How the Chemical Properties of Polysaccharides Make It Possible to Design Various Types of Organic–Inorganic Composites for Catalytic Applications
Abstract
1. Introduction
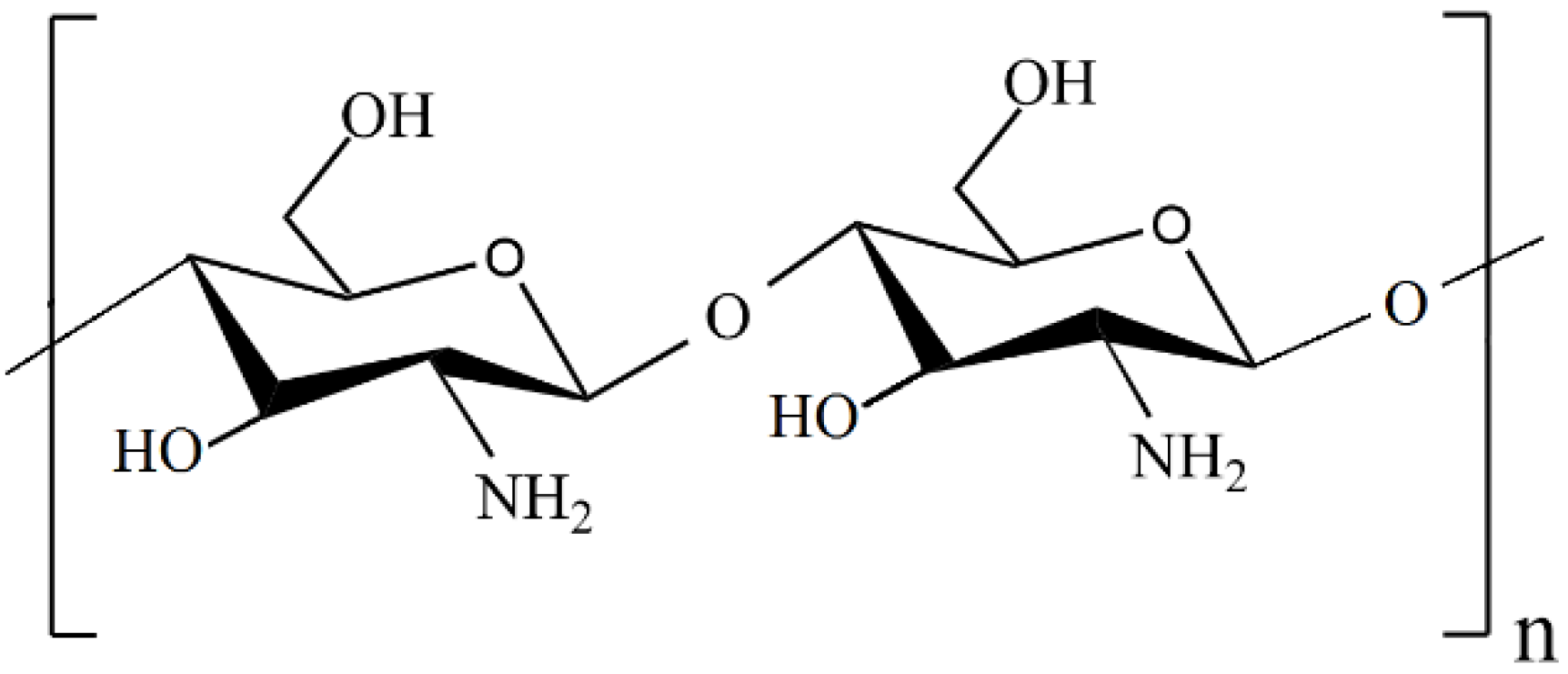
2. Chitosan-Based Nanocomposites
2.1. Chitosan as a Catalyst Support or Stabilizing Agent
2.2. Chitosan-Modified Catalysts on Inorganic Supports
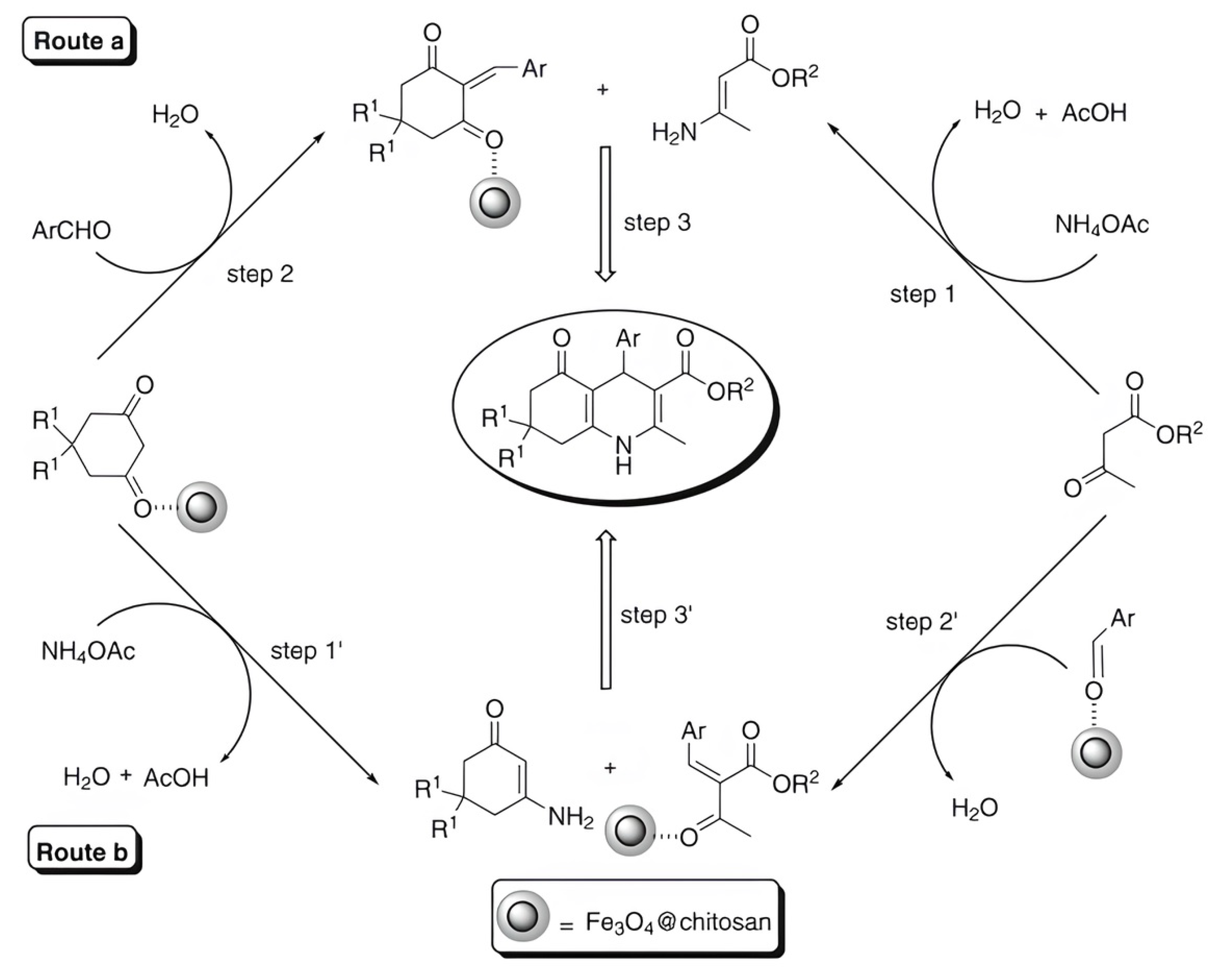
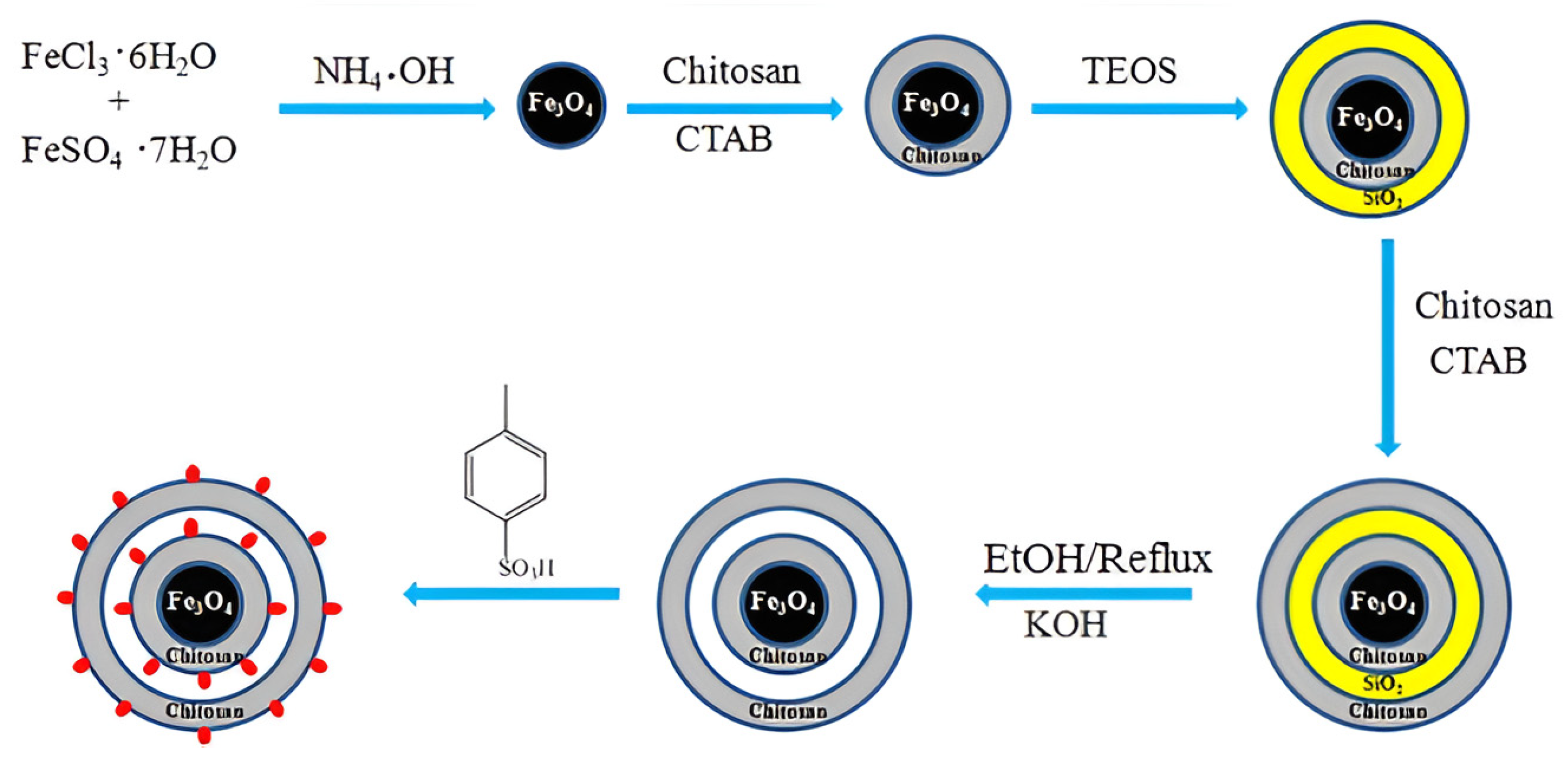
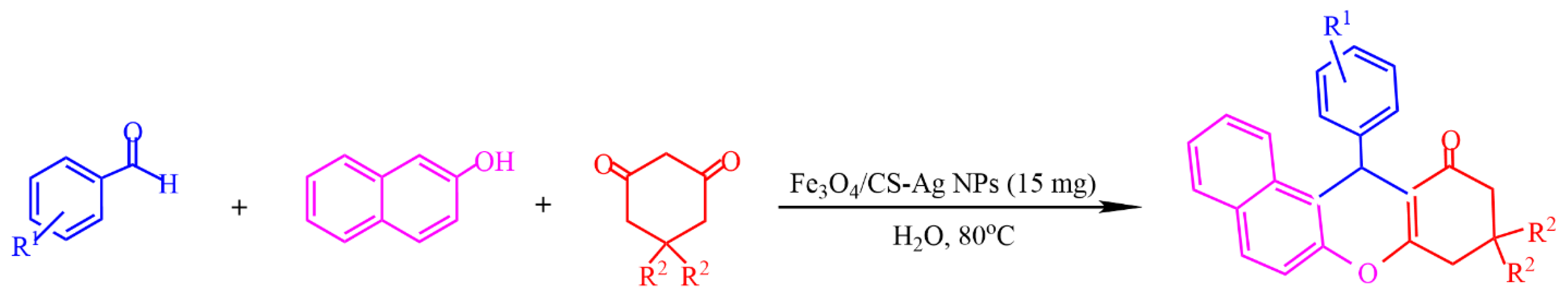
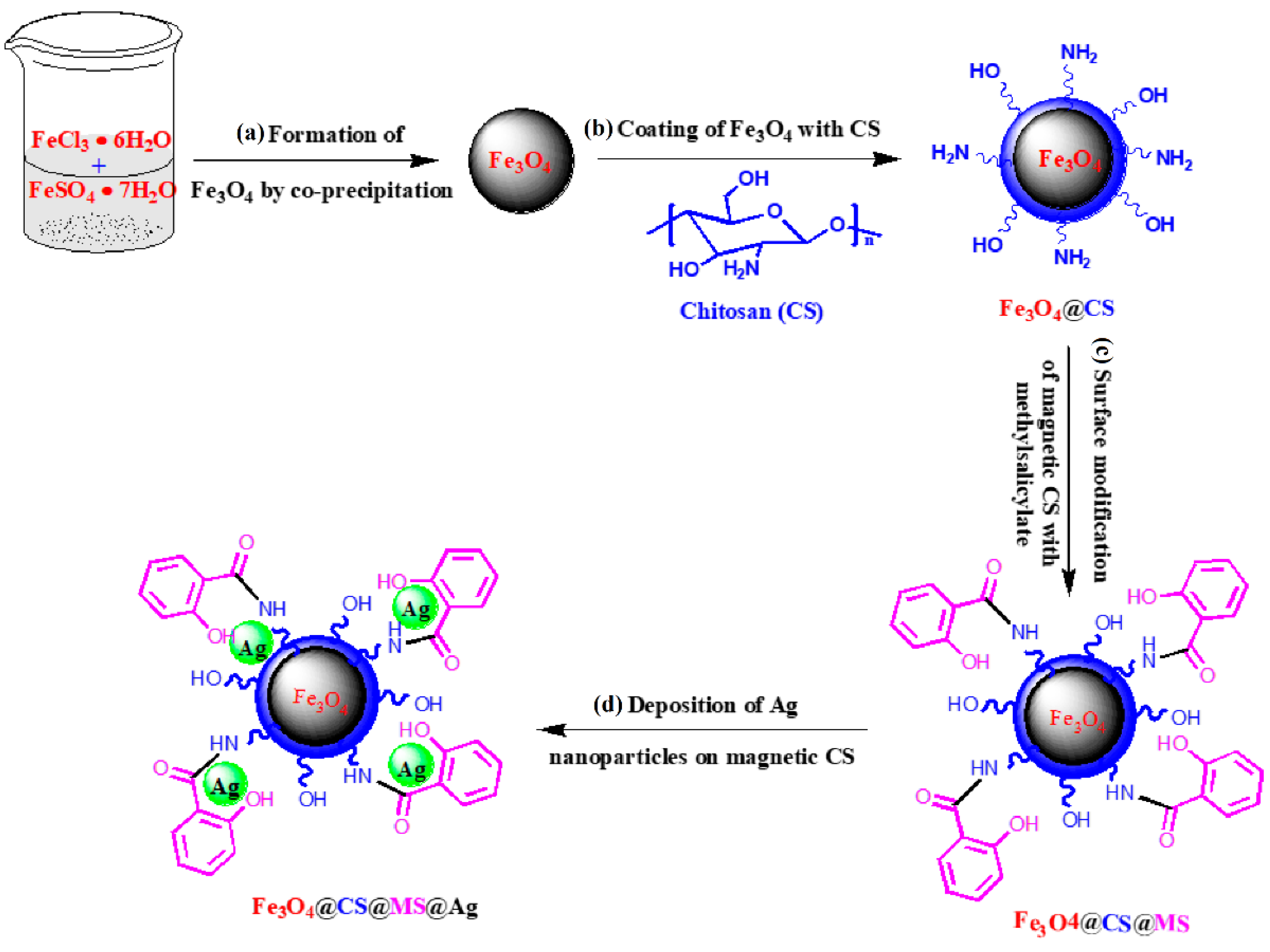
2.3. Chitosan-Based Magnetic Catalysts
2.3.1. Chitosan as a Catalyst
2.3.2. Metal Catalysts on Magnetic Chitosan
2.3.3. Enzymatic Catalysts on Magnetic Chitosan
2.4. Chitosan/Metal–Organic Framework Catalysts
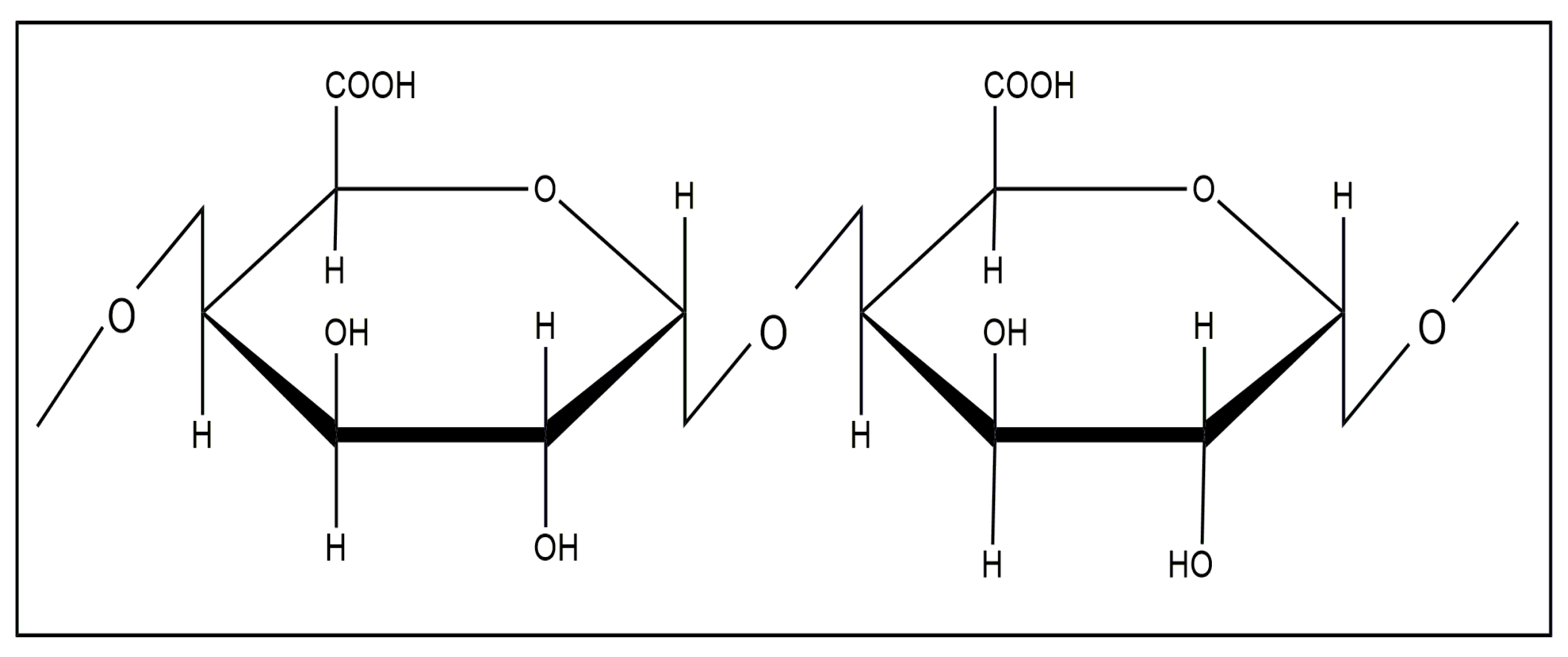
3. Pectin-Based Nanocomposites
3.1. Pectin and Pectin–Inorganic Composites as Catalysts
3.2. Metal Nanoparticles Stabilized with Pectin
3.3. Pectin-Based Magnetic Catalysts
4. Cellulose-Based Nanocomposites
4.1. Cellulose as a Catalyst Support and Stabilizing Agent
4.2. Cellulose-Based Magnetic Catalysts
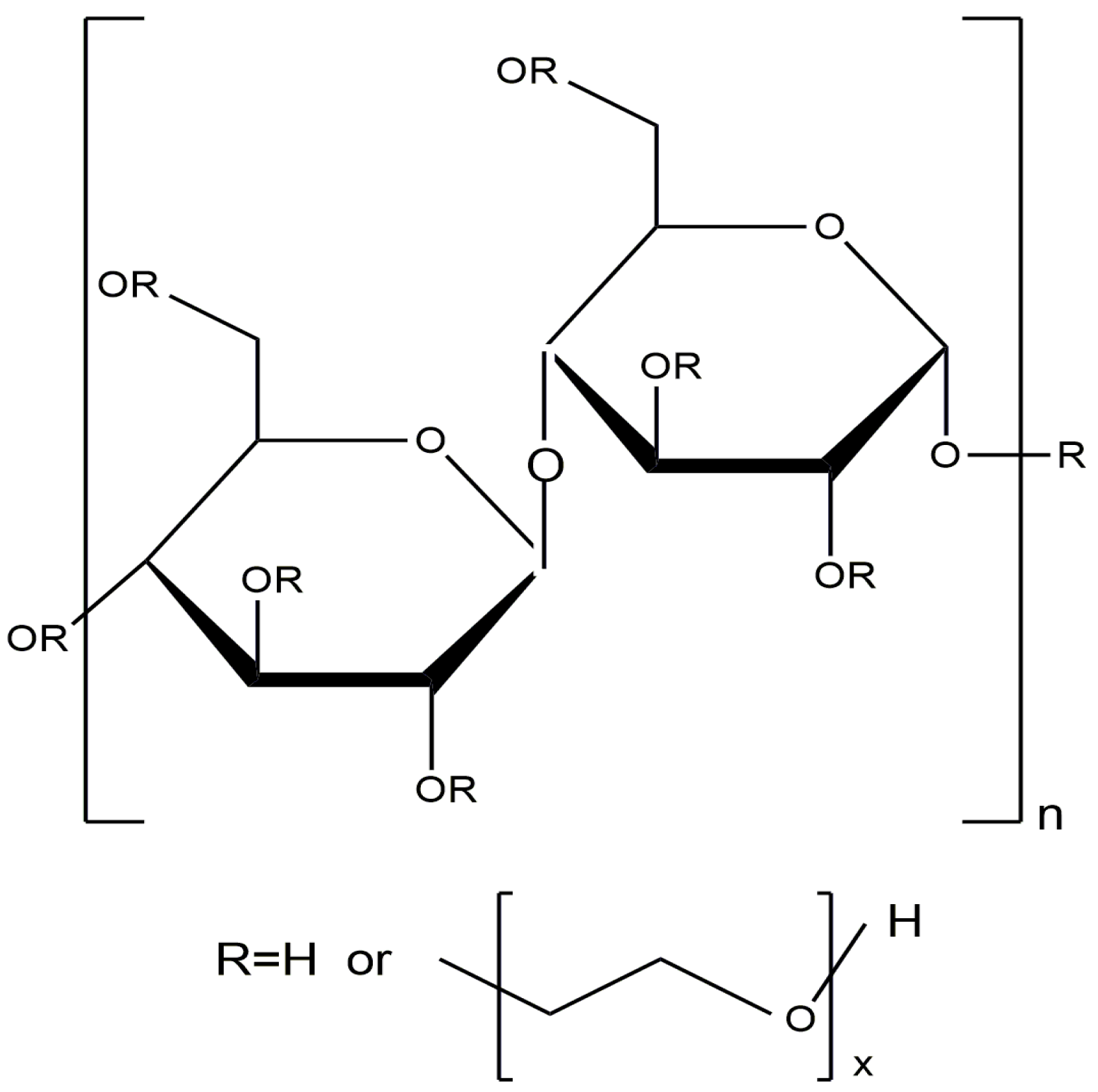
4.3. HEC-Based Nanocomposites
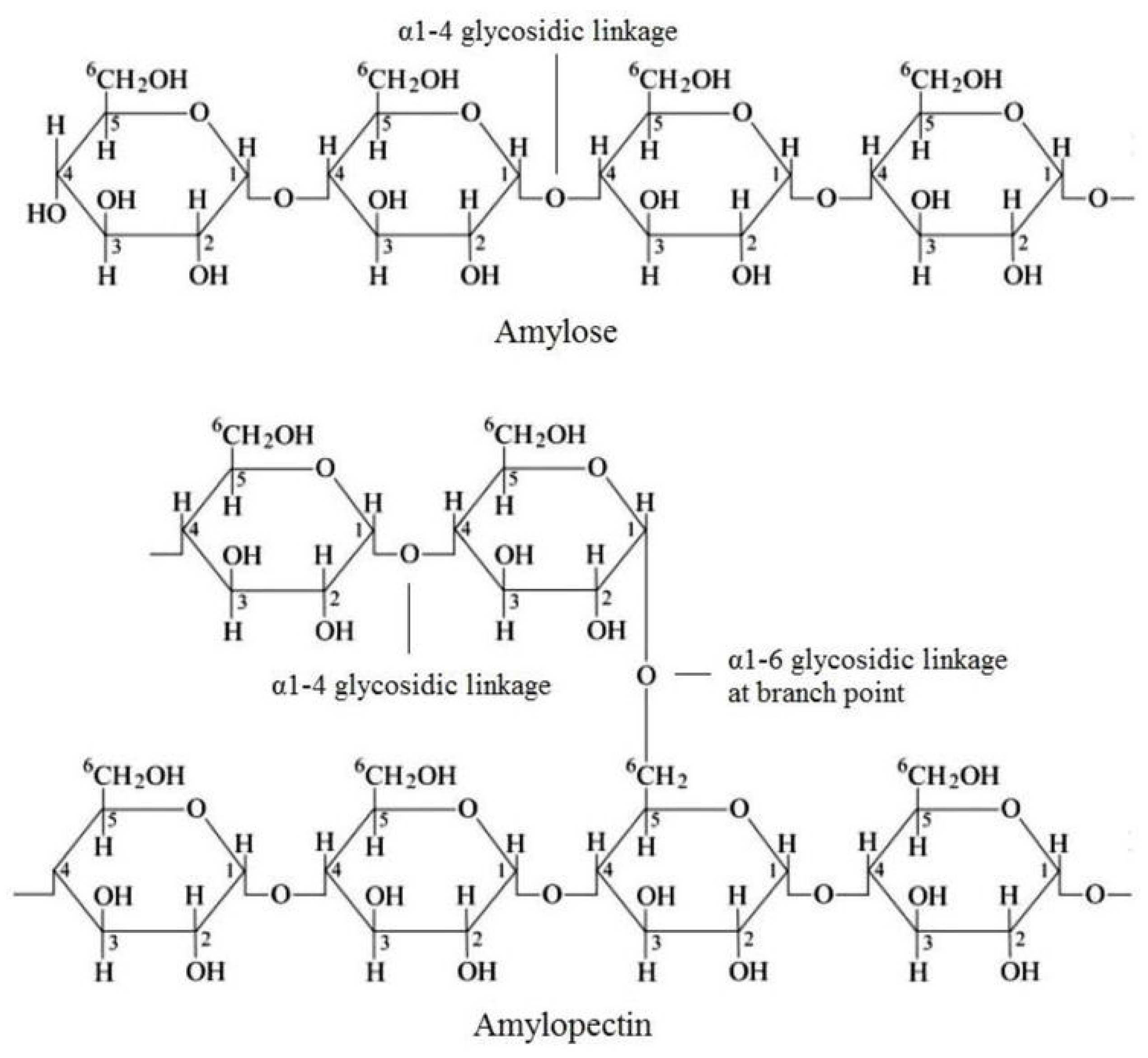
5. Starch-Based Nanocomposites
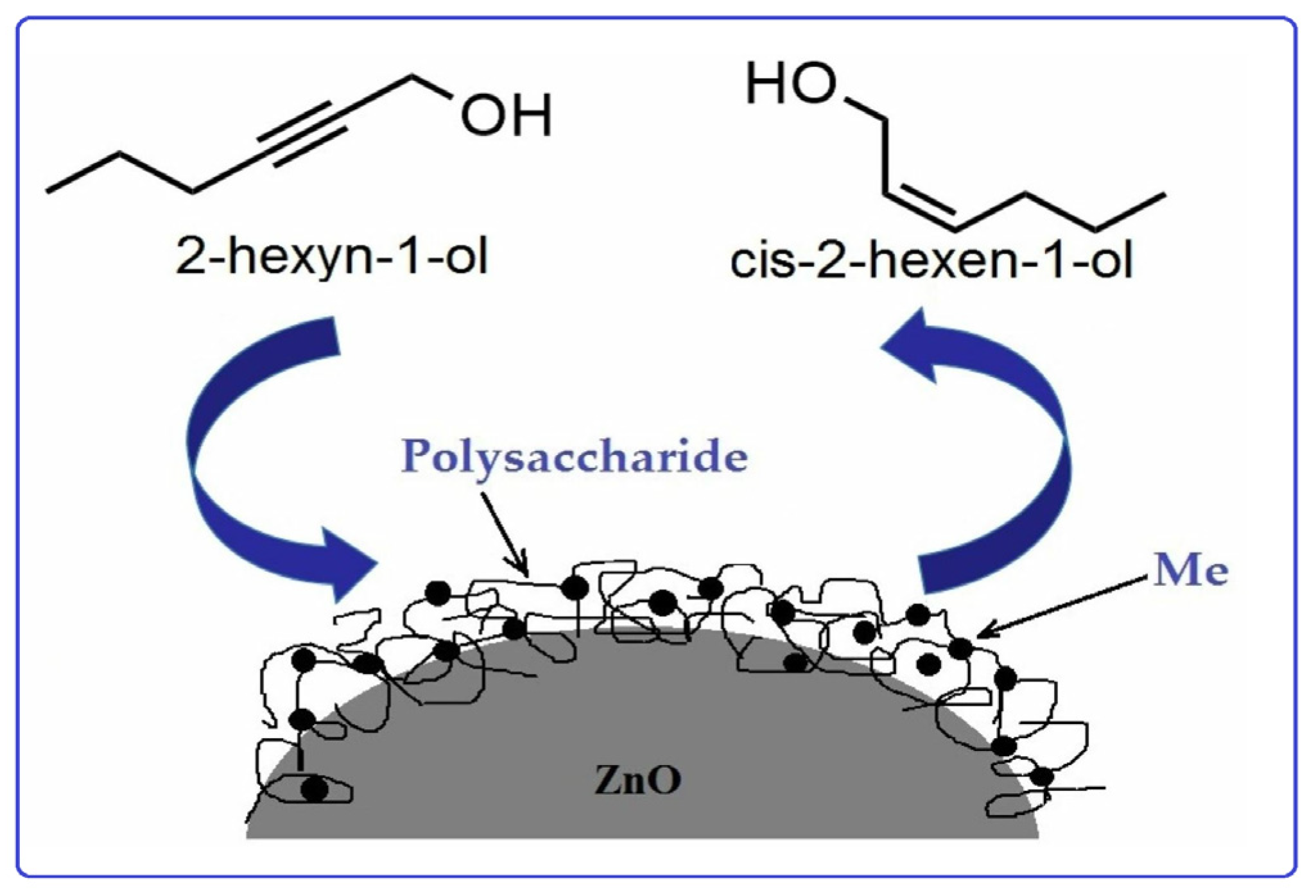
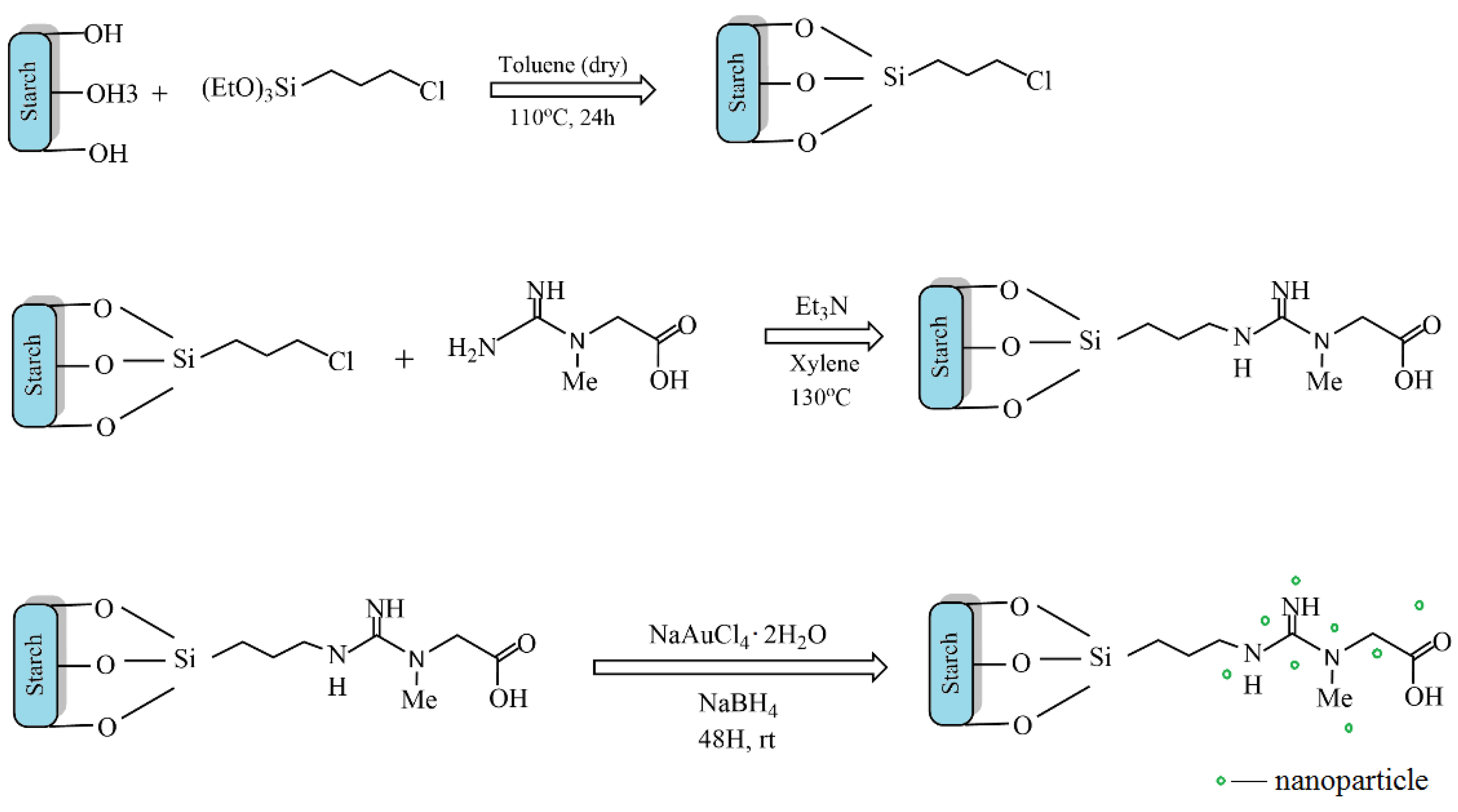
5.1. Metal Nanoparticles Stabilized with Starch
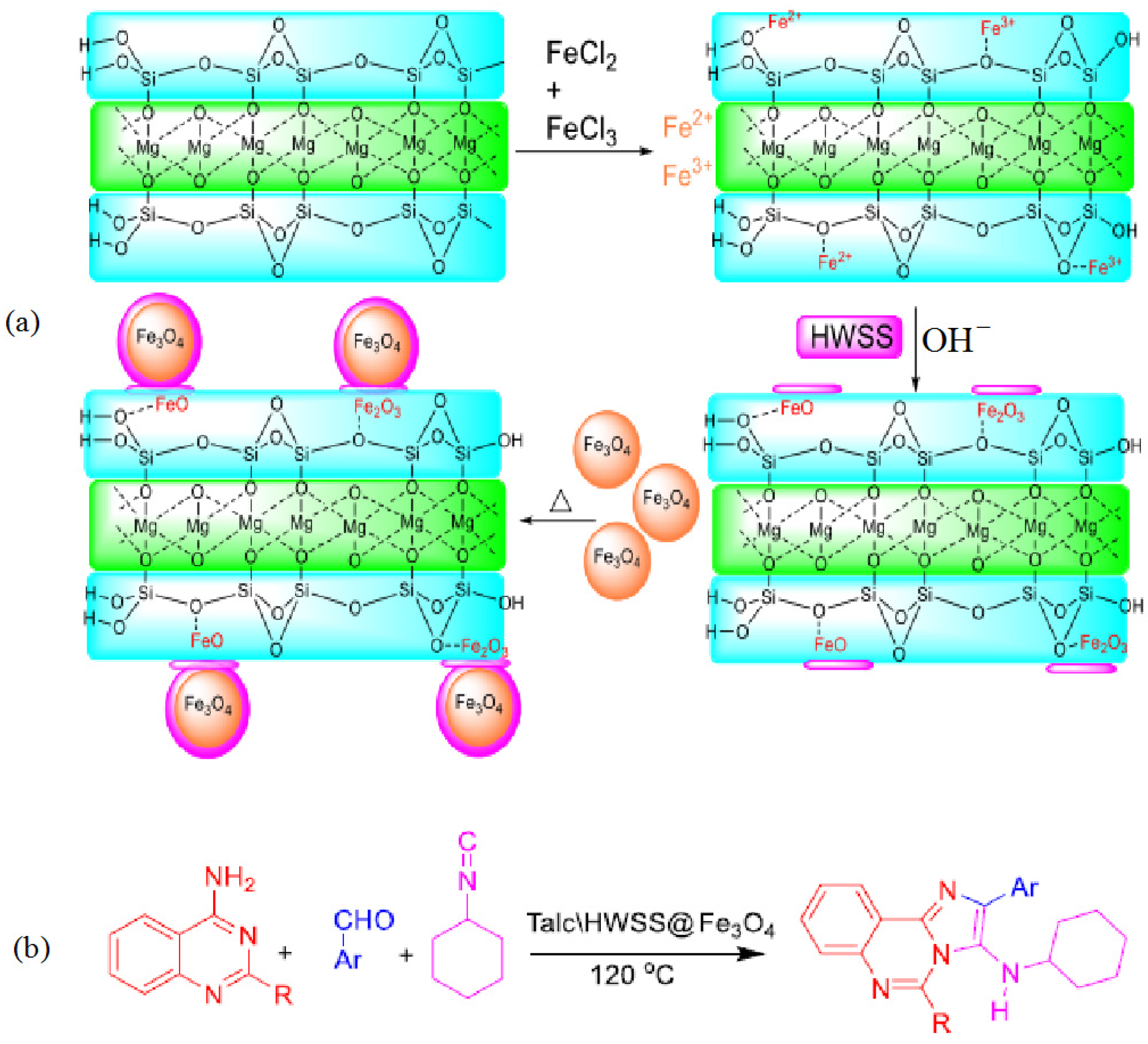
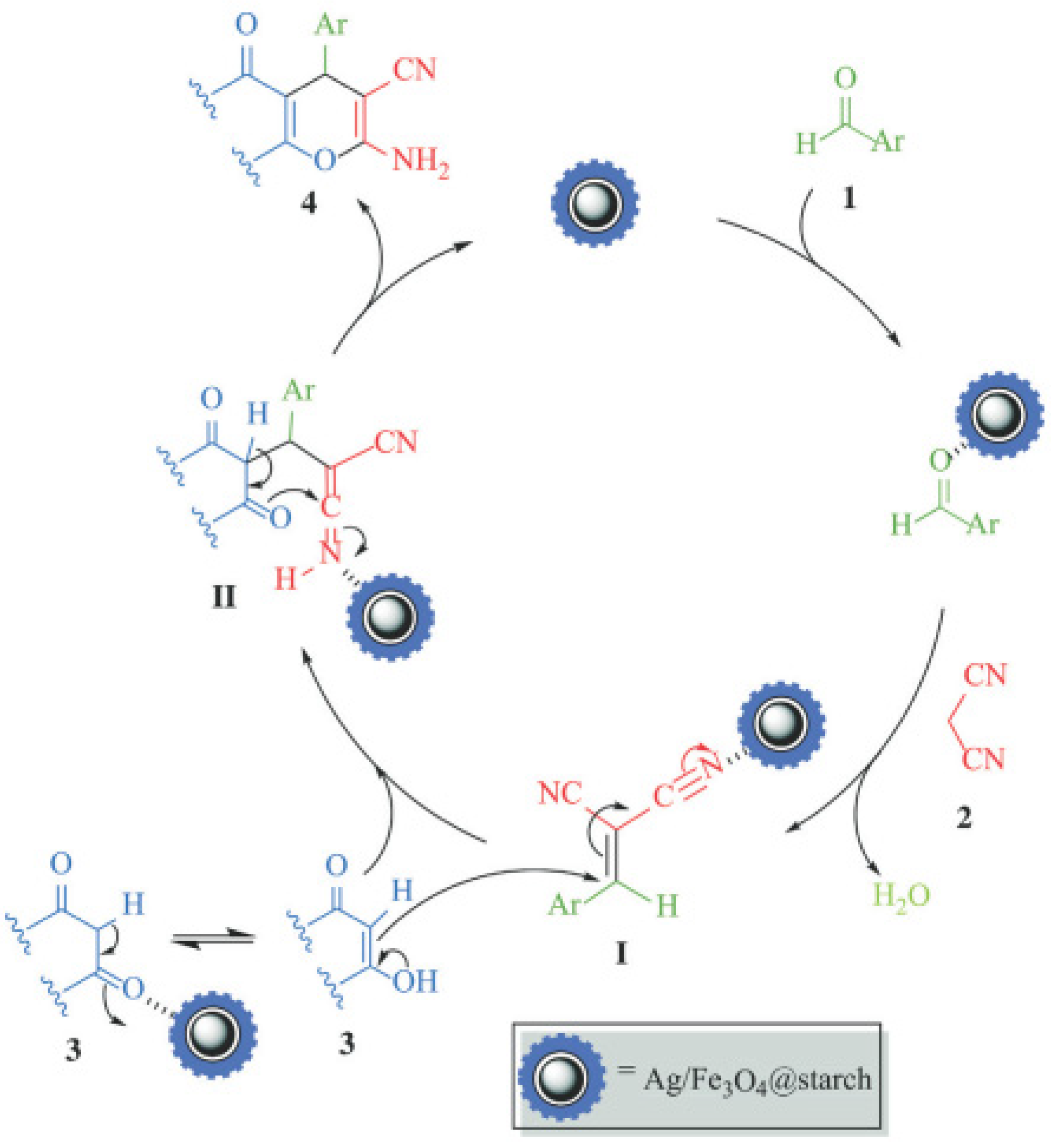
5.2. Starch-Based Magnetic Catalysts
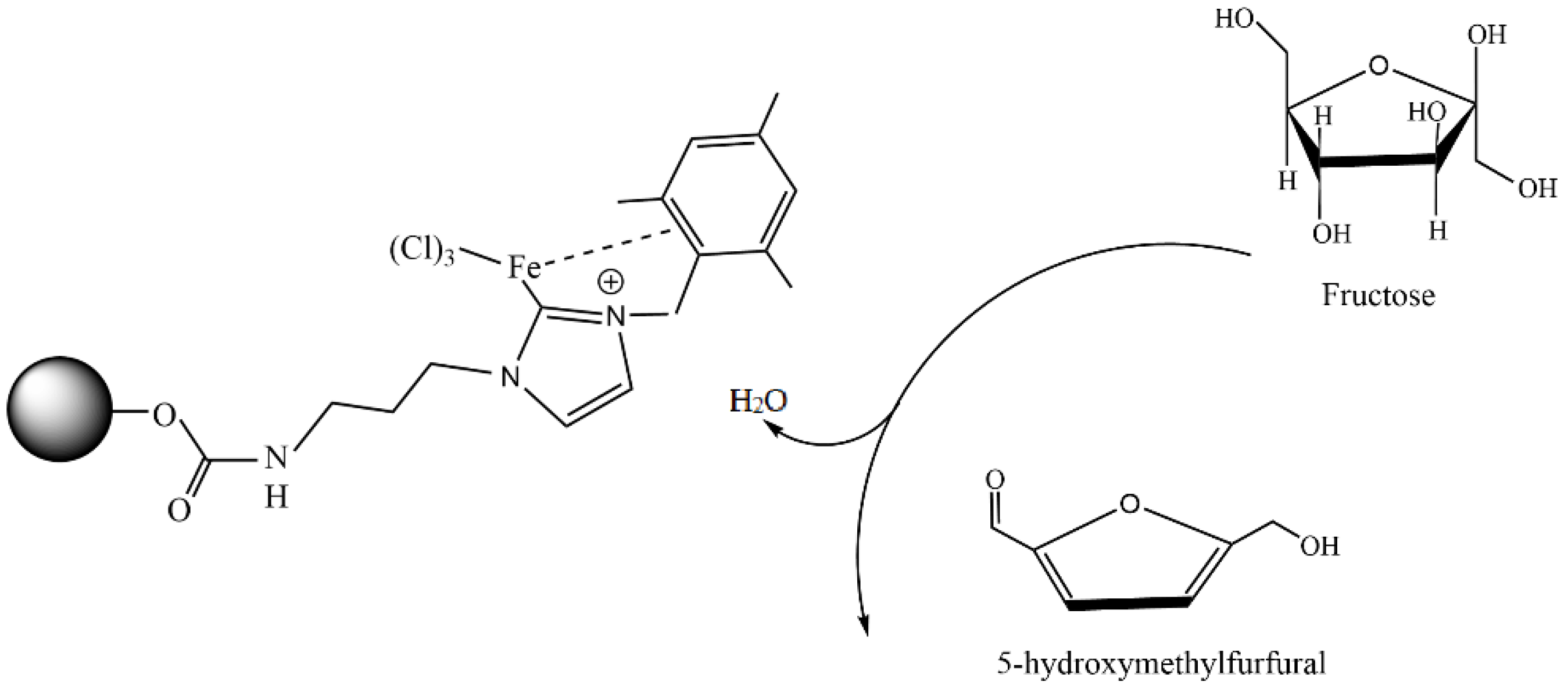
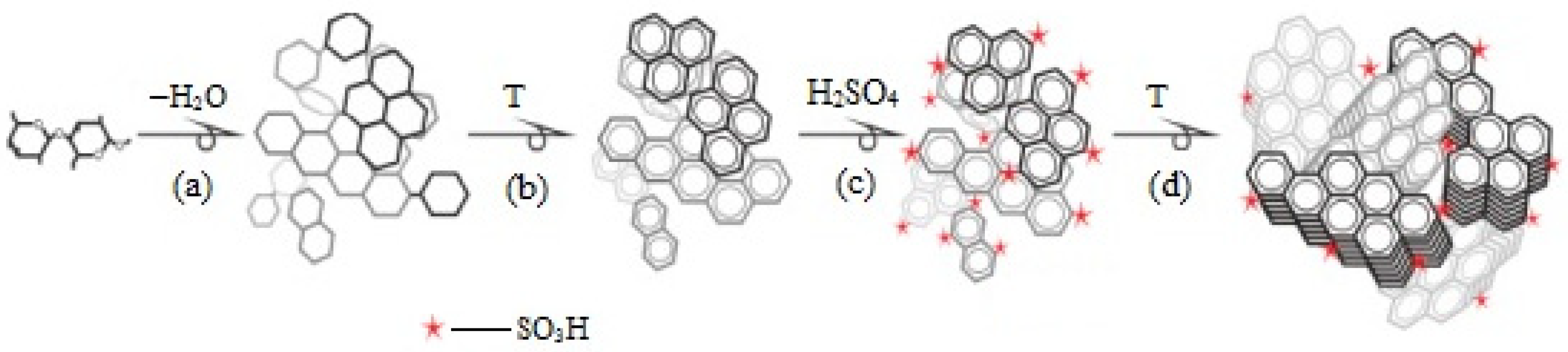
5.3. Starch as Carbon Precursor in the Synthesis of a Carbon-Based Catalyst
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Díaz-Montes, E. Polysaccharides: Sources, Characteristics, Properties, and Their Application in Biodegradable Films. Polysaccharides 2022, 3, 480–501. [Google Scholar] [CrossRef]
- Adrian, M.G.; Mihaela, M.; Cristian, V.D. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Muhammad, N.; Jost, N. Polysaccharides: Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–23719. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, Y.; Chen, J.; Lu, C. A review for natural polysaccharides with anti-pulmonary fibrosis properties, which may benefit to patients infected by 2019-nCoV. Carbohydr. Polym. 2020, 247, 116740. [Google Scholar] [CrossRef]
- Shunxiang, X.; Laibao, Z.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Baba, H.S.; Belhadri, M. Rheological properties of biopolymers drilling fluids. J. Pet. Sci. Eng. 2009, 67, 84–90. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of Polysaccharides in Food, Digestion and Health. Crit. Rev. Food Sci. Nutr. 2015, 57, 237–253. [Google Scholar] [CrossRef]
- Wu, Q.; Cheng, N.; Fang, D.; Wang, H.; Rahman, F.; Hao, H.; Zhang, Y. Recent advances on application of polysaccharides in cosmetics. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100004. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Microbial Polysaccharide Gums as Used in Cosmetics. Int. J. Toxicol. 2016, 35 (Suppl. S1), 5S–49S. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Jin, X.; Huang, C.; Xiang, Z. The Application of Polysaccharides and Their Derivatives in Pigment, Barrier, and Functional Paper Coatings. Polymers 2020, 12, 1837. [Google Scholar] [CrossRef]
- Ganesarajan, D.; Simon, L.; Tamrakar, S.; Kiziltas, A.; Mielewski, D.; Behabtu, N.; Lenges, C. Hybrid composites with engineered polysaccharides for automotive lightweight. Compos. Part C Open Access 2022, 7, 100222. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Chen, Z.; Chen, Y.; Chen, H. Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review. Polymers 2017, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Quignard, F.; Di Renzo, F.; Guibal, E. From natural polysaccharides to materials for catalysis, adsorption, and remediation. Top. Curr. Chem. 2010, 294, 165–197. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.F.; Gularte, M.S.; Quadrado, R.F.N.; Biajoli, A.F.P.; Fajardo, A.R. Copper species supported in polysaccharide-based materials: From preparation to application in catalysis. Catal. Rev. 2023, 65, 52–117. [Google Scholar] [CrossRef]
- Oshrat, L.-O.; Shira, B.; Shlomov, B.; Wolfson, A. Renewable Polysaccharides as Supports for Palladium Phosphine Catalysts. Polymers 2018, 10, 659. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Li, W.; Pei, X.; Ye, D. Chitosan derived efficient and stable Pd nano-catalyst for high efficiency hydrogenation. Int. J. Biol. Macromol. 2023, 241, 124615. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Tabibian, B.; Shaklein, S.; Tzur, E.; Wolfson, A. Acceleration of transfer-hydrogenation of cyclohexene with palladium catalysts in the presence of polysaccharides. ACG Publ. 2020, 13, 138–145. [Google Scholar] [CrossRef]
- Schüßler, S.; Blaubach, N.; Stolle, A.; Cravotto, G.; Ondruschka, B. Application of a cross-linked Pd–chitosan catalyst in liquid-phase-hydrogenation using molecular hydrogen. Appl. Catal. A Gen. 2012, 445–446, 231–238. [Google Scholar] [CrossRef]
- Reddy, K.P.; Swetha, C.; Murugadoss, A. Pd/Chitosan Nanoparticle Catalysts Prepared by Solid Mortar Grinding for Hydrogenation of Nitroarenes. ACS Sustain. Chem. Eng. 2023, 11, 1643–1654. [Google Scholar] [CrossRef]
- Nagaraja, K.; Hemalatha, D.; Ansar, S.; Hwan, O.T. Novel, Biosynthesis of Palladium Nanoparticles using Strychnos Potatorum Polysaccharide as a Green sustainable approach and their effective Catalytic Hydrogenation of 4-Nitrophenol. Int. J. Biol. Macromol. 2023, 253, 126983. [Google Scholar] [CrossRef] [PubMed]
- Zharmagambetova, A.K.; Talgatov, E.T.; Auyezkhanova, A.S.; Bukharbayeva, F.U.; Jumekeyeva, A.I. Polysaccharide-Stabilized PdAg Nanocatalysts for Hydrogenation of 2-Hexyn-1-ol. Catalysts 2023, 13, 1403. [Google Scholar] [CrossRef]
- Zharmagambetova, A.; Auyezkhanova, A.; Talgatov, E.; Jumekeyeva, A.; Buharbayeva, F.; Akhmetova, S.; Myltykbayeva, Z.; Nieto, J.M.L. Synthesis of polymer protected Pd–Ag/ZnO catalysts for phenylacetylene hydrogenation. J. Nanopart. Res. 2022, 24, 236. [Google Scholar] [CrossRef]
- Mahmoud, N.; Nasrin, S.; Zahra, N.; Bidgoli, S.; Sadat, N.; Fahimeh, S. Recent progresses in the application of cellulose, starch, alginate, gum, pectin, chitin and chitosan based (nano)catalysts in sustainable and selective oxidation reactions: A review. Carbohydr. Polym. 2020, 241, 116353. [Google Scholar] [CrossRef]
- Wolfson, A.; Stamker, E.; Levy-Ontman, O. Recyclable Pd-based polysaccharide catalyst for aerobic oxidation of benzyl alcohol. J. Appl. Polym. Sci. 2021, 139, 51517. [Google Scholar] [CrossRef]
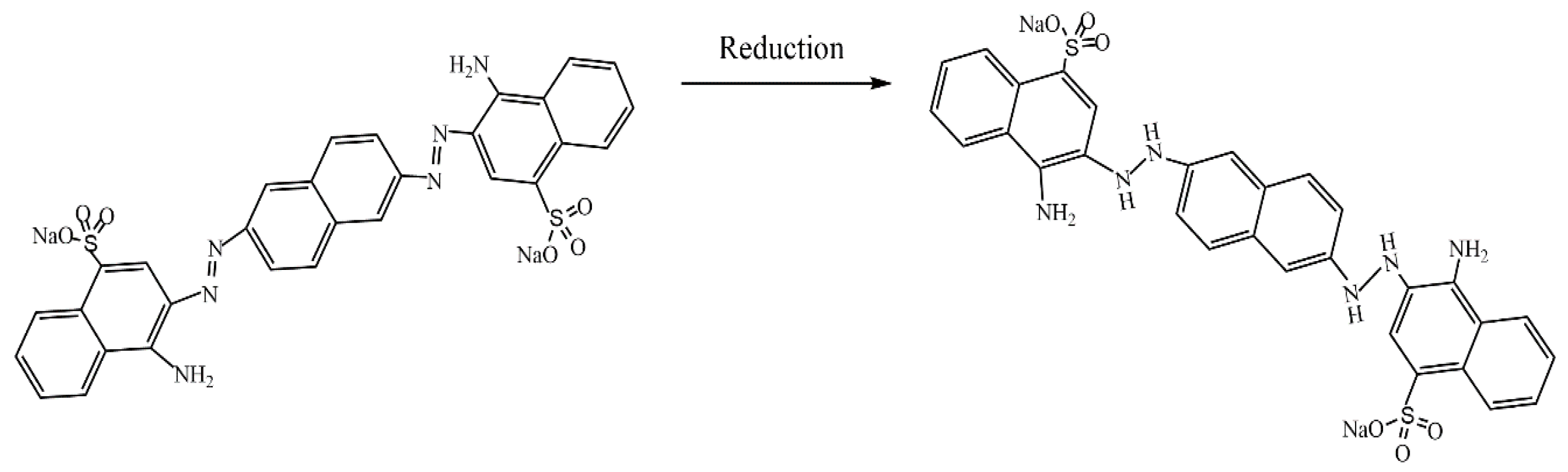
- Henrique, P.M.; Rafael, F.N.; Thiago, A.L.; Bernardo, A.; Fajardo, A.R. Polysaccharide/Fe(III)-porphyrin hybrid film as catalyst for oxidative decolorization of toxic azo dyes: An approach for wastewater treatment. Arab. J. Chem. 2020, 13, 5923–5938. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Balu, A.M.; Romero, A.A.; Luque, R.; Beilstein, J. New bio-nanocomposites based on iron oxides and polysaccharides applied to oxidation and alkylation reactions. Beilstein J. Org. Chem. 2017, 13, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized Chitosan as a Green, Recyclable, Biopolymer-Supported Catalyst for the [3 + 2] Huisgen Cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 5916–5920. [Google Scholar] [CrossRef]
- Molnár, Á. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Tabassum, S.; Zahoor, A.F.; Ahmad, S.; Noreen, R.; Khan, S.G.; Ahmad, H. Cross-coupling reactions towards the synthesis of natural products. Mol. Divers. 2021, 26, 647–689. [Google Scholar] [CrossRef]
- Fatemeh, R. Recent Advances in the Application of Chitosan and Chitosan Derivatives as Bio Supported Catalyst in the Cross Coupling Reactions. Curr. Org. Chem. 2019, 23, 390–408. [Google Scholar] [CrossRef]
- Adi, W.; Oshrat, L. Recent Developments in the Immobilization of Palladium Complexes on Renewable Polysaccharides for Suzuki–Miyaura Cross-Coupling of Halobenzenes and Phenylboronic Acids. Catalysts 2020, 10, 136. [Google Scholar] [CrossRef]
- Le, V.D.; Le, T.C.; Chau, V.; Le, N.T.; Dang, C.T.; Vo, T.; Nguyen, T.D.; Nguyen, T. Palladium nanoparticles in situ synthesized on Cyclea barbata pectin as a heterogeneous catalyst for Heck coupling in water, the reduction of nitrophenols and alkynes. New J. Chem. 2021, 10, 4746–4755. [Google Scholar] [CrossRef]
- Adi, W.; Shira, B.; Oshrat, L. Study of Pd-based catalysts within red algae-derived polysaccharide supports in a Suzuki cross-coupling reaction. RSC Adv. 2018, 8, 37939–37948. [Google Scholar] [CrossRef]
- Adi, W.; Oshrat, L. Development and application of palladium nanoparticles on renewable polysaccharides as catalysts for the Suzuki cross-coupling of halobenzenes and phenylboronic acids. Mol. Catal. 2020, 493, 111048. [Google Scholar] [CrossRef]
- Dohendou, M.; Pakzad, K.; Nezafat, Z.; Nasrollahzadeh, M.; Dekamin, M.G. Progresses in chitin, chitosan, starch, cellulose, pectin, alginate, gelatin and gum based (nano)catalysts for the Heck coupling reactions: A review. Int. J. Biol. Macromol. 2021, 192, 771–819. [Google Scholar] [CrossRef]
- El-Araby, A.; Janati, W.; Ullah, R.; Ercisli, S.; Errachildi, F. Chitosan, chitosan derivatives, and chitosan-based nanocomposites: Eco-friendly materials for advanced applications (a review). Front. Chem. 2023, 11, 1327426. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wilson, L.D. An Overview of the Design of Chitosan-Based Fiber Composite Materials. J. Compos. Sci. 2021, 5, 160. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules 2021, 11, 1599. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Furtos, G.; Grabska, S.; Michalska, M. Structure and Interactions in Chitosan Composites. Key Eng. Mater. 2016, 672, 257–260. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jó’zwik-Pruska, J.; Wi’sniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future-Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yadav, S.; Pramanik, J.; Sivamaruthi, B.S.; Jayeoye, T.J.; Prajapati, B.G.; Chaiyasut, C. Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications. Polymers 2023, 15, 3150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahi, T.; Gu, W.; Li, B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [PubMed]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Muniglia, L. Enzymatic synthesis of chitosan derivatives and their potential applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N. Chitin- and Chitosan-Based Composite Materials. Biomimetics 2022, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chen, B.-Y.; Den, W. Chitosan as a Natural Polymer for Heterogeneous Catalysts Support: A Short Review on Its Applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef]
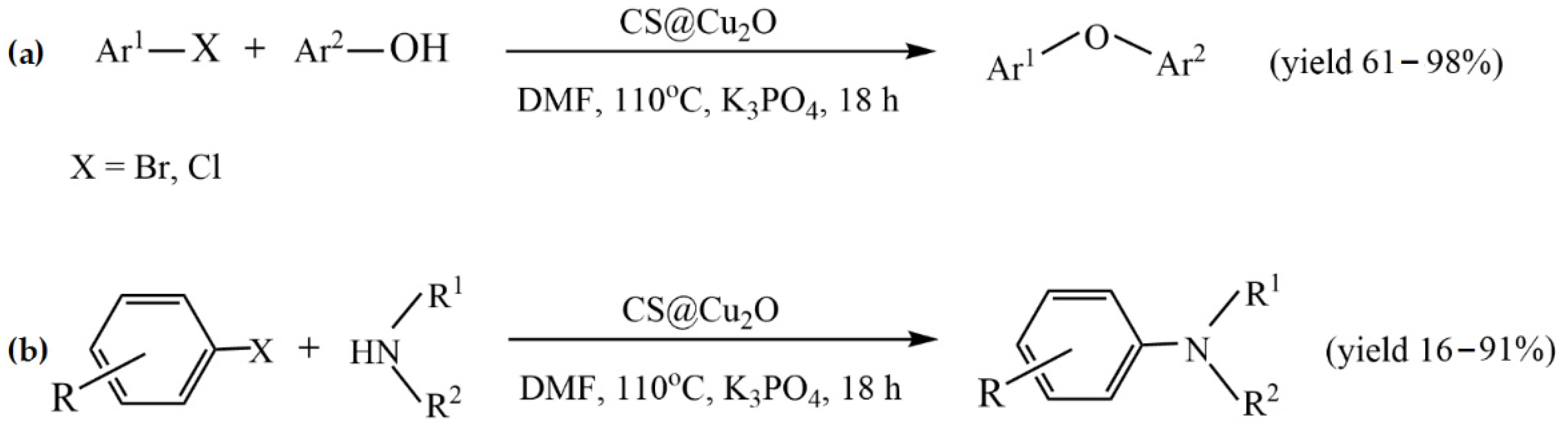
- Qian, C.; Zhu, W.; Liu, J.; Wang, X.; Qiu, L. Chitosan@Cu2O as A Facile, Efficient and Reusable Catalyst for Ligand Free C-O and C-N Coupling. Chin. J. Org. Chem. 2019, 39, 1695–1703. [Google Scholar] [CrossRef]
- Reddy, K.P.; Meerakrishna, R.S.; Shanmugam, P.; Satpati, B.; Murugadoss, A. Rapid Gram-Scale Synthesis of Au/Chitosan Nanoparticles catalysts by Using Solid Mortar Grinding. New J. Chem. 2021, 45, 438–446. [Google Scholar] [CrossRef]
- Grandini, C.P.; Schmitt, C.R.; Duarte, F.A.; Rosa, D.S.; Rosa, C.H.; Rosa, G.R. New sustainable and robust catalytic supports for palladium nanoparticles generated from chitosan/cellulose film and corn stem biochar. Environ. Sci. Pollut. Res. 2023, 30, 6068–6079. [Google Scholar] [CrossRef]
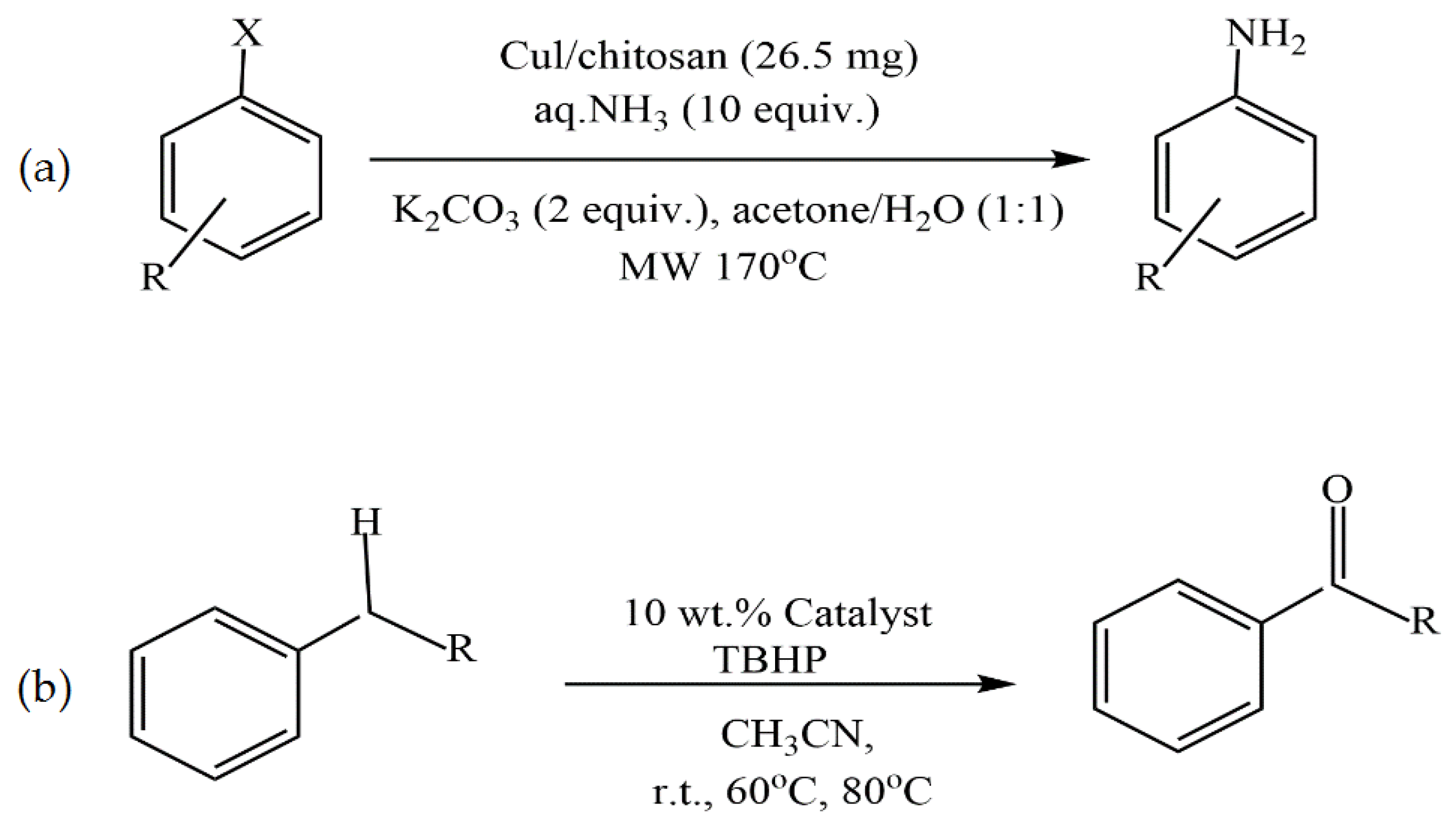
- Kanarat, J.; Bunchuay, T.; Chutimasakul, T.; Limprasart, W.; Unlum, J.; Tantirungrotechai, J. Copper-Chitosan Beads as Efficient and Recyclable Heterogeneous Catalysts for C-H Oxidation and C-X Amination. Chem. Sel. 2022, 7, 202202517. [Google Scholar] [CrossRef]
- Correia, L.M.; Campelo, N.S.; Albuquerque, R.F.; Cavalcante, C.L.; Cecilia, J.A.; Rodríguez-Castellón, E.; Guibal, E.; Vieira, R.S. Calcium/chitosan spheres as catalyst for biodiesel production. Polym. Int. 2015, 64, 242–249. [Google Scholar] [CrossRef]
- Shao, L.; Qi, C. Chitosan microspheres-supported palladium species as an efficient and recyclable catalyst for Mizoroki–Heck reaction. New J. Chem. 2017, 41, 8156–8165. [Google Scholar] [CrossRef]
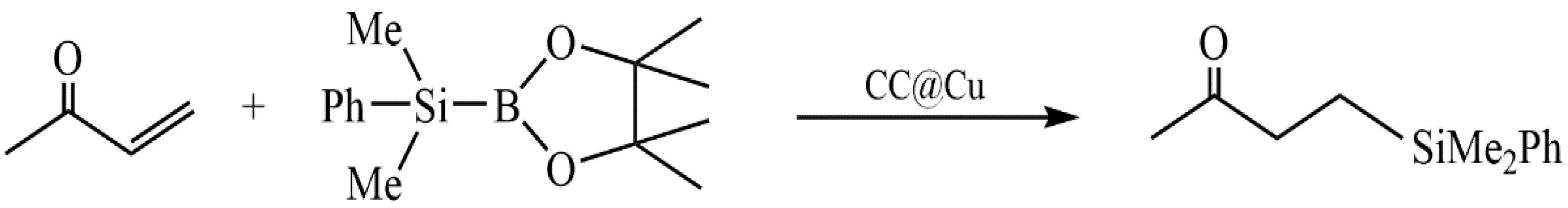
- Wang, W.; Xiao, Z.; Huang, C.; Zheng, K.; Luo, Y.; Dong, Y.; Shen, Z.; Li, W.; Qin, C. Preparation of Modified Chitosan Microsphere-Supported Copper Catalysts for the Borylation of α,β-Unsaturated Compounds. Polymers 2019, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
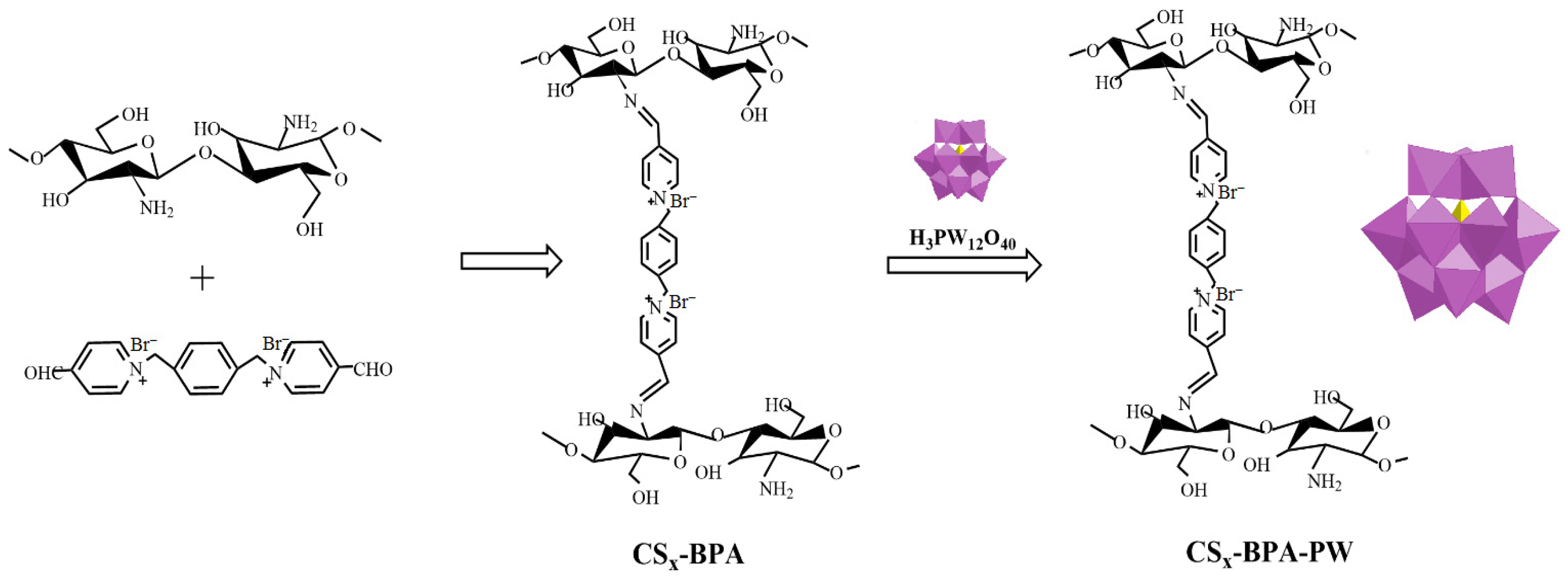
- Li, J.; Zhang, C.; Jiang, P.; Leng, Y. Cross-linked chitosan supporting polyoxometalates catalyst with adjustable redox property for H2O2-based oxidation reactions. Catal. Commun. 2017, 94, 13–17. [Google Scholar] [CrossRef]
- Ríos-Caloch, G.; Santes, V.; Escobar, J.; Pérez-Romo, P.; Díaz, L.; Lartundo-Rojas, L. Effect of Chitosan on the Performance of NiMoP-Supported Catalysts for the Hydrodesulfurization of Dibenzothiophene. J. Nanomater. 2016, 2016, 4047874. [Google Scholar] [CrossRef]
- Sun, K.; Liu, Y.; Zhang, T.; Zhou, J. Modification of Pillared Intercalated Montmorillonite Clay as Heterogeneous Pd Catalyst Supports. Molecules 2023, 28, 7638. [Google Scholar] [CrossRef] [PubMed]
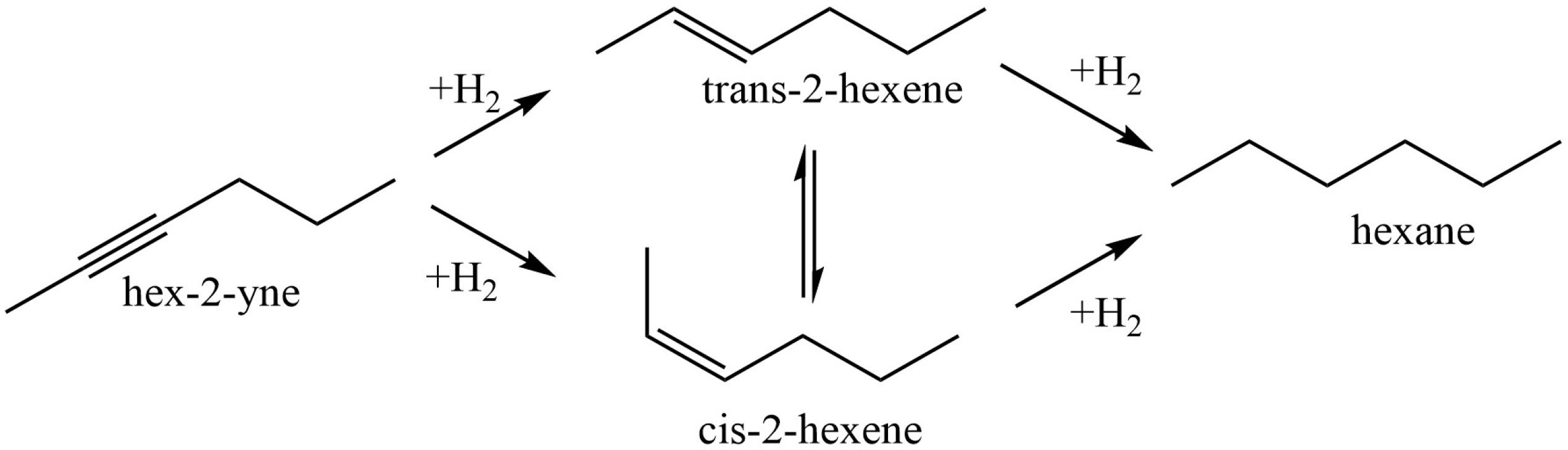
- Zharmagambetova, A.K.; Auyezkhanova, A.S.; Talgatov, E.T.; Jumekeyeva, A.I. Chitosan-Modified Palladium Catalysts in Hydrogenation of n-Hex-2-Yne. Theor. Exp. Chem. 2021, 57, 371–376. [Google Scholar] [CrossRef]
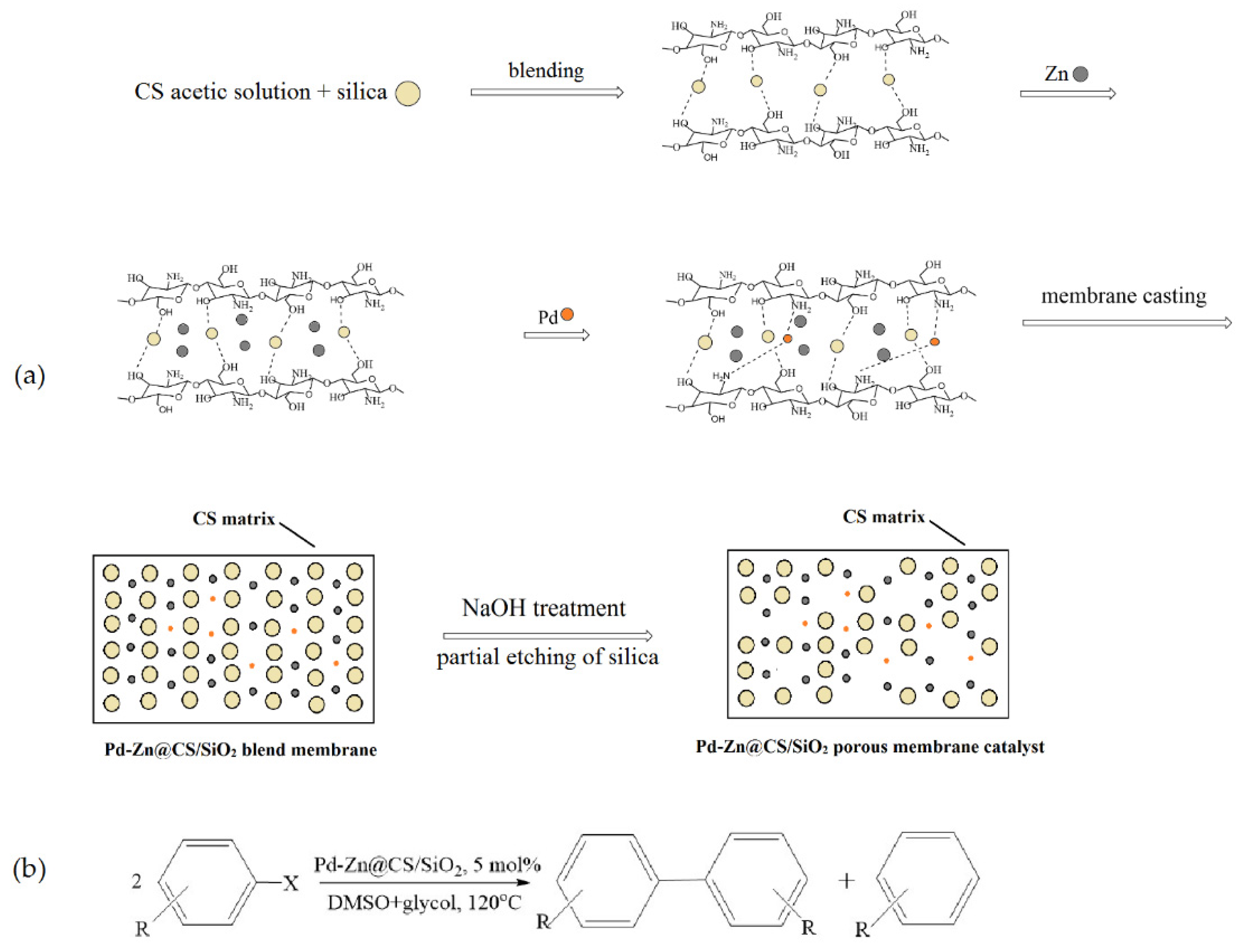
- Liu, Q.; Xu, M.; Wang, Y.; Feng, R.; Yang, Z.; Zuo, S.; Qi, C.; Zeng, M. Co-immobilization of Pd and Zn nanoparticles in chitosan/silica membranes for efficient, recyclable catalysts used in Ullmann reaction. Int. J. Biol. Macromol. 2017, 105, 575–583. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Shao, Y.; He, F.; Wu, M.; Ni, H.; Zheng, Y.; Sun, Y. Chitosan–silica nanoparticles catalyst (M@CS–SiO2) for the degradation of 1,1-dimethylhydrazine. Res. Chem. Intermed. 2019, 45, 1721–1735. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Bakhsh, E.M.; Asiri, A.M.; Sobahi, R.A. Synthesis and characterization of metal nanoparticles templated chitosan-SiO2 catalyst for the reduction of nitrophenols and dyes. Carbohydr. Polym. 2018, 192, 217–230. [Google Scholar] [CrossRef]
- Ma, L.; Su, Y.; Chen, J.; Xu, J. Silica/Chitosan Core–Shell Hybrid-Microsphere-Supported Pd Catalyst for Hydrogenation of Cyclohexene Reaction. Ind. Eng. Chem. Res. 2017, 56, 12655–12662. [Google Scholar] [CrossRef]
- Talgatov, E.T.; Auyezkhanova, A.S.; Seitkalieva, K.S.; Tumabayev, N.Z.; Akhmetova, S.N.; Zharmagambetova, A.K. Co-precipitation synthesis of mesoporous maghemite for catalysis application. J. Porous. Mater. 2020, 27, 919–927. [Google Scholar] [CrossRef]
- Talgatov, E.T.; Auyezkhanova, A.S.; Seitkalieva, K.S.; Akhmetova, S.N.; Zharmagambetova, A.K. Effect of the Size of Polyacrylamide-Stabilized Palladium Nanoparticles Supported on γ-Fe2O3 on Their Catalytic Properties in the Hydrogenation of Phenylacetylene. Theor. Exp. Chem. 2019, 55, 331–336. [Google Scholar] [CrossRef]
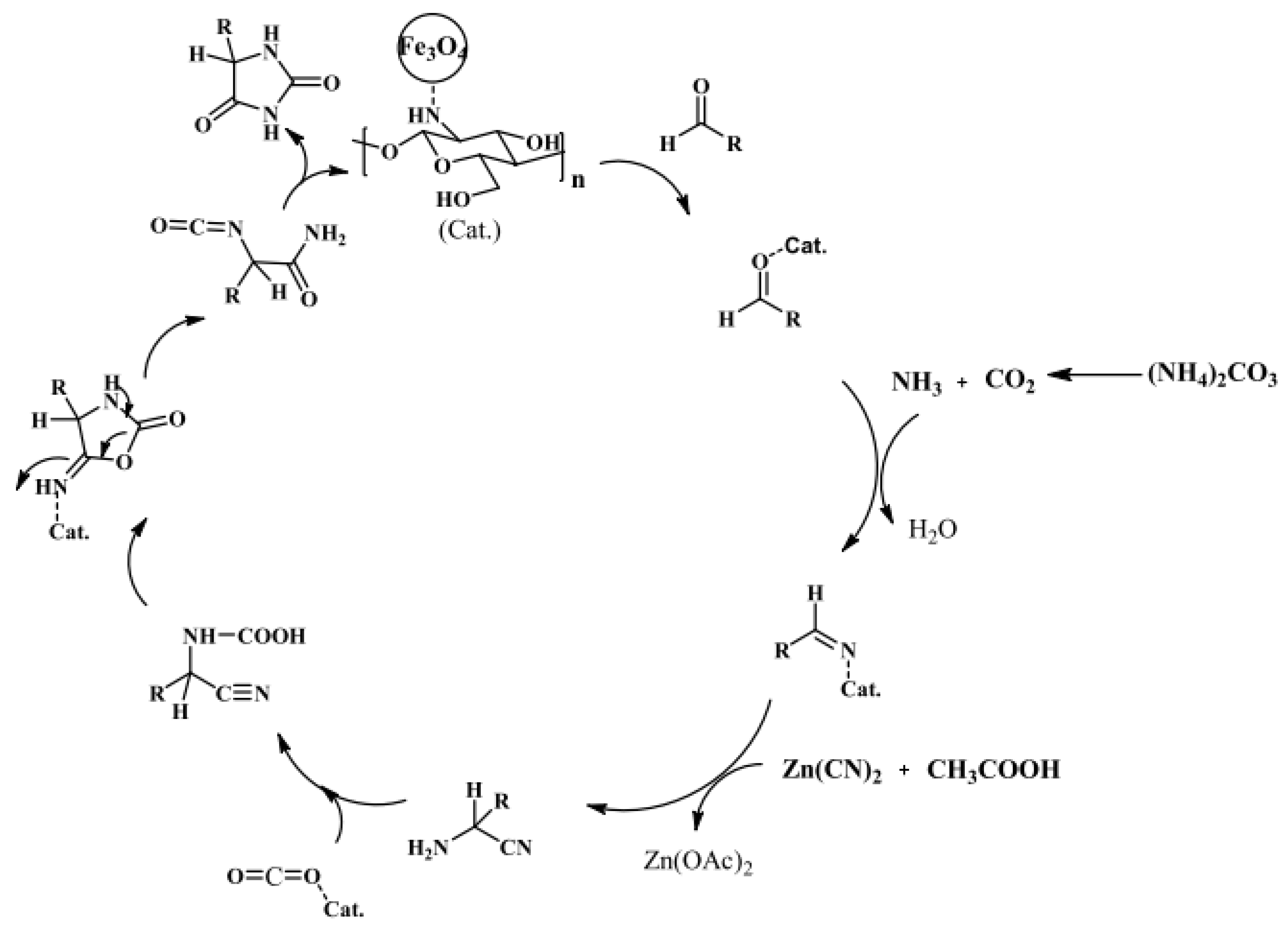
- Safari, J.; Javadian, L. Fe3O4-chitosan nanoparticles as a robust magnetic catalyst for efficient synthesis of 5-substituted hydantoins using zinc cyanide. Iran. J. Catal. 2016, 6, 57–64. [Google Scholar]
- Hasan, K.; Joseph, R.G.; Patole, S.P.; Al-Qawasmeh, R.A. Development of magnetic Fe3O4-chitosan immobilized Cu(II) Schiff base catalyst: An efficient and reusable catalyst for microwave assisted one-pot synthesis of propargylamines via A3 coupling. Catal. Commun. 2023, 174, 106588. [Google Scholar] [CrossRef]
- Maleki, A.; Kamalzare, M.; Aghaei, M. Efficient one-pot four-component synthesis of 1,4-dihydropyridines promoted by magnetite/chitosan as a magnetically recyclable heterogeneous nanocatalyst. J. Nanostruct. Chem. 2015, 5, 95–105. [Google Scholar] [CrossRef]
- Wang, A.; Li, H.; Pan, H.; Zhang, H.; Xu, F.; Yu, Z.; Yang, S. Efficient and green production of biodiesel catalyzed by recyclable biomass derived magnetic acids. Fuel Process. Technol. 2018, 181, 259–267. [Google Scholar] [CrossRef]
- Mohammadi, R.; Eidi, E.; Ghavami, M.; Kassaee, M.Z. Chitosan synergistically enhanced by successive Fe3O4 and silver nanoparticles as a novel green catalyst in one-pot, three-component synthesis of tetrahydrobenzo[α]xanthene-11-ones. J. Mol. Catal. A Chem. 2014, 393, 309–316. [Google Scholar] [CrossRef]
- Rahimzadeh, G.; Tajbakhsh, M.; Ali Ayati, M.D. Heteropolyacid coupled with cyanoguanidine decorated magnetic chitosan as an efficient catalyst for the synthesis of pyranochromene derivatives. Sci. Rep. 2022, 12, 17027. [Google Scholar] [CrossRef]
- Hasan, K.; Shehadi, I.A.; Al-Bab, N.D.; Elgamouz, A. Magnetic Chitosan-Supported Silver Nanoparticles: A Heterogeneous Catalyst for the Reduction of 4-Nitrophenol. Catalysts 2019, 9, 839. [Google Scholar] [CrossRef]
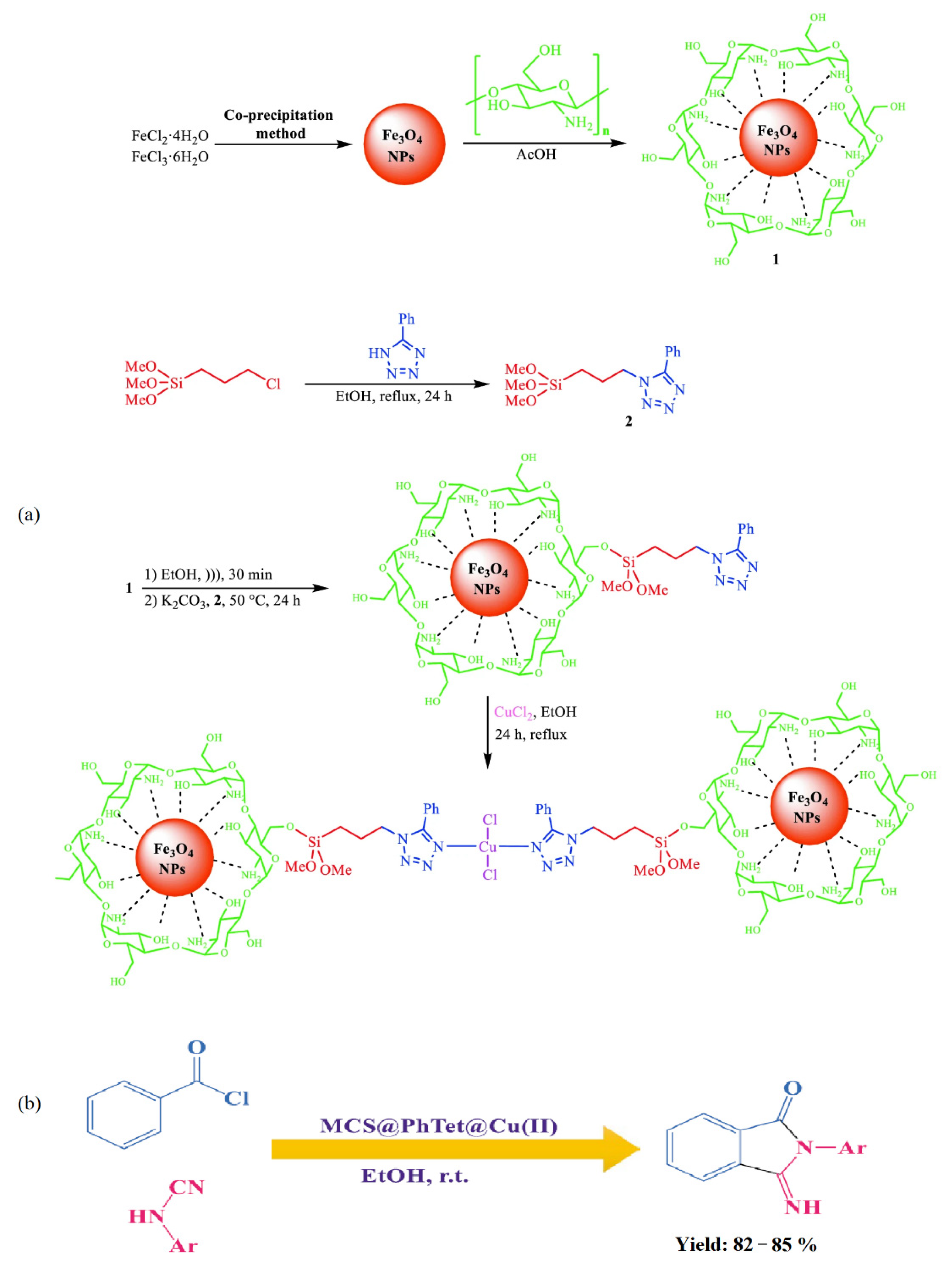
- Nasrollahzadeh, M.; Shafiei, N.; Orooji, Y. Magnetic chitosan stabilized Cu(II)-tetrazole complex: An effective nanocatalyst for the synthesis of 3-imino-2-phenylisoindolin-1-one derivatives under ultrasound irradiation. Sci. Rep. 2022, 12, 6724. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.S.; de Farias, B.S.; Junior, T.R.S.C.; Pinto, L.A.d.A.; Diaz, P.S. Chitosan–based nanofibers for enzyme immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef]
- Ramírez, J.; Buestán, L.; López-Maldonado, E.A.; Pinos-Vélez, V. Preparation and Physicochemical Characterization of Biodiesel from Recycled Vegetable Oil in Cuenca, Ecuador by Transesterification Catalyzed by KOH and NaOH. Eng 2023, 4, 954–963. [Google Scholar] [CrossRef]
- Cubides-Roman, D.C.; Pérez, V.H.; de Castro, H.F.; Orrego, C.E.; Giraldo, O.H.; Silveira, E.G.; David, G.F. Ethyl esters (biodiesel) production by Pseudomonas fluorescens lipase immobilized on chitosan with magnetic properties in a bioreactor assisted by electromagnetic field. Fuel 2017, 196, 481–487. [Google Scholar] [CrossRef]
- Chen, G.; Liu, J.; Qi, Y.; Yao, J.; Yan, B. Biodiesel production using magnetic whole-cell biocatalysts by immobilization of Pseudomonas mendocina on Fe3O4-chitosan microspheres. Biochem. Eng. J. 2016, 113, 86–92. [Google Scholar] [CrossRef]
- Miri, S.; Ravula, A.; Akhtarian, S.; Davoodi, S.M.; Brar, S.K.; Martel, R.; Rouissi, T. Immobilized cold-active enzymes onto magnetic chitosan microparticles as a highly stable and reusable carrier for p-xylene biodegradation. Front. Environ. Eng. 2023, 2, 1341816. [Google Scholar] [CrossRef]
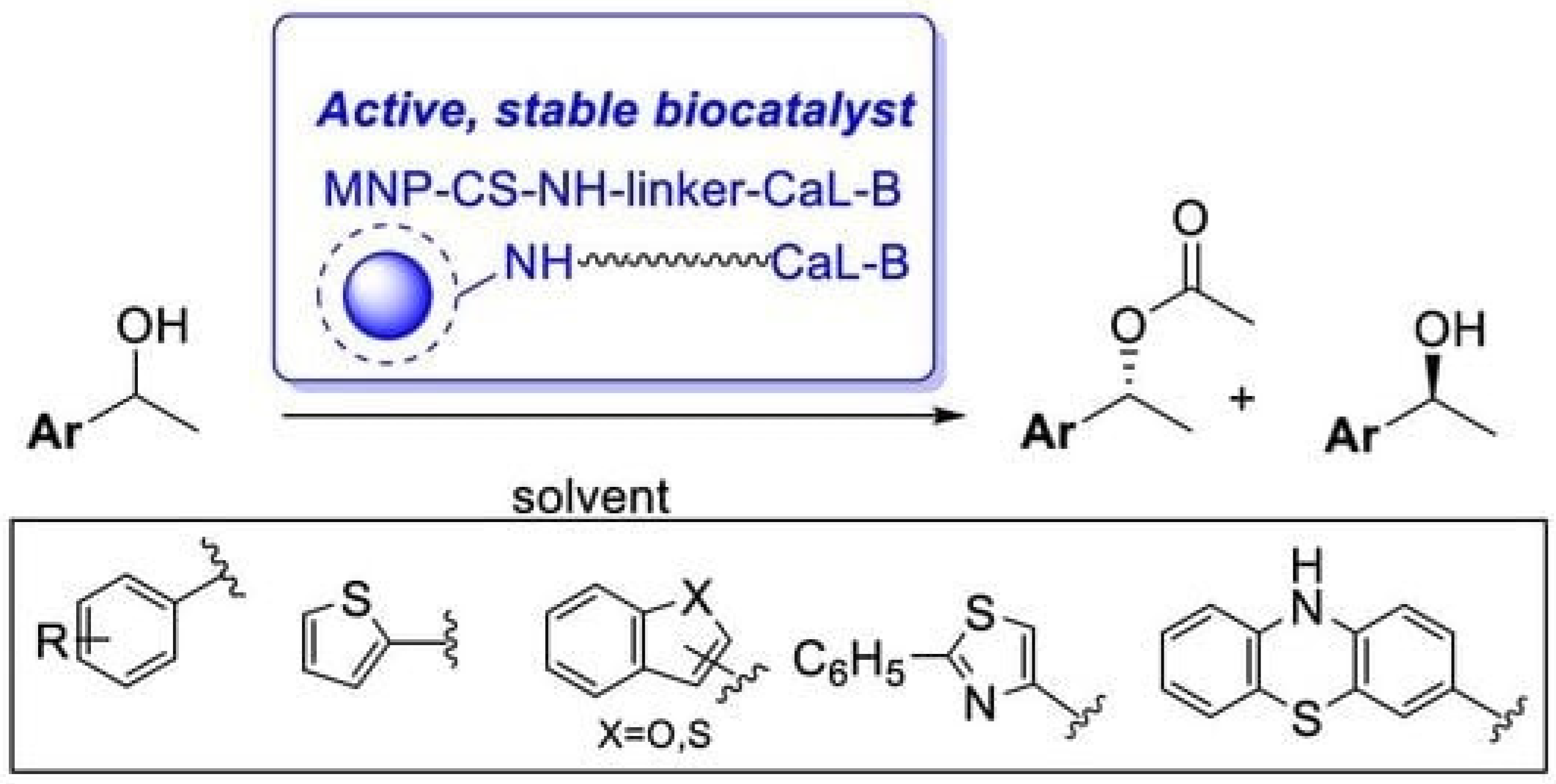
- Spelmezan, C.G.; Bencze, L.C.; Katona, G.; Irimie, F.D.; Paizs, C.; Toșa, M.I. Efficient and Stable Magnetic Chitosan-Lipase B from Candida Antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols. Molecules 2020, 25, 350. [Google Scholar] [CrossRef] [PubMed]
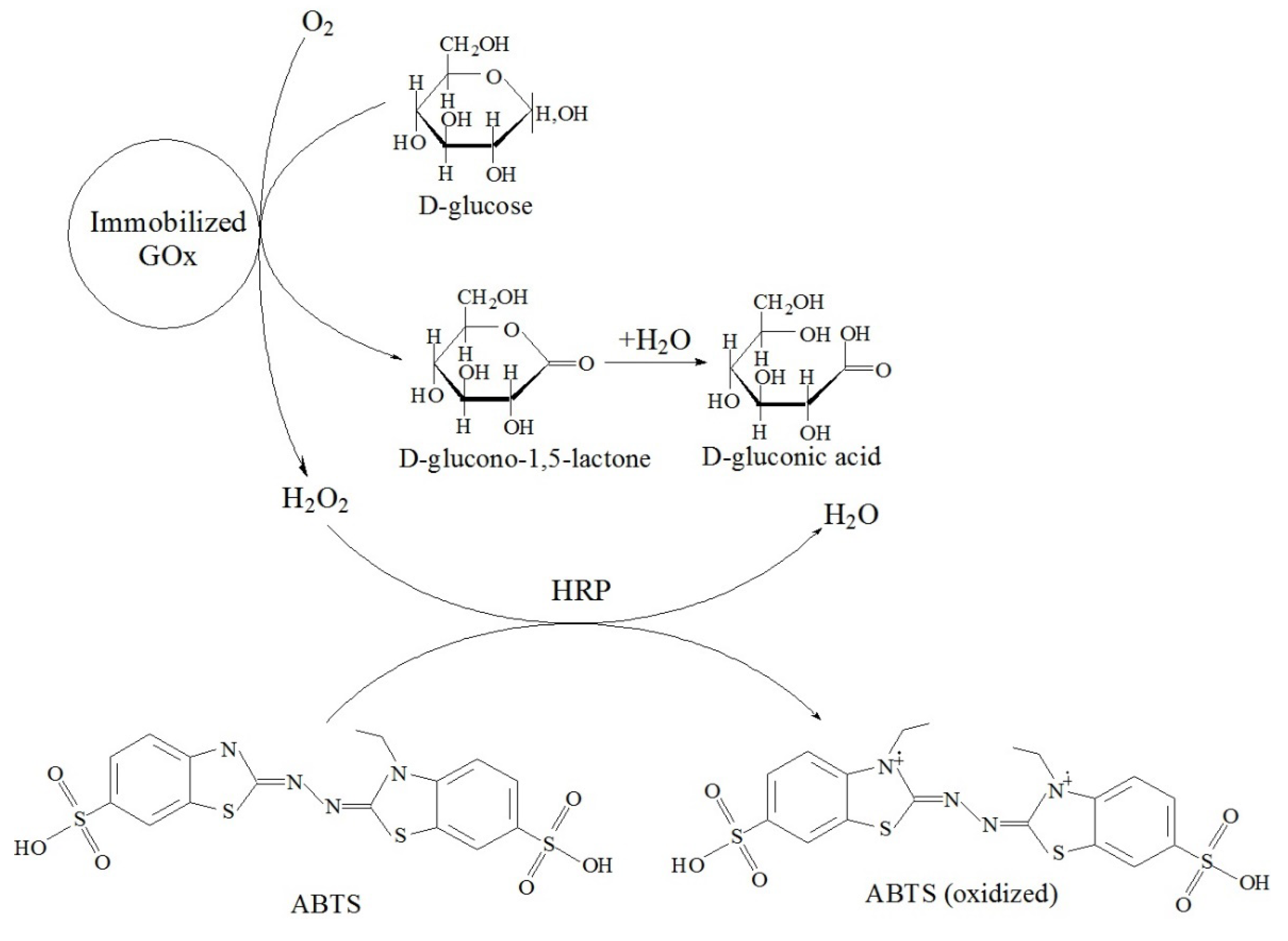
- Tikhonov, B.B.; Lisichkin, D.R.; Sulman, A.M.; Sidorov, A.I.; Bykov, A.V.; Lugovoy, Y.V.; Karpenkov, A.Y.; Bronstein, L.M.; Matveeva, V.G. Magnetic Nanoparticle Support with an Ultra-Thin Chitosan Layer Preserves the Catalytic Activity of the Immobilized Glucose Oxidase. Nanomaterials 2024, 14, 700. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Jacob, M.M.; Dayanandan, N.; Chinthala, M.; Ponnuchamy, M.; Vo, D.-V.N.; Appunni, S.; Gajendhran, A.S. Chitosan/metal organic frameworks for environmental, energy, and bio-medical applications: A review. Mater. Adv. 2023, 4, 5920–5947. [Google Scholar] [CrossRef]
- Anderson, S.L.; Stylianou, K.C. Biologically derived metal organic frameworks. Coord. Chem. Rev. 2017, 349, 102–128. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sirajudheen, P.; Sajna, V.P.; Park, C.M.; Meenakshi, S. Construction of ternary (1D/2D/3D) Fe2O3-supported micro pillared Cu-based MOF on chitosan with improved photocatalytic behavior on removal of paraquat. Environ. Sci. Pollut. Res. 2023, 30, 24876–24889. [Google Scholar] [CrossRef]
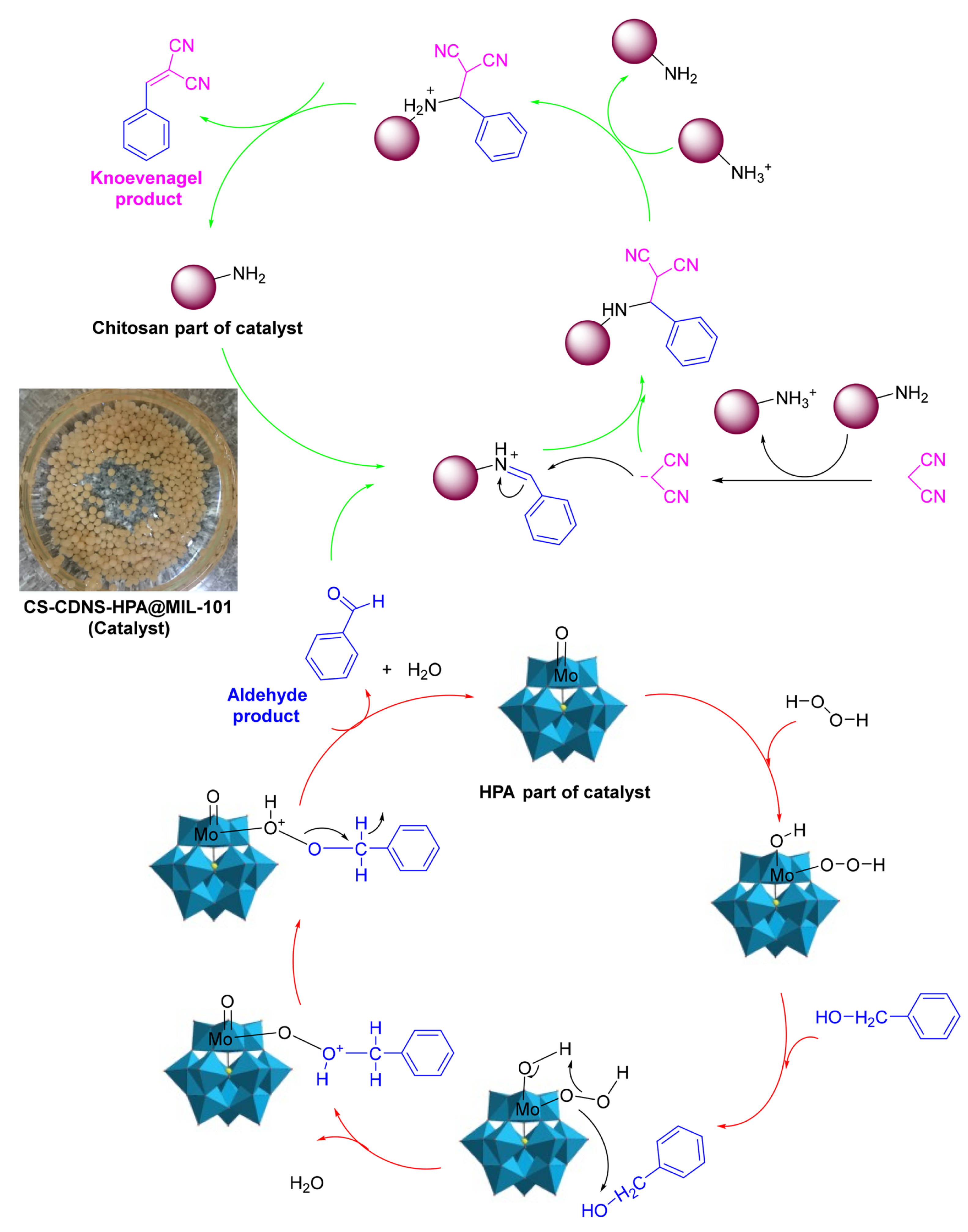
- Sadjadi, S.; Abedian-Dehaghani, N.; Heydari, A.; Heravi, M.M. Chitosan bead containing metal-organic framework encapsulated heteropolyacid as an efficient catalyst for cascade condensation reaction. Sci. Rep. 2023, 13, 2797. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Chen, Z.; Hu, J. Metal-organic framework grown in situ on chitosan microspheres as robust host of palladium for heterogeneous catalysis: Suzuki reaction and the p-nitrophenol reduction. Int. J. Biol. Macromol. 2022, 206, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Ara, R.; Yasmin, F.; Suchi, M.; Zzaman, W. Microwave and ultrasound assisted extraction techniques with citric acid of pectin from Pomelo (Citrus maxima) peel. Meas. Food 2024, 13, 100135. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, W.; Liao, X.; Hu, X.; Wu, J.; Wang, X. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT-Food Sci. Technol. 2017, 79, 640–646. [Google Scholar] [CrossRef]
- Sarah, M.; Hanum, F.; Rizky, M.; Hisham, M.F. Microwave-assisted extraction of pectin from cocoa peel. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012079. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Abu-Salem, F.M.; Helmy Azab, D.E.-S. A Comparative Study of Pectin Green Extraction Methods from Apple Waste: Characterization and Functional Properties. Int. J. Food Sci. 2022, 2022, 2865921. [Google Scholar] [CrossRef] [PubMed]
- Riyamol; Chengaiyan, J.G.; Rana, S.S.; Ahmad, F.; Haque, S.; Capanoglu, E. Recent Advances in the Extraction of Pectin from Various Sources and Industrial Applications. ACS Omega 2023, 8, 46309–46324. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and applications of pectin in food, biomedical, and pharmaceutical industry: A review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Tran, N.T.K.; Nguyen, V.B.; Tran, T.V.; Nguyen, T.T.T. Microwave-assisted extraction of pectin from jackfruit rags: Optimization, physicochemical properties and antibacterial activities. Food Chem. 2023, 418, 135807. [Google Scholar] [CrossRef]
- Pattarapisitporn, A.; Noma, S.; Klangpetch, W.; Demura, M.; Hayashi, N. Extraction of citrus pectin using pressurized carbon dioxide and production of its oligosaccharides. Food Biosci. 2024, 57, 103584. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, S. The effect of degree of esterification of pectin on the interaction between pectin and wheat gluten protein. Food Hydrocoll. 2023, 136, 108272. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Archut, A.; Eichhorn, M.; Kastner, H. Pectin—Plant protein systems and their application. Food Hydrocoll. 2021, 118, 106783. [Google Scholar] [CrossRef]
- Caroço, R.F.; Kim, B.; Santacoloma, P.A.; Abildskov, J.; Lee, J.H.; Huusom, J.K. Analysis and model-based optimization of a pectin extraction process. J. Food Eng. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Ripoll, C.S.S.; Hincapié-Llanos, G.A. Evaluation of sources and methods of pectin extraction from fruit and Vegetable wastes: A Systematic Literature Review (SLR). Food Biosci. 2023, 51, 102278. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Microwave-assisted extraction of pectin from grape pomace. Sci. Rep. 2022, 12, 12722. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Ranganathan, V.; Arora, A.; Macfarlane, D.R.; Patti, A.F. Lemon juice based extraction of pectin from mango peels: Waste to wealth by sustainable approaches. ACS Sustain. Chem. Eng. 2016, 4, 5915–5920. [Google Scholar] [CrossRef]
- Hu, W.; Cheng, H.; Wu, D.; Chen, J.; Ye, X.; Chen, S. Enhanced extraction assisted by pressure and ultrasound for targeting RG-I enriched pectin from citrus peel wastes: A mechanistic study. Food Hydrocoll. 2022, 133, 107778. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Amiri-Rigi, A.; Abdoun-Ouallouche, K.; Aberkane, L.; Djefal-Kerrar, A.; Mahomoodally, M.F.; Emmambux, M.N. Assessing the bioactivity, cytotoxicity, and rheological properties of pectin recovered from citrus peels. Food Biosci. 2022, 46, 101550. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Rahimifard, S.; Matharu, A.S.; Dugmore, T.I.J. Life-Cycle Assessment of Microwave-Assisted Pectin Extraction at Pilot Scale. ACS Sustain. Chem. Eng. 2019, 7, 5167–5175. [Google Scholar] [CrossRef]
- Abou-Elseoud, W.S.; Hassan, E.A.; Hassan, M.L. Extraction of pectin from sugar beet pulp by enzymatic and ultrasound-assisted treatments. Carbohydr. Polym. Technol. Appl. 2021, 2, 100042. [Google Scholar] [CrossRef]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT-Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Deslis, S.; Fabiano-Tixier, A.-S.; Pierre, F.-X.; Bily, A.; Chemat, F. Towards a “dry” bio-refinery without solvents or added water using microwaves and ultrasound for total valorization of fruit and vegetable by-products. Green Chem. 2016, 18, 3106–3115. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Martínez-Sabando, J.; Coin, F.; Melillo, J.H.; Goyanes, S.; Cerveny, S. A Review of Pectin-Based Material for Applications in Water Treatment. Materials 2023, 16, 2207. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N. Biological Properties and Biomedical Applications of Pectin and Pectin-Based Composites: A Review. Molecules 2023, 28, 7974. [Google Scholar] [CrossRef]
- Jandosov, J.; Alavijeh, M.; Sultakhan, S.; Baimenov, A.; Bernardo, M.; Sakipova, Z.; Azat, S.; Lyubchyk, S.; Zhylybayeva, N.; Naurzbayeva, G.; et al. Activated Carbon/Pectin Composite Enterosorbent for Human Protection from Intoxication with Xenobiotics Pb(II) and Sodium Diclofenac. Molecules 2022, 27, 2296. [Google Scholar] [CrossRef]
- Abouzeid, R.; El-Kader, A.H.A.; Salama, A.; Fahmy, T.Y.A.; El-Sakhawy, M. Preparation and properties of novel biocompatible pectin/silica calcium phosphate hybrids. Cellul. Chem. Technol. 2022, 56, 371–378. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Pectin modified metal nanoparticles and their application in property modification of biosensors. Carbohydr. Polym. Technol. Appl. 2021, 2, 100164. [Google Scholar] [CrossRef]
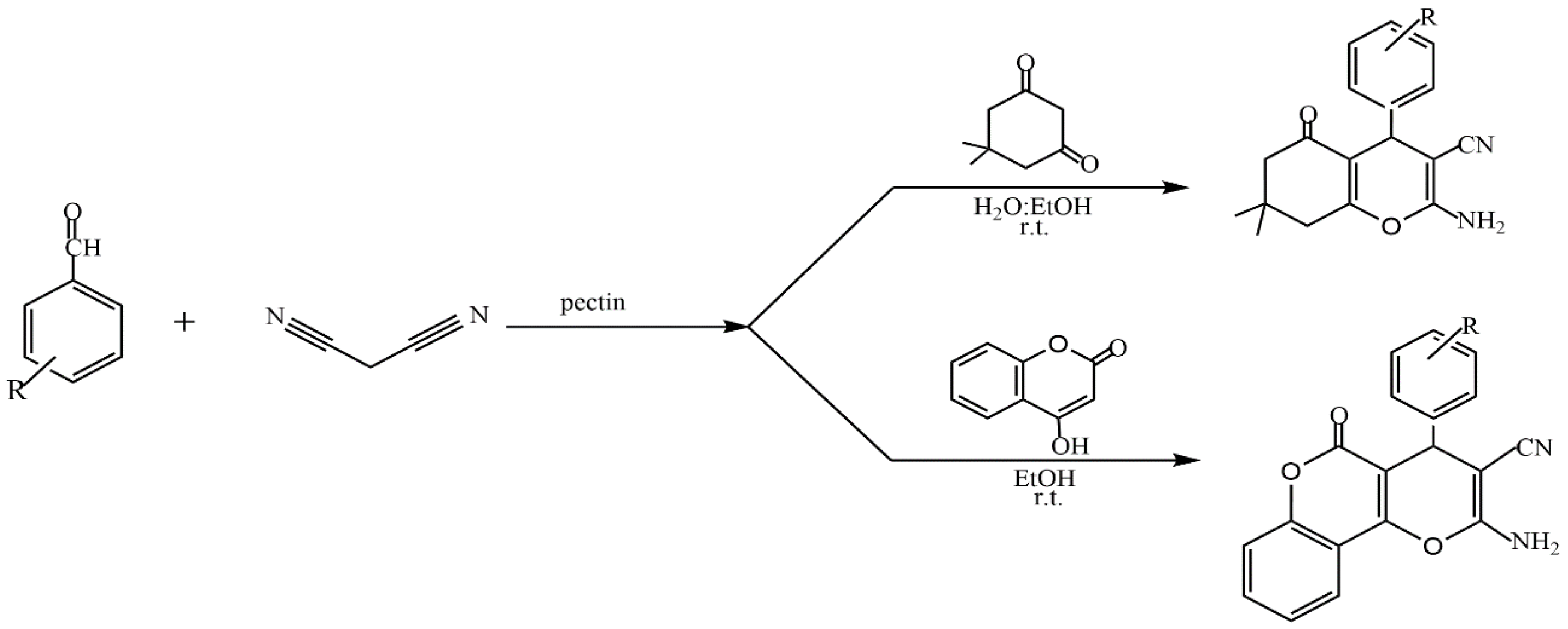
- Kangani, M.; Hazeri, N.; Maghsoodlou, M.T. A Mild and Environmentally Benign Synthesis of Tetrahydrobenzo[b]pyrans and Pyrano[c]chromenes Using Pectin as a Green and Biodegradable Catalyst. J. Chin. Chem. Soc. 2016, 63, 896–901. [Google Scholar] [CrossRef]
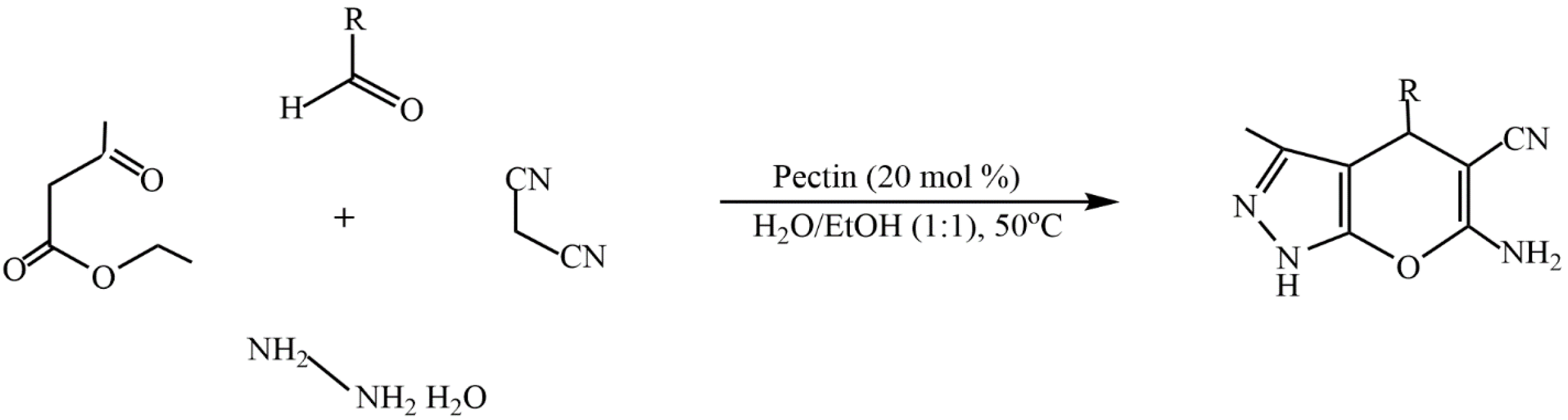
- Mohamadpour, F. Pectin as a natural biopolymer catalyst promoted green synthesis of dihydropyrano[2,3-c]pyrazole derivatives in aqueous ethanol media. Indian J. Chem. 2022, 61, 405–410. [Google Scholar] [CrossRef]
- Albano, G.; Petri, A.; Aronica, L.A. Palladium Supported on Bioinspired Materials as Catalysts for C–C Coupling Reactions. Catalysts 2023, 13, 210. [Google Scholar] [CrossRef]
- Attallah, O.A.; Rabee, M. A pectin/chitosan/zinc oxide nanocomposite for adsorption/photocatalytic remediation of carbamazepine in water samples. RSC Adv. 2020, 10, 40697–40708. [Google Scholar] [CrossRef]
- Fiedot-Toboła, M.; Dmochowska, A.; Jędrzejewski, R.; Stawiński, W.; Kryszak, B.; Cybińska, J. Pectin-organophilized ZnO nanoparticles as sustainable fillers for high-density polyethylene composites. Int. J. Biol. Macromol. 2021, 182, 1832–1842. [Google Scholar] [CrossRef]
- Khazaei, A.; Rahmati, S.; Hekmatian, Z.; Saeednia, S. A green approach for the synthesis of palladium nanoparticles supported on pectin: Application as a catalyst for solvent-free Mizoroki–Heck reaction. J. Mol. Catal. A Chem. 2013, 372, 160–166. [Google Scholar] [CrossRef]
- Nazeri, M.T.; Javanbakht, S.; Nabi, M.; Shaabani, A. Copper phthalocyanine-conjugated pectin via the Ugi four-component reaction: An efficient catalyst for CO2 fixation. Carbohydr. Polym. 2022, 283, 119144. [Google Scholar] [CrossRef] [PubMed]
- Acosta, P.I.; Campedelli, R.R.; Correa, E.L.; Bazani, H.A.G.; Nishida, E.N.; Souza, B.S.; Mora, J.R. Efficient production of biodiesel by using a highly active calcium oxide prepared in presence of pectin as heterogeneous catalyst. Fuel 2020, 271, 117651. [Google Scholar] [CrossRef]
- Baran, T. Pd(0) nanocatalyst stabilized on a novel agar/pectin composite and its catalytic activity in the synthesis of biphenyl compounds by Suzuki-Miyaura cross coupling reaction and reduction of o-nitroaniline. Carbohydr. Polym. 2018, 195, 45–52. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Jiang, W.; Ma, G.; Zangeneh, M.M. In situ decorated Au NPs on pectin-modified Fe3O4 NPs as a novel magnetic nanocomposite (Fe3O4/Pectin/Au) for catalytic reduction of nitroarenes and investigation of its anti-human lung cancer activities. Int. J. Biol. Macromol. 2020, 163, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- ASTM D6751-20A; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2020.
- Yarmohammadi, N.; Ghadermazi, M.; Derikvand, Z.; Mozafari, R. In situ synthesis of bimetallic γ-Fe2O3/Cu nanoparticles over pectin hydrogel obtained from biomass resource (orange peel) as a reusable green catalyst for oxidation and C-S cross-coupling reactions. Chem. Pap. 2022, 76, 4289–4307. [Google Scholar] [CrossRef]
- Baran, T. Highly recoverable, reusable, cost-effective, and Schiff base functionalized pectin supported Pd(II) catalyst for microwave-accelerated Suzuki cross-coupling reactions. Int. J. Biol. Macromol. 2019, 127, 232–239. [Google Scholar] [CrossRef]
- Dolinska, J.; Holdynski, M.; Pieta, P.; Lisowski, W.; Ratajczyk, T.; Palys, B.; Jablonska, A.; Opallo, M. Noble Metal Nanoparticles in Pectin Matrix. Preparation, Film Formation, Property Analysis, and Application in Electrocatalysis. ACS Omega 2020, 5, 23909–23918. [Google Scholar] [CrossRef]
- Ponce, S.; Gangotena, P.A.; Anthony, C.; Rodriguez, Y.; Bazani, H.A.G.; Keller, M.H.; Souza, B.S.; Vizuete, K.; Debut, A.; Mora, J.R. Aluminum-based catalysts prepared in the presence of pectin for low-energy biodiesel production. Fuel 2024, 361, 130691. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Seitkalieva, K.S.; Talgatov, E.T.; Auezkhanova, A.S.; Dzhardimalieva, G.I.; Pomogailo, A.D. Polymer-modified supported palladium catalysts for the hydrogenation of acetylene compounds. Kinet. Catal. 2016, 57, 360–367. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Auyezkhanova, A.S.; Talgatov, E.T.; Akhmetova, S.N.; Tumabayev, N.Z.; Rafikova, K.S. Polysaccharide-Stabilized Nanocatalysts in Hydrogenation of Phenylacetylene. Theor. Exp. Chem. 2020, 56, 39–45. [Google Scholar] [CrossRef]
- Noval, V.E.; Carriazo, J.G. Fe3O4-TiO2 and Fe3O4-SiO2 Core-shell Powders Synthesized from Industrially Processed Magnetite (Fe3O4) Microparticles. Mater. Res. 2019, 22, 3. [Google Scholar] [CrossRef]
- Zhang, W.; Veisi, H.; Sharifi, R.; Salamat, D.; Karmakar, B.; Hekmati, M.; Hemmati, S.; Zhang, Z.; Su, Q.; Zangeneh, M.M. Fabrication of Pd NPs on Pectin-modified Fe3O4 NPs: A magnetically nanocatalyst for C–C and C–N cross coupling reactions and investigating the cardiovascular protective effects. Int. J. Biol. Macromol. 2020, 160, 1252–1262. [Google Scholar] [CrossRef]
- Ghamari Kargar, P.; Ghasemi, M.; Bagherzade, G. Copper (II) supported on a post-modified magnetic pectin Fe3O4@PectinImidazoleSO3H-Cu(II): An efficient biopolymer-based catalyst for selective oxidation of alcohols with aqueous TBHP. Sci. Iran. 2022, 29, 1338–1350. [Google Scholar] [CrossRef]
- Xue, W.; Yang, G.; Karmakar, B.; Gao, Y. Sustainable synthesis of Cu NPs decorated on pectin modified Fe3O4 nanocomposite: Catalytic synthesis of 1-substituted-1H-tetrazoles and in-vitro studies on its cytotoxicity and anti-colorectal adenocarcinoma effects on HT-29 cell lines. Arab. J. Chem. 2021, 14, 103306. [Google Scholar] [CrossRef]
- Siuki, H.K.; Bagherzade, G.; Kargar, P.G. A Green Method for Synthesizing Nickel Nanoparticles Supported by Magnetized Pectin: Applied as a Catalyst for Aldehyde Synthesis as a Precursor in Xanthan Synthesis. ChemistrySelect 2020, 5, 13537–13544. [Google Scholar] [CrossRef]
- Doustkhah, E.; Heidarizadeh, M.; Rostamnia, S.; Hassankhani, A.; Kazemi, B.; Liu, X. Copper immobilization on carboxylic acid-rich Fe3O4-Pectin: Cu2+@Fe3O4-Pectin a superparamagnetic nanobiopolymer source for click reaction. Mater. Lett. 2018, 216, 139–143. [Google Scholar] [CrossRef]
- Li, S.; He, G.; Huang, J. Self-assembly on natural cellulose: Towards high-efficient catalysts. Curr. Opin. Colloid Interface Sci. 2023, 63, 101655. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Guo, X.-Y.; Liu, B.; Zhang, J.-L.; Gao, X.-H.; Ma, Q.-X.; Fan, S.-B.; Zhao, T.-S. Cellulose modified iron catalysts for enhanced light olefins and linear C5+ α-olefins from CO hydrogenation. Fuel 2021, 294, 120504. [Google Scholar] [CrossRef]
- Naeimi, A.; Ardakani, M.H. Natural bentonite/cellulose/vanadium novel complex nanopolymer complex catalyst: Synthesis, characterization, and oxidation activity of several organic compounds. Carbohydr. Polym. Technol. Appl. 2024, 7, 100439. [Google Scholar] [CrossRef]
- El Allaoui, B.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Cellulose beads supported CoFe2O4: A novel heterogeneous catalyst for efficient rhodamine B degradation via advanced oxidation processes. Int. J. Biol. Macromol. 2024, 259, 128893. [Google Scholar] [CrossRef] [PubMed]
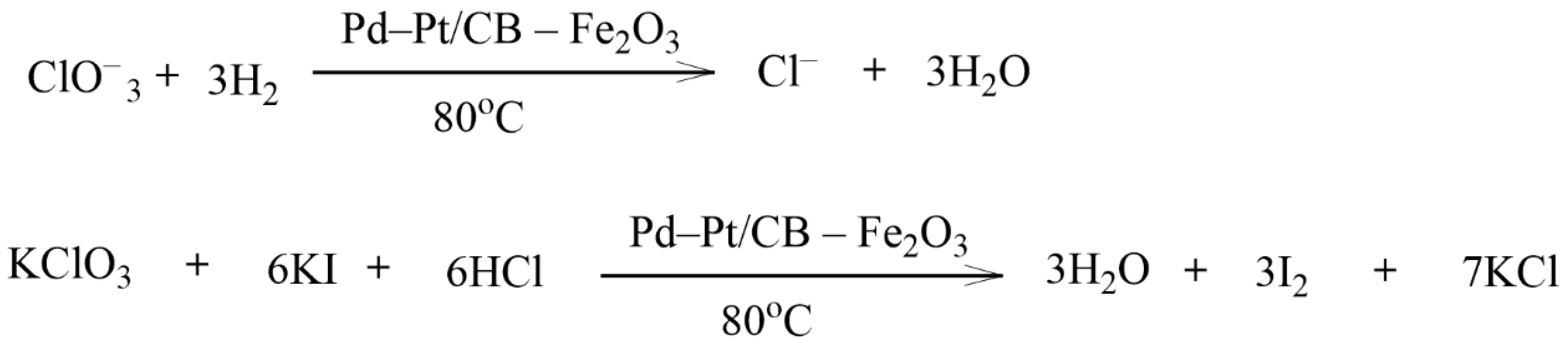
- Sikora, E.; Katona, K.K.; Muránszky, G.; Bánhidi, O.; Kristály, F.; Szabó, J.T.; Windisch, M.; Fiser, B.; Vanyorek, L. Cellulose-based catalyst design for efficient chlorate reduction. Arab. J. Chem. 2021, 14, 103202. [Google Scholar] [CrossRef]
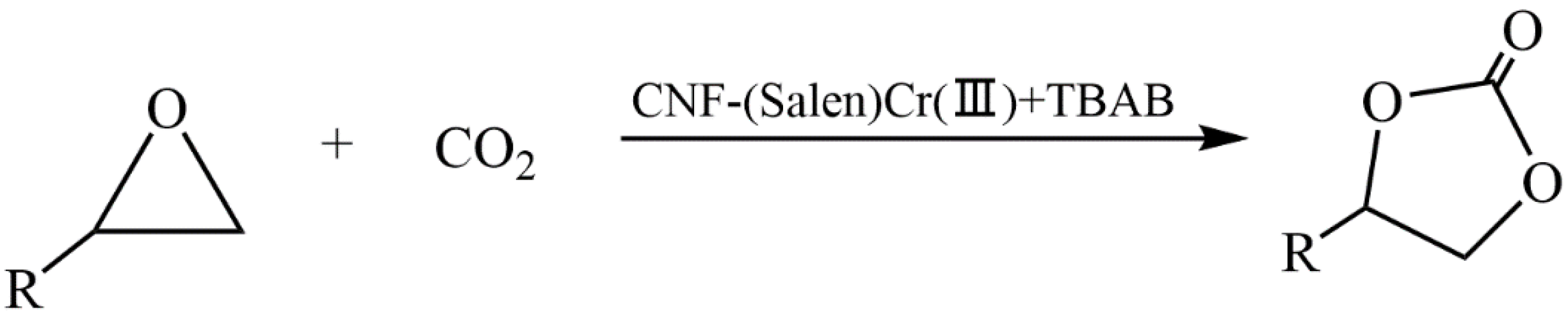
- Liu, Y.; Li, S.; Yu, X.; Chen, Y.; Tang, X.; Hu, T.; Shi, L.; Pudukudy, M.; Shan, S.; Zhi, Y. Cellulose nanofibers (CNF) supported (Salen)Cr(III) composite as an efficient heterogeneous catalyst for CO2 cycloaddition. Mol. Catal. 2023, 547, 113344. [Google Scholar] [CrossRef]
- Gia-Thien Ho, T.; Truong, D.P.T.; Nguyen, H.B.; Do, B.L.; Dinh, T.A.; Ton-That, P.; Nguyen, T.T.V.; Truong, T.B.T.; Huynh, K.P.H.; Tri, N. Green synthesized nano-silver/cellulose aerogel as a robust active and recyclable catalyst towards nitrophenol hydrogenation. Chem. Eng. J. 2023, 471, 144604. [Google Scholar] [CrossRef]
- El Idrissi, N.; Belachemi, L.; Merle, N.; Zinck, P.; Kaddami, H. Comprehensive preparation and catalytic activities of Co/TEMPO-cellulose nanocomposites: A promising green catalyst. Carbohydr. Polym. 2022, 295, 119765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, B.; Zhang, Z.; Zhao, X.; Li, W.; Lia, B.; Zhu, L. Preparation of chitosan/cellulose composite copper catalyst for green synthesis in the construction of C–Si bonds in aqueous phase. Green Chem. 2023, 25, 6253–6262. [Google Scholar] [CrossRef]
- Zhang, L.; Long, S.; Jiao, H.; Liu, Z.; Zhang, P.; Lei, A.; Gong, W.; Pei, X. Cellulose derived Pd nano-catalyst for efficient catalysis. RSC Adv. 2022, 12, 18676–18684. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, L.; Shi, Z.; Ma, J.; Wang, Z. Cellulose template designed porous ZnO based catalysts with different valence copper for solar photocatalytic CO2 conversion. Ind. Crops Prod. 2022, 186, 115223. [Google Scholar] [CrossRef]
- Li, D.; Lu, G.; Cai, C. Modified cellulose with tunable surface hydrophilicity/hydrophobicity as a novel catalyst support for selective reduction of nitrobenzene. Catal. Commun. 2020, 137, 105949. [Google Scholar] [CrossRef]
- Bahsis, L.; El Ayouchia, H.B.; Anane, H.; Benhamou, K.; Kaddami, H.; Julve, M.; Stiriba, S.-E. Cellulose-copper as bio-supported recyclable catalyst for the clickable azide-alkyne [3 + 2] cycloaddition reaction in water. Int. J. Biol. Macromol. 2018, 119, 849–856. [Google Scholar] [CrossRef]
- Prekob, Á.; Hajdu, V.; Muránszky, G.; Fiser, B.; Sycheva, A.; Ferenczi, T.; Viskolcz, B.; Vanyorek, L. Application of carbonized cellulose-based catalyst in nitrobenzene hydrogenation. Mater. Today Chem. 2020, 17, 100337. [Google Scholar] [CrossRef]
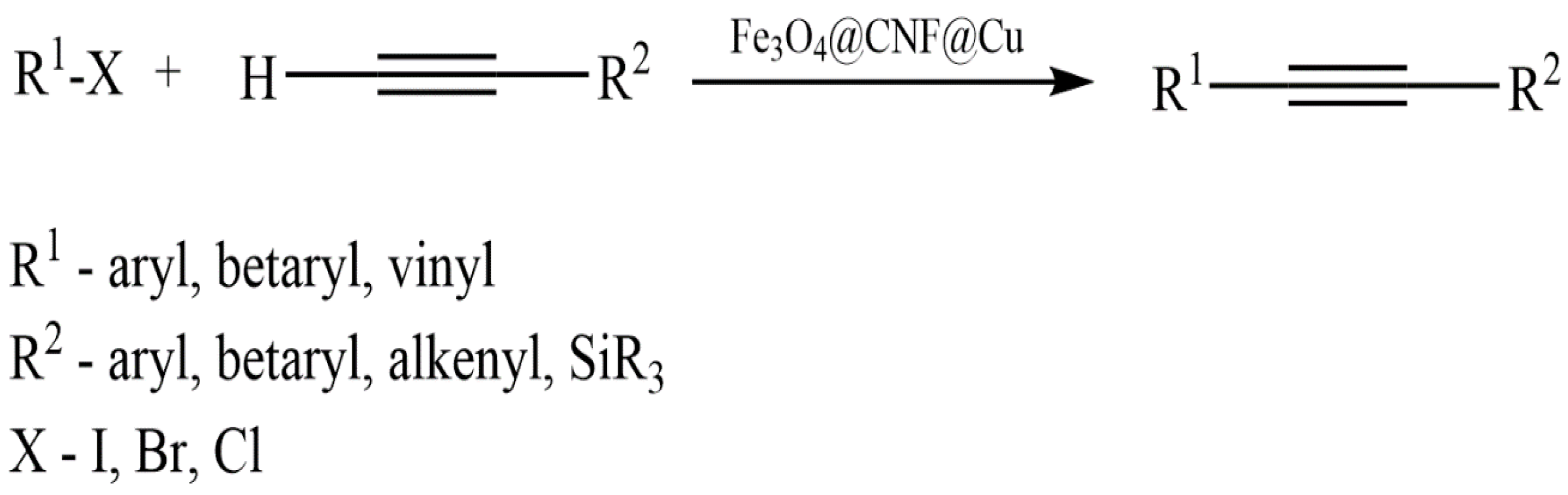
- Fan, X.; Lin, D.; Xu, Z.; Li, Y. Pd/Cu bimetallic catalyst immobilized on PEI capped cellulose-polyamidoamine dendrimer: Synthesis, characterization, and application in Sonogashira reactions for the synthesis of alkynes and benzofurans. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129206. [Google Scholar] [CrossRef]
- Dewan, A.; Sarmah, M.; Bhattacharjee, P.; Bharali, P.; Thakur, A.J.; Bora, U. Sustainable nano fibrillated cellulose supported in situ biogenic Pd nanoparticles as heterogeneous catalyst for C–C cross coupling reactions. Sustainable. Chem. Pharm. 2021, 23, 100502. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Zhao, D.; Chen, W.; Li, H.; Hou, C. Preparation of Magnetic CuFe2O4@Ag@ZIF-8 Nanocomposites with Highly Catalytic Activity Based on Cellulose Nanocrystals. Molecules 2020, 25, 124. [Google Scholar] [CrossRef]
- Almajidi, Y.Q.; Majeed, A.A.; Ali, E.; Abdullaev, S.; Koka, N.A.; Bisht, Y.S.; Fenjan, M.N.; Alawadi, A.; Alsalamy, A.; Saleh, L.H. A versatile magnetic nanocomposite based on cellulose-cyclodextrin hydrogel embedded with graphene oxide and Cu2O nanoparticles for catalytic application. Int. J. Biol. Macromol. 2024, 260, 129367. [Google Scholar] [CrossRef] [PubMed]
- Kargar, P.G.; Len, C.; Luque, R. Cu/cellulose-modified magnetite nanocomposites as a highly active and selective catalyst for ultrasound-promoted aqueous O-arylation Ullmann and sp-sp2 Sonogashira cross-coupling reactions. Sustainable. Chem. Pharm. 2022, 27, 100672. [Google Scholar] [CrossRef]
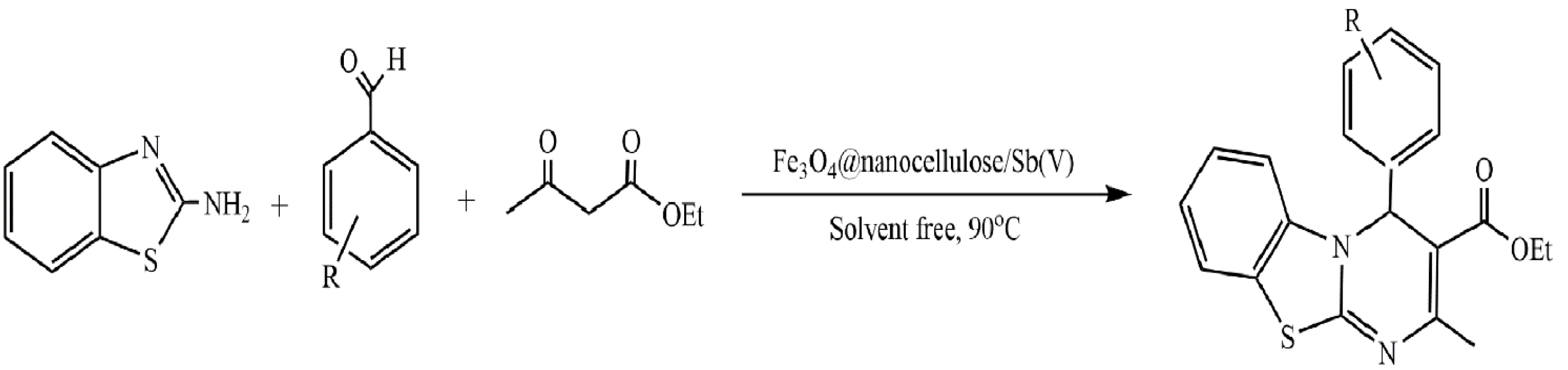
- Hosseinikhah, S.S.; Mirjalili, B.B.F. Fe3O4@NCs/Sb(V): As a Cellulose Based Nano-Catalyst for the Synthesis of 4H-Pyrimido [2,1-b]benzothiazoles. Polycycl. Aromat. Compd. 2022, 42, 1013–1022. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis and characterization of superabsorbent hydrogels based on hydroxyethylcellulose and acrylic acid. Carbohydr. Polym. 2017, 166, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meunier, D.M.; Partain, E.M. Molecular weight distribution characterization of hydrophobe-modified hydroxyethyl cellulose by size-exclusion chromatography. J. Chromatogr. A 2014, 1359, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, L.; Xu, W.; Fan, L.; Nie, M. Preparation and characterization of aminated hyaluronic acid/oxidized hydroxyethyl cellulose hydrogel. Carbohydr. Polym. 2018, 199, 170–177. [Google Scholar] [CrossRef]
- Dong, Y.; Bi, J.; Zhang, S.; Zhu, D.; Meng, D.; Ming, S.; Qin, K.; Liu, Q.; Li, T. Palladium supported on N-Heterocyclic carbene functionalized hydroxyethyl cellulose as a novel and efficient catalyst for the Suzuki reaction in aqueous media. Appl. Surf. Sci. 2020, 531, 147392. [Google Scholar] [CrossRef]
- Grządka, E.; Matusiak, J.; Paszkiewicz, M. Factors influencing the stability of the 2-hydroxyethyl cellulose/alumina system. Cellulose 2018, 25, 2839–2847. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Adsorption and corrosion inhibition behavior of hydroxyethylcellulose and synergistic surfactants additives for carbon steel in 1 M HCl. Carbohyd. Polym. 2017, 156, 202–214. [Google Scholar] [CrossRef]
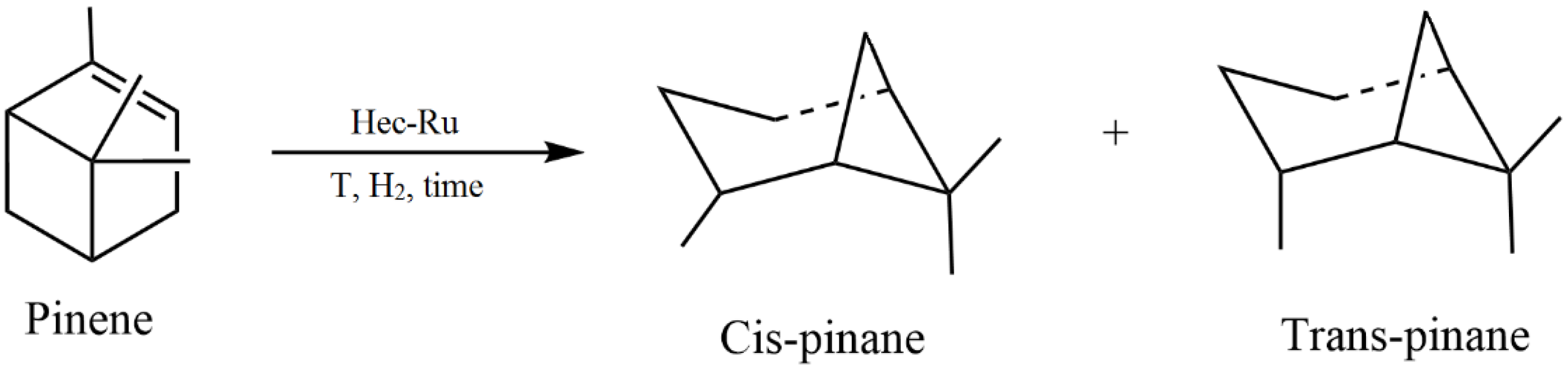
- Xie, C.; Qu, L.; Yu, H.; Yu, F.; Yuan, B.; Yu, S.; Nie, S. Synthesis of Ru nanoparticles with hydroxyethyl cellulose as stabilizer for high-efficiency reduction of α-pinene. Cellulose 2019, 26, 8059–8071. [Google Scholar] [CrossRef]
- Unlu, D.; Ilgen, O.; Hilmioglu, N.D. Biodiesel additive ethyl levulinate synthesis by catalytic membrane: SO4−2/ZrO2 loaded hydroxyethyl cellulose. Chem. Eng. J. 2016, 302, 260–268. [Google Scholar] [CrossRef]
- Khoo, P.S.; Ilyas, R.A.; Uda, M.N.A.; Hassan, S.A.; Nordin, A.H.; Norfarhana, A.S.; Ab Hamid, N.H.; Rani, M.S.A.; Abral, H.; Norrrahim, M.N.F.; et al. Starch-Based Polymer Materials as Advanced Adsorbents for Sustainable Water Treatment: Current Status, Challenges, and Future Perspectives. Polymers 2023, 15, 3114. [Google Scholar] [CrossRef] [PubMed]
- Egharevba, O.H. Chemical Properties of Starch and Its Application in the Food Industry; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/68437 (accessed on 5 August 2019).
- Tan, W.T.; Jusoh, M.; Zakaria, Z.Y. Starch-derived Solid Acid Catalyst for Biodiesel Production: A Mini Review. Chem. Eng. Trans. 2021, 89, 487–492. [Google Scholar] [CrossRef]
- Wongmanee, K.; Khuanamkam, S.; Chairam, S. Gold nanoparticles stabilized by starch polymer and their use as catalyst in homocoupling of phenylboronic acid. J. King Saud Univ. 2017, 29, 547–552. [Google Scholar] [CrossRef]
- Cao, F.; Lu, S.; Wang, L.; Zheng, M.; Quek, S.Y. Modified porous starch for enhanced properties: Synthesis, characterization and applications. Food Chem. 2023, 415, 135765. [Google Scholar] [CrossRef]
- Jafari, S.; Khodaensaf, F.; Delattre, C.; Bazargan, V.; Lukova, P. Mesoporous Starch Cryoaerogel Material as an Emerging Platform for Oral Drug Delivery: Synthesis and In Vitro Evaluation. Gels 2023, 9, 623. [Google Scholar] [CrossRef]
- Luque, R.; Clark, J.H.; Yoshida, K.; Gai, P.L. Efficient aqueous hydrogenation of biomass platform molecules using supported metal nanoparticles on Starbons®. Chem. Commun. 2009, 35, 5305–5307. [Google Scholar] [CrossRef]
- Duman, S.; Özhava, D. Green Approaches to Dehydrogenation of DMAB Catalyzed by Starch Stabilized Ru(0), Cu(0) and Ni(0) Nanoparticles in the Absence of a Solvent. Chem. Sel. 2023, 8, e202204606. [Google Scholar] [CrossRef]
- Dasgupta, N.; Nayak, M.A.; Gauthier, M. Starch-Stabilized Iron Oxide Nanoparticles for the Photocatalytic Degradation of Methylene Blue. Polysaccharides 2022, 3, 655–670. [Google Scholar] [CrossRef]
- Chikunov, A.S.; Taran, O.P.; Pyshnaya, I.A.; Parmon, V.N. Colloidal FeIII, MnIII, CoIII, and CuII Hydroxides Stabilized by Starch as Catalysts of Water Oxidation Reaction with One Electron Oxidant Ru(bpy)33+. Chem. Phys. Chem. 2019, 20, 410–421. [Google Scholar] [CrossRef]
- Gholinejad, M.; Dasvarz, N.; Shojafar, M.; Sansano, J.M. Starch Functionalized Creatine for Stabilization of Gold Nanoparticles: Efficient Heterogeneous Catalyst for the Reduction of Nitroarenes. Inorganica Chim. Acta 2019, 495, 118965. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Z.; Lu, X. Facile synthesis of starch-based nanoparticle stabilized Pickering emulsion: Its pH-responsive behavior and application for recyclable catalysis. Green Chem. 2018, 20, 1538–1550. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Rad-Moghadam, K.; Mehrdad, M.; Rouhi, S. Starch mediates and cements densely magnetite-coating of talc, giving an efficient nano-catalyst for three-component synthesis of imidazo [1,2-c]quinazolines. Sci. Rep. 2024, 14, 666. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.; Poursattar, A.; Zamani, A. A Novel Biopolymer-based Nanomagnetic Catalyst for the Synthesis of 4H-pyran and Tetrahydro-4H-chromene Derivatives. S. Afr. J. Chem. 2020, 73, 55–63. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Ansari, S.; Hamedifar, H.; Mahdavi, M. The use of magnetic starch as a support for an ionic liquid-β-cyclodextrin based catalyst for the synthesis of imidazothiadiazolamine derivatives. Int. J. Biol. Macromol. 2019, 135, 453–461. [Google Scholar] [CrossRef]
- Budarin, V.; Clark, J.H.; Hardy, J.E.; Luque, R.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New Starch-Derived Mesoporous Carbonaceous Materials with Tunable Properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786. [Google Scholar] [CrossRef]
- Matharu, A.S.; Ahmed, S.; Almonthery, B.; Macquarrie, D.J.; Lee, Y.-S.; Kim, Y. Starbon/High-Amylose Corn Starch-Supported N-Heterocyclic Carbene–Iron(III) Catalyst for Conversion of Fructose into 5-Hydroxymethylfurfural. ChemSusChem 2018, 11, 716–725. [Google Scholar] [CrossRef]
- Clohessy, J.; Kwapinski, W. Carbon-Based Catalysts for Biodiesel Production—A Review. Appl. Sci. 2020, 10, 918. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.; Mou, L.; Wu, M.; Wang, Y.; Cai, W.; Tan, F. Preparation of the porous carbon-based solid acid from starch for efficient degradation of chitosan to D-glucosamine. Int. J. Biol. Macromol. 2022, 209, 1629–1637. [Google Scholar] [CrossRef]
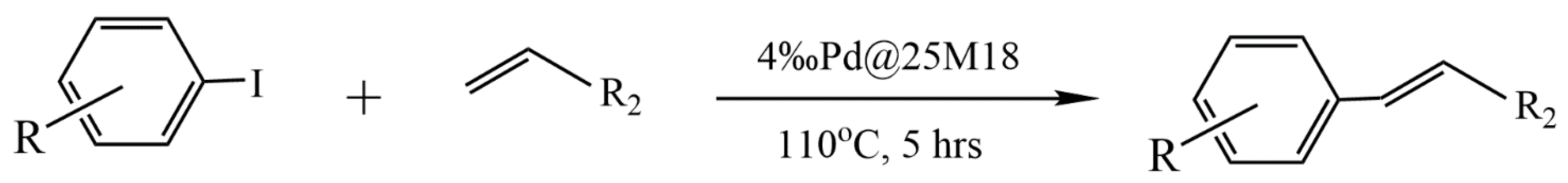




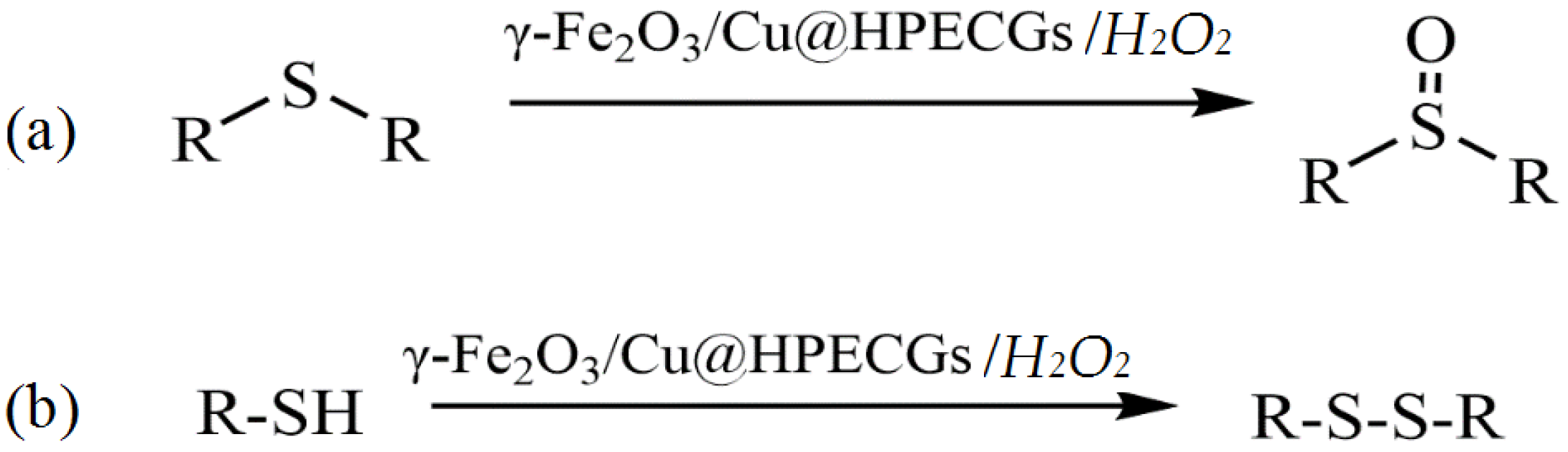
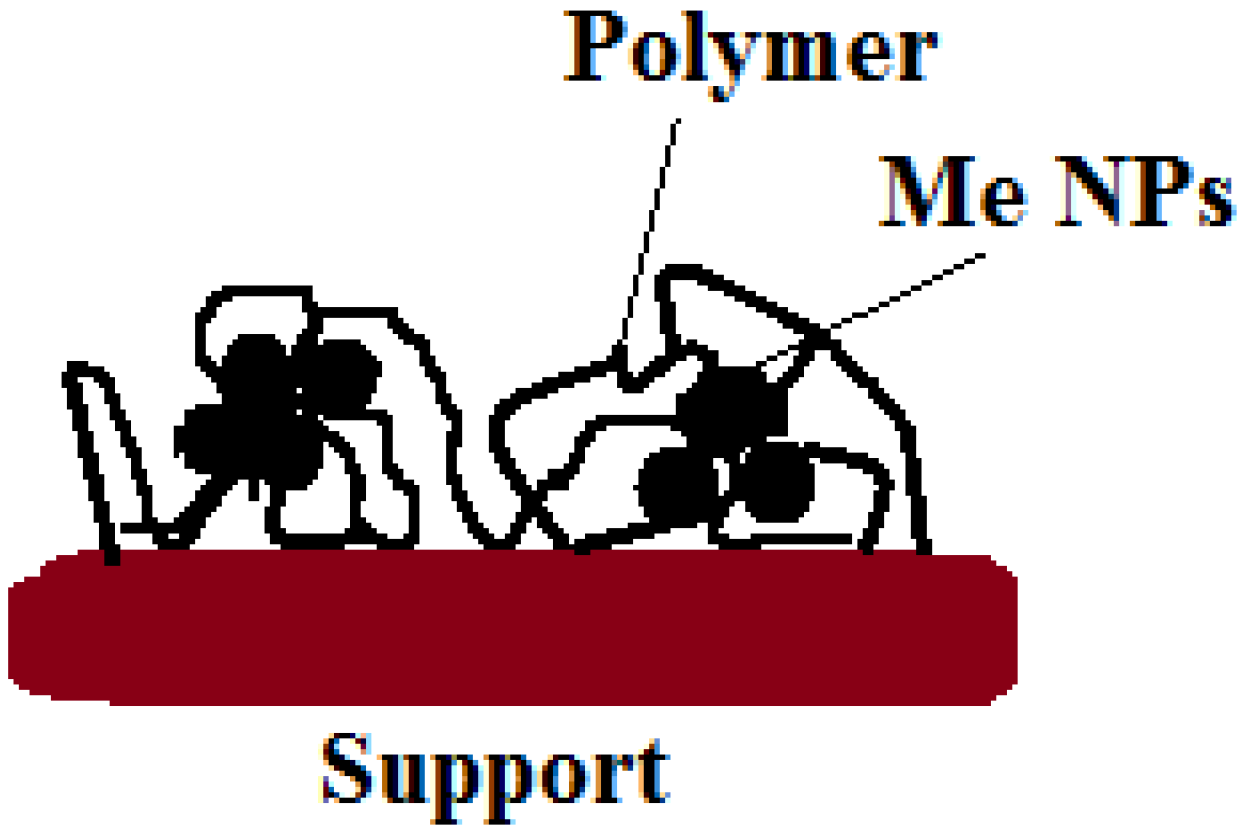
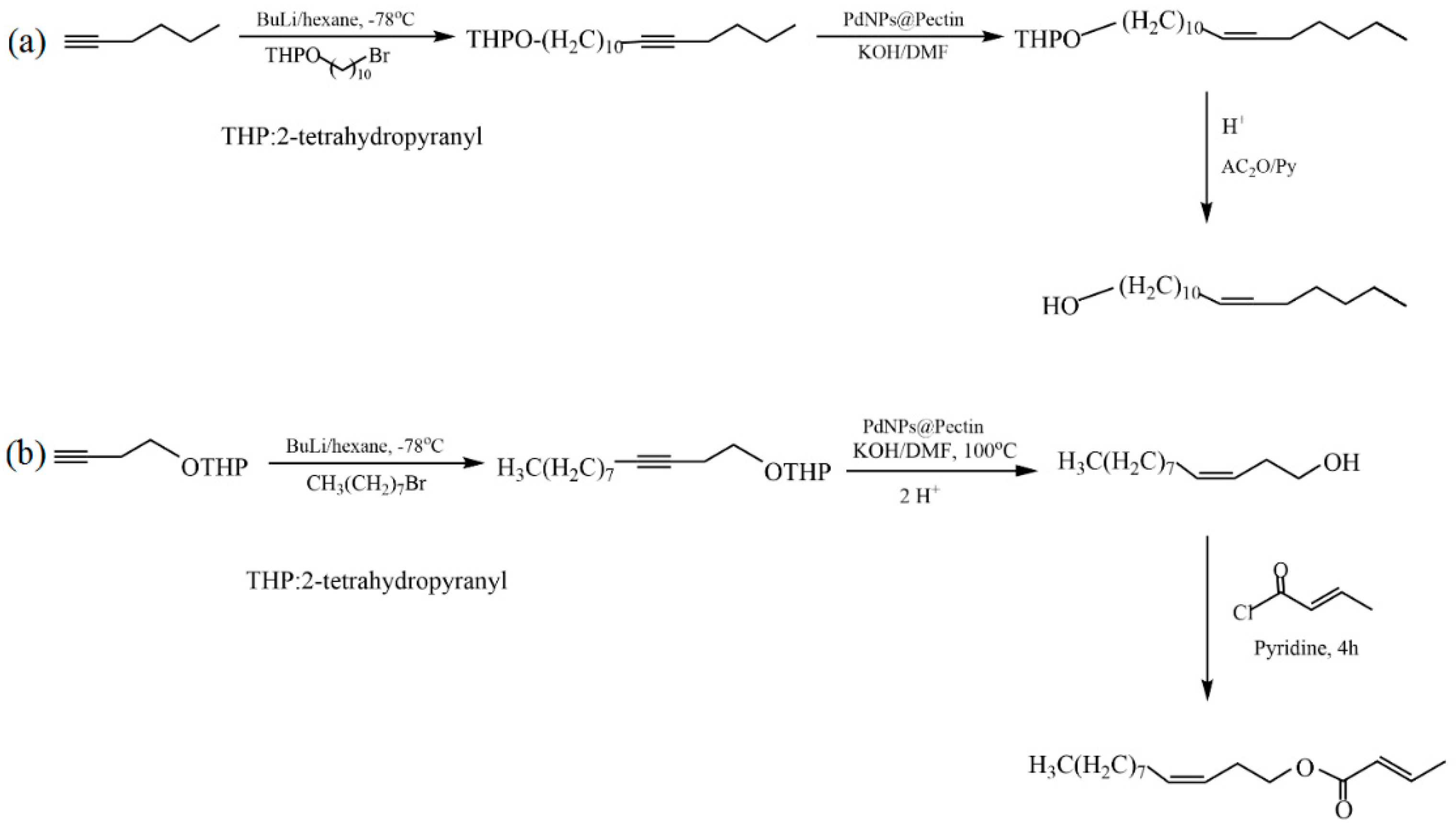

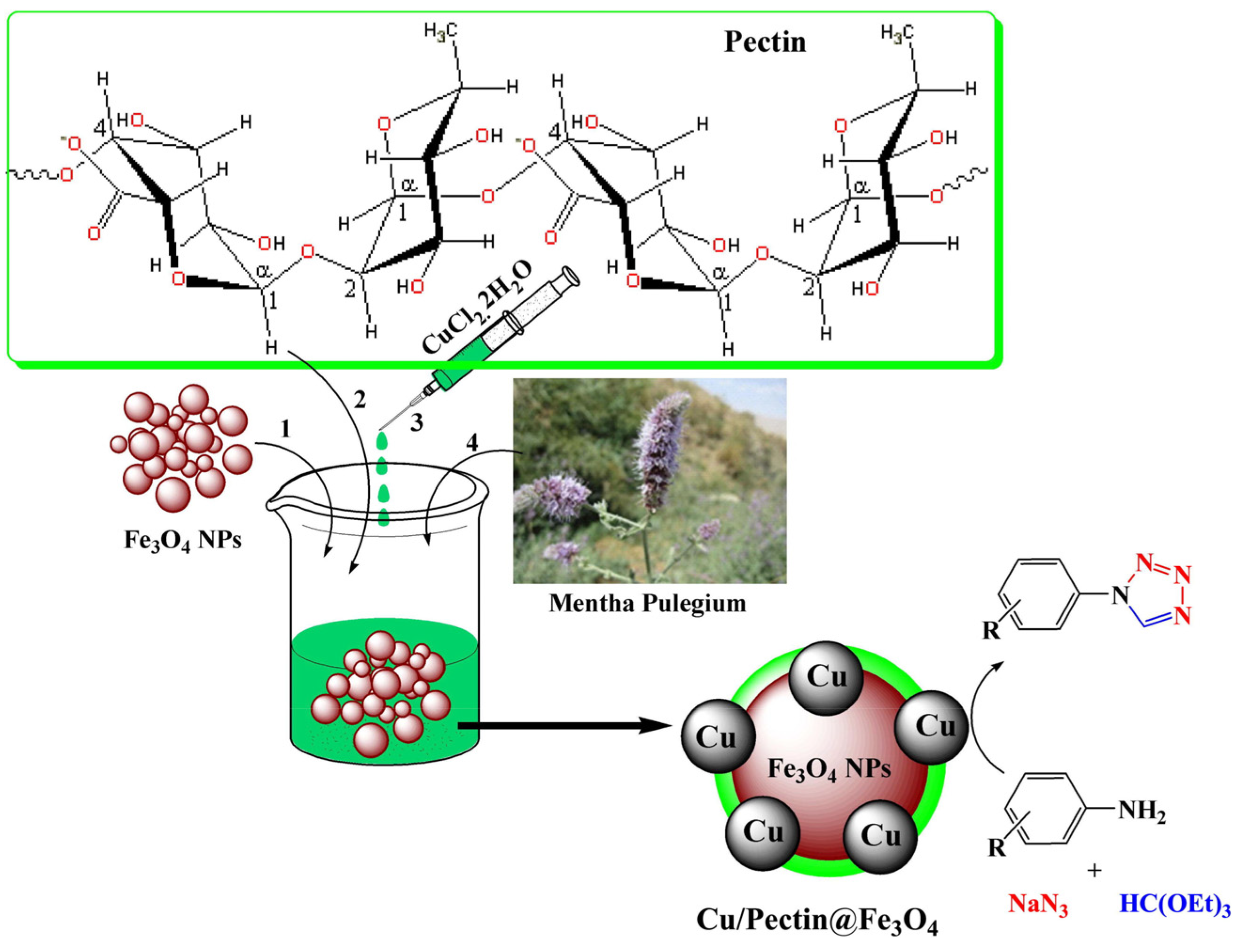


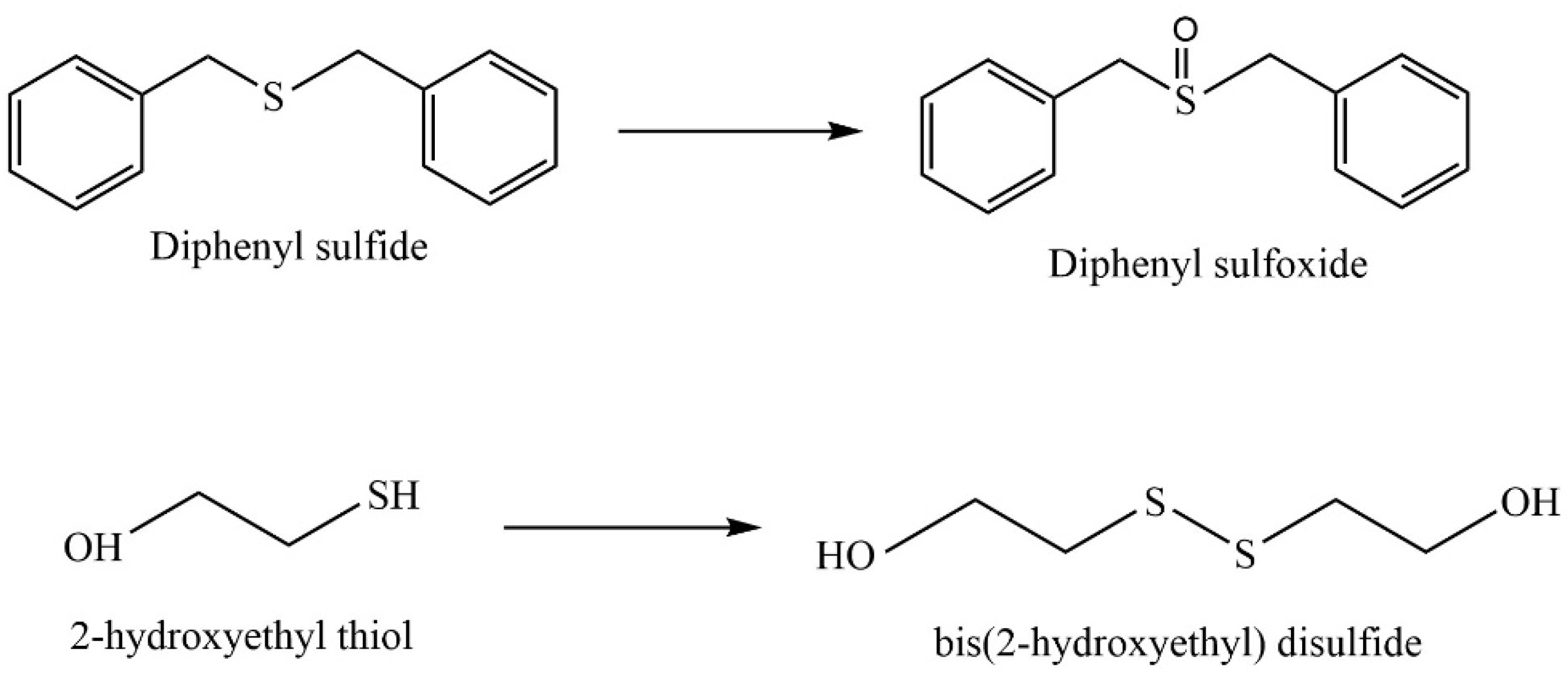


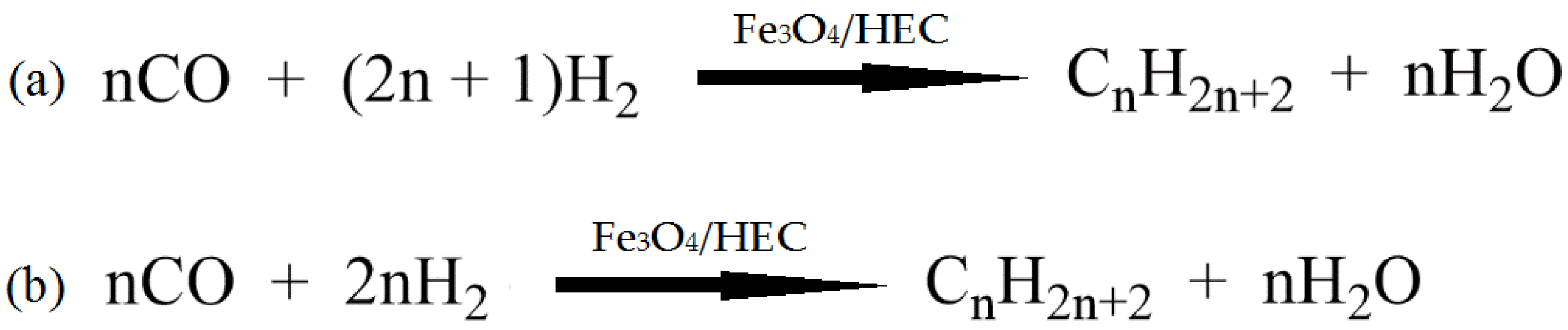

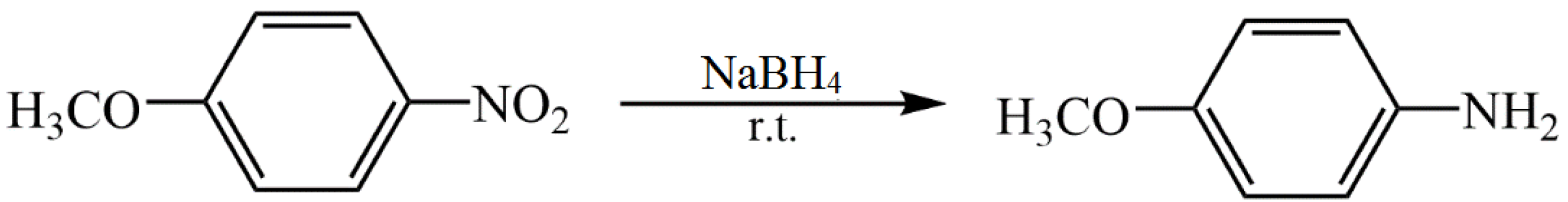

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetova, S.; Zharmagambetova, A.; Talgatov, E.; Auyezkhanova, A.; Malgazhdarova, M.; Zhurinov, M.; Abilmagzhanov, A.; Jumekeyeva, A.; Kenzheyeva, A. How the Chemical Properties of Polysaccharides Make It Possible to Design Various Types of Organic–Inorganic Composites for Catalytic Applications. Molecules 2024, 29, 3214. https://doi.org/10.3390/molecules29133214
Akhmetova S, Zharmagambetova A, Talgatov E, Auyezkhanova A, Malgazhdarova M, Zhurinov M, Abilmagzhanov A, Jumekeyeva A, Kenzheyeva A. How the Chemical Properties of Polysaccharides Make It Possible to Design Various Types of Organic–Inorganic Composites for Catalytic Applications. Molecules. 2024; 29(13):3214. https://doi.org/10.3390/molecules29133214
Chicago/Turabian StyleAkhmetova, Sandugash, Alima Zharmagambetova, Eldar Talgatov, Assemgul Auyezkhanova, Makpal Malgazhdarova, Murat Zhurinov, Arlan Abilmagzhanov, Aigul Jumekeyeva, and Alima Kenzheyeva. 2024. "How the Chemical Properties of Polysaccharides Make It Possible to Design Various Types of Organic–Inorganic Composites for Catalytic Applications" Molecules 29, no. 13: 3214. https://doi.org/10.3390/molecules29133214
APA StyleAkhmetova, S., Zharmagambetova, A., Talgatov, E., Auyezkhanova, A., Malgazhdarova, M., Zhurinov, M., Abilmagzhanov, A., Jumekeyeva, A., & Kenzheyeva, A. (2024). How the Chemical Properties of Polysaccharides Make It Possible to Design Various Types of Organic–Inorganic Composites for Catalytic Applications. Molecules, 29(13), 3214. https://doi.org/10.3390/molecules29133214





