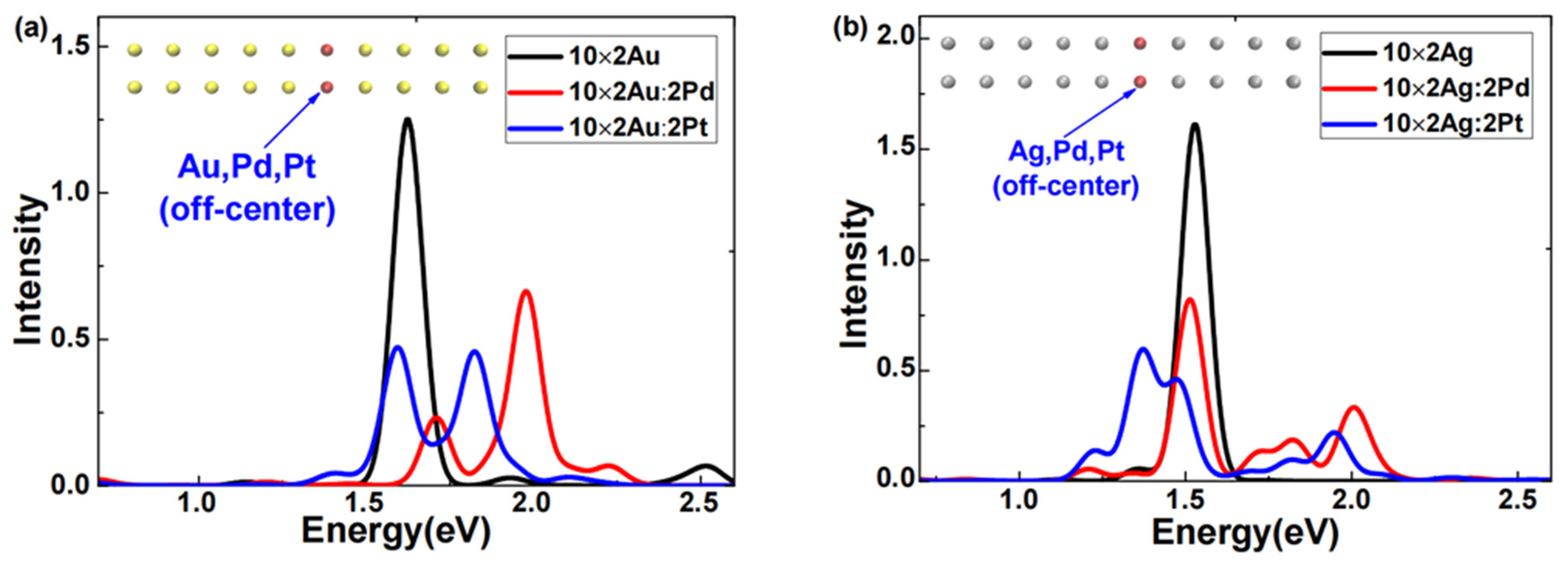
2.1. Discrepancies in Absorption Spectra Induced by Off-Center Doping
The excited-state absorption spectra of 10 × 2 Au or 10 × 2 Ag atomic arrays doped with Pd or Pt off-center were obtained via LR-TDDFT calculations, as illustrated in
Figure 1.
Figure 1a shows that after the Pd or Pt doping, the absorption spectra of the 10 × 2 gold atomic array exhibited two distinct features. First, for either Pd or Pt doping, the plasmon peaks of the gold atomic array broadened into two clearly separated peaks. Second, for Pd-doped gold atomic arrays, both broadened plasmon peaks blueshifted, and the peak of higher intensity had a greater shift, which was remarkably consistent with the findings of Conley et al. [
19] using RT-TDDFT analysis. Regarding the Pt doping, the 10 × 2 gold atomic array exhibited two plasmon peaks of comparable intensity; one peak slightly redshifted, the other significantly blueshifted, and they were situated between the two broadened plasmon peaks of the Pd-doped gold atomic arrays. In
Figure 1b, after the Pd or Pt doping, the absorption spectra of the silver atomic array also displayed two distinct features. First, for either Pd or Pt doping, the plasmon peaks of the silver atomic array broadened. Second, for the Pd-doped silver atomic arrays, both broadened plasmon peaks slightly redshifted, whereas, for the Pt-doped silver atomic arrays, the higher-intensity peak significantly redshifted. This pronounced redshift of the plasmon peak of Pt-doped silver atomic arrays was particularly notable since previous doping response studies [
17,
19,
23] typically observed blueshifts in broadened peaks.
Combining the characteristics of the absorption spectra in
Figure 1a,b, we can conclude that for both Pd and Pt doping, the plasmon peaks of gold or silver atomic arrays broadened, which was consistent with the findings of Conley et al. [
19] using RT-TDDFT analysis. However, there were substantial differences in the broadening and shifting of the plasmon peaks. For example, in Pt-doped silver atomic arrays, the higher-intensity plasmon peak significantly redshifted. This phenomenon clearly demonstrated that doping the same atomic species into different systems or doping different atoms into the same system results in markedly different effects. These sensitive differences in weak doping responses present new opportunities and challenges for the rational control of the optical properties and applications of nanoscale systems.
2.2. Analysis of the Electronic Structure of Atomic Arrays
To comprehend the aforementioned sensitivity to weak doping responses, we analyzed the electronic structure before and after the weak doping of atomic arrays.
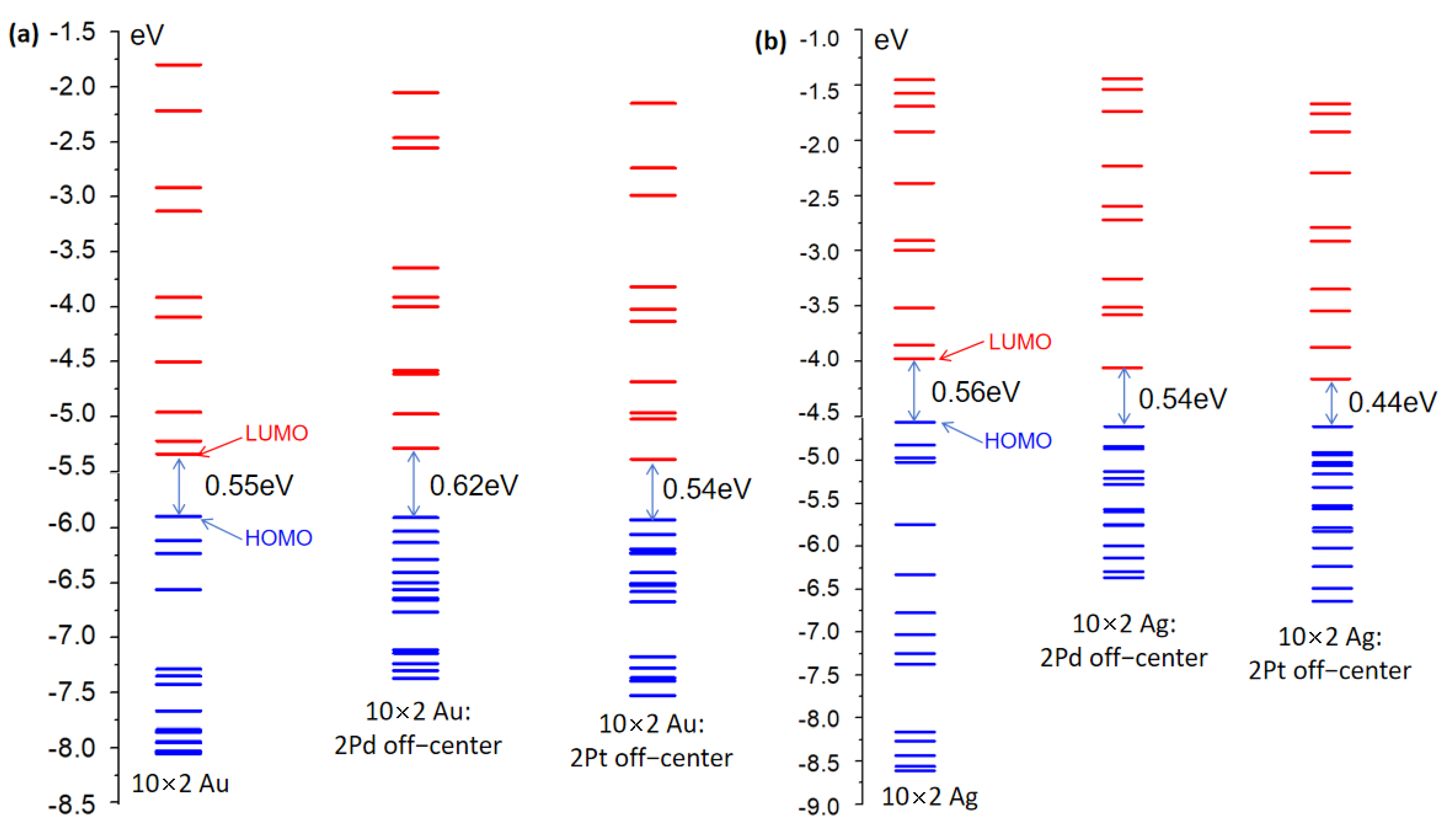
Initially, the electronic structures of the undoped 10 × 2 Au and 10 × 2 Ag atomic arrays were considered. The 10 × 2 Au and 10 × 2 Ag atomic arrays consisted of 20 Au and Ag atoms, respectively, as illustrated in
Figure 1. Orbital energy diagrams of the 10 × 2 Au and 10 × 2 Ag atomic arrays were computed and included 15 occupied orbitals and 10 unoccupied orbitals, as shown in
Figure 2. In the electronic structures of the gold and silver atomic arrays, molecular orbitals primarily constituted of 4d orbitals were fully occupied, while those composed of 5s and 5p orbitals were partially occupied.
Figure 2a,b show that the 10 × 2 Au and 10 × 2 Ag atomic arrays had nearly identical band gaps (energy difference between HOMO and LUMO orbitals) (0.55 eV and 0.56 eV, respectively), but there were substantial disparities in the energy distributions of the 15 occupied and 10 unoccupied orbitals. The
supplementary information in Tables S1 and S2 reveals that in the range of 15 occupied orbitals, the HOMO-4 of the 10 × 2 Au atomic array constituted a localized d-band, whereas the localized d-band appeared at HOMO-10 for the 10 × 2 Ag atomic array. The energy variations in the range of 15 occupied orbitals for the 10 × 2 Au and 10 × 2 Ag atomic arrays were 2.16 eV and 4.06 eV, respectively, with a difference of 1.19 eV. Hence, the d-band orbitals of the 10 × 2 Au atomic array occurred in a higher energy range than those of the 10 × 2 Ag atomic array. For the 10 unoccupied orbitals, the energy variations for the 10 × 2 Au and 10 × 2 Ag atomic arrays were 3.54 eV and 2.53 eV, respectively, with a difference of 1.01 eV. Hence, the 10 × 2 Au atomic array had a larger gap between unoccupied orbitals than the 10 × 2 Ag atomic array.
We considered the electronic structure of the 10 × 2 Au and 10 × 2 Ag atomic arrays doped with Pd and Pt atoms. For the 10 × 2 Pd-doped Au and 10 × 2 Ag atomic arrays, three distinct effects were observed. First, the change in the HOMO-LUMO gap differed: there was an increase of 0.07 eV for the 10 × 2 Au array and a decrease of 0.02 eV for the 10 × 2 Ag array upon Pd doping. Second, the gaps between unoccupied orbitals significantly decreased for 15 orbitals, whereas the gaps for the remaining 10 orbitals exhibited minor changes. There was also irregularity in the differences between pairs of unoccupied orbitals. Third, and most importantly, the
supporting information in Tables S1–S3 shows that in the Pd-doped 10 × 2 Au array, the HOMO-3 orbital exhibited localized d-band characteristics, whereas HOMO-3 corresponded to the ∑
4 orbital of the Pd dopant atom. Similarly, in the Pd-doped 10 × 2 Ag array, HOMO-5 corresponded to a hybridized orbital, HOMO-5 corresponded to the ∑
4 orbital of the Pd dopant atom, and HOMO-6 exhibited localized d-band characteristics. Thus, Pd doping of the 10 × 2 Au and 10 × 2 Ag atomic arrays caused d-band orbitals to appear at higher energy levels. Additionally, orbitals that corresponded to the dopant Pd atom and hybridized orbitals with ambiguous nodal structures [
24] emerged.
Next, the 10 × 2 Pt-doped Au and Ag atomic arrays were examined. Upon Pt doping, three distinct effects were observed on the 10 × 2 Au and 10 × 2 Ag atomic arrays. First, the HOMO-LUMO gap decreased for both arrays by 0.01 eV and 0.12 eV for the 10 × 2 Au and Ag arrays, respectively. The gap reduction in the 10 × 2 Ag array upon Pt doping was substantial, which caused a significant redshift in the plasmon excitations (this is further supported by specific data in the subsequent section). Second, the gaps between unoccupied orbitals significantly decreased, whereas the gaps for the remaining 10 orbitals exhibited similar changes to those upon Pd doping. Third, and also significantly, based on
Table S1, in the Pt-doped 10 × 2 Au array, HOMO-1 corresponded to a hybridized orbital and HOMO-3 corresponded to the ∑
4 orbital of the Pd dopant atom, but clear d-band characteristics were only evident at HOMO-12. In the Pt-doped 10 × 2 Ag array, HOMO-2 corresponded to a hybridized orbital, HOMO-4 corresponded to the ∑
4 orbital of the Pd dopant atom, and no orbitals with localized d-band characteristics were observed in the range of the 15 unoccupied orbitals examined. Upon Pt doping, in both 10 × 2 Au and 10 × 2 Ag atomic arrays, the orbitals with pronounced localized d-band characteristics only appeared at relatively lower energy levels in the occupied orbitals.
In summary, contrasting the undoped 10 × 2 Au and 10 × 2 Ag atomic arrays revealed d-band orbitals at higher energy levels in the occupied orbitals of the 10 × 2 Au array, and there were larger gaps between unoccupied orbitals. Furthermore, comparing the Pd- and Pt-doped 10 × 2 Au and 10 × 2 Ag atomic arrays revealed the appearance of orbitals that corresponded to the dopant atoms in the occupied orbitals. However, the distinction was that in the Pd-doped 10 × 2 Au and 10 × 2 Ag atomic arrays, d-band orbitals emerged at relatively higher energy levels in the occupied orbitals, whereas, in the Pt-doped arrays, the orbitals with pronounced d-band characteristics appeared at relatively lower energy levels in the occupied orbitals. These findings and their specific impact on the absorption spectra were further corroborated and validated through a subsequent analysis of Kohn–Sham (K-S) orbital transition contributions.
2.3. Analysis of Kohn–Sham Orbital Transition Contributions
To comprehend the disparities in the absorption spectra of 10 × 2 atomic arrays perturbed by the off-central doping of Pd or Pt and substantiate and validate the findings of the aforementioned electronic structure analysis,
Table 1 presents the transition contributions to the principal excitations that formed the plasmon peaks and the K-S orbital statistics.
From
Table 1, in the undoped gold atomic array (10 × 2 Au), the primary excitations of the plasmon peak occurred from ground state S
0 to excited state S
19, with an excitation energy of 1.62 eV. These excitations predominantly involved four single-particle transitions with contributions exceeding 5%. In the transition with the highest contribution, the occupation orbital HOMO-3 transitioned to the unoccupied orbital LUMO+1, which accounted for 71.4% of the transition contribution. Following the methodology in reference [
24], the variation in orbital nodal structures was examined. The occupation orbital HOMO-3 exhibited six longitudinal nodes and zero transverse nodes. The unoccupied orbital LUMO+1 exhibited seven longitudinal nodes and zero transverse nodes. Consequently, the difference in longitudinal nodal counts between these orbitals for the primary transition was 1. This result is completely consistent with the calculations of Conley et al. [
19] using the RT-TDDFT method, where the difference in longitudinal nodal counts was consistently 1. The third-highest contribution from the occupation orbital HOMO-6 (d-band) represented a typical localized d-type orbital. In bulk materials, bands composed of sp-type orbitals are called conduction bands and those composed of d-type orbitals are called valence bands. Therefore, for the undoped gold atomic array (10 × 2 Au), the transition from ground state S
0 to excited state S
19 involved a minor proportion of interband single-particle transitions, which blueshifted the plasmon peak [
8]. This result partially elucidates the relatively smaller excitation energy observed in the undoped silver atomic array (10 × 2 Ag) under identical unperturbed conditions.
It is noteworthy that the energy positions of d orbitals in Au and Ag differed. Yet, why was the excitation energy of the 10 × 2 Au pure gold atomic array and 10 × 2 Ag silver atomic array only 0.09 eV apart? It is known that plasmons are defined as collective oscillations of conduction electrons, and their frequency depends not only on the geometric constraints of sp valence electrons and the energy positions of d electrons but also on the hybridization between d and sp electrons [
24]. In the pure system discussed here, 10 × 2 Au(Ag), the single particles primarily involved in collective excitations were sp valence electrons (as shown in
Table 1: the weight of HOMO-6 with d-orbital characteristics participating in excitations in the 10 × 2 Au system was only 9.2%, whereas, in the 10 × 2 Ag system, single particles without d-orbital characteristics participated in excitations). Therefore, the energy of plasmons mainly depended on the system’s geometric size (geometric constraints), leading to very close absorption peaks of plasmons. This further illustrates that plasmons are collective oscillations of conduction electrons.
The present study investigated the Pd-doped gold atom array 10 × 2 Au:2Pd with two plasmon peaks. According to
Table 1, the primary excitations that contributed to the low-energy peak involved transitions from ground state S
0 to excited state S
51, with an excitation energy of 1.71 eV. There were three main single-particle transitions with over 5% contribution. The transition with the highest contribution was from the occupied orbital HOMO-5 to the unoccupied orbital LUMO+3 and represented 54.8% of the total transition. The HOMO-5 orbital lacked transverse nodes and had six longitudinal nodes, whereas the LUMO+3 orbital lacked transverse nodes and had seven longitudinal nodes, which resulted in a longitudinal node difference of 1. Similarly, the primary excitations that contributed to the high-energy peak involved transitions from ground state S
0 to excited state S
65, with an excitation energy of 1.98 eV. There were four main single-particle transitions with over 5% contribution. The transition with the highest contribution was from the occupied orbital HOMO-2 to the unoccupied orbital LUMO+5 and represented 42.1% of the total transition. The HOMO-2 orbital lacked transverse nodes and had three longitudinal nodes, whereas the LUMO+5 orbital lacked transverse nodes and had eight longitudinal nodes, which resulted in a longitudinal node difference of 5. The correlation between longitudinal node difference and excitation energy supports the findings of [
24].
Similarly, the Pt-doped gold atom array 10 × 2 Au:2Pt exhibited two plasmon peaks, as depicted in
Table 1. The primary excitations that constituted the low-energy peak involved transitions from ground state S
0 to excited state S
53, with an excitation energy of 1.60 eV. There were five main single-particle transitions with over 5% contribution. The transition with the highest contribution was from the occupied orbital HOMO-6 to the unoccupied orbital LUMO+1 and represented 43.0% of the total transition contribution. The HOMO-6 orbital lacked transverse nodes and had six longitudinal nodes, whereas the LUMO+1 orbital lacked transverse nodes and had seven longitudinal nodes, so the longitudinal node difference was 1, which was similar to the node difference for the undoped 10 × 2 gold atom array. The excitation energy difference between low-energy and high-energy peaks was only 0.02 eV. For the high-energy peak, the longitudinal node difference was 5, so its excitation energy was 0.22 eV higher than that of the low-energy peak.
Therefore, the differences in the absorption spectra of the 10 × 2 gold atom arrays with off-central Pd or Pt doping were consistent with the findings of Conley et al. [
19] In the 10 × 2 silver atom array with off-central Pd or Pt doping, the shifts of the two peaks were different from those observed in the Pd-doped 10 × 2 Au:2Pd gold atom array. In the case of 10 × 2 Ag:2Pd, the low-energy peak experienced almost no shift (excitation energy difference of only 0.02 eV). Furthermore, the high-energy peak primarily involved transitions from ground state S
0 to excited state S
56, with an excitation energy of 1.99 eV, which closely matched the observed high-energy peak of 1.98 eV for the Pd-doped 10 × 2 Au:2Pd.gold atom array. Similarly, Pt doping induced a significant redshift in the plasmon peak of the 10 × 2 Ag:2Pt silver atom array. The main excitations that caused the redshift involved transitions from ground state S
0 to excited state S
35, with an excitation energy of 1.37 eV, including four main transition pairs with over 5% contribution. However, an explanation that was only based on the difference in node structures failed to account for the observed redshift phenomenon. Therefore, an analysis from the electron transfer perspective was warranted.
In conclusion, the LR-TDDFT theoretical calculations for the Pd-doped 10 × 2 gold atom array exhibited a blueshift in the plasmon absorption peak, which is consistent with the findings of Conley et al. However, for the Pt-doped 10 × 2 silver atom array, the plasmon peak exhibited a redshift, which a purely nodal comparison of transition contributions could not reasonably explain. Hence, an analysis from the electron transfer perspective was necessary for a comprehensive understanding.
2.4. Analysis of the Inter-Fragment Electron Transfer between Fragments
By computing excited-state electronic distribution, one can gain insight into the characteristics of electron transfer. The following methods can be used: subtracting the excited-state density from the ground-state density, calculating the difference between excited-state atomic charges and ground-state atomic charges, etc. The amount of electron transfer from fragment
R to
S fragment during the electronic excitation process can be quantified as follows in Equation (1) [
25]:
is the proportion of excited electrons attributed to fragment
R and
is the proportion attributed to the destination fragment
S. A greater proportion of electrons attributed to fragment
S corresponds to a greater transfer from
R to
S. After defining the unidirectional electron transfer between fragments, one can define the net electron transfer between two fragments as the difference between transfers in both directions, as shown in Equation (2) [
25]. Additionally, one can define the net electron change for a particular fragment as the sum of net electron transfers between that fragment and all other fragments, as shown in Equation (3) [
25].
The diagonal elements of matric
Q in the equation formally represent the “amount of electron transfer from a fragment to itself”. Physically, this value can be interpreted as the extent to which electrons are redistributed in the fragment due to excitation. To date, the literature has effectively used this method. For example, Zhao et al. [
26] used electron transfer analysis to analyze excited-state electron distributions, which proved highly effective in understanding the photonic and photocatalytic properties of materials.
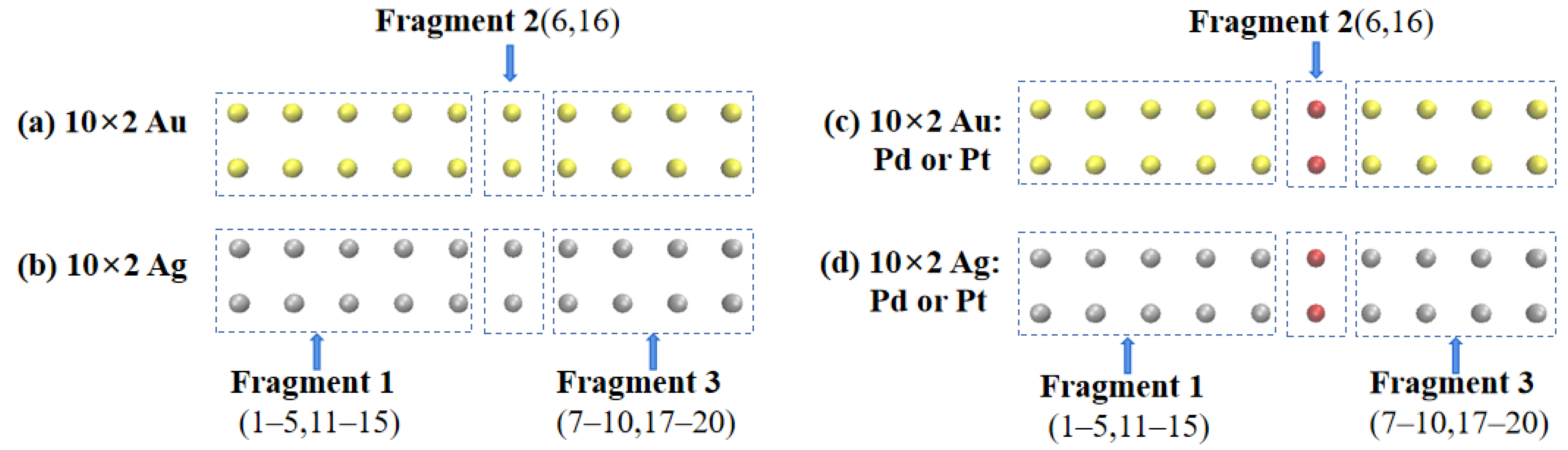
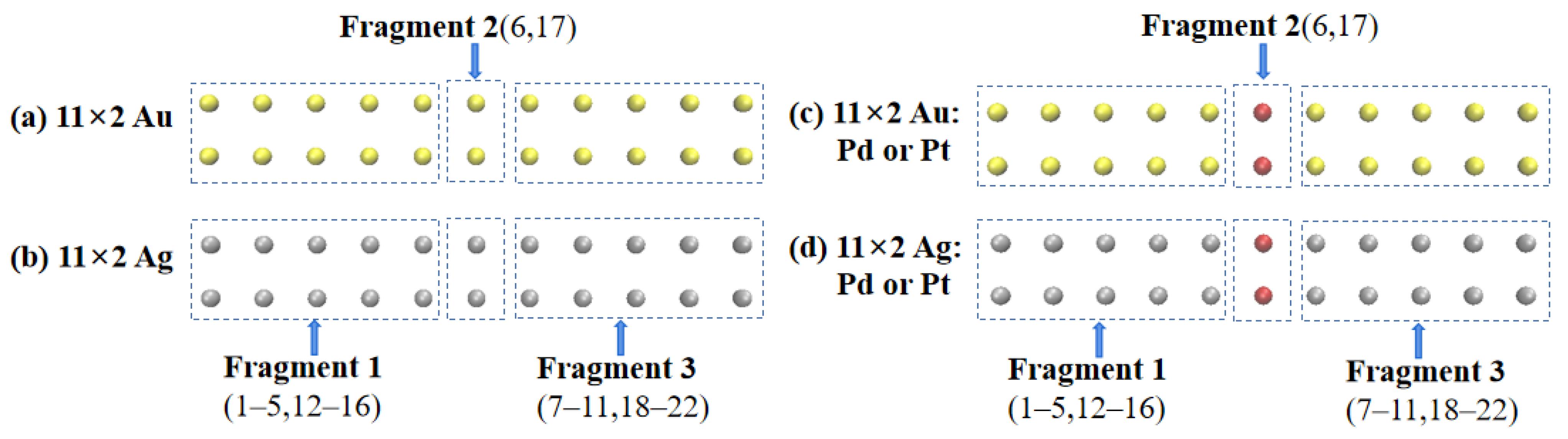
In this study, the off-center doped atomic array was decomposed into three fragments: Fragment 1 consisted of gold or silver atoms with atomic numbers of 1–5 and 11–15; Fragment 3 consisted of gold or silver atoms with atomic numbers of 7–10 and 17–20; Fragment 2 consisted of gold or silver atoms with atomic numbers 6 and 16 when not doped and palladium or platinum atoms when doped, as shown in
Figure 3. The net electron transfer among the three fragments for each system was computed and is summarized in
Table 2. To visualize the net electron transfer among the fragments,
Figure 4 presents the net electron transfer during the partial array excitations.
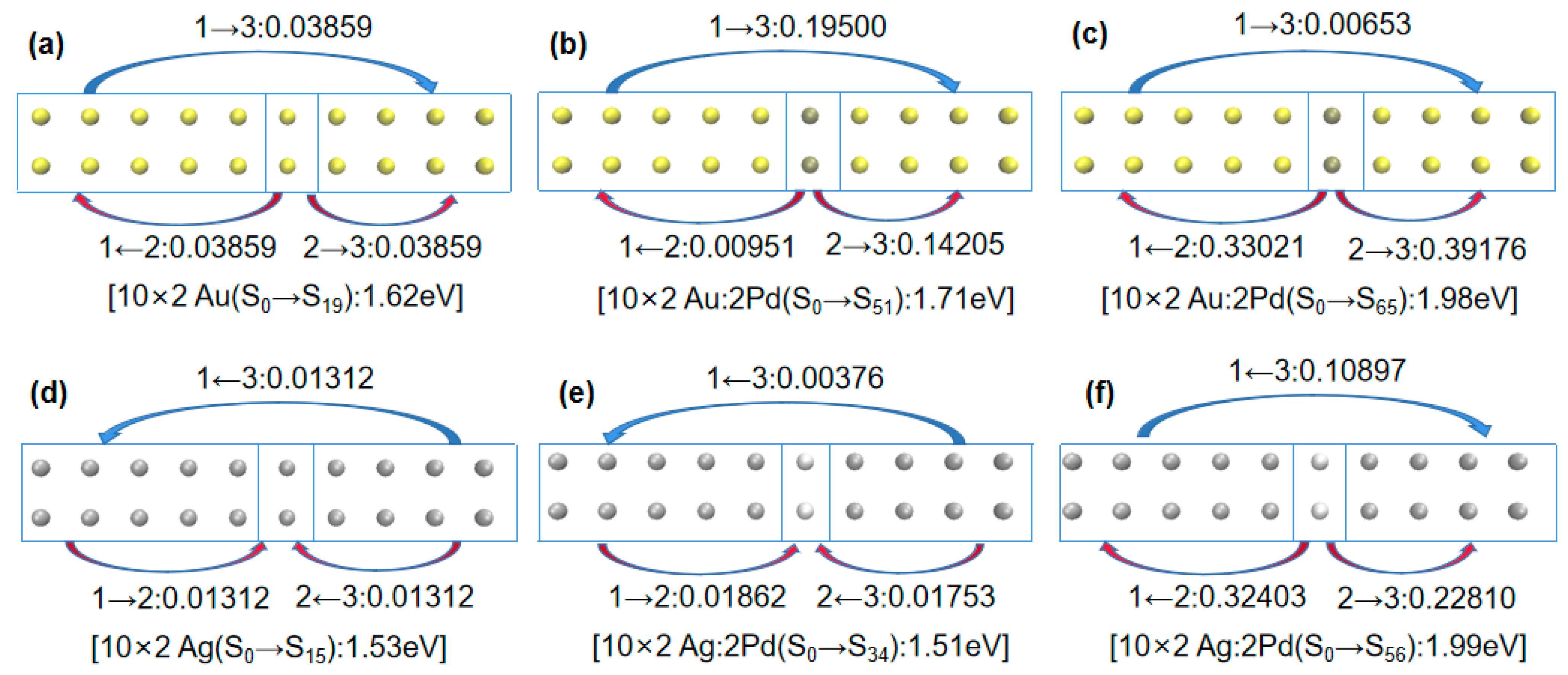
Combining
Figure 3 and
Figure 4 with
Table 2 reveals that in the undoped scenario, the electron transfer between the gold atomic array (10 × 2 Au) and silver atomic array (10 × 2 Ag) among the three fragments was minimal. For example, in
Figure 4a, transitioning from ground state S
0 to excited state S
19 in the gold atomic array (10 × 2 Au) involved a 0.03859 e transfer from Fragment 2 to Fragment 3. However, when palladium (Pd) was doped into the gold atomic array (10 × 2 Au:2Pd), as depicted in
Figure 4b, the transition from ground state S
0 to excited state S
51 involved a 0.14205 e transfer from Fragment 2 to Fragment 3. In
Figure 4c, the transitioning from ground state S
0 to excited state S
65 involved Fragment 2 transferring 0.33021 e and 0.39176 e to Fragment 1 and 3, respectively. Thus, when more electrons were transferred to the adjacent fragments due to the palladium doping, the excitation energy increased. Similar conclusions were drawn for platinum (Pt) doping in the gold atomic array (10 × 2 Au:2Pt), as indicated in
Table 2.
Next, we examined Pd and Pt doping in a 10 × 2 silver atomic array. Combining
Figure 1b and
Table 2 reveals that in the 10 × 2 Ag:2Pd array, the absorption spectrum broadened into two plasmon peaks. Specifically, the plasmon peak from ground state S
0 to excited state S
34 was very close in position to that without doping. Comparing
Figure 4d,e, they also had similar electron transfer characteristics; Fragment 1 and 3 transferred electrons to Fragment 2 and Fragment 3 transferred electrons to Fragment 1. However, there was a significant blueshift in the plasmon peak from ground state S
0 to excited state S
56 in
Figure 4f, which indicated a much larger electron transfer from Fragment 2 (Pd atoms) to Fragment 1 and 3. Thus, the amount and direction of electron transfer in the same system determined the relative magnitude of excitation energy.
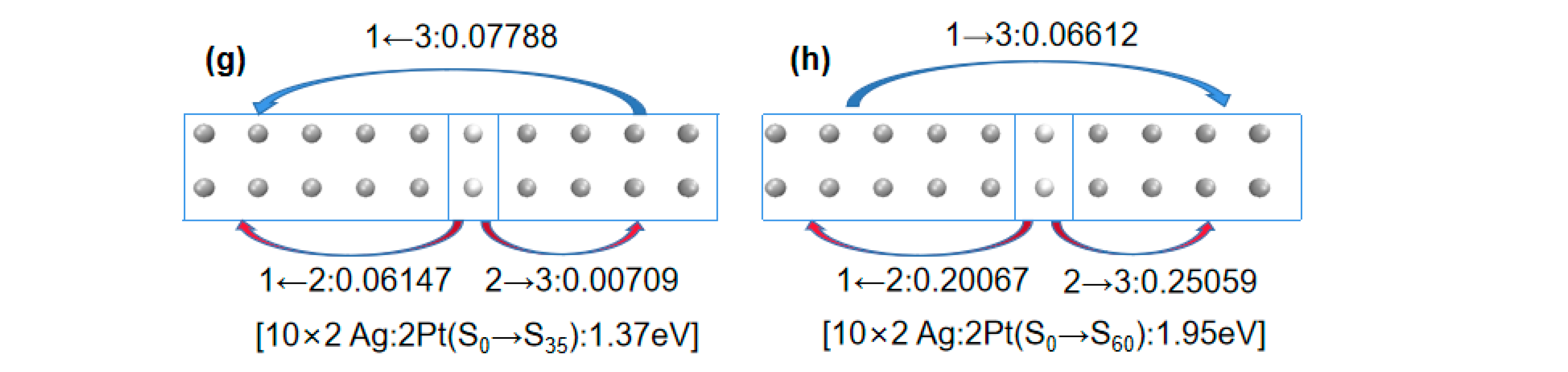
In particular, in the 10 × 2 Ag:2Pt array, a redshift occurred in the plasmon peak from ground state S0 to excited state S35. The aforementioned analysis, using the difference in electron wave function node numbers did not provide a reasonable explanation, so electron transfer analysis was used.
First, considering the electron transfer direction, a comparison of
Figure 4e,g shows that Fragment 1 and 3 transferred electrons to Fragment 2 in the 10 × 2 Ag:2Pd array, whereas Fragment 2 transferred electrons to Fragment 1 and 3 in the 10 × 2 Ag:2Pt array. Thus, they had different electron distributions during excitation. For identical numbers of electrons, a further distribution corresponds to a smaller Coulomb interaction. Therefore, for the broadened low-energy peaks in the 10 × 2 Pd- and Pt-doped silver atomic arrays, the energy of the low-energy peak was lower for the Pt doping (1.37 eV) than for the Pd doping (1.51 eV).
Second, considering the electron transfer amount, a comparison of
Figure 4g,h shows that in the 10 × 2 Ag:2Pt array, from ground state S
0 to excited state S
35, Fragment 2 transferred 0.06147 e and 0.00709 e to Fragment 1 and 3, respectively. However, from ground state S
0 to excited state S
60, Fragment 2 transferred 0.20067 e and 0.25059 e to Fragment 1 and 3, respectively. Clearly, similar to the patterns observed for the Pd- and Pt-doped gold atomic arrays, for the same system, a greater electron transfer transition corresponded to greater excitation energy.
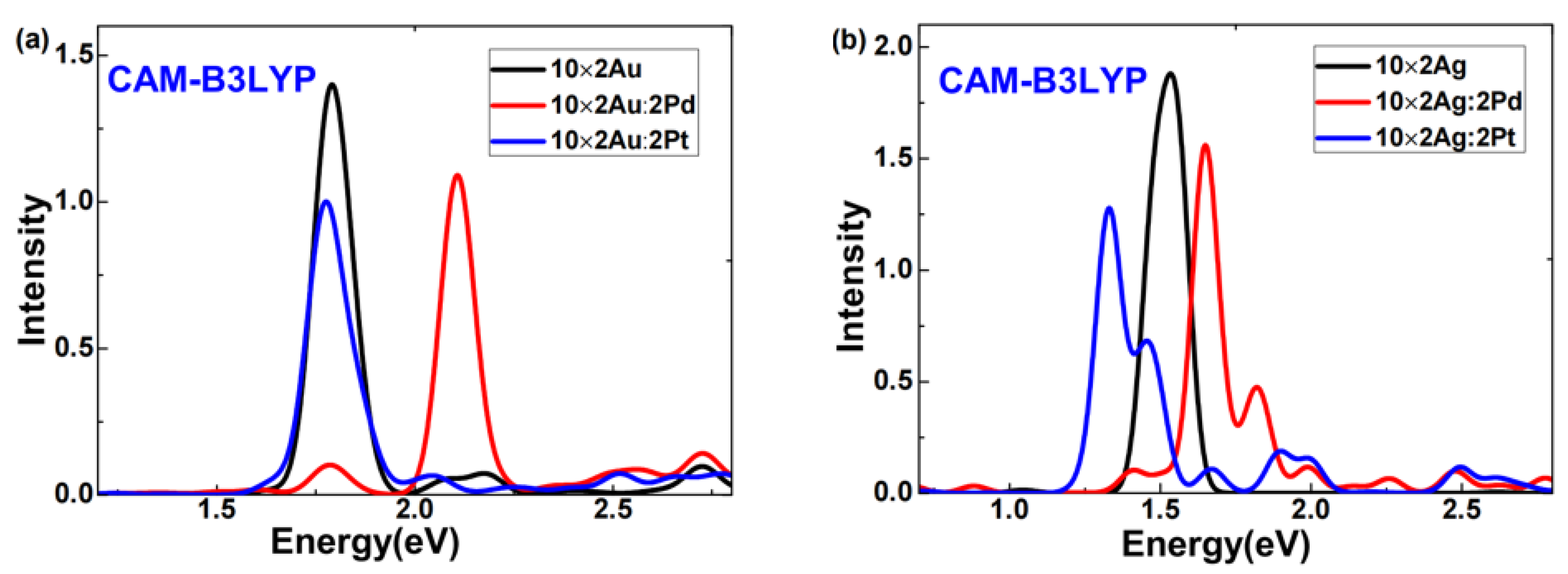
Due to concerns that calculations with the GGA functional might overestimate electron transfer [
27], we also employed the CAM-B3LYP functional to investigate pure and doped 10 × 2 Au (Ag) atomic arrays, aiming to assess whether B3PW91 functional calculations yielded unrealistic electron transfers. This study presents absorption spectra of pure and doped 10 × 2 Au (Ag) atomic arrays computed using the CAM-B3LYP functional combined with LANL2DZ, as shown in
Figure 5. Comparing
Figure 1 and
Figure 5 reveals that while there were differences between spectra obtained using CAM-B3LYP and B3PW91 functionals, the trends in the blue shift and red shift of plasmon peaks after doping were consistent. Here, our focus is on examining whether B3PW91 functional calculations produced unrealistic electron transfers; hence, we do not discuss differences in plasmon peaks obtained from the two functionals.
Firstly, consider the doping of Pd and Pt in the gold atomic array. From
Table 3, in the 10 × 2 Ag:2Pd array, the absorption spectrum showed two plasmonic peaks. Specifically, from the ground state s
0 to the excited state S
37, it exhibited similar electron transfer characteristics to the pure 10 × 2 gold atomic array from s
0 to S
18: minimal electron transfer between Fragments. However, from s
0 to S
74, Fragment 2 transferred electrons to Fragment 1 and 3, with values of 0.33021 e and 0.39176 e, respectively. This indicates that more electron transfer from Fragment 2 (Pd atoms) to Fragment 1 and 3 led to a more pronounced blue shift of the plasmon peak. In the 10 × 2 Ag:2Pt array, similar patterns in electron transfer and plasmon peak shifts were observed. This suggests that both functionals described the electron transfer and plasmon peak shifts equivalently.
Next, combining
Table 2 and
Table 3, we discuss whether B3PW91 functional calculations overestimated electron transfer. For the pure 10 × 2 Au atomic array, the B3PW91 functional calculated the electron transfer from Fragment 2 to Fragment 1 and 3 as 0.03859 e, while the CAM-B3LYP functional calculated it as 0.01160 e. Clearly, B3PW91 functional indeed overestimated electron transfer. However, for the pure 10 × 2 Ag atomic array, the B3PW91 functional calculated the electron transfer from Fragment 1 and 3 to Fragment 2 as 0.01312 e, whereas the CAM-B3LYP functional calculated it as 0.03942 e. This shows that the B3PW91 functional calculations underestimated electron transfer. Comparing other doping scenarios in
Table 2 and
Table 3 reveals that relative to the CAM-B3LYP functional, the B3PW91 functional can overestimate, underestimate, or approximate electron transfer, but the trend in electron transfer is generally consistent. Thus, the results of CAM-B3LYP functional calculations confirmed that using the B3PW91 functional to study the relationship between electron transfer and plasmon peak shifts is at least qualitatively feasible.
In summary, the broadening of plasmon peaks into different energy levels or the blue- or redshifts upon doping a certain system depends on the direction and magnitude of the electron transfer and is intricately linked to their competitive effects. Analysis based on the difference in the number of orbital nodes contributed by the transitions may sometimes prove ineffective in explaining these phenomena. However, introducing electron transfer analysis can offer another perspective to better understand the causes of excitation energy variations. CAM-B3LYP functional calculations also indicated that analyzing the relationship between electron transfer and plasmon peak shifts using the B3PW91 functional in this study was feasible.
2.5. Electron Transfer Analysis for Symmetric Center Doping
From the above discussion, the distinctiveness of asymmetric doping responses can be well explained by electron transfer analysis. To verify the universality of the electron transfer analysis in doping responses, we examined the differences in the plasmonic responses of 11 × 2 arrays of gold and silver atoms with center doping using electron transfer analysis.
First, using the same method, we obtained the excited states of symmetrically center-doped Pd or Pt in 11 × 2 gold and silver atom arrays.
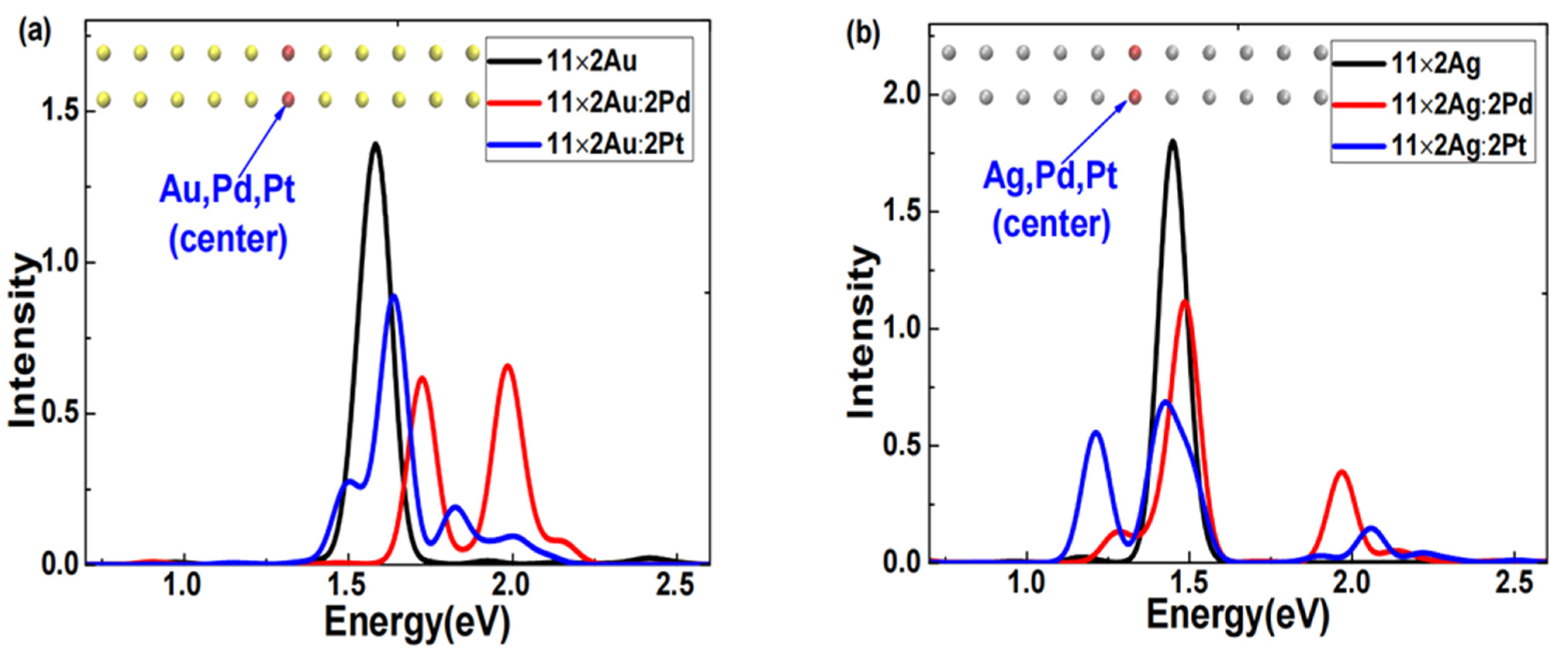
Figure 6 shows the absorption spectra broadened with Gaussian functions.
Figure 6a shows two distinct features in the absorption spectra of the doped 11 × 2 gold atom array: the plasmon peak of the Pd-doped array broadened into two significantly blueshifted and stronger peaks; the plasmon peak of the Pt-doped array also broadened into multiple peaks, but only one was stronger and slightly blueshifted.
Figure 6b shows two distinct features in the absorption spectra of the doped silver atom array: the plasmon peaks of both Pd- and Pt-doped silver atom arrays broadened into two clearly separated and stronger peaks; both plasmon peaks of the Pd-doped array exhibited varying degrees of blueshift, whereas one of the plasmon peaks of the Pt-doped array significantly redshifted. This result illustrates that the modulation effects produced by doping the same element into different arrays or doping different elements into the same array can significantly vary under center-symmetric doping conditions.
Next, to understand the differences in the excited-state absorption spectra of 11 × 2 gold and silver atom arrays that were symmetrically center-doped with Pd or Pt as described above, we decomposed the doped arrays into three Fragments: Fragment 1, consisting of gold or silver atoms with atomic numbers 1–5 and 12–16; Fragment 3, consisting of gold or silver atoms with atomic numbers 7–11 and 18–22; and Fragment 2, consisting of atoms with atomic numbers 6 and 17, as depicted in
Figure 7.
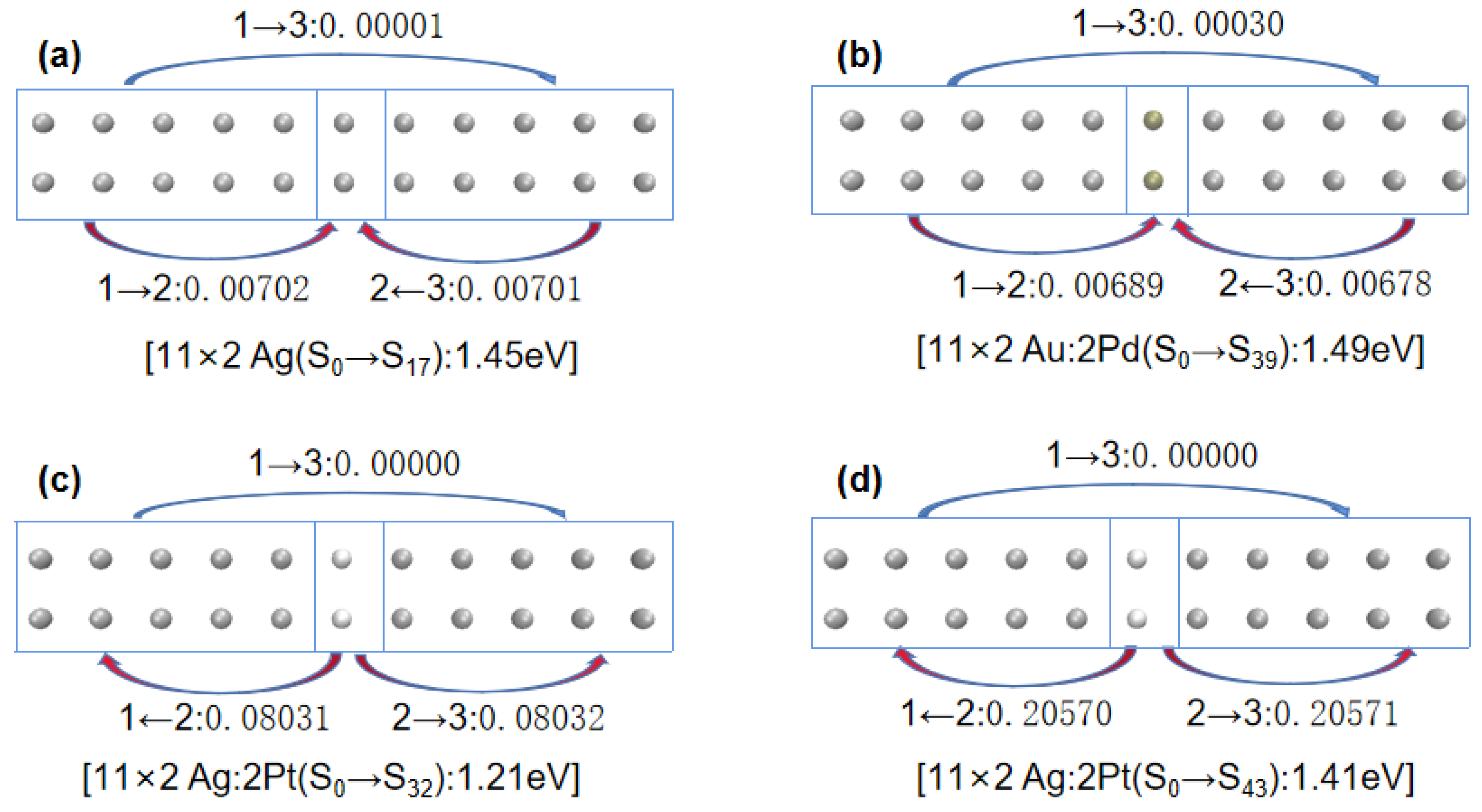
Table 3 shows the net electron transfer amounts between Fragments. To more intuitively understand the net electron transfer amounts among the three Fragments,
Figure 8 illustrates selected array excitation electron net transfer models.
Combining
Figure 7 and
Figure 8, and
Table 4, when Pd-doped 11 × 2 Au:2Pd atomic array transitioned from ground state S
0 to excited state S
59, the electron transfers from Fragment 1 and 3 to Fragment 2 were 0.02820 e and 0.02819 e, respectively. However, from ground state S
0 to excited state S
76, the electron transfers from Fragment 2 to Fragment 1 and 3 were 0.011257 e and 0.011261 e, respectively. When more electrons transferred from the doped Pd atoms to the two adjacent Fragments, the excitation energy increased. For the Pt-doped 11 × 2 Au:2Pt atomic array, there was minimal electron transfer between Fragments from ground state S
0 to excited state S
62, which was comparable to the undoped 11 × 2 Au atomic array. Hence, its excitation energy was very close to that of the undoped 11 × 2 Au atomic array (with a difference of only 0.04 eV).
For the 11 × 2 Ag:2Pd array, the electron transfer of the main excited states corresponded to the conclusions for the 11 × 2 Au:2Pd configuration, where more electrons transferring from the doped Pd atoms to the neighboring Fragments resulted in higher excitation energies. However, the situation was notably different for the 11 × 2 Ag:2Pt array. By transitioning from ground state S0 to excited state S32, Fragment 2 simultaneously transferred 0.08031 e and 0.08032 e to Fragment 1 and 3, respectively. Similarly, by transitioning from S0 to excited state S43, Fragment 2 transferred 0.20570 e and 0.20571 e to Fragment 1 and 3, respectively. More transferred electrons from the doped Pt atoms to the adjacent Fragments corresponded to greater excitation energy.
In particular, concerning the observed redshift phenomena in the last two main plasmon peaks after the Pt doping in the 11 × 2 Ag:2Pt array, similar interpretations could be made for the 10 × 2 Ag:2Pt array based on the direction and quantity of electron transfer. First, regarding the electron transfer direction, a comparison of
Figure 8a,c reveals that for 11 × 2 Ag:2Pt, the low-energy peak transitions from the ground state (S
0) to the excited state (S
32) involved electron transfers toward the ends of the array; for undoped 11 × 2 Ag, the plasmon peaks transitioned from the ground state (S
0) to the excited state (S
17), with electron transfers toward the center of the array. When the electron distribution is relatively distant under equivalent electron numbers, the Coulomb interaction diminishes and results in relatively lower excitation energies. Second, regarding the electron transfer quantity, for the 11 × 2 Ag:2Pt array, the high-energy peak transitions from the ground state (S
0) to the excited state (S
42) involved electron transfers toward the ends of the array, with a substantial increase in transferred electron quantity, which led to relatively greater excitation energies.
Thus, the electron transfer analysis of symmetrically centered doping responses obtained similar conclusions to the off-center doping responses: in the same system, the quantity and direction of electron transfer between doped atoms and neighboring arrays determined the broadening direction, shifting direction, and magnitudes of the plasmon peaks in each excitation process.