Imidazoquinoline Derivatives as Potential Inhibitors of InhA Enzyme and Mycobacterium tuberculosis
Abstract
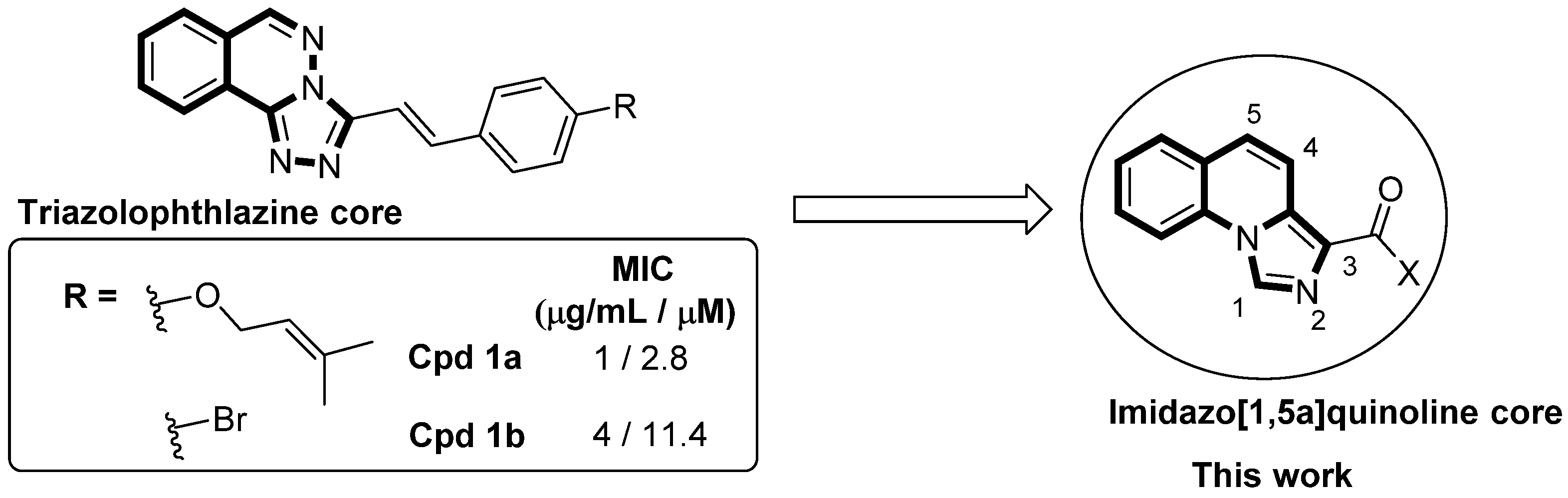
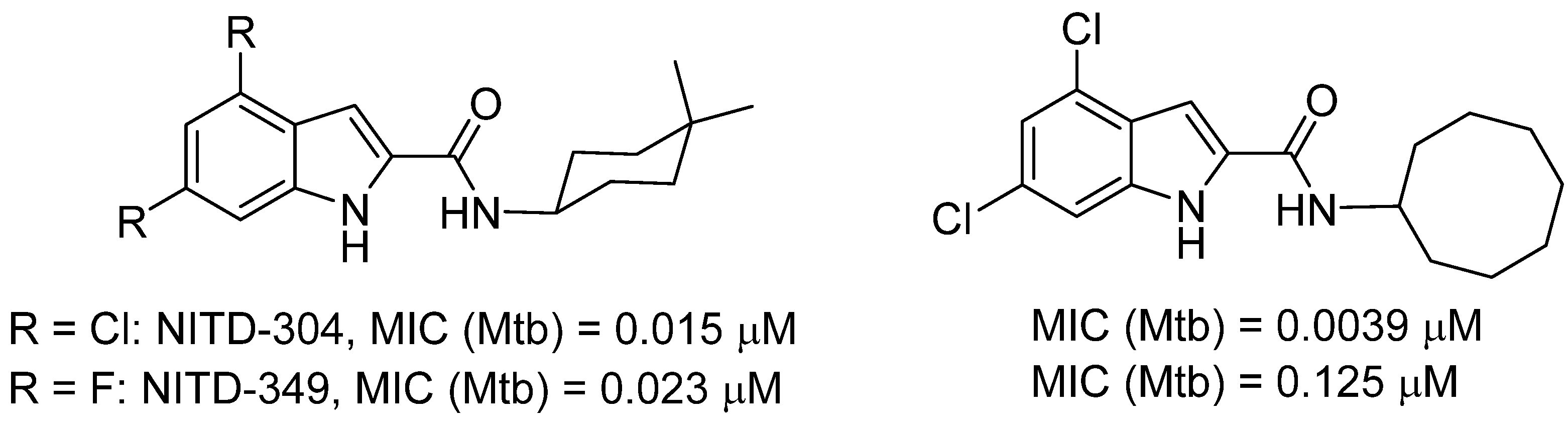
1. Introduction
2. Results
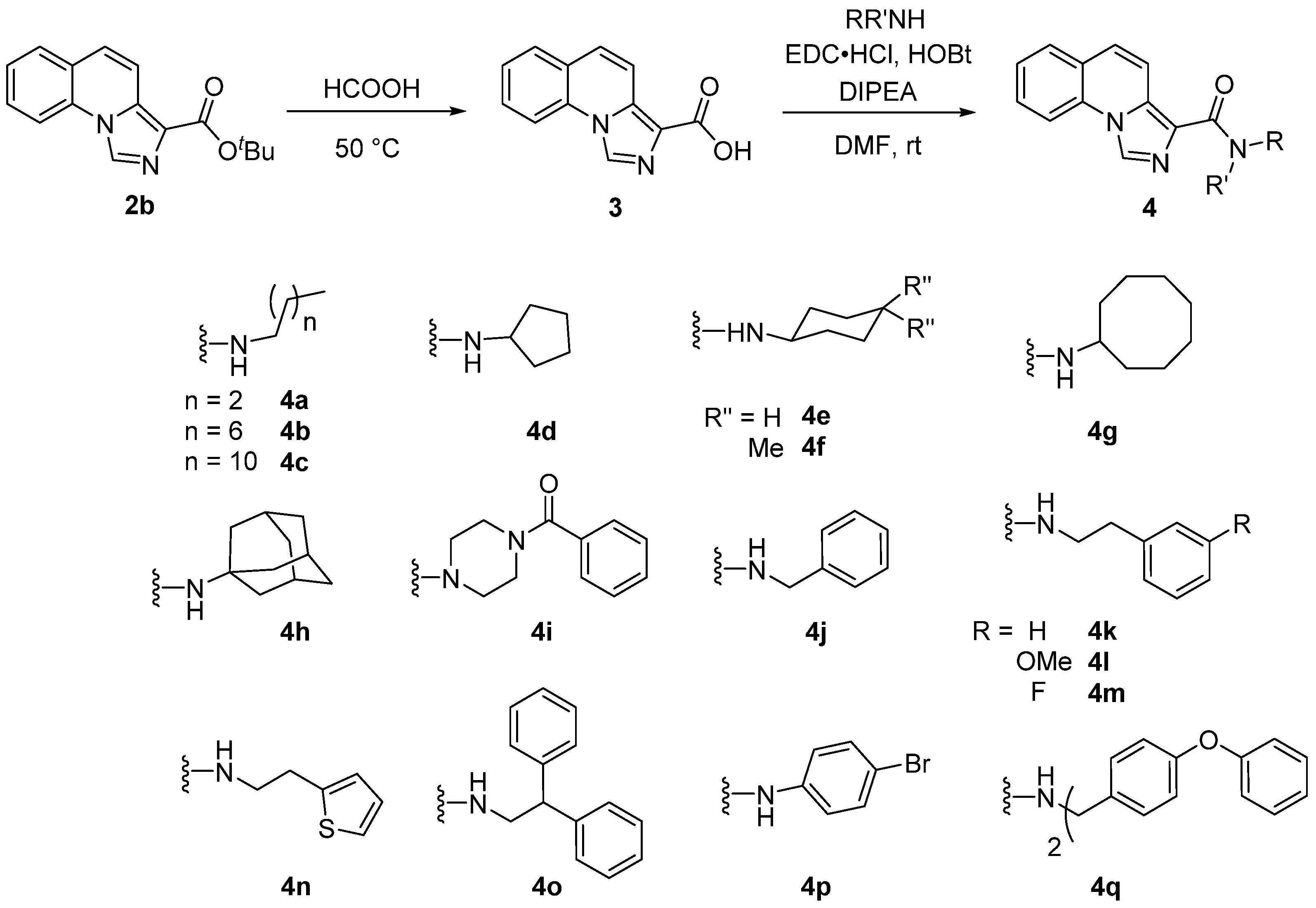
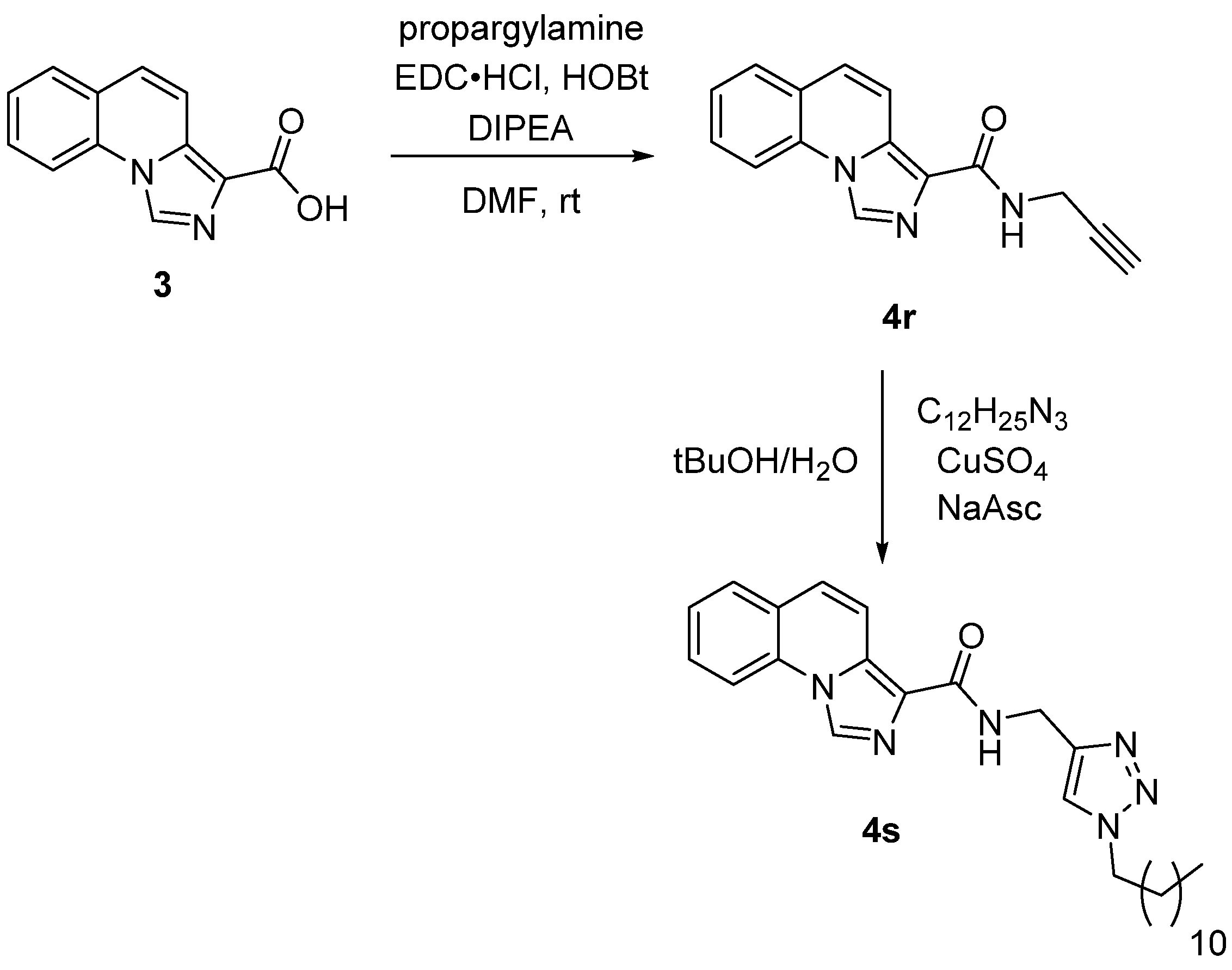
2.1. Chemistry
2.2. Biological Activity
2.2.1. InhA Inhibition
2.2.2. Inhibition of MTB Growth
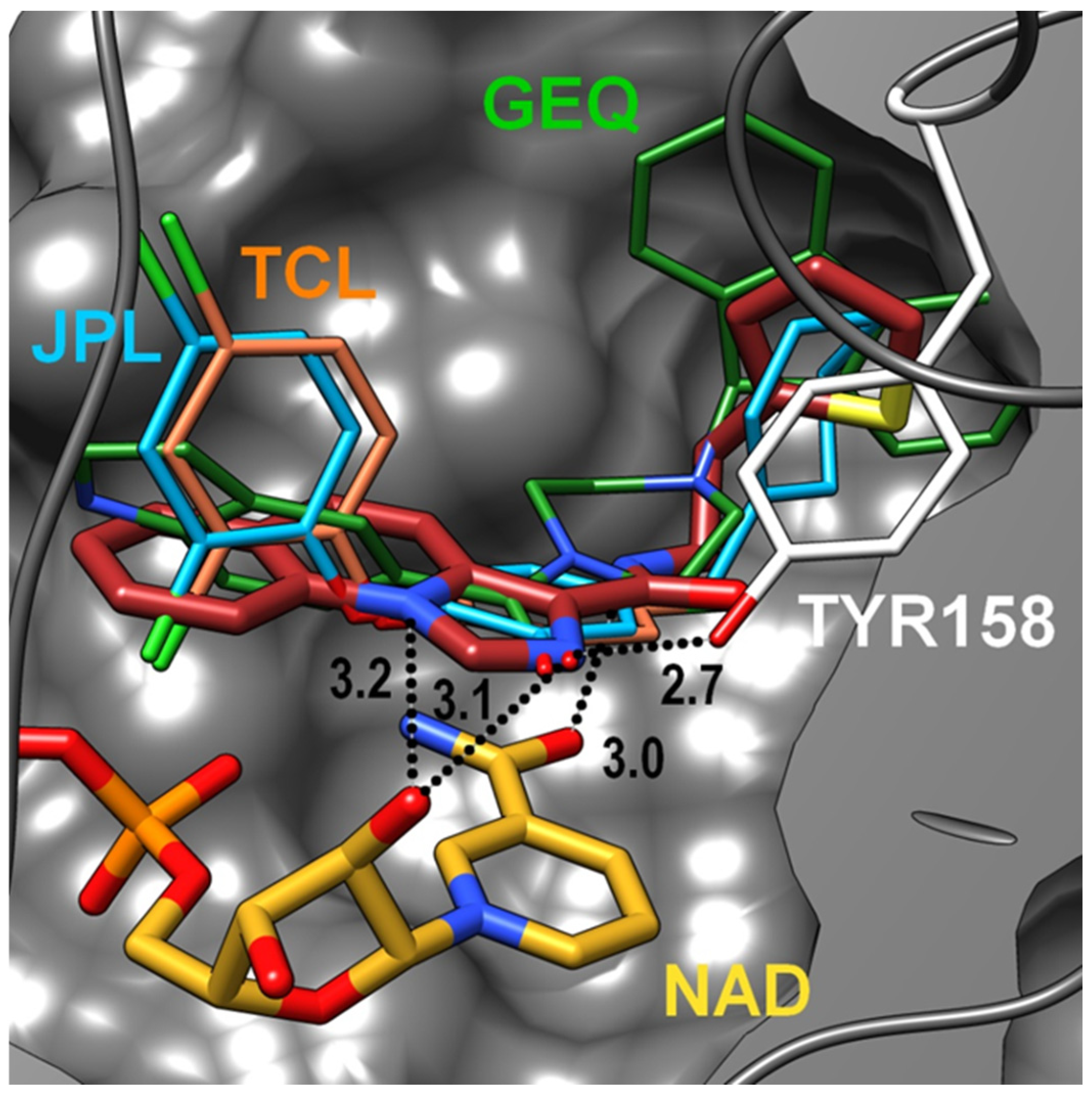
2.3. Docking Studies
3. Experimental
3.1. Materials and Methods
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of Imidazole Compounds
3.2.2. General Procedure for the Coupling of the Different Amines with Compound 3 (4a–4s)
3.2.3. Purity of the Compounds
3.3. Molecular Docking Studies of Compound 4n
3.3.1. Molecular Graphics
3.3.2. Molecular Docking
3.4. Evaluation of InhA Enzyme Inhibition
3.5. Minimal Inhibitory Concentration Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023; WHO: Geneva, Switzerland, 2023; pp. 1–75. [Google Scholar]
- Kakkar, A.K.; Dahiya, N. Bedaquiline for the treatment of resistant tuberculosis: Promises and Pitfalls. Tuberculosis 2014, 94, 357–362. [Google Scholar] [CrossRef]
- Keam, S.J. Pretomanid: First approval. Drugs 2019, 79, 1797–1803. [Google Scholar] [CrossRef]
- Ryan, N.J.; Lo, J.H. Delamanid: First global approval. Drugs 2014, 74, 1041–1045. [Google Scholar] [CrossRef]
- Boyne, M.E.; Sullivan, T.J.; amEnde, C.W.; Lu, H.; Gruppo, V.; Heaslip, D.; Amin, A.G.; Chatterjee, D.; Lenaerts, A.; Tonge, P.J.; et al. Targeting fatty acid biosynthesis for the development of novel chemotherapeutics against Mycobacterium tuberculosis: Evaluation of A-ring-modified diphenyl ethers as high-affinity InhA inhibitors. Antimicrob. Agents Chemother. 2007, 51, 3562–3567. [Google Scholar] [CrossRef]
- Pan, P.; Knudson, S.E.; Bommineni, G.; Li, H.J.; Lai, C.T.; Liu, N.; Garcia-Diaz, M.; Simmerling, C.; Patil, S.S.; Slayden, R.A.; et al. Time-dependent diaryl ether inhibitors of InhA: Structure-activity relationship studies of enzyme inhibition, antibacterial activity, and in vivo efficacy. ChemMedChem 2014, 9, 776–791. [Google Scholar] [CrossRef]
- Rodriguez, F.; Saffon, N.; Sammartino, J.C.; Degiacomi, G.; Pasca, M.R.; Lherbet, C. First triclosan-based macrocyclic inhibitors of InhA enzyme. Bioorg. Chem. 2020, 95, 103498. [Google Scholar] [CrossRef]
- Stec, J.; Vilcheze, C.; Lun, S.; Perryman, A.L.; Wang, X.; Freundlich, J.S.; Bishai, W.; Jacobs, W.R., Jr.; Kozikowski, A.P. Biological evaluation of potent triclosan-derived inhibitors of the enoyl-acyl carrier protein reductase InhA in drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis. ChemMedChem 2014, 9, 2528–2537. [Google Scholar] [CrossRef]
- Chebaiki, M.; Delfourne, E.; Tamhaev, R.; Danoun, S.; Rodriguez, F.; Hoffmann, P.; Grosjean, E.; Goncalves, F.; Azéma-Despeyroux, J.; Pál, A.; et al. Discovery of new diaryl ether inhibitors against Mycobacterium tuberculosis targeting the minor portal of InhA. Eur. J. Med. Chem. 2023, 259, 115646. [Google Scholar] [CrossRef]
- Tamhaev, R.; Grosjean, E.; Ahamed, H.; Chebaiki, M.; Rodriguez, F.; Recchia, D.; Degiacomi, G.; Pasca, M.R.; Maveyraud, L.; Mourey, L.; et al. Exploring the plasticity of the InhA substrate-binding site using new diaryl ether inhibitors. Bioorg. Chem. 2024, 143, 107032. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Kanhed, A.M.; Chandrasekaran, B.; Palkar, M.B.; Agrawal, N.; Lherbet, C.; Hampannavar, G.A.; Karpoormath, R. Discovery of novel N-methyl carbazole tethered rhodanine derivatives as direct inhibitors of Mycobacterium tuberculosis InhA. Bioorg. Med. Chem. Lett. 2019, 29, 2338–2344. [Google Scholar] [CrossRef]
- Chollet, A.; Mori, G.; Menendez, C.; Rodriguez, F.; Fabing, I.; Pasca, M.R.; Madacki, J.; Kordulakova, J.; Constant, P.; Quemard, A.; et al. Design, synthesis and evaluation of new GEQ derivatives as inhibitors of InhA enzyme and Mycobacterium tuberculosis growth. Eur. J. Med. Chem. 2015, 101, 218–235. [Google Scholar] [CrossRef]
- Kuo, M.R.; Morbidoni, H.R.; Alland, D.; Sneddon, S.F.; Gourlie, B.B.; Staveski, M.M.; Leonard, M.; Gregory, J.S.; Janjigian, A.D.; Yee, C.; et al. Targeting tuberculosis and malaria through inhibition of Enoyl reductase: Compound activity and structural data. J. Biol. Chem. 2003, 278, 20851–20859. [Google Scholar] [CrossRef]
- Hartkoorn, R.C.; Sala, C.; Neres, J.; Pojer, F.; Magnet, S.; Mukherjee, R.; Uplekar, S.; Boy-Rottger, S.; Altmann, K.H.; Cole, S.T. Towards a new tuberculosis drug: Pyridomycin—Nature’s isoniazid. EMBO Mol. Med. 2012, 4, 1032–1042. [Google Scholar] [CrossRef]
- Kienle, M.; Eisenring, P.; Stoessel, B.; Horlacher, O.P.; Hasler, S.; van Colen, G.; Hartkoorn, R.C.; Vocat, A.; Cole, S.T.; Altmann, K.H. Synthesis and structure-activity relationship studies of C2-modified analogs of the antimycobacterial natural product pyridomycin. J. Med. Chem. 2020, 63, 1105–1131. [Google Scholar] [CrossRef]
- Manjunatha, U.H.; Rao, S.P.S.; Kondreddi, R.R.; Noble, C.G.; Camacho, L.R.; Tan, B.H.; Ng, S.H.; Ng, P.S.; Ma, N.L.; Lakshminarayana, S.B.; et al. Direct inhibitors of InhA are active against Mycobacterium tuberculosis. Sci. Transl. Med. 2015, 7, 269ra263. [Google Scholar] [CrossRef]
- Menendez, C.; Rodriguez, F.; Ribeiro, A.L.; Zara, F.; Frongia, C.; Lobjois, V.; Saffon, N.; Pasca, M.R.; Lherbet, C.; Baltas, M. Synthesis and evaluation of α-ketotriazoles and α,β-diketotriazoles as inhibitors of Mycobacterium tuberculosis. Eur. J. Med. Chem. 2013, 69, 167–173. [Google Scholar] [CrossRef]
- Suresh, A.; Srinivasarao, S.; Agnieszka, N.; Ewa, A.K.; Alvala, M.; Lherbet, C.; Chandra Sekhar, K.V.G. Design and synthesis of 9H-fluorenone based 1,2,3-triazole analogues as Mycobacterium tuberculosis InhA inhibitors. Chem. Biol. Drug Des. 2018, 91, 1078–1086. [Google Scholar] [CrossRef]
- Vau, D.; Krykun, S.; Pasca, M.R.; Frongia, C.; Lobjois, V.; Chassaing, S.; Lherbet, C.; Baltas, M. Triazolophthalazines—Easily accessible compounds with potent antitubercular activity. ChemMedChem 2016, 11, 1078–1089. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Georgescu, F.; Georgescu, E.; Caira, M.R. Pyrroloquinolines, imidazoquinolines, and pyrroloquinazolines with a bridgehead nitrogen. Adv. Het. Chem. 2019, 129, 155–244. [Google Scholar] [CrossRef]
- Capelli, A.; Giuliani, G.; Anzini, M.; Riitano, D.; Giorgi, G.; Vomero, S. Design, synthesis, and structure-affinity relationship studies in NK1 receptor ligands based on azole-fused quinolinecarboxamide moieties. Bioorg. Med. Chem. 2008, 16, 6850–6859. [Google Scholar] [CrossRef]
- Cappelli, A.; Anzini, M.; Castriconi, F.; Grisci, G.; Paolino, M.; Braile, C.; Valenti, S.; Giuliani, G.; Vomero, S.; Di Capua, A.; et al. Design, Synthesis, and Biological Evaluation of Imidazo[1,5-a]quinoline as Highly Potent Ligands of Central Benzodiazepine Receptors. J. Med. Chem. 2016, 59, 3353–3372. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wan, C.; Yang, Y.; Zha, Z.; Wang, Z. The synthesis of imidazo[1,5-a]quinolones via a decarboxylative cyclization under metal-free conditions. RSC Adv. 2018, 8, 23058–23065. [Google Scholar] [CrossRef] [PubMed]
- CCDC-2358967 (4g) and CCDC-2358968 (4h) Contain the Supplementary Crystallographic Data for this Paper. These Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 4 June 2024).
- Kondreddi, R.R.; Jiricek, J.; Rao, S.P.S.; Lakshminarayana, S.B.; Camacho, L.R.; Rao, R.; Herve, M.; Bifani, P.; Ma, N.L.; Kuhen, K.; et al. Design, synthesis, and biological evaluation of indole-2-carboxamides: A promising class of antituberculosis agents. J. Med. Chem. 2013, 56, 8849e8859. [Google Scholar] [CrossRef] [PubMed]
- Franz, N.D.; Belardinelli, J.M.; Kaminski, M.A.; Dunn, L.C.; de Moura, V.C.N.; Blaha, M.A.; Truong, D.D.; Li, W.; Jackson, M.; North, E.J. Design, synthesis and evaluation of indole-2-carboxamides with pan anti-mycobacterial activity. Bioorg. Med. Chem. 2017, 25, 3746e3755. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, J.S.; Wang, F.; Vilcheze, C.; Gulten, G.; Langley, R.; Schiehser, G.A.; Jacobus, D.P.; Jacobs, W.R.; Sacchettini, J.C. Triclosan Derivatives: Towards Potent Inhibitors of Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis. ChemMedChem 2009, 4, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E.J. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, N.; Shindyalov, I.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Huang, C.C.; Ferrin, T.E.J. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinform. 2006, 7, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Rozwarski, D.A.; Vilchèze, C.; Sugantino, M.; Bittman, R.; Sacchettini, J.C. Crystal structure of the Mycobacterium tuberculosis enoyl-ACP reductase, InhA, in complex with NAD and a C16 fatty acyl substrate. J. Biol. Chem. 1999, 274, 15582–15589. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H.J. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Joshi, S.D.; Dixit, S.R.; Basha, J.; Kulkarni, V.H.; Aminabhavi, T.M.; Nadagouda, M.N.; Lherbet, C. Pharmacophore mapping, molecular docking, chemical synthesis of some novel pyrrolyl benzamide derivatives and evaluation of their inhibitory activity against enoyl-ACP reductase (InhA) and Mycobacterium tuberculosis. Bioorg. Chem. 2018, 81, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Degiacomi, G.; Gianibbi, B.; Recchia, D.; Stelitano, G.; Truglio, G.I.; Marra, P.; Stamilla, A.; Makarov, V.; Chiarelli, L.R.; Manetti, F.; et al. CanB, a druggable cellular target in Mycobacterium tuberculosis. ACS Omega 2023, 8, 25209–25220. [Google Scholar] [CrossRef] [PubMed]
- Bruker AXS Inc. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G. M ShelXT, Acta Crystallogr. Sect. A; University of Göttingen: Göttingen, Germany, 2015. [Google Scholar]
- Sheldrick, G. M ShelXT, Acta Crystallogr. Sect. C; University of Göttingen: Göttingen, Germany, 2015. [Google Scholar]


| Cpd | % Inhibition at 50 μM | Purity (%) by LC-MS |
|---|---|---|
| 2a | 26 | ND a |
| 2b | 17 | ND a |
| 4a | 40 | >99 |
| 4b | 29 | >99 |
| 4c | 16 | 98 |
| 4d | 0 | >99 |
| 4e | 23 | >99 |
| 4f | 10 | >99 |
| 4g | 19 | >99 |
| 4h | 4 | 97 |
| 4i | 9 | 94 |
| 4j | 31 | >99 |
| 4k | 13 | >99 |
| 4l | 7 | 98 |
| 4m | 8 | 97 |
| 4n | 66 (24) b | >99 |
| 4o | 16 | 99 |
| 4p | 15 | 98 |
| 4q | 34 | 97 |
| 4r | 49 | >99 |
| 4s | 33 | 97 |
| Triclosan | 98 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, P.; Azéma-Despeyroux, J.; Goncalves, F.; Stamilla, A.; Saffon-Merceron, N.; Rodriguez, F.; Degiacomi, G.; Pasca, M.R.; Lherbet, C. Imidazoquinoline Derivatives as Potential Inhibitors of InhA Enzyme and Mycobacterium tuberculosis. Molecules 2024, 29, 3076. https://doi.org/10.3390/molecules29133076
Hoffmann P, Azéma-Despeyroux J, Goncalves F, Stamilla A, Saffon-Merceron N, Rodriguez F, Degiacomi G, Pasca MR, Lherbet C. Imidazoquinoline Derivatives as Potential Inhibitors of InhA Enzyme and Mycobacterium tuberculosis. Molecules. 2024; 29(13):3076. https://doi.org/10.3390/molecules29133076
Chicago/Turabian StyleHoffmann, Pascal, Joëlle Azéma-Despeyroux, Fernanda Goncalves, Alessandro Stamilla, Nathalie Saffon-Merceron, Frédéric Rodriguez, Giulia Degiacomi, Maria Rosalia Pasca, and Christian Lherbet. 2024. "Imidazoquinoline Derivatives as Potential Inhibitors of InhA Enzyme and Mycobacterium tuberculosis" Molecules 29, no. 13: 3076. https://doi.org/10.3390/molecules29133076
APA StyleHoffmann, P., Azéma-Despeyroux, J., Goncalves, F., Stamilla, A., Saffon-Merceron, N., Rodriguez, F., Degiacomi, G., Pasca, M. R., & Lherbet, C. (2024). Imidazoquinoline Derivatives as Potential Inhibitors of InhA Enzyme and Mycobacterium tuberculosis. Molecules, 29(13), 3076. https://doi.org/10.3390/molecules29133076










