Enhanced Cataluminescence Sensor Based on SiO2/MIL-53(Al) for Detecting Isobutylaldehyde
Abstract
1. Introduction
2. Results and Discussion
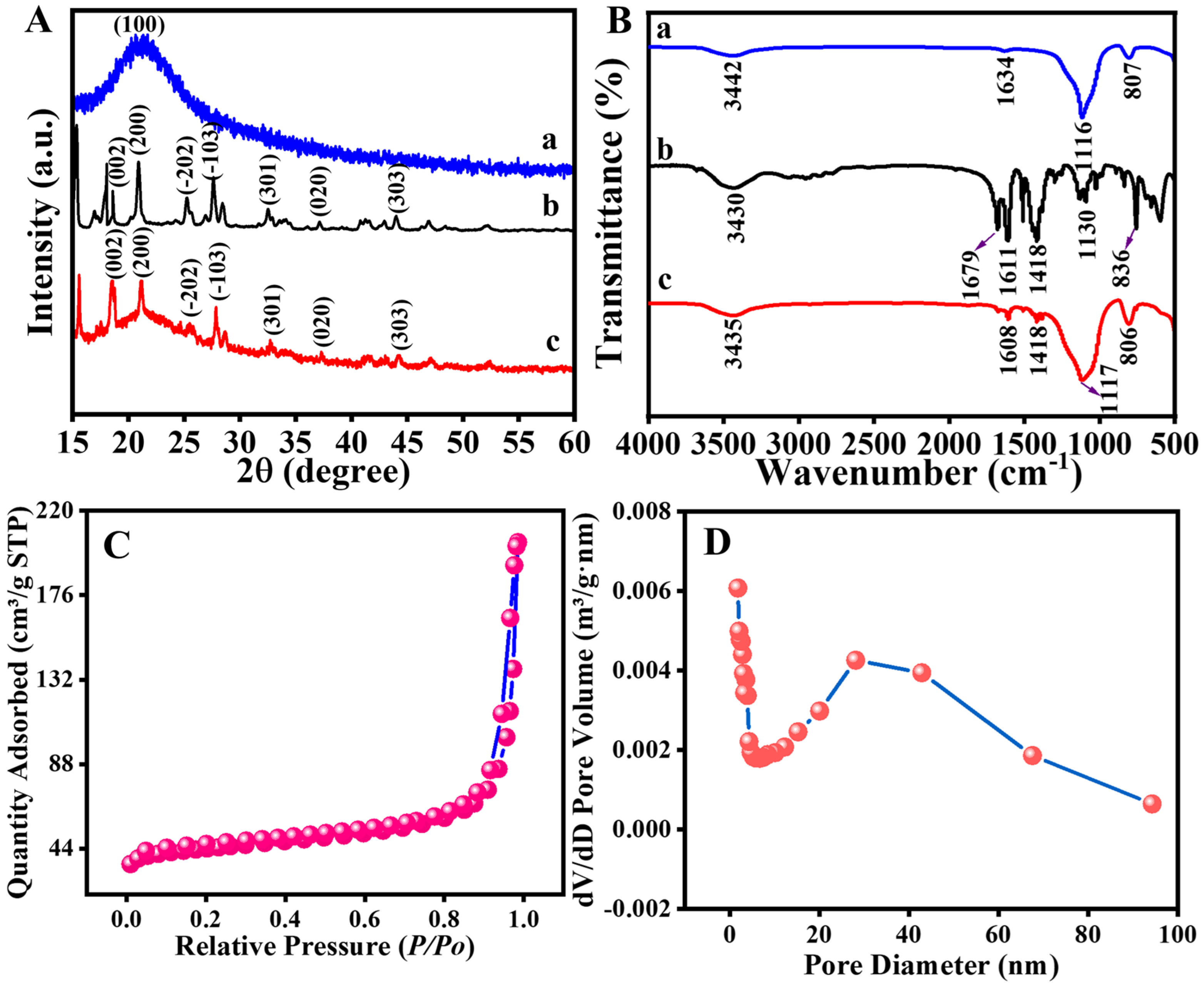
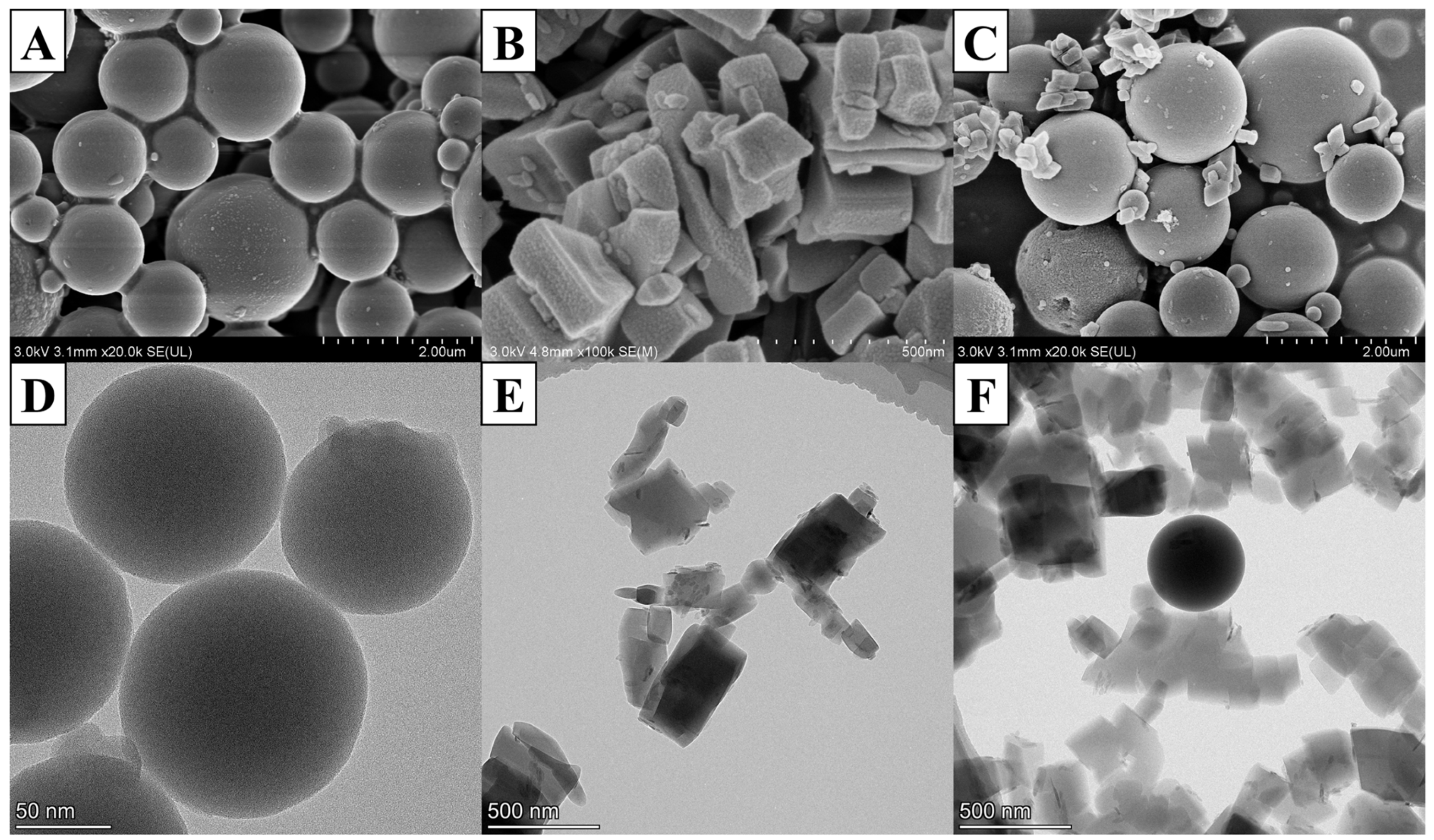
2.1. Material Characterization
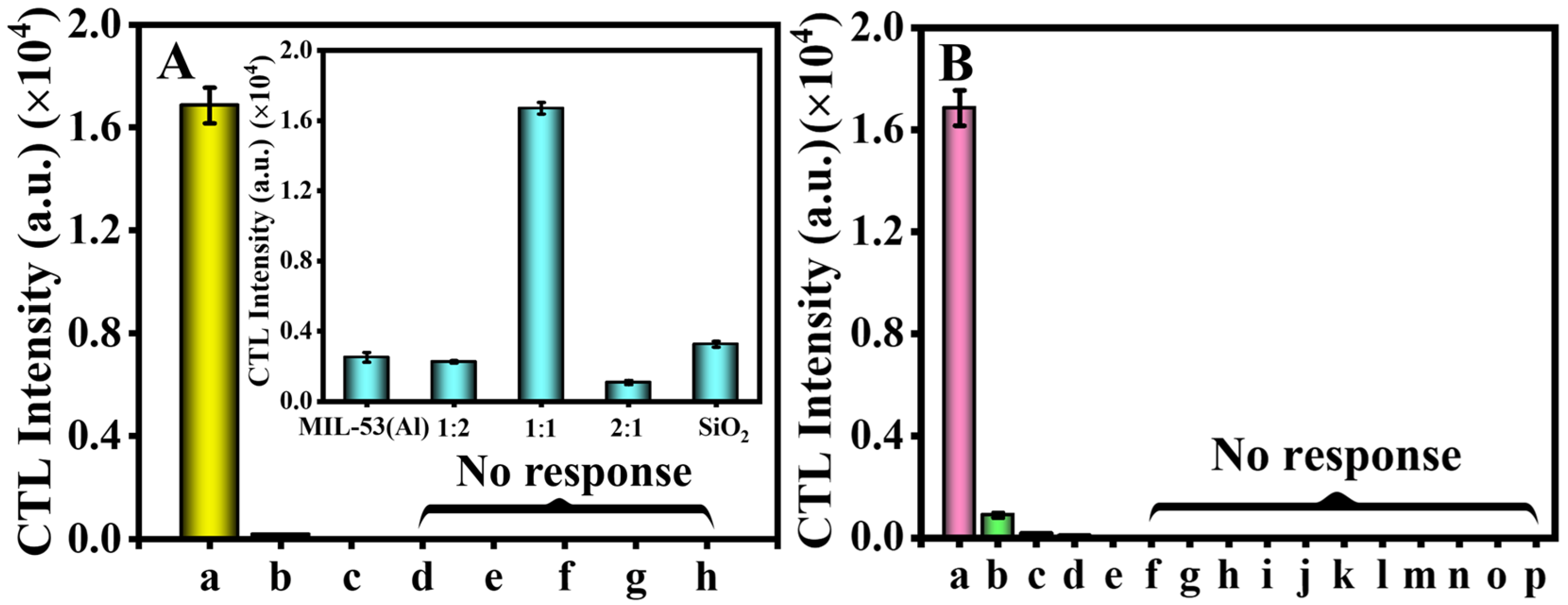
2.2. Selection of the Sensing Material and Gases
2.3. Optimization of CTL Conditions
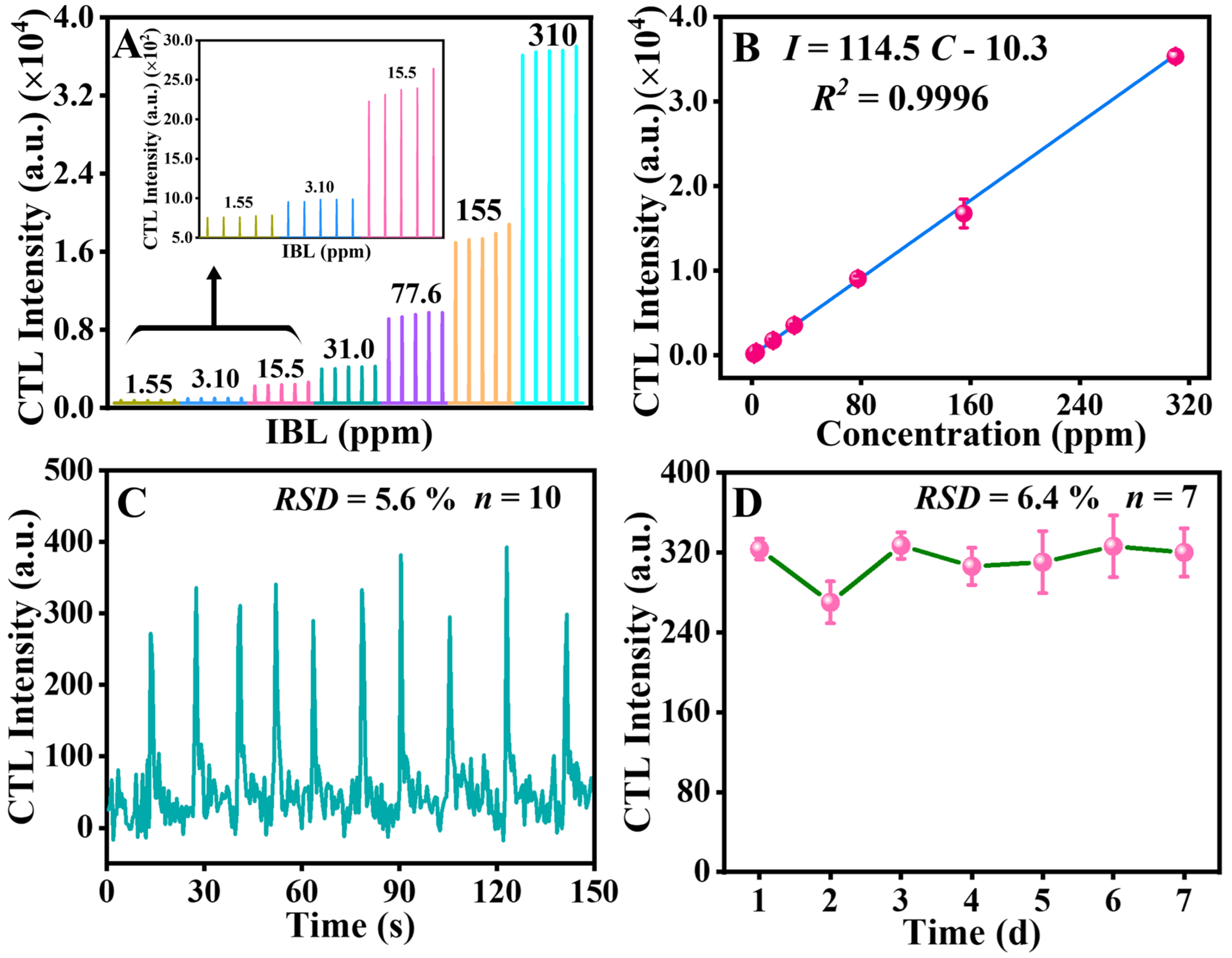
2.4. Method of Analysis and Application
2.5. Sensing Mechanism
3. Materials and Methods
3.1. Chemical Reagents and Materials
3.2. Preparation of SiO2/MIL-53(Al) Composites
3.3. Instrumentation and Analysis
3.4. Computation of Sensing Mechanism
3.5. Chromatographic Mass Spectrometry Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atsumi, S.; Higashide, W.; Liao, J.C. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 2009, 27, 1177–1180. [Google Scholar] [CrossRef]
- Xi, L.J.; Zhang, J.; Wu, R.X.; Wang, T.; Ding, W. Characterization of the volatile compounds of zhenba bacon at different process stages using GC–MS and GC–IMS. Foods 2021, 10, 2869. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, A.B.; Abdollahi, A. Dispersive liquid–liquid microextraction for the high performance liquid chromatographic determination of aldehydes in cigarette smoke and injectable formulations. J. Hazard. Mater. 2013, 6, 254–255. [Google Scholar]
- Ma, Y.X.; Li, H.; Peng, S.; Wang, L.Y. Highly selective and sensitive fluorescent paper sensor for nitroaromatic explosive detection. Anal. Chem. 2012, 84, 8415–8421. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Covington, J.A.; Udrea, F.; Gardner, J.W. Identification and quantification of different vapours using a single polymer chemoresistor and the novel dual transient temperature modulation technique. Sens. Actuators B Chem. 2009, 141, 370–380. [Google Scholar] [CrossRef]
- Breysse, M.; Claudel, B.; Faure, L.; Guenin, M.; Williajis, R.J.J. Chemiluminescence during the catalysis of carbon monoxide oxidation on a thoria surface. J. Catal. 1976, 45, 137–144. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, Y.; Wang, Y.; Zhou, Q.; Zheng, Y.G.; Chen, Y.F.; Zhang, Q.C. A highly sensitive and selective isobutyraldehyde sensor based on nanosized Sm2O3 particles. J. Anal. Methods Chem. 2020, 2020, 5205724–5205732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Yan, W.; Jiang, L.; Zheng, Y.G.; Wang, J.; Zhang, R. Synthesis of nano-praseodymium oxide for cataluminescence sensing of acetophenone in exhaled breath. Molecules 2019, 24, 4275. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.L.; He, Y.G.; Zhang, Y.X.; Yin, B.Q.; Ali, F. Detection and determination of harmful gases in confined spaces for the internet of things based on cataluminescence sensor. Sens. Actuators B Chem. 2019, 296, 126686–126694. [Google Scholar] [CrossRef]
- Yang, F.X.; Gu, C.X.; Liu, B.N.; Hou, C.J.; Zhou, K.W. Pt-activated Ce4La6O17 nanocomposites for formaldehyde and carbon monoxide sensor at low operating temperature. J. Alloys Compd. 2019, 787, 173–179. [Google Scholar] [CrossRef]
- Li, M.; Hu, Y.F.; Li, G.K. A study on the cataluminescence of propylene oxide on FeNi layered double hydroxides/graphene oxide. New J. Chem. 2021, 45, 11823–11830. [Google Scholar] [CrossRef]
- Zhang, R.K.; Wang, D.; Wu, Y.J.; Hu, Y.H.; Chen, J.Y.; He, J.C.; Wang, J.X. A cataluminescence sensor based on NiO nanoparticles for sensitive detection of acetaldehyde. Molecules 2020, 25, 1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Zhou, Q.; Wang, Y.; Wu, Y.; Jiang, L.; Zheng, Y.G.; Liang, J. The development of a simple yet excellent cataluminescence sensor based on nano-Dy2O3 for the rapid monitoring of cyclopentanone. Meas. Sci. Technol. 2020, 31, 095102. [Google Scholar] [CrossRef]
- Pan, F.K.; Sun, B.; Tang, Z.; Zhu, S.G. A fast response cataluminescence ether gas sensor based on GO/Mo2TiC2Tx at low working temperature. RSC Adv. 2022, 12, 8361–8367. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.Q.; Yan, S.G.; Sun, M.X.; Song, H.J.; Zhang, L.C.; Hu, J.X.; Lv, Y. Efficient cataluminescence sensor towards (NH4)2S based on graphitic carbon nitride by nitrogen vacancy modulation. Sens. Actuators B Chem. 2023, 375, 132890–132897. [Google Scholar] [CrossRef]
- Xiong, S.Q.; Yan, S.G.; Zhang, L.C.; Lv, Y. Fluorine functionalized graphitic carbon nitride for cataluminescence sensing of H2S. Sens. Actuators B Chem. 2021, 339, 129855–129863. [Google Scholar] [CrossRef]
- Fois, M.; Cox, T.; Ratcliffe, N.; Costello, B. Rare earth doped metal oxide sensor for the multimodal detection of volatile organic compounds (VOCs). Sens. Actuators B Chem. 2021, 330, 129264–129272. [Google Scholar] [CrossRef]
- Pu, S.R.; Pan, Y.; Zhang, L.C.; Lv, Y. Online evaluation of the catalytic performance of MnO2 and its application in H2S cataluminescence sensing. Anal. Chim. Acta 2021, 1180, 338883–338890. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.Y.; Yi, X.F.; Song, H.J.; Lv, Y. Cataluminescence sensing of carbon disulfide based on CeO2 hierarchical hollow microspheres. Anal. Bioanal. Chem. 2018, 410, 5113–5122. [Google Scholar] [CrossRef]
- Li, L.; Hu, Y.; Deng, D.Y.; Song, H.J.; Lv, Y. Highly sensitive cataluminescence gas sensors for 2-butanone based on g-C3N4 sheets decorated with CuO nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8831–8841. [Google Scholar] [CrossRef]
- Wei, C.D.; Song, H.J.; Huang, Z.L.; Zhang, L.C.; Li, L.; Lv, Y. Ozone-Activated cataluminescence sensor system for dichloroalkanes based on silica nanospheres. ACS Sens. 2021, 6, 2893–2901. [Google Scholar] [CrossRef]
- Hu, J.X.; Zhang, L.C.; Song, H.J.; Su, Y.Y.; Lv, Y. Ozone-inducted ratiometric cataluminescence for aromatic compounds discrimination based on Eu, Tb co-doped MgO. Sens. Actuators B Chem. 2021, 327, 128939–128948. [Google Scholar] [CrossRef]
- Yang, F.X.; Zhang, W.J.; Zhao, Y.X.; Ji, Y.Z.; Liu, B.N.; Zhou, K.W. Optimization of working conditions by response surface methodology of sulfur dioxide gas sensors based on Au/CoO-2La2WO6 nanoparticles. Anal. Chem. 2020, 5, 11145–11151. [Google Scholar] [CrossRef]
- Shi, Z.X.; Li, G.K.; Hu, Y.F. Cataluminescence sensor based on Pt/NU-901 nanocomposite for rapid capture, catalysis and detection of acetone in exhaled breath. Anal. Chim. Acta 2022, 1206, 339787–339796. [Google Scholar] [CrossRef]
- Li, Q.Y.; Sun, M.X.; Zhang, L.C.; Song, H.J.; Lv, Y. A novel Ce(IV)-MOF-based cataluminescence sensor for detection of hydrogen sulfide. Sens. Actuators B Chem. 2022, 362, 131746–131753. [Google Scholar] [CrossRef]
- Huang, X.Y.; Huang, Z.L.; Zhang, L.C.; Liu, R.; Lv, Y. Highly efficient cataluminescence gas sensor for acetone vapor based on UIO-66 metal-organic frameworks as preconcentrator. Sens. Actuators B Chem. 2020, 312, 127952–127959. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Lu, X.T.; Xia, L.; Zhong, Y.H.; Li, G.K.; Hu, Y. Cobalt doped nitrogenous porous carbon derived from covalent organic framework as cataluminescence catalyst for rapid determination of n-hexane in edible oil. Talanta 2021, 232, 122428–122436. [Google Scholar] [CrossRef]
- Huang, X.Y.; Song, H.J.; Zhang, L.C.; Deng, D.Y.; Lv, Y. ZnO nanoparticle-decorated CeO2 nanospheres for cataluminescence sensing of H2S. ACS Appl. Nano Mater. 2021, 4, 9557–9565. [Google Scholar] [CrossRef]
- Huang, X.Y.; Yan, S.G.; Deng, D.Y.; Zhang, L.C.; Liu, R.; Lv, Y. Novel strategy for engineering the metal-oxide@MOF core@shell architecture and its applications in cataluminescence sensing. ACS Appl. Mater. Interfaces 2021, 13, 3471–3480. [Google Scholar] [CrossRef]
- Song, H.J.; Zhang, L.C.; He, C.L.; Qu, Y.; Tian, Y.F.; Lv, Y. Graphene sheets decorated with SnO2 nanoparticles: In situ synthesis and highly efficient materials for cataluminescence gas sensors. J. Mater. Chem. A 2011, 21, 5972–5977. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, Y.; Hong, S. Tailor-made mesoporous SiO2/Au thin flm with a substitutable interface for highly sensitive and selective room-temperature gas detection of VOCs. Sens. Actuators B Chem. 2023, 73, 132763–132792. [Google Scholar]
- Ge, W.; Zhang, X.H.; Ge, X.T.; Liu, K. Synthesis of α-Fe2O3/SiO2 nanocomposites for the enhancement of acetone sensing performance. Mater. Res. Bull. 2021, 141, 111379–111388. [Google Scholar] [CrossRef]
- Rafat, M.; Joo, Y.; Cho, K.; Park, S.; Park, K.; Oh, W. Comparative and efficient ammonia gas sensing study with self-assembly-synthesized metal oxide-SiC fiber-based mesoporous SiO2 composites. ACS. Omega 2022, 7, 37933–37942. [Google Scholar] [CrossRef] [PubMed]
- Komal, P.; Shilpa, P.; Pankaj, R.; Tansir, A.; Aftab, A.P.K.; Quyet, V.L.; Van, H.N.; Chaudhery, M.H.; Pardeep, S. Recent advances in Metal Organic Framework (MOF)-based hierarchical composites for water treatment by adsorptional photocatalysis: A review. Environ. Res. 2023, 222, 115349–115363. [Google Scholar]
- Sohrabi, H.; Ghasemzadeh, S.; Ghoreishi, Z.; Majidi, M.R.; Yoon, Y.; Dizge, N.; Khataee, A. Metal-organic frameworks (MOF)-based sensors for detection of toxic gases: A review of current status and future prospects. Mater. Chem. Phys. 2023, 299, 127512–127525. [Google Scholar] [CrossRef]
- Usman, M.; Ali, M.; Al-Maythalony, B.A.; Ghanem, A.S.; Saadi, O.W.; Ali, M.; Jafar Mazumder, M.A.; Abdel-Azeim, S.; Habib, M.A.; Yamani, Z.H.; et al. Highly efficient permeation and separation of gases with metal-organic frameworks confined in polymeric nanochannels. ACS Appl. Mater. Interfaces 2020, 12, 49992–50001. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.Y.; Zhang, Y.; Zhang, F.; Zhang, S.X.; Wang, Z.G.; Jin, J. MOF nanosheet-based mixed matrix membranes with metal-organic coordination interfacial interaction for gas separation. ACS Appl. Mater. Interfaces 2020, 12, 49101–49110. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose–metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted Applications: A review. Coord. Chem. Rev. 2022, 451, 214263–214303. [Google Scholar] [CrossRef]
- Khalil, I.E.; Fonseca, J.; Reithofer, M.R.; Eder, T.; Chin, J.M. Tackling orientation of metal-organic frameworks (MOFs): The quest to enhance MOF performance. Coord. Chem. Rev. 2023, 481, 215043–215074. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, D.; Yin, J.B.; Shang, Y.X.; Du, J.; Kang, Z.X.; Wang, R.M.; Chen, Y.L.; Sun, D.F.; Jiang, J.Z. An ultrafast responsive NO2 gas sensor based on a hydrogen-bonded organic framework material. Chem. Commun. 2020, 56, 703–706. [Google Scholar] [CrossRef]
- Wu, F.; Ye, J.H.; Cao, Y.L.; Wang, Z.Y.; Miao, T.T.; Shi, Q. Recent advances in fluorescence sensors based on DNA-MOF hybrids. Luminescence 2020, 35, 440–446. [Google Scholar] [CrossRef]
- Pu, S.R.; Pan, Y.; Zhang, L.C.; Lv, Y. Recent advances in chemiluminescence and cataluminescence for the detection of volatile sulfur compounds. Appl. Spectrosc. Rev. 2023, 58, 401–427. [Google Scholar] [CrossRef]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Ferey, G. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chemistry 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, N.; Askari, S.; Fouladitajar, A.; Akbari, I. Facile synthesis of hierarchically structured MIL-53 (Al) with superior properties using an environmentally-friendly ultrasonic method for separating lead ions from aqueous solutions. Sci. Rep. 2022, 12, 2649–2665. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Jiang, S.; Ji, S.; Shi, D.; Cheng, H. Metal-organic framework MIL-53 (Al): Synthesis, catalytic performance for the Friedel-Crafts acylation, and reaction mechanism. Sci. China Chem. 2015, 58, 1544–1552. [Google Scholar] [CrossRef]
- Kandilioti, G.; Siokou, A.; Papaefthimiou, V.; Kennou, S.; Gregoriou, V.G. Molecular composition and orientation of interstitial versus surface silicon oxides for Si (111)/SiO2 and Si (100)/SiO2 interfaces using FT-IR and X-ray photoelectron spectroscopies. Appl. Spectrosc. 2003, 57, 628–635. [Google Scholar] [CrossRef]
- Rahmani, E.; Rahmani, M. Al-based MIL-53 metal organic framework (MOF) as the new catalyst for Friedel-Crafts alkylation of benzene. Ind. Eng. Chem. Res. 2018, 57, 169–178. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wu, J.P. Performance of a Metal-Organic framework MIL-53(Al)-supported cobalt catalyst in the CO catalytic oxidation reaction. J. Phys. Chem. C 2014, 30, 715–722. [Google Scholar]
- Wu, Y.Y.; Zhang, S.C.; Na, N.; Wang, X.; Zhang, X.R. A novel gaseous ester sensor utilizing chemiluminescence on nano-sized SiO2. Sens. Actuators B Chem. 2007, 126, 461–466. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Juan, A.; Damiani, D.; Pistonesi, C. Zirconocene interaction with MAO on (111) and (100) silica surfaces. Macromol. Theory Simul. 2000, 9, 381–387. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Qu, Y.X.; Ren, H. Density functional theory studies on ethanol physisorption onultr afine silica. Acta Phys. Chim. Sin. 2006, 22, 820–825. [Google Scholar]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. Vaspkit: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 7, 108033–108051. [Google Scholar] [CrossRef]
- Togo, A.; Ba, F.; Anaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 2008, 8, 134106–134114. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.C.; Eng, Y.X.; Llen, C.; Wan, C.Z.; Olosskiy, B.; Li, M.F.; Zhao, Z.P.; Wang, Y.L.; Sun, H.T.; et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Custodio, M.J.A.; Caamano, F.C.; Lage, M.A.; Almeida, P.J.; Lorenzo, R.A.; Carro, A.M. GC–MS determination of malondialdehyde, acrolein, and 4-hydroxy-2-no-nenal by ultrasound-assisted dispersive liquid-liquid microextraction in beverages. Food Chem. 2022, 384, 132530. [Google Scholar] [CrossRef]

| Sample ID | Mixture | Detected Values (ppm) | Spiked Concentration (ppm) | Detected Values (ppm) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | IBL | - | 7.70 | 7.39 | 95.1 | 8.5 |
| Cyclohexanone | 77.0 | |||||
| Butanal | 77.0 | |||||
| 2 | IBL | - | 7.70 | 7.82 | 101 | 5.2 |
| Isobutanol | 77.0 | |||||
| Ethyl acetate | 77.0 | |||||
| 3 | IBL | - | 7.70 | 7.67 | 98.6 | 4.8 |
| N-propanal | 77.0 | |||||
| Methylallyl aldehyde | 77.0 | |||||
| 4 | IBL | - | 7.70 | 7.17 | 92.4 | 5.9 |
| Diethyl ether | 77.0 | |||||
| Butanal | 77.0 | |||||
| Methylallyl aldehyde | 77.0 | |||||
| 5 | IBL | - | 7.70 | 7.29 | 93.8 | 6.4 |
| 2-butoxy ethanol | 77.0 | |||||
| Styrene | 77.0 | |||||
| Methyl isobutyl ketone | 77.0 | |||||
| 3-methyl-2-butene-1-ol | 77.0 | |||||
| 6 | Volatile gas in a refrigerated display cabinet | 4.25 ± 0.372 | 1.50 | 5.12 | 83.4 | 8.2 |
| 3.10 | 7.26 | 97.3 | 7.3 | |||
| 6.20 | 10.6 | 105 | 5.5 | |||
| 7 | Volatile gas in medicine locker display cabinet | 8.01 ± 0.248 | 4.00 | 11.4 | 92.2 | 9.4 |
| 8.00 | 15.9 | 98.8 | 6.1 | |||
| 16.0 | 24.1 | 100 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Long, X.; Tang, S.; Jiang, L.; Ban, Z.; Chen, Y.; Zhang, R. Enhanced Cataluminescence Sensor Based on SiO2/MIL-53(Al) for Detecting Isobutylaldehyde. Molecules 2024, 29, 3287. https://doi.org/10.3390/molecules29143287
Zhang Q, Long X, Tang S, Jiang L, Ban Z, Chen Y, Zhang R. Enhanced Cataluminescence Sensor Based on SiO2/MIL-53(Al) for Detecting Isobutylaldehyde. Molecules. 2024; 29(14):3287. https://doi.org/10.3390/molecules29143287
Chicago/Turabian StyleZhang, Qianchun, Xixi Long, Shan Tang, Li Jiang, Zhaoru Ban, Yanju Chen, and Runkun Zhang. 2024. "Enhanced Cataluminescence Sensor Based on SiO2/MIL-53(Al) for Detecting Isobutylaldehyde" Molecules 29, no. 14: 3287. https://doi.org/10.3390/molecules29143287
APA StyleZhang, Q., Long, X., Tang, S., Jiang, L., Ban, Z., Chen, Y., & Zhang, R. (2024). Enhanced Cataluminescence Sensor Based on SiO2/MIL-53(Al) for Detecting Isobutylaldehyde. Molecules, 29(14), 3287. https://doi.org/10.3390/molecules29143287







