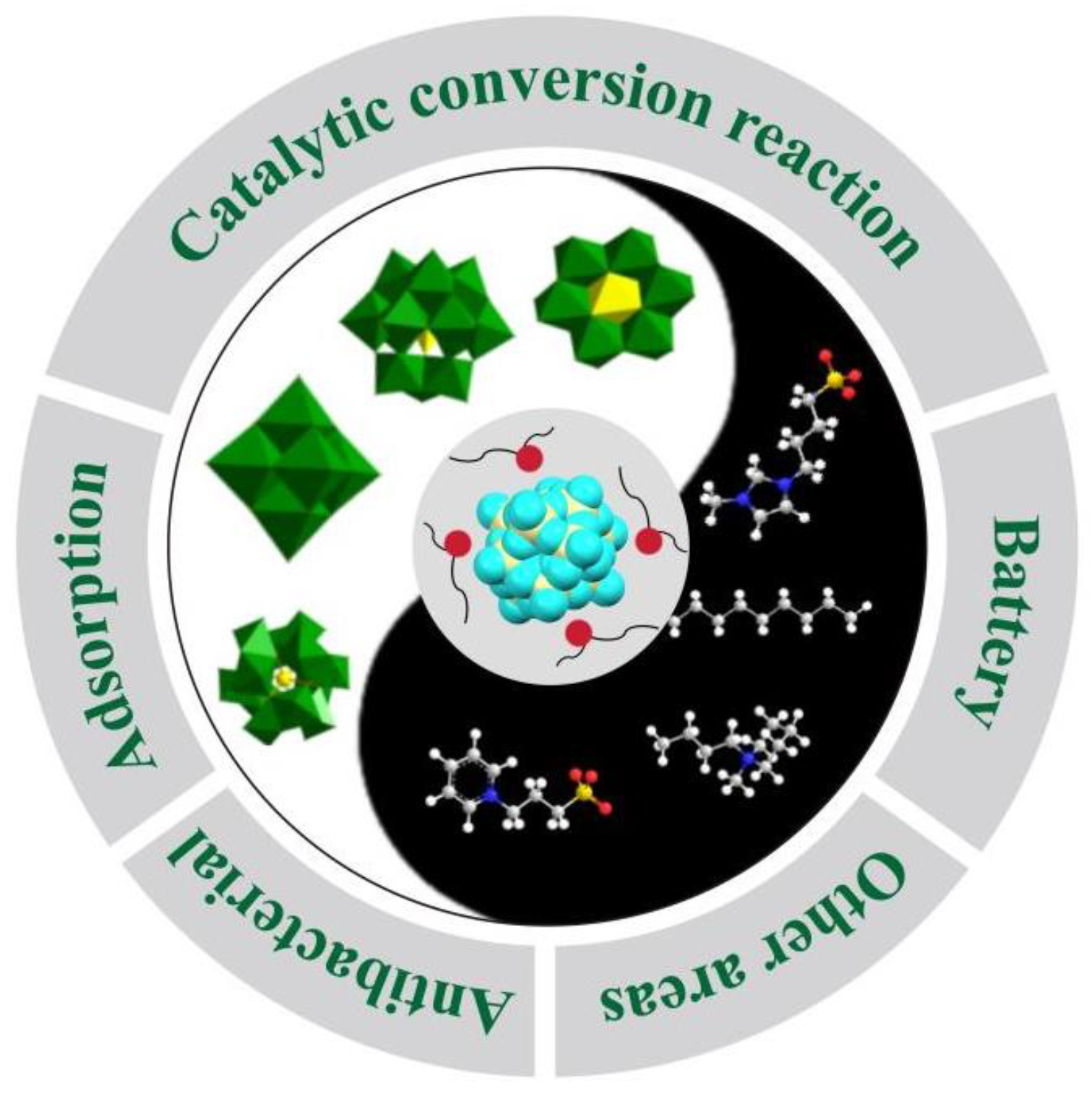
Recent Advances on the Functionalities of Polyoxometalate-Based Ionic Liquids
Abstract
1. Introduction
2. The Applications of POM-ILs in the Oxidation Reactions
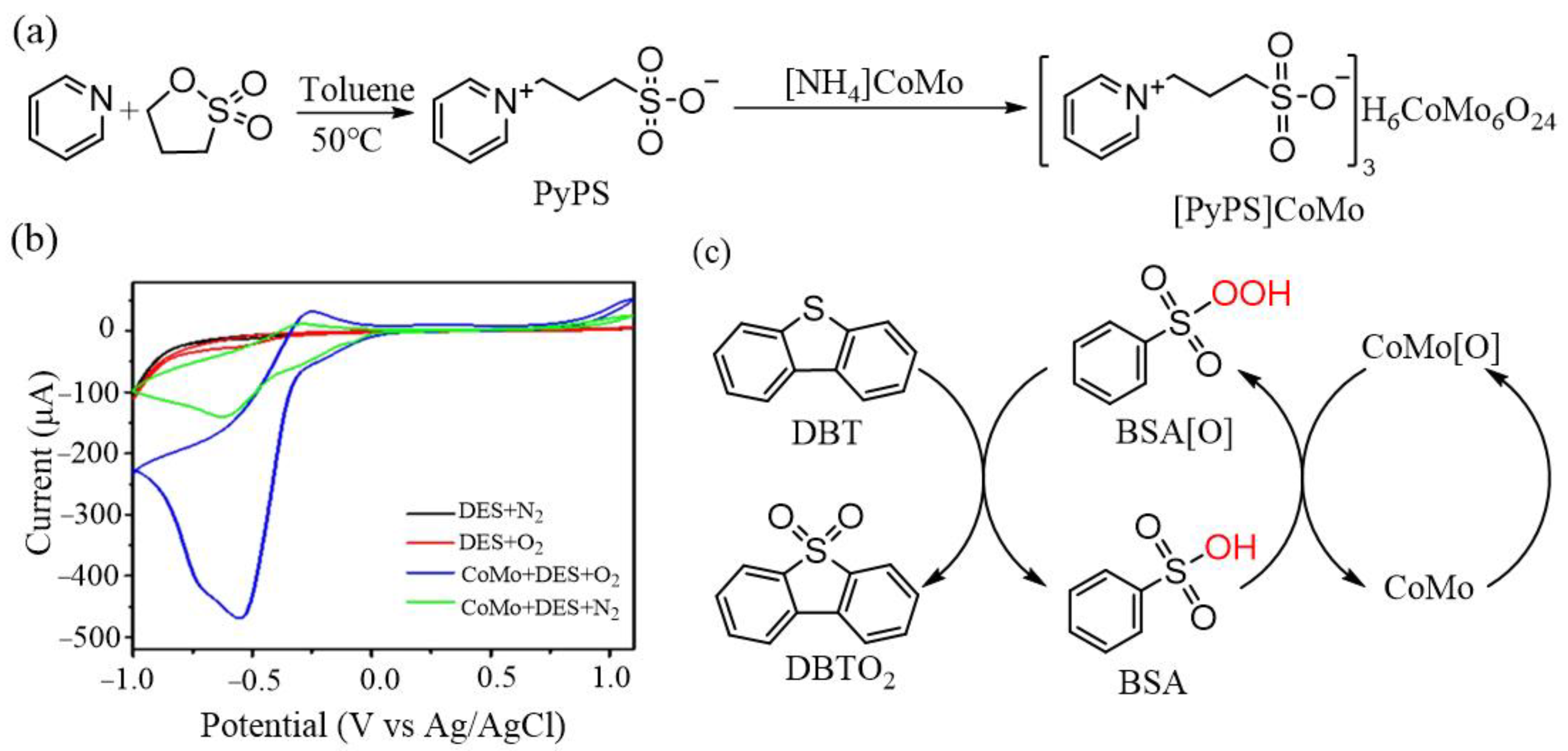
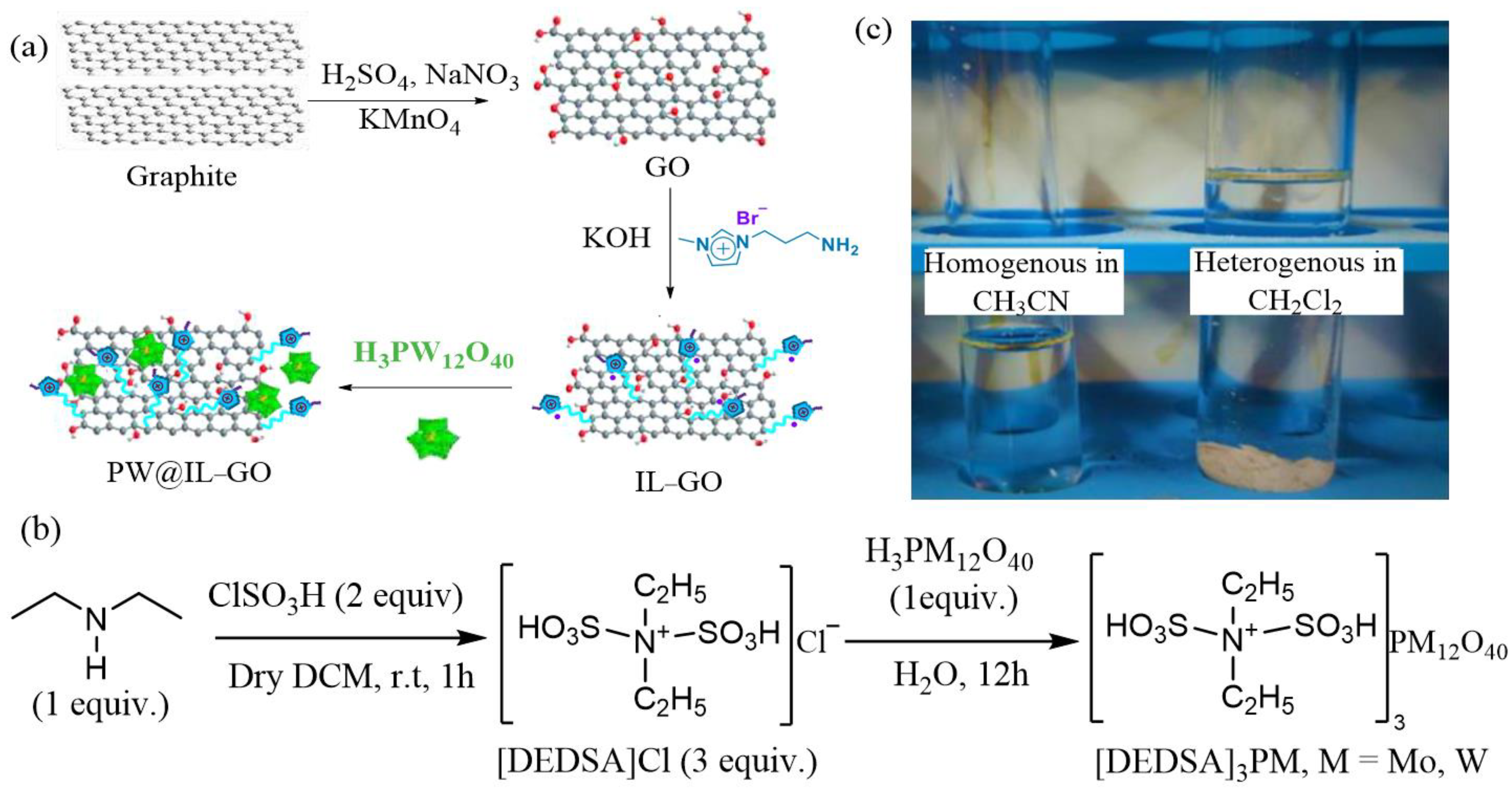
2.1. Oxidative Desulfurization
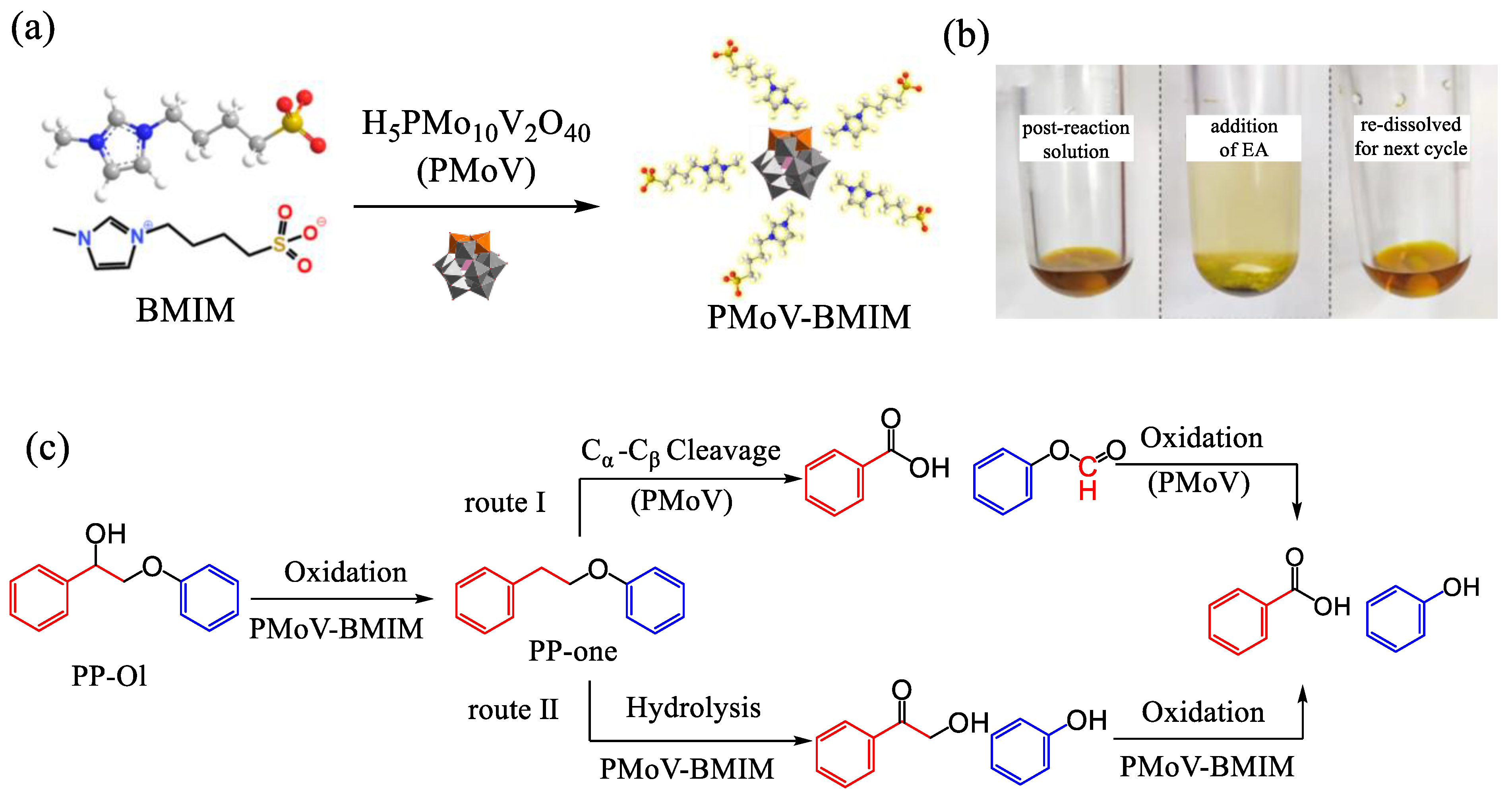
2.2. Alcohol Oxidation
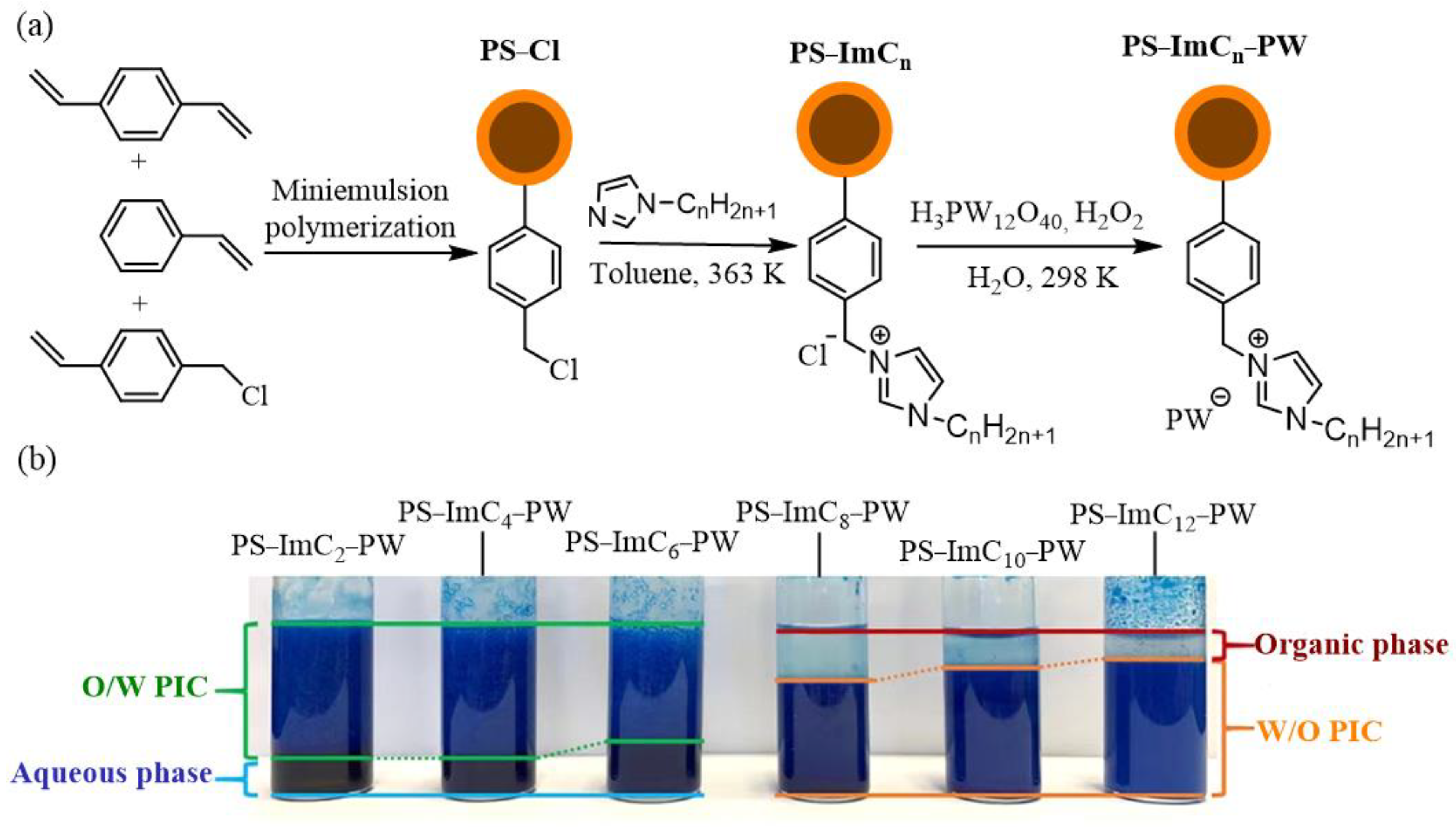
2.3. Olefin Epoxidation
2.4. Oxidative Degradation of Herbicides
3. The Applications of POM-ILs in the Lysis Reaction
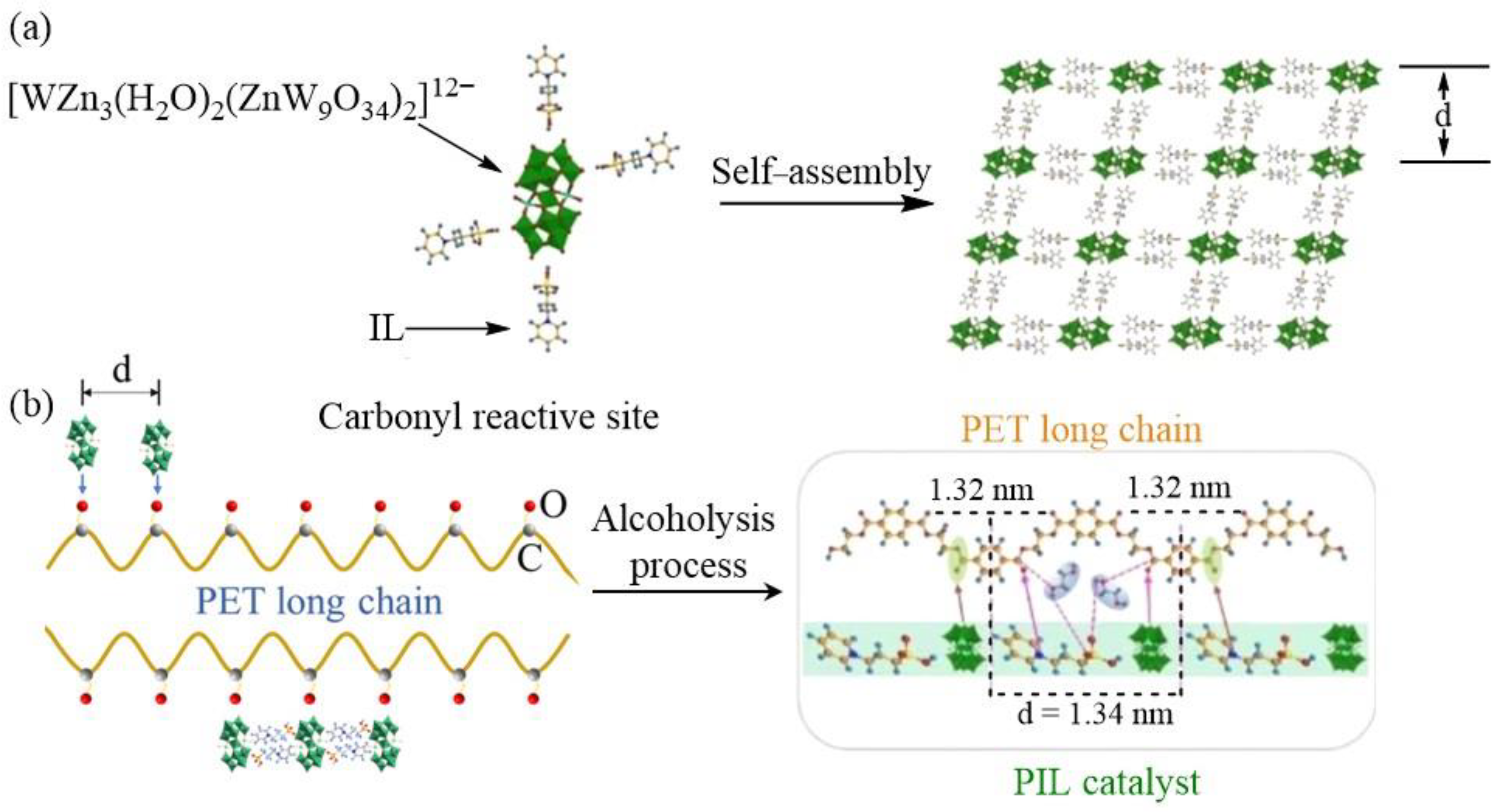
3.1. Degradable Polyethylene Terephthalate
3.2. Lysis of Lignocellulose
4. The Applications of POM-ILs in Other Catalytic Reactions
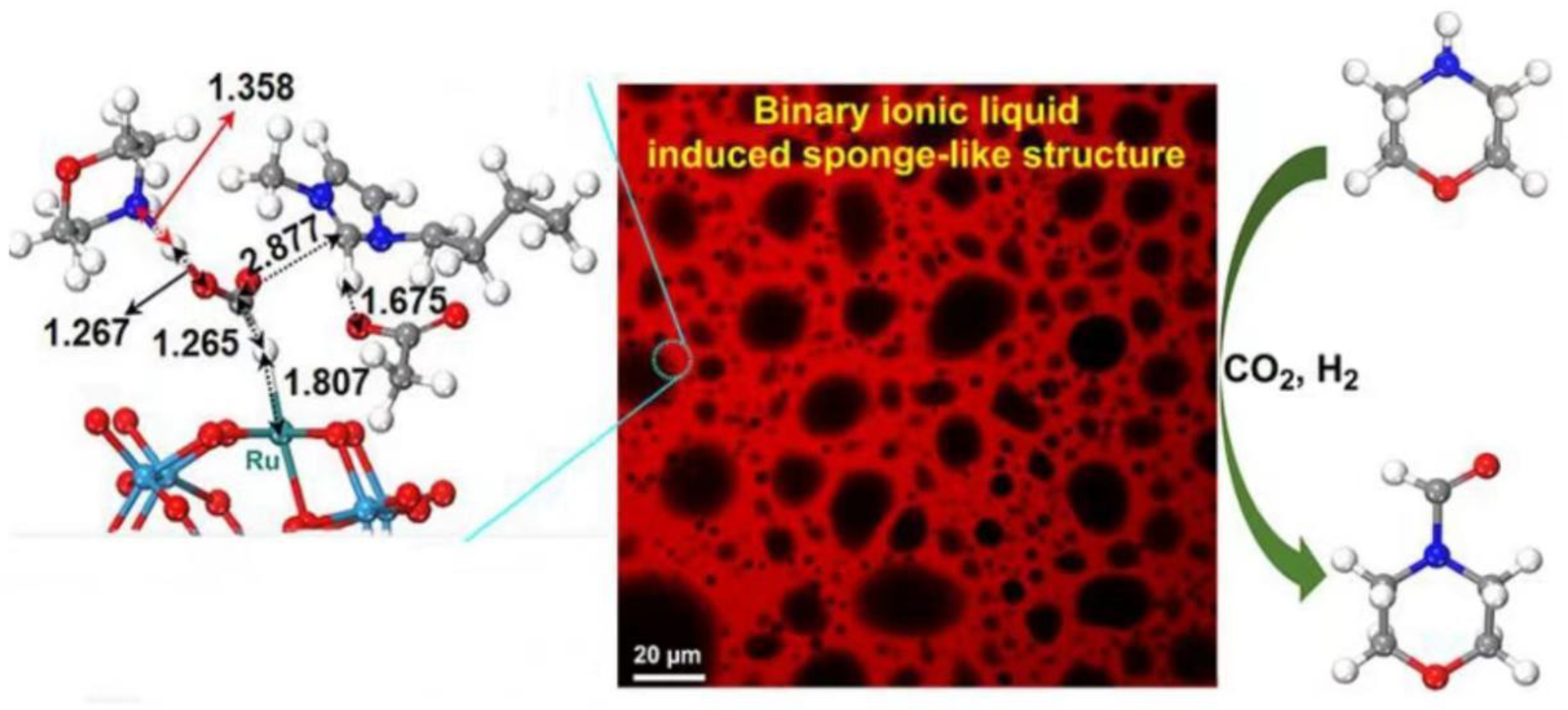
4.1. Formylation
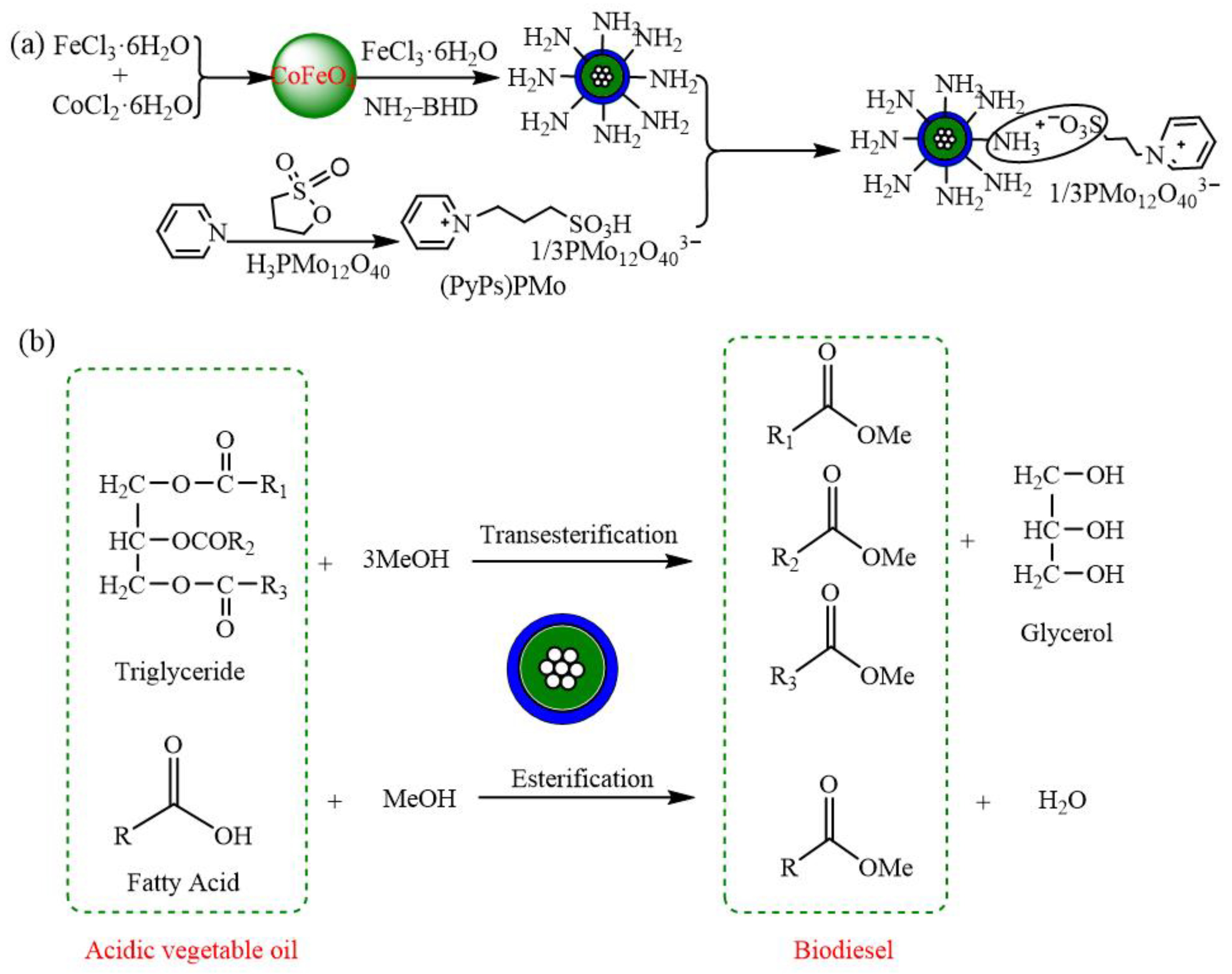
4.2. Esterification Reaction
4.3. Catalytic Synthesis of Aspirin and Paracetamol
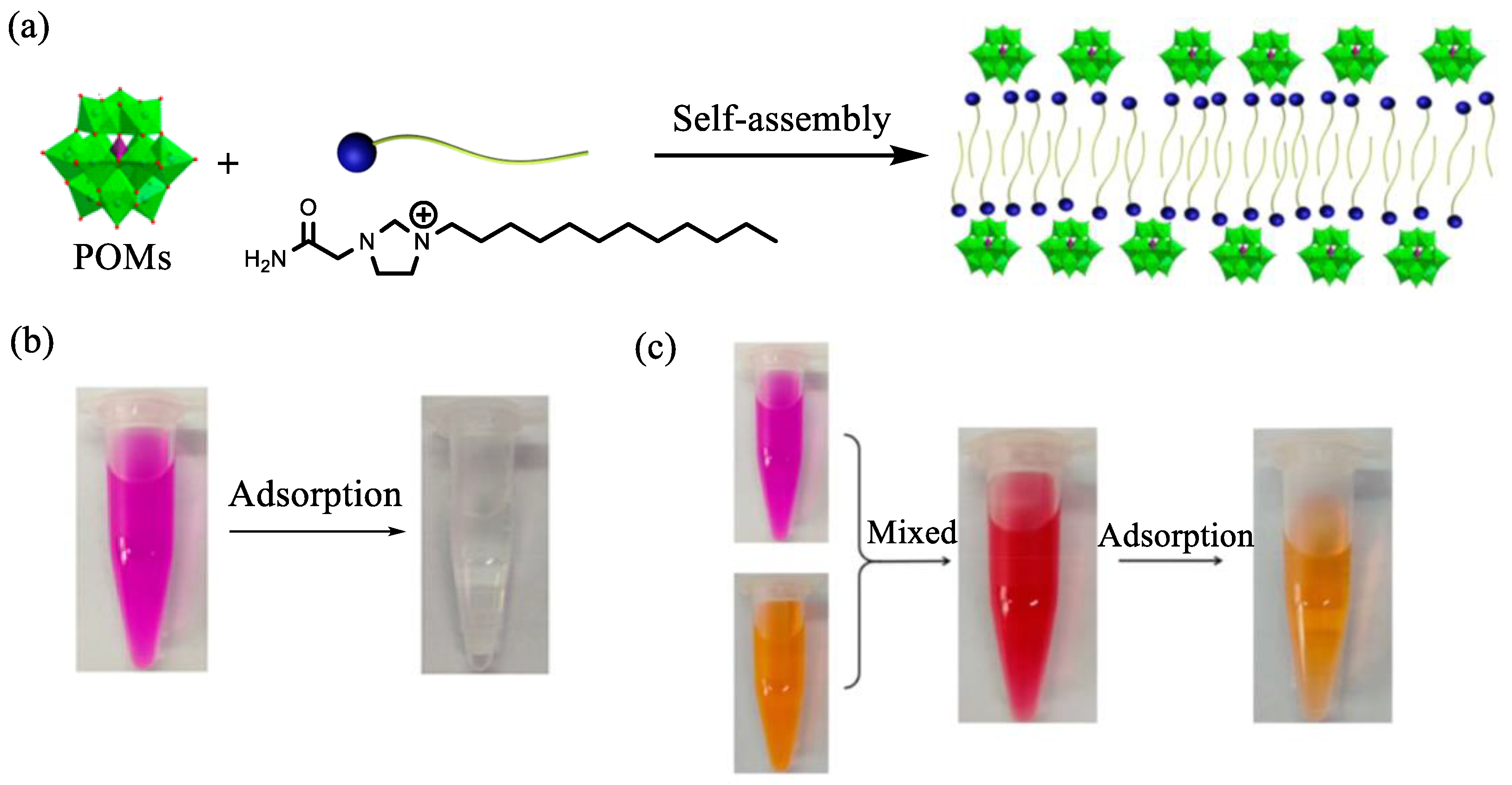
5. The Applications of POM-ILs in Adsorption
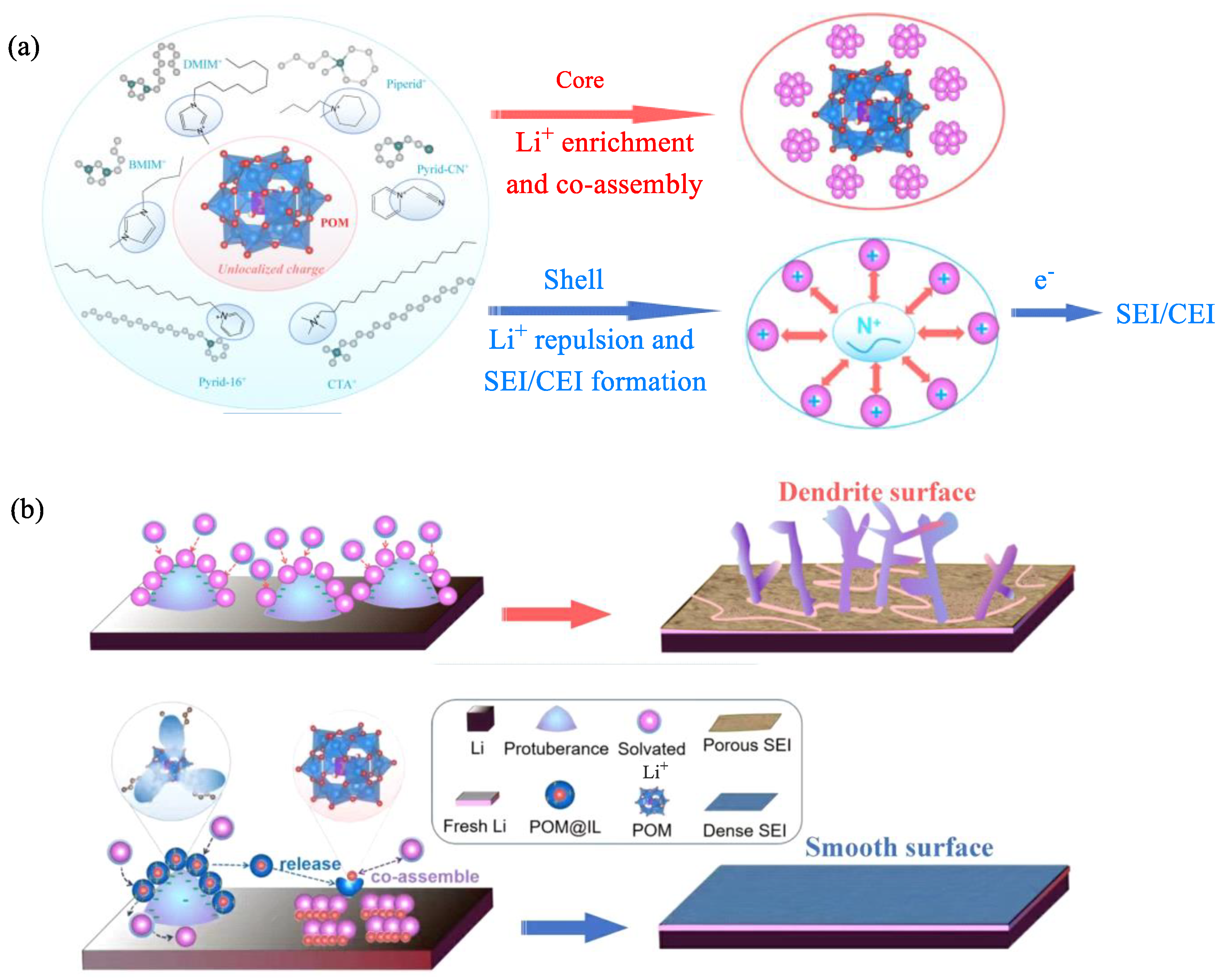
6. The Applications of POM-ILs in Lithium-Ion Batteries
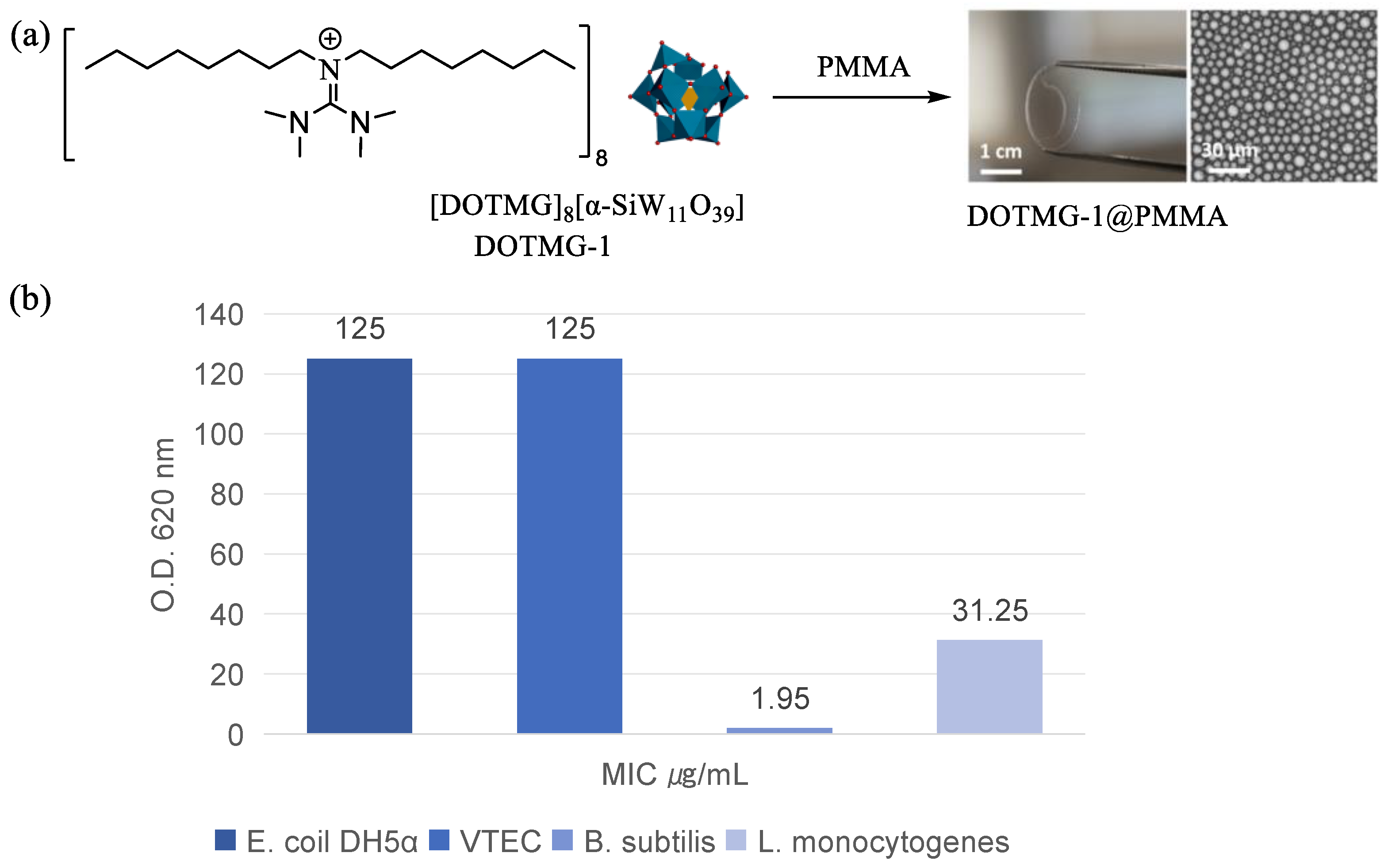
7. The Applications of POM-ILs in Antibacterial
8. The Applications of POM-ILs in Other Areas
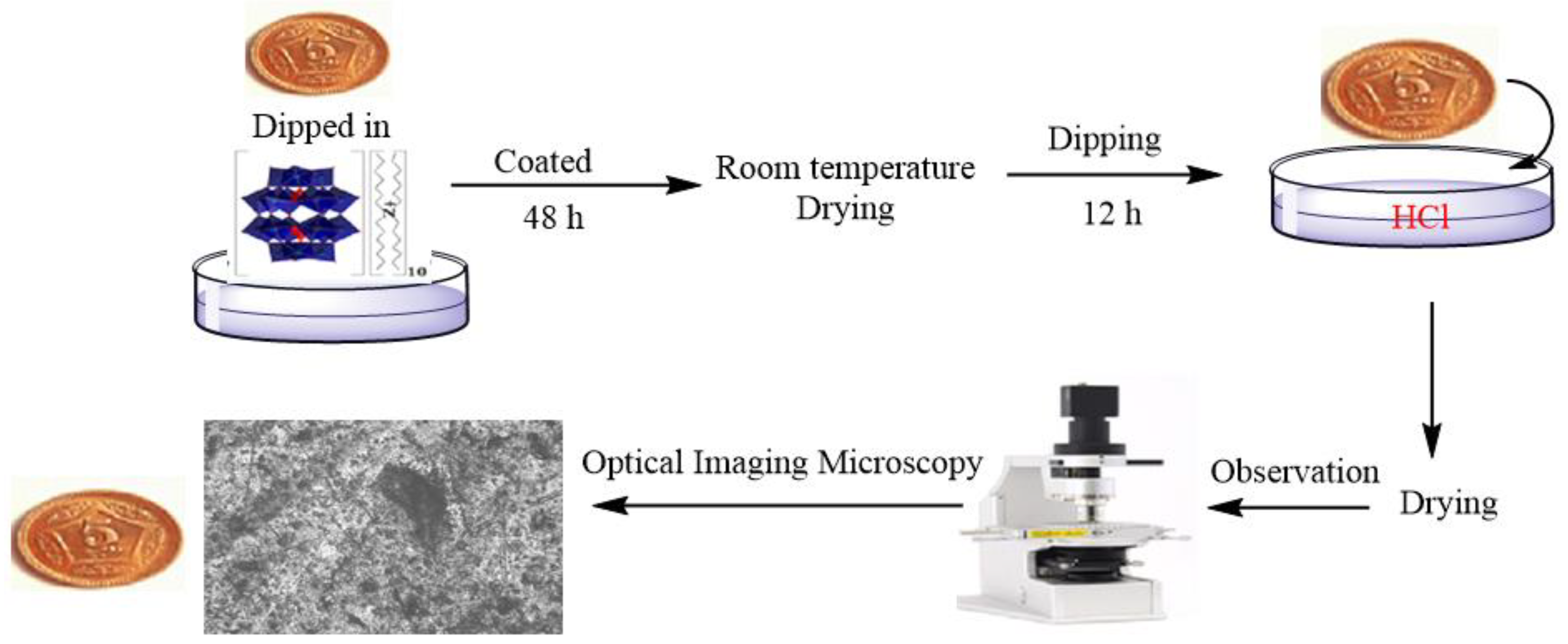
8.1. Anticorrosion
8.2. Solar Cells
9. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DES | Deep eutectic solvents |
| PyPS | 3-(Pyridine-1-ium-1-yl) propane-1-sulfonate |
| CoMo | Co(OH)6Mo6O183− |
| MIM | 1-Alkyl-3-methylimidazolium |
| GO | Graphene oxide |
| DBT | p-Dibenzothiophene |
| Vim | 1-Vinyl-3-amylimidazolium |
| PM | H3PM12O40 (M = Mo, W) |
| DEDSA | Diethyldisulphoammonium |
| DBDSA | Dibutyldisulfoammonium |
| PET | Polyethylene terephthalate |
| BMIM | 1-Butyl-3-methylimidazolium |
| Py | Pyridinium |
| TEAPs | 1-(3-sulfonic group) triethylamine |
| MIMPs | 1-methyl-3-(3-sulfopropyl)imidazolium |
| PMoV | H5PMo10V2O40 |
| BSMIM | Butylsulfonate-3-methylimidazolium |
| PANI | Polyaniline |
| SAILEPs | Surface-active ionic liquid-encapsulated polyoxometalate |
| DDVAC | N,N-dimethyl-dodecyl-(4-vinylbenzyl) ammonium chloride |
| Co4PW | Na10[Co4(H2O)2(PW9O34)2] |
| NCA | LiNi0.8Co0.15Al0.05O2 |
| RGO | Reduced GO |
| DOTMG | N,N,N′,N′-tetramethyl-N″,N″-dioctylguanidinum |
| MIC | Minimum inhibitory concentration |
| PMMA | Poly(methylmethacrylate) |
| SiW11 | [α-SiW11O39]8− |
| P44412, P44414, and P66614 | tribu-tyldodecyl, tributyltetradecyl, and trihexyltetradecyl |
| P2W17 | K10[α2-P2W17O61]∙20H2O |
| P2V3W15 | K8HP2W15V3O62·9H2O |
| TAC | tris(dihexylamino)cyclopropylene |
| PSCs | Perovskite solar cells |
| Li-TFSI | lithium bistrifluoromethane sulfonimide |
| Spiro-OMeTAD | 2,2′,7′-tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene |
References
- Walden, P. Molecular Weights and Electrical Conductivity of Several Fused Salts. Bull. Acad. Imp. Sci. St. Petersbourg 1914, 8, 405–422. [Google Scholar]
- Imam, H.T.; Krasňan, V.; Rebroš, M.; Marr, A.C. Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules 2021, 26, 4791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, X.; Yao, H.; Zhou, Q.; Xin, J.; Lu, X.; Zhang, S. Degradation of poly(ethylene terephthalate) catalyzed by metal-free choline-based ionic liquids. Green Chem. 2020, 22, 3122–3131. [Google Scholar] [CrossRef]
- Silva, R.M.A.; Montes-Campos, H.; Lobo Ferreira, A.I.M.C.; Bakis, E.; Santos, L.M.N.B.F. Thermodynamic Study of Alkylsilane and Alkylsiloxane-Based Ionic Liquids. J. Phys. Chem. B 2024, 128, 3742–3754. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, O.; Kultin, D.; Kustov, L. Advanced research and prospects on polymer ionic liquids: Trends, potential and application. Green Chem. 2023, 25, 9001–9019. [Google Scholar] [CrossRef]
- Deng, H.; Wang, X.; Chen, J.; Zhao, J.; Jiang, Z.; Tian, Z.; Du, P.; Li, Y. Experimental and molecular dynamics study of fuel desulfurization process using deep eutectic solvent. J. Environ. Chem. Eng. 2023, 11, 110277. [Google Scholar] [CrossRef]
- El-hoshoudy, A.N.; Soliman, F.S.; Abd El-Aty, D.M. Extractive desulfurization using choline chloride-based DES/molybdate nanofluids; Experimental and theoretical investigation. J. Mol. Liq. 2020, 318, 114307. [Google Scholar] [CrossRef]
- Wang, H.; Kang, X.; Han, B. Electrocatalysis in deep eutectic solvents: From fundamental properties to applications. Chem. Sci. 2024, 15, 9949–9976. [Google Scholar] [CrossRef] [PubMed]
- Protsenko, V.S.; Bobrova, L.S.; Korniy, S.A.; Danilov, F.I. Electrochemical synthesis and characterization of electrocatalytic materials for hydrogen production using Cr(III) baths based on a deep eutectic solvent. Mater. Lett. 2022, 313, 131800. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rew. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Li, D.; Ma, P.; Niu, J.; Wang, J. Recent advances in transition-metal-containing Keggin-type polyoxometalate-based coordination polymers. Coord. Chem. Rev. 2019, 392, 49–80. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Yu, H.; Han, S.; Wei, Y. Recent Advances of Anderson-Type Polyoxometalates as Catalysts Largely for Oxidative Transformations of Organic Molecules. Molecules 2022, 27, 5212. [Google Scholar] [CrossRef] [PubMed]
- Anyushin, A.V.; Vanhaecht, S.; Parac-Vogt, T.N. A Bis-organosilyl-Functionalized Wells–Dawson Polyoxometalate as a Platform for Facile Amine Postfunctionalization. Inorg. Chem. 2020, 59, 10146–10152. [Google Scholar] [CrossRef]
- Wang, C.; Dai, Z.; Zhang, Q.; Li, X.; Ma, M.; Shi, Z.; Zhang, J.; Liu, Q.; Chen, H. A bifunctional biomineralized polyoxometalate enabling efficient Non-Inflammatory NIR-II photothermal tumor therapy. Chem. Eng. J. 2024, 490, 151601. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Dong, B.X.; Lan, Y.Q. Polyoxometalate-Based Compounds for Photo- and Electrocatalytic Applications. Angew. Chem. Int. Ed. 2020, 59, 20779–20793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, T.; Shao, H.; Li, F.; Li, D.; Yang, Y.; Yu, H.; Dong, X. First one-dimensional Cu2ZnSnS4-based gas sensor and enhanced performance at room temperature by polyoxometalate electron acceptor. Sens. Actuator B-Chem. 2023, 380, 133405. [Google Scholar] [CrossRef]
- Goura, J.; Bassil, B.S.; Bindra, J.K.; Rutkowska, I.A.; Kulesza, P.J.; Dalal, N.S.; Kortz, U. FeIII48-Containing 96-Tungsto-16-Phosphate: Synthesis, Structure, Magnetism and Electrochemistry. Chem.-Eur. J. 2020, 26, 15821–15824. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, J.; Li, B.; Wu, L. Organic-Cation Modulated Assembly Behaviors of a Ureidopyrimidone-Grafting Cluster. Molecules 2023, 28, 3677. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, F.; Liu, X.; Li, B. Cations Modulated Assembly of Triol-Ligand Modified Cu-Centered Anderson-Evans Polyanions. Molecules 2022, 27, 2933. [Google Scholar] [CrossRef]
- Chang, T.; Qu, D.; Li, B.; Wu, L. Organic/Inorganic Species Synergistically Supported Unprecedented Vanadomolybdates. Molecules 2022, 27, 7447. [Google Scholar] [CrossRef]
- Guan, W.; Wang, G.; Li, B.; Wu, L. Organic macrocycle-polyoxometalate hybrids. Coord. Chem. Rev. 2023, 481, 215039. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Raman, K.; Herrera, R.; Zhang, Q.; Archer, L.A.; Giannelis, E.P. A Liquid Derivative of 12-Tungstophosphoric Acid with Unusually High Conductivity. J. Am. Chem. Soc. 2004, 126, 15358–15359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, Z.; Hou, W.; Wang, Q.; Wang, J. Polyoxometalate-based phase transfer catalysis for liquid–solid organic reactions: A review. Catal. Sci. Technol. 2015, 5, 4324–4335. [Google Scholar] [CrossRef]
- Gao, Y.; Choudhari, M.; Such, G.K.; Ritchie, C. Polyoxometalates as chemically and structurally versatile components in self-assembled materials. Chem. Sci. 2022, 13, 2510–2527. [Google Scholar] [CrossRef]
- Misra, A.; Kozma, K.; Streb, C.; Nyman, M. Beyond Charge Balance: Counter-Cations in Polyoxometalate Chemistry. Angew. Chem. Int. Ed. 2019, 59, 596–612. [Google Scholar] [CrossRef]
- Ahmadian, M.; Anbia, M. Oxidative Desulfurization of Liquid Fuels Using Polyoxometalate-Based Catalysts: A Review. Energy Fuels 2021, 35, 10347–10373. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Mehrvarz, E.; Taghipour, A. Polyoxometalate as an effective catalyst for the oxidative desulfurization of liquid fuels: A critical review. Rev. Chem. Eng. 2020, 36, 831–858. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, S.; Jin, Q.; Zhao, J. Review on oxidative desulfurization of fuel by supported heteropolyacid catalysts. J. Ind. Eng. Chem. 2020, 82, 1–16. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, S. Polyoxometalate as an Effective Catalyst for Catalytic Lignin into Value-Added Molecules. ChemCatChem 2024, 16, e202301204. [Google Scholar] [CrossRef]
- Qiao, Y.; Shi, E.; Wei, X.; Hou, Z. Ionic liquid-stabilized metal oxoclusters: From design to catalytic application. Green Chem. 2024, 26, 5127–5149. [Google Scholar] [CrossRef]
- Berardi, S.; Carraro, M.; Sartorel, A.; Modugno, G.; Bonchio, M. Hybrid Polyoxometalates: Merging Organic and Inorganic Domains for Enhanced Catalysis and Energy Applications. Isr. J. Chem. 2011, 51, 259–274. [Google Scholar] [CrossRef]
- Han, Z.; Bond, A.M.; Zhao, C. Recent trends in the use of polyoxometalate-based material for efficient water oxidation. Sci. China Chem. 2011, 54, 1877–1887. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Ma, R.; He, L.-N. Polyoxometalate-based ionic liquids-promoted CO2 conversion. Sci. China Chem. 2016, 59, 507–516. [Google Scholar] [CrossRef]
- Martinetto, Y.; Pégot, B.; Roch-Marchal, C.; Cottyn-Boitte, B.; Floquet, S. Designing Functional Polyoxometalate-Based Ionic Liquid Crystals and Ionic Liquids. Eur. J. Inorg. Chem. 2019, 2020, 228–247. [Google Scholar] [CrossRef]
- Nogueira, L.S.; Ribeiro, S.; Granadeiro, C.M.; Pereira, E.; Feio, G.; Cunha-Silva, L.; Balula, S.S. Novel polyoxometalate silica nano-sized spheres: Efficient catalysts for olefin oxidation and the deep desulfurization process. Dalton Trans. 2014, 43, 9518–9528. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Leal, B.C.; Lozano, P.; Monteiro, A.L.; Migowski, P.; Scholten, J.D. Ionic Liquids in Metal, Photo-, Electro-, and (Bio) Catalysis. Chem. Rew. 2024, 124, 5227–5420. [Google Scholar] [CrossRef]
- Liang, J.; Wang, W.; Wu, W.; Wu, M.; Hua, J.; Liu, Y.; Liu, C. Cationic Exchange of Evans-Showell Polyoxometalate to Construct Efficient Hydrodesulfurization Catalyst. ChemistrySelect 2023, 8, e202300004. [Google Scholar] [CrossRef]
- Lin, R.; Pan, H.; Xu, W.; Zhang, L.; Wang, X.; Zhang, J.; Chen, K. Hydrodesulfurization of benzothiophene on Ni2P surface. Energy Explor. Exploit. 2020, 38, 2711–2728. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Li, H.; Wei, Y.; Fu, Y.; Liao, W.; Zhu, L.; Chen, G.; Zhu, W.; Li, H. Tuning the electrophilicity of vanadium-substituted polyoxometalate based ionic liquids for high-efficiency aerobic oxidative desulfurization. Appl. Catal. B-Environ. 2020, 271, 118936. [Google Scholar] [CrossRef]
- Xing, X.-X.; Guo, H.-L.; He, T.-M.; An, X.; Li, H.-P.; Zhu, W.-S.; Li, H.-M.; Pang, J.-Y.; Dang, D.-B.; Bai, Y. Tungstovanadate-Based Ionic Liquid Catalyst [C2(MIM)2]2VW12O40 Used in Deep Desulfurization for Ultraclean Fuel with Simultaneous Recovery of the Sulfone Product. ACS Sustain. Chem. Eng. 2022, 10, 11533–11543. [Google Scholar] [CrossRef]
- de Rink, R.; Klok, J.B.M.; van Heeringen, G.J.; Sorokin, D.Y.; ter Heijne, A.; Zeijlmaker, R.; Mos, Y.M.; de Wilde, V.; Keesman, K.J.; Buisman, C.J.N. Increasing the Selectivity for Sulfur Formation in Biological Gas Desulfurization. Environ. Sci. Technol. 2019, 53, 4519–4527. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, L.; Zhao, Q.; Peng, Y. The effect of biofilm growth on the sulfur oxidation pathway and the synergy of microorganisms in desulfurization reactors under different pH conditions. J. Hazard. Mater. 2022, 432, 128638. [Google Scholar] [CrossRef]
- Li, J.; Lei, X.-J.; Tang, X.-D.; Zhang, X.-P.; Wang, Z.-Y.; Jiao, S. Acid Dicationic Ionic Liquids as Extractants for Extractive Desulfurization. Energy Fuels 2019, 33, 4079–4088. [Google Scholar] [CrossRef]
- Cheng, H.; Cui, Y.; Ge, Z.; Wang, R.; Qin, Z.; Chen, L.; Qi, Z. Insight into the mechanism of tuned extractive desulfurization by aqueous tetrabutylphosphonium bromide. Sep. Purif. Technol. 2021, 262, 118342. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, H.; Ye, Z.; Huang, Q.; Chen, X. Adsorption desulfurization performance and adsorption-diffusion study of B2O3 modified Ag-CeOx/TiO2-SiO2. J. Hazard. Mater. 2019, 362, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Zhao, J.; Liu, Y.; Liu, C. Ultra-deep desulfurization by reactive adsorption desulfurization on copper-based catalysts. J. Energy Chem. 2019, 29, 8–16. [Google Scholar] [CrossRef]
- Piera, J.; Bäckvall, J.E. Catalytic Oxidation of Organic Substrates by Molecular Oxygen and Hydrogen Peroxide by Multistep Electron Transfer—A Biomimetic Approach. Angew. Chem. Int. Ed. 2008, 47, 3506–3523. [Google Scholar] [CrossRef]
- Chi, M.; Su, T.; Sun, L.; Zhu, Z.; Liao, W.; Ren, W.; Zhao, Y.; Lü, H. Biomimetic oxygen activation and electron transfer mechanism for oxidative desulfurization. Appl. Catal. B-Environ. 2020, 275, 119134. [Google Scholar] [CrossRef]
- Xing, X.-X.; Guo, H.-L.; Feng, T.; He, T.-M.; Zhu, W.-S.; Li, H.-M.; Pang, J.-Y.; Bai, Y.; Dang, D.-B. Design and Synthesis of Amphiphilic Catalyst [C16mim]5VW12O40Br and Its Application in Deep Desulfurization with Superior Cyclability at Room Temperature. Inorg. Chem. 2023, 62, 5780–5790. [Google Scholar] [CrossRef]
- Mohammadi-Nejati, F.; Shahhosseini, S. Covalent immobilization of POM-based ILs on magnetic graphene oxide for efficient catalytic oxidative desulfurization of model fuel under solvent-free and moderate reaction conditions. Fuel Process. Technol. 2023, 252, 107980. [Google Scholar] [CrossRef]
- Mao, S.-X.; Zhou, Q.-H.; Guo, H.-L.; Du, M.; Zhu, W.-S.; Li, H.-M.; Pang, J.-Y.; Dang, D.-B.; Bai, Y. Porous phosphomolybdate-based poly(ionic liquid) hybrids with reversible water absorption for enhancement of oxidative desulfurization. Fuel 2023, 333, 126392. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, L.; Gao, R.; Hu, G.; Zhao, J. Deep desulfurization of fuels using supported ionic liquid-polyoxometalate hybrid as catalyst: A comparison of different types of ionic liquids. J. Hazard. Mater. 2021, 401, 123267. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lv, Z.; Gao, R.; Hu, G.; Zhao, J. Dawson type polyoxometalate based-poly ionic liquid supported on different carbon materials for high-efficiency oxidative desulfurization with molecular oxygen as the oxidant. New J. Chem. 2020, 44, 20358–20366. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, L.; Liu, Z.; Gao, R.; Hu, G.; Zhao, J. Poly(ionic liquid)–polyoxometalate/graphene oxide composites as catalysts for deep desulfurization. New J. Chem. 2022, 46, 756–766. [Google Scholar] [CrossRef]
- Li, S.-W.; Wang, W.; Zhao, J.-S. The quantity and type of ILs needed to form magnetic-heteropolyacid mesoporous catalysts and their highly performance for DBT removal. Sustain. Energ. Fuels 2020, 4, 2422–2437. [Google Scholar] [CrossRef]
- Mao, S.-X.; Song, J.-Y.; Zhu, W.-S.; Li, H.-M.; Pang, J.-Y.; Dang, D.-B.; Bai, Y. Heterogeneous oxidative desulfurization of fuels using amphiphilic mesoporous phosphomolybdate-based poly(ionic liquid) over a wide temperature range. Fuel 2023, 352, 128982. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480. [Google Scholar] [CrossRef]
- Wang, J.-X.; Zhou, X.-T.; Han, Q.; Guo, X.-X.; Liu, X.-H.; Xue, C.; Ji, H.-B. Efficient and selective oxidation of alcohols to carbonyl compounds at room temperature by a ruthenium complex catalyst and hydrogen peroxide. New J. Chem. 2019, 43, 19415–19421. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, M.; Yang, C.; Chen, Y.; Tan, R.; Yin, D. Alcohols selective oxidation with H2O2 catalyzed by robust heteropolyanions intercalated in ionic liquid-functionalized graphene oxide. Mater. Chem. Phys. 2020, 256, 123681. [Google Scholar] [CrossRef]
- Kashyap, N.; Das, S.; Borah, R. Solvent responsive self-separation behaviour of Brønsted acidic ionic liquid-polyoxometalate hybrid catalysts on H2O2 mediated oxidation of alcohols. Polyhedron 2021, 196, 114993. [Google Scholar] [CrossRef]
- Zhang, S.; Hong, B.; Fan, Z.; Lu, J.; Xu, Y.; Pera-Titus, M. Aquivion–Carbon Composites with Tunable Amphiphilicity for Pickering Interfacial Catalysis. ACS Appl. Mater. Interfaces 2018, 10, 26795–26804. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, Y.; Wang, Y.; Wu, F.; Zhou, Z.; Zhang, Z. Polyoxometalate-Supported Ionic Liquid@Core–Shell Polymer Nanoparticles: Novel Pickering Interfacial Catalysts for Efficient and Safe Epoxidation of Allyl Chloride with Low-Concentration H2O2. ACS Sustain. Chem. Eng. 2023, 11, 12934–12945. [Google Scholar] [CrossRef]
- Kashyap, N.; Kalita, S.; Bora, D.B.; Das, S.; Yashmin, F.; Guha, A.K.; Borah, R. A mechanistic study on solar energized degradation of herbicide into value-added product using -SO3H functionalized ionic liquid-polyoxometalate based heterogeneous catalyst in aqueous medium. J. Mol. Struct. 2024, 1311, 138372. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Recent Developments in the Chemical Recycling of Postconsumer Poly(ethylene terephthalate) Waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar] [CrossRef]
- Liao, Z.; Duan, Y.; Guo, L.; Zheng, R.; Wang, L.; Chen, Y.; Zhang, L.; Qian, X. Preparation of a heteropoly acid ionic liquid and its application in the catalytic degradation of bottle-grade PET. New J. Chem. 2023, 47, 4337–4345. [Google Scholar] [CrossRef]
- Fang, P.; Zheng, X.; Zhang, R.; Xu, J.; Yan, D.; Zhou, Q.; Xin, J.; Shi, C.; Xia, S.; Lu, X. Accurate Layer Spacing Matching of Polyoxometalate (POM) Anion-based Ionic Liquids (ILs) to Promote PET Alcoholysis. ChemCatChem 2023, 15, e202200712. [Google Scholar] [CrossRef]
- Wang, M.; Wang, F. Catalytic Scissoring of Lignin into Aryl Monomers. Adv. Mater. 2019, 31, 1901866. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Gupta, N.K.; Sels, B.; Ralph, J.; Shuai, L. Protection Strategies Enable Selective Conversion of Biomass. Angew. Chem. Int. Ed. 2020, 59, 11704–11716. [Google Scholar] [CrossRef]
- Xin, X.; Li, Z.; Chi, M.; Zhang, M.; Dong, Y.; Lv, H.; Yang, G.-Y. A recoverable polyoxometalate-ionic liquid catalyst for selective cleavage of lignin β-O-4 models under mild conditions. Green Chem. 2023, 25, 2815–2824. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, R.; Zhang, H.; Li, F.; Long, J.; Jiang, L.; Li, X. One-pot production of diethyl maleate via catalytic conversion of raw lignocellulosic biomass. Green Chem. 2021, 23, 10116–10122. [Google Scholar] [CrossRef]
- Dai, X.; Wang, B.; Wang, A.; Shi, F. Amine formylation with CO2 and H2 catalyzed by heterogeneous Pd/PAL catalyst. Chin. J. Catal. 2019, 40, 1141–1146. [Google Scholar] [CrossRef]
- Liao, H.; Chen, M.; Ma, Y.; Peng, Q.; Wei, X.; Hou, Z. Solvent-Assisted Ruthenium Complex Catalyzes Hydrogenation and the Reductive Amination of Carbon Dioxide. Ind. Eng. Chem. Res. 2022, 61, 15156–15168. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Sun, M.-J.; Liu, C.-G. CO oxidation on the phosphotungstic acid supported Rh single–atom catalysts via Rh–assisted Mans–van Krevelen mechanism. Mol. Catal. 2019, 462, 37–45. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, C.; Jiang, Y.; Wei, X.; Liu, Y.; Liao, H.; Wang, H.; Dai, S.; An, P.; Hou, Z. Ruthenium Single-Atom Anchored in Polyoxometalate-Ionic Liquids for N-Formylation of Amines with CO2 and H2. ACS Catal. 2023, 13, 10295–10308. [Google Scholar] [CrossRef]
- de Lima, A.L.; Ronconi, C.M.; Mota, C.J.A. Heterogeneous basic catalysts for biodiesel production. Catal. Sci. Technol. 2016, 6, 2877–2891. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Rafiee, E.; Eavani, S. Heterogenization of heteropoly compounds: A review of their structure and synthesis. RSC Adv. 2016, 6, 46433–46466. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Synthesis of heterogenized polyoxometalate-based ionic liquids with Brönsted-Lewis acid sites: A magnetically recyclable catalyst for biodiesel production from low-quality oils. J. Ind. Eng. Chem. 2020, 87, 162–172. [Google Scholar] [CrossRef]
- Jung, S.; Tsukuda, Y.; Kawashima, R.; Ishiki, T.; Matsumoto, A.; Nakaniwa, A.; Takagi, M.; Noguchi, T.; Imai, N. Convenient synthesis of acetaminophen analogues containing α-amino acids and fatty acids via their mixed carbonic carboxylic anhydrides in aqueous organic solvent. Tetrahedron Lett. 2013, 54, 5718–5720. [Google Scholar] [CrossRef]
- Majedi, A.; Davar, F.; Abbasi, A. Sucrose-mediated sol–gel synthesis of nanosized pure and S-doped zirconia and its catalytic activity for the synthesis of acetyl salicylic acid. J. Ind. Eng. Chem. 2014, 20, 4215–4223. [Google Scholar] [CrossRef]
- Maleki, A.; Azizi, M.; Emdadi, Z. A novel poly(ethyleneoxide)-based magnetic nanocomposite catalyst for highly efficient multicomponent synthesis of pyran derivatives. Green Chem. Lett. Rev. 2018, 11, 573–582. [Google Scholar] [CrossRef]
- Nasiri, E.; Kooshki, F.; Kooti, M.; Rezaeinasab, R. Functionalized nanomagnetic graphene by ion liquid containing phosphomolybdic acid for facile and fast synthesis of paracetamol and aspirin. Appl. Organomet. Chem. 2021, 35, e6413. [Google Scholar] [CrossRef]
- Duan, F.; Liu, X.; Qu, D.; Li, B.; Wu, L. Polyoxometalate-Based Ionic Frameworks for Highly Selective CO2 Capture and Separation. CCS Chem. 2021, 3, 2676–2687. [Google Scholar] [CrossRef]
- Mohammadi, M.D.; Abbas, F.; Louis, H.; Mathias, G.E.; Unimuke, T.O. Trapping of CO, CO2, H2S, NH3, NO, NO2, and SO2 by polyoxometalate compound. Comput. Theor. Chem. 2022, 1215, 113826. [Google Scholar] [CrossRef]
- Ranjbari, S.; Ayati, A.; Niknam Shahrak, M.; Tanhaei, B.; Hamidi Tabrizi, S. Design of [BmIm]3PW12O40 Ionic Liquid Encapsulated-ZIF-8 Nanocomposite for Cationic Dye Adsorptive Removal: Modeling by Response Surface Methodology. Ind. Eng. Chem. Res. 2023, 62, 4636–4645. [Google Scholar] [CrossRef]
- Qi, L.; Gong, Y.; Fang, M.; Jia, Z.; Cheng, N.; Yu, L. Surface-Active Ionic-Liquid-Encapsulated Polyoxometalate Nanospheres: Construction, Self-Assembly, Adsorption Behavior, and Application for Dye Removal. ACS Appl. Nano Mater. 2020, 3, 375–383. [Google Scholar] [CrossRef]
- Yang, J.; Chu, N.; Chen, X. Preparation of Polyoxometalate-Based Composite by Solidification of Highly Active Cobalt-Containing Polytungstate on Polymeric Ionic Liquid for the Efficient Isolation of Proteinase K. Molecules 2023, 28, 3307. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rew. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Eshetu, G.G.; Judez, X.; Li, C.; Rodriguez-Martínez, L.M.; Armand, M. Electrolyte Additives for Lithium Metal Anodes and Rechargeable Lithium Metal Batteries: Progress and Perspectives. Angew. Chem. Int. Ed. 2018, 57, 15002–15027. [Google Scholar] [CrossRef]
- Meng, J.; Lei, M.; Lai, C.; Wu, Q.; Liu, Y.; Li, C. Lithium Ion Repulsion-Enrichment Synergism Induced by Core–Shell Ionic Complexes to Enable High-Loading Lithium Metal Batteries. Angew. Chem. Int. Ed. 2021, 60, 23256–23266. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chu, Y.; Wang, Y.; Fang, Z.; Liu, Z.; Deng, Y.; Dong, Q.; Hao, Z. Nanohybridization of Keggin polyoxometalate clusters and reduced graphene oxide for lithium-ion batteries. J. Nanopart. Res. 2021, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, G.; Luan, D.; Yuan, Q.; Wei, Y.; Wang, X. Antibacterial Adhesion of Borneol-Based Polymer via Surface Chiral Stereochemistry. ACS Appl. Mater. Interfaces 2014, 6, 19371–19377. [Google Scholar] [CrossRef]
- Lin, W.; Ni, Y.; Pang, J. Microfluidic spinning of poly (methyl methacrylate)/konjac glucomannan active food packaging films based on hydrophilic/hydrophobic strategy. Carbohydr. Polym. 2019, 222, 114986. [Google Scholar] [CrossRef]
- Enderle, A.G.; Franco-Castillo, I.; Atrián-Blasco, E.; Martín-Rapún, R.; Lizarraga, L.; Culzoni, M.J.; Bollini, M.; de la Fuente, J.M.; Silva, F.; Streb, C.; et al. Hybrid Antimicrobial Films Containing a Polyoxometalate-Ionic Liquid. ACS Appl. Polym. Mater. 2022, 4, 4144–4153. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, Y.; Zhang, B. Phosphonium-based ionic liquids as antifungal agents for conservation of heritage sandstone. RSC Adv. 2022, 12, 1922–1931. [Google Scholar] [CrossRef]
- Eyssautier-Chuine, S.; Franco-Castillo, I.; Misra, A.; Hubert, J.; Vaillant-Gaveau, N.; Streb, C.; Mitchell, S.G. Evaluating the durability and performance of polyoxometalate-ionic liquid coatings on calcareous stones: Preventing biocolonisation in outdoor environments. Sci. Total Environ. 2023, 884, 163739. [Google Scholar] [CrossRef]
- Herrmann, S.; Kostrzewa, M.; Wierschem, A.; Streb, C. Polyoxometalate Ionic Liquids as Self-Repairing Acid-Resistant Corrosion Protection. Angew. Chem. Int. Ed. 2014, 53, 13596–13599. [Google Scholar] [CrossRef]
- Majeed, I.; Ahmad, Z.; AlMasoud, N.; Alomar, T.S.; Hussain, S.; Asif, H.M.; Mansoor, F.; Nazar, Z.; El-Bahy, Z.M. Preparation of polyoxometalate ionic liquids (POM-ILs) coated on metal coins for anticorrosion activity. Polyhedron 2023, 243, 116577. [Google Scholar] [CrossRef]
- Curnow, O.J.; Senthooran, R. Ionic liquid Keggin polyoxometallates with the tris(dihexylamino)cyclopropenium cation. Polyhedron 2023, 233, 116318. [Google Scholar] [CrossRef]
- Cruz, H.; Pinto, A.L.; Lima, J.C.; Branco, L.C.; Gago, S. Application of polyoxometalate-ionic liquids (POM-ILs) in dye-sensitized solar cells (DSSCs). Mater. Lett.-X 2020, 6, 100033. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, J.; Yang, Y.; Dong, Y.; Wang, J.; Wang, W.; Lin, K.; Xia, D. Dual-functional POM@IL complex modulate hole transport layer properties and interfacial charge dynamics for highly efficient and stable perovskite solar cells. Chin. Chem. Lett. 2024, 35, 108933. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, B. Recent Advances on the Functionalities of Polyoxometalate-Based Ionic Liquids. Molecules 2024, 29, 3216. https://doi.org/10.3390/molecules29133216
Wang H, Li B. Recent Advances on the Functionalities of Polyoxometalate-Based Ionic Liquids. Molecules. 2024; 29(13):3216. https://doi.org/10.3390/molecules29133216
Chicago/Turabian StyleWang, Hongxue, and Bao Li. 2024. "Recent Advances on the Functionalities of Polyoxometalate-Based Ionic Liquids" Molecules 29, no. 13: 3216. https://doi.org/10.3390/molecules29133216
APA StyleWang, H., & Li, B. (2024). Recent Advances on the Functionalities of Polyoxometalate-Based Ionic Liquids. Molecules, 29(13), 3216. https://doi.org/10.3390/molecules29133216






