Integrating UPLC-Q-TOF-MS and Network Pharmacology to Explore the Potential Mechanisms of Paeonia lactiflora Pall. in the Treatment of Blood Stasis Syndrome
Abstract
1. Introduction
2. Results and Discussion
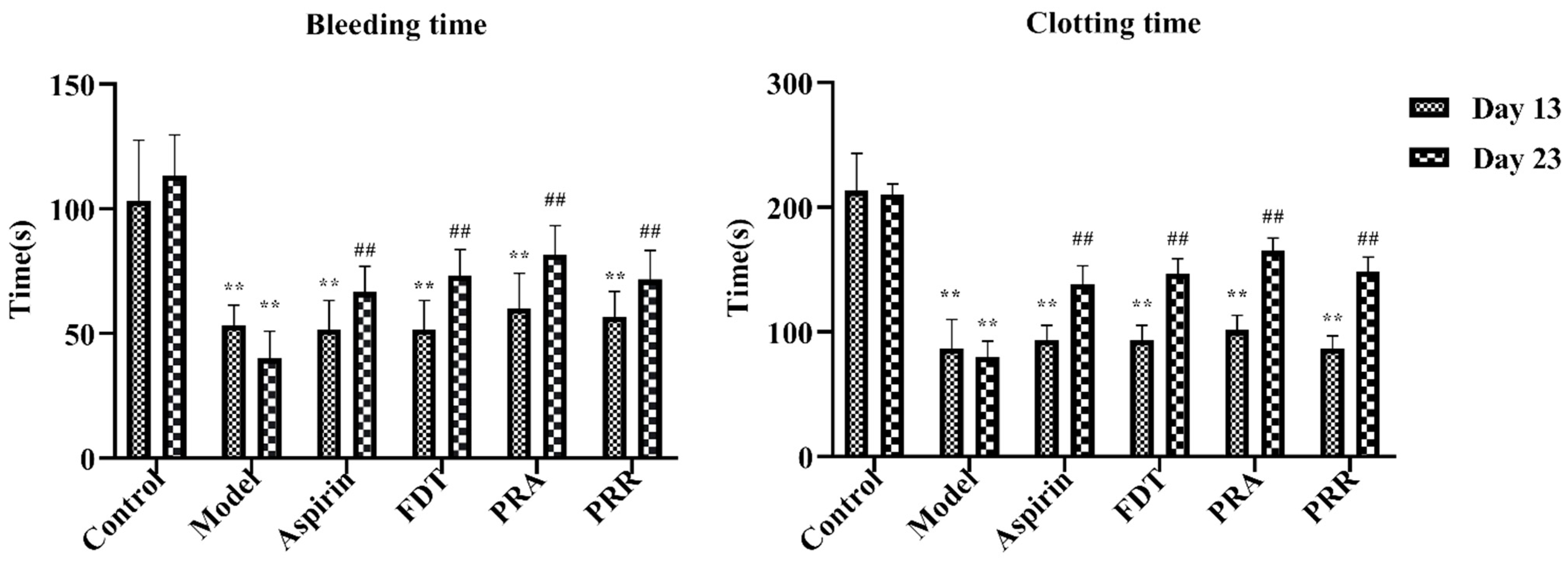
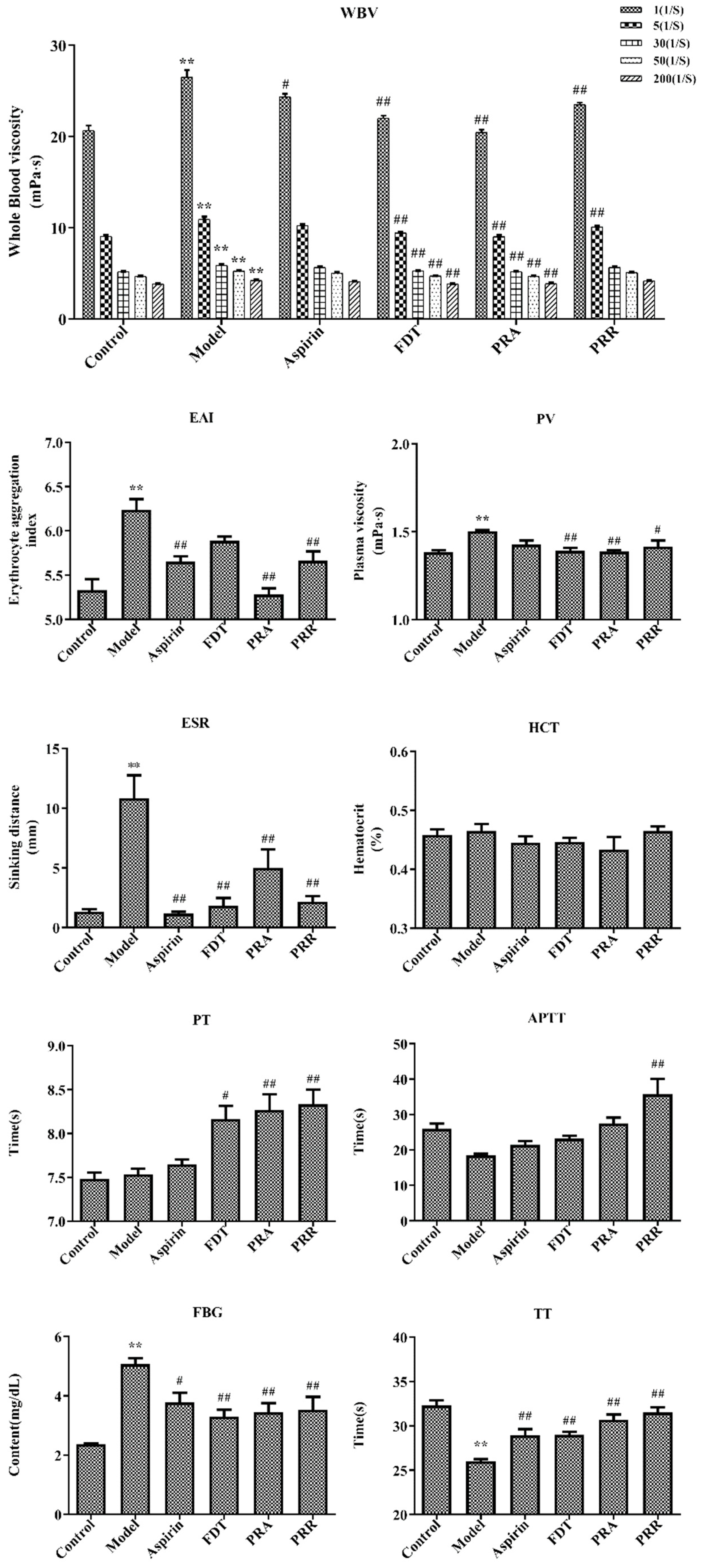
2.1. Evaluation of Model
2.2. Hemorheology and Evaluation of Related Functional Parameters
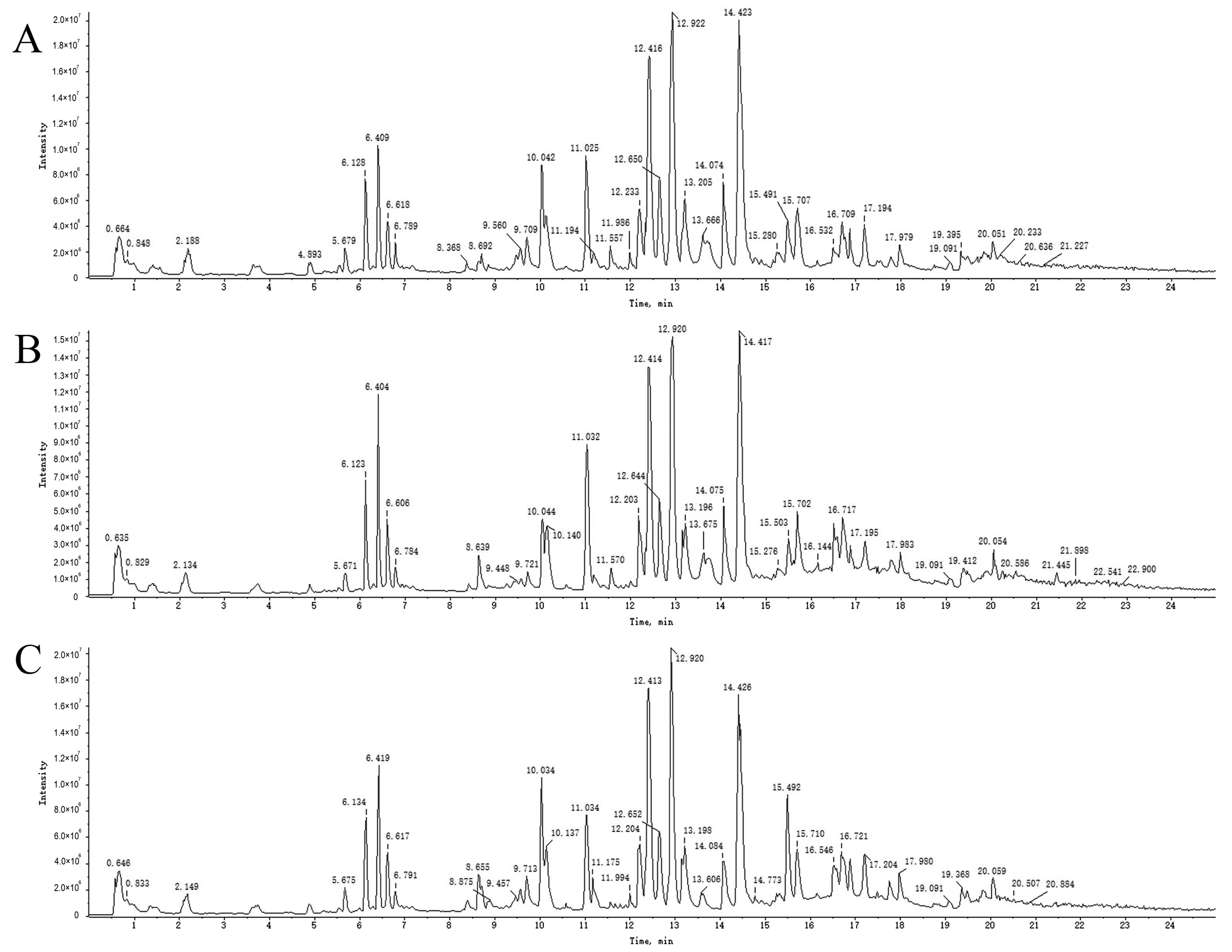
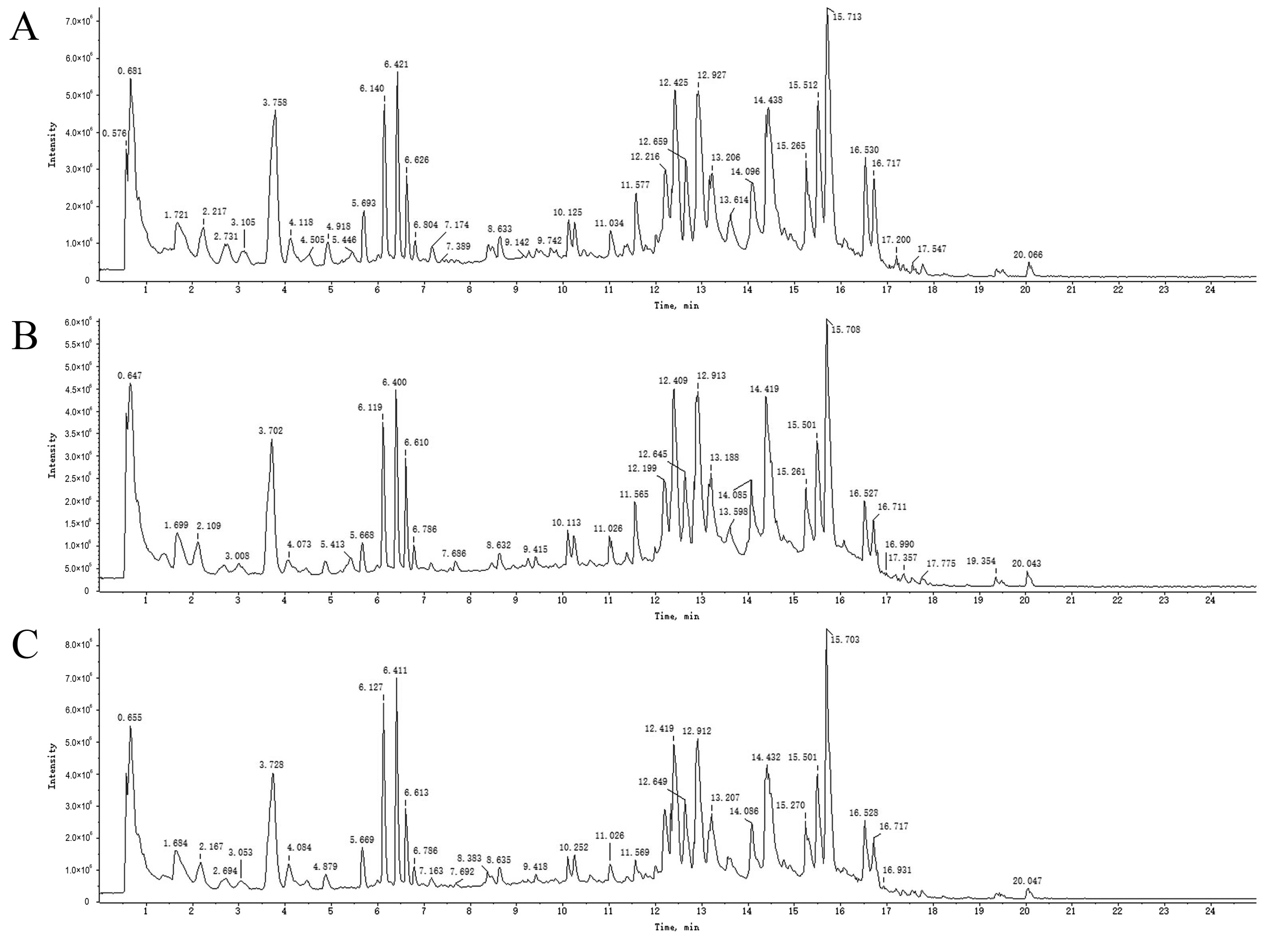
2.3. UPLC-Q-TOF-MS Analysis
2.4. Analysis and Identification of Compounds in Drug-Containing Serum
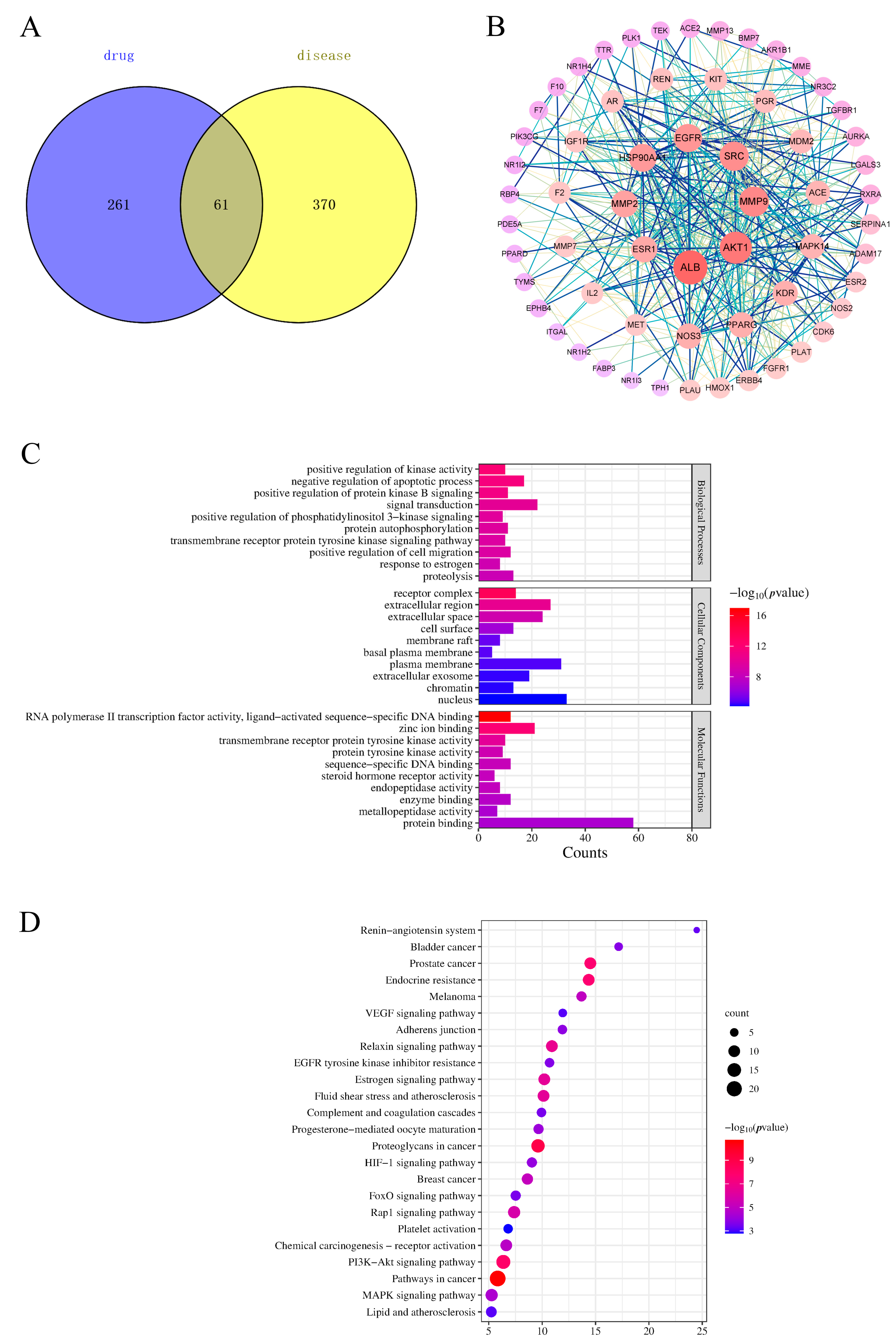
2.5. Target Screening of Components Entering the Blood
2.6. Protein–Protein Interaction (PPI) Network Analysis
2.7. Functional Enrichment Analysis and Pathway Enrichment Analysis
2.8. “Component–Target–Pathway” Network Analysis
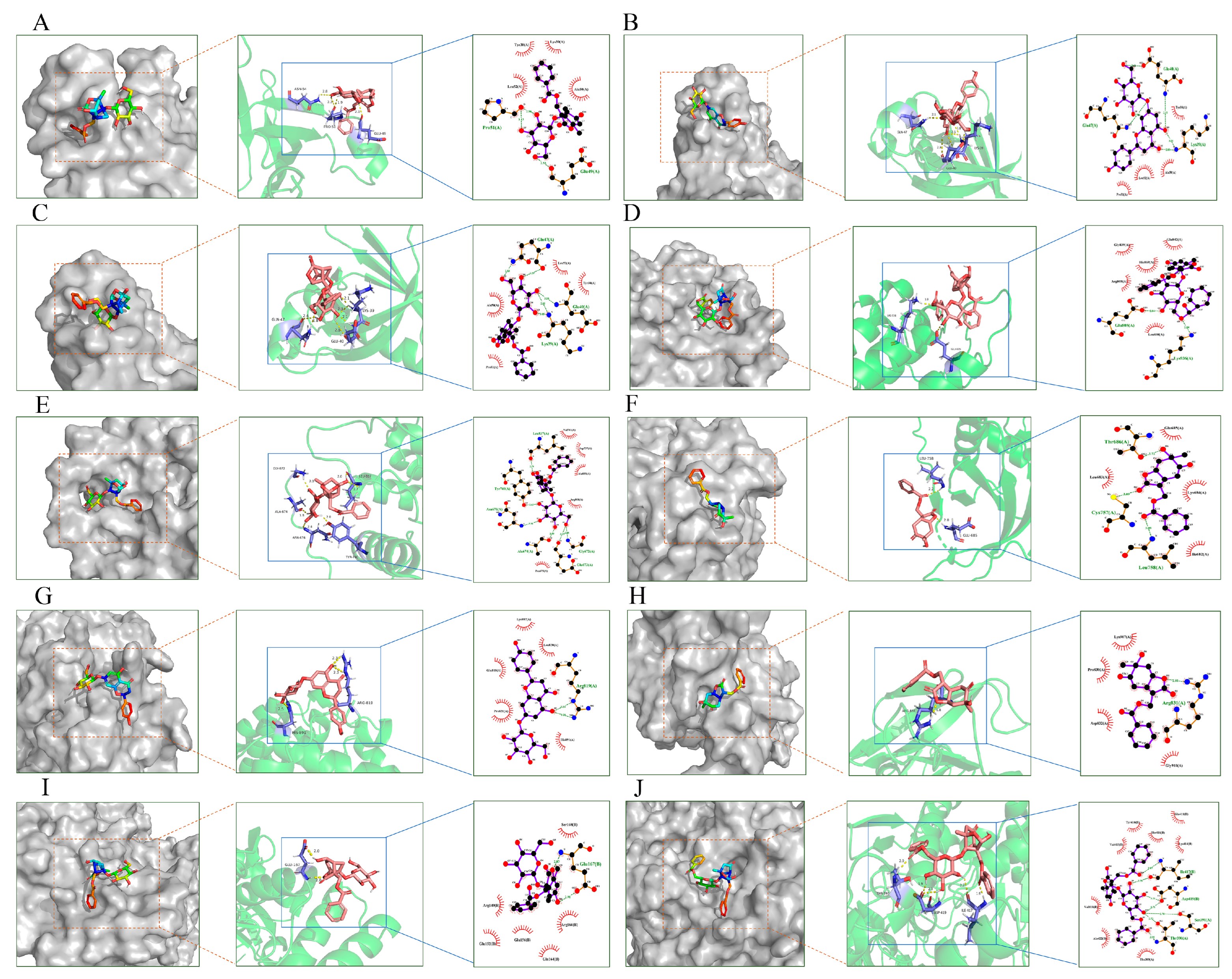
2.9. Molecular Docking Validation Analysis
3. Materials and Methods
3.1. Reagents and Materials
3.1.1. Reagents
3.1.2. Plant Materials
3.2. Animals and Treatment
3.3. Herbal Preparation
3.4. Experimental Model and Drug Administration
3.5. Hemorheological and Coagulation Parameters
3.6. Detection and Identification of Blood Components
3.6.1. Preparation of Medicated and Blank Sera
3.6.2. Chromatographic Conditions
3.6.3. MS Conditions
3.6.4. Data Processing and Compound Identification
3.7. Network Pharmacology Analysis
3.7.1. Active Components and Targets
3.7.2. Construction of Protein–Protein Interaction Networks
3.7.3. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Analyses
3.7.4. Construction of the ‘Component–Target–Pathway’ Network
3.7.5. Molecular Docking
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Shen, J.; Wang, Z.Q.; Liu, S.S.; Liu, Q.; Li, Y.; He, C.N.; Xiao, P.G. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Chen, H.W.; Li, J.; Wu, Q.J. Efficacy, Chemical Constituents, and Pharmacological Actions of Radix Paeoniae Rubra and Radix Paeoniae Alba. Front. Pharmacol. 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; pp. 108–165. [Google Scholar]
- Xie, P.Y.; Cui, L.L.; Shan, Y.; Kang, W.Y. Antithrombotic Effect and Mechanism of Radix Paeoniae Rubra. Biomed. Res. Int. 2017, 2017, 9475074. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.Y.; Zhao, N.N.; Du, X.Y.; Bai, L.Y.; Liu, J.K. The protective effect of peony extract on acute myocardial infarction in rats. Phytomedicine 2011, 18, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.T.; Hao, M.; Tong, H.J.; Su, L.L.; Fei, C.H.; Gu, W.; Mao, J.; Lu, T.L.; Mao, C.Q. Screening of blood-activating active components from Curcuma wenyujin Y.H. Chen et C. Ling rhizome based on spectrum-effect relationship analysis and network pharmacology. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1188, 123022. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Wu, J.X.; Lu, R.G.; Liu, X.; Chin, B.; Zhu, H.J.; Yin, C.L.; Cheng, B.; Wu, Z.; Chen, X.J.; et al. Dynamic urinary metabolomics analysis based on UHPLC-Q-TOF/MS to investigate the potential biomarkers of blood stasis syndrome and the effects of Danggui Sini decoction. J. Pharm. Biomed. Anal. 2020, 179, 112986. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Ji, D.; Li, L.; Su, L.L.; Gu, W.; Gu, L.Y.; Wang, Q.H.; Lu, T.L.; Mao, C.Q. Mechanism of Curcuma wenyujin Rhizoma on Acute Blood Stasis in Rats Based on a UPLC-Q/TOF-MS Metabolomics and Network Approach. Molecules 2018, 24, 82. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Niu, M.Z.; Zhang, S.Q.; Zhang, B.; Yang, K.; Li, S. Interpretation of Network Pharmacology Evaluation Method Guidance. Chin. Tradit. Herbal. Drugs 2021, 52, 4119–4129. [Google Scholar]
- Wu, J.S.; Zhang, F.Q.; Li, Z.Z.; Jin, W.Y.; Shi, Y. Integration strategy of network pharmacology in Traditional Chinese Medicine: A narrative review. J. Tradit. Chin. Med. 2022, 42, 479–486. [Google Scholar]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Zhang, L.L.; Sheng, F.Y.; He, Y.; Yang, Y.; Hu, Y.F.; Li, W.; Li, P.; Wu, M.Y.; Gong, Y.; Zhang, Y.M.; et al. Buxue Yimu Pills improve angiogenesis and blood flow in experimental zebrafish and rat models. J. Ethnopharmacol. 2022, 289, 115002. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, M.; Zhao, Y.H.; Jiang, X.; Wang, M.; Zhang, Y.X.; Zhao, C.J. Rapid characterization of chemical constituents of Shaoyao Gancao decoction using UHPLC coupled with Fourier transform ion cyclotron resonance mass spectrometry. RSC Adv. 2020, 10, 29528–29535. [Google Scholar] [CrossRef]
- Yao, C.S.; Shen, Y.H.; Xu, Y.L. The Chemical Constituents of Coleus Forskohlii. Nat. Prod. Res. Dev. 2002, 14, 1–6. [Google Scholar]
- Liu, E.H.; Qi, L.W.; Li, B.; Peng, Y.B.; Li, P.; Li, C.Y.; Cao, J. High-speed separation and characterization of major constituents in Radix Paeoniae Rubra by fast high-performance liquid chromatography coupled with diode-array detection and time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2009, 23, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Zhu, S.; Ge, Y.W.; Toume, K.; Wang, Z.T.; Batkhuu, J.; Komatsu, K. Characterization and quantification of monoterpenoids in different types of peony root and the related Paeonia species by liquid chromatography coupled with ion trap and time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2016, 129, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.D.; Jiang, L.L.; Li, H.Y.; Yan, P.F.; Zhang, Y.L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules 2016, 21, 1362. [Google Scholar] [CrossRef]
- Riaz, N.; Malik, A.; Rehman, A.U.; Ahmed, Z.; Muhammad, P.; Nawaz, S.A.; Siddiqui, J.; Choudhary, M.I. Lipoxygenase inhibiting and antioxidant oligostilbene and monoterpene galactoside from Paeonia emodi. Phytochemistry 2004, 65, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Y.; Cao, G.; Wu, X.; Chen, X.C.; Cai, B.C. Ultra-performance liquid chromatography coupled with high-resolution quadrupole time-of-flight mass spectrometry analysis of the impact of bran-processing on the chemical profile of Radix Paeoniae Alba (Baishao). Nat. Prod. Res. 2015, 29, 776–779. [Google Scholar] [CrossRef]
- Pei, K.; Duan, Y.; Cai, H.; Tu, S.C.; Qiao, F.X.; Song, X.Q.; Liu, X.; Cao, G.; Fan, K.L.; Cai, B.C. Ultra-high-performance liquid chromatography-quadrupole/time of flight mass spectrometry combined with statistical analysis for rapidly revealing the influence of sulfur-fumigated Paeoniae Radix Alba on the chemical constituents of Si Wu Tang. Anal. Methods 2015, 7, 9442–9451. [Google Scholar] [CrossRef]
- Lu, Z.Y. Study on chemical constituents from the flower and the seed cake of Paeonia rockii. Henan Univ. Sci. Tec. 2014. [Google Scholar] [CrossRef]
- Sivaraman, B.; Latour, R.A. The adherence of platelets to adsorbed albumin by receptor-mediated recognition of binding sites exposed by adsorption-induced unfolding. Biomaterials 2010, 31, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. Albumin as a zinc carrier: Properties of its high-affinity zinc-binding site. Biochem. Soc. Trans. 2008, 36, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.M.; Anderson, N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002, 14, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell. Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.C.; Deak, M.; Alessi, D.R.; van Aalten, D.M. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr. Biol. 2002, 12, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Milburn, C.C.; Deak, M.; Kelly, S.M.; Price, N.C.; Alessi, D.R.; Van Aalten, D.M. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem. J. 2003, 375, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef] [PubMed]
- Lung, D.K.; Reese, R.M.; Alarid, E.T. Intrinsic and Extrinsic Factors Governing the Transcriptional Regulation of ESR1. Horm. Cancer. 2020, 11, 129–147. [Google Scholar] [CrossRef]
- De Kock, L.; Freson, K. The (Patho)Biology of SRC Kinase in Platelets and Megakaryocytes. Medicina 2020, 56, 633. [Google Scholar] [CrossRef]
- Zuehlke, A.D.; Beebe, K.; Neckers, L.; Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chem. 2020, 20, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Belotti, D.; Paganoni, P.; Manenti, L.; Garofalo, A.; Marchini, S.; Taraboletti, G.; Giavazzi, R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: Implications for ascites formation. Cancer Res. 2003, 63, 5224–5229. [Google Scholar] [PubMed]
- Janssens, S.P.; Shimouchi, A.; Quertermous, T.; Bloch, D.B.; Bloch, K.D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J. Biol. Chem. 1992, 267, 14519–14522. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Du, P.; Wang, J.J. Paeoniflorin ameliorates acute myocardial infarction of rats by inhibiting inflammation and inducible nitric oxide synthase signaling pathways. Mol. Med. Rep. 2015, 12, 3937–3943. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol. Pharm. Bull. 2011, 34, 1785–1788. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.S.; Zhang, C.L.; Liang, L.C.; Wei, L.; Wang, H.; Zhou, F.K.; Li, R.J.; Zou, D.H.; Huang, X.H.; Liu, J. Identification of Potential Key Genes Involved in the Carotid Atherosclerosis. Clin. Interv. Aging. 2021, 16, 1071–1084. [Google Scholar] [CrossRef]
- Huo, Z.H.; Kong, Y.; Meng, M.; Cao, Z.F.; Zhou, Q.S. Atorvastatin enhances endothelial adherens junctions through promoting VE-PTP gene transcription and reducing VE-cadherin-Y731 phosphorylation. Vasc. Pharmacol. 2019, 117, 7–14. [Google Scholar] [CrossRef]
- Foley, J.H. Examining coagulation-complement crosstalk: Complement activation and thrombosis. Thromb. Res. 2016, 141 (Suppl. S2), S50–S54. [Google Scholar] [CrossRef]
- Conway, E.M. Complement-coagulation connections. Blood Coagul. Fibrinolysis. 2018, 29, 243–251. [Google Scholar] [CrossRef]
- Yun, S.H.; Sim, E.H.; Goh, R.Y.; Park, J.I.; Han, J.Y. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed. Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef]
- Seyed Ahmad, R.; Nooshin, S.; Amir Reza, M. The crosstalk between VEGF signaling pathway and long non-coding RNAs in neovascular retinal diseases: Implications for anti-VEGF therapy. Gene Rep. 2022, 27, 101541. [Google Scholar]
- Xin, C.G.; Zhu, C.N.; Jin, Y.; Li, H. Discovering the role of VEGF signaling pathway in mesendodermal induction of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2021, 553, 58–64. [Google Scholar] [CrossRef]
- Ye, X.; Ren, H.; Zhang, M.; Sun, Z.; Jiang, A.C.; Xu, G. ERK1/2 Signaling Pathway in the Release of VEGF from Müller Cells in Diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3481–3489. [Google Scholar] [CrossRef]
- Wang, J.P.; Fu, X.J.; Jiang, C.; Yu, L.; Wang, M.H.; Han, W.; Liu, L.; Wang, J. Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signaling pathway in a rat model of vascular dementia. Behav. Brain Res. 2014, 265, 171–180. [Google Scholar] [CrossRef]
- Zhu, J.; Xue, X.O.; He, Z.P.; Yan, P.J.; Zhang, J.W.; Sun, H.Y. Effects of Wudan Pills on Endometrial Angiogenesis and ERK -VEGF/MMP -9 Pathway in Rats with Endometriosis of Cold Congelation and Blood Stasis. WJIM 2022, 17, 645–651. [Google Scholar]
- Ye, X.; Ren, H.; Jiang, T.; Zhang, T.; Li, G. Effect of Diabetes Blood-Stasis Syndrome and Xuefu Zhuyu Decoction on ERK1/2-VEGF Signal Pathway in Rat Retina Müller Cells. Histol. Histopathol. 2022, 37, 757–767. [Google Scholar] [PubMed]
- Xu, J.J.; Xu, F.; Wang, W.; Zhang, Y.F.; Hao, B.Q.; Shang, M.Y.; Liu, G.X.; Li, Y.L.; Yang, S.B.; Wang, X.; et al. Elucidation of the Mechanisms and Effective Substances of Paeoniae Radix Rubra Against Toxic Heat and Blood Stasis Syndrome with a Stage-Oriented Strategy. Front. Pharmacol. 2022, 13, 842839. [Google Scholar] [CrossRef]
- Ma, F.X.; Xue, P.F.; Wang, Y.Y.; Wang, Y.N.; Xue, S.Y. Research progress of serum pharmacochemistry of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 2017, 42, 1265–1270. [Google Scholar] [PubMed]
- Zhang, Y.; Zhou, X.; Sun, L. Effect of Total Glucosides of Paeony on Imiquimod-Induced Psoriatic Skin Lesions by Regulating VEGF. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Editorial Board of the Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1979; Volume 27, p. 51. [Google Scholar]
- Dang, X.; Miao, J.J.; Chen, A.Q.; Li, P.; Chen, L.; Liang, J.R.; Xie, R.M.; Zhao, Y. The Antithrombotic Effect of RSNK in Blood-Stasis Model Rats. J. Ethnopharmacol. 2015, 173, 266–272. [Google Scholar] [CrossRef]
- Li, C.; Hu, M.; Jiang, S.; Liang, Z.; Wang, J.; Liu, Z.; Wang, H.-M.D.; Kang, W. Evaluation Procoagulant Activity and Mechanism of Astragalin. Molecules 2020, 25, 177. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.H.; Xu, X.K.; Yang, J.S.; Gao, Y.; Xin, J.Y.; Chen, W.; Zhang, L.; Li, J.L.; Wang, J.; Wei, Y.P.; et al. Identification of chemical components and rat serum metabolites in Danggui Buxue decoction based on UPLC-Q-TOF-MS, the UNIFI platform and molecular networks. RSC Adv. 2023, 13, 32778–32785. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, H.M.; Xiong, L.L.; Jin, H.L.; Liu, Y.F.; Shen, A.J.; Liang, X.M. Monoterpenoids from Paeoniae Radix Rubra based on UPLC-Q-TOF-MS. Zhongguo Zhong Yao Za Zhi 2023, 48, 1005–1013. [Google Scholar] [PubMed]
- Chen, L.; Liang, W.; Zhang, K.; Wang, Z.; Cheng, W.; Li, W. To Elucidate the Mechanism of “Scrophulariae Radix-Fritillaria” in Goiter by Integrated Metabolomics and Serum Pharmaco-Chemistry. Front. Pharmacol. 2024, 15, 1206718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wan, D.; Li, Y.; Wang, S.; Zhou, X.; Sefidkon, F.; Yang, X. UPLC-MS Analysis, Quantification of Compounds, and Comparison of Bioactivity of Methanol Extract and Its Fractions from Qiai (Artemisia argyi Lévl. et Van.). Molecules 2023, 28, 2022. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Wang, X.; Pan, C.; Gong, J.; Liu, X.; Li, H. Enhancing the Enrichment of Pharmacophore-Based Target Prediction for the Polypharmacological Profiles of Drugs. J. Chem. Inf. Model. 2016, 56, 1175–1183. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 Update: A Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Consortium, U. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Stein, T.I.; Dahary, D.; Fishilevich, S.; Lancet, D. Practical Guide to Life Science Databases; Abugessaisa, I., Kasukawa, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 27–56. [Google Scholar]
- Hamosh, A.; Amberger, J.S.; Bocchini, C.; Scott, A.F.; Rasmussen, S.A. Online Mendelian Inheritance in Man (OMIM): Victor McKusick’s magnum opus. Am. J. Med. Genet. Part A 2021, 185, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Assenov, Y.; Ramírez, F.; Schelhorn, S.E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2008, 24, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Zhang, Z.; Cheng, M.; Mei, Z.; Li, X.; Liu, P.; Diao, L.; Ma, Y.; Jiang, P.; et al. BATMAN-TCM 2.0: An Enhanced Integrative Database for Known and Predicted Interactions between Traditional Chinese Medicine Ingredients and Target Proteins. Nucleic Acids Res. 2024, 52, D1110–D1120. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. Methods Mol. Biol. 2017, 1607, 627–641. [Google Scholar]
- Gaillard, T. Evaluation of AutoDock and AutoDock Vina on the CASF-2013 Benchmark. J. Chem. Inf. Model. 2018, 58, 1697–1706. [Google Scholar] [CrossRef]
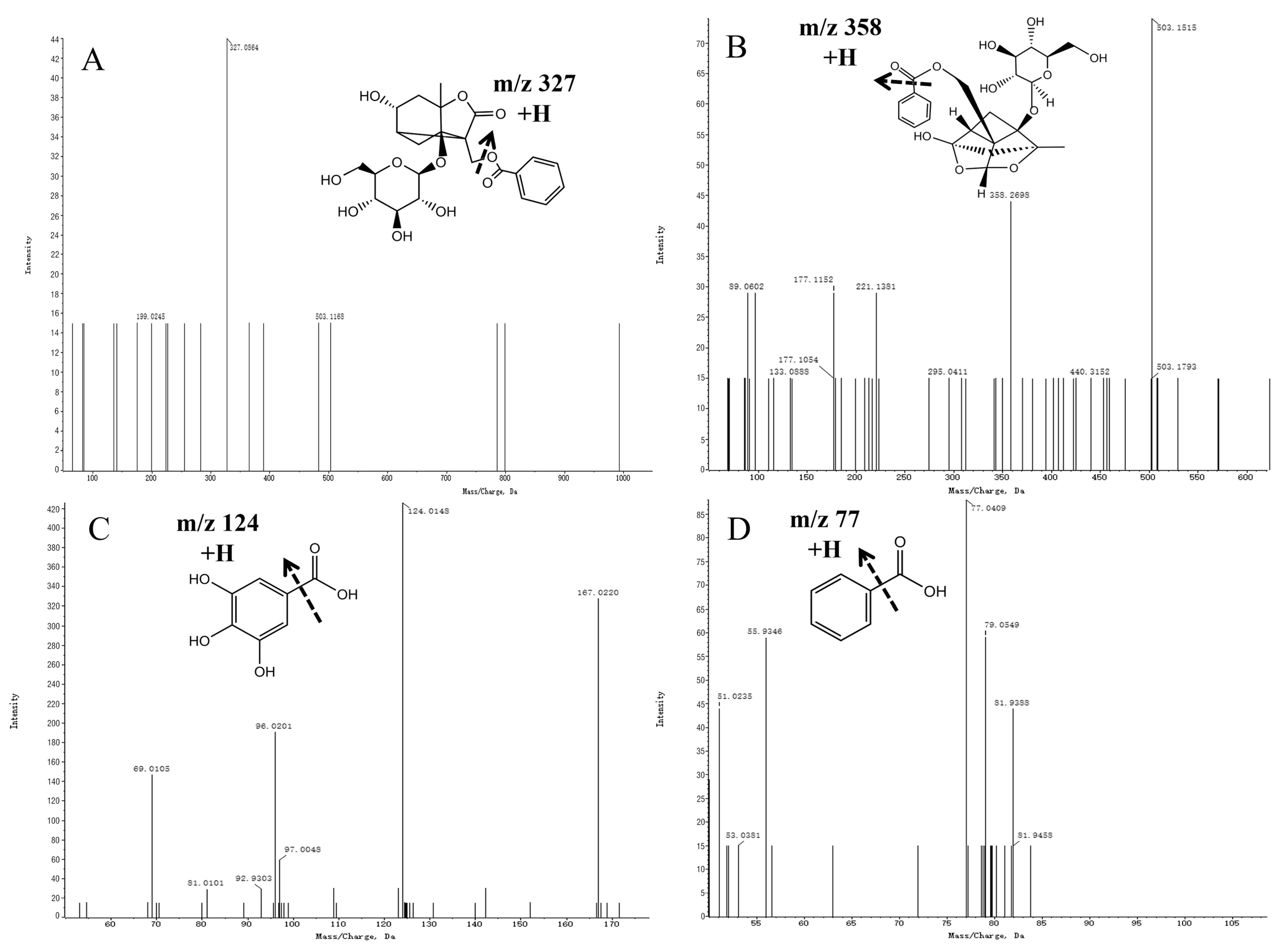

| No. | tR (min) | Formula | Error (ppm) | Ion Mode | m/z | Fragment Ions (m/z) | PRA | PRR | Identification | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.98 | C21H22O10 | −2.72 | M + H, M + Na | 434.1918 | 375.1155, 104.1070, 86.0974 | + | − | Naringenin-7-O-glucoside | [14] |
| 2 | 2.26 | C10H14O4 | −0.62 | M + H-H2O, M + Na | 181.0857 | 152.0656, 135.0864, 77.0362 | + | + | Guaiphenesin | [15] |
| 3 | 3.91 | C23H28O11 | −0.39 | M + H, M + Na | 503.1152 | 327.0864 | + | + | Albiflorin | [16,17] |
| 4 | 2.68 | C23H28O12 | −1.22 | M + Na | 519.1511 | 281.0583 | + | + | Paeonin C | [18,19] |
| 5 | 8.05 | C30H32O12 | 0.28 | M + Na | 607.3274 | 607.3274 | + | + | Paeonidaninol A | [20] |
| 6 | 3.93 | C17H18O6 | 1.01 | M + H-H2O, M + H | 319.1076 | 111.0189, 209.0936, 93.0094 | + | + | (+)-Paeonilactone C | [14] |
| 7 | 5.63 | C7H6O2 | −4.09 | M + H | 123.0428 | 81.9458, 79.0622, 77.0409, 51.0235, 50.0160 | + | + | Benzoic acid | [21] |
| 8 | 1.00 | C7H6O5 | −3.18 | M-H | 169.0137 | 124.0148, 97.0048, 69.0105 | + | + | Gallic acid | [16,21] |
| 9 | 2.03 | C8H8O5 | −2.46 | M-H | 183.0049 | 123.0103, 109.0167, 95.0118 | + | − | 5-Hydroxyisovanillic acid | [22] |
| 10 | 5.61 | C23H28O11 | −1.62 | M + H-H2O, M + Na | 503.3051 | 440.3152, 358.2698, 295.0411, 221.1381, 177.1152, 133.0888, 89.0602 | − | + | Paeoniflorin | [16,17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Du, Q.; Shi, S.; Lv, J.; Zhang, W.; Ge, D.; Xing, L.; Yu, N. Integrating UPLC-Q-TOF-MS and Network Pharmacology to Explore the Potential Mechanisms of Paeonia lactiflora Pall. in the Treatment of Blood Stasis Syndrome. Molecules 2024, 29, 3019. https://doi.org/10.3390/molecules29133019
Ma M, Du Q, Shi S, Lv J, Zhang W, Ge D, Xing L, Yu N. Integrating UPLC-Q-TOF-MS and Network Pharmacology to Explore the Potential Mechanisms of Paeonia lactiflora Pall. in the Treatment of Blood Stasis Syndrome. Molecules. 2024; 29(13):3019. https://doi.org/10.3390/molecules29133019
Chicago/Turabian StyleMa, Mengzhen, Qianqian Du, Suying Shi, Jiahui Lv, Wei Zhang, Dezhu Ge, Lihua Xing, and Nianjun Yu. 2024. "Integrating UPLC-Q-TOF-MS and Network Pharmacology to Explore the Potential Mechanisms of Paeonia lactiflora Pall. in the Treatment of Blood Stasis Syndrome" Molecules 29, no. 13: 3019. https://doi.org/10.3390/molecules29133019
APA StyleMa, M., Du, Q., Shi, S., Lv, J., Zhang, W., Ge, D., Xing, L., & Yu, N. (2024). Integrating UPLC-Q-TOF-MS and Network Pharmacology to Explore the Potential Mechanisms of Paeonia lactiflora Pall. in the Treatment of Blood Stasis Syndrome. Molecules, 29(13), 3019. https://doi.org/10.3390/molecules29133019





