High-Yield Production of Dihydroxyacetone from Crude Glycerol in Fed-Batch Cultures of Gluconobacter oxydans
Abstract
1. Introduction
2. Results
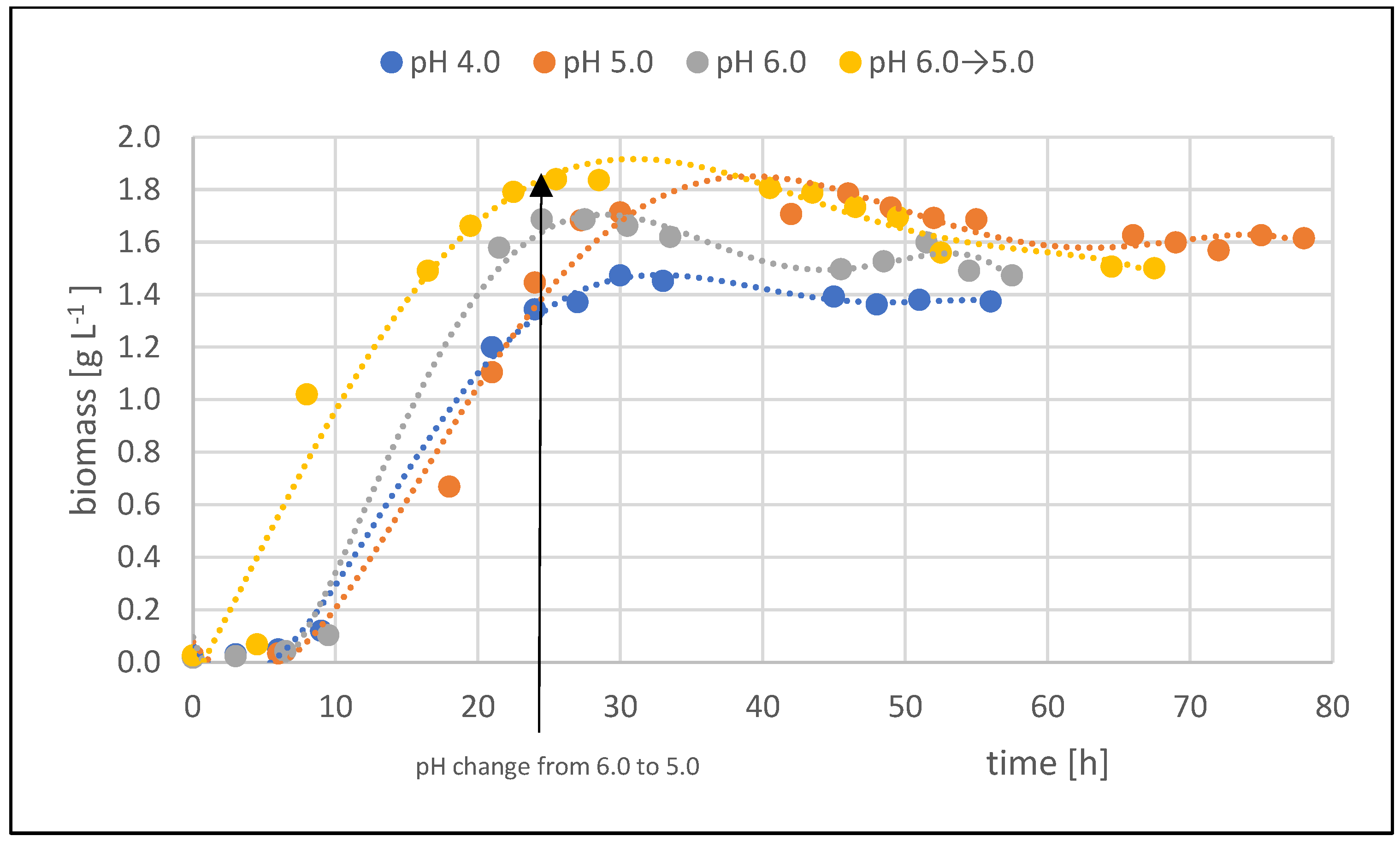
2.1. The Influence of the pH of the Culture Medium on DHA Production
2.2. The Influence of Crude Glycerol Concentration on the Efficiency of Obtaining Dihydroxyacetone by Bioconversion
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Culture Media
4.2. Crude Glycerol
4.3. Preparation of Inoculum
4.4. Bioreactor Process
4.5. Analytical Methods
4.6. Calculation of Kinetic Parameters of Culture
4.7. Statistical Analysis
5. Conclusions
- -
- Crude glycerol is a valuable raw material for obtaining DHA by bioconversion using the Gluconobacter oxydans LMG 1385.
- -
- The pH of the culture medium significantly impacted the efficiency of the DHA production process using the Gluconobacter oxydans LMG 1385. The optimal pH of the medium during fed-batch cultures was 5.0.
- -
- The most suitable method for producing DHA from crude glycerol generated in biodiesel production is fed-batch cultivation of the Gluconobacter oxydans LMG 1385 with the feed based on the level of oxygen saturation of the culture medium. However, a change in the control value is required during the bioconversion stage.
- -
- In bioconversion of crude glycerol to DHA, three stages can be distinguished: multiplication of bacterial biomass, initiation of glycerol to DHA, and intensive bioconversion of glycerol to DHA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD-FAO. Agricultural Outlook 2018–2027; OECD/FAO: Paris, France, 2018. [Google Scholar]
- Meesters, P.A.E.P.; Huijberts, G.N.M.; Eggink, G. High-cell-density cultivations of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Wen, Z.; Pyle, D.J.; Athalye, S.K. Glycerol waste from biodiesel manufacturing. In Microbial Conversions of Raw Glycerol; Aggelis, G., Ed.; Nova Science: New York, NY, USA, 2009; pp. 1–7. [Google Scholar]
- Schieck, S.J.; Shurson, G.C.; Kerr, B.J.; Johnston, L.J. Evaluation of glycerol, a biodiesel coproduct, in grow-finish pig diets to support growth and pork quality. J. Anim. Sci. 2010, 88, 3927–3935. [Google Scholar] [CrossRef]
- Bohon, M.D.; Metzger, B.A.; Linak, W.P.; King, C.J.; Roberts, W.L. Glycerol combustion and emissions. Proc. Combust. Inst. 2011, 33, 2717–2724. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Kumar, R.P.; Elavazhagan, S.; Barathiraja, B.; Jayakumar, M.; Varjani, S.J. Utilization of Crude Glycerol from Biodiesel Industry for the Production of Value-Added Bioproducts. In Waste to Wealth, Energy, Environment, and Sustainability; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 69–70. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Marcinkiewicz, M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol. Lett. 2009, 31, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Habe, H.; Shimada, Y.; Fukuoka, T.; Kitamoto, D.; Itagaki, M.; Watanabe, K.; Yanagishita, H.; Sakaki, K. Production of Glyceric Acid by Gluconobacter sp. NBRC3259 Using Raw Glycerol. Biosci. Biotechnol. Biochem. 2009, 73, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Rymowicz, W. Continuous production of citric acid from raw glycerol by Yarrowia lipolytica in cell recycle cultivation. Chem. Pap. 2011, 65, 119–123. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour. Technol. 2002, 82, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Belser, E.; Zülli, F. Self-tanning Based on Stimulation of Melanin Biosynthesis. Cosmet. Toilet. 2007, 122, 55–62. [Google Scholar]
- Choquenet, B.; Couteau, C.; Paparis, E.; Coiffard, L.J.M. Foundations and self-tanning products: Do they provide any protection from the sun? J. Dermatol. 2009, 36, 587–591. [Google Scholar] [CrossRef]
- Misterska, M.; Szulczyńska-Gabor, J.; Żaba, R. Etiopatogeneza, obraz kliniczny i leczenie bielactwa. Post. Dermatol. Alergol. 2009, 4, 212–223. [Google Scholar]
- Obeid, O.A.; Jamal, Z.M.; Hwalla, N.; Emery, P.W. The effect of glutamine and dihydroxyacetone supplementation on food intake, weight gain, and postprandial glycogen synthesis in female Zucker rats. Nutrition 2006, 22, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Ślepokura, K.; Lis, T. Crystal structures of dihydroxyacetone and its derivatives. Carbohydr. Res. 2004, 339, 1995–2007. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Jain, S.R.; Kumar, A. Microbial production of dihydroxyacetone. Biotechnol. Adv. 2008, 26, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.R.; Zawaneh, P.N.; Putnam, D. Poly(carbonate-ester)s of Dihydroxyacetone and Lactic Acid as Potential Biomaterials. Biomacromolecules 2011, 12, 977–986. [Google Scholar] [CrossRef]
- Liu, Y.P.; Sun, Y.; Tan, C.; Li, H.; Zheng, X.J.; Jin, K.Q.; Wang, G. Efficient production of dihydroxyacetone from biodiesel-derived crude glycerol by newly isolated Gluconobacter frateurii. Bioresour. Technol. 2013, 142, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Liebminger, S.; Hofbauer, R.; Siebenhofer, M.; Nyanhongo, G.S.; Guebitz, G.M. Microbial conversion of crude glycerol to dihydroxyacetone. Waste Biomass Valor. 2014, 5, 781–787. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Moholkar, V.S. Optimization of 1,3-dihydroxyacetone production from crude glycerol by immobilized Gluconobacter oxydans MTCC 904. Bioresour. Technol. 2016, 216, 1058–1065. [Google Scholar] [CrossRef]
- Zheng, X.; Jin, K.; Zhang, L.; Liu, Y. Effects of oxygen transfer coefficient on dihydroxyacetone production from crude glycerol. Braz. J. Microbiol. 2016, 47, 129–135. [Google Scholar] [CrossRef]
- Stasiak-Różańska, L.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A.; Lipińska, E. Utilization of a waste glycerol fraction using and reusing immobilized Gluconobacter oxydans ATCC 621 cell extract. Electron. J. Biotechnol. 2017, 27, 44–48. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Moholkar, V.S. Kinetic analysis of dihydroxyacetone production from crude glycerol by immobilized Gluconobacter oxydans MTCC 904. Bioresour. Technol. 2016, 216, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Padhi, S.K.; Moholkar, V.S. Process optimization and analysis of product inhibition kinetics of crude glycerol fermentation for 1,3-dihydroxyacetone production. Bioresour. Technol. 2017, 244, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.C.; Zheng, Y.G.; Shen, Y.C. Dissolved-oxygen-stat fed-batch fermentation of 1,3-dihydroxyacetone from glycerol by Gluconobacter oxydans ZJB09112. Biotechnol. Bioprocess Eng. 2010, 15, 651–656. [Google Scholar] [CrossRef]
- Hu, Z.C.; Zheng, Y.G. Enhancement of 1,3-dihydroxyacetone production by a UV-induced mutant of Gluconobacter oxydans with DO control strategy. Appl. Biochem. Biotechnol. 2011, 165, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Albin, A.; Bader, J.; Mast-Gerlach, E.; Stahl, E. Improving fermentation and biomass formation of Gluconobacter oxydans. J. Biotech. 2007, 131, 133–187. [Google Scholar] [CrossRef]
- Bauer, R. Optimierung der Mikrobiellen Herstellung von Dihydroxyaceton. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2005. [Google Scholar]
- Bauer, R.; Hekmat, D. Development of a transient segregated mathematical model of the semi continuous microbial production process of dihydroxyacetone. Biotechnol. Prog. 2006, 22, 278–284. [Google Scholar] [CrossRef]
- Hekmat, D.; Bauer, R.; Neff, V. Optimization of the microbial synthesis of dihydroxyacetone in a semi-continuous repeated-fed-batch process by in situ immobilization of Gluconobacter oxydans. Process Biochem. 2007, 42, 71–76. [Google Scholar] [CrossRef]
- Ripoll, M.; Jackson, E.; Trelles, J.A.; Betancor, L. Dihydroxyacetone production via heterogeneous biotransformations of crude glycerol. J. Biotechnol. 2021, 47, 102–109. [Google Scholar] [CrossRef]
- Liu, X.; Jensen, P.R.; Workman, M. Bioconversion of crude glycerol feedstocks into ethanol by Pachysolen tannophilus. Bioresour. Technol. 2012, 104, 579–586. [Google Scholar] [CrossRef]
- Hu, Z.C.; Liu, Z.Q.; Zheng, Y.G.; Shen, Y.C. Production of 1,3 dihydroxyacetone from glycerol by Gluconobacter oxydans ZJB09112. J. Microbiol. Biotechnol. 2010, 20, 340–345. [Google Scholar] [CrossRef]
- Zeng, W.; Shan, X.; Liu, L.; Zhou, J. Efficient 1,3-dihydroxyacetone biosynthesis in Gluconobacter oxydans using metabolic engineering and a fed-batch strategy. Bioresour. Bioprocess. 2022, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Stasiak-Różańska, L.; Błażejak, S. Production of dihydroxyacetone from an aqueous solution of glycerol in the reaction catalyzed by immobilized cell preparation of acetic acid bacteria Gluconobacter oxydans ATCC 621. Eur. Food Res. Technol. 2012, 235, 1125–1132. [Google Scholar] [CrossRef]
- Navrátil, M.; Tkáč, J.; Švitel, J.; Danielsson, B.; Šturdik, E. Monitoring of the bioconversion of glycerol to dihydroxyacetone with immobilized Gluconobacter oxydans cell using thermometric flow injection analysis. Process Biochem. 2001, 36, 1045–1052. [Google Scholar] [CrossRef]
- Raška, J.; Skopal, F.; Komers, K.; Machek, J. Kinetics of glycerol biotransformation to dihydroxyacetone by immobilized Gluconobacter oxydans and effect of reaction conditions. Collect. Czech Chem. Commun. 2007, 72, 1269–1283. [Google Scholar] [CrossRef]
- Li, M.; Wu, J.; Liu, X.; Lin, J.; Wei, D.; Chen, H. Enhanced production of dihydroxyacetone from glycerol by over expression of glycerol dehydrogenase in an alcohol-deficient mutant of Gluconobacter oxydans. Bioresour. Technol. 2010, 101, 8294–8299. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, D.; Bauer, R.; Fricke, J. Optimization of the microbial synthesis of dihydroxyacetone from glycerol with Gluconobacter oxydans. Bioprocess. Biosyst. Eng. 2003, 26, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Katsikis, N.; Varga, S.; Hekmat, D. Study of the inhibitory effect of the product dihydroxyacetone on Gluconobacter oxydans in a semi continuous two-stage repeated-fed-batch process. Bioprocess. Biosyst. 2005, 5, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.C.; Zheng, Y.G.; Shen, Y.C. Use of glycerol for producing 1,3 dihydroxyacetone by Gluconobacter oxydans in an airlift bioreactor. Bioresour. Technol. 2011, 102, 7177–7182. [Google Scholar] [CrossRef]
- PN-EN 14110; Produkty Przetwarzania Olejów i Tłuszczów—Estry Metylowe Kwasów Tłuszczowych (FAME)—Oznaczanie Zawartości Metanolu, KT 92 Nasiona Roślin Oleistych, Tłuszczów Roślinnych i Zwierzęcych oraz ich Produktów Ubocznych. Comite Europeen de Normalisation: Warszawa, Poland, 2004.
- European Pharmacopoeia; Council of Europe, Stationery Office: Strasburg, France, 2008.
- ISO 2464; Crude Glycerine for Industrial Use—Calculation of Matter (Organic) Non-Glycerol (MONG). ISO: London, UK, 1973.
- AOCS Surplus Method Ea 2-38; Ash, Alkalinity or Acidity and Sodium Chloride. AOCS: Champaign, IL, USA, 1973.
- ISO 12937; Petroleum Products—Determination of Water—Coulometric Kart Fischer Titration Method. International Organization for Standardization: Geneva, Switzerland, 2000.
- Tkáč, J.; Navrátil, M.; Šturdík, E.; Gemeiner, P. Monitoring of dihydroxyacetone production during oxidation of glycerol by immobilized Gluconobacter oxydans cells with an enzyme biosensor. Enzyme Microb. Technol. 2001, 28, 383–388. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Zhou, C. HPLC Methods for Determination of Dihydroxyacetone and Glycerol in Fermentation Broth and Comparison with a Visible Spectrophotometric Method to Determine Dihydroxyacetone. J. Chromatogr. Sci. 2008, 46, 912–916. [Google Scholar] [CrossRef]
- Leśniak, W. Badania Nad Fermentacją Kwasu Cytrynowego Metodą Wgłębną. Ph.D. Thesis, WSE-WSR, Wroclaw-Poznań, Poland, 1972. [Google Scholar]


| Symbols | Unit | Parameters | Set the pH Value of the Culture Medium | |||
|---|---|---|---|---|---|---|
| 4.0 | 5.0 | 6.0 | 6.0 → 5.0 | |||
| Set parameters | ||||||
| t | h | Cultivation time | 51 | 69 | 54.5 | 67.5 |
| S0 | g·L−1 | Initial concentration of crude glycerol in the culture medium | 70.0 | 70.0 | 70.0 | 70.0 |
| Result parameters | ||||||
| SK | g·L−1 | The final concentration of crude glycerol in the culture medium | 37.8 ± 1.13 | 0.0 | 53.1 ± 1.59 | 31.2 ± 0.93 |
| RS | g·L−1·h−1 | Average volumetric consumption rate of crude glycerol | 2.90 ± 0.09 | 2.7 ± 0.080 | 2.13 ± 0.06 | 2.72 ± 0.08 |
| XK | g·L−1 | Final biomass concentration in the culture medium | 1.38 ± 0.04 | 1.60 ± 0.05 | 1.49 ± 0.04 | 1.51 ± 0.04 |
| RX | g·L−1·h−1 | Average volumetric growth rate of biomass | 0.03 ± 0.001 | 0.02 ± 0.001 | 0.03 ± 0.001 | 0.02 ± 0.001 |
| PK | g·L−1 | Final DHA concentration in the culture medium | 118.9 ± 3.50 | 175.8 ± 5.20 | 91.6 ± 2.70 | 138.8 ± 4.16 |
| RP | g·L−1·h−1 | Average volumetric rate of bioconversion of crude glycerol to DHA | 2.33 ± 0.07 | 2.55 ± 0.07 | 1.68 ± 0.05 | 2.06 ± 0.06 |
| QP | g·g−1·h−1 | The average specific rate of bioconversion of crude glycerol to DHA | 1.69 ± 0.05 | 1.59 ± 0.04 | 1.13 ± 0.03 | 1.37 ± 0.04 |
| YX/Sw | % (m/m) | Total biomass yield | 0.7 ± 0.012 | 0.9 ± 0.026 | 0.9 ± 0.027 | 0.7 + 0.022 |
| YP/Sw | % (m/m) | Total DHA yield | 64.1 ± 1.92 | 94.3 ± 2.80 | 54.2 ± 1.62 | 64.7 ± 1.94 |
| Kef | g·L−1·h−1 | DHA production efficiency factor | 1.5 | 2.4 | 0.9 | 1.3 |
| Symbols | Unit | Parameters | Initial Concentration of Crude Glycerol in Fed-Batch Cultures | ||||
|---|---|---|---|---|---|---|---|
| 40.0 | 55.0 | 70.0 | 85.0 | 100.0 | |||
| Set parameters | |||||||
| t | h | Cultivation time | 48 | 44 | 69 | 45 | 69 |
| tZ | h | Start time of crude glycerol feeding | 15 | 20 | 26.75 | 27 | 24 |
| Result parameters | |||||||
| SK | g·L−1 | The final concentration of crude glycerol in the culture medium | 33.4 ± 1.00 | 14.3 ± 0.41 | 0.0 | 3.2 ± 0.09 | 7.8 ± 0.23 |
| RS | g·L−1·h−1 | Average volumetric consumption rate of crude glycerol | 3.42 ± 0.10 | 3.26 ± 0.09 | 2.70 ± 0.08 | 3.20 ± 0.09 | 2.68 ± 0.08 |
| QS | g·g−1·h−1 | Average specific consumption rate of crude glycerol | 1.99 ± 0.05 | 1.82 ± 0.05 | 1.69 ± 0.05 | 2.31 ± 0.06 | 1.85 ± 0.05 |
| XK | g·dm−1 | Final biomass concentration in the culture medium | 1.72 ± 0.05 | 1.79 ± 0.05 | 1.60 ± 0.04 | 1.38 ± 0.04 | 1.45 ± 0.04 |
| RX | g·L−1·h−1 | Average volumetric growth rate of biomass | 0.04 ± 0.001 | 0.04 ± 0.001 | 0.02 ± 0.001 | 0.03 ± 0.001 | 0.02 ± 0.001 |
| PK | g·L−1 | Final DHA concentration in the culture medium | 125.3 ± 3.75 | 123.1 ± 3.68 | 175.8 ± 5.20 | 123.0 ± 3.67 | 145.5 ± 4.35 |
| RP | g·L−1·h−1 | Average volumetric rate of bioconversion of crude glycerol to DHA | 2.61 ± 0.07 | 2.80 ± 0.08 | 2.55 ± 0.07 | 2.73 ± 0.08 | 2.11 ± 0.06 |
| QP | g·g−1·h−1 | The average specific rate of bioconversion of crude glycerol to DHA | 1.52 ± 0.04 | 1.56 ± 0.04 | 1.59 ± 0.04 | 1.98 ± 0.05 | 1.46 ± 0.04 |
| YX/Sw | % (m/m) | Total biomass yield | 0.8 ± 0.02 | 1.1 ± 0.03 | 0.9 ± 0.02 | 0.9 ± 0.02 | 0.8 ± 0.02 |
| YP/Sw | % (m/m) | Total DHA yield | 63.4 ± 1.90 | 78.1 ± 2.31 | 94.3 ± 2.80 | 83.6 ± 2.50 | 75.6 ± 2.26 |
| Kef | g·L−1·h−1 | DHA production efficiency factor | 1.7 | 2.2 | 2.4 | 2.3 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, K.; Garncarek, Z. High-Yield Production of Dihydroxyacetone from Crude Glycerol in Fed-Batch Cultures of Gluconobacter oxydans. Molecules 2024, 29, 2932. https://doi.org/10.3390/molecules29122932
Górska K, Garncarek Z. High-Yield Production of Dihydroxyacetone from Crude Glycerol in Fed-Batch Cultures of Gluconobacter oxydans. Molecules. 2024; 29(12):2932. https://doi.org/10.3390/molecules29122932
Chicago/Turabian StyleGórska, Katarzyna, and Zbigniew Garncarek. 2024. "High-Yield Production of Dihydroxyacetone from Crude Glycerol in Fed-Batch Cultures of Gluconobacter oxydans" Molecules 29, no. 12: 2932. https://doi.org/10.3390/molecules29122932
APA StyleGórska, K., & Garncarek, Z. (2024). High-Yield Production of Dihydroxyacetone from Crude Glycerol in Fed-Batch Cultures of Gluconobacter oxydans. Molecules, 29(12), 2932. https://doi.org/10.3390/molecules29122932







