α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines
Abstract
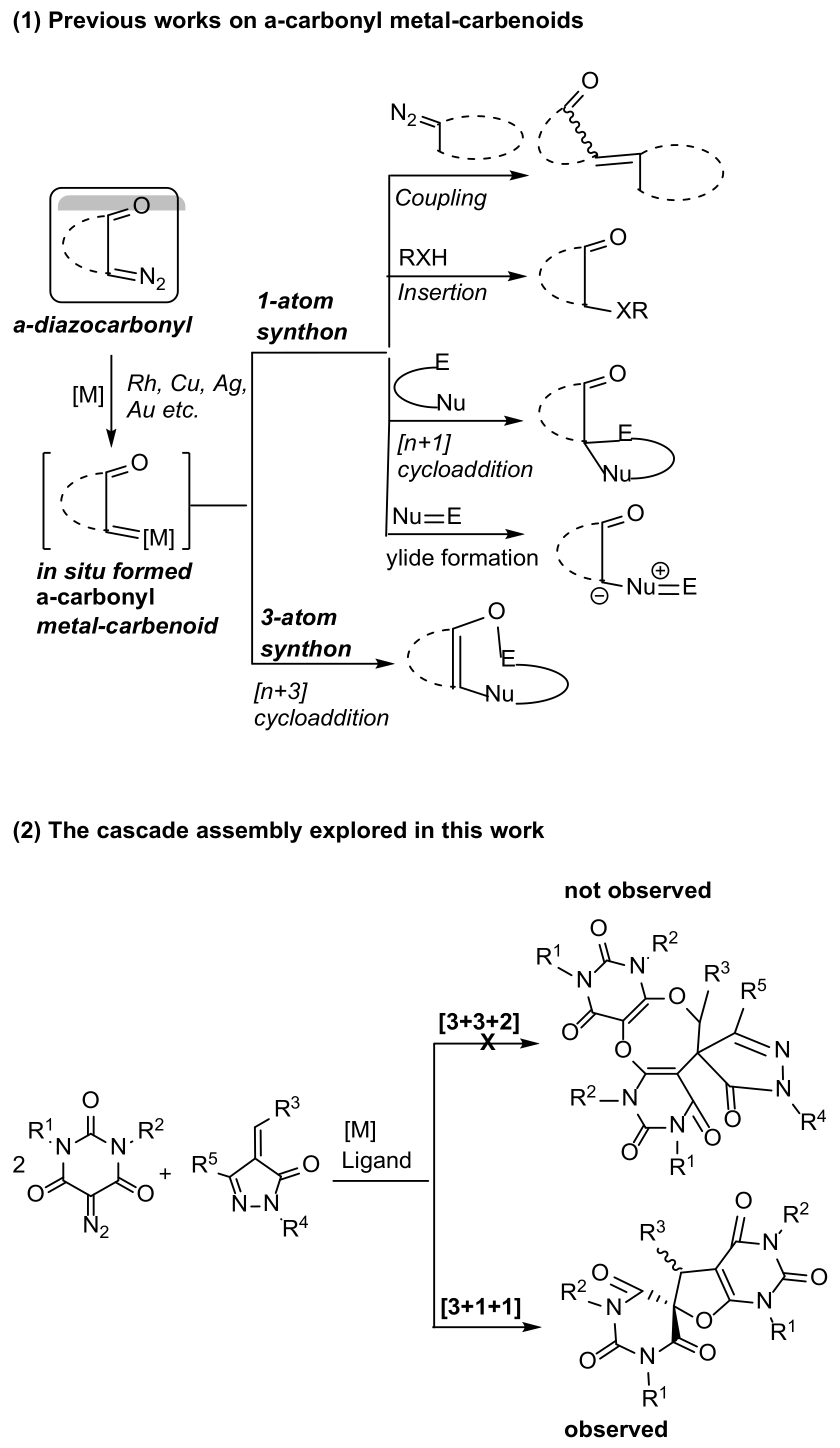
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for Cascade Assembly Reaction
3.2. Gram-Scale Synthesis of Compound 3a

3.3. Characterization of Product 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Clercq, E. Highly potent and selective inhibition of varicella-zoster virus replication by bicyclic furo [2,3-d]pyrimidine nucleoside analogues. Med. Res. Rev. 2003, 23, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Dutta, L.; Sharma, M.; Bhuyan, P.J. Regioisomeric synthesis of dihydrofuro [2,3-d]pyrimidines in a diastereoselective manner involving nitrogen ylides in one-pot three-component reaction. Tetrahedron 2016, 72, 6654–6660. [Google Scholar] [CrossRef]
- Olyaei, A.; Sadeghpour, M. Review on synthetic approaches towards barbituric acids-based furo [2,3-d] pyrimidines. J. Heterocycl. Chem. 2023, 60, 1838–1863. [Google Scholar] [CrossRef]
- Sayed, H.H.; Abbas, H.A.; Morsi, E.M.; Amr Ael, G.; Abdelwahad, N.A. Antimicrobial activity of some synthesized glucopyranosyl-pyrimidine carbonitrile and fused pyrimidine systems. Acta Pharm. 2010, 60, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.; Giofre, S.V.; Garozzo, A.; Bisignano, B.; Corsaro, A.; Chiacchio, M.A. Synthesis and biological evaluation of furopyrimidine N,O-nucleosides. Bioorg. Med. Chem. 2013, 21, 5688–5693. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.I.; Lee, S.H.; Cheong, C.S. A facile synthesis of some substituted furopyrimidine derivatives. J. Heterocycl. Chem. 2009, 43, 1129–1133. [Google Scholar] [CrossRef]
- Kaczmarek, R.; Twardy, D.J.; Olson, T.L.; Korczynski, D.; Andrei, G.; Snoeck, R.; Dolot, R.; Wheeler, K.A.; Dembinski, R. Extension of furopyrimidine nucleosides with 5-alkynyl substituent: Synthesis, high fluorescence, and antiviral effect in the absence of free ribose hydroxyl groups. Eur. J. Med. Chem. 2021, 209, 112884. [Google Scholar] [CrossRef]
- Han, J.; Kaspersen, S.J.; Nervik, S.; Norsett, K.G.; Sundby, E.; Hoff, B.H. Chiral 6-aryl-furo[2,3-d]pyrimidin-4-amines as EGFR inhibitors. Eur. J. Med. Chem. 2016, 119, 278–299. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Das, U. Studies in Pyrimidine-Annelated Heterocycles1 by Tandem Cyclization: Regioselective Synthesis of [6,6]Pyranopyran by Intramolecular [1,6] Michael Addition. J. Org. Chem. 1998, 63, 9997–10000. [Google Scholar] [CrossRef]
- Gangjee, A.; Li, W.; Yang, J.; Kisliuk, R.L. Design, Synthesis, and Biological Evaluation of Classical and Nonclassical 2-Amino-4-oxo-5-substituted-6-methylpyrrolo[3,2-d]pyrimidines as Dual Thymidylate Synthase and Dihydrofolate Reductase Inhibitors. J. Med. Chem. 2008, 51, 68–76. [Google Scholar] [CrossRef]
- Gangjee, A.; Vidwans, A.; Elzein, E.; McGuire, J.J.; Queener, S.F.; Kisliuk, R.L. Synthesis, Antifolate, and Antitumor Activities of Classical and Nonclassical 2-Amino-4-oxo-5-substituted-pyrrolo [2,3-d]pyrimidines. J. Med. Chem. 2001, 44, 1993–2003. [Google Scholar] [CrossRef]
- Janeba, Z.; Balzarini, J.; Andrei, G.; Snoeck, R.; Clercq, E.D.; Robins, M.J. Synthesis and Biological Evaluation of Acyclic 3-[(2-Hydroxyethoxy)methyl] Analogues of Antiviral Furo- and Pyrrolo[2,3-d]pyrimidine Nucleosides. J. Med. Chem. 2005, 48, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagin, A.N.; Elinson, M.N.; Dorofeeva, E.O.; Zaimovskaya, T.A.; Stepanov, N.O.; Gorbunov, S.V.; Belyakov, P.A.; Nikishin, G.I. Electrocatalytic and chemical assembling of N,N′-dialkylbarbituric acids and aldehydes: Efficient cascade approach to the spiro-[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′-(1′H,3H,3′H)-pentone framework. Tetrahedron 2012, 68, 1198–1206. [Google Scholar] [CrossRef]
- Teimouri, M.B.; Akbari-Moghaddam, P.; Motaghinezhad, M. Urotropine–bromine promoted synthesis of functionalized oxaspirotricyclic furopyrimidines via a domino Knoevenagel condensation/Michael addition/α-bromination/Williamson cycloetherification sequence in water. Tetrahedron 2013, 69, 6804–6809. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Aleali, F.; Lashgari, N. Recent applications of barbituric acid in multicomponent reactions. RSC Adv. 2016, 6, 50895–50922. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Stepanov, N.O.; Belyakov, P.A.; Nikishin, G.I. Cascade assembly of N,N′-dialkylbarbituric acids and aldehydes: A simple and efficient one-pot approach to the substituted 1,5-dihydro-2H,2′H-spiro(furo[2,3-d]pyrimidine-6,5′-pyrimidine)-2,2′,4,4′,6′(1′H,3H,3′H)-pentone framework. Tetrahedron Lett. 2010, 51, 6598–6601. [Google Scholar] [CrossRef]
- Elinson, M.N.; Merkulova, V.M.; Ilovaisky, A.I.; Nikishin, G.I. Cascade Assembling of Isatins and Barbituric Acids: Facile and Efficient Way to 2′′H-Dispiro[indole-3,5′-furo[2,3-d]pyrimidine-6′,5′′-pyrimidine]-2,2′,2′′,4′,4′′,6′′-(1H,1′H,1′′H,3′H,3′′H)-hexone Scaffold. J. Heterocycl. Chem. 2013, 50, 1236–1241. [Google Scholar] [CrossRef]
- Teimouria, M.B.; Moghaddamb, P.A. Molecular iodine-catalysed tandem synthesis of oxospirotricyclic furopyrimidines in water. J. Chem. Res. 2016, 40, 196–198. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Mi, Y.H.; Zhao, H.W.; Song, X.Q.; Li, J.T.; Zhang, F. Rh(II)-Catalyzed Homocoupling/[4+1] Cycloaddition Cascade of Diazobarbiturates with Diazopyrazolones to Prepare Spirobarbiturates. Adv. Synth. Catal. 2024, 366, 2596–2601. [Google Scholar] [CrossRef]
- Yin, Z.; He, Y.; Chiu, P. Application of (4+3) cycloaddition strategies in the synthesis of natural products. Chem. Soc. Rev. 2018, 47, 8881–8924. [Google Scholar] [CrossRef]
- Xia, Y.; Qiu, D.; Wang, J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. [Google Scholar] [CrossRef] [PubMed]
- Roose, T.R.; Verdoorn, D.S.; Mampuys, P.; Ruijter, E.; Maes, B.U.W.; Orru, R.V.A. Transition metal-catalysed carbene- and nitrene transfer to carbon monoxide and isocyanides. Chem. Soc. Rev. 2022, 51, 5842–5877. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Z.; Goh, J.; Maraswami, M.; Jia, Z.; Tian, J.S.; Loh, T.P. Recent Advances in Alkenyl sp2 C-H and C-F Bond Functionalizations: Scope, Mechanism, and Applications. Chem. Rev. 2022, 122, 17479–17646. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, J. Gold-catalyzed transformations of alpha-diazocarbonyl compounds: Selectivity and diversity. Chem. Soc. Rev. 2016, 45, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with alpha-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Duffy, R.; Ratnikov, M.; Zhou, L. Catalytic Carbene Insertion into C-H Bonds. Chem. Rev. 2010, 110, 704–724. [Google Scholar] [CrossRef]
- Requejo, M.M.; Perez, P.J. Coinage Metal Catalyzed C-H Bond Functionalization of Hydrocarbons. Chem. Rev. 2008, 108, 3379–3394. [Google Scholar] [CrossRef]
- Yadagiri, D.; Anbarasan, P. Catalytic Functionalization of Metallocarbenes Derived from alpha-Diazocarbonyl Compounds and Their Precursors. Chem. Rev. 2021, 21, 3872–3883. [Google Scholar]
- Sebastian, D.; Satishkumar, S.; Pradhan, P.; Yang, L.; Lakshman, M.K. General Approach to N(6),C5′-Difunctionalization of Adenosine. J. Org. Chem. 2022, 87, 18–39. [Google Scholar] [CrossRef]
- Luo, X.; Chen, G.; He, L.; Huang, X. Amination of Diazocarbonyl Compounds: N-H Insertion under Metal-Free Conditions. J. Org. Chem. 2016, 81, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, X.; Huang, J.; Qian, Y.; Xu, X.; Kang, Z.; Hu, W. Enantioselective Propargylation of Oxonium Ylide with alpha-Propargylic-3-Indolymethanol: Access to Chiral Propargylic Indoles. Org. Lett. 2022, 24, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Kidonakis, M.; Stratakis, M. Au Nanoparticle-Catalyzed Insertion of Carbenes from alpha-Diazocarbonyl Compounds into Hydrosilanes. Org. Lett. 2018, 20, 4086–4089. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Yu, S.; Liu, Z.; Zhang, Y. Rh-Catalyzed Coupling of Acrylic/Benzoic Acids with alpha-Diazocarbonyl Compounds: An Alternative Route for alpha-Pyrones and Isocoumarins. Org. Lett. 2022, 24, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Yu, S.; Liu, Z.; Xu, Z.; Zhang, Y. Synthesis of Furans via Rhodium(III)-Catalyzed Cyclization of Acrylic Acids with alpha-Diazocarbonyl Compounds. J. Org. Chem. 2022, 87, 11979–11988. [Google Scholar] [CrossRef]
- Guo, Y.; Empel, C.; Pei, C.; Atodiresei, I.; Fallon, T.; Koenigs, R.M. Photochemical Cyclopropanation of Cyclooctatetraene and (Poly-)unsaturated Carbocycles. Org. Lett. 2020, 22, 5126–5130. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liu, X.; Feng, X. Asymmetric Catalytic Rearrangements with alpha-Diazocarbonyl Compounds. Acc. Chem. Res. 2022, 55, 415–428. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Zhang, X.; Fan, X. Rh(III)-Catalyzed Cascade Reactions of Sulfoxonium Ylides with alpha-Diazocarbonyl Compounds: An Access to Highly Functionalized Naphthalenones. Org. Lett. 2019, 21, 2541–2545. [Google Scholar] [CrossRef]
- Alavi, S.; Lin, J.B.; Grover, H.K. Copper-Catalyzed Annulation of Indolyl alpha-Diazocarbonyl Compounds Leads to Structurally Rearranged Carbazoles. Org. Lett. 2021, 23, 5559–5564. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Xing, D.; Jing, C.; Zhou, J.; Wang, C.; Wang, D.; Hu, W. Facile synthesis of 3-aryloxindoles via bronsted acid catalyzed Friedel-Crafts alkylation of electron-rich arenes with 3-diazooxindoles. Org. Lett. 2014, 16, 2934–2937. [Google Scholar] [CrossRef]
- Yu, X.; Yu, S.; Xiao, J.; Wan, B.; Li, X. Rhodium(III)-catalyzed azacycle-directed intermolecular insertion of arene C-H bonds into alpha-diazocarbonyl compounds. J. Org. Chem. 2013, 78, 5444–5452. [Google Scholar] [CrossRef]
- Rao, C.; Mai, S.; Song, Q. Rh(ii)/phosphine-cocatalyzed synthesis of dithioketal derivatives from diazo compounds through simultaneous construction of two different C-S bonds. Chem. Commun. 2018, 54, 5964–5967. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, S. Chemodivergent Synthesis of Oxazoles and Oxime Ethers Initiated by Selective C-N/C-O Formation of Oximes and Diazo Esters. Org. Lett. 2021, 23, 8549–8553. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.X.; Niu, Y.; Qi, Z.C.; Yang, S.D. Ir(III)-Catalyzed C-H Functionalization of Triphenylphosphine Oxide toward 3-Aryl Oxindoles. J. Org. Chem. 2020, 85, 14527–14536. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Yang, X.; Xie, F.; Li, X. Divergent Access to 1-Naphthols and Isocoumarins via Rh(III)-Catalyzed C-H Activation Assisted by Phosphonium Ylide. Org. Lett. 2017, 19, 3410–3413. [Google Scholar] [CrossRef]
- Happy, S.; Junaid, M.; Yadagiri, D. Reactivity of quinone methides with carbenes generated from alpha-diazocarbonyl compounds and related compounds. Chem. Commun. 2023, 59, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhao, Y.; Li, H.; Zheng, Y.; Lian, P.; Wan, X. [3 + 3]-Cycloaddition of alpha-Diazocarbonyl Compounds and N-Tosylaziridines: Synthesis of Polysubstituted 2 H-1,4-Oxazines through Synergetic Catalysis of AgOTf/Cu(OAc)2. Org. Lett. 2019, 21, 2356–2359. [Google Scholar] [CrossRef]
- DeAngelis, A.; Dmitrenko, O.; Fox, J.M. Rh-catalyzed intermolecular reactions of cyclic alpha-diazocarbonyl compounds with selectivity over tertiary C-H bond migration. J. Am. Chem. Soc. 2012, 134, 11035–11043. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xu, X.; Wojtas, L.; Kim, M.M.; Zhang, X.P. Regioselective synthesis of multisubstituted furans via metalloradical cyclization of alkynes with alpha-diazocarbonyls: Construction of functionalized alpha-oligofurans. J. Am. Chem. Soc. 2012, 134, 19981–19984. [Google Scholar] [CrossRef]
- Chen, Z.S.; Duan, X.H.; Zhou, P.X.; Ali, S.; Luo, J.Y.; Liang, Y.M. Palladium-catalyzed divergent reactions of alpha-diazocarbonyl compounds with allylic esters: Construction of quaternary carbon centers. Angew. Chem. Int. Ed. 2012, 124, 1399–1403. [Google Scholar] [CrossRef]
- Austeri, M.; Rix, D.; Zeghida, W.; Lacour, J. CpRu-Catalyzed O-H Insertion and Condensation Reactions of α-Diazocarbonyl Compounds. Org. Lett. 2011, 13, 1394–1397. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, X.; Zhang, X.; Fan, X. Synthesis of Spiro[benzo[d][1,3]oxazine-4,4′-isoquinoline]s via [4+1+1] Annulation of N-Aryl Amidines with Diazo Homophthalimides and O2. Org. Lett. 2022, 24, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, Y.; Zhang, X.; Fan, X. Selective Synthesis of Pyrazolonyl Spirodihydroquinolines or Pyrazolonyl Spiroindolines under Aerobic or Anaerobic Conditions. Org. Lett. 2022, 24, 9473–9478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, M.; Lu, P.; Wang, Y. Rh(III)-Catalyzed Reaction of 4-Diazoisochroman-3-imines with (2-Formylaryl)boronic Acids To Access a Straightforward Construction of 5H-Isochromeno[3,4-c]isoquinolines. J. Org. Chem. 2023, 88, 13544–13552. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Li, B.; Zhang, X.; Fan, X. Expeditious Synthesis of Spiroindoline Derivatives via Tandem C(sp2)-H and C(sp3)-H Bond Functionalization of N-Methyl-N-nitrosoanilines. Org. Lett. 2024, 26, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.W.; Yoshikai, N. Copper-Catalyzed Coupling of 2-Siloxy-1-alkenes and Diazocarbonyl Compounds: Approach to Multisubstituted Furans, Pyrroles, and Thiophenes. J. Org. Chem. 2016, 81, 5566–5573. [Google Scholar] [CrossRef] [PubMed]
- Kantin, G.; Dar’in, D.; Krasavin, M. RhII-Catalyzed Cycloaddition of α-Diazo Homophthalimides and Nitriles Delivers Oxazolo[5,4-c]isoquinolin-5(4H)-one Scaffold. Eur. J. Org. Chem. 2018, 2018, 4857–4859. [Google Scholar] [CrossRef]
- Inyutina, A.; Kantin, G.; Dar In, D.; Krasavin, M. Diastereoselective Formal [5 + 2] Cycloaddition of Diazo Arylidene Succinimides-Derived Rhodium Carbenes and Aldehydes: A Route to 2-Benzoxepines. J. Org. Chem. 2021, 86, 13673–13683. [Google Scholar] [CrossRef]
- Huang, Z.; He, Y.; Wang, L.; Li, J.; Xu, B.H.; Zhou, Y.G.; Yu, Z. Copper-Catalyzed [4+1] Annulation of Enaminothiones with Indoline-Based Diazo Compounds. J. Org. Chem. 2022, 87, 4424–4437. [Google Scholar] [CrossRef]
- He, X.; Liu, K.; Yan, S.; Wang, Y.; Jiang, Y.; Zhang, X.; Fan, X. Synthesis of 1,7-Fused Indolines Tethered with Spiroindolinone Based on C-H Activation Strategy with Air as a Sustainable Oxidant. J. Org. Chem. 2024, 89, 1880–1897. [Google Scholar] [CrossRef]
- Guranova, N.I.; Dar’in, D.; Kantin, G.; Novikov, A.S.; Bakulina, O.; Krasavin, M. Rh(II)-Catalyzed Spirocyclization of alpha-Diazo Homophthalimides with Cyclic Ethers. J. Org. Chem. 2019, 84, 4534–4542. [Google Scholar] [CrossRef]
- Gecht, M.; Kantin, G.; Dar’in, D.; Krasavin, M. A novel approach to biologically relevant oxazolo[5,4-d]pyrimidine-5,7-diones via readily available diazobarbituric acid derivatives. Tetrahedron Lett. 2019, 60, 151120. [Google Scholar] [CrossRef]
- Best, D.; Burns, D.J.; Lam, H.W. Direct Synthesis of 5-Aryl Barbituric Acids by Rhodium(II)-Catalyzed Reactions of Arenes with Diazo Compounds. Angew. Chem. Int. Ed. 2015, 54, 7410–7413. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, Y.R. Efficient Synthesis of Spirobarbiturates and Spirothiobarbiturates Bearing Cyclopropane Rings by Rhodium(II)-Catalyzed Reactions of Cyclic Diazo Compounds. Bull. Korean Chem. Soc. 2013, 34, 1735–1740. [Google Scholar] [CrossRef]
- Torán, R.; Miguélez, R.; Sanz-Marco, A.; Vila, C.; Pedro, J.R.; Blay, G. Asymmetric Addition and Cycloaddition Reactions with Ylidene-Five-Membered Heterocycles. Adv. Synth. Catal. 2021, 23, 5196–5234. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, X.; Feng, X. Recent Advances in Metal-Catalyzed Asymmetric 1,4-Conjugate Addition (ACA) of Nonorganometallic Nucleophiles. Chem. Rev. 2018, 118, 7586–7656. [Google Scholar] [CrossRef]
- Zhan, G.; Du, W.; Chen, Y.C. Switchable divergent asymmetric synthesis via organocatalysis. Chem. Soc. Rev. 2017, 46, 1675–1692. [Google Scholar] [CrossRef] [PubMed]
- CCDC 2309187 (3a) contains the Supplementary Crystallographic Data for this Paper. These Data can be Obtained Free of Charge from The Cambridge Crystallographic Data Center. X-ray Single Crystal Structures of 3a was shown with Thermal Ellipsoils Shown at the 50% Probability Level. Available online: http://www.ccdc.camac.uk/data_request/cif (accessed on 1 April 2023).
- Albright, H.; Davis, A.J.; Gomez-Lopez, J.L.; Vonesh, H.L.; Quach, P.K.; Lambert, T.H.; Schindler, C.S. Carbonyl–Olefin Metathesis. Chem. Rev. 2021, 15, 9359–9406. [Google Scholar] [CrossRef]
- Hoveyda, A.H.; Qin, C.; Sui, X.Z.; Liu, Q.; Li, X.; Nikbakht, A. Taking Olefin Metathesis to the Limit: Stereocontrolled Synthesis of Trisubstituted Alkenes. Acc. Chem. Res. 2023, 18, 2426–2446. [Google Scholar] [CrossRef]
- Lozano-Vila, A.M.; Monsaert, S.; Bajek, A.; Verpoort, F. Ruthenium-Based Olefin Metathesis Catalysts Derived from Alkynes. Chem. Rev. 2010, 110, 4865–4909. [Google Scholar] [CrossRef]
- Kumar, V.; Scilabra, P.; Politzer, P.; Terraneo, G.; Daolio, A.; Fernandez-Palacio, F.; Murray, J.S.; Resnati, G. Tetrel and Pnictogen Bonds Complement Hydrogen and Halogen Bonds in Framing the Interactional Landscape of Barbituric Acids. Cryst. Growth Des. 2020, 21, 642–652. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Yuan, C.; Wang, G.P.; Zhu, S.F.; Wu, Y.; Wang, B.; Sun, Z.; Xiao, Y.; Zhou, Q.L.; et al. Enantioselective Synthesis of Spirobarbiturate-Cyclohexenes through Phosphine-Catalyzed Asymmetric [4 + 2] Annulation of Barbiturate-Derived Alkenes with Allenoates. Org. Lett. 2016, 18, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Temprado, M.; Roux, M.V.; Ros, F.; Notario, R.; Segura, M.; Chickos, J.S. Thermophysical Study of Several Barbituric Acid Derivatives by Differential Scanning Calorimetry (DSC). J. Chem. Eng. Data 2010, 56, 263–268. [Google Scholar] [CrossRef]
- Fakhraian, H.; Nafari, Y. Preparative, mechanistic and tautomeric investigation of 1-phenyl and 1-methyl derivative of 3-methyl-5-pyrazolone. J. Chem. Sci. 2021, 133, 40. [Google Scholar] [CrossRef]
- Tonga, M. Tunable optical properties of push-pull chromophores: End group effect. Tetrahedron Lett. 2020, 61, 152205. [Google Scholar] [CrossRef]
- Yu, J.; Xu, J.; Li, J.; Jin, Y.; Xu, W.; Yu, Z.; Lv, Y. A continuous-flow procedure for the synthesis of 4-Benzylidene-pyrazol-5-one derivatives. J. Flow Chem. 2018, 8, 29–34. [Google Scholar] [CrossRef]
- Aljohani, F.A.; El-Hag, M.; El-Manawaty, M.A. An Efficient One-pot Synthesis of Certain StereoselectiveSpiro [pyrazole-4,5′-isoxazoline]-5-one Derivatives: In vitro Evaluation of Antitumor Activities, Molecular Docking and In silico ADME Predictions. Chem. Res. Chin. Univ. 2022, 38, 1073–1082. [Google Scholar] [CrossRef]
- Khairnar, P.V.; Su, Y.H.; Edukondalu, A.; Lin, W. Enantioselective Synthesis of Spiropyrazolone-Fused Cyclopenta[c]chromen-4-ones Bearing Five Contiguous Stereocenters via [3+2] Cycloaddition. J. Org. Chem. 2021, 86, 12326–12335. [Google Scholar] [CrossRef]
- Zhao, C.; Shi, K.; He, G.; Gu, Q.; Ru, Z.; Yang, L.; Zhong, G. NHC-Catalyzed Asymmetric Formal [4 + 2] Annulation to Construct Spirocyclohexane Pyrazolone Skeletons. Org. Lett. 2019, 21, 7943–7947. [Google Scholar] [CrossRef]
- Sheibani, H.; Babaie, M. Three-Component Reaction to Form 1,4-Dihydropyrano [2,3-c]-pyrazol-5-yl Cyanides. Synth. Commun. 2009, 40, 257–265. [Google Scholar] [CrossRef]
- Awasthi, A.; Yadav, P.; Kumar, V.; Tiwari, D.K. α-Amino Acids Mediated C–C Double Bonds Cleavage in Diastereoselective Synthesis of Aza-Spirocyclic Pyrazolones. Adv. Synth. Catal. 2020, 362, 4378–4383. [Google Scholar] [CrossRef]
- Shindalkar, S.S.; Madje, B.R.; Hangarge, R.V.; Patil, P.T.; Dongare, M.K. Borate Zirconia Mediated Knoevenagel Condensation Reaction in Water. J. Korean Chem. Soc. 2005, 49, 377–380. [Google Scholar] [CrossRef][Green Version]


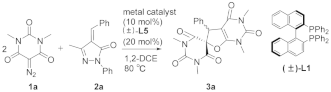 | |||
|---|---|---|---|
| Entry | [M] [f] | Time (h) | Yield [b] (%) |
| 1 [c] | Rh2(OAc)4 | 6 | 40 |
| 2 [d] | Rh2(OAc)4 | 6 | 65 |
| 3 | Rh2(OAc)4 | 6 | 75 |
| 4 | Ph3PAuCl | 6 | NR [e] |
| 5 | (CH3CN)4·CuBF4 | 6 | NR [e] |
| 6 | DPPE·NiCl2 | 8 | NR [e] |
| 7 | DPPE·PdCl2 | 8 | NR [e] |
| 8 | Pd(DPPE)2 | 8 | NR [e] |
| 9 | Pd2(dba)3 | 6 | trace |
| 10 | (F3CSO2)NAg | 6 | trace |
| 11 | Ru(OAc)3 | 6 | 21 |
| 12 | [Rh(COD)2]BF4 | 6 | NR [e] |
| 13 | [Rh3O(OAc)6(H2O)3]OAc | 6 | NR [e] |
| 14 | Rh2(esp)2 | 6 | 93 |
| 15 [g] | Rh2(esp)2 | 6 | 25 |
| 16 [h] | Rh2(esp)2 | 6 | 16 |
| 17 [i] | Rh2(esp)2 | 6 | trace |
| 18 [j] | Rh2(esp)2 | 6 | NR [e] |
 | |||||
|---|---|---|---|---|---|
| Entry | Ligand | Solvent | Time (h) | Yield [b] (%) | ee [d] (%) |
| 1 | - | 1,2-DCE | 6 | trace | - |
| 2 | (±)-L2 | 1,2-DCE | 6 | NR [c] | - |
| 3 | (±)-L3 | 1,2-DCE | 6 | 45 | - |
| 4 | (±)-L4 | 1,2-DCE | 6 | 67 | - |
| 5 | dppf | 1,2-DCE | 6 | 68 | - |
| 6 | dppb | 1,2-DCE | 6 | 77 | - |
| 7 | (±)-L5 | 1,2-DCE | 8 | 82 | - |
| 8 | (±)-L1 | 1,2-DCE | 6 | 93 | - |
| 9 | (±)-L1 | PhCF3 | 6 | NR [c] | - |
| 10 | (±)-L1 | HFIP | 6 | NR [c] | - |
| 11 | (±)-L1 | C6F6 | 6 | NR [c] | - |
| 12 | (±)-L1 | CHCl3 | 6 | NR [c] | - |
| 13 | (S)-L2 | 1,2-DCE | 6 | 45 | 0 |
| 14 | (R, R)-L3 | 1,2-DCE | 6 | 67 | 0 |
| 15 | (R)-L5 | 1,2-DCE | 6 | 93 | 0 |
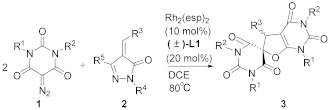 | |||||
|---|---|---|---|---|---|
| Entry | 1 (R1, R2) | 2 (R3, R4, R5) | 3 | Time (h) | Yield [b] (%) |
| 1 | 1a (Me, Me) | 2a (Ph, Ph, Me) | 3a | 6 | 93 |
| 2 | 1a (Me, Me) | 2b (Ph, Me, Me) | 3a | 6 | 86 |
| 3 | 1a (Me, Me) | 2c (Ph, Me, H) | 3a | 6 | 96 |
| 4 | 1a (Me, Me) | 2d (Ph, Ph, Ph) | 3a | 6 | 57 |
| 5 | 1a (Me, Me) | 2e (Me, Ph, Me) | 3b | 6 | 38 |
| 6 | 1a (Me, Me) | 2f (Priso, Ph, Me) | - | 6 | NR [c] |
| 7 | 1a (Me, Me) | 2g  | - | 6 | NR [c] |
| 8 | 1a (Me, Me) | 2h (2-thiophenyl, Ph, Me) | - | 6 | NR [c] |
| 9 | 1a (Me, Me) | 2i (5-benzofuranyl, Ph, Me) | 3c | 6 | 56 |
| 10 | 1b (cyclohexyl, cyclohexyl) | 2c (Ph, Me, H) | - | 6 | NR [c] |
| 11 | 1c (Bn, Bn) | 2c (Ph, Me, H) | 3d | 6 | 88 |
| 12 | 1d (Et, Et) | 2c (Ph, Me, H) | 3e | 6 | 91 |
| 13 | 1e (Priso, Priso) | 2c (Ph, Me, H) | 3f | 6 | 47 |
| 14 | 1f (Butert, Butert) | 2c (Ph, Me, H) | - | 6 | NR [c] |
| 15 | 1g (4-MeC6H4, 4-MeC6H4) | 2c (Ph, Me, H) | - | 6 | NR [c] |
| 16 | 1a (Me, Me) | 2j (4-MeC6H4, Ph, Me) | 3g | 3 | 68 |
| 17 | 1a (Me, Me) | 2k (4-BrC6H4, Ph, Me) | 3h | 3 | 66 |
| 18 | 1a (Me, Me) | 2l (3-ClC6H4, Ph, Me) | 3i | 3 | 72 |
| 19 | 1a (Me, Me) | 2m (2-naphthyl, Ph, Me) | 3j | 6 | 78 |
| 20 | 1a (Me, Me) | 2n (4- F3CC6H4, Ph, Me) | 3k | 6 | 80 |
| 21 | 1a (Me, Me) | 2o (4-MeOC6H4, Ph, Me) | 3l | 3 | 48 |
| 22 | 1a (Me, Me) | 2p (4-O2NC6H4, Ph, Me) | 3m | 6 | 62 |
| 23 | 1a (Me, Me) | 2q (2-BrC6H4, Ph, Me) | 3n | 6 | 66 |
| 24 | 1a (Me, Me) | 2r (3-MeC6H4, Ph, Me) | 3o | 6 | 90 |
| 25 | 1d (Et, Et) | 2j (4-MeC6H4, Ph, Me) | 3p | 6 | 82 |
| 26 | 1d (Et, Et) | 2k (4-BrC6H4, Ph, Me) | 3q | 6 | 95 |
| 27 | 1d (Et, Et) | 2l (3-ClC6H4, Ph, Me) | 3r | 6 | 93 |
| 28 | 1d (Et, Et) | 2m (2-naphthyl, Ph, Me) | 3s | 6 | 80 |
| 29 | 1d (Et, Et) | 2o (4-MeOC6H4, Ph, Me) | 3t | 6 | 65 |
| 30 | 1d (Et, Et) | 2p (4-O2NC6H4, Ph, Me) | 3u | 6 | 90 |
| 31 | 1d (Et, Et) | 2q (2-BrC6H4, Ph, Me) | 3v | 6 | 63 |
| 32 | 1d (Et, Et) | 2r (3-MeC6H4, Ph, Me) | 3w | 6 | 73 |
| 33 | 1h (Me,Bn) | 2a (Ph, Ph, Me) | - | 6 | NR [c] |
| 34 | 1h (Me, Bn) | 2c (Ph, Me, H) | - | 6 | NR [c] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Mi, Y.-H.; Wang, K.; Zhao, H.-W. α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines. Molecules 2024, 29, 3178. https://doi.org/10.3390/molecules29133178
Zhang Y, Mi Y-H, Wang K, Zhao H-W. α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines. Molecules. 2024; 29(13):3178. https://doi.org/10.3390/molecules29133178
Chicago/Turabian StyleZhang, Yue, Yu-Hang Mi, Kuo Wang, and Hong-Wu Zhao. 2024. "α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines" Molecules 29, no. 13: 3178. https://doi.org/10.3390/molecules29133178
APA StyleZhang, Y., Mi, Y.-H., Wang, K., & Zhao, H.-W. (2024). α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines. Molecules, 29(13), 3178. https://doi.org/10.3390/molecules29133178





