Design, Synthesis, and Antifungal Activities of Phenylpyrrole Analogues Based on Alkaloid Lycogalic Acid
Abstract
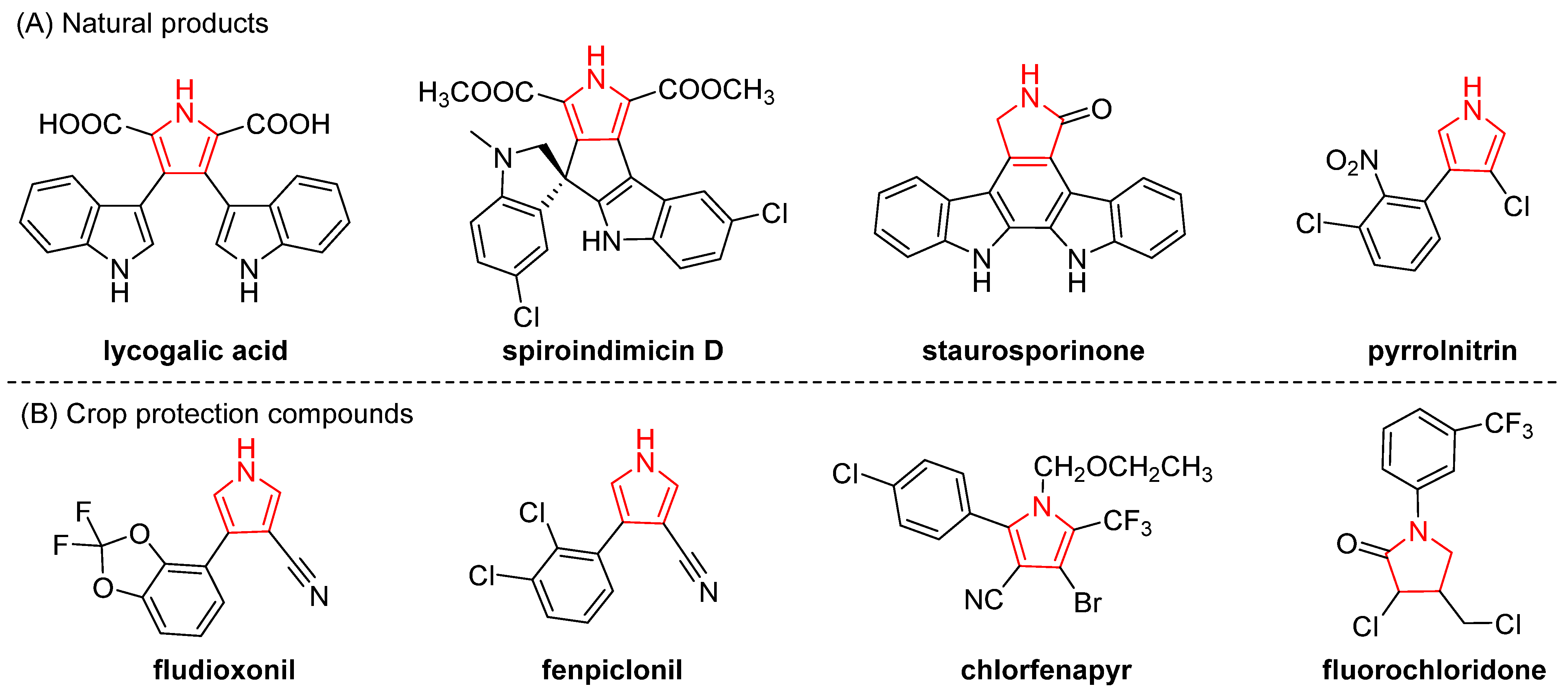
1. Introduction
2. Results
2.1. Chemicals
2.2. In Vitro Antifungal Activities of Target Compounds 5 and 8a–8s
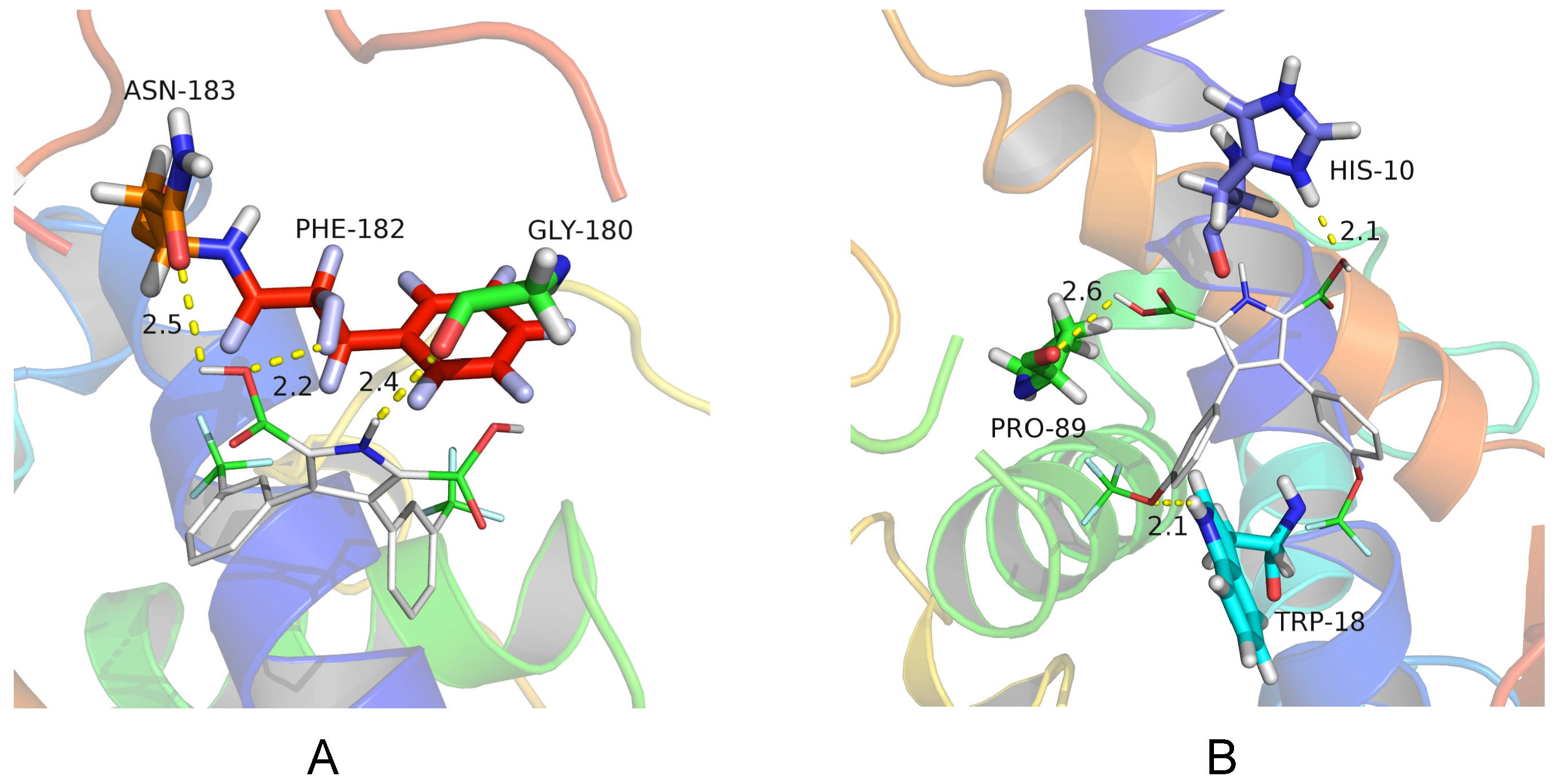
2.3. Molecular Docking Research
3. Discussion
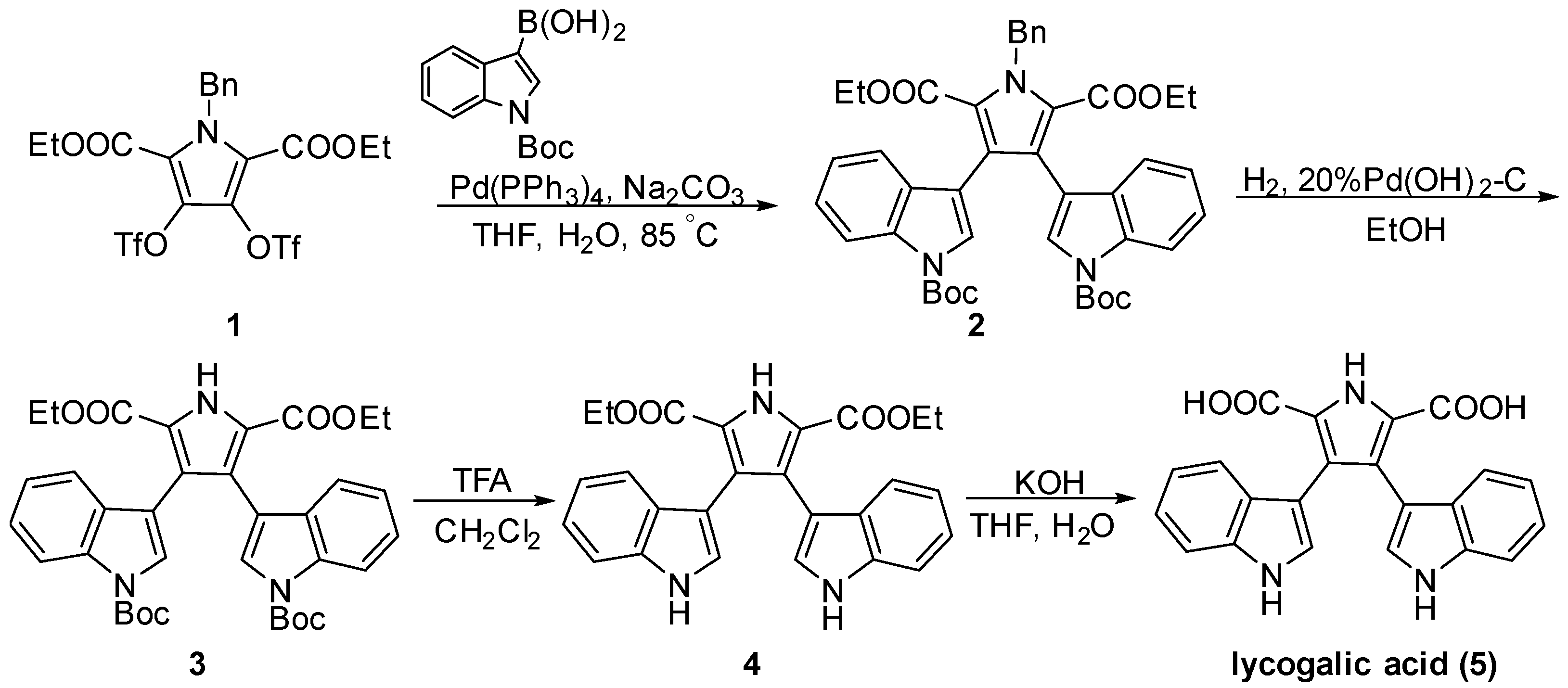
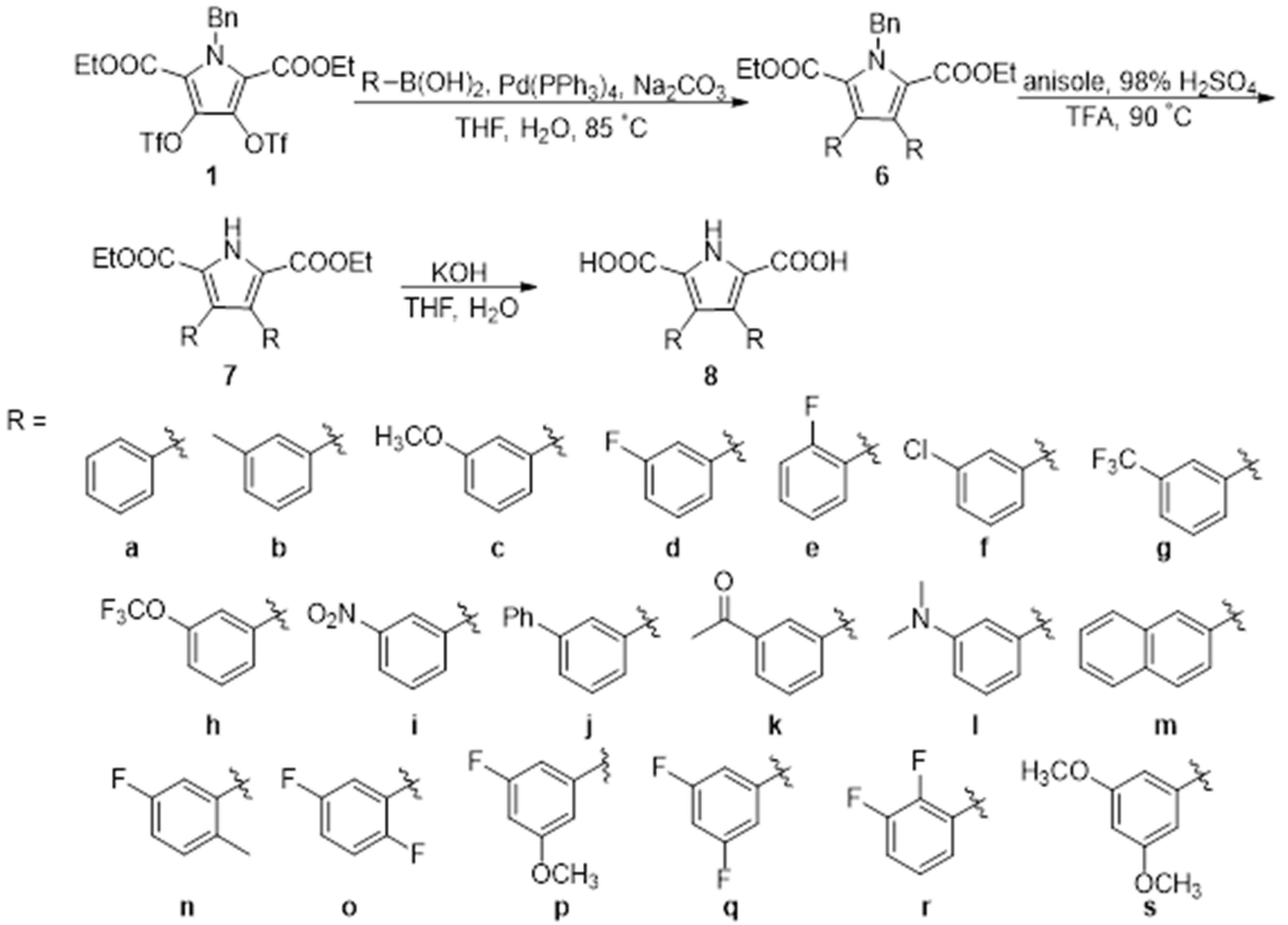
3.1. Synthesis
3.2. Structure–Activity Relationship (SAR) Analysis for the Antifungal Activity
3.3. Molecular Docking
4. Materials and Methods
4.1. Chemicals and Instruments
4.2. Synthetic Procedures
4.2.1. Synthesis of Compound 2–5
4.2.2. Synthesis of Compounds 6–8
4.3. In Vitro Target Compounds against Seven Phytopathogenic Fungi
4.4. Calculation Procedures for Molecular Docking Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, R.A.C.; Naidu, R.A. Global dimensions of plant virus diseases: Current status and future perspectives. Annu. Rev. Virol. 2019, 6, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dou, M.L.; Xia, Y.Y.; Hu, Z.; Zhang, B.J.; Bai, Y.X.; Xie, J.; Liu, Q.F.; Xie, C.P.; Lu, D.D.; et al. Photostable 1-trifluoromethyl cinnamyl alcohol derivatives designed as potential fungicides and bactericides. J. Agric. Food Chem. 2021, 69, 5435–5445. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y. Special issue: Relationship between insect vectors and plant pathogens. Arch. Insect Biochem. Physiol. 2023, 112, e21999. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.R.; Do Couto Santos, R. Pest control: Can chitinases help to reduce pesticide use? J. Agric. Food Chem. 2019, 67, 8071–8073. [Google Scholar] [CrossRef] [PubMed]
- Lisiecki, K.; Lemańczyk, G.; Piesik, D.; Mayhew, C.A. Screening winter wheat genotypes for resistance traits against Rhizoctonia cerealis and Rhizoctonia solani infection. Agriculture 2022, 12, 1981. [Google Scholar] [CrossRef]
- Geng, X.X.; Gao, Z.; Zhao, L.; Zhang, S.F.; Wu, J.; Yang, Q.H.; Liu, S.H.; Chen, X.H. Comparative transcriptome analysis of resistant and susceptible wheat in response to Rhizoctonia cerealis. BMC Plant Biol. 2022, 22, 235. [Google Scholar] [CrossRef]
- Wu, T.C.; Zhu, X.L.; Lü, L.J.; Chen, X.Y.; Xu, G.B.; Zhang, Z.Y. The wheat receptor-like cytoplasmic kinase TaRLCK1B is required for host immune response to the necrotrophic pathogen Rhizoctonia cerealis. J. Integr. Agric. 2020, 19, 2616–2627. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, C.J.; Luo, X.F.; Li, A.P.; Zhang, S.Y.; An, J.X.; Zhang, Z.J.; Ma, Y.; Zhang, B.Q.; Liu, Y.Q. Design, synthesis, and biological evaluation of novel berberine derivatives against phytopathogenic fungi. Pest Manag. Sci. 2022, 78, 4361–4376. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; He, F.; Song, B.A.; Wu, J. Future direction of agrochemical development for plant disease in China. Food Energy Secur. 2021, 10, e293. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Feitosa, B.F.; de Alcântara, C.M.; de Lima, A.B.S.; Silva, A.S.; Araújo, A.D.; Cavalcanti, M.T.; Mori, E.; Araújo, I.M.; de Farias, P.A.M.; Wilairatana, P.; et al. Bioactive natural products for chemical control of microorganisms: Scientific prospecting (2001-2021) and systematic review. Molecules 2022, 27, 5917. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.K.; Kong, L.L.; Yuan, T.Y.; Li, W.; Zhang, W.; Hou, B.Y.; Lu, Y.; Du, G.H. The strategies and techniques of drug discovery from natural products. Pharmacol. Ther. 2020, 216, 107686. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef]
- Yan, Y.M.; Li, X.; Zhang, C.H.; Lv, L.J.; Gao, B.; Li, M.H. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- van Pee, K.H.; Ligon, J.M. Biosynthesis of pyrrolnitrin and other phenylpyrrole derivatives by bacteria. Nat. Prod. Rep. 2000, 17, 157–164. [Google Scholar] [CrossRef]
- Chollet, J.F.; Rocher, F.; Jousse, C.; Delétage-Grandon, C.; Bashiardes, G.; Bonnemain, J.L. Synthesis and phloem mobility of acidic derivatives of the fungicide fenpiclonil. Pest Manag. Sci. 2004, 60, 1063–1072. [Google Scholar] [CrossRef]
- Kilani, J.; Fillinger, S. Phenylpyrroles: 30 years, two molecules and (nearly) no resistance. Front. Microbiol. 2016, 7, 2014. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Su, J.; Wang, Z.S.; Hou, C.X.; Wu, P.C.; Xing, Y.; Li, X.S.; Zhu, X.L.; Lu, Y.C.; Xu, L.J. Synthesis, design and three-dimensional quantitative structure sctivity relationship (3D-QSAR) research of phenylpyrrole fungicides. Chin. J. Org. Chem. 2021, 41, 3560–3570. [Google Scholar] [CrossRef]
- Črešnar, B.; Petrič, Š. Cytochrome P450 enzymes in the fungal kingdom. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 29–35. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, S.; Huo, J.Q.; Sun, S.S.; Wang, Y.N.; Yang, H.W.; Zhao, S.Y.; Tang, L.F.; Han, L.J.; Zhang, J.L.; et al. Design, synthesis, and evaluation of novel 4-chloropyrazole-based pyridines as potent fungicide candidates. J. Agric. Food Chem. 2022, 70, 9327–9336. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.C.; Hargrove, T.Y.; Zhao, B.; Wawrzak, Z.; Goldstone, J.V.; Nes, W.D.; Kelly, S.L.; Waterman, M.R.; Stegeman, J.J.; Lepesheva, G.I. Concerning P450 evolution: Structural analyses support bacterial origin of sterol 14α-demethylases. Mol. Biol. Evol. 2021, 38, 952–967. [Google Scholar] [CrossRef]
- Monk, B.C.; Tomasiak, T.M.; Keniya, M.V.; Huschmann, F.U.; Tyndall, J.D.A.; O’Connell, J.D.; Cannon, R.D.; McDonald, J.G.; Rodriguez, A.; Finer-Moore, J.S.; et al. Architecture of a single membrane spanning cytochrome P450 suggests constraints that orient the catalytic domain relative to a bilayer. Proc. Natl. Acad. Sci. USA 2014, 111, 3865–3870. [Google Scholar] [CrossRef]
- Howard-Jones, A.R.; Walsh, C.T. Staurosporine and rebeccamycin aglycones are assembled by the oxidative action of StaP, StaC, and RebC on chromopyrrolic acid. J. Am. Chem. Soc. 2006, 128, 12289–12298. [Google Scholar] [CrossRef]
- Chae, C.S.; Park, J.S.; Chung, S.C.; Kim, T.I.; Lee, S.H.; Yoon, K.M.; Shin, J.; Oh, K.B. Production of chromopyrrolic acid by coexpression of inkOD in a heterologous host Streptomyces albus. Bioorganic Med. Chem. Lett. 2009, 19, 1581–1583. [Google Scholar] [CrossRef]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef]
- Knölker, H.J.; Reddy, K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4428. [Google Scholar] [CrossRef]
- Hao, Y.K.; He, H.F.; Zhou, P.; Niu, K.K.; Song, H.J.; Liu, Y.X.; Zhang, J.J.; Hu, D.Y.; Wang, Q.M.; Song, B.A. Discovery of indoloazepinone analogues as novel antiviral, antiphytopathogenic fungus, and insecticidal agents. ACS Agric. Sci. Technol. 2022, 2, 761–768. [Google Scholar] [CrossRef]
- Chen, W.; Hao, H.L.; Zhang, C.L.; Shen, Y.M. Synthesis and antitumor activity of lycogarubin C and lycogalic acid. Chin. J. Org. Chem. 2014, 34, 797–803. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Wawrzak, Z.; Liu, J.L.; Nes, W.D.; Waterman, M.R.; Lepesheva, G.I. Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14α-demethylase (CYP51) from Leishmania infantum. J. Biol. Chem. 2011, 286, 26838–26848. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Sugiyama, A.; Purqon, A.; Nagao, H.; Nishikawa, K. Binding free energy calculation and structural analysis for antigen-antibody complex. AIP Conf. Proc. 2006, 832, 566–569. [Google Scholar]
- Li, W.X.; Liu, S. A biomimetic approach to lycogarubin C, lynamicin D and related analogues. Org. Biomol. Chem. 2024, 22, 2558–2561. [Google Scholar] [CrossRef]
- Zhou, N.N.; Xie, T.; Liu, L.; Xie, Z.X. Cu/Mn co-oxidized cyclization for the synthesis of highly substituted pyrrole derivatives from amino acid esters: A strategy for the biomimetic syntheses of lycogarubin C and chromopyrrolic acid. J. Org. Chem. 2014, 79, 6061–6068. [Google Scholar] [CrossRef] [PubMed]
- Yasui, E.; Tsuda, J.; Ohnuki, S.; Nagumo, S. Selective mono-reduction of pyrrole-2,5 and 2,4-dicarboxylates. Chem. Pharm. Bull. 2016, 64, 1262–1267. [Google Scholar] [CrossRef][Green Version]
- María, D.S.; Farrán, M.A.; García, M.A.; Pinilla, E.; Torres, M.R.; Elguero, J.; Claramunt, R.M. Synthetic hosts for molecular recognition of ureas. J. Org. Chem. 2011, 76, 6780–6788. [Google Scholar] [CrossRef]
- Zong, K.; Reynolds, J.R. 3,4-Alkylenedioxypyrroles: Functionalized derivatives as monomers for new electron-rich conducting and electroactive polymers. J. Org. Chem. 2001, 66, 6873–6882. [Google Scholar] [CrossRef]
- Zhao, H.P.; Liu, Y.X.; Cui, Z.P.; Beattie, D.; Gu, Y.C.; Wang, Q.M. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.M. Studies on the Resistance Mechanism of Rhizoctonia cerealis to Tebuconazole; Shandong Agricultural University: Taian, China, 2006; pp. 101–102. [Google Scholar]

| Compound | Inhibition Rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| F.C. 2 | C.H. 2 | P.P. 2 | R.C. 2 | A.S. 2 | P.G. 2 | S.S. 2 | |
| 5 | 20 ± 1 | 32 ± 1 | 49 ± 1 | 65 ± 1 | 25 ± 1 | 29 ± 1 | 75 ± 2 |
| 8a | 26 ± 1 | 18 ± 1 | 0 | 26 ± 1 | 11 ± 1 | 29 ± 1 | 70 ± 1 |
| 8b | 36 ± 1 | 24 ± 1 | 33 ± 1 | 79 ± 1 | 6 ± 1 | 14 ± 1 | 79 ± 1 |
| 8c | 21 ± 1 | 38 ± 1 | 31 ± 1 | 53 ± 1 | 13 ± 1 | 50 ± 1 | 74 ± 1 |
| 8d | 20 ± 1 | 18 ± 1 | 20 ± 1 | 66 ± 1 | 14 ± 1 | 43 ± 1 | 70 ± 1 |
| 8e | 31 ± 1 | 26 ± 1 | 12 ± 1 | 62 ± 1 | 8 ± 1 | 14 ± 1 | 37 ± 1 |
| 8f | 36 ± 1 | 24 ± 1 | 66 ± 1 | 79 ± 1 | 19 ± 1 | 29 ± 1 | 79 ± 1 |
| 8g | 32 ± 1 | 47 ± 1 | 57 ± 1 | 92 ± 1 | 13 ± 1 | 43 ± 1 | 79 ± 1 |
| 8h | 43 ± 1 | 44 ± 1 | 49 ± 1 | 91 ± 1 | 19 ± 1 | 69 ± 1 | 84 ± 1 |
| 8i | 68 ± 1 | 44 ± 1 | 71 ± 1 | 82 ± 1 | 31 ± 1 | 50 ± 1 | 69 ± 1 |
| 8j | 63 ± 1 | 35 ± 1 | 56 ± 1 | 78 ± 1 | 13 ± 1 | 43 ± 1 | 50 ± 1 |
| 8k | 32 ± 1 | 18 ± 1 | 49 ± 1 | 20 ± 1 | 19 ± 1 | 43 ± 1 | 34 ± 1 |
| 8l | 30 ± 1 | 15 ± 1 | 66 ± 1 | 75 ± 1 | 13 ± 1 | 79 ± 1 | 65 ± 1 |
| 8m | 36 ± 1 | 41 ± 1 | 61 ± 1 | 82 ± 1 | 25 ± 1 | 50 ± 1 | 79 ± 1 |
| 8n | 34 ± 1 | 35 ± 1 | 49 ± 1 | 71 ± 1 | 6 ± 1 | 29 ± 1 | 61 ± 1 |
| 8o | 23 ± 1 | 12 ± 1 | 49 ± 1 | 36 ± 1 | 19 ± 1 | 14 ± 1 | 73 ± 1 |
| 8p | 27 ± 1 | 12 ± 1 | 62 ± 1 | 46 ± 1 | 6 ± 1 | 14 ± 1 | 74 ± 1 |
| 8q | 45 ± 1 | 50 ± 1 | 49 ± 1 | 66 ± 1 | 19 ± 1 | 14 ± 1 | 77 ± 1 |
| 8r | 30 ± 1 | 35 ± 1 | 49 ± 1 | 66 ± 1 | 31 ± 1 | 43 ± 1 | 77 ± 1 |
| 8s | 29 ± 1 | 24 ± 1 | 41 ± 1 | 40 ± 1 | 6 ± 1 | 43 ± 1 | 77 ± 1 |
| carbendazim | 100 | 52 ± 1 | 100 | 100 | 13 ± 1 | 100 | 93 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhou, Z.; Wang, T.; Lu, A. Design, Synthesis, and Antifungal Activities of Phenylpyrrole Analogues Based on Alkaloid Lycogalic Acid. Molecules 2024, 29, 3150. https://doi.org/10.3390/molecules29133150
Zhang S, Zhou Z, Wang T, Lu A. Design, Synthesis, and Antifungal Activities of Phenylpyrrole Analogues Based on Alkaloid Lycogalic Acid. Molecules. 2024; 29(13):3150. https://doi.org/10.3390/molecules29133150
Chicago/Turabian StyleZhang, Shuaiheng, Zhenghong Zhou, Tienan Wang, and Aidang Lu. 2024. "Design, Synthesis, and Antifungal Activities of Phenylpyrrole Analogues Based on Alkaloid Lycogalic Acid" Molecules 29, no. 13: 3150. https://doi.org/10.3390/molecules29133150
APA StyleZhang, S., Zhou, Z., Wang, T., & Lu, A. (2024). Design, Synthesis, and Antifungal Activities of Phenylpyrrole Analogues Based on Alkaloid Lycogalic Acid. Molecules, 29(13), 3150. https://doi.org/10.3390/molecules29133150








