Modulation of the Human Erythrocyte Antioxidant System by the 5- and 6-Membered Heterocycle-Based Nitroxides
Abstract
1. Introduction
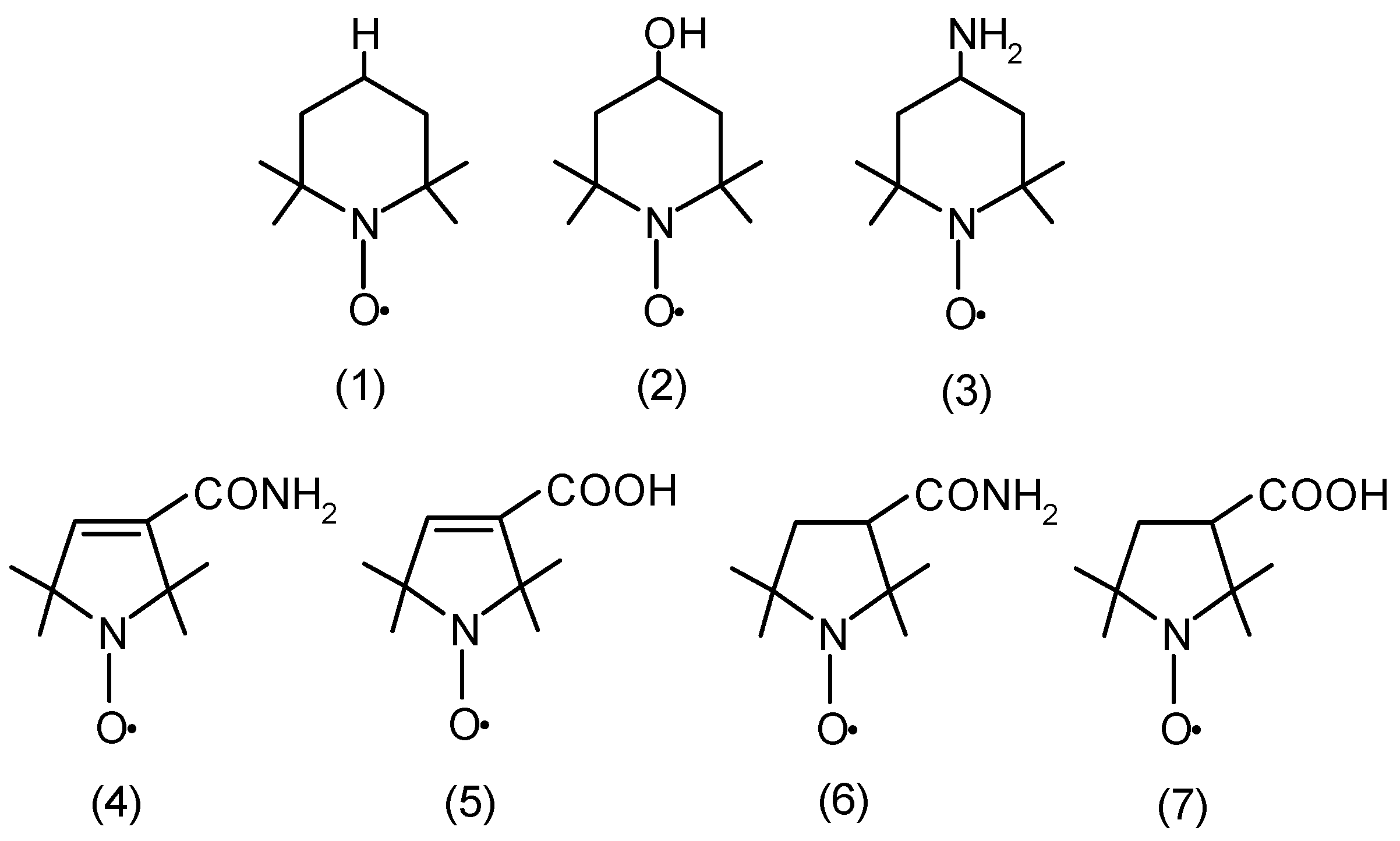
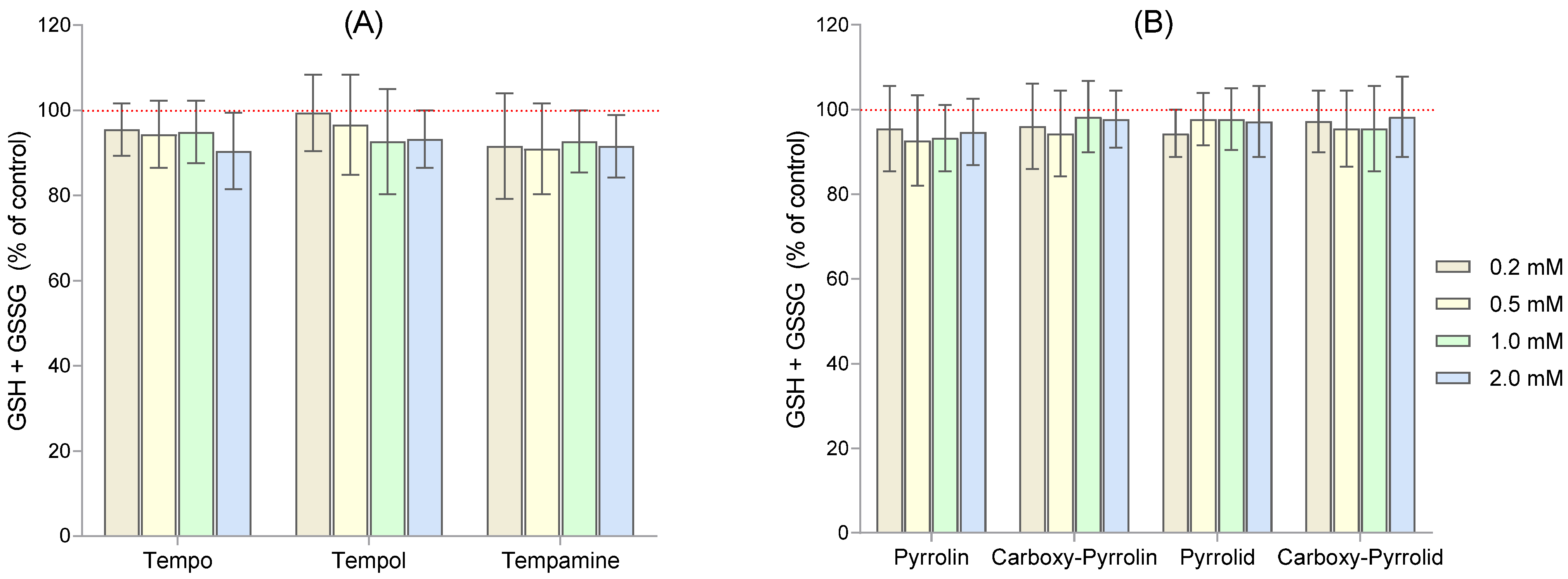
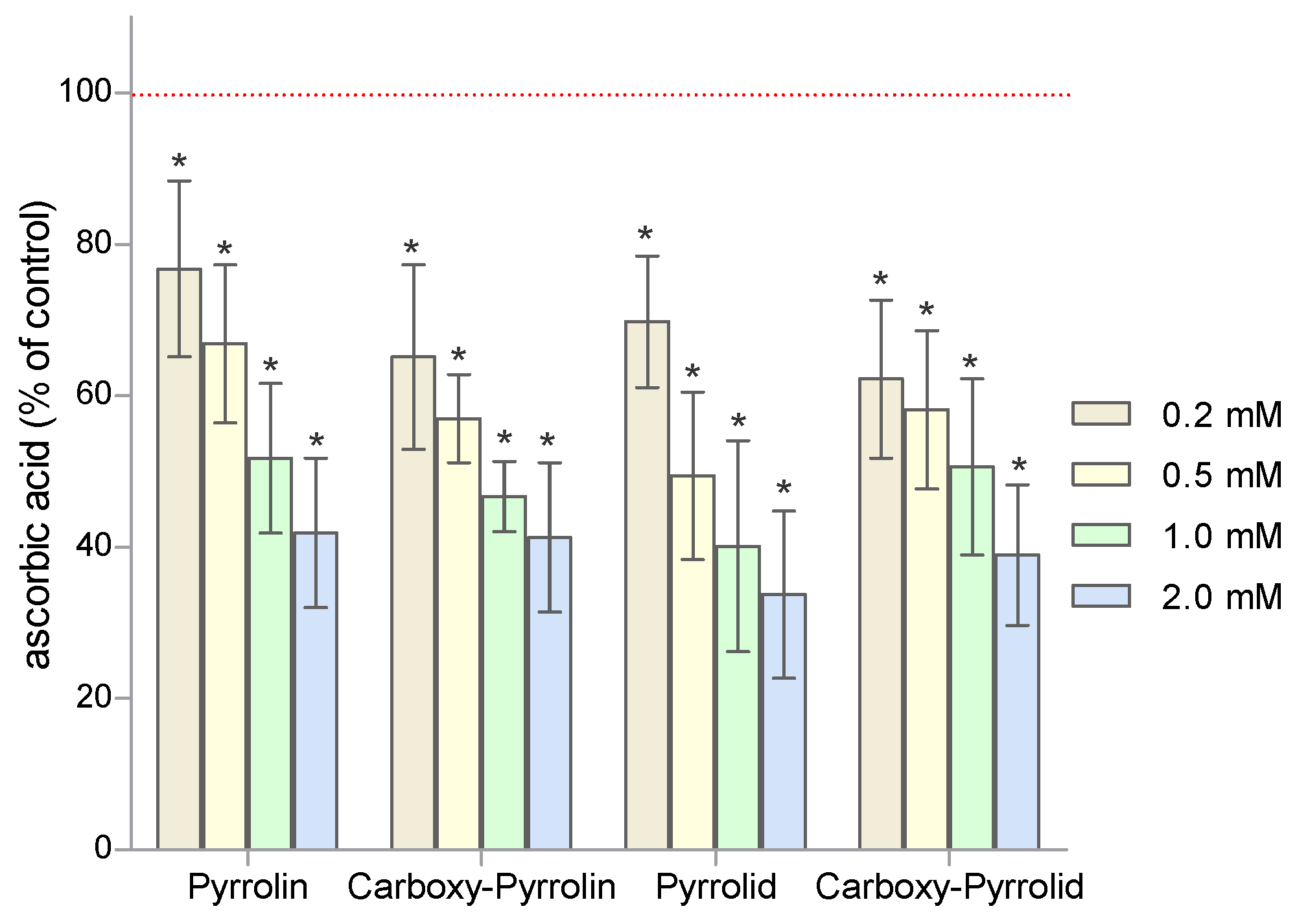
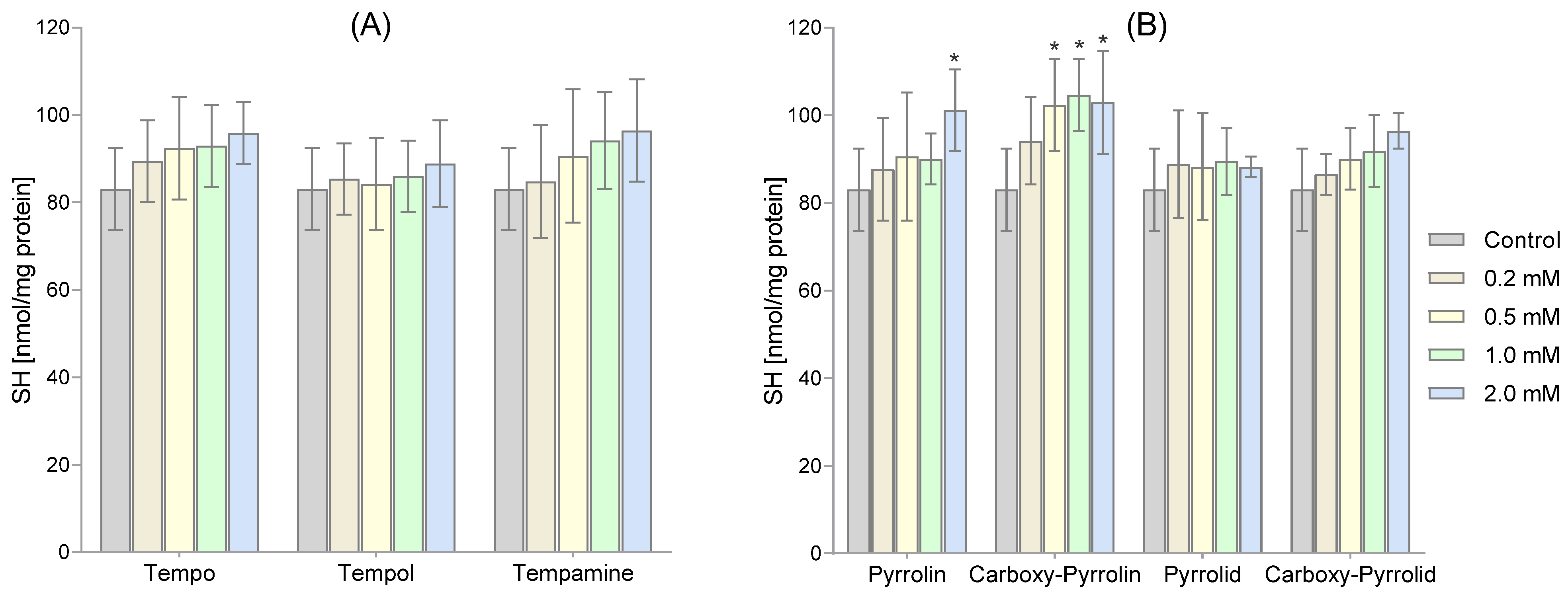
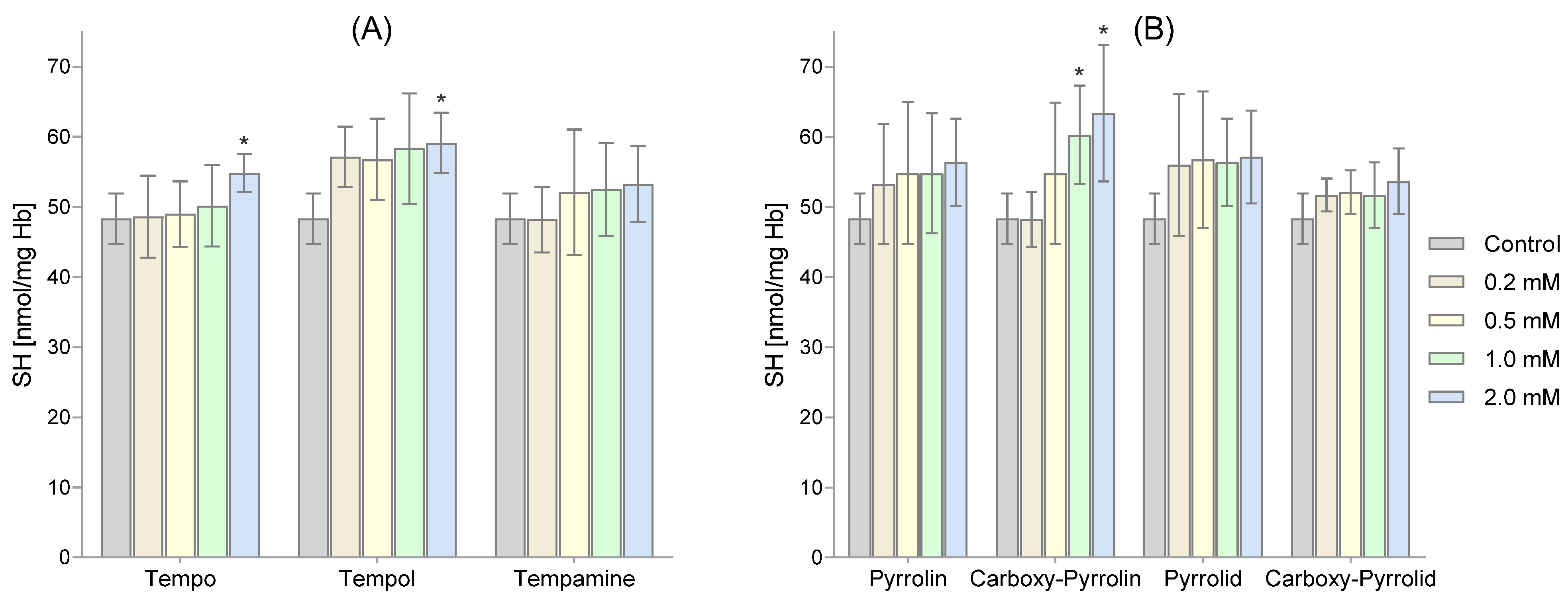
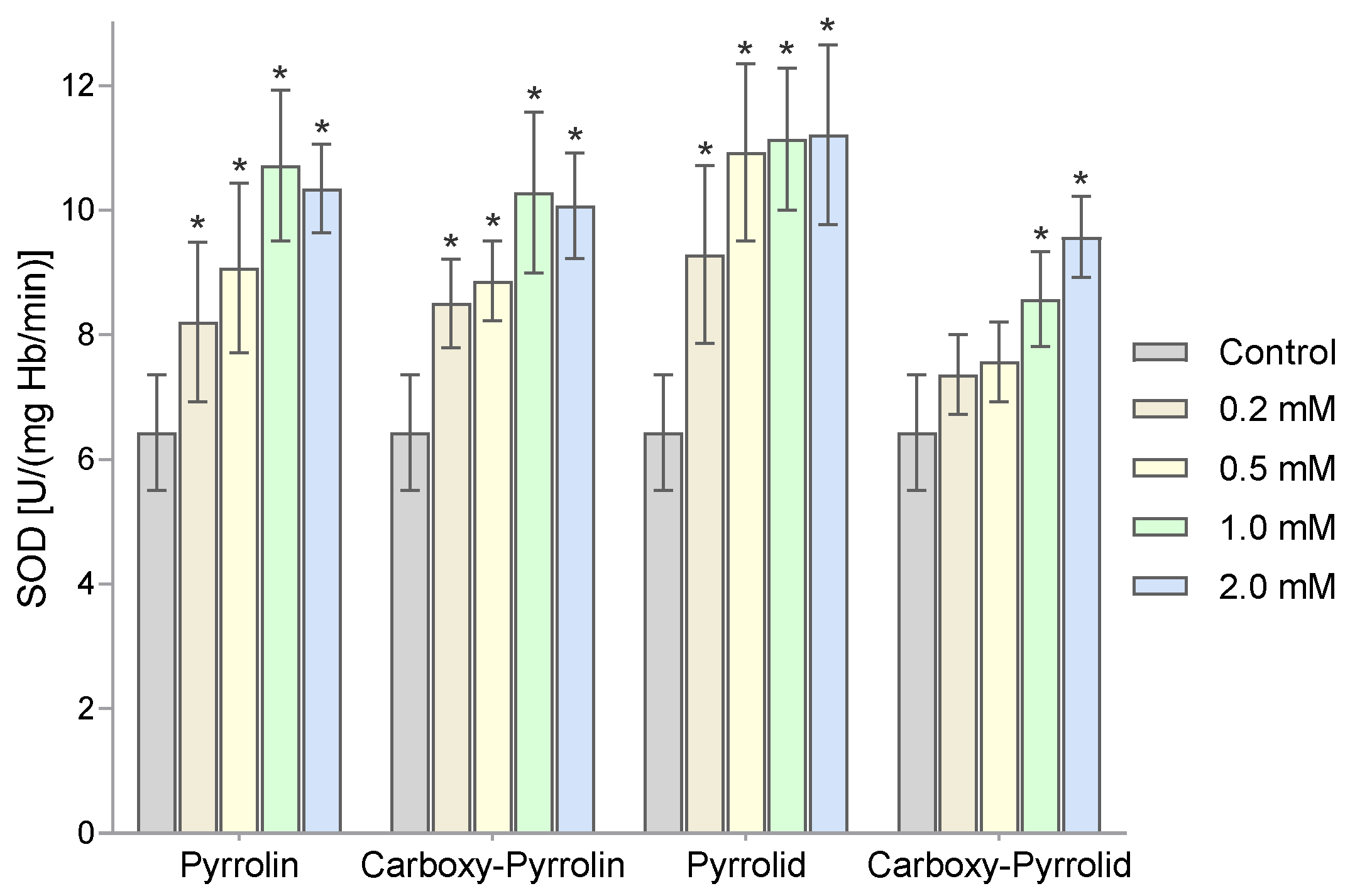
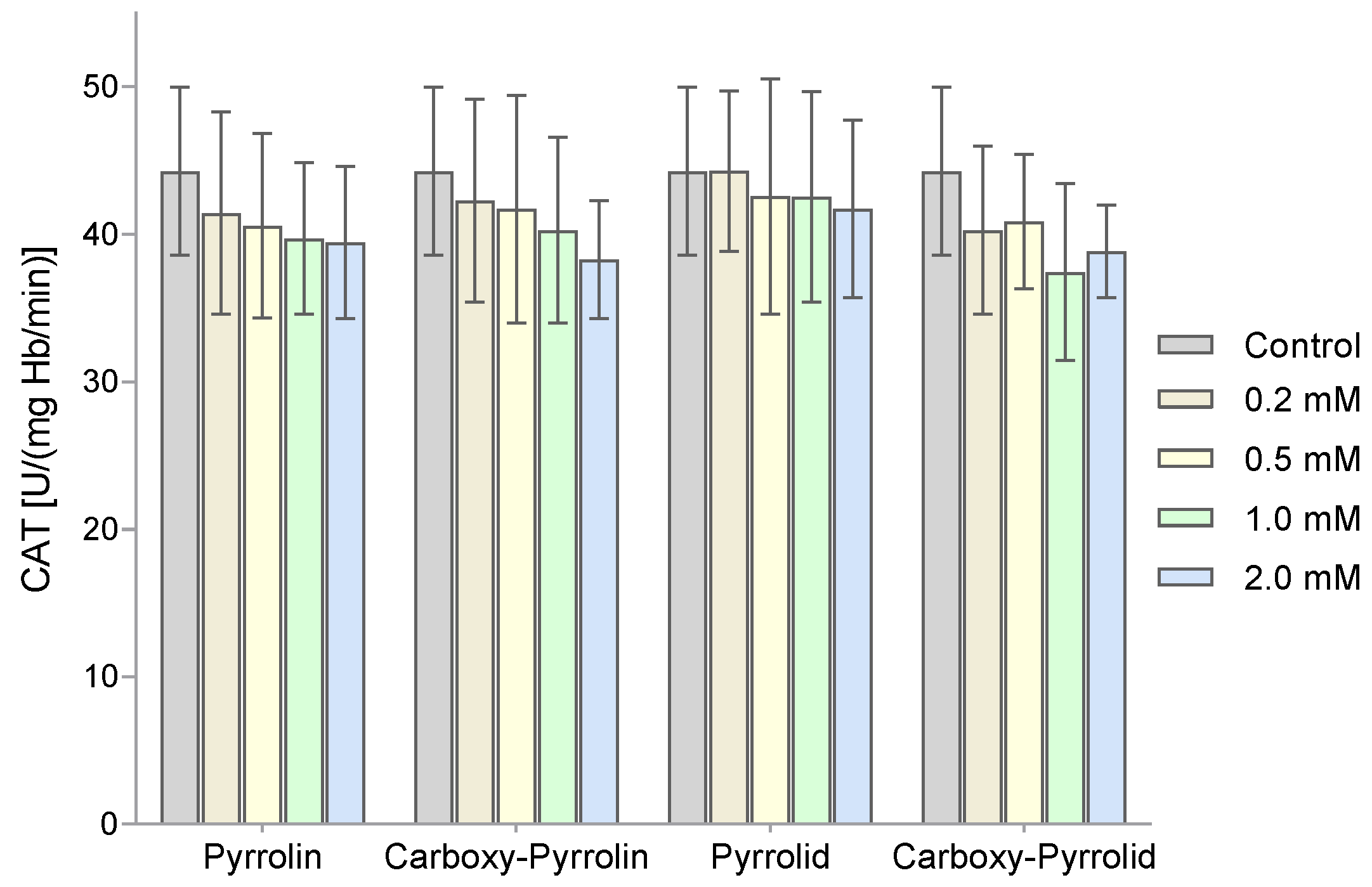
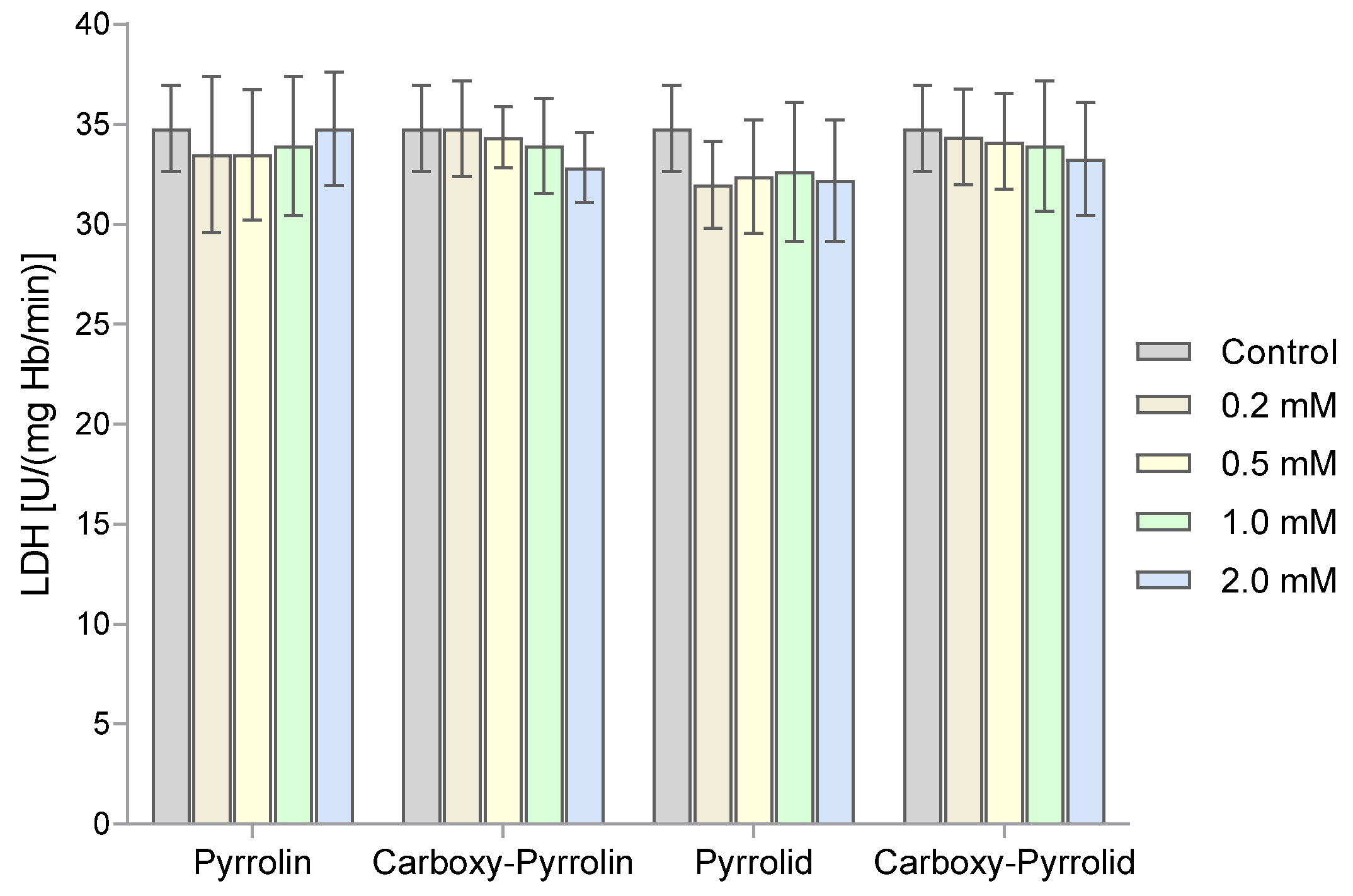
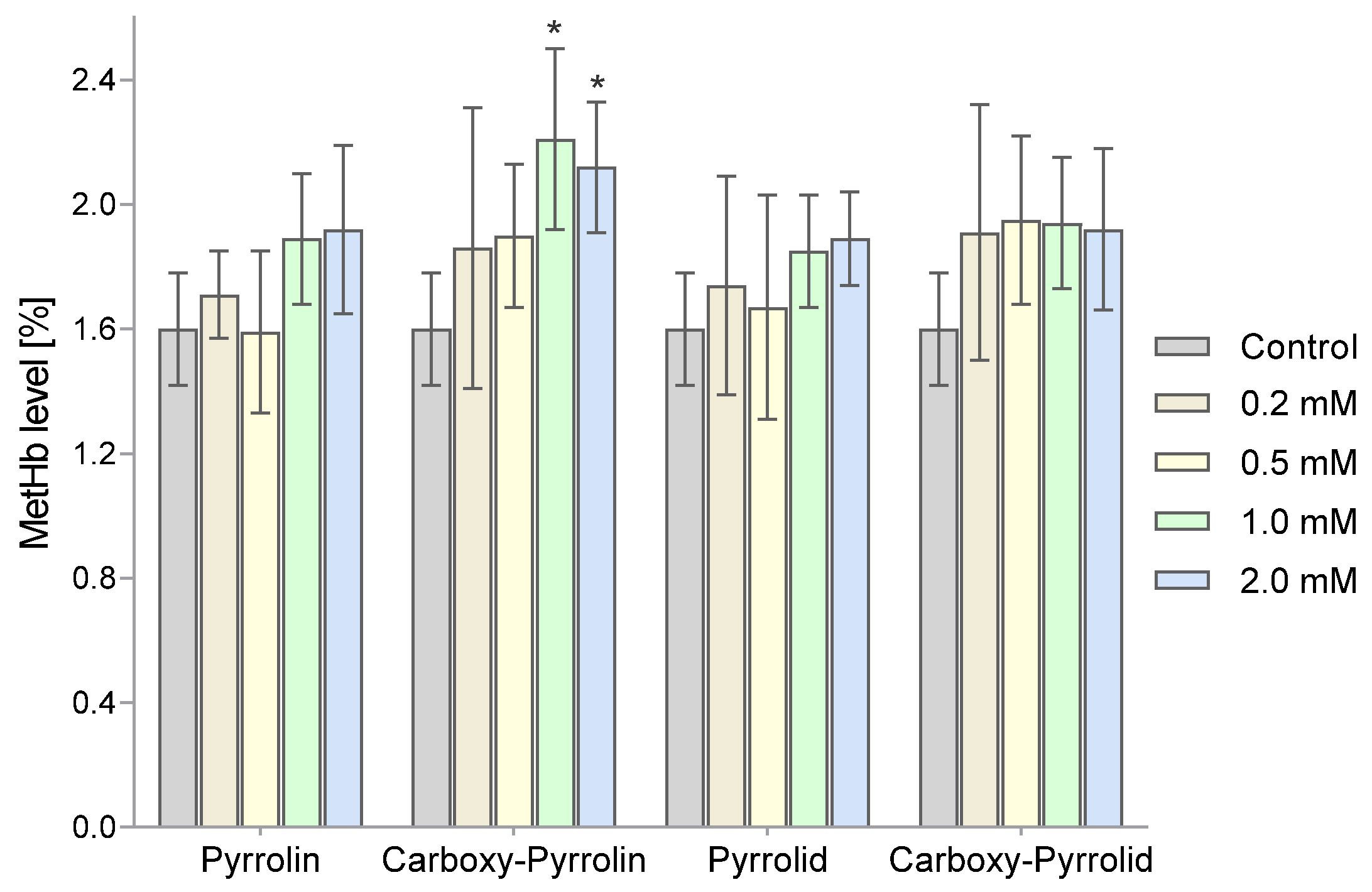
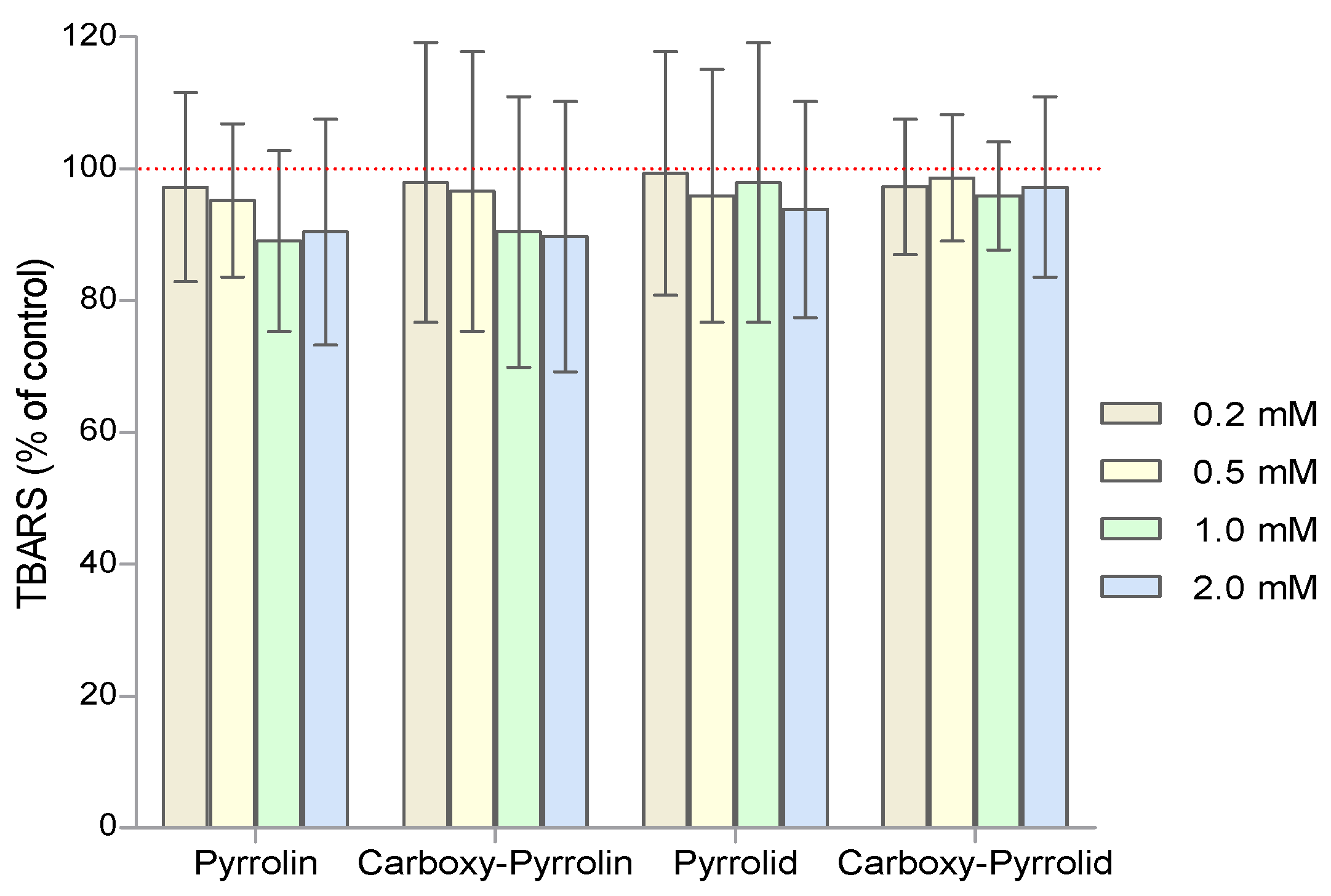
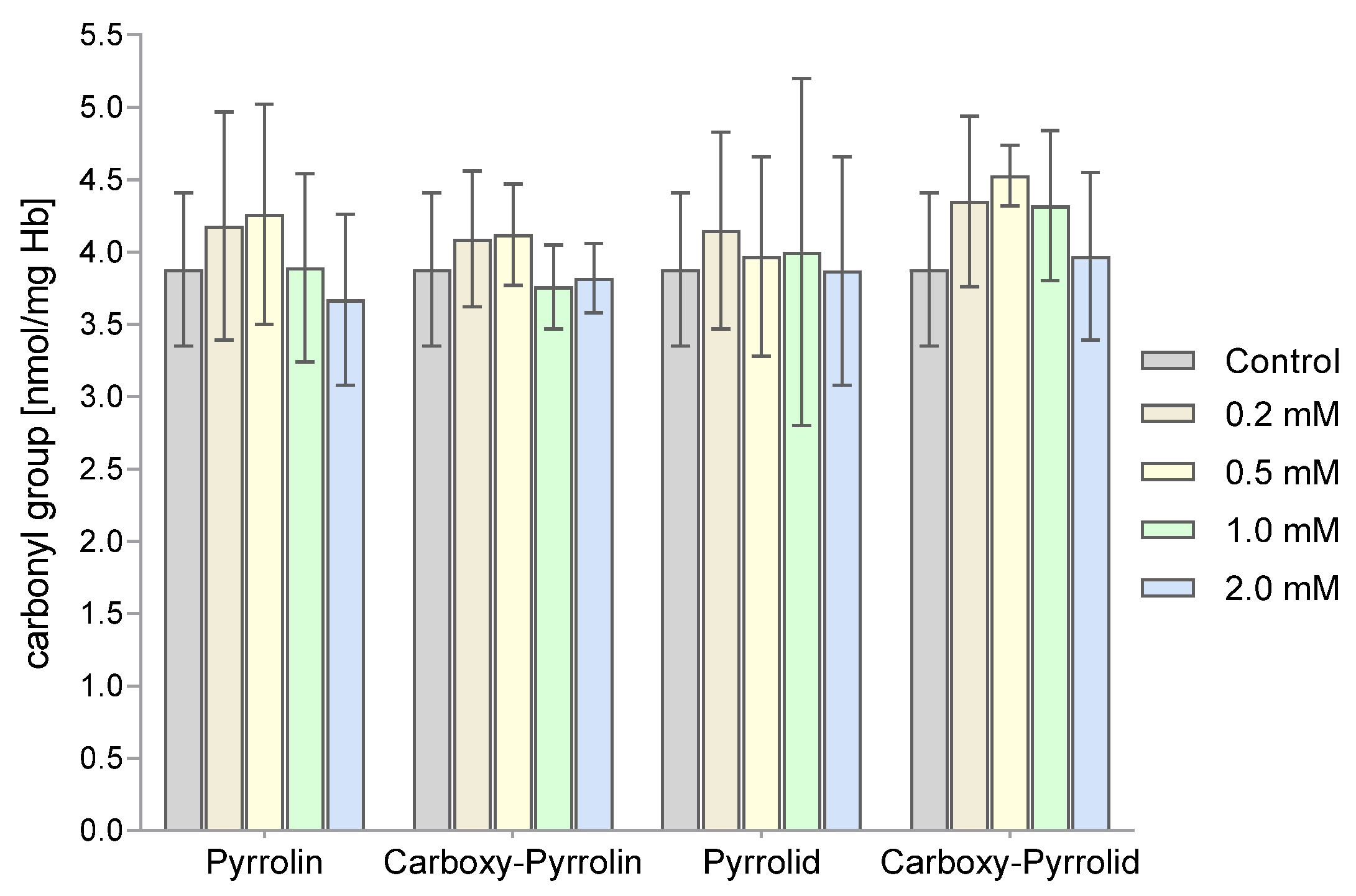
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Material
4.3. Hemolysate and RBC Membrane Preparation
4.4. Determination of Total Glutathione Concentration (GSH + GSSG) in Hemolysate
4.5. Determination of Ascorbate Concentration in Hemolysate
4.6. Determination of the Concentration of Thiol Groups in RBC Membranes and Hemolysate Proteins
4.7. Superoxide Dismutase Activity Assay
4.8. Determination of Catalase Activity
4.9. Lactate Dehydrogenase Activity Assay
4.10. Determination of the Degree of Hemoglobin Oxidation (% MetHb)
4.11. Determination of the Thiobarbituric Acid-Reactive Substances
4.12. Determination of the Concentration of Carbonyl Groups
4.13. Statistical Analysis of the Obtained Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | ascorbic acid |
| Carboxy-Pyrrolin | 3-carboxy-2,2,5,5-tetramethylpyrroline-1-oxyl |
| Carboxy-Pyrrolid | 3-carboxy-2,2,5,5-tetramethylpyrrolidine-1-oxyl |
| CAT | catalase |
| DHA | dehydroascorbate |
| GSH | glutathione |
| GSSG | oxidized glutathione |
| GPx | glutathione peroxidase |
| GRx | glutathione reductase |
| GT | glutathione transferase |
| Grx | glutaredoxin |
| G6PD | glucose-6-phosphate dehydrogenase |
| Hb | hemoglobin |
| LDH | lactate dehydrogenase |
| Mb | myoglobin |
| MetHb | methemoglobin |
| NAD+ | nicotinamide adenine dinucleotide oxidized form |
| NADH | nicotinamide adenine dinucleotide reduced form |
| RBCs | red blood cells (erythrocytes) |
| TBARSs | thiobarbituric acid-reactive substances |
| Tempo | 2,2,6,6-tetramethylpiperidine-1-oxyl |
| Tempol | 4- hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl |
| Tempamine | 4-amine-2,2,6,6-tetramethylpiperidine-1-oxyl |
| Pyrrolin | 3-carbamoyl-2,2,5,5-tetramethylpyrroline-1-oxyl |
| Pyrrolid | 3-carbamoyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl |
| SOD | superoxide dismutase |
| Trx | thioredoxin |
References
- Ouari, O. Nitroxides: Synthesis, Properties and Applications, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2021; ISBN 9781788019668. [Google Scholar]
- Berliner, L.J. (Ed.) Spin Labeling: Theory and Applications; Academic Press: New York, NY, USA; London, UK, 1976. [Google Scholar]
- Krishna, M.C.; Samuni, A. Nitroxides as antioxidants. Methods Enzymol. 1994, 234, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Free Nitroxyl Radicals; Rozantsev, E., Ed.; Springer: New York, NY, USA, 2013; ISBN 9781475707106. [Google Scholar]
- Berliner, L.J. (Ed.) Spin Labeling 2: Theory and Applications; Academic Press: New York, NY, USA; London, UK, 1979. [Google Scholar]
- Kocherginsky, N.; Swartz, H.M. Nitroxide Spin Labels: Reactions in Biology and Chemistry; CRC Press: Boca Raton, FL, USA, 1995; ISBN 084934204X. [Google Scholar]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.-I.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef]
- Belkin, S.; Mehlhorn, R.J.; Hideg, K.; Hankovsky, O.; Packer, L. Reduction and destruction rates of nitroxide spin probes. Arch. Biochem. Biophys. 1987, 256, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Bartosz, G. Nitroxide reduction in human red blood cells. Curr. Top. Biophys. 1996, 60–65. [Google Scholar]
- Glebska, J.; Gwozdzinski, K. Oxygen-dependent reduction of nitroxides by ascorbic acid and glutathione. EPR investigations. Curr. Top. Biophys. 1998; 75–82. [Google Scholar]
- Goldstein, S.; Samuni, A. Kinetics and mechanism of peroxyl radical reactions with nitroxides. J. Phys. Chem. A 2007, 111, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Haidasz, E.A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K.U.; Valgimigli, L.; Pratt, D.A. Acid Is Key to the Radical-Trapping Antioxidant Activity of Nitroxides. J. Am. Chem. Soc. 2016, 138, 5290–5298. [Google Scholar] [CrossRef]
- Samuni, A.; Goldstein, S.; Russo, A.; Mitchell, J.B.; Krishna, M.C.; Neta, P. Kinetics and mechanism of hydroxyl radical and OH-adduct radical reactions with nitroxides and with their hydroxylamines. J. Am. Chem. Soc. 2002, 124, 8719–8724. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ishigaki, H.; Okada, H.; Suyama, S. New Method in Free Radical Chemistry Using 2,4-Diphenyl-4-methyl-1-pentene as Radical Trapping Agent. Polym. J. 1997, 29, 366–369. [Google Scholar] [CrossRef]
- Genovese, D.; Baschieri, A.; Vona, D.; Baboi, R.E.; Mollica, F.; Prodi, L.; Amorati, R.; Zaccheroni, N. Nitroxides as Building Blocks for Nanoantioxidants. ACS Appl. Mater. Interfaces 2021, 13, 31996–32004. [Google Scholar] [CrossRef]
- Glebska, J.; Pulaski, L.; Gwozdzinski, K.; Skolimowski, J. Structure-activity relationship studies of protective function of nitroxides in Fenton system. Biometals 2001, 14, 159–170. [Google Scholar] [CrossRef]
- Glebska, J.; Gwozdzinski, K. Nitroxides as protectors against oxidative damage induced by the Fenton system. Curr. Top. Biophys. 2000, 57–63. [Google Scholar]
- Goldstein, S.; Samuni, A.; Merenyi, G. Reactions of nitric oxide, peroxynitrite, and carbonate radicals with nitroxides and their corresponding oxoammonium cations. Chem. Res. Toxicol. 2004, 17, 250–257. [Google Scholar] [CrossRef]
- Samuni, A.; Krishna, C.M.; Riesz, P.; Finkelstein, E.; Russo, A. A novel metal-free low molecular weight superoxide dismutase mimic. J. Biol. Chem. 1988, 263, 17921–17924. [Google Scholar] [CrossRef]
- Krishna, M.C.; Samuni, A.; Taira, J.; Goldstein, S.; Mitchell, J.B.; Russo, A. Stimulation by nitroxides of catalase-like activity of hemeproteins. Kinetics and mechanism. J. Biol. Chem. 1996, 271, 26018–26025. [Google Scholar] [CrossRef]
- Wilson, M.T.; Reeder, B.J. The peroxidatic activities of Myoglobin and Hemoglobin, their pathological consequences and possible medical interventions. Mol. Aspects Med. 2022, 84, 101045. [Google Scholar] [CrossRef]
- Czepas, J.; Koceva-Chyła, A.; Gwoździński, K.; Jóźwiak, Z. Different effectiveness of piperidine nitroxides against oxidative stress induced by doxorubicin and hydrogen peroxide. Cell Biol. Toxicol. 2008, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Koceva-Chyła, A.; Gwoździński, K.; Kochman, A.; Stolarska, A.; Jóźwiak, Z. Effects of pyrroline and pyrrolidine nitroxides on lipid peroxidation in heart tissue of rats treated with doxorubicin. Cell. Mol. Biol. Lett. 2003, 8, 179–183. [Google Scholar] [PubMed]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as Antioxidants and Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef] [PubMed]
- Tabaczar, S.; Czepas, J.; Koceva-Chyla, A.; Kilanczyk, E.; Piasecka-Zelga, J.; Gwozdzinski, K. The effect of the nitroxide pirolin on oxidative stress induced by doxorubicin and taxanes in the rat brain. J. Physiol. Pharmacol. 2017, 68, 295–308. [Google Scholar]
- Krainz, T.; Gaschler, M.M.; Lim, C.; Sacher, J.R.; Stockwell, B.R.; Wipf, P. A Mitochondrial-Targeted Nitroxide Is a Potent Inhibitor of Ferroptosis. ACS Cent. Sci. 2016, 2, 653–659. [Google Scholar] [CrossRef]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Black, H.D.; Xu, W.; Hortle, E.; Robertson, S.I.; Britton, W.J.; Kaur, A.; New, E.J.; Witting, P.K.; Chami, B.; Oehlers, S.H. The cyclic nitroxide antioxidant 4-methoxy-TEMPO decreases mycobacterial burden in vivo through host and bacterial targets. Free Radic. Biol. Med. 2019, 135, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zareba, M.; Widomska, J.; Burke, J.M.; Subczynski, W.K. Nitroxide free radicals protect macular carotenoids against chemical destruction (bleaching) during lipid peroxidation. Free Radic. Biol. Med. 2016, 101, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Lahiani, A.; Hidmi, A.; Katzhendler, J.; Yavin, E.; Lazarovici, P. Novel Synthetic PEGylated Conjugate of α-Lipoic Acid and Tempol Reduces Cell Death in a Neuronal PC12 Clonal Line Subjected to Ischemia. ACS Chem. Neurosci. 2016, 7, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Krishna, M.C. Nitroxides as radiation protectors. Mil. Med. 2002, 167, 49–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, H.V.-T.; Chen, Q.; Paletta, J.T.; Harvey, P.; Jiang, Y.; Zhang, H.; Boska, M.D.; Ottaviani, M.F.; Jasanoff, A.; Rajca, A.; et al. Nitroxide-Based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. ACS Cent. Sci. 2017, 3, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Fu, T.C.; Funk, A.; London, R.E. Differential clearance of nitroxide MRI contrast agents from rat cerebral ventricles. Brain Res. Bull. 1995, 36, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Yu, L.-E.; Wang, J.-Y. Nitroxide antioxidant as a potential strategy to attenuate the oxidative/nitrosative stress induced by hydrogen peroxide plus nitric oxide in cultured neurons. Nitric Oxide 2016, 54, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive Oxygen Species and Their Involvement in Red Blood Cell Damage in Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef]
- Yang, S.; Jensen, M.K.; Rimm, E.B.; Willett, W.; Wu, T. Erythrocyte superoxide dismutase, glutathione peroxidase, and catalase activities and risk of coronary heart disease in generally healthy women: A prospective study. Am. J. Epidemiol. 2014, 180, 901–908. [Google Scholar] [CrossRef]
- Klein, R.; Nagy, O.; Tóthová, C.; Chovanová, F. Clinical and Diagnostic Significance of Lactate Dehydrogenase and Its Isoenzymes in Animals. Vet. Med. Int. 2020, 2020, 5346483. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Ying, M.; Jin, C.; Li, J.; Hu, X. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct. Target. Ther. 2021, 6, 242. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. The Cellular and Organismal Effects of Nitroxides and Nitroxide-Containing Nanoparticles. Int. J. Mol. Sci. 2024, 25, 1446. [Google Scholar] [CrossRef] [PubMed]
- Tabaczar, S.; Domeradzka, K.; Czepas, J.; Piasecka-Zelga, J.; Stetkiewicz, J.; Gwoździński, K.; Koceva-Chyła, A. Anti-tumor potential of nitroxyl derivative Pirolin in the DMBA-induced rat mammary carcinoma model: A comparison with quercetin. Pharmacol. Rep. 2015, 67, 527–534. [Google Scholar] [CrossRef]
- Bujak-Pietrek, S.; Pieniazek, A.; Gwozdzinski, K.; Gwozdzinski, L. The Effect of Piperidine Nitroxides on the Properties of Metalloproteins in Human Red Blood Cells. Molecules 2023, 28, 6174. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Grahame, D.A.; Samuni, A.; Mitchell, J.B.; Russo, A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci. USA 1992, 89, 5537–5541. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Ojima, N.; Kikugawa, K. Conversion of nitroxide radicals by phenolic and thiol antioxidants. Free Radic. Res. 1997, 27, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Nothig-Laslo, V.; Bobst, A.M. Reinvestigation of the oxidation properties of nitroxides. Croat. Chem. Acta 1991, 64, 1. [Google Scholar]
- Bujak, S.; Gwozdzinski, K. Nitroxides lead to reduced level of glutathione in red blood cells. Medimond Int. Proc. 2003, 105–108. [Google Scholar]
- Balcerczyk, A.; Łuczak, K.; Soszyński, M.; Bartosz, G. Prooxidative effects of TEMPO on human erythrocytes. Cell Biol. Int. 2004, 28, 585–591. [Google Scholar] [CrossRef]
- Bujak, S.; Gwozdzinski, K. The effect of stable nitroxide radicals on the activity of glutathione-dependent enzymes: Reductase, peroxidase and transferase in human erythrocytes. Medimond Int. Proc. 2006, 71–74. [Google Scholar]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic. Biol. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Orrico, F.; Villar, S.F.; López, A.C.; Silva, N.; Donzé, M.; Thomson, L.; Denicola, A. Oxidants and Antioxidants in the Redox Biochemistry of Human Red Blood Cells. ACS Omega 2023, 8, 147–168. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009, 4, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Ledesma, S.; Randall, L.M.; Parsonage, D.; Dalla Rizza, J.; Karplus, P.A.; Poole, L.B.; Denicola, A.; Ferrer-Sueta, G. Differential Kinetics of Two-Cysteine Peroxiredoxin Disulfide Formation Reveal a Novel Model for Peroxide Sensing. Biochemistry 2018, 57, 3416–3424. [Google Scholar] [CrossRef]
- Lee, T.-H.; Kim, S.-U.; Yu, S.-L.; Kim, S.H.; Park, D.S.; Moon, H.-B.; Dho, S.H.; Kwon, K.-S.; Kwon, H.J.; Han, Y.-H.; et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 2003, 101, 5033–5038. [Google Scholar] [CrossRef]
- Matte, A.; Bertoldi, M.; Mohandas, N.; An, X.; Bugatti, A.; Brunati, A.M.; Rusnati, M.; Tibaldi, E.; Siciliano, A.; Turrini, F.; et al. Membrane association of peroxiredoxin-2 in red cells is mediated by the N-terminal cytoplasmic domain of band 3. Free Radic. Biol. Med. 2013, 55, 27–35. [Google Scholar] [CrossRef]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Gallogly, M.M.; Starke, D.W.; Mieyal, J.J. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid. Redox Signal. 2009, 11, 1059–1081. [Google Scholar] [CrossRef] [PubMed]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress-The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxidative Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef]
- John, S.; Kale, M.; Rathore, N.; Bhatnagar, D. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem. 2001, 12, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Slimen, S.; Saloua, E.F.; Najoua, G. Oxidative stress and cytotoxic potential of anticholinesterase insecticide, malathion in reproductive toxicology of male adolescent mice after acute exposure. Iran. J. Basic Med. Sci. 2014, 17, 522–530. [Google Scholar] [PubMed]
- Scott, M.D.; Wagner, T.C.; Chiu, D.T. Decreased catalase activity is the underlying mechanism of oxidant susceptibility in glucose-6-phosphate dehydrogenase-deficient erythrocytes. Biochim. Biophys. Acta 1993, 1181, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.; Chan, P.C. Re-evaluation of the kinetics of lactate dehydrogenase-catalyzed chain oxidation of nicotinamide adenine dinucleotide by superoxide radicals in the presence of ethylenediaminetetraacetate. J. Biol. Chem. 1976, 251, 3841–3844. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Bielski, B.H. Enzyme-catalyzed free radical reactions with nicotinamide adenine nucleotides. II. Lactate dehydrogenase-catalyzed oxidation of reduced nicotinamide adenine dinucleotide by superoxide radicals generated by xanthine oxidase. J. Biol. Chem. 1974, 249, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Petrat, F.; Bramey, T.; Kirsch, M.; de Groot, H. Initiation of a superoxide-dependent chain oxidation of lactate dehydrogenase-bound NADH by oxidants of low and high reactivity. Free Radic. Res. 2005, 39, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, G.; Gabbianelli, R.; Damiani, E.; Santroni, A.M.; Fedeli, D.; Wozniak, M.; Greci, L. The effect of indolinic and quinolinic nitroxide radicals on trout erythrocytes exposed to oxidative stress. Free Radic. Res. 1998, 28, 507–516. [Google Scholar] [CrossRef]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Drabkin, D.L. Spectrophotometric studies; the crystallographic and optical properties of the hemoglobin of man in comparison with those of other species. J. Biol. Chem. 1946, 164, 703–723. [Google Scholar] [CrossRef]
- Akerboom, T.P.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Omaye, S.T.; Turnbull, J.D.; Sauberlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Egwim, I.O.; Gruber, H.J. Spectrophotometric measurement of mercaptans with 4,4′-dithiodipyridine. Anal. Biochem. 2001, 288, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 1972, 247, 6960–6962. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, F.; Ladue, J.S. Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biol. Med. 1955, 90, 210–213. [Google Scholar] [CrossRef]
- Stocks, J.; Dormandy, T.L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br. J. Haematol. 1971, 20, 95–111. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwozdzinski, K.; Bujak-Pietrek, S.; Pieniazek, A.; Gwozdzinski, L. Modulation of the Human Erythrocyte Antioxidant System by the 5- and 6-Membered Heterocycle-Based Nitroxides. Molecules 2024, 29, 2941. https://doi.org/10.3390/molecules29122941
Gwozdzinski K, Bujak-Pietrek S, Pieniazek A, Gwozdzinski L. Modulation of the Human Erythrocyte Antioxidant System by the 5- and 6-Membered Heterocycle-Based Nitroxides. Molecules. 2024; 29(12):2941. https://doi.org/10.3390/molecules29122941
Chicago/Turabian StyleGwozdzinski, Krzysztof, Stella Bujak-Pietrek, Anna Pieniazek, and Lukasz Gwozdzinski. 2024. "Modulation of the Human Erythrocyte Antioxidant System by the 5- and 6-Membered Heterocycle-Based Nitroxides" Molecules 29, no. 12: 2941. https://doi.org/10.3390/molecules29122941
APA StyleGwozdzinski, K., Bujak-Pietrek, S., Pieniazek, A., & Gwozdzinski, L. (2024). Modulation of the Human Erythrocyte Antioxidant System by the 5- and 6-Membered Heterocycle-Based Nitroxides. Molecules, 29(12), 2941. https://doi.org/10.3390/molecules29122941





