Synergistic Effect of UiO-66 Directly Grown on Kombucha-Derived Bacterial Cellulose for Dye Removal
Abstract
1. Introduction
2. Results and Discussion
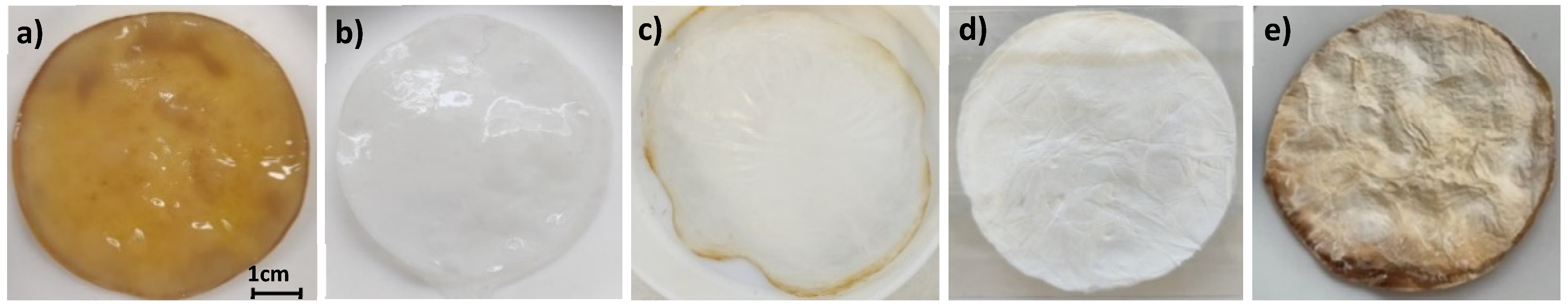
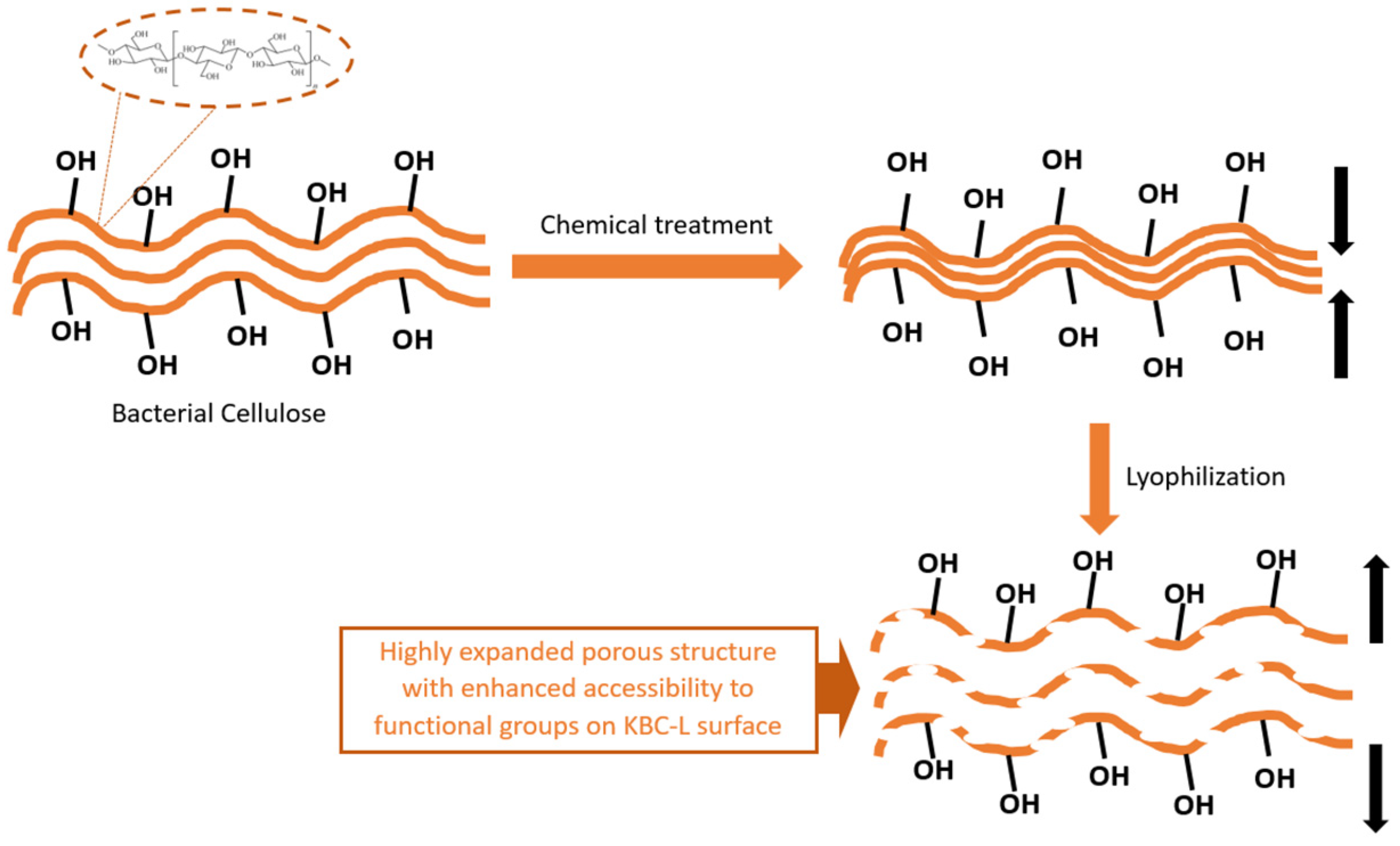
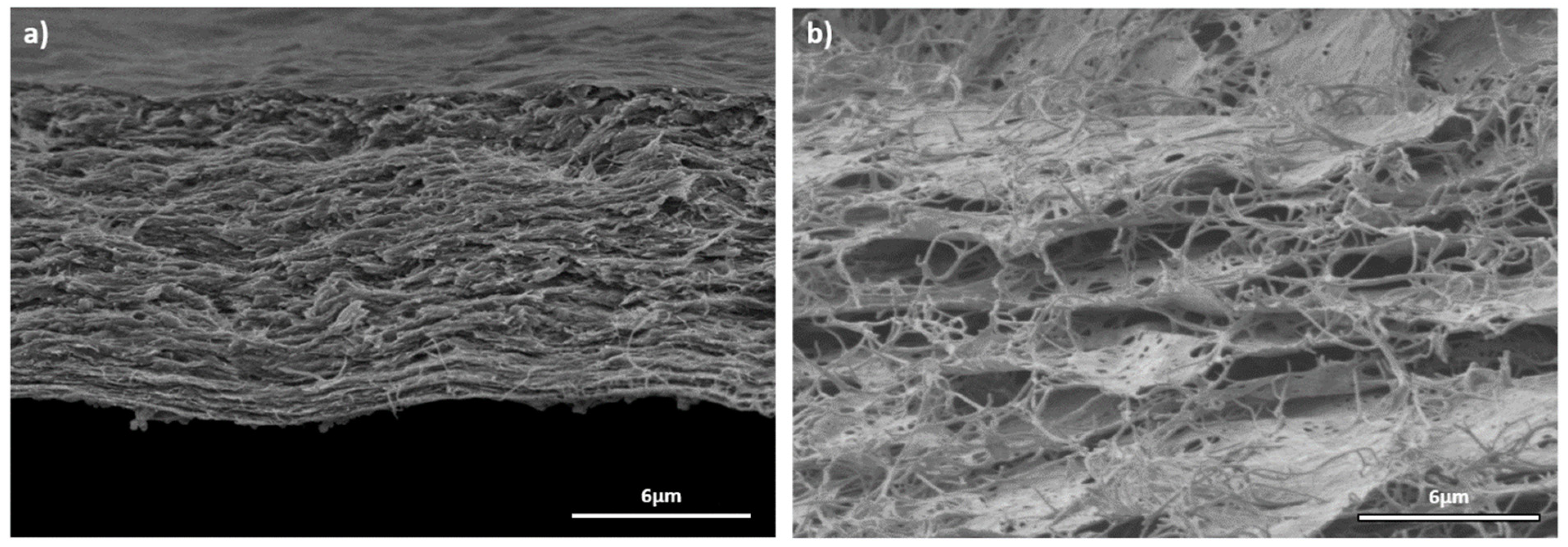
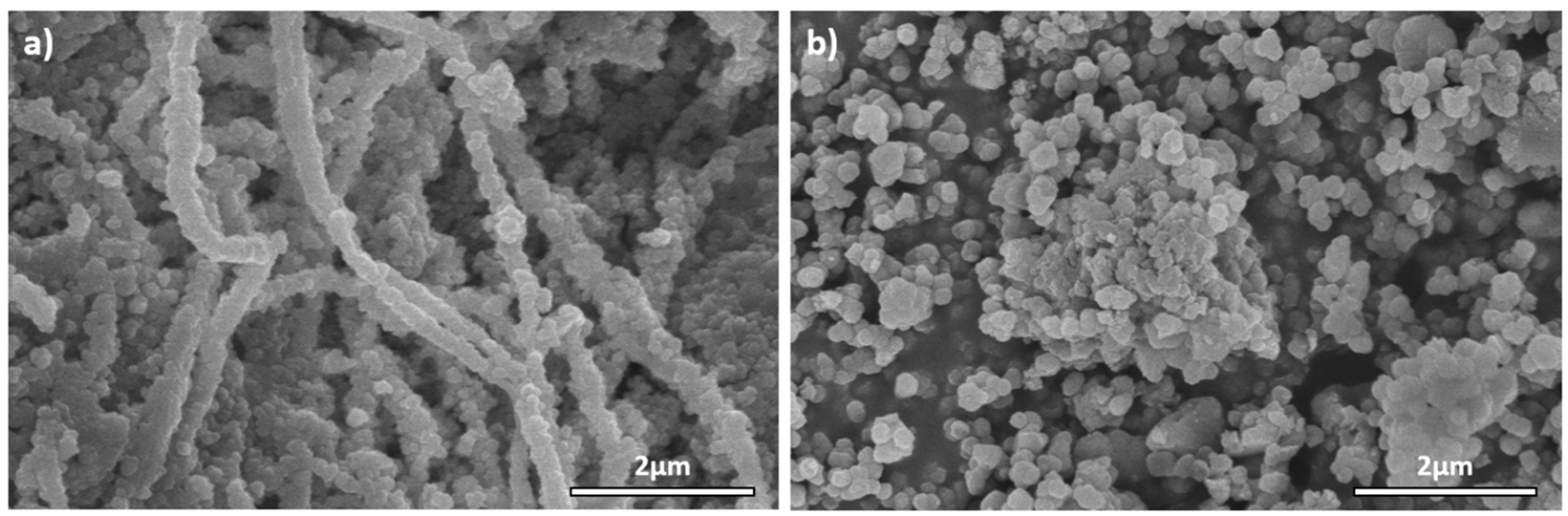
2.1. Physicochemical Characterization of the Materials
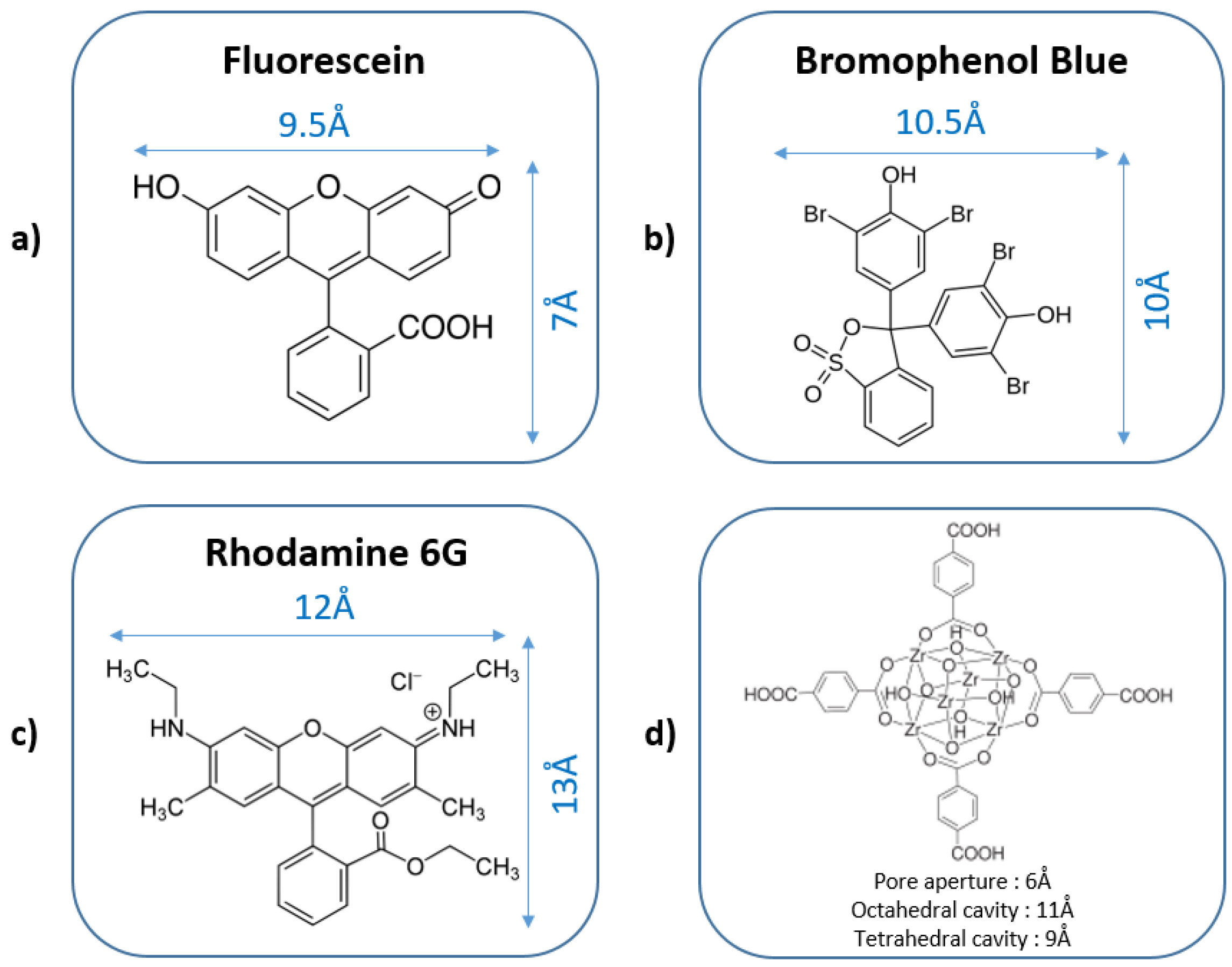
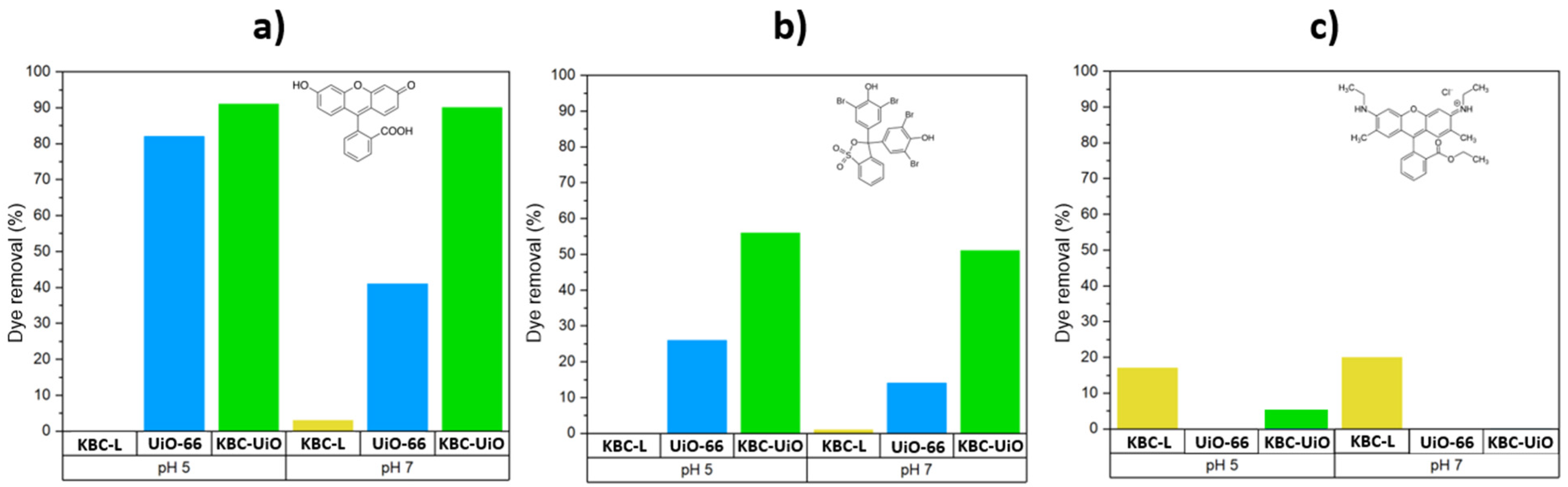
2.2. Dyes Adsorption Performance—Effects of pH and Ionic Strength on Dye Adsorption
3. Materials and Methods
3.1. Formation of KBC Membranes
3.1.1. Growth of Kombucha Biofilms
3.1.2. Chemical Cleaning Treatment of the SCOBY
3.1.3. Drying/Stabilization of Cellulosic Membranes
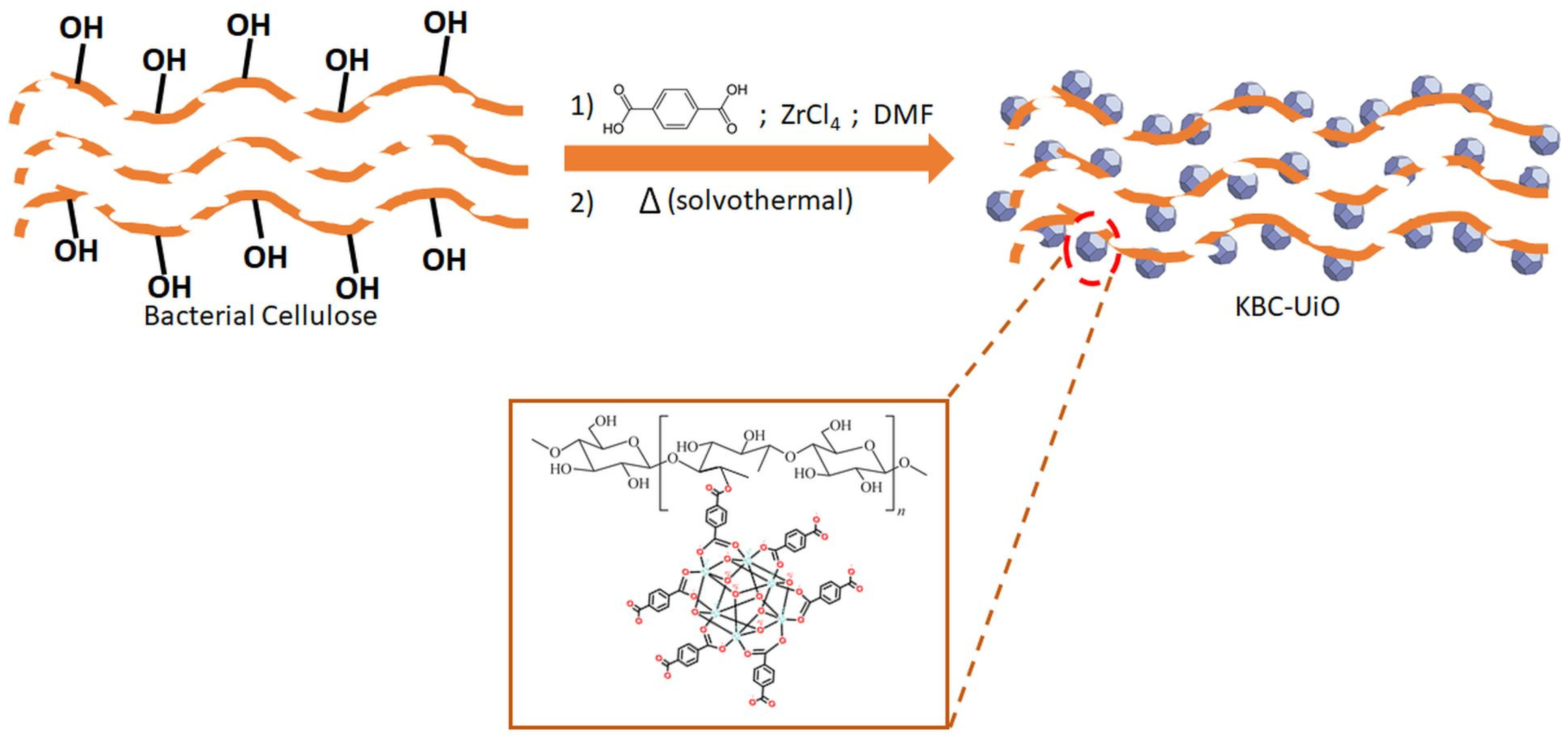
3.2. Formation of KBC-UiO Composite Membranes
3.3. Physicochemical Characterization of Materials
3.4. Dye Adsorption Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, B.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q.; Zheng, S. Development of Polymeric and Polymer-Based Hybrid Adsorbents for Pollutants Removal from Waters. Chem. Eng. J. 2009, 151, 19–29. [Google Scholar] [CrossRef]
- Nouar, F.; Mouchaham, G.; Serre, C. Metal-Organic Frameworks (MOFs)—Fabrication, propriétés et applications. Étude Et Propriétés Des Métaux 2021, M 4 795, 1–13. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective Adsorption of Cationic Dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Esteve, F.; Maireles, M.; Iglesias, D.; Martín, N.; Zanatta, M.; Sans, V.; Cirujano, F.G.; García-Verdugo, E. One-Pot Growth of Metal-Organic Frameworks on Polymers for Catalytic Performance Enhancement in the CO2 Cycloaddition to Epoxides. J. CO2 Util. 2023, 78, 102636. [Google Scholar] [CrossRef]
- Eagleton, A.M.; Ambrogi, E.K.; Miller, S.A.; Vereshchuk, N.; Mirica, K.A. Fiber Integrated Metal-Organic Frameworks as Functional Components in Smart Textiles. Angew. Chem. Int. Ed. 2023, 62, e202309078. [Google Scholar] [CrossRef] [PubMed]
- Bechelany, M.; Drobek, M.; Vallicari, C.; Abou Chaaya, A.; Julbe, A.; Miele, P. Highly Crystalline MOF-Based Materials Grown on Electrospun Nanofibers. Nanoscale 2015, 7, 5794–5802. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Liao, T.; Liang, X.; Chen, W.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Two-Linker MOFs-Based Glass Fiber Paper Monolithic Adsorbent for Atmospheric Water Harvesting in Arid Climates. J. Clean. Prod. 2022, 373, 133838. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Rahman, M.A.; Dzarfan Othman, M.H.; Jaafar, J.; Aziz, A.A. Preparation, Characterizations and Performance Evaluations of Alumina Hollow Fiber Membrane Incorporated with UiO-66 Particles for Humic Acid Removal. J. Membr. Sci. 2018, 563, 162–174. [Google Scholar] [CrossRef]
- Demirel, Ö.H.; Rijnaarts, T.; De Wit, P.; Wood, J.A.; Benes, N.E. Electroforming of a Metal–Organic Framework on Porous Copper Hollow Fibers. J. Mater. Chem. A 2019, 7, 12616–12626. [Google Scholar] [CrossRef]
- Lee, D.T.; Zhao, J.; Oldham, C.J.; Peterson, G.W.; Parsons, G.N. UiO-66-NH2 Metal–Organic Framework (MOF) Nucleation on TiO2, ZnO, and Al2O3 Atomic Layer Deposition-Treated Polymer Fibers: Role of Metal Oxide on MOF Growth and Catalytic Hydrolysis of Chemical Warfare Agent Simulants. ACS Appl. Mater. Interfaces 2017, 9, 44847–44855. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose–Metal Organic Frameworks (CelloMOFs) Hybrid Materials and Their Multifaceted Applications: A Review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Mei, C.; Sun, J.; Lee, J.; Wu, Q.; Hubbe, M.A.; Li, M.-C. Recent Advances in Metal Organic Framework and Cellulose Nanomaterial Composites. Coord. Chem. Rev. 2022, 461, 214496. [Google Scholar] [CrossRef]
- Tu, K.; Ding, Y.; Keplinger, T. Review on Design Strategies and Applications of Metal-Organic Framework-Cellulose Composites. Carbohydr. Polym. 2022, 291, 119539. [Google Scholar] [CrossRef]
- Guo, R.; Cai, X.; Liu, H.; Yang, Z.; Meng, Y.; Chen, F.; Li, Y.; Wang, B. In Situ Growth of Metal–Organic Frameworks in Three-Dimensional Aligned Lumen Arrays of Wood for Rapid and Highly Efficient Organic Pollutant Removal. Environ. Sci. Technol. 2019, 53, 2705–2712. [Google Scholar] [CrossRef]
- Mai, T.; Wang, P.-L.; Yuan, Q.; Ma, C.; Ma, M.-G. In Situ Anchoring Zn-Doped ZIF-67 on Carboxymethylated Bacterial Cellulose for Effective Indigo Carmine Capture. Nanoscale 2021, 13, 18210–18217. [Google Scholar] [CrossRef] [PubMed]
- Au-Duong, A.-N.; Lee, C.-K. Flexible Metal–Organic Framework-Bacterial Cellulose Nanocomposite for Iodine Capture. Cryst. Growth Des. 2018, 18, 356–363. [Google Scholar] [CrossRef]
- Hou, X.; Sun, L.; Hu, Y.; An, X.; Qian, X. De-Doped Polyaniline as a Mediating Layer Promoting In-Situ Growth of Metal–Organic Frameworks on Cellulose Fiber and Enhancing Adsorptive-Photocatalytic Removal of Ciprofloxacin. Polymers 2021, 13, 3298. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lin, L.; Xu, T.; Liu, J.; Tang, M.; Wang, Z. In-Situ Fabrication of MOF@CoP Hybrid Bifunctional Electrocatalytic Nanofilm on Carbon Fibrous Membrane for Efficient Overall Water Splitting. Int. J. Hydrogen Energy 2024, 49, 1446–1457. [Google Scholar] [CrossRef]
- KarzarJeddi, M.; Laitinen, O.; Mahkam, M.; Liimatainen, H. Zwitterionic Hybrid Aerobeads of Binary Metal Organic Frameworks and Cellulose Nanofibers for Removal Anionic Pollutants. Mater. Des. 2020, 196, 109106. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; El-Mehalmey, W.A.; Haikal, R.R.; Safy, M.E.A.; Amin, M.; Shatla, H.R.; Karakalos, S.G.; Alkordi, M.H. Tuning the Chemical Environment within the UiO-66-NH2 Nanocages for Charge-Dependent Contaminant Uptake and Selectivity. Inorg. Chem. 2019, 58, 15078–15087. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, L.-F. Ultraviolet Spectra and Structure of Zinc-Cellulose Complexes in Zinc Chloride Solution. J. Appl. Polym. Sci. 1999, 71, 1441–1446. [Google Scholar] [CrossRef]
- Navya, P.V.; Gayathri, V.; Samanta, D.; Sampath, S. Bacterial Cellulose: A Promising Biopolymer with Interesting Properties and Applications. Int. J. Biol. Macromol. 2022, 220, 435–461. [Google Scholar] [CrossRef]
- Ma, X.; Lou, Y.; Chen, X.-B.; Shi, Z.; Xu, Y. Multifunctional Flexible Composite Aerogels Constructed through In-Situ Growth of Metal-Organic Framework Nanoparticles on Bacterial Cellulose. Chem. Eng. J. 2019, 356, 227–235. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.; Nistor, C.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Sederavičiūtė, F.; Bekampienė, P.; Domskienė, J. Effect of Pretreatment Procedure on Properties of Kombucha Fermented Bacterial Cellulose Membrane. Polym. Test. 2019, 78, 105941. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Bechtel, C.W.; Park, J.; Jiang, D.; Bashammakh, M.A.; Pereault, F.; Zodrow, K.R. Living Filtration Membranes Demonstrate Antibiofouling Properties. ACS EST Water 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Ayyappan, V.G.; Vhatkar, S.S.; Bose, S.; Sampath, S.; Das, S.K.; Samanta, D.; Mandal, A.B. Incorporations of Gold, Silver and Carbon Nanomaterials to Kombucha-Derived Bacterial Cellulose: Development of Antibacterial Leather-like Materials. J. Indian Chem. Soc. 2022, 99, 100278. [Google Scholar] [CrossRef]
- Barud, H.S.; Regiani, T.; Marques, R.F.C.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J.L. Antimicrobial Bacterial Cellulose-Silver Nanoparticles Composite Membranes. J. Nanomater. 2011, 2011, 721631. [Google Scholar] [CrossRef]
- Strem Catalog. UiO-66 Zirconium Building Bricks for Stable Metal Organic Frameworks. Available online: https://www.strem.com/uploads/resources/documents/uio-66.pdf (accessed on 16 December 2023).
- Friebe, S.; Geppert, B.; Steinbach, F.; Caro, J. Metal–Organic Framework UiO-66 Layer: A Highly Oriented Membrane with Good Selectivity and Hydrogen Permeance. ACS Appl. Mater. Interfaces 2017, 9, 12878–12885. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.-Q.; Tsai, F.-C.; Xie, L.; Zhang, K.-D.; Liu, H.-L.; Ma, N.; Shi, D.; Jiang, T. Ligands-Coordinated Zr-Based MOF for Wastewater Treatment. Nanomaterials 2018, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, L.; Wang, Y.; Zhang, X.-F.; Hao, D.; Feng, Y.; Yao, J. Lightweight UiO-66/Cellulose Aerogels Constructed through Self-Crosslinking Strategy for Adsorption Applications. Chem. Eng. J. 2019, 371, 138–144. [Google Scholar] [CrossRef]
- Cui, J.; Xu, X.; Yang, L.; Chen, C.; Qian, J.; Chen, X.; Sun, D. Soft Foam-like UiO-66/Polydopamine/Bacterial Cellulose Composite for the Removal of Aspirin and Tetracycline Hydrochloride. Chem. Eng. J. 2020, 395, 125174. [Google Scholar] [CrossRef]
- Bowden, N.B. Nanomaterials-Based Membranes Increase Flux and Selectivity to Enable Chemical Separations. ACS Appl. Nano Mater. 2020, 3, 9538–9541. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Osmen, S.; Reimhult, E. Cellulosic Biofilm Formation of Komagataeibacter in Kombucha at Oil-Water Interfaces. Biofilm 2022, 4, 100071. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Song, S.; Zhao, X. Selective Adsorption and Separation of Dyes from Aqueous Solution by a Zirconium-based Porous Framework Material. Appl. Organomet. Chem. 2021, 35, e6314. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Haddad, S.; Sacca, S.; Orellana-Tavra, C.; Fairen-Jimenez, D.; Forgan, R.S. Selective Surface PEGylation of UiO-66 Nanoparticles for Enhanced Stability, Cell Uptake, and pH-Responsive Drug Delivery. Chem 2017, 2, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nam, S.-N.; Jang, A.; Jang, M.; Park, C.M.; Son, A.; Her, N.; Heo, J.; Yoon, Y. Review of Adsorption–Membrane Hybrid Systems for Water and Wastewater Treatment. Chemosphere 2022, 286, 131916. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wang, D.; Grady, T.L. A Comparison of Kombucha SCOBY Bacterial Cellulose Purification Methods. SN Appl. Sci. 2020, 2, 240. [Google Scholar] [CrossRef]
- Eggensperger, C.G.; Giagnorio, M.; Holland, M.C.; Dobosz, K.M.; Schiffman, J.D.; Tiraferri, A.; Zodrow, K.R. Sustainable Living Filtration Membranes. Environ. Sci. Technol. Lett. 2020, 7, 213–218. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Thangaraj, P.; Sathishkumar, M.; Binupriya, A.R.; Swaminathan, K.; Yun, S.E. Preservation of Kombucha Tea—Effect of Temperature on Tea Components and Free Radical Scavenging Properties. J. Agric. Food Chem. 2008, 56, 9064–9071. [Google Scholar] [CrossRef]
- Tang, K.Y.; Heng, J.Z.X.; Lin, M.; Li, Z.; Ye, E.; Loh, X.J. Kombucha SCOBY Waste as a Catalyst Support. Chem. Asian J. 2021, 16, 2939–2946. [Google Scholar] [CrossRef]
- BB Kombucha Home Page. Available online: https://bbkombucha.fr/ (accessed on 13 December 2023).
- Li, J.; Tan, S.; Xu, Z. Anisotropic Nanocellulose Aerogel Loaded with Modified UiO-66 as Efficient Adsorbent for Heavy Metal Ions Removal. Nanomaterials 2020, 10, 1114. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Chen, J.P.; Li, K. Superior Removal of Arsenic from Water with Zirconium Metal-Organic Framework UiO-66. Sci. Rep. 2015, 5, 16613. [Google Scholar] [CrossRef]
- Ma, J.; Hu, J.; Tang, Y.; Gu, H.; Jiang, M.; Zhang, J. In-situ preparation of hollow cellulose nanocrystals/zeolitic imidazolate framework hybrid microspheres derived from Pickering emulsion. J. Colloid Interface Sci. 2020, 572, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Meng, X.; Liu, C.; Lu, M.; Liu, J.; Dai, L.; Wang, W.; Zhao, W.; Xiong, C.; Ni, Y. Carbohydrates-rich corncobs supported metal-organic frameworks as versatile biosorbents for dye removal and microbial inactivation. Carbohydr. Polym. 2019, 222, 115042. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhu, M.; Zhu, Y.; Zhao, Y.; Yang, M.; Miao, Z.; Ren, H.; Ma, Q.; Qian, L. Zeolitic imidazolate framework-67 functionalized cellulose hybrid aerogel: An environmentally friendly candidate for dye removal. Cellulose 2020, 27, 2161–2172. [Google Scholar] [CrossRef]
- Hidayat, A.R.P.; Zulfa, L.L.; Widyanto, A.R.; Abdullah, R.; Kusumawati, Y.; Ediati, R. Selective adsorption of anionic and cationic dyes on mesoporous UiO-66 synthesized using a template-free sonochemistry method: Kinetic, isotherm and thermodynamic studies. RSC Adv. 2023, 13, 12320–12343. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Kadhi, N.S. Studies on Adsorption of Fluorescein Dye from Aqueous Solutions Using Wild Herbs. Int. J. Anal. Chem. 2020, 9, 8019274. [Google Scholar] [CrossRef]



| Qt (mg·g−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluorescein | Bromophenol Blue | Rhodamine 6G | |||||||
| KBC-L | UiO-66 | KBC-UiO | KBC-L | UiO-66 | KBC-UiO | KBC-L | UiO-66 | KBC-UiO | |
| pH 5 | 0 | 8.4 | 9.4 | 0 | 7.3 | 15.9 | 2.0 | 0 | 0.6 |
| pH 7 | 0.3 | 4.5 | 9.7 | 0.2 | 4.3 | 15.0 | 2.4 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Joly, P.; Gallois, A.; Bosc-Rouessac, F.; Drobek, M.; Julbe, A. Synergistic Effect of UiO-66 Directly Grown on Kombucha-Derived Bacterial Cellulose for Dye Removal. Molecules 2024, 29, 3057. https://doi.org/10.3390/molecules29133057
Plaza-Joly P, Gallois A, Bosc-Rouessac F, Drobek M, Julbe A. Synergistic Effect of UiO-66 Directly Grown on Kombucha-Derived Bacterial Cellulose for Dye Removal. Molecules. 2024; 29(13):3057. https://doi.org/10.3390/molecules29133057
Chicago/Turabian StylePlaza-Joly, Pierre, Arthur Gallois, Florence Bosc-Rouessac, Martin Drobek, and Anne Julbe. 2024. "Synergistic Effect of UiO-66 Directly Grown on Kombucha-Derived Bacterial Cellulose for Dye Removal" Molecules 29, no. 13: 3057. https://doi.org/10.3390/molecules29133057
APA StylePlaza-Joly, P., Gallois, A., Bosc-Rouessac, F., Drobek, M., & Julbe, A. (2024). Synergistic Effect of UiO-66 Directly Grown on Kombucha-Derived Bacterial Cellulose for Dye Removal. Molecules, 29(13), 3057. https://doi.org/10.3390/molecules29133057







