Exploring Recent Developments in Graphene-Based Cathode Materials for Fuel Cell Applications: A Comprehensive Overview
Abstract
1. Introduction
2. Enhanced Catalytic Performance
2.1. Native Catalytic Capability
The Presence of Oxygen Vacancies
2.2. Observable Catalytic Activity
2.2.1. Nanoarchitecture
2.2.2. Three-Dimensional Structure Arrangement
3. Exceptional Long-Term Durability
3.1. Chemical Durability
3.2. Stability under Thermal Conditions
3.2.1. Chemical Modification through Doping
3.2.2. Integrated Cathode Materials
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Cao, S.; Kosonen, R.; Hamdy, M. Multi-objective optimisation of an interactive buildings-vehicles energy sharing network with high energy flexibility using the Pareto archive NSGA-II algorithm. Energy Convers. Manag. 2020, 218, 113017–113036. [Google Scholar] [CrossRef]
- Arat, H.T.; Surer, M.G.; Gokpinar, S.; Aydin, K. Conceptual design analysis for a lightweight aircraft with a fuel cell hybrid propulsion system. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 45, 46–60. [Google Scholar]
- Petrescu, R.V.V.; Machin, A.; Fontanez, K. Hydrogen for aircraft and propulsion. Int. J. Hydrogen Energy 2020, 45, 20740–20764. [Google Scholar] [CrossRef]
- Ugurlu, A. An emission analysis study of hydrogen powered vehicle. Clean Technol. 2022, 4, 908–930. [Google Scholar] [CrossRef]
- Contreras, A.; Yigit, S.; Ozay, K.; Veziroglu, T.N. Hydrogen as aviation fuel: A comparison with hydrocarbon fuels. Int. J. Hydrogen Energy 1998, 22, 1053–1060. [Google Scholar] [CrossRef]
- Verstraete, D. Long range transport aircraft using hydrogen fuel. Int. J. Hydrogen Energy 2020, 478, 228649–228666. [Google Scholar] [CrossRef]
- Mohanty, D.; Chao, K.Y.; Li, T.N.; Lee, S.W.; Tseng, C.J.; Hung, I.-M. Microstructure of hydrogen electrode catalyst layer materials for solid oxide electrolysis cells. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Bodner, M.; Hacker, V. A brief review of Poly(Vinyl Alcohol)-based anion exchange membranes for alkaline fuel cells. Adv. Energy Mater. 2022, 14, 3565. [Google Scholar] [CrossRef]
- Alnaqbi, H.; Sayed, E.T.; Al-Asheh, S.; Bahaa, A.; Alawadhi, H.; Abdelkareem, M.A. Current progression in graphene-based membranes for low temperature fuel cells. Int. J. Hydrogen Energy 2024, 52, 800–842. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Lin, B.Y.; Kirk, D.W.; Thorpe, S.J. Performance of alkaline fuel cells: A possible future energy system? J. Power Sources 2006, 161, 474–483. [Google Scholar] [CrossRef]
- Cuneo, A.; Zaccaria, V.; Tuker, D.; Sore, A. Gas turbine size optimization in a hybrid system considering SOFC degradation. Appl. Energy 2018, 79, 855–864. [Google Scholar] [CrossRef]
- Hauch, A.; Kungas, R.; Blenow, P.; Hasen, A.B.; Hasen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, 6118–6126. [Google Scholar] [CrossRef] [PubMed]
- Hossiain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Chen, K.; Lü, Z.; Chen, X.; Ai, N.; Huang, X.; Du, X.; Su, W. Development of LSM-based cathodes for solid oxide fuel cells based on YSZ films. J. Power Sources 2007, 172, 742–748. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1995, 76, 563–588. [Google Scholar] [CrossRef]
- Corigliano, O.; Pagnotta, L.; Fragiacomo, P. On the Technology of Solid Oxide Fuel Cell (SOFC) Energy Systems for Stationary Power Generation: A Review. Sustainability 2022, 14, 15276. [Google Scholar] [CrossRef]
- Singhal, S.C.; Kendall, K. High-Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–22. [Google Scholar]
- Mogensen, M.; Kammer, K. Conversion of Hydrocarbons in Solid Oxide Fuel Cells. Annu. Rev. Mater. Res. 2003, 33, 321–331. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar]
- Tao, Z.; Fu, M.; Liu, Y.; Gao, Y.; Tong, H.; Hu, W.; Lei, L.; Bi, L. High-performing proton-conducting solid oxide fuel cells with triple-conducting cathode of Pr0.5Ba0.5(Co0.7Fe0.3)O3-δ tailored with W. Int. J. Hydrogen Energy 2022, 47, 1947–1953. [Google Scholar] [CrossRef]
- Lei, L.; Tao, Z.; Hong, T.; Wang, X.; Chen, F. A highly active hybrid catalyst modified (La0.60Sr0.40)0.95Co0.20Fe0.80O3-δ cathode for proton conducting solid oxide fuel cells. J. Power Sources 2018, 389, 1–7. [Google Scholar] [CrossRef]
- Tong, H.; Fu, M.; Yang, Y.; Chen, F.; Tao, Z. A novel self-assembled cobalt-free perovskite composite cathode with triple-conduction for intermediate proton conducting solid oxide fuel cells. Advanced Functional Materials. Adv. Func. Mater. 2022, 32, 2209695. [Google Scholar] [CrossRef]
- Aguadero, A.; Fawcett, L.; Taub, S.; Woolley, R.; Wu, K.-T.; Xu, N.; Kilner, J.A.; Skinner, S.J. Materials development for intermediate-temperature solid oxide electrochemical devices. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Campo, F.J.G.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. J. Mater. Sci. 2012, 47, 3925–5471. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Kotomin, E.A.; Merkle, R.; Mastrikov, Y.A.; Maier, J. Combined theoretical and experimental analysis of processes determining cathode performance in solid oxide fuel cells. Phys. Chem. Chem. Phys. 2013, 15, 5443–5471. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Kleis, J.; Rossmeisl, J.; Shao-Horn, Y.; Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 2021, 45, 15797–15831. [Google Scholar] [CrossRef]
- Li, G.; He, B.; Ling, Y.; Xu, J.; Zhao, L. Highly active YSB infiltrated LSCF cathode for proton conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2015, 40, 13576–13582. [Google Scholar] [CrossRef]
- Kumar, A.; Vibbu, V.; Bassar, J.M.; Nohl, L.; Haart, L.G.J.D.; Bouvet, M.; Eichel, R.-A. Ammonia as a potential energy vector in the Burgeoning Hydrogen economy. ChemElectroChem 2024, e2023300845. [Google Scholar] [CrossRef]
- Lai, X.; Jin, C.; Yi, W.; Han, X.; Feng, X.; Zheng, Y.; Ouyang, M. Mechanism, modeling, detection, and prevention of the internal short circuit in lithium-ion batteries: Recent advances and perspectives. Energy Storag. Mater. 2021, 35, 470–499. [Google Scholar] [CrossRef]
- Munoz-Garcia, A.B.; Ritzmann, A.M.; Pavone, M.; Keith, J.A.; Carter, E.A. Oxygen transport in perovskite-type solid oxide fuel cell materials: Insights from quantum mechanics. Acc. Chem. Res. 2014, 47, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.B.; Lane, J.A.; Steele, B.C.H. Electrode kinetics of porous mixed-conducting oxygen electrode. J. Electrochem. Soc. 2019, 143, 3554–3564. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Ma, J.; Yin, Y.; Fronzi, M.; Wang, X.; Bi, L. Tailoring electronic structure of perovskite cathode for proton-conducting solid oxide fuel cells with high performance. J. Power Sources 2021, 489, 229486. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Pavone, M. First-principles design of new electrodes for proton-conducting solid-oxide electrochemical cells: A-site doped Sr2Fe1.5Mo0.5O6-δ perovskite. Chem. Mater. 2016, 28, 490–500. [Google Scholar] [CrossRef]
- Kreuer, K.; Münch, W.; Traub, U.; Maier, J. On proton transport in perovskite-type oxides and plastic hydroxides. Ber. Bunsen Ges. Phys. Chem. 1998, 102, 552–559. [Google Scholar] [CrossRef]
- Tao, Z.; Xu, X.; Bi, L. Density functional theory calculations for cathode materials of proton-conducting solid oxide fuel cells: A mini-review. Electrochem. Commun. 2021, 129, 107072. [Google Scholar] [CrossRef]
- Zakaria, Z.; Awang Mat, Z.; Abu Hassan, S.H.; Boon Kar, Y. A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int. J. Energy Res. 2019, 44, 594–611. [Google Scholar] [CrossRef]
- Tu, B.; Yin, Y.; Zhang, F.; Su, X.; Lyu, X.; Cheng, M. High performance of direct methane-fuelled solid oxide fuel cell with samarium modified nickel-based anode. Int. J. Hydrogen Energy 2020, 45, 27587–27596. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Jamaludin, A.S.; Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Members 2020, 10, 99–116. [Google Scholar] [CrossRef]
- Rahumi, O.; Sobolev, A.; Rath, M.K.; Borodianskiy, K. Nanostructured engineering of nickel cermet anode for solid oxide fuel cell using inkjet printing. J. Eur. Ceram. Soc. 2021, 41, 4528–4536. [Google Scholar] [CrossRef]
- Liu, F.H. Hydrogen-Metal Systems: Hydride Forming Alloys. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 3953–3969. [Google Scholar]
- Nash, D.; Aklil, D.; Jonson, E.; Gazey, V.R. Ortisi, 4.05—Hydrogen Storage: Compressed Gas. Compr. Renew. Energy 2012, 37, 2679–2682. [Google Scholar]
- da Rosa, A.V. Fundamentals of Renew Energy Processes; Elsevier Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Su, H.; Hu, Y. Recent advances in graphene-based materials for fuel cell applications. Energy Sci. Eng. 2021, 9, 958–983. [Google Scholar] [CrossRef]
- Dwivedi, S. Graphene based electrodes for hydrogen fuel cells: A comprehensive review. Hydrog. Energy 2022, 47, 41848–41877. [Google Scholar] [CrossRef]
- Akhter, R.; Hussain, S.; Maktedar, S.S. Advanced graphene-based (photo & electro) catalysts for sustainable & clean energy technologies. New. J. Chem. 2024, 48, 437–505. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Urade, A.R.; Lahiri, I.; Suresh, K. S Graphene Properties, Synthesis and Applications: A Review. JOM 2023, 75, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by graphene-based materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hu, Y.H. How magical is magic-angle graphene? Matter 2020, 2, 1106–1114. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef]
- Liu, M.; Dai, Y.; Maryam, B.; Cui, J.; Liu, X. Graphene Used for Energy Conversion and Storage by Electrochemistry: A Brief Global Overview. Curr. Nanosci. 2024, 20, 2–17. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Z.; Zhang, Q.; Guan, J. Progress in nonmetal-doped graphene electrocatalysts for the oxygen reduction reaction. ChemSusChem 2019, 12, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Gautam, A.; Rai, V. Graphene-based bipolar plates for polymer electrolyte membrane fuel cells. Front. Mater. Sci. 2019, 13, 217–241. [Google Scholar] [CrossRef]
- Hu, S.; Lozada-Hidalgo, M.; Wang, F.C. Proton transport through one-atom-thick crystals. Nature 2014, 516, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qiao, J.; Yang, L.; Zhang, J. A Review of Graphene-Based Nanostructural Materials for Both Catalyst Supports and Metal-Free Catalysts in PEM Fuel Cell Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 1301523–1301547. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, M. Drawing conclusions from graphene. Phys. World 2006, 19, 33–38. [Google Scholar] [CrossRef]
- Banerjee, A.; Deutschmann, O. Elementary kinetics of the oxygen reduction reaction on LSM-YSZ composite cathodes. J. Catal. 2017, 346, 30–49. [Google Scholar] [CrossRef]
- Shishkin, M.; Ziegler, T. Direct modeling of the electrochemistry in the three-phase boundary of solid oxide fuel cell anodes by density functional theory: A critical overview. Phys. Chem. Chem. Phys. 2014, 16, 1798–1808. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Motola, M.; Babar, Z.U.D.; Li, C.J.; Li, C.X. Recent progress of perovskite-based electrolyte materials for solid oxide fuel cells and performance optimizing strategies for energy storage applications. Mater. Res. Bull. 2022, 146, 111612. [Google Scholar] [CrossRef]
- Harilal, M.; Krishnan, S.G. Nanocarbons and electric double-layer capacitors. In Supercapacitors; Elsevier: Amsterdam, The Netherlands, 2024; pp. 17–43. [Google Scholar]
- Salahdin, O.D.; Sayadi, H.; Solanki, R.; Parra, R.M.R.; Al-Thamir, M.; Jalil, A.T.; Kianfar, E. Graphene and carbon structures and nanomaterials for energy storage. Appl. Phys. A Mater. Sci. Process. 2022, 128, 703. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, R.; Chen, W. Graphene-supported nanoelectrocatalysts for fuel cells: Synthesis, properties, and applications. Chem. Rev. 2014, 114, 5117–5160. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Wang, X. Graphene-based nanomaterials for catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. [Google Scholar] [CrossRef]
- Soo, L.T.; Loh, K.S.; Mohamad, A.B.; Daud, W.R.W.; Wong, W.Y. An overview of the electrochemical performance of modified graphene used as an electrocatalyst and as a catalyst support in fuel cells. Appl. Catal. A Gen. 2015, 497, 198–210. [Google Scholar] [CrossRef]
- Adil, S.F.; Ashraf, M.; Khan, M.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Tahir, M.N. Advances in graphene/inorganic nanoparticle composites for catalytic applications. Chem. Rec. 2022, 22, e202100274. [Google Scholar] [CrossRef] [PubMed]
- Julkapli, N.M.; Bagheri, S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrogen Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Poetzsch, D.; Merkle, R.; Maier, J. Oxygen reduction at dense thin-film microelectrodes on a proton-conducting electrolyte. J. Electrochem. Soc. 2015, 162, F939–F950. [Google Scholar] [CrossRef]
- Kumuk, B.; Alemu, M.A.; Ilbas, M. Investigation of the effect of ion transition type on performance in solid oxide fuel cells fueled hydrogen and coal gas. Int. J. Hydrogen Energy 2022, 47, 3409–3415. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; Section 1.3, Chemical Bonds in Biochemistry; Freeman W H: New York, NY, USA, 2002. [Google Scholar]
- Li, Y.; Zhao, D.; Wang, Y.; Xue, R.; Shen, Z.; Li, X. The mechanism of hydrogen storage in carbon materials. Int. J. Hydrogen Energy 2007, 32, 2513–2517. [Google Scholar] [CrossRef]
- Liang, S.; Hao, P.C.; Shi, P.Y. The Power of Single-Atom Catalysis. Chem. Cat. Chem. 2015, 7, 2559–2567. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of grapheme. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, Y.; Hu, Y.; Li, C.; Wu, P.; Wei, S.; Cai, C. Pd@Pt Core−Shell Nanostructures with Controllable Composition Synthesized by a Microwave Method and Their Enhanced Electrocatalytic Activity toward Oxygen Reduction and Methanol Oxidation. J. Phys. Chem. C 2010, 114, 11861–11867. [Google Scholar] [CrossRef]
- Wang, L.; Tian, C.; Wang, H.; Ma, Y.; Wang, B.; Fu, H. Mass Production of Graphene via an in Situ Self-Generating Template Route and Its Promoted Activity as Electrocatalytic Support for Methanol Electroxidization. J. Phys. Chem. C 2010, 114, 8727–8733. [Google Scholar] [CrossRef]
- Chan, K.T.; Neaton, J.B.; Cohen, M.L. First-principles study of metal adatom adsorption on grapheme. Phys. Rev. 2008, 77, 235430. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Guan, P.; Liu, C.; Huang, H.; Xue, F.; Dong, X.; Pennycook, S.J.; Chisholm, M.F. Catalytically active single-atom niobium in graphitic layers. Nat. Commun. 2013, 4, 1924. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Z.; Yu, G.; Chen, W.; Chen, Z. CO Catalytic Oxidation on Iron-Embedded Graphene: Computational Quest for Low-Cost Nanocatalysts. J. Phys. Chem. C 2010, 114, 6250–6254. [Google Scholar] [CrossRef]
- Song, E.H.; Wen, Z.; Jiang, Q. CO Catalytic Oxidation on Copper-Embedded Graphene. J. Phys. Chem. C 2011, 115, 3678–3683. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Q.; Shan, M.; Jiao, Z.; Zhou, X.; Ling, C.; Yan, Z. Adsorption and Catalytic Activation of O2 Molecule on the Surface of Au-Doped Graphene under an External Electric Field. J. Phys. Chem. C 2012, 116, 19918–19924. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom Catalysis Using Pt/Graphene Achieved through Atomic Layer Deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Z.; Dai, X. A theoretical simulation on the catalytic oxidation of CO on Pt/grapheme. Phys. Chem. Chem. Phys. 2012, 14, 16566–16572. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Yan, Z.; Zhang, X.; Jia, J.; Wu, H. Boron-/Fe-codoped graphene as high-activity single-atom catalyst. Theor. Chem. Acc. 2017, 136, 90. [Google Scholar] [CrossRef]
- Ye, L.; Ran, R.; Yao, Z.; Shao, Z.; Jin, W.; Xu, N.; Anh, J. Evaluation of Ba0.5Sr0.5Co0.8Fe0.2O3-δ as a potential cathode for an anode supported proton conducting solid-oxide fuel cell. J. Power Sources 2008, 180, 15–22. [Google Scholar]
- Lin, Y.; Ran, R.; Zhang, C.; Cai, R.; Shao, Z. Performance of PrBaCo2O5+δ as a proton-conducting solid-oxide fuel cell cathode. J. Phys. Chem. 2010, 114, 3764–3772. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, F.; Chen, F.; Liu, M. Investigation of A-site deficient Ba0.9Co0.7Fe0.2Nb0.1O3-δ cathode for proton conducting electrolyte based solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 8431–8436. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium cobalt ferrite perovskite electrodes of solid oxide fuel cells—A review. Int. J. Hydrogen Energy 2019, 44, 7448–7493. [Google Scholar]
- Wang, Y.; Yang, Z.; Lu, F.; Jin, C.; Wu, J.; Shen, M.; Yang, R.; Chen, F. Carbon-coating functionalized La0.6Sr1.4MnO4+δ layered perovskite oxide: Enhanced catalytic activity for the oxygen reduction reaction. RSC Adv. 2015, 5, 974–980. [Google Scholar] [CrossRef]
- Li, Q.; Guo, M.; Wang, K.; Wei, Z.; Du, G.; Zhang, G.; Chen, N. LaSr2Mn2O7 Ruddlesden-Popper manganites for oxygen reduction and electrochemical capacitors. J. Rare Earths 2020, 38, 763–769. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium-ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef]
- Acik, M.; Mattevi, C.; Gong, C.; Lee, G.; Cho, K.; Chhowalla, M.; Chabal, Y.J. The Role of Intercalated Water in Multilayered Graphene Oxide. ACS Nano 2010, 4, 5861–5868. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Solangi, N.H.; Mubarak, N.M.; Karri, R.R.; Mazari, S.A.; Koduru, J.R. Recent development of graphene and MXene-based nanomaterials for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2024, 73, 905–931. [Google Scholar] [CrossRef]
- Wakata, K.; Karim, M.R.; Islam, M.S.; Ohtani, R.; Nakamura, M.; Koinuma, M.; Hayami, S. Superionic Conductivity in Hybrid of 3-Hydroxypropanesulfonic Acid and Graphene Oxide. Chem. Asian J. 2016, 12, 194–197. [Google Scholar] [CrossRef]
- Shukla, G.; Pandey, R.P.; Shahi, V.K. Temperature resistant phosphorylated graphene oxide-sulphonated polyimide composite cation exchange membrane for water desalination with improved performance. J. Membr. Sci. 2016, 520, 972–982. [Google Scholar] [CrossRef]
- Ko, T.; Kim, K.; Lim, M.Y.; Nam, S.Y.; Kim, T.H.; Kim, S.K.; Lee, J.C. Sulfonated poly(arylene ether sulfone) composite membranes having poly(2,5-benzimidazole)-grafted graphene oxide for fuel cell applications. J. Mater. Chem. A 2015, 3, 20595–20606. [Google Scholar] [CrossRef]
- Cao, L.; Sun, Q.; Gao, Y.; Liu, L.; Shi, H. Novel acid-base hybrid membrane based on amine-functionalized reduced graphene oxide and sulfonated polyimide for vanadium redox flow battery. Electrochim. Acta 2015, 158, 24–34. [Google Scholar] [CrossRef]
- Dai, W.; Shen, Y.; Li, Z.; Yu, L.; Xi, J.; Qiu, X. SPEEK/Graphene oxide nanocomposite membranes with superior cyclability for highly efficient vanadium redox flow battery. J. Mater. Chem. A 2014, 2, 12423–12432. [Google Scholar] [CrossRef]
- He, Y.; Tong, C.; Geng, L.; Liu, L.; Lü, C. Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide. J. Membr. Sci. 2014, 458, 36–46. [Google Scholar] [CrossRef]
- Karim, M.R.; Takehira, H.; Matsui, T.; Murashima, Y.; Ohtani, R.; Nakamura, M.; Hayami, S. Graphene and Graphene Oxide as Super Materials. Curr. Inorg. Chem. 2014, 4, 191–219. [Google Scholar] [CrossRef]
- Kumar, R.; Mamlouk, M.; Scott, K. Sulfonated polyether ether ketone—Sulfonated graphene oxide composite membranes for polymer electrolyte fuel cells. RSC Adv. 2014, 4, 617–623. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Zhang, H.; Zhang, T.; Zhang, B.; Cao, S.; Liu, J. Polydopamine-modified graphene oxide nanocomposite membrane for proton exchange membrane fuel cell under anhydrous conditions. J. Mater. Chem. A 2014, 2, 9548–9558. [Google Scholar] [CrossRef]
- Pshenichnyuk, I.A.; Coto, P.B.; Leitherer, S.; Thoss, M. Charge Transport in Pentacene–Graphene Nanojunctions. J. Phys. Chem. Lett. 2013, 4, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Burzurí, E.; Island, J.O.; Díaz-Torres, R.; Fursina, A.; González-Campo, A.; Roubeau, O.; Teat, S.J.; Aliaga-Alcalde, N.; Ruiz, E.; van der Zant, H.S. Sequential Electron Transport and Vibrational Excitations in an Organic Molecule Coupled to Few-Layer Graphene Electrodes. ACS Nano 2016, 10, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Weckbecker, D.; Pedro, B.C.; Thoss, M. Controlling the conductance of a graphene molecule nanojunction by proton transfer. Nano Lett. 2017, 17, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Stefanucci, G.; Kurth, S. Steady-State Density Functional Theory for Finite Bias Conductances. Nano Lett. 2015, 15, 8020–8025. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ying, Z.; Xu, A.; Cheng, Y. Unraveling the Water-Mediated Proton Conduction Mechanism along the Surface of Graphene Oxide. Chem. Mater. 2020, 32, 6062–6069. [Google Scholar] [CrossRef]
- Bollella, P.; Ludwig, R.; Gorton, L. Cellobiose dehydrogenase: Insights on the nanostructuration of electrodes for improved development of biosensors and biofuel cells. Appl. Mater. Today 2017, 9, 319–332. [Google Scholar] [CrossRef]
- Ramesh, A.; Balakrishna, P.; Dhanaprabhu, S.S.; Ravanan, A.; Maniraj, J. Enzyme-modified electrodes for biofuel cells: A comprehensive review. Mater. Today Proc. 2021, 46, 3495–3501. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, D.; Haotian, Z.; Du, J.; Li, K.; Zang, H.Y.; Tan, H.; Wang, Y.; Xing, W.; Li, Y. A bismuth oxide/graphene oxide nanocomposite membrane showing super proton conductivity and low methanol permeability. Chem. Sci. 2019, 10, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Hu, Y.H. 3D graphene: Synthesis, properties, and solar cell applications. Chem. Commun. 2023, 59, 6660–6673. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, J.; Baumann, D.; Peng, L.; Xu, Y.; Shakir, I.; Huang, Y.; Duan, X. Hierarchical 3D electrodes for electrochemical energy storage. Nat. Rev. Mater. 2019, 4, 45–60. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Sun, H.; Gao, C. Superstructured Assembly of Nanocarbons: Fullerenes, Nanotubes, and Graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xing, M.; Zhang, J. Recent advances in three-dimensional graphene-based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Chang, D.; Xu, Z.; Gao, C. A Review on Graphene Fibers: Expectations, Advances, and Prospects. Adv. Mater. 2020, 32, 1902664–19026675. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, H. 3D Graphene Materials from the Reduction of CO2. Acc. Mater. Res. 2021, 2, 48–58. [Google Scholar] [CrossRef]
- Feng, H.P.; Tang, L.; Zeng, G.; Zhou, Y.; Deng, Y.; Ren, X.; Liang, C.; Wei, M.; Yu, J.F. Core-shell nanomaterials: Applications in energy storage and conversion. Adv. Colloid Interface Sci. 2019, 267, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, J.; Liu, S.; Zhang, Q.; Liu, Y.; Tan, Y.; Luo, S.; Guo, F.; Ma, J.; Li, P.; et al. Electrospinning of Neat Graphene Nanofibers. Adv. Fiber Mater. 2022, 4, 268–279. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, C.; Zou, Y.; Lianmg, J.; Cheng, Q.; Sun, L. Low-temperature synthesis of sea urchin-like Co-Ni oxide on graphene oxide for supercapacitor electrodes. J. Mater. Sci. Technol. 2020, 55, 223–230. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, S.; Hu, Y.H. 3D Graphene Materials: From Understanding to Design and Synthesis Control. Chem. Rev. 2020, 120, 10336–10453. [Google Scholar] [CrossRef]
- Shao, Z.; Ni, M. Fuel cells: Materials needs and advances. MRS Bull. 2024, 49, 451–463. [Google Scholar] [CrossRef]
- Finnerty, C.T.K.; Menon, A.; Conway, K.; Lee, D.; Nelson, M.; Urban, J.J.; Sedlak, D.; Mi, B. Interfacial Solar Evaporation by a 3D Graphene Oxide Stalk for Highly Concentrated Brine Treatment. Environ. Sci. Technol. 2021, 55, 15435–15445. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, M.S.; Fukuda, M.; Yagyu, J.; Feng, Z.; Sekine, Y.; Lindoy, L.F.; Ohyama, J.; Hayami, S. High Proton Conductivity of 3D Graphene Oxide Intercalated with Aromatic Sulfonic Acids. Chem. Plus Chem. 2022, 87, e202200003. [Google Scholar]
- Youssef, M.E.; Amin, R.S.; El-Khatib, K.M. Development and performance analysis of PEMFC stack based on bipolar plates fabricated employing different designs. J. Chem. 2018, 11, 609–614. [Google Scholar] [CrossRef]
- Wei, Y.; Qian, T.; Liu, J.; Guo, X.; Gong, Q.; Liu, Z.; Tian, B.; Qiao, J. Novel composite Nafion membranes modified with copper phthalocyanine tetrasulfonic acid tetrasodium salt for fuel cell application. J. Mater. 2019, 5, 252–257. [Google Scholar] [CrossRef]
- Yin, B.; Liang, R.; Liang, X.; Fu, D.; Wang, L.; Sun, G. Construction of Stable Wide-Temperature-Range Proton Exchange Membranes by Incorporating a Carbonized Metal–Organic Frame into Polybenzimidazoles and olyacrylamide Hydrogels. Small 2021, 17, 2103214. [Google Scholar] [CrossRef]
- Zhou, Y.; Clive, H.Y.; Hu, Y.H.; Wang, C.; Cheng, X.; Wai, C.M.; Yang, M.; Lin, Y. Making ultrafine and highly-dispersive multimetallic nanoparticles in three-dimensional graphene with supercritical fluid as excellent electrocatalyst for oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 18628–18638. [Google Scholar] [CrossRef]
- Peng, J.; Huang, J.; Jiang, C.; Xu, Y.W.; Wu, X.L.; Li, X. Generalized spatial–temporal fault location method for solid oxide fuel cells using LSTM and causal inference. IEEE Trans. Transp. Electrif. 2022, 8, 4583–4594. [Google Scholar] [CrossRef]
- Golkhatmi, S.Z.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools, Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shukla, G.; Manohar, M.; Shahi, V.K. Graphene oxide based nanohybrid proton exchange membranes for fuel cell applications: An overview. Adv. Colloid Interface Sci. 2017, 240, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, K.; Karim, M.R.; Ogata, C.; Tateishi, H.; Taniguchi, T.; Koinuma, M.; Hayami, S.; Matsumoto, Y. Optimization of proton conductivity in graphene oxide by filling sulfate ions. Chem. Commun. 2014, 50, 14527–14530. [Google Scholar] [CrossRef] [PubMed]
- Wakata, K.; Islam, M.S.; Karim, M.R.; Hatakeyama, K.; Rabin, N.N.; Ohtani, R.; Nakamura, M.; Koinuma, M.; Hayami, S. Role of hydrophilic groups in acid intercalated graphene oxide as a superionic conductor. RSC Adv. 2017, 7, 21901–21905. [Google Scholar] [CrossRef]
- Shudo, Y.; Karim, M.R.; Ohtani, M.; Nakamura, M.; Hayami, S. Hybrids from the π−π Stacking of Graphene Oxide and Aromatic Sulfonic Compounds for Improved Proton Conductivity. ChemElectroChem 2018, 5, 238–241. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Matsui, T.; Takehira, H.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T.; Nakamura, T.; Noro, S.; et al. Graphene Oxide Nanosheet with High Proton Conductivity. J. Am. Chem. Soc. 2013, 135, 8097–8100. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and its electrochemistry—An update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.K.; Chen, C.L.; Chen, Q.W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2014, 43, 2841–2857. [Google Scholar] [CrossRef] [PubMed]
- Poh, H.L.; Šimek, P.; Sofer, Z.; Tomandl, I.; Pumera, M. Boron and nitrogen doping of graphene via thermal exfoliation of graphite oxide in a BF3 or NH3 atmosphere: Contrasting properties. J. Mater. Chem. A 2013, 1, 13146–13153. [Google Scholar] [CrossRef]
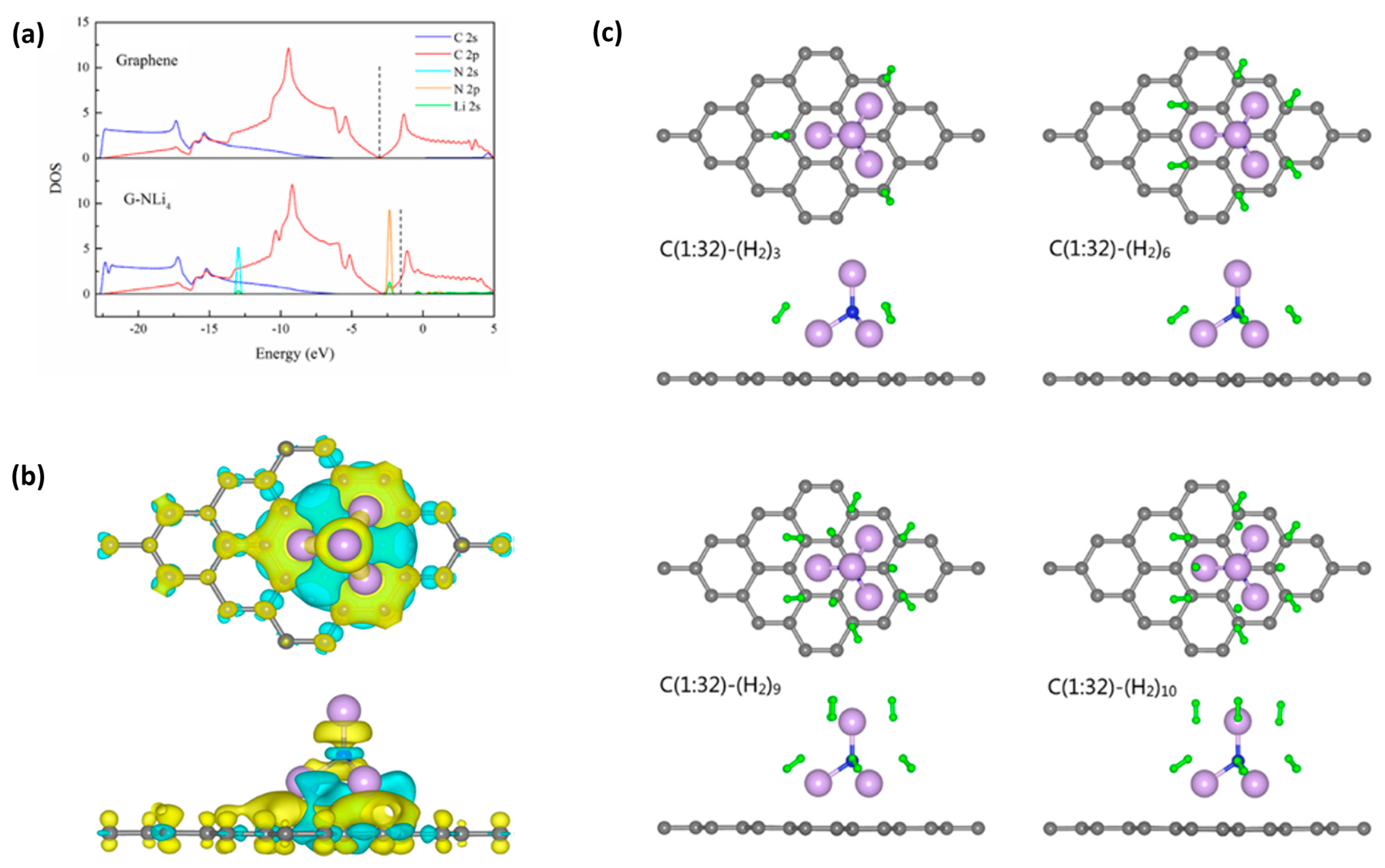
- Qi, H.; Wang, X.; Chen, H. Superalkali NLi4 decorated graphene: A promising hydrogen storage material with high reversible capacity at ambient temperature. Int. J. Hydrogen Energy 2021, 46, 232254–232262. [Google Scholar] [CrossRef]
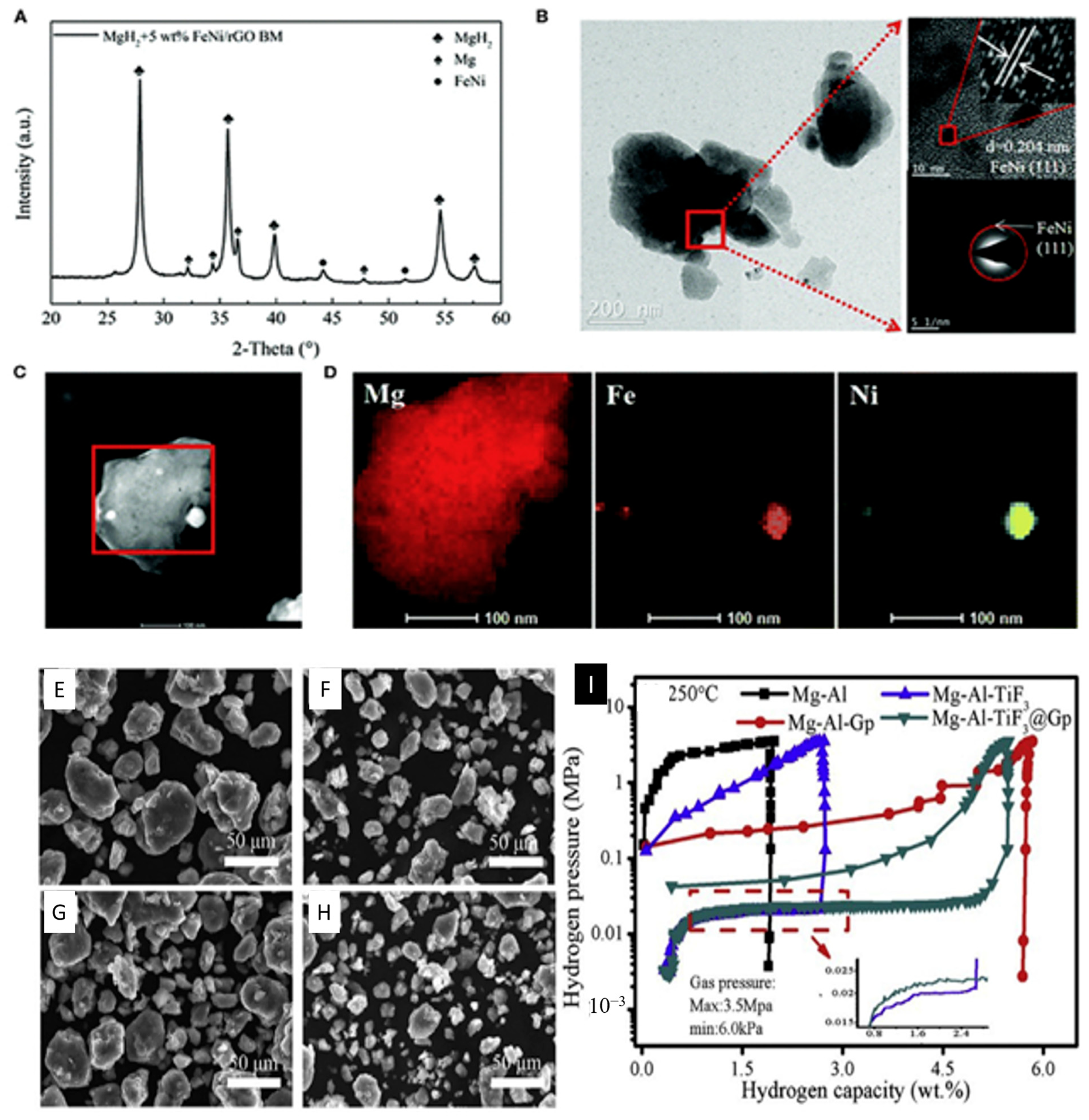
- Feng, D.; Zhou, D.; Zhao, Z.; Zhai, T.; Yuan, Z.; Sun, H.; Ren, H.; Zhang, Y. Progress of graphene and loaded transition metals on Mg-based hydrogen storage alloys. Int. J. Hydrogen Energy 2021, 46, 33468–33485. [Google Scholar] [CrossRef]
- Dong, S.; Lv, E.; Wang, J.; Li, C.; Ma, K.; Gao, Z.; Yang, W.; Ding, Z.; Wu, C.; Gates, I.D. Construction of transition metal-decorated boron doped twin-graphene for hydrogen storage: A theoretical prediction. Fuel 2021, 304, 121351–121359. [Google Scholar] [CrossRef]
- Samantaray, S.S.; Sangeetha, V.; Abinaya, S.; Ramaprabhu, S. Diatom frustule-graphene based nanomaterial for room temperature hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 764–773. [Google Scholar] [CrossRef]
- Bonab, S.S.Y.; Kouzehgar, H.; Tabrizi, A.T.; Aghajani, H. Assessment of the effect of electrophoretic deposition parameters on hydrogen storage performance of graphene oxide layer applied on nickel foam. Int. J. Hydrogen Energy 2022, 47, 2491–2499. [Google Scholar] [CrossRef]
- Fan, X.; Yuan, W.; Zhang, D.H.; Li, C.M. Heteropolyacid-mediated self-assembly of heteropolyacid-modified pristine graphene supported Pd nanoflowers for superior catalytic performance toward formic acid oxidation. ACS Appl. Energy Mater. 2018, 1, 411–420. [Google Scholar] [CrossRef]
- Feng, J.J.; Chen, S.S.; Chen, X.L.; Zhang, X.F.; Wang, A.J. One-pot fab rication of reduced graphene oxide supported dendritic core shell gold@gold-palladium nanoflowers for glycerol oxidation. J. Colloid Interface Sci. 2018, 509, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Benipal, N.; Wang, H.; Chadderdon, D.J.; Jiang, Y.; Wei, W.; Hu, Y.H.; Li, W. Metal-catalyst-free carbohy drazide fuel cells with three-dimensional graphene anodes. ChemSusChem 2015, 8, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Ahmad, K.; Li, H.; Gassoumi, A.; Raza, R.; Saleem, M.; Jafri, S.H.M.; Abbas, G. Synergistic Electrochemical Properties of Graphene Incorporated LCZ-Oxide Cathode for Low Temperature Solid Oxide Fuel Cell. Crystals 2023, 13, 434–445. [Google Scholar] [CrossRef]
- Ahmad, K.; Ahmad, M.A.; Raza, R.; Khan, M.A.; Abbas, G. Synthesis and electrical characterizations of graphene oxide incorporated nanocomposite cathode materials for low temperature SOFCs. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
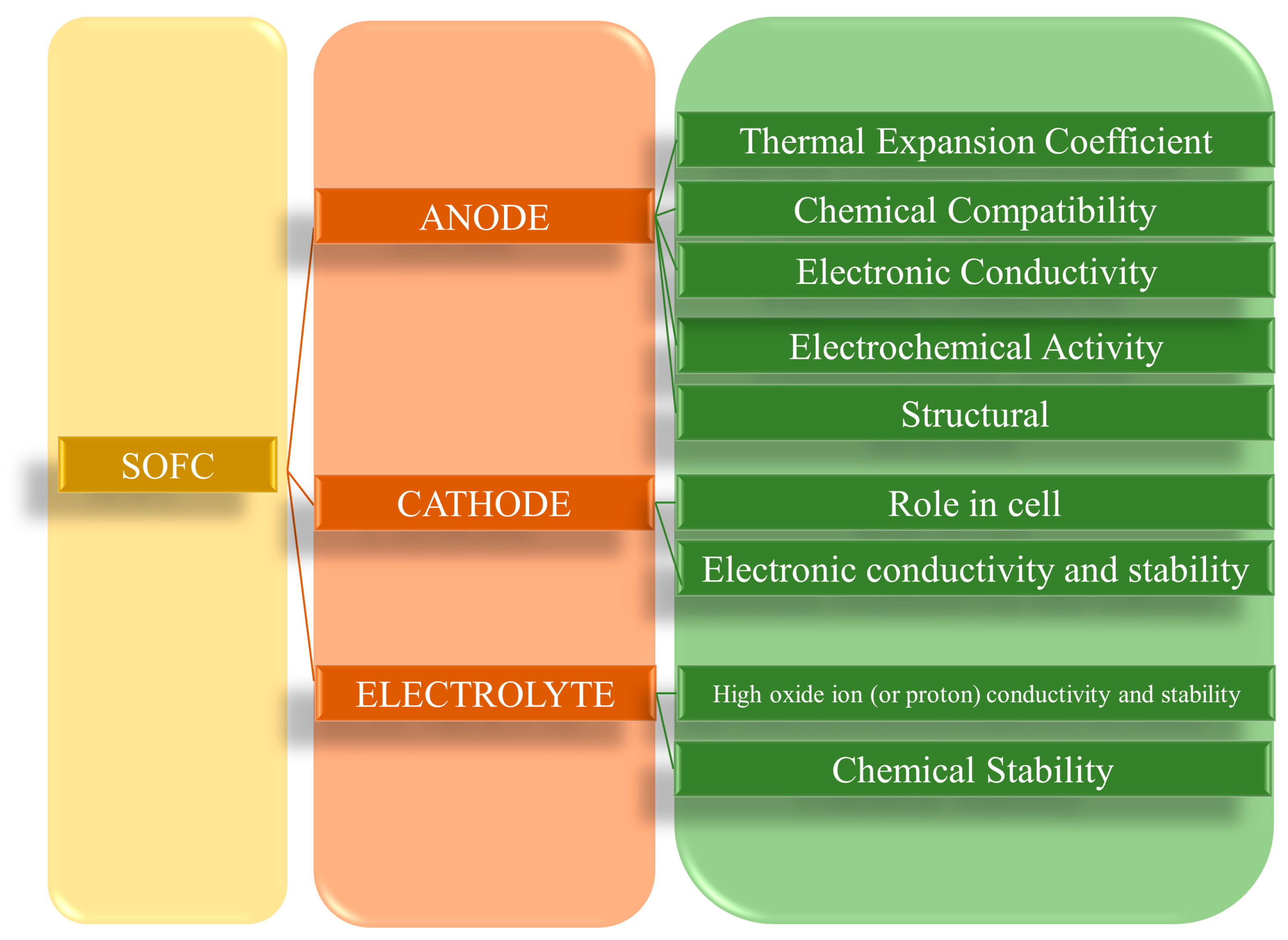
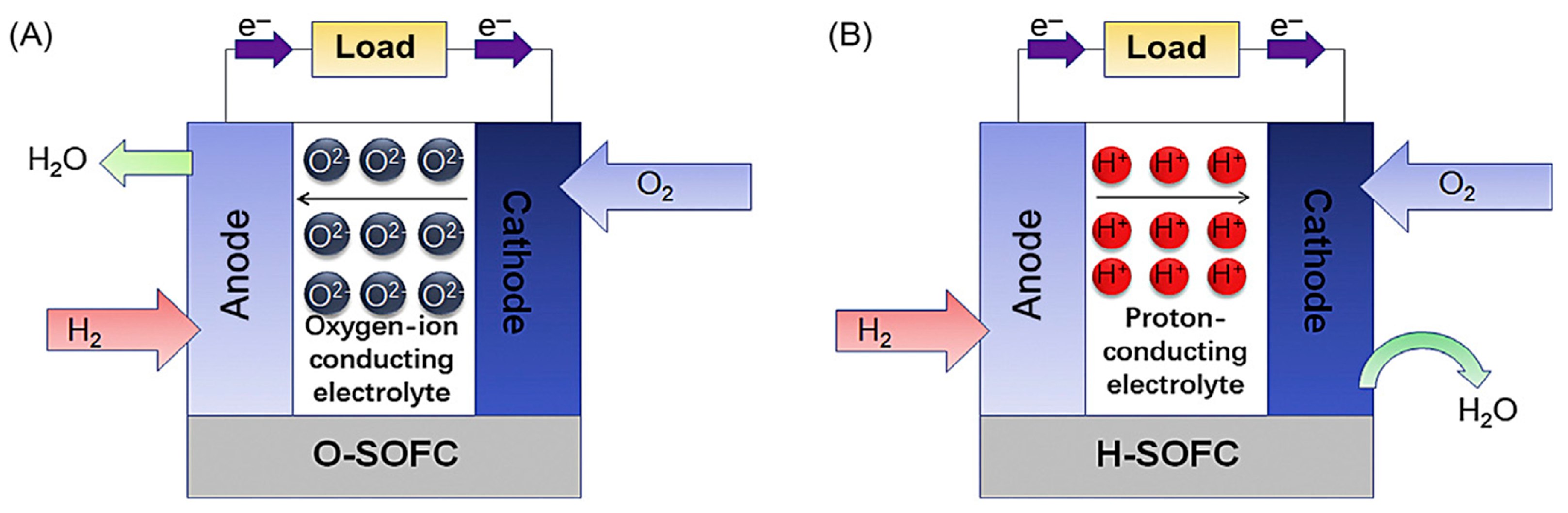


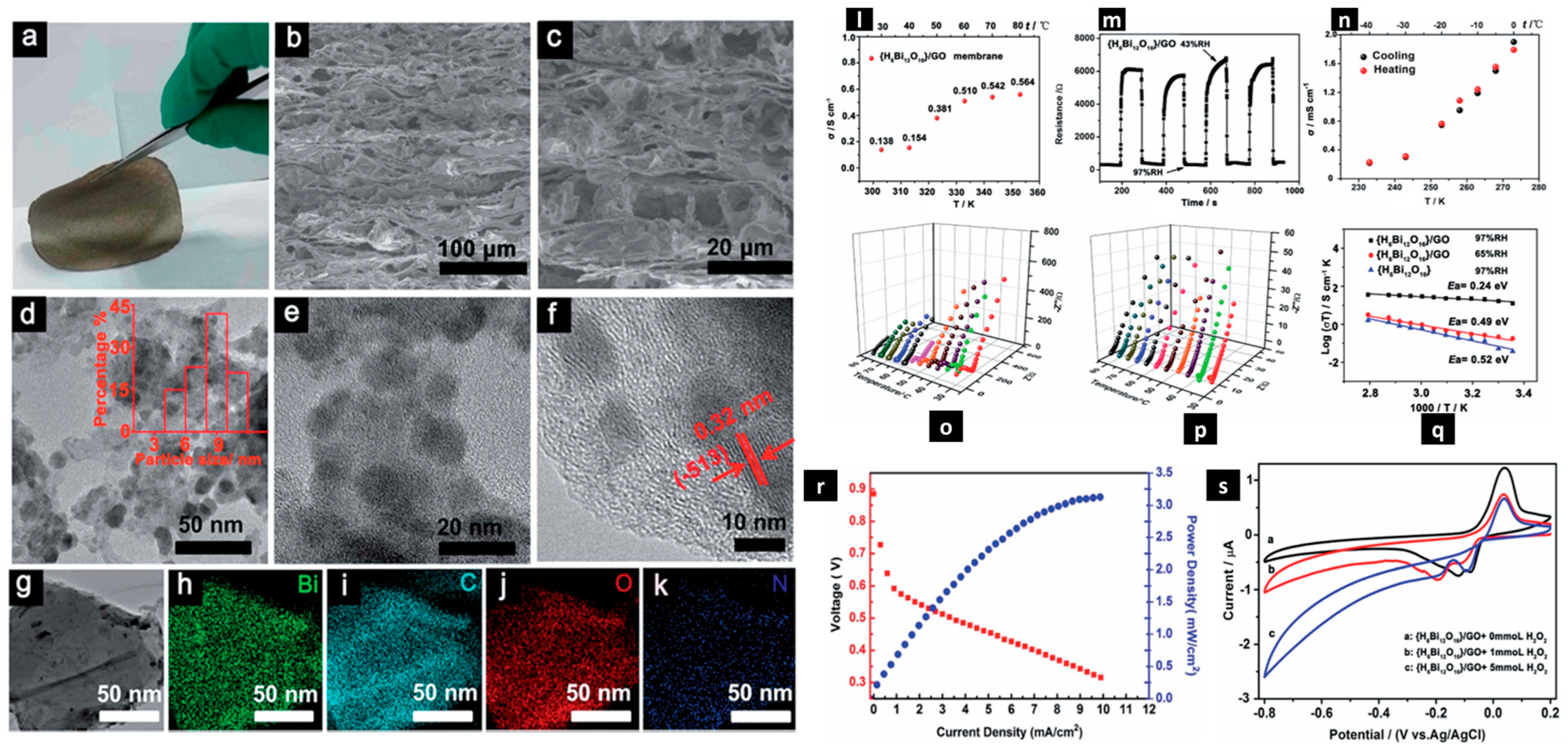

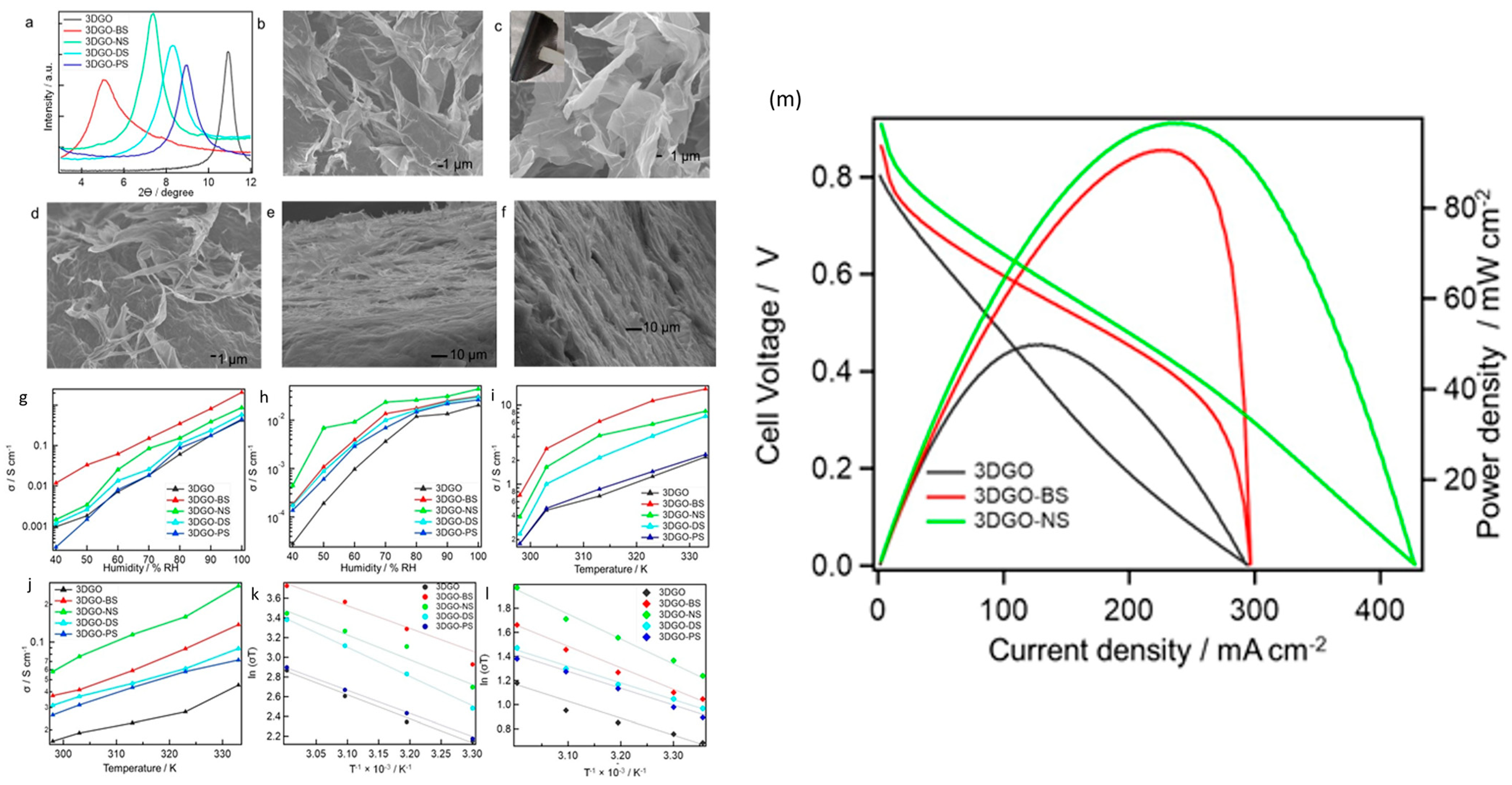

| Fuel Cell Type | Power Range | Temperature Range (°C) | Typical Fuel | Electrolyte Type | Efficiency | Life Span (h) | Ref. |
|---|---|---|---|---|---|---|---|
| SOFC | ≤1 MW (up to 250 kW) | 500–1000 | Hydrogen, Methanol, Hydrocarbons | Porous Ceramic Material | 50–60% | 20,000–80,000 | [10] |
| PEMFC | ≤1 MW (up to 200 kW) | 110–180 | Hydrogen | Water-based Polymer Membrane | 45–55% | 60,000–80,000 | [10] |
| PAFC | ≤11 MW (100–400 kW) | 150–220 | Hydrogen, LNG, Methanol | Phosphoric Acid | 30–42% | 40,000–60,000 | [11] |
| AFC | ≤500 kW (up to 200 kW) | 60–200 | Hydrogen | Potassium Hydroxide | 40–50% | 5000–8000 | [12] |
| MCFC | ≤1 MW (up to 250 kW) | 650–800 | Hydrogen, Methanol, Hydrocarbons | Molten Carbonate Salt | 43–55% | 15,000–30,000 | [11] |
| Graphene-Based Cathode Material | Performance | Ref. |
|---|---|---|
| NiLi4 graphene | storage capacity of hydrogen, high reversibility of hydrogen, 10.75 wt% hydrogen storage | [144] |
| Graphene-catalyzed Mg-based hydrogen storage alloys | high energy density, high hydrogen storage capacity, fast hydrogen uptake and discharge kinetics, low thermodynamic stability | [145] |
| Boron-doped twin graphene | improved hydrogen storage capacity (gravimetric density of 4.95 wt%) | [146] |
| Au@AuPd-rGO | enhanced electrocatalytic activity and durability | [150] |
| LCZ oxide graphene | power density of 2675 W m−2 | [152] |
| GO La0.3Sr0.7Fe0.4Ti0.6O3-δ | power density of 362 mWcm−2, specific resistance of 0.02 × 10−4 Ωm2 | [153] |
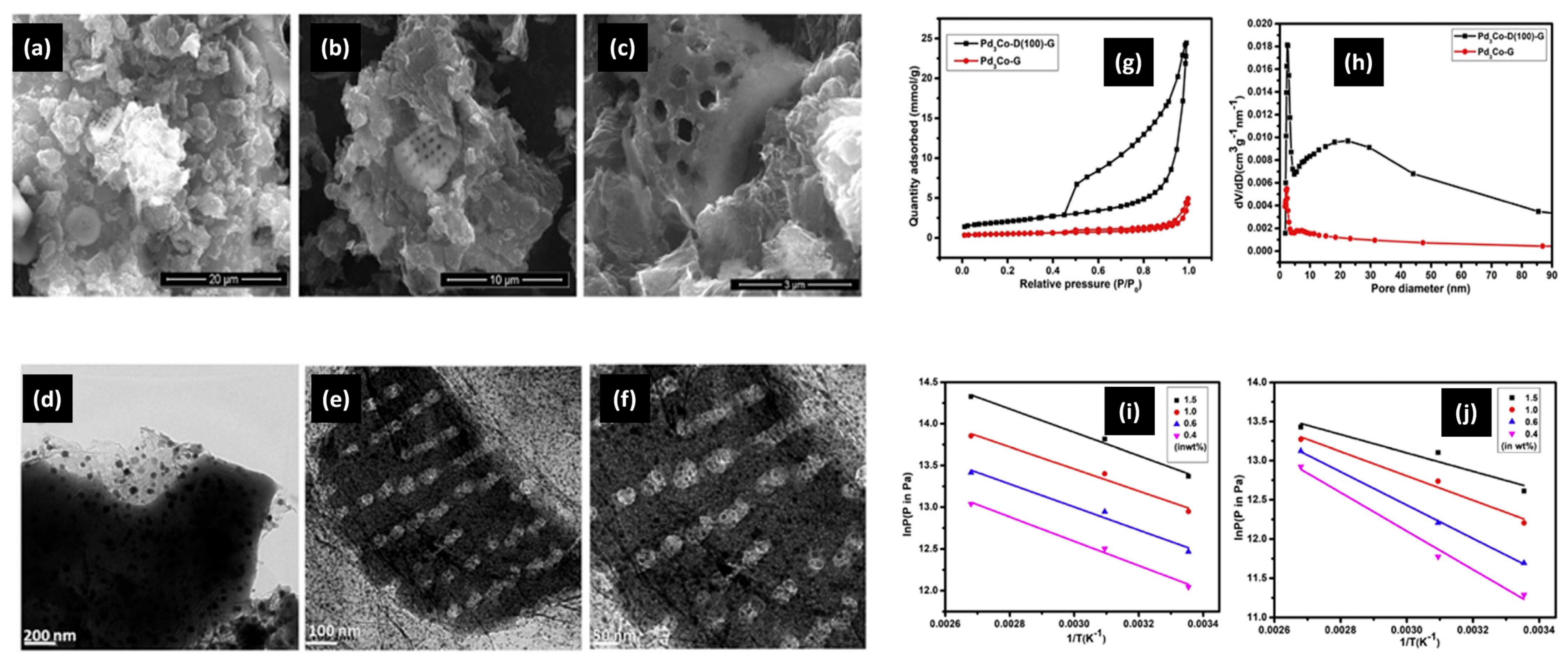
| Pd3Co-D(100)-G | pore volume of 0.84 × 10−6 m3g−1, surface area of 163.25 m2g−1 | [147] |
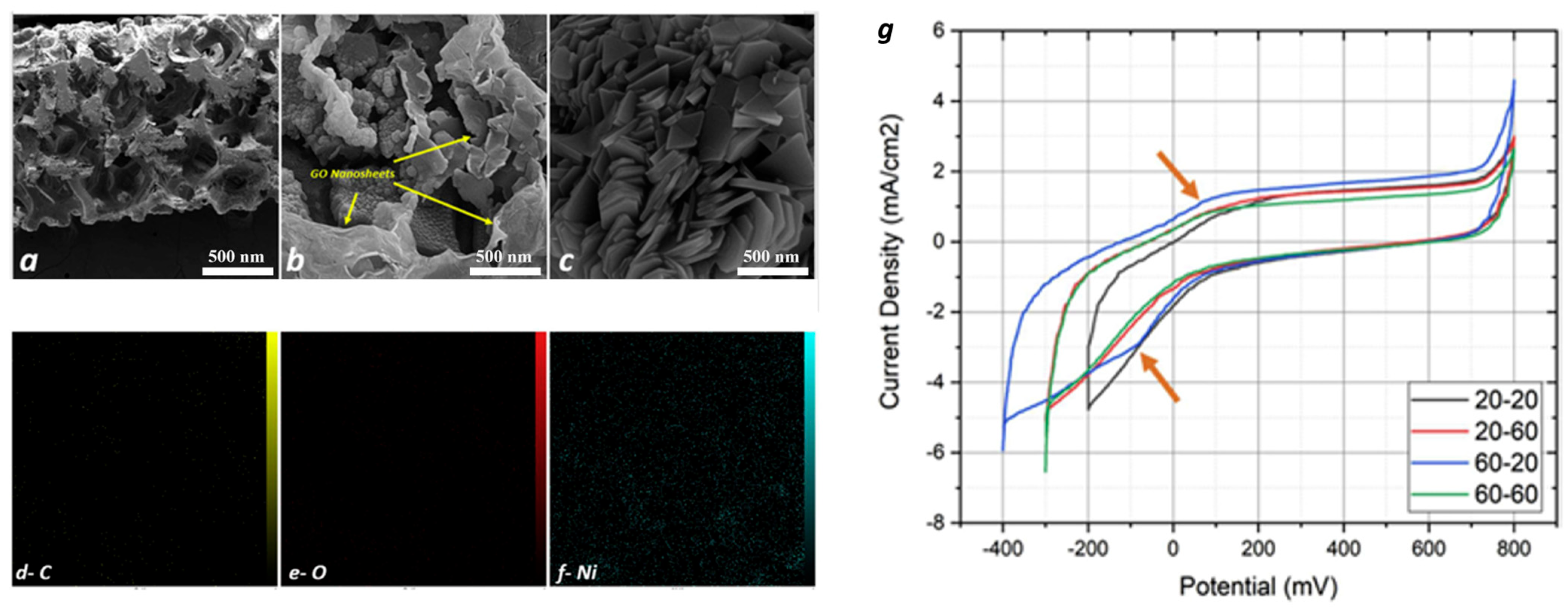
| GO Ni foam | hydrogen storage capacity of 50.9 Ah kg−1 | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samantaray, S.; Mohanty, D.; Satpathy, S.K.; Hung, I.-M. Exploring Recent Developments in Graphene-Based Cathode Materials for Fuel Cell Applications: A Comprehensive Overview. Molecules 2024, 29, 2937. https://doi.org/10.3390/molecules29122937
Samantaray S, Mohanty D, Satpathy SK, Hung I-M. Exploring Recent Developments in Graphene-Based Cathode Materials for Fuel Cell Applications: A Comprehensive Overview. Molecules. 2024; 29(12):2937. https://doi.org/10.3390/molecules29122937
Chicago/Turabian StyleSamantaray, Somya, Debabrata Mohanty, Santosh Kumar Satpathy, and I-Ming Hung. 2024. "Exploring Recent Developments in Graphene-Based Cathode Materials for Fuel Cell Applications: A Comprehensive Overview" Molecules 29, no. 12: 2937. https://doi.org/10.3390/molecules29122937
APA StyleSamantaray, S., Mohanty, D., Satpathy, S. K., & Hung, I.-M. (2024). Exploring Recent Developments in Graphene-Based Cathode Materials for Fuel Cell Applications: A Comprehensive Overview. Molecules, 29(12), 2937. https://doi.org/10.3390/molecules29122937









