Here, after detailing the calculation of uncertainties, this study compared these values with those in the literature. Finally, the risk of false conformity assessment applied to the isotopic analysis of C1 carbon in the exploratory assessment of natural gas was presented.
4.1. Calculation of Expanded Uncertainties by LPU (Law of Propagation of Uncertainties)
To verify the expanded uncertainty, a second internal secondary standard was analyzed, stored in a B1 cylinder of a mixture of 70% mol/mol of methane with 30% mol/mol of ethane, supplied by the company Air Liquide.
The first step is the calculation of the standard uncertainty of the ratios of the CO
2 experimental areas:
The next step is to calculate the combined standard uncertainty of
,
from the available information of
(certificate) and the
value from the literature.
value for this standard was −34.4 ± 0.00002, k = 2, for a confidence level of 95.4%.
Calculation of standard uncertainties for carbon abundances,
and
:
Calculation of standard uncertainties for oxygen ratios:
and
:
Calculation of standard uncertainties for carbon abundances,
and
:
Calculation of standard uncertainties for carbon abundances,
and
:
Calculation of standard uncertainties for theoretical CO
2 areas,
and
:
Calculation of standard uncertainties for CO
2 correction factors,
and
:
Calculation of standard uncertainties for corrected CO
2 areas,
and
, specifically for methane:
Calculation of standard uncertainty for corrected oxygen ratios,
:
Calculation of the standard uncertainty of
:
Calculation of the standard uncertainty of
:
Calculation of the standard uncertainty
:
From the information in
Table 2, the same calculation is performed for ethane, propane, and CO
2,
Table 5:
The next step is to combine the standard uncertainties of the mathematical models,
Table 5, with their respective intermediate precision,
Table 3. As predicted in the literature, this latter contribution, the measurement of dispersion, expressed as intermediate precision, is one of the most relevant contributions to the measurement uncertainty [
51]. These standard uncertainty values are the final combined standard uncertainties,
Table 6.
Finally, the expanded uncertainties are calculated based on Equation (29) for a 95.45% confidence level when considering infinite degrees of freedom (
k = 2), Equation (27). These uncertainties are shown in
Table 7, associated with the δ
13C values of the compounds of interest.
The uncertainty results shown in
Table 7 are more significant than that of 0.5‰, historically recommended by the manufacturer of the mass spectrometer for the analytical technique considered in this study. This relative increase suggests greater caution in geochemical interpretations that consider carbon isotopic ratio values using the compounds previously listed.
4.2. Comparison with Literature Data
It is important to note that the results of the calculated uncertainties presented in this report,
Table 7, comprise two main contributions: one from the equipment and the established procedures, and the other from the calculation procedures outlined in this study.
Regarding natural gas analyses, it is common to spread the information that the measurement uncertainty for carbon isotopes in a natural gas matrix is around 0.5‰. This information is not exactly incorrect, but it is based on an incomplete approach. In fact, the experience of several laboratories shows that this is a reasonable value for a natural gas sample matrix when it is carried out in modern equipment, i.e., gas chromatography–isotope ratio mass spectrometer (GC-IRMS) for the analysis of carbon isotopes performed under minimal quality control conditions. The lack of reliability of this information derives from the fact that this uncertainty ignores a set of error sources that are propagated only after instrumental analysis, which are numerical corrections applied to the measured result.
In many case studies presented in the literature, gases interpreted to have the same origin and/or the same thermal maturity have, at the same time, a difference in their carbon isotopic composition for C
1 greater than 0.5‰. In the analysis and interpretation of large isotopic gas data sets, interlaboratory variability and other uncertainties (for example, related to gas sampling and handling) are considered to have the least influence on the determination of general isotopic composition trends associated with the various natural geochemical processes [
52]. The uncertainty in measuring the carbon isotopic ratios presented here suggests that the geochemical approach generally recognizes a compositional variability that is beyond the commonly reported 0.5‰ value.
In a general way for the considered technique, the possible variabilities that contribute to the analytical uncertainties can be summarized in the following topics [
18]: (i) sample: heterogeneity, matrix variations; (ii) sample preparation: weighing (except for gases), extraction, derivatization, collection, storage; (iii) instrumental analysis: conversion to CO
2, chromatographic separation, transfer through capillaries and valves; (iv) data collection by the equipment: ion current, electronic fluctuations, ion current ratios; (v) integration: software problems, background, time change, baseline choice; (vi) calculation of δ values: correction for
17O; (vii) correction on δ values: blank correction, linearity, electronic drift, memory effect; and (viii) scale calibration: adjustment in control chart, normalization procedures.
Comparing the expanded uncertainty calculated in this study (1.7–1.8‰) with the others shown in
Table 1, there are clearly higher and lower values. This divergence should be seen with caution since it is not part of a systematic study and has no basis to clarify the differences found, as they may have different sources, such as intrinsic differences between matrices and the calculation methods used by each author, which is fundamental to understand for isotopes and may even differ for instruments of different manufacturers. However, it is worth noting that, of all the studies that applied the expanded methodology and that explicitly reported the inclusion of the uncertainty of the correction methods, only two had the greatest uncertainties found below 1.0‰. It is also important to note that there are other corrections that were not applied in this study, such as the blank corrections or the electronic drift. Their contributions to the accuracy and precision of the work should be appreciated in the future.
The correction method related to the contribution of
17O, highlighted in this study, has already been reviewed by several authors [
53,
54]. There are basically two types of correction: those based on Craig’s method (1957) [
55], further developed by Santrock (1985) [
31], called SSH, and the method proposed by IUPAC [
34]. A review of these methods, including the problems found in the correction approach, is given by [
34,
53]. The authors detail the limitations of the SSH model and why a linear approximation method would be superior. However, commercial software does not adopt this IUPAC directive—the most recent methodology—and continues to apply the calculation method based on Craig (1957) [
55] and Santrock et al. (1985) [
35], the oldest method, which apparently introduces greater errors. The discussion of all those methods is beyond the scope of this work. However, this fact must be considered in future studies.
It should also be pointed out that the continuous flow (CF) and dual inlet techniques, both widely used in the literature and in several laboratories currently, have important differences between them. The former leads to higher background values, in addition to other problems, such as the requirement for a reference gas with higher pressure and more residual water in the system, degrading the reproducibility of the results. However, it has advantages that compensate for such limitations, such as the greater ease in the preparation step and injection of samples, the ability to measure more compounds from a complex mixture in the same injection, and the relatively lower required amounts of sample [
10,
56,
57].
The discussions raised so far can be summarized in the following topics: (i) there is no uniformity in the presentation of stable isotope results; (ii) the matrices differences should be discussed considering a physical–chemical approach in such a way that uncertainties, in general, could be better understood and thus lowered; (iii) despite repeated attempts by international bodies such as IUPAC, there is still no consensus about the best 17O correction method; (iv) isotopic corrections are not applied in a standardized way. Issues like linearity correction, blank correction, and normalization are applied without clear criteria. Steps that could be followed regardless of the considered isotope technique or analytical laboratory; and (v) the uncertainty arising from calculations and subsequent corrections is often neglected, which does not directly affect the quality control of the analysis laboratory (when not included in the routine), but affects the derived application, such as food quality control or geological interpretation.
From the evaluation and comparison of the results obtained in this study with the ones reported in the literature, one can conclude that the uncertainty values and methodology presented here are reasonable and should be considered when used for interpretations of any nature.
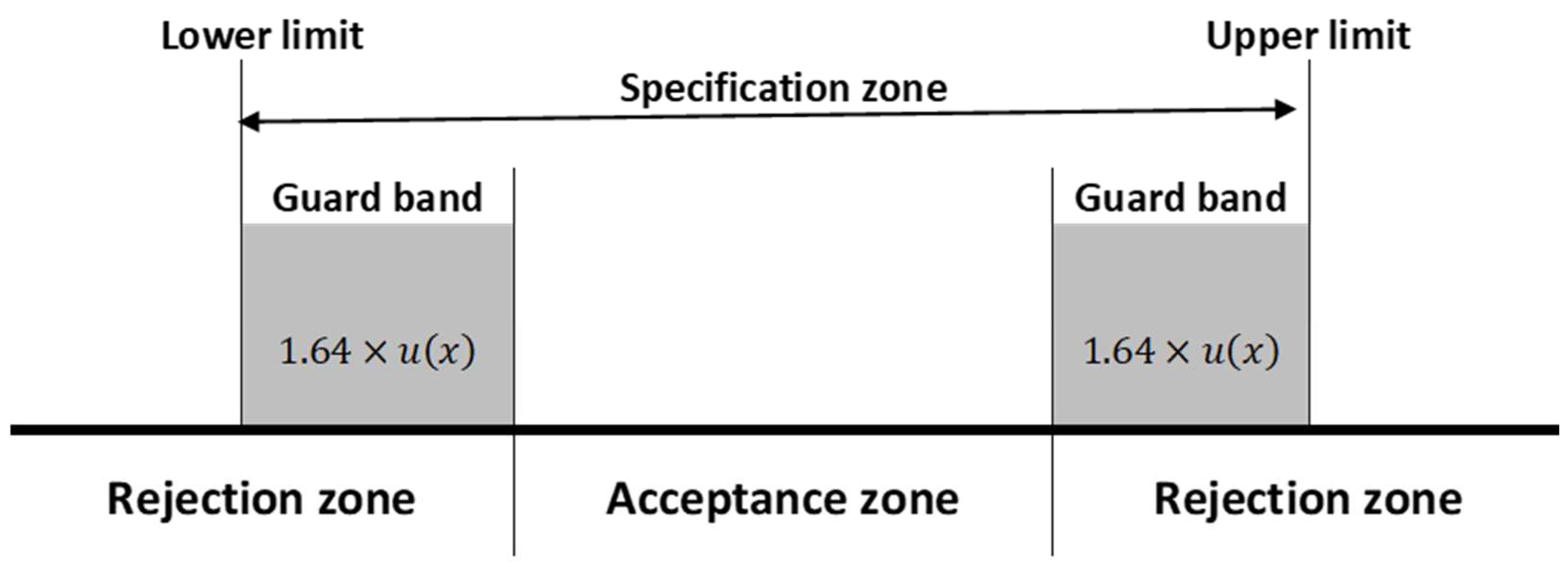
The uncertainty range in the measurement of the carbon isotopic composition in gases (e.g., 1.8‰ for C1), although considerably more significant than the one usually reported in the geochemical literature (0.5‰), might have a limited impact on the set of interpretations, generally supported by general trends in the variation of isotopic compositions from many data and case studies.
The typically recognized compositional fields characterized by natural gases from different origins (biogenic, thermogenic, and abiotic) are usually distinguished by their typical isotopic range of compositions. However, it is known that there is an overlapping of isotopic values, and some limits are poorly defined from the typical biogenic (e.g., 70‰), thermogenic (e.g., 40‰) or abiotic (e.g., 10‰) gas compositions (e.g., C
1 in Etiope and Lollar, 2013) [
58]. In these scenarios, either the diagnosis of the origin or the estimates of mixtures between different end members would be affected by such an uncertainty range. In the case of studies involving fractionation factors (e.g., equilibrium temperature), those biases would be more noticeable, making the application tool useless. The uncertainty value of 1.8‰ (for C
1) should be considered in the geochemical analysis and interpretation, notably in the study of specific cases, such as in areas of exploratory boundaries, where the compositional background of gas occurrences are still unknown or when isotopic measurements plot in intermediate domains, e.g., between −55‰ and −50‰, the approximate limit between biogenic and thermogenic gases [
59,
60]. In these scenarios, the diagnosis of origin and calculation of the approximate proportions of each contribution can be affected by such an uncertainty range.