Enhancing Performance of Organic Pollutant Degradation via Building Heterojunctions with ZnO Nanowires and Na Doped Conjugated 2,4,6-Triaminopyrimidin-g-C3N4
Abstract
1. Introduction
2. Results and Discussion
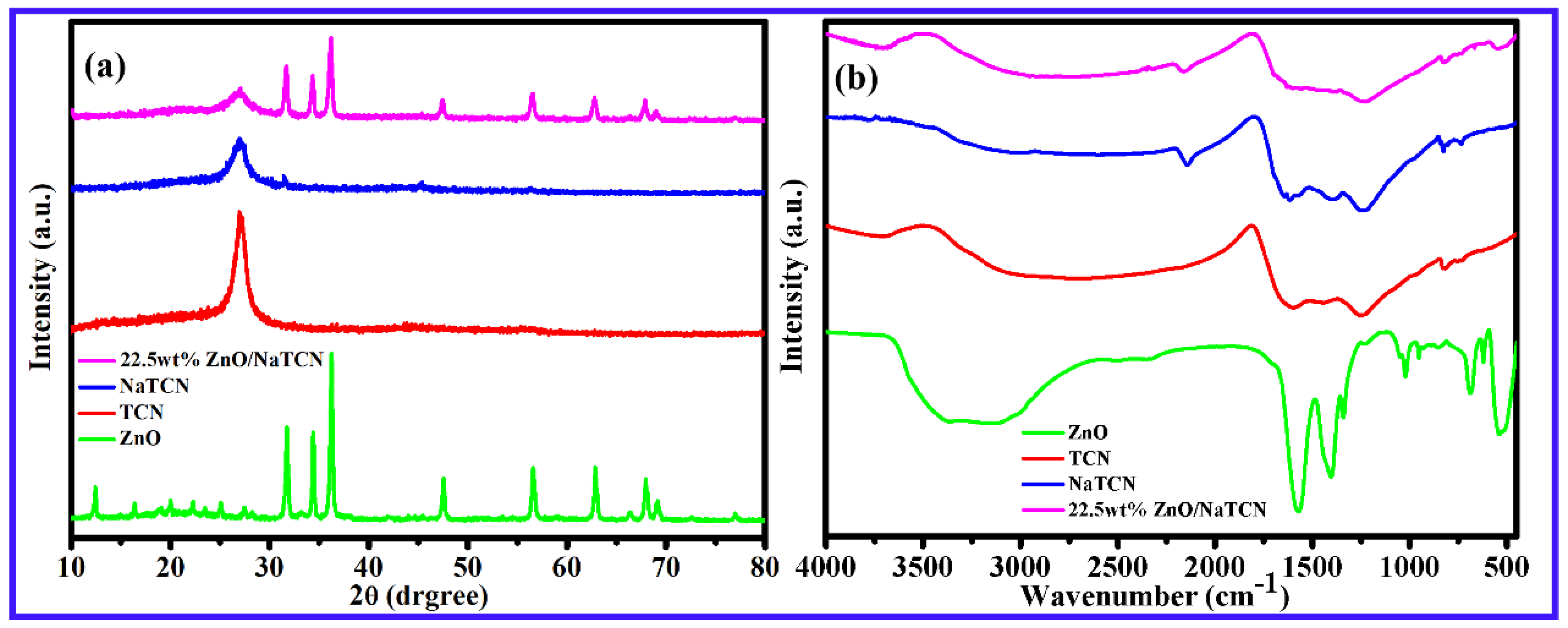
2.1. Morphological and Structural Characterization
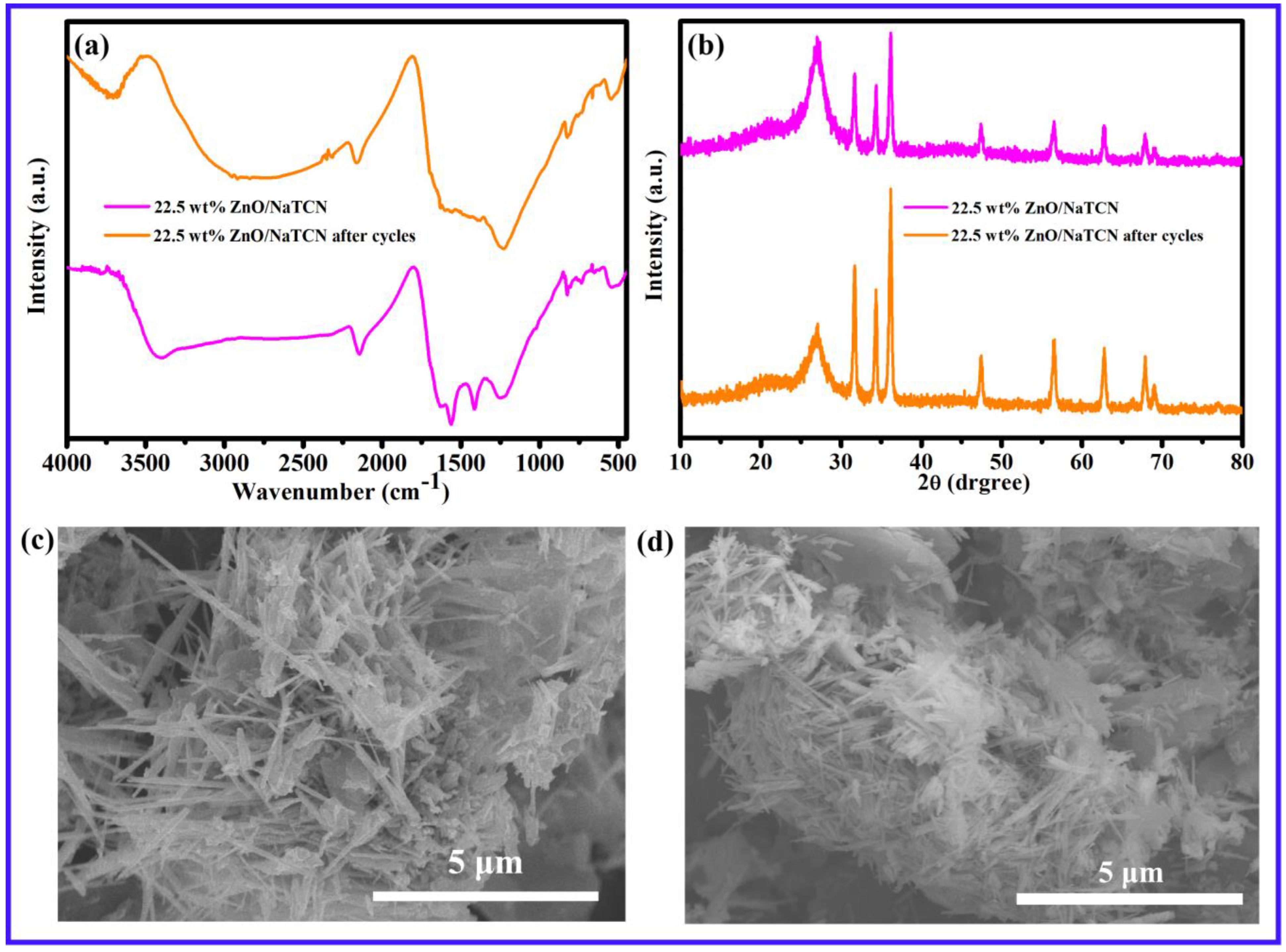
2.2. Evaluation of Photocatalytic Degradation Performance
3. Methodology
3.1. Materials
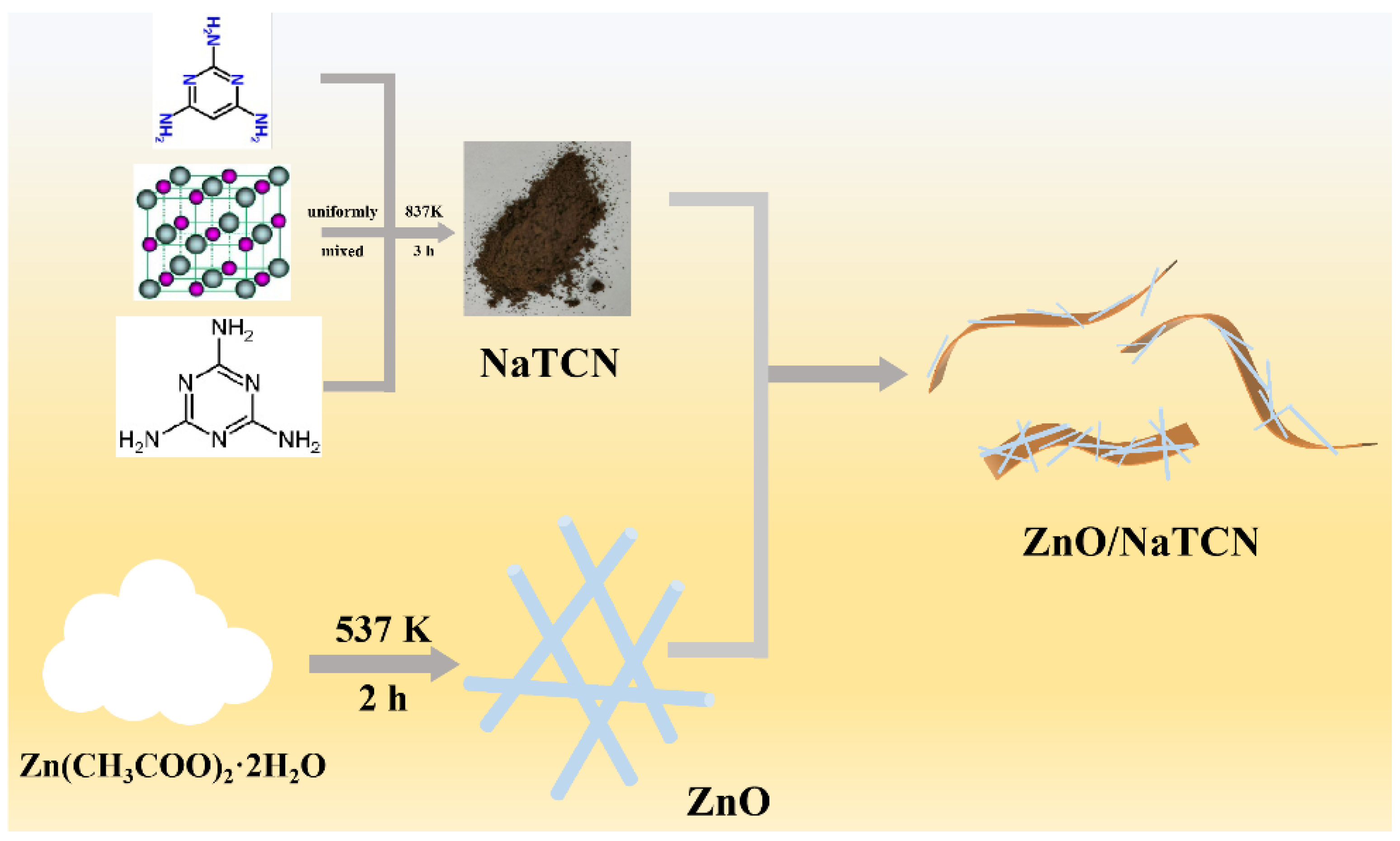
3.2. Preparation of TCN and NaTCN
3.3. Preparation of ZnO Nanowires (ZnO NWs)
3.4. Preparation of ZnO NWs/Na-Doped TAP-CN (ZnO/NaTCN)
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Fan, D.; Li, Z.; Cheng, Y.; Yang, X.; Zhang, T. A Review on the Bioinspired Photocatalysts and Photocatalytic Systems. Adv. Sustain. Syst. 2022, 6, 2100477. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Chao, D. Organic terpyridine molecule as an efficient cocatalyst for metal–free CO2 photoreduction mediated by mesoporous graphitic carbon nitride. Chem. Eng. J. 2022, 429, 132348. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Y.M.; König, B. Energy-and atom-efficient chemical synthesis with endergonic photocatalysis. Nat. Rev. Chem. 2022, 6, 745–755. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Sun, X.; Ji, H.; Liu, W.; Cai, Z. Construction of Z-scheme Ag/AgVO3/carbon-rich g-C3N4 heterojunction for enhanced photocatalytic degradation of sulfamethiadiazole: DFT calculation and mechanism study. Chem. Eng. J. 2022, 433, 133604. [Google Scholar] [CrossRef]
- Shi, W.; Hao, C.; Fu, Y.; Guo, F.; Tang, Y.; Yan, X. Enhancement of synergistic effect photocatalytic/persulfate activation for degradation of antibiotics by the combination of photo-induced electrons and carbon dots. Chem. Eng. J. 2022, 433, 133741. [Google Scholar] [CrossRef]
- Dalal, C.; Garg, A.K.; Jain, N.; Naziruddin, A.; Prajapati, R.; Choudhary, S.; Sonkar, S. Sunlight-assisted photocatalytic degradation of azo-dye using zinc-sulfide embedded reduced graphene oxide. Sol. Energy 2023, 251, 315–324. [Google Scholar] [CrossRef]
- Liang, C.; Li, C.; Zhu, Y.; Du, X.; Yao, C.; Ma, Y.; Zhao, J. Recent advances of photocatalytic degradation for BTEX: Materials, operation, and mechanism. Chem. Eng. J. 2023, 455, 140461. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.; Rawal, J.; Lee, S.; In, I.; Park, S. A review on MXene synthesis, stability, and photocatalytic applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Dihom, H.R.; Al-Shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Khamidun, M.H.B. Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. J. Water Process Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.; Yan, J.; Liu, Y.; Jiang, H.; Zhang, H.; Tang, J.; Liu, Q. Research progresses on the application of perovskite in adsorption and photocatalytic removal of water pollutants. J. Hazard. Mater. 2023, 442, 130024. [Google Scholar] [CrossRef]
- Hannachi, E.; Slimani, Y.; Nawaz, M.; Sivakumar, R.; Trabelsi, Z.; Vignesh, R.; Akhtar, S.; Almessiere, M.A.; Baykal, A.; Yasin, G. Preparation of cerium and yttrium doped ZnO nanoparticles and tracking their structural, optical, and photocatalytic performances. J. Rare Earths 2023, 41, 682–688. [Google Scholar] [CrossRef]
- Wu, Y.; Altuner, E.E.; Tiri, R.N.E.H.; Bekmezci, M.; Gulbagca, F.; Aygun, A.; Xia, C.; Le, Q.V.; Sen, F.; Karimi-Maleh, H. Hydrogen generation from methanolysis of sodium borohydride using waste coffee oil modified zinc oxide nanoparticles and their photocatalytic activities. Int. J. Hydrogen Energy 2023, 48, 6613–6623. [Google Scholar] [CrossRef]
- Manojkumar, U.; Kaliannan, D.; Srinivasan, V.; Balasubramanian, B.; Kamyab, H.; Mussa, Z.; Palaniyappan, J.; Mesbah, M.; Chelliapan, S.; Palaninaicker, S. Green synthesis of zinc oxide nanoparticles using Brassica oleracea var. botrytis leaf extract: Photocatalytic, antimicrobial and larvicidal activity. Chemosphere 2023, 323, 138263. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Gu, X.; Zhao, Y.; Qi, K.; Yan, Y. S-scheme CuInS2/ZnS heterojunctions for the visible light-driven photocatalytic degradation of tetracycline antibiotic drugs. J. Taiwan Inst. Chem. Eng. 2023, 142, 104679. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Liang, Q.; Zhou, M.; Yao, C.; Xu, S.; Li, Z. Core-shell ZIF-8@MIL-68 (In) derived ZnO nanoparticles-embedded In2O3 hollow tubular with oxygen vacancy for photocatalytic degradation of antibiotic pollutant. J. Hazard. Mater. 2021, 414, 125395. [Google Scholar] [CrossRef]
- Fu, Y.; Ren, Z.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Xing, L.; Ma, J.; Wang, H.; Xue, X. Direct Z-scheme heterojunction of ZnO/MoS2 nanoarrays realized by flowing-induced piezoelectric field for enhanced sunlight photocatalytic performances. Appl. Catal. B Environ. 2021, 285, 119785. [Google Scholar] [CrossRef]
- Kong, J.Z.; Zhai, H.F.; Zhang, W.; Wang, S.S.; Zhao, X.R.; Li, M.; Li, H.; Li, A.D.; Wu, D. Visible light-driven photocatalytic performance of N-doped ZnO/g-C3N4 nanocomposites. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Selvam, N.C.S. Enhanced visible light-driven photocatalytic performance of ZnO-g-C3N4 coupled with graphene oxide as a novel ternary nanocomposite. J. Hazard. Mater. 2015, 299, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, Y.; Cheng, X.; Ma, J.; Li, L.; Fan, X.; Ding, Y.; Han, Y.; Tao, R. K+-Doped ZnO/g-C3N4 heterojunction: Controllable preparation, efficient charge separation, and excellent photocatalytic VOC degradation performance. Ind. Eng. Chem. Res. 2021, 61, 187–197. [Google Scholar] [CrossRef]
- Cui, J.; Xu, C.; Jin, Z.; Liu, H.; Hu, R.; Liu, F. Visible light photocatalysis: Efficient Z-scheme LaFeO3/g-C3N4/ZnO photocatalyst for phenol degradation. Environ. Sci. Pollut. Res. 2023, 30, 96875–96890. [Google Scholar] [CrossRef]
- Vignesh, K.; Kang, S.; Kwak, B.S.; Kang, M. Meso-porous ZnO nano-triangles@ graphitic-C3N4 nano-foils: Fabrication and Recyclable photocatalytic activity. Sep. Purif. Technol. 2015, 147, 257–265. [Google Scholar] [CrossRef]
- Habibollahi, Z.; Peyravi, M.; Khalili, S.; Jahanshahi, M. ZnO-based ternary nanocomposite for decolorization of methylene blue by photocatalytic dynamic membrane. Mater. Today Chem. 2022, 23, 100748. [Google Scholar] [CrossRef]
- Javed, M.; Qamar, M.A.; Shahid, S.; Shahid, S.; Alsaab, H.O.; Asif, S. Highly efficient visible light active Cu-ZnO/S-gC3N4 nanocomposites for efficient photocatalytic degradation of organic pollutants. RSC Adv. 2021, 11, 37254–37267. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, Z.; Zou, Y.; Chen, J.; Shi, J. The progress of g-C3N4 in photocatalytic H2 evolution: From fabrication to modification. Coord. Chem. Rev. 2024, 500, 215489. [Google Scholar] [CrossRef]
- Hao, P.; Chen, Z.; Yan, Y.; Shi, W.; Guo, F. Recent advances, application and prospect in g-C3N4-based S-scheme heterojunction photocatalysts. Sep. Purif. Technol. 2024, 330, 125302. [Google Scholar] [CrossRef]
- Rai, R.S.; Bajpai, V.; Khan, M.I.; Khan, M.; Elboughdiri, N.; Shanableh, A.; Luque, R. An eco-friendly approach on green synthesis, bio-engineering applications, and future outlook of ZnO nanomaterial: A critical review. Environ. Res. 2023, 221, 114807. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Naveen, K.V.; Zhang, X.; Han, K.; Wang, M. Research progress on chitosan-zinc oxide nanocomposites fabrication, characterization, biomedical and environmental applications. Coord. Chem. Rev. 2023, 496, 215398. [Google Scholar] [CrossRef]
- Zelekew, O.A.; Haitosa, H.H.; Chen, X.; Wu, Y. Recent progress on plant extract-mediated biosynthesis of ZnO-based nanocatalysts for environmental remediation: Challenges and future outlooks. Adv. Colloid Interface Sci. 2023, 317, 102931. [Google Scholar] [CrossRef]
- Renita, A.A.; Sathish, S.; Kumar, P.S.; Prabu, D.; Manikandan, N.; Iqbal, A.M.; Rajesh, G.; Rangasamy, G. Emerging aspects of metal ions-doped zinc oxide photocatalysts in degradation of organic dyes and pharmaceutical pollutants—A review. J. Environ. Manag. 2023, 344, 118614. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, S.; Dileepan, A.G.B. Treatment of landfill leachate using photocatalytic based advanced oxidation process-a critical review. J. Environ. Manag. 2023, 345, 118794. [Google Scholar] [CrossRef] [PubMed]
- Dineshbabu, N.; Jayaprakash, R.N.; Karuppasamy, P.; Arun, T.; Vijaya, J.J.; Nimshi, R.E.; Pandian, M.S.; Packiam, S.M.; Ramasamy, P. Investigation on Tetracycline degradation and bactericidal properties of binary and ternary ZnO/NiO/g-C3N4 composites prepared by a facile co-precipitation method. J. Environ. Chem. Eng. 2022, 10, 107368. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, J.; Ren, J.; Chen, H.; Tian, X.; Feng, C.; Li, C.; Zhang, J.; Tang, X.; Hou, X. ZnO@g-C3N4 photocatalyst with switchable carrier transfer mechanism between type-II and S-scheme through S doping. J. Alloys Compd. 2024, 982, 173756. [Google Scholar] [CrossRef]
- Qiu, S.; Li, J. High-Efficiency Ag-Modified ZnO/g-C3N4 Photocatalyst with 1D-0D-2D Morphology for Methylene Blue Degradation. Molecules 2024, 29, 2182. [Google Scholar] [CrossRef]
- Cui, J.; Lu, X.; Guo, M.; Zhang, M.; Sun, L.; Xiong, J.; Zhang, R.; Li, X.; Qiao, Y.; Li, D.; et al. Construction of a g-C3N4-driven photocatalytic system for boosted biomass-derived alcohol oxidation: A promising route towards sustainable biomass valorization. Catal. Sci. Technol. 2023, 13, 940–957. [Google Scholar] [CrossRef]
- Zhao, B.; Zhong, W.; Chen, F.; Wang, P.; Bie, C.; Yu, H. High-crystalline g-C3N4 photocatalysts: Synthesis, structure modulation, and H2-evolution application. Chin. J. Catal. 2023, 52, 127–143. [Google Scholar] [CrossRef]

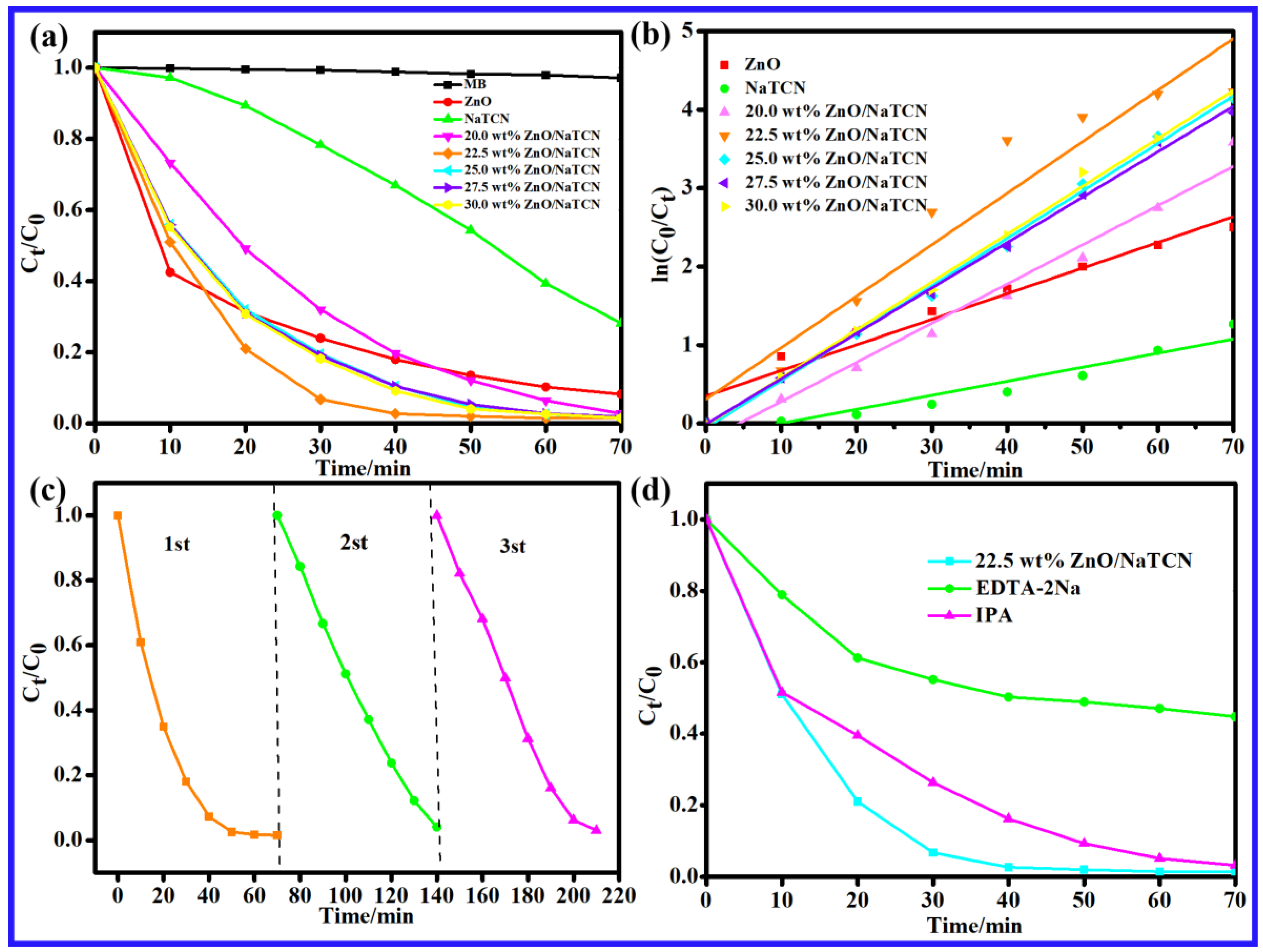
| y = ln(C0/Ct) | R2 | Degradation Rate (%) | |
|---|---|---|---|
| ZnO | y = 0.03264x + 0.34991 | 0.9472 | 91.81% |
| NaTCN | y = 0.0179x − 0.1772 | 0.90563 | 71.83% |
| 20.0 wt% ZnO/NaTCN | y = 0.04995x − 0.21918 | 0.97607 | 97.22% |
| 22.5 wt% ZnO/NaTCN | y = 0.06566x + 0.30978 | 0.91801 | 98.54% |
| 25.0 wt% ZnO/NaTCN | y = 0.06045x − 0.05791 | 0.99636 | 98.42% |
| 27.5 wt% ZnO/NaTCN | y = 0.05789x − 0.00893 | 0.99734 | 98.10% |
| 30.0 wt% ZnO/NaTCN | y = 0.06097x − 0.02469 | 0.9965 | 98.48% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ruan, Z.; Yang, X.; Huang, Y.; Xing, J. Enhancing Performance of Organic Pollutant Degradation via Building Heterojunctions with ZnO Nanowires and Na Doped Conjugated 2,4,6-Triaminopyrimidin-g-C3N4. Molecules 2024, 29, 3240. https://doi.org/10.3390/molecules29133240
Liu Z, Ruan Z, Yang X, Huang Y, Xing J. Enhancing Performance of Organic Pollutant Degradation via Building Heterojunctions with ZnO Nanowires and Na Doped Conjugated 2,4,6-Triaminopyrimidin-g-C3N4. Molecules. 2024; 29(13):3240. https://doi.org/10.3390/molecules29133240
Chicago/Turabian StyleLiu, Ziyi, Zixin Ruan, Xiaojie Yang, Yaqiong Huang, and Jun Xing. 2024. "Enhancing Performance of Organic Pollutant Degradation via Building Heterojunctions with ZnO Nanowires and Na Doped Conjugated 2,4,6-Triaminopyrimidin-g-C3N4" Molecules 29, no. 13: 3240. https://doi.org/10.3390/molecules29133240
APA StyleLiu, Z., Ruan, Z., Yang, X., Huang, Y., & Xing, J. (2024). Enhancing Performance of Organic Pollutant Degradation via Building Heterojunctions with ZnO Nanowires and Na Doped Conjugated 2,4,6-Triaminopyrimidin-g-C3N4. Molecules, 29(13), 3240. https://doi.org/10.3390/molecules29133240





