Modified Halloysite as an Adsorbent for the Removal of Cu(II) Ions and Reactive Red 120 Dye from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of the Halloysite Samples
2.2. Adsorption Study
2.2.1. Effect of Adsorbent Dose
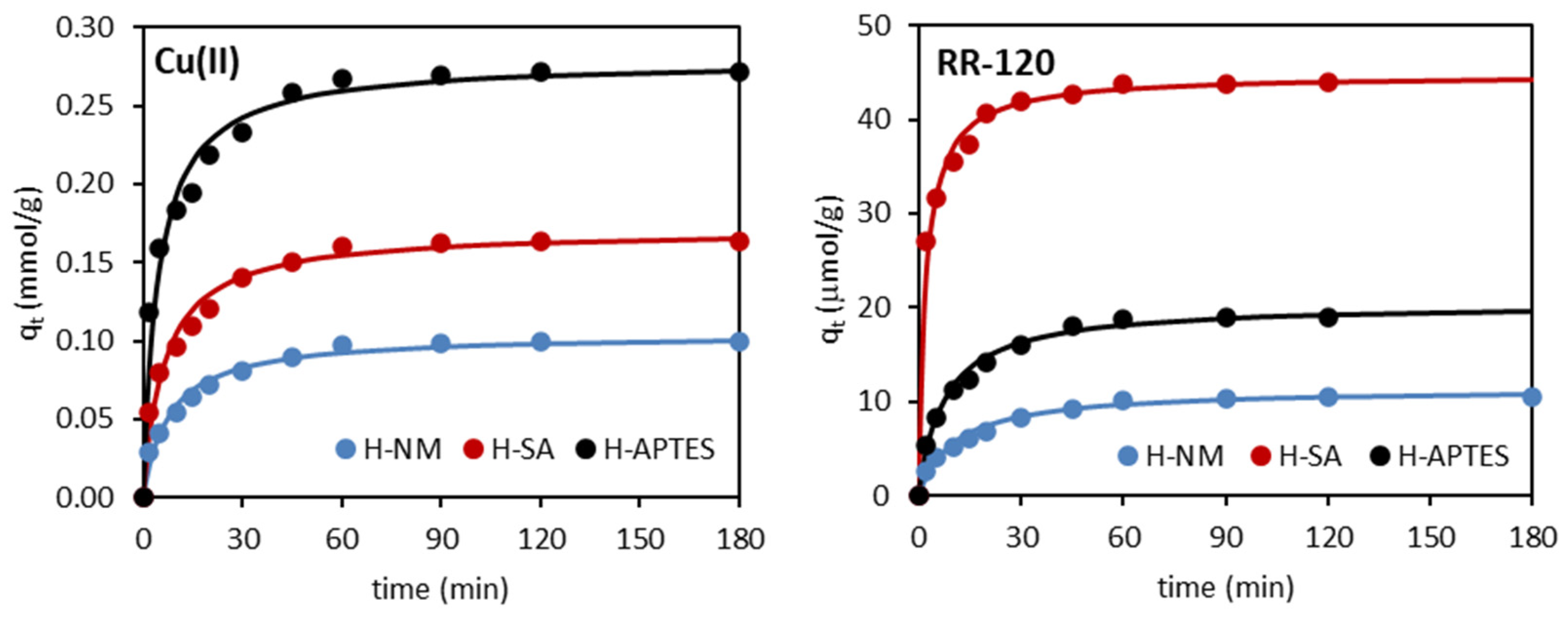
2.2.2. Adsorption Kinetics
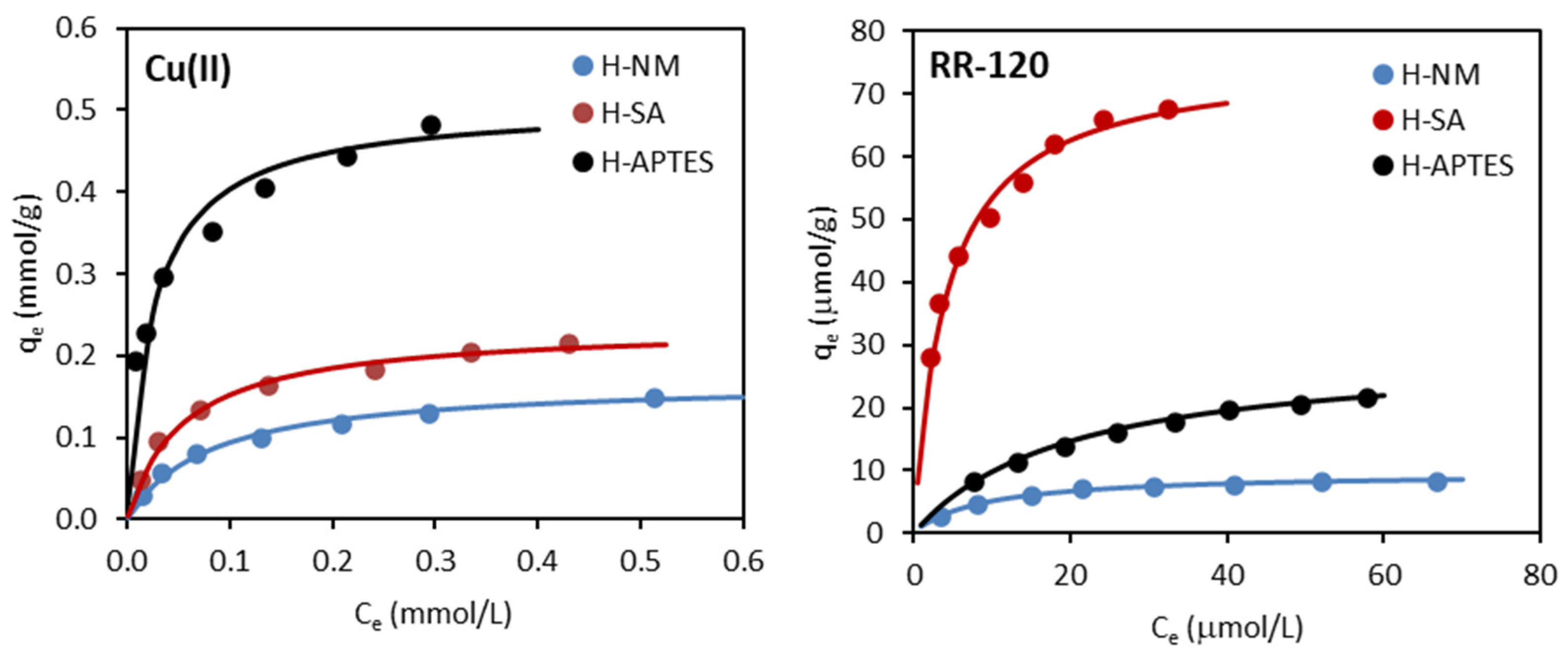
2.2.3. Adsorption Isotherms
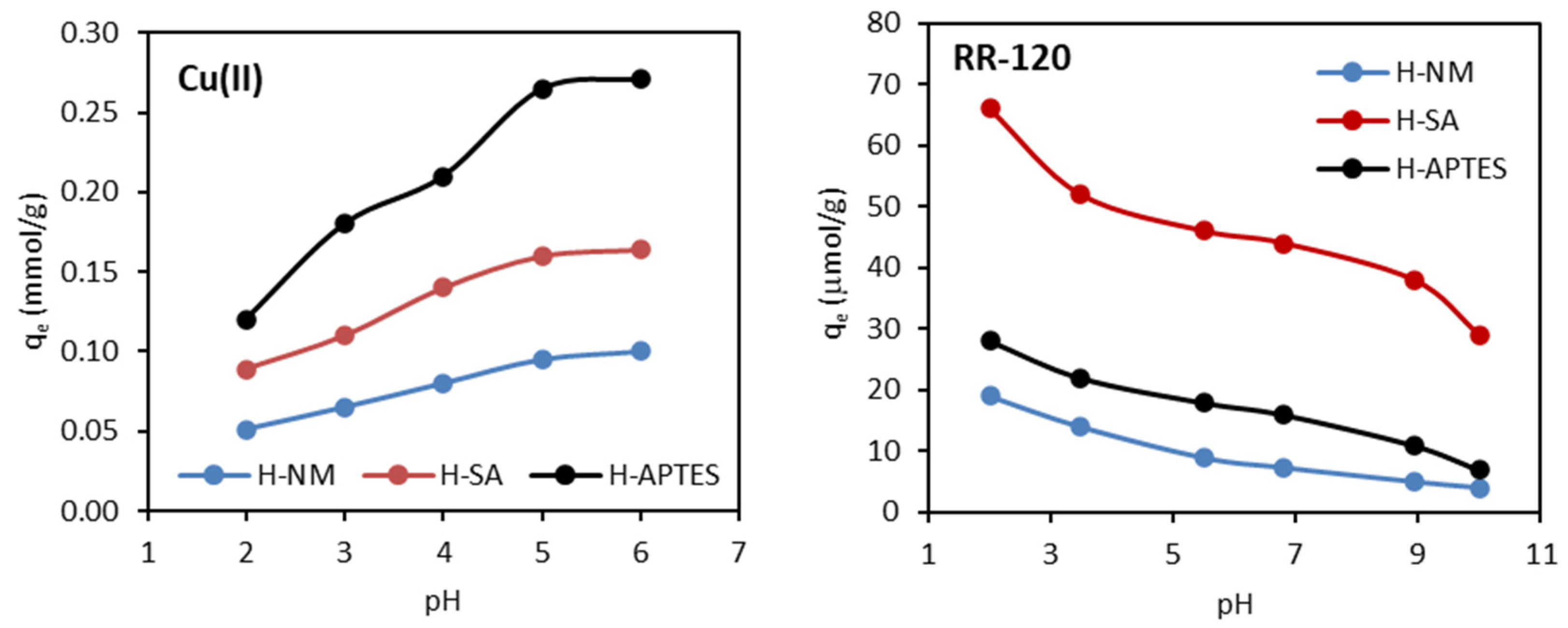
2.2.4. Effect of Solution pH
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation and Characterization of Adsorbents
3.3. Bath Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rafique, M.; Hajra, S.; Tahir, M.B.; Gillani, S.S.A.; Irshad, M. A review on sources of heavy metals, their toxicity and removal technique using physico-chemical processes from wastewater. Environ. Sci. Pollut. Res. 2022, 29, 16772–16781. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Mrabet, B.S.; Harfi, S. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile dyeing industry environmental impacts and remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphil, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Crini, N.M. Conventional and non conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 195–213. [Google Scholar] [CrossRef]
- Iravani, R.; An, C.; Adamian, Y.; Mohammadi, M. A review on the use of nanoclay adsorbents in environmental pollution control. Water Air Soil Pollut. 2022, 233, 109. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.H.; Bhatti, H.M.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Yu, L.; Wang, H.; Zhang, Y.; Zhang, B.; Liu, J. Recent advances in halloysite nanotube derived composites for water treatment. Environ. Sci. Nano 2015, 3, 28–44. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, J.; Yu, G.; Núñez-Delgado, A.; Kornaros, M. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Wilson, I.; Keeling, J. Global occurrence, geology and characteristics of tubular halloysite deposits. Clay Miner. 2016, 51, 309–324. [Google Scholar] [CrossRef]
- Cheng, C.; Song, W.; Zhao, Q.; Zhang, H. Halloysite nanotubes in polymer science: Purification, characterization, modification and applications. Nanotechnol. Rev. 2020, 9, 323–344. [Google Scholar] [CrossRef]
- Biddeci, G.; Spinelli, G.; Colomba, P.; Di Blasi, F. Review nanomaterials: A review about halloysite nanotubes, properties, and application in the biological field. Int. J. Mol. Sci. 2022, 23, 11518. [Google Scholar] [CrossRef] [PubMed]
- Matusik, J.; Wścisło, A. Enhanced heavy metal adsorption on functionalized nanotubular halloysite interlayer grafted with aminoalcohols. Appl. Clay Sci. 2014, 100, 50–59. [Google Scholar] [CrossRef]
- Mellouk, S.; Cherifi, S.; Sassi, M.; Marouf-Khelifa, K.; Bengueddach, A.; Schott, J.; Khelifa, A. Intercalation of halloysite from Djebel Debagh (Algeria) and adsorption of copper ions. Appl. Clay Sci. 2009, 44, 230–236. [Google Scholar] [CrossRef]
- Mellouk, S.; Belhakem, A.; Marouf-Khelifa, K.; Schott, J.; Khelifa, A. Cu(II) adsorption by halloysites intercalated with sodium acetate. J. Colloid Interf. Sci. 2011, 360, 716–724. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Legocka, I.; Kuśmierek, K.; Świątkowski, A. Modified polymers filler as an effective adsorbent of Cu(II) ions from an aqueous solution. Polimery 2021, 66, 239–244. [Google Scholar] [CrossRef]
- Bessaha, F.; Marouf-Khelifa, K.; Batonneau-Gener, I.; Khelifa, A. Characterization and application of heat-treated and acid-leached halloysites in the removal of malachite green: Adsorption, desorption, and regeneration studies. Desalin. Water Treat. 2015, 57, 14609–14621. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Wierzbicka, E.; Legocka, I. Enhanced adsorption of Direct Orange 26 dye in aqueous solutions by modified halloysite. Physicochem. Probl. Miner. Process. 2020, 56, 693–701. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Kuśmierek, K.; Świątkowski, A.; Legocka, I. Efficient Rhodamine B dye removal from water by acid- and organo-modified halloysites. Minerals 2022, 12, 350. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.; Mishra, K.G.; Dash, S.K. Adsorption of copper(II) on NH2-MCM-41 and its application for epoxidation of styrene. Ind. Eng. Chem. Res. 2012, 51, 2235–2246. [Google Scholar] [CrossRef]
- Shirendev, N.; Bat-Amgalan, M.; Kano, N.; Kim, H.-J.; Gunchin, B.; Ganbat, B.; Yunden, G. A natural zeolite developed with 3-aminopropyltriethoxysilane and adsorption of Cu(II) from aqueous media. Appl. Sci. 2022, 12, 11344. [Google Scholar] [CrossRef]
- Wei, J.; Chen, S.; Li, Y.; He, Z.; Geng, L.; Liao, L. Aqueous Cu(II) ion adsorption by amino functionalized mesoporous silica KIT-6. RSC Adv. 2020, 10, 20504–20514. [Google Scholar] [CrossRef]
- Li, H.; Xiao, D.; He, H.; Lin, R.; Zuo, P. Adsorption behavior and adsorption mechanism of Cu(II) ions on amino-functionalized magnetic nanoparticles. Trans. Nonferrous Met. Soc. China 2013, 23, 2657–2665. [Google Scholar] [CrossRef]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Râpa, M.; Turcanu, A.A.; Matei, E.; Predescu, A.M.; Pantilimon, M.C.; Coman, G.; Predescu, C. Adsorption of copper (II) from aqueous solutions with alginate/clay hybrid materials. Materials 2021, 14, 7187. [Google Scholar] [CrossRef]
- Nassef, E.; Mahmoud, A.; Salah, H.; El-taweel, Y. Removal of copper ions from liquid wastes using adsorption technique. Int. J. Res. Ind. Eng. 2017, 6, 522–562. [Google Scholar] [CrossRef]
- Zendelska, A.; Golomeova, M.; Blazev, K.; Krstev, B.; Golomeov, B.; Krstev, A. Adsorption of copper ions from aqueous solutions on natural zeolite. Environ. Prot. Eng. 2015, 41, 17–36. [Google Scholar] [CrossRef]
- Djebbar, M.; Djafri, F. Adsorption of Cu(II) on natural and treated clays. Water Qual. Res. J. 2016, 51, 26–32. [Google Scholar] [CrossRef]
- Bourliva, A.; Sikalidis, A.K.; Papadopoulou, L.; Betsiou, M.; Michailidis, K.; Sikalidis, C.; Filippidis, A. Removal of Cu2+ and Ni2+ ions from aqueous solutions by adsorption onto natural palygorskite and vermiculite. Clay Miner. 2018, 53, 1–15. [Google Scholar] [CrossRef]
- Wang, X.-S.; Wang, J.; Sun, C. Removal of copper(II) ions from aqueous solutions using natural kaolinite. Adsorpt. Sci. Technol. 2006, 24, 517–530. [Google Scholar] [CrossRef]
- Zenasni, M.A.; Benfarhi, S.; Merlin, A.; Molina, S.; George, B.; Meroufel, B. Adsorption of Cu(II) on maghnite from aqueous solution: Effects of pH, initial concentration, interaction time and temperature. Nat. Sci. 2012, 4, 856–868. [Google Scholar] [CrossRef][Green Version]
- Langama, P.L.M.; Anguile, J.J.; Ndong, A.N.M.M.; Bouraima, A.; Bissielou, C. Adsorption of copper (ii) ions in aqueous solution by a natural mouka smectite and an activated carbon prepared from kola nut shells by chemical activation with zinc chloride (ZnCl2). Open J. Inorg. Chem. 2021, 11, 131–144. [Google Scholar] [CrossRef]
- Anna, B.; Kleopas, M.; Constantine, S.; Anestis, F.; Maria, B. Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: Study in mono- and multi-metal systems. Environ. Earth Sci. 2014, 73, 5435–5444. [Google Scholar] [CrossRef]
- Ouakouak, A.; Rihani, K.; Youcef, L.; Hamdi, N.; Guergazi, S. Adsorption characteristics of Cu (II) onto CaCl2 pretreated algerian bentonite. Mater. Res. Express 2020, 7, 025045. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Wang, A. Removal of Cu(II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard. Mater. 2007, 149, 346–354. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Ahmed, M.J.; Ojukwu, V.E.; Danish, M.; Stylianou, M.; Ighalo, J.O. A comprehensive review on adsorption of Reactive Red 120 dye using various adsorbents. J. Mol. Liq. 2024, 394, 123719. [Google Scholar] [CrossRef]
- Hossein, M.A.; Behzad, H. Removal of Reactive Red 120 and Direct Red 81 dyes from aqueous solutions by pumice. Res. J. Chem. Environ. 2012, 16, 62–68. [Google Scholar]
- Aragaw, T.A.; Alene, A.N. A comparative study of acidic, basic, and reactive dyes adsorption from aqueous solution onto kaolin adsorbent: Effect of operating parameters, isotherms, kinetics, and thermodynamics. Emerg. Contam. 2022, 8, 59–74. [Google Scholar] [CrossRef]
- Kittinaovarat, S.; Kansomwan, P.; Jiratumnukul, N. Chitosan/modified montmorillonite beads and adsorption Reactive Red 120. Appl. Clay Sci. 2010, 48, 87–91. [Google Scholar] [CrossRef]
- Errais, E.; Duplay, J.; Darragi, F.; M’Rabet, I.; Aubert, A.; Huber, F.; Morvan, G. Efficient anionic dye adsorption on natural untreated clay: Kinetic study and thermodynamic parameters. Desalination 2011, 275, 74–81. [Google Scholar] [CrossRef]
- Al Rubai, H.F.; Hassan, A.K.; Sultan, M.S.; Abood, W.M. Kinetics of adsorption of Reactive Red 120 using bentonite modified by CTAB and study the effect of salts. Nat. Environ. Pollut. Technol. 2021, 20, 281–289. [Google Scholar] [CrossRef]
- Nejib, A.; Joelle, D.; Fadhila, A.; Sophie, G.; Malika, T.-A. Adsorption of anionic dye on natural and organophilic clays: Effect of textile dyeing additives. Desalin. Water Treat. 2015, 54, 1754–1769. [Google Scholar] [CrossRef]
- Tabak, A.; Baltas, N.; Afsin, B.; Emirik, M.; Caglar, B.; Eren, E. Adsorption of Reactive Red 120 from aqueous solutions by cetylpyridinium-bentonite. J. Chem. Technol. Biotechnol. 2010, 85, 1199–1207. [Google Scholar] [CrossRef]
- Ay, C.; Sarpaşar, Z. Using zeolite and Fe3O4@zeolite composites in removal of Reactive Red 120 from wastewater: Isotherm, kinetic, thermodynamic and adsorption behaviors. J. Dispers. Sci. Technol. 2023, 44, 370–381. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, J.H.; Moon, D.H.; Shin, H.J. Adsorption and precipitation of anionic dye Reactive Red 120 from aqueous solution by aminopropyl functionalized magnesium phyllosilicate. Korean J. Chem. Eng. 2019, 36, 101–108. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Sprynskyy, M.; Świątkowski, A. Raw lignite as an effective low-cost adsorbent to remove phenol and chlorophenols from aqueous solutions. Sep. Sci. Technol. 2020, 55, 1741–1751. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, M.R. Effects of pH on isotherms modeling for Cu(II) ions adsorption using maple wood sawdust. Chem. Eng. J. 2009, 149, 273–280. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.-W.; Feng, W.; Yao, Z.-Y. Cu(II) Adsorption from aqueous solution onto poly(acrylic acid/chestnut shell pigment) hydrogel. Water 2022, 14, 3500. [Google Scholar] [CrossRef]
- Navaei, A.; Yazdani, M.; Alidadi, H.; Dankoob, M.; Bonyadi, Z.; Dehghan, A.; Hosseini, A. Biosorption of Reactive Red 120 dye from aqueous solution using Saccharomyces cerevisiae: RSM analysis, isotherms and kinetic studies. Desalin. Water Treat. 2019, 171, 418–427. [Google Scholar] [CrossRef]
- Çelekli, A.; Yavuzatmaca, M.; Bozkurt, H. Modeling the removal of Reactive Red 120 on pistachio husk. Clean 2010, 38, 173–180. [Google Scholar] [CrossRef]
- Çelekli, A.; Ilgün, G.; Bozkurt, H. Sorption equilibrium, kinetic, thermodynamic, and desorption studies of Reactive Red 120 on Chara contraria. Chem. Eng. J. 2012, 191, 228–235. [Google Scholar] [CrossRef]
- Hosseini, A.R.; Khorshid, A.R.; Mahvi, A.H. Decolorisation of Reactive Red 120 dye by using Single-Walled Carbon Nanotubes in aqueous solutions. J. Chem. 2013, 2013, 938374. [Google Scholar] [CrossRef]
- Çelekli, A.; Al-Nuaimi, A.I.; Bozkurt, H. Adsorption kinetic and isotherms of Reactive Red 120 on Moringa oleifera seed as an eco-friendly process. J. Mol. Struct. 2019, 1195, 168–178. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Sabar, S. Adsorption and mechanism study for reactive red 120 dye removal by cross-linked chitosan-epichlorohydrin biobeads. Desalin. Water Treat. 2019, 164, 378–387. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Wen, H.-Y.; Wen, J.-C.; Gollakota, A.R.K.; Shu, H.-M.; Reddy, G.M. Characterization of protonated amine modified lotus (Nelumbo nucifera) stem powder and its application in the removal of textile (Reactive Red 120) dye from liquid phase. J. Mol. Liq. 2021, 338, 116486. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Ghosh, G.C.; Akter, M.N.; Audhikary, K.; Islam, M.S.; Ghosh, P.; Zaman, S.; Habib, A.; Kabir, A.H.M.E. Biosorption of Reactive Red 120 dye from aqueous solutions by using Mahagoni (Swietenia mahagoni) wood and bark charcoal: Equilibrium, and kinetic studies. Pollution 2021, 7, 905–921. [Google Scholar] [CrossRef]
- Wieczorek, M.; Tomaszewska, J.; Bajda, T.; Długosz, J. Effect of calcinated halloysite on structure and properties of rigid poly(vinyl chloride) composites. Chem. Process Eng. 2022, 43, 383–404. [Google Scholar] [CrossRef]
- Filice, S.; Bongiorno, C.; Libertino, S.; Compagnini, G.; Gradon, L.; Iannazzo, D.; La Magna, A.; Scalese, S. Structural Characterization and Adsorption Properties of Dunino Raw Halloysite Mineral for Dye Removal from Water. Materials 2021, 14, 3676. [Google Scholar] [CrossRef]
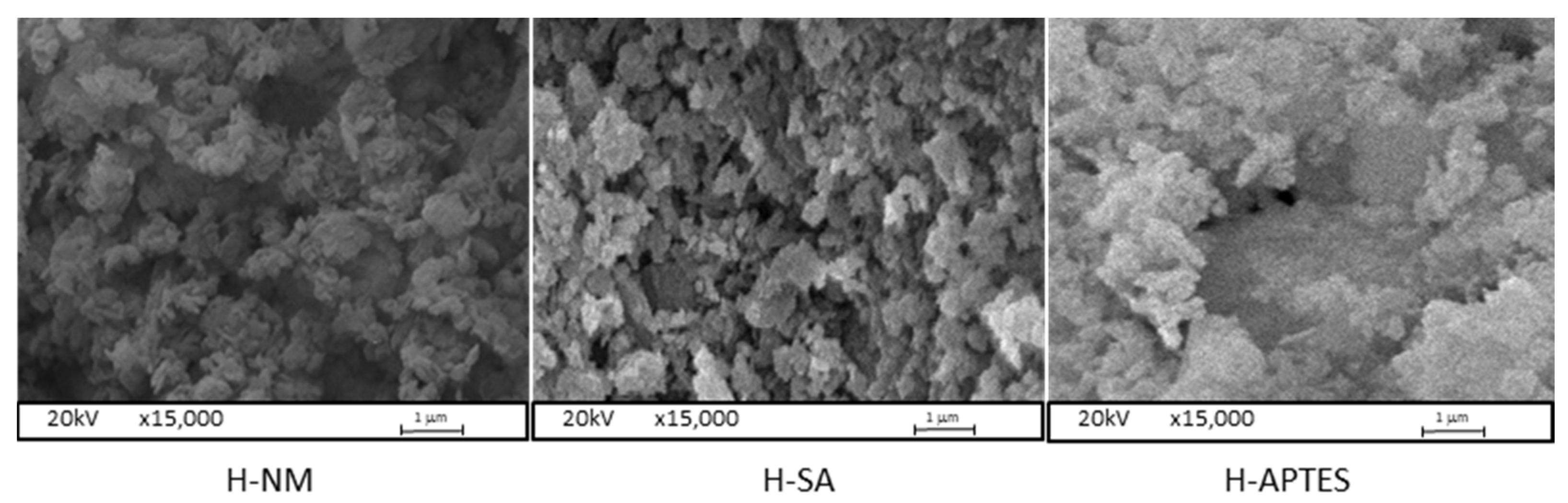
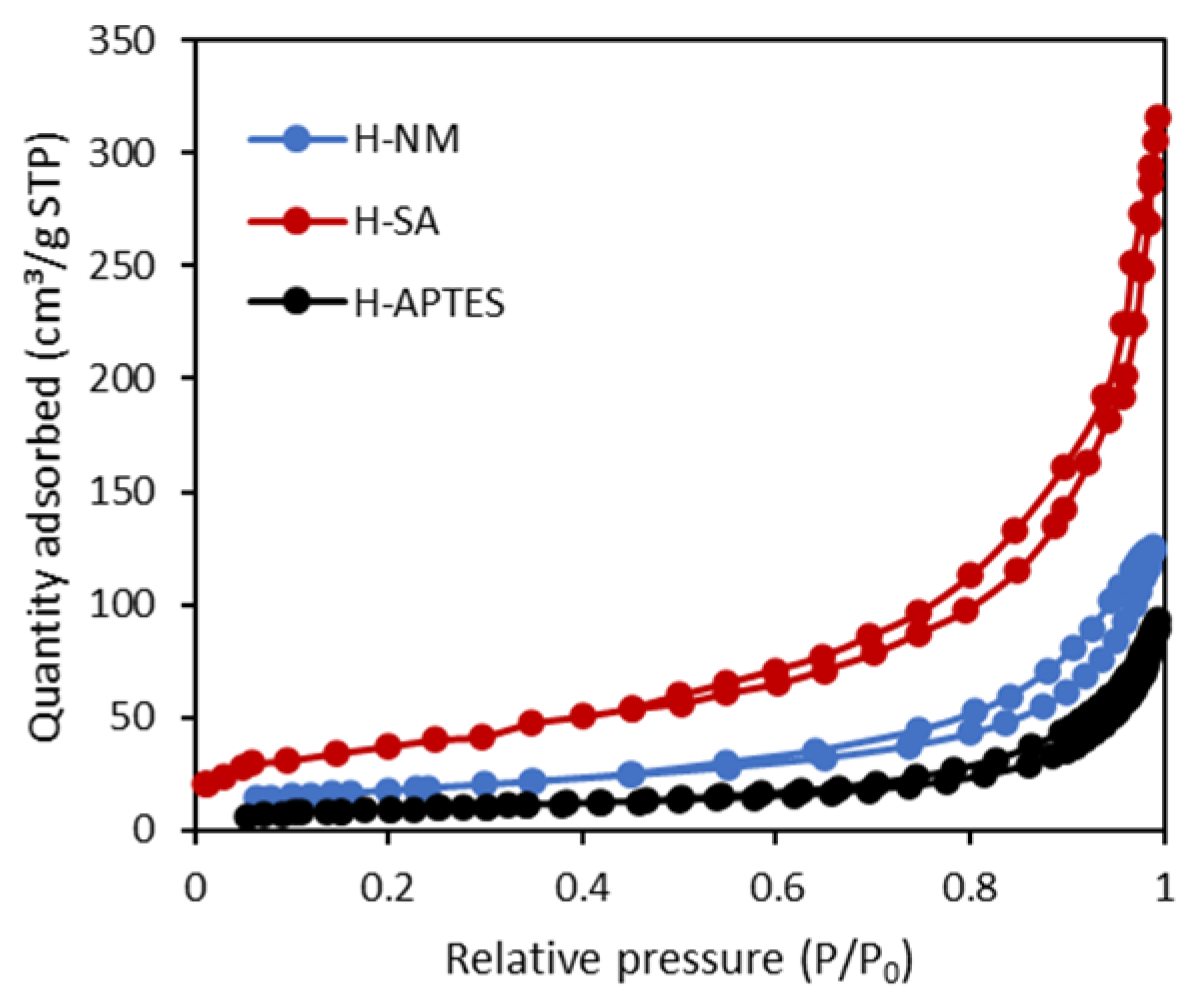
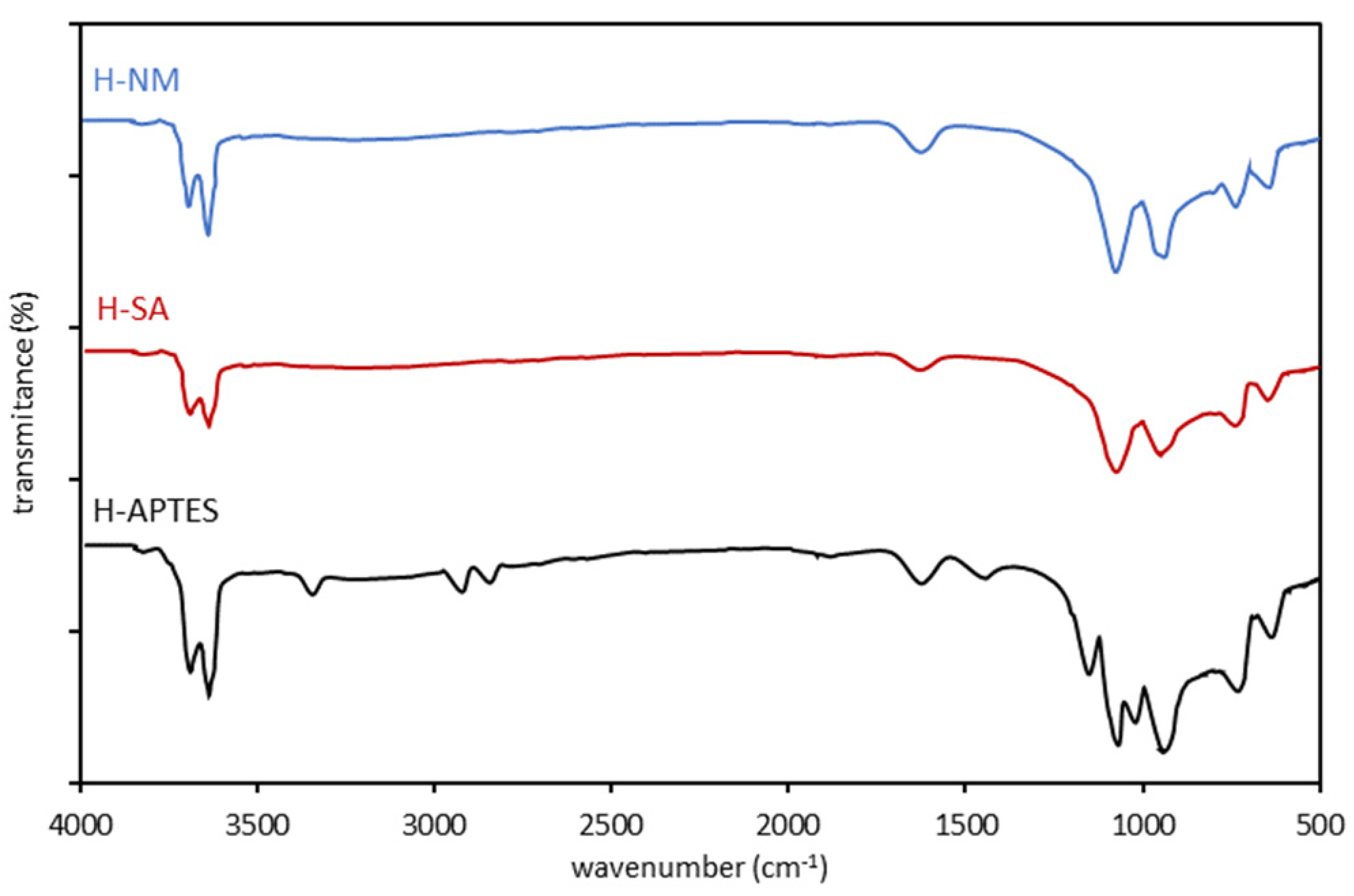
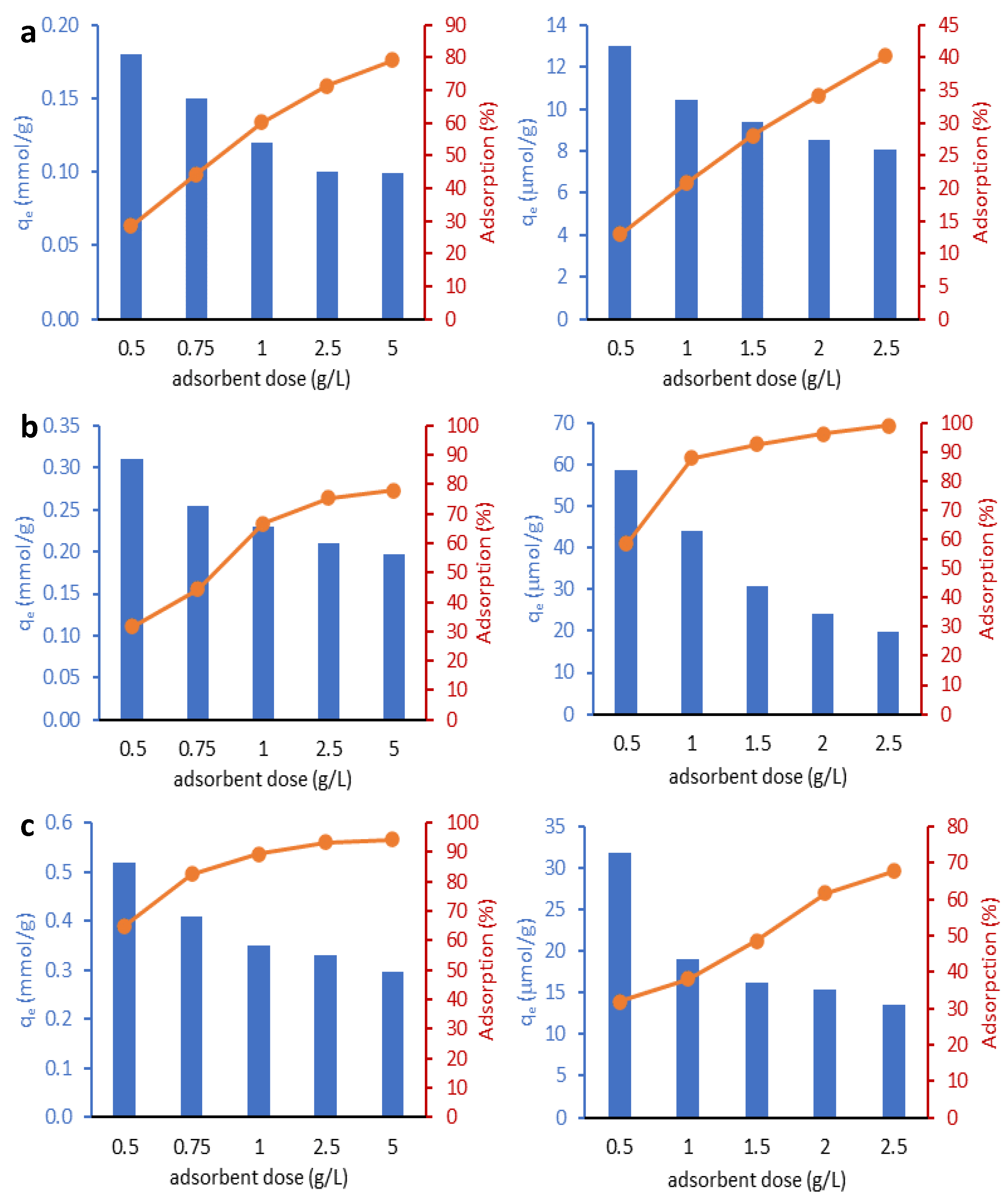
| Adsorbent | C | O | Al | Si | Fe | Ti | N |
|---|---|---|---|---|---|---|---|
| at.% | |||||||
| H-NM | - | 63.5 | 16.0 | 18.4 | 1.8 | 0.3 | - |
| H-SA | - | 63.2 | 15.7 | 19.2 | 1.7 | 0.2 | - |
| H-APTES | 28.9 | 36.9 | 12.9 | 16.7 | 0.9 | 0.4 | 3.3 |
| Adsorbent | SBET (m2/g) | Vt (cm3/g) | Vmi (cm3/g) | Vme (cm3/g) |
|---|---|---|---|---|
| H-NM | 53 | 0.217 | 0.019 | 0.198 |
| H-SA | 129 | 0.399 | 0.057 | 0.342 |
| H-APTES | 34 | 0.108 | 0.0147 | 0.093 |
| H-NM | H-SA | H-APTES | ||||
|---|---|---|---|---|---|---|
| Temp. (°C) | Δm (%) | Temp. (°C) | Δm (%) | Temp. (°C) | Δm (%) | |
| End of drying | 180 | 2.1 | 200 | 2.6 | 185 | 9.5 |
| Temp. of max of DTG | 448 | 7.2 | 459 | 9.5 | 472 | 19.5 |
| End of weight loss | 680 | 9.4 | 700 | 11.6 | 715 | 25.8 |
| H-NM | H-SA | H-APTES |
|---|---|---|
| Wavenumber (cm−1) | ||
| 3697 3622 1634 1108 913 791 753 | 3696 3621 1634 1107 911 791 752 | 3697 3622 3295 2930 2886 1631 1484 1140 1108 1089 913 791 753 |
| Kinetic Model | Adsorbent/Adsorbate | ||
|---|---|---|---|
| H-NM | H-SA | H-APTES | |
| Cu(II) | |||
| qe exp (mmol/g) | 0.101 | 0.164 | 0.271 |
| Pseudo-first-order | |||
| k1 (1/min) | 0.0420 | 0.0428 | 0.0490 |
| qe1 cal (mmol/g) | 0.051 | 0.104 | 0.151 |
| R2 | 0.948 | 0.966 | 0.986 |
| Pseudo-second-order | |||
| k2 (g/mmol∙min) | 1.211 | 0.913 | 0.790 |
| qe2 cal (mmol/g) | 0.105 | 0.171 | 0.279 |
| R2 | 0.999 | 0.999 | 0.998 |
| RR-120 | |||
| qe exp (µmol/g) | 10.42 | 43.96 | 19.03 |
| Pseudo-first-order | |||
| k1 (1/min) | 0.0843 | 0.0615 | 0.0608 |
| qe1 cal (µmol/g) | 15.73 | 25.01 | 17.87 |
| R2 | 0.905 | 0.911 | 0.921 |
| Pseudo-second-order | |||
| k2 (g/µmol∙min) | 0.0083 | 0.0104 | 0.0064 |
| qe2 cal (µmol/g) | 11.42 | 44.84 | 19.05 |
| R2 | 0.997 | 0.999 | 0.999 |
| Isotherm Model | Adsorbent/Adsorbate | ||
|---|---|---|---|
| H-NM | H-SA | H-APTES | |
| Cu(II) | |||
| Freundlich | |||
| KF ((mmol/g)(L/mmol)1/n) | 0.230 | 0.329 | 0.675 |
| 1/n | 0.449 | 0.397 | 0.264 |
| R2 | 0.943 | 0.944 | 0.990 |
| Langmuir | |||
| qm (mmol/g) | 0.169 | 0.236 | 0.507 |
| KL (L/mmol) | 12.50 | 18.16 | 39.01 |
| R2 | 0.995 | 0.996 | 0.995 |
| Temkin | |||
| bT (kJ/mol) | 72.87 | 53.39 | 29.78 |
| AT (L/g) | 151.5 | 233.9 | 966.6 |
| R2 | 0.961 | 0.965 | 0.921 |
| RR-120 | |||
| Freundlich | |||
| KF ((µmol/g)(L/µmol)1/n) | 1.750 | 24.04 | 3.140 |
| 1/n | 0.406 | 0.316 | 0.487 |
| R2 | 0.941 | 0.973 | 0.987 |
| Langmuir | |||
| qm (µmol/g) | 9.640 | 75.76 | 29.33 |
| KL (L/µmol) | 0.111 | 0.241 | 0.051 |
| R2 | 0.999 | 0.997 | 0.997 |
| Temkin | |||
| bT (kJ/mol) | 1.198 | 0.169 | 0.361 |
| AT (L/g) | 1.047 | 3.406 | 0.400 |
| R2 | 0.989 | 0.992 | 0.994 |
| Adsorbent | qm (mg/g) | Ref. |
|---|---|---|
| H-NM | 10.73 * | This paper |
| H-SA | 14.98 * | This paper |
| H-APTES | 32.19 * | This paper |
| alginate/kaolin | 0.339 | [28] |
| bentonite clay | 0.406 | [29] |
| alginate/montmorillonite | 0.680 | [28] |
| clinoptilolite | 5.269 | [30] |
| zeolite modified with APTES | 6.176 | [24] |
| natural montmorillonite | 12.22 | [31] |
| palygorskite | 12.53 | [32] |
| urea modified halloysite (H-Mo) | 13.87 | [17] |
| melamine modified halloysite (H-Me) | 15.24 | [17] |
| H2SO4-activated montmorillonite | 15.40 | [31] |
| kaolinite | 16.79 | [33] |
| maghnite | 21.78 | [34] |
| natural smectite | 28.82 | [35] |
| natural Ca-bentonite | 32.26 | [36] |
| vermiculite | 32.68 | [32] |
| CH3COONa-intercalated halloysite | 52.30 | [16] |
| CaCl2 pretreated Algerian bentonite | 55.47 | [37] |
| acid-activated palygorskite | 93.02 | [38] |
| Adsorbent | qm (mg/g) | Ref. |
|---|---|---|
| H-NM | 12.90 * | This paper |
| H-SA | 101.4 * | This paper |
| H-APTES | 39.24 * | This paper |
| pumice | 0.320 | [40] |
| kaolin | 1.090 | [41] |
| chitosan-montmorillonite composite | 5.608 | [42] |
| natural untreated clay (Tunisia) | 29.94 | [43] |
| CTAB-modified bentonite | 51.28 | [44] |
| raw Fouchana clay | 54.64 | [45] |
| CTAB-modified bentonite | 81.97 | [46] |
| zeolite | 145.9 | [47] |
| Fe3O4@zeolite composite | 154.3 | [47] |
| HDTMA-bromide modified clay | 163.9 | [45] |
| functionalized magnesium phyllosilicate | 229.9 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuśmierek, K.; Świątkowski, A.; Wierzbicka, E.; Legocka, I. Modified Halloysite as an Adsorbent for the Removal of Cu(II) Ions and Reactive Red 120 Dye from Aqueous Solutions. Molecules 2024, 29, 3099. https://doi.org/10.3390/molecules29133099
Kuśmierek K, Świątkowski A, Wierzbicka E, Legocka I. Modified Halloysite as an Adsorbent for the Removal of Cu(II) Ions and Reactive Red 120 Dye from Aqueous Solutions. Molecules. 2024; 29(13):3099. https://doi.org/10.3390/molecules29133099
Chicago/Turabian StyleKuśmierek, Krzysztof, Andrzej Świątkowski, Ewa Wierzbicka, and Izabella Legocka. 2024. "Modified Halloysite as an Adsorbent for the Removal of Cu(II) Ions and Reactive Red 120 Dye from Aqueous Solutions" Molecules 29, no. 13: 3099. https://doi.org/10.3390/molecules29133099
APA StyleKuśmierek, K., Świątkowski, A., Wierzbicka, E., & Legocka, I. (2024). Modified Halloysite as an Adsorbent for the Removal of Cu(II) Ions and Reactive Red 120 Dye from Aqueous Solutions. Molecules, 29(13), 3099. https://doi.org/10.3390/molecules29133099








