Paracetamol. Struct. Chem. 2022, 33, 1365–1378. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* Basis Set for Third-row Atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 15. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01 2016; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Funes-Ardoiz, I.; Robert, S. Paton GoodVibes; Version 2.0.3 (v2.0.3); Zenodo: Meyrin, Switzerland, 2018; Available online: https://zenodo.org/records/1435820 (accessed on 14 June 2024).
- Luchini, G.; Alegre-Requena, J.V.; Funes-Ardoiz, I.; Paton, R.S. GoodVibes: Automated Thermochemistry for Heterogeneous Computational Chemistry Data. F1000Res 2020, 9, 291. [Google Scholar] [CrossRef]
- Staudinger, H. Über Polymerisation. Berichte Dtsch. Chem. Ges. 1920, 53, 1073–1085. [Google Scholar] [CrossRef]
- Wittig, G.; Geissler, G. Zur Reaktionsweise Des Pentaphenyl-phosphors Und Einiger Derivate. Justus Liebigs Ann. Chem. 1953, 580, 44–57. [Google Scholar] [CrossRef]
- Pintér, I.; Kovács, J.; Tóth, G. Synthesis of Sugar Ureas via Phosphinimines. Carbohydr. Res. 1995, 273, 99–108. [Google Scholar] [CrossRef]
- Kovács, J.; Pintér, I.; Messmer, A.; Tóth, G.; Duddeck, H. A New Route to Cyclic Urea Derivatives of Sugars via Phosphinimines. Carbohydr. Res. 1987, 166, 101–111. [Google Scholar] [CrossRef]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E. (Lucy); Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic. Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef] [PubMed]
- Hocquet, A.; Langgård, M. An Evaluation of the MM+ Force Field. J. Mol. Model. 1998, 4, 94–112. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Guarnieri, F.; Still, W.C. A Rapidly Convergent Simulation Method: Mixed Monte Carlo/Stochastic Dynamics. J. Comput. Chem. 1994, 15, 1302–1310. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. How Accurate Are the Minnesota Density Functionals for Noncovalent Interactions, Isomerization Energies, Thermochemistry, and Barrier Heights Involving Molecules Composed of Main-Group Elements? J. Chem. Theory Comput. 2016, 12, 4303–4325. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-Consistent Perturbation Theory of Diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Tantillo, D.J. Chemical Shift Repository. Available online: http://cheshirenmr.info/ (accessed on 14 June 2024).

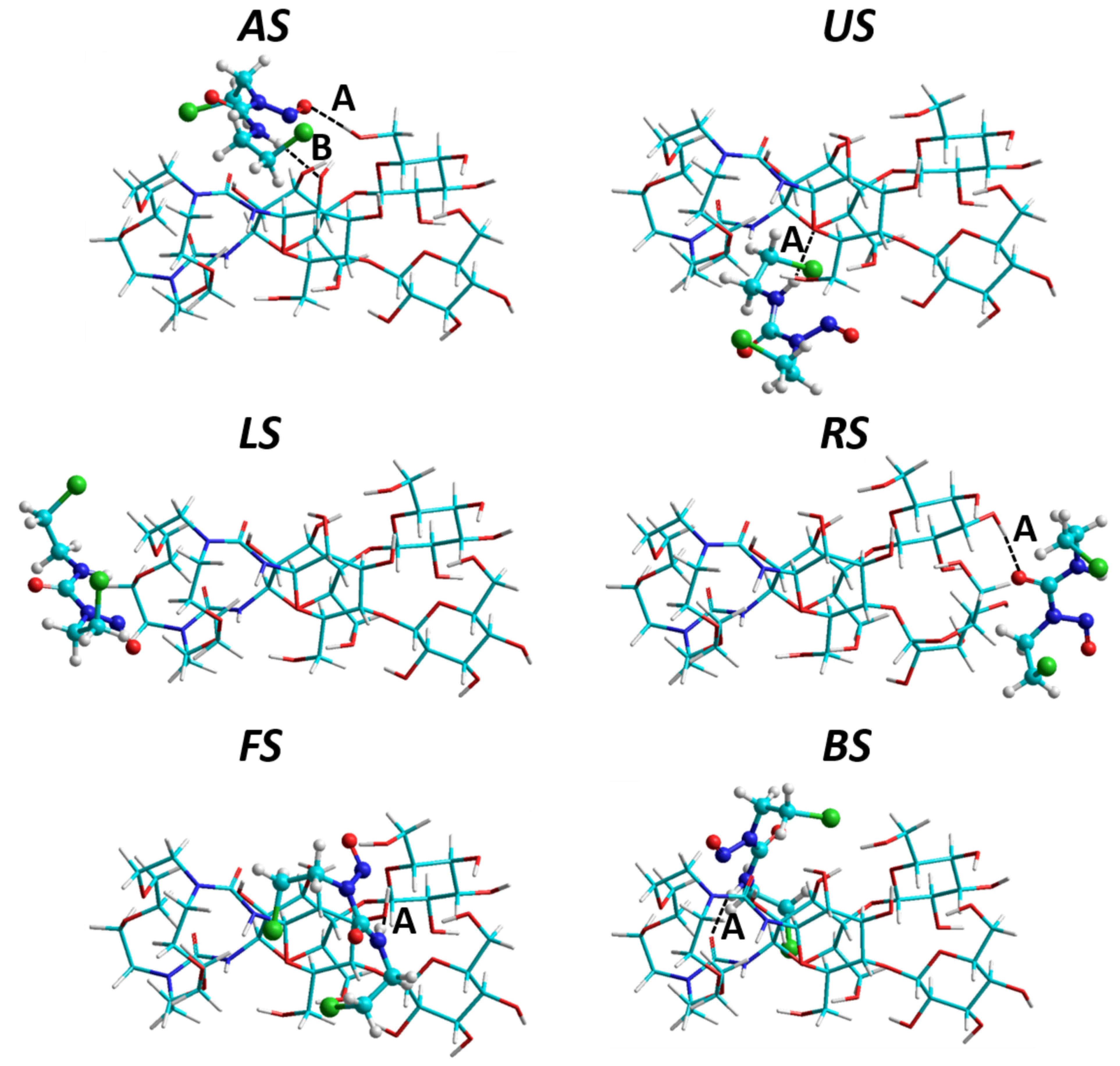
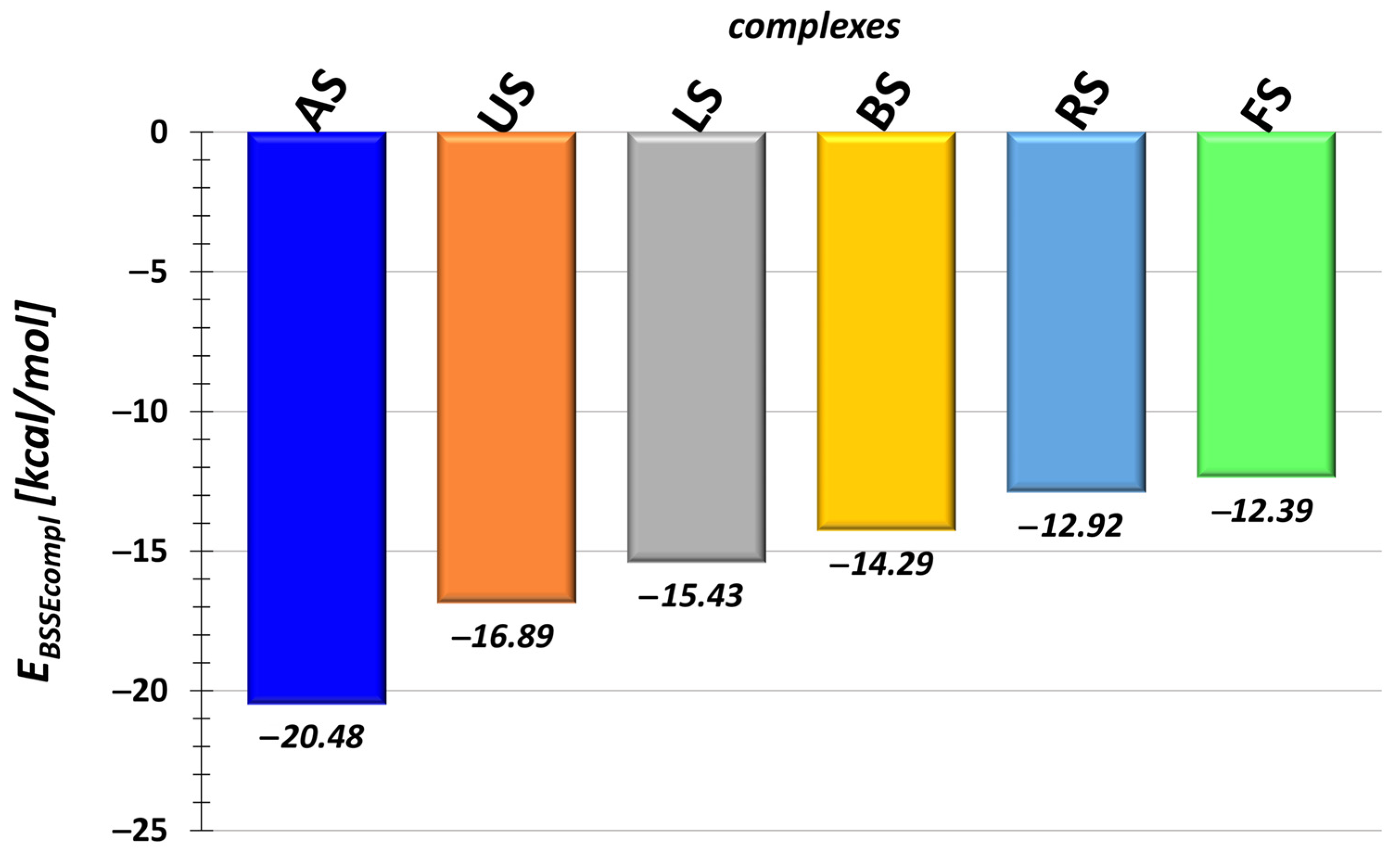
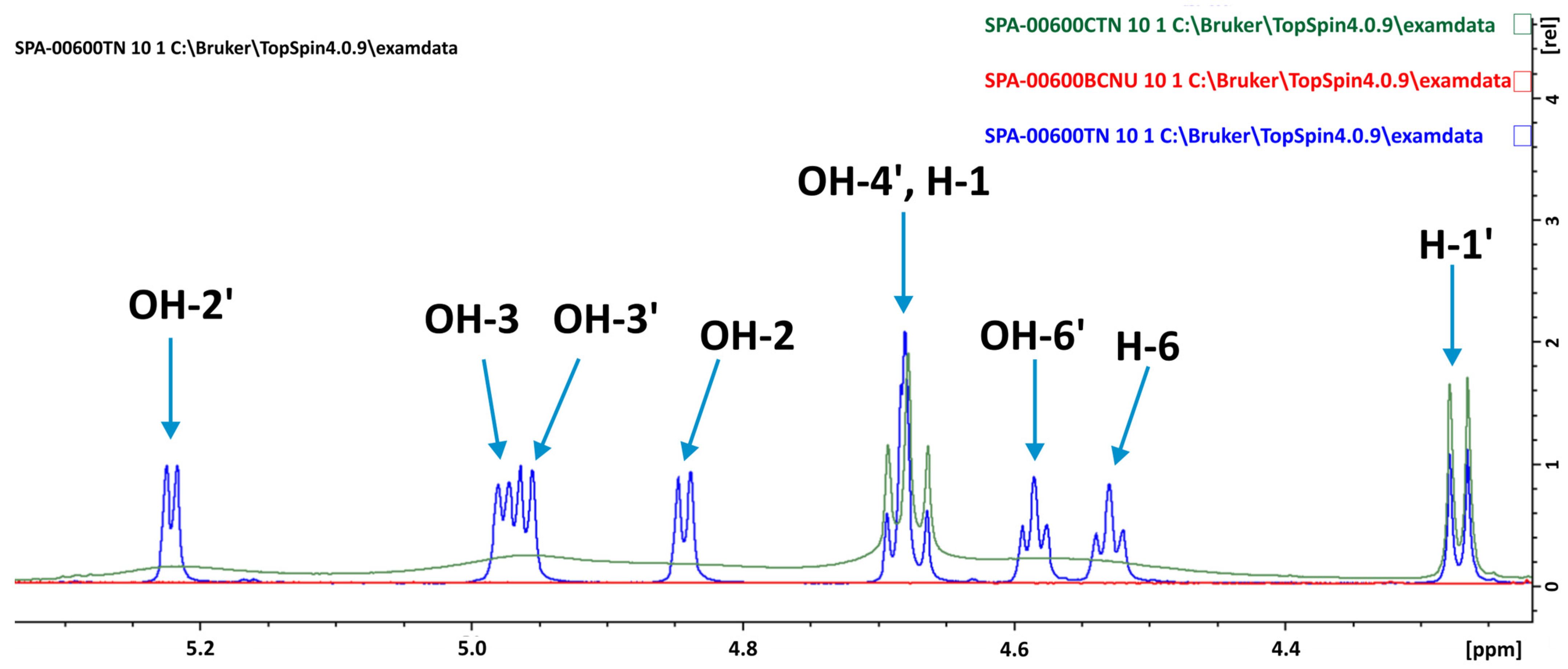

| Molecule | EdefTN | EdefBCNU | Etot | |||
|---|---|---|---|---|---|---|
| AS | −27.26 | 1.86 | 4.92 | 6.78 | −8.98 | −18.75 |
| US | −21.25 | 1.56 | 2.80 | 4.36 | −5.88 | −15.47 |
| LS | −17.98 | 0.48 | 2.08 | 2.55 | −5.14 | −14.10 |
| BS | −17.43 | 0.53 | 2.61 | 3.14 | −4.07 | −12.76 |
| RS | −21.50 | 7.08 | 1.50 | 8.58 | −0.92 | −10.70 |
| FS | −15.25 | 0.55 | 2.32 | 2.86 | −3.18 | −11.33 |
| TN in Complex | BCNU in Complex | ||||||||
|---|---|---|---|---|---|---|---|---|---|
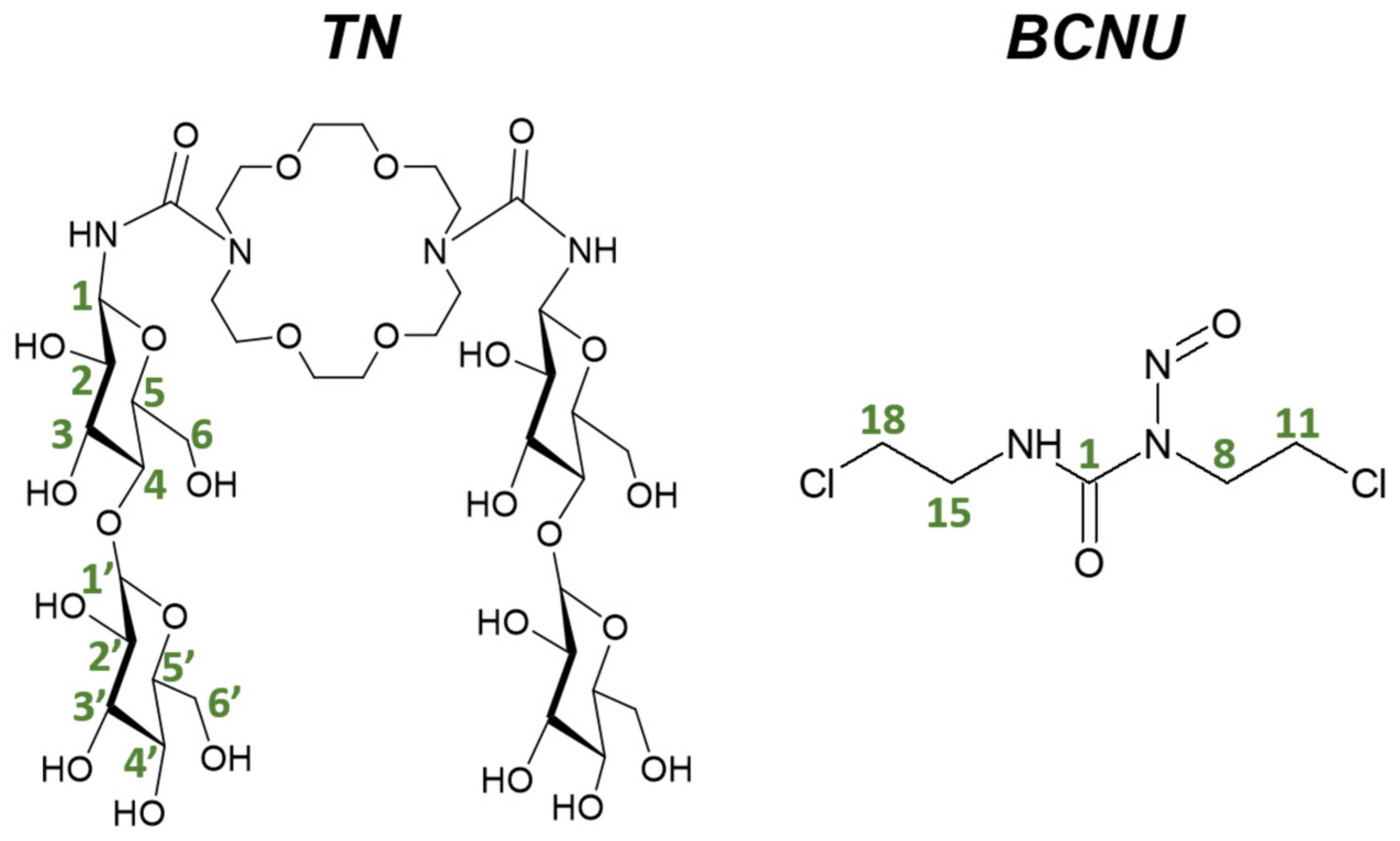
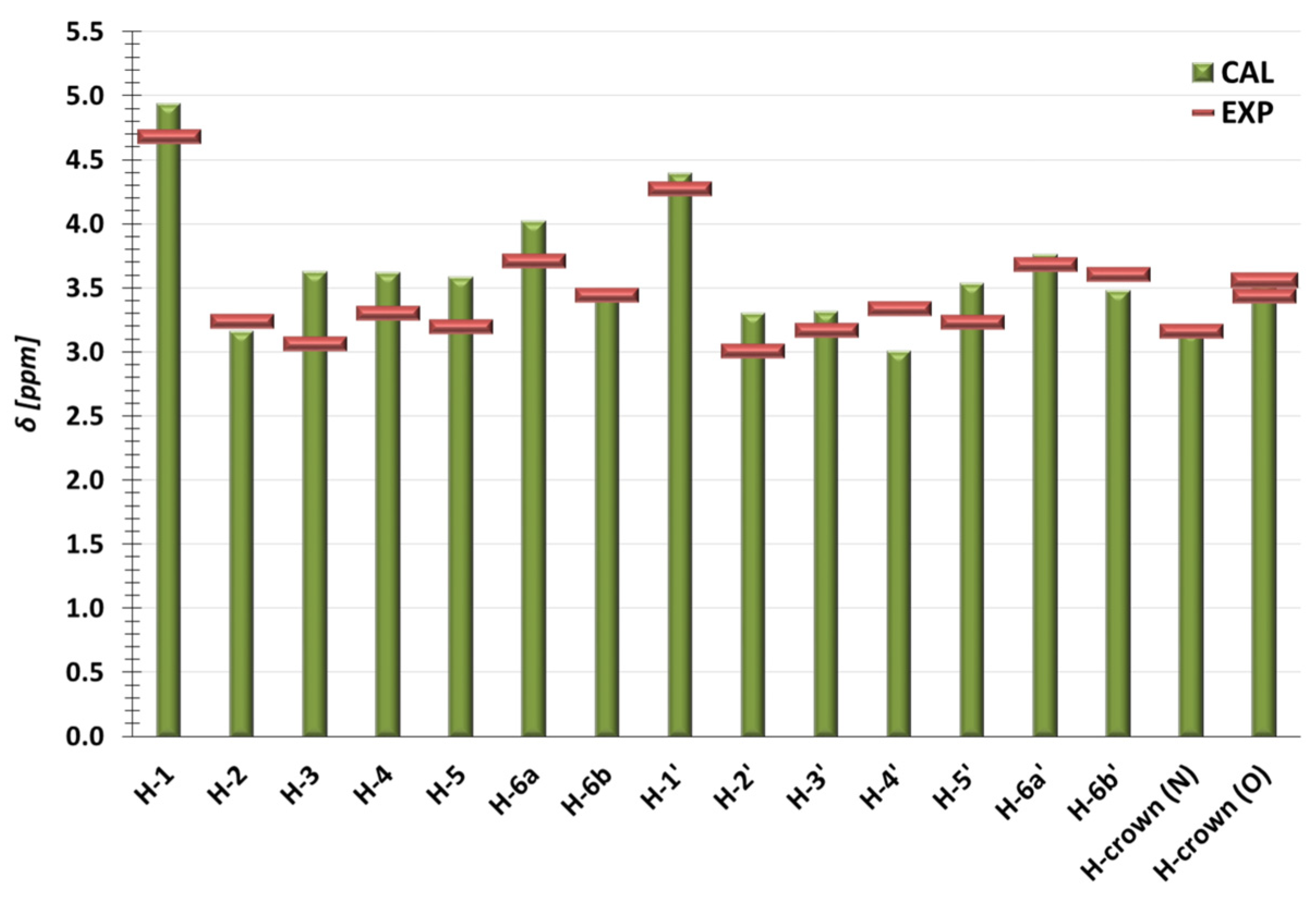
| Number of Atoms (Figure 1) | δCAL of TN in AS | δEXP of TN in Complex | ΔδCAL | ΔδEXP | Number of Atoms (Figure 1) | δCAL of BCNU in AS | δEXP of BCNU in Complex | ΔδCAL | ΔδEXP |
| H-1 | 5.11 | 4.68 | 0.17 | −0.002 | NH | 7.67 | 8.96 | 0.63 | −0.0012 |
| H-2 | 3.14 | 3.23 | −0.02 | −0.0014 | H-8 | 3.87 | 3.62 | −0.09 | −0.001 |
| H-3 | 3.75 | 3.06 | 0.13 | −0.0005 | H-11 | 3.71 | 3.76 | 0.34 | −0.0008 |
| H-4 | 3.57 | 3.29 | −0.05 | −0.0006 | H-15 | 3.21 | 3.76 | −0.27 | 0.1295 |
| H-5 | 3.62 | 3.23 | 0.03 | 0.035 | H-18 | 3.99 | 4.10 | 0.33 | 0.0008 |
| H-6a | 4.04 | 3.69 | 0.02 | −0.0121 | |||||
| H-6b | 3.41 | 3.44 | −0.05 | −0.0029 | |||||
| H-1′ | 4.40 | 4.27 | 0.01 | −0.0001 | |||||
| H-2′ | 3.32 | 3.06 | 0.02 | 0.0525 | |||||
| H-3′ | 3.41 | 3.16 | 0.09 | −0.0042 | |||||
| H-4′ | 3.07 | 3.34 | 0.06 | 0.0075 | |||||
| H-5′ | 3.67 | 3.23 | 0.14 | −0.001 | |||||
| H-6a’ | 3.76 | 3.69 | 0.00 | 0.0119 | |||||
| H-6b’ | 3.50 | 3.60 | 0.02 | 0.0002 | |||||
| Cell Lines | Carmustine | TN:BCNU |
|---|---|---|
| CCD-Co18 | 63.09 ± 4.4 | 199.52 ± 5.9 |
| HT29 | 56.23 ± 3.3 | 158.49 ± 7.8 |
| MCF10A | 282.84 ± 6.2 | - |
| MCF7 | 27.18 ± 1.4 | 89.12 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoelm, M.; Porwański, S.; Jóźwiak, P.; Krześlak, A. Combined Theoretical and Experimental Investigations: Design, Synthesis, Characterization, and In Vitro Cytotoxic Activity Assessment of a Complex of a Novel Ureacellobiose Drug Carrier with the Anticancer Drug Carmustine. Molecules 2024, 29, 3359. https://doi.org/10.3390/molecules29143359
Hoelm M, Porwański S, Jóźwiak P, Krześlak A. Combined Theoretical and Experimental Investigations: Design, Synthesis, Characterization, and In Vitro Cytotoxic Activity Assessment of a Complex of a Novel Ureacellobiose Drug Carrier with the Anticancer Drug Carmustine. Molecules. 2024; 29(14):3359. https://doi.org/10.3390/molecules29143359
Chicago/Turabian StyleHoelm, Marta, Stanisław Porwański, Paweł Jóźwiak, and Anna Krześlak. 2024. "Combined Theoretical and Experimental Investigations: Design, Synthesis, Characterization, and In Vitro Cytotoxic Activity Assessment of a Complex of a Novel Ureacellobiose Drug Carrier with the Anticancer Drug Carmustine" Molecules 29, no. 14: 3359. https://doi.org/10.3390/molecules29143359
APA StyleHoelm, M., Porwański, S., Jóźwiak, P., & Krześlak, A. (2024). Combined Theoretical and Experimental Investigations: Design, Synthesis, Characterization, and In Vitro Cytotoxic Activity Assessment of a Complex of a Novel Ureacellobiose Drug Carrier with the Anticancer Drug Carmustine. Molecules, 29(14), 3359. https://doi.org/10.3390/molecules29143359








