Blue Phosphorescent Pt(II) Compound Based on Tetradentate Carbazole/2,3′-Bipyridine Ligand and Its Application in Organic Light-Emitting Diodes
Abstract
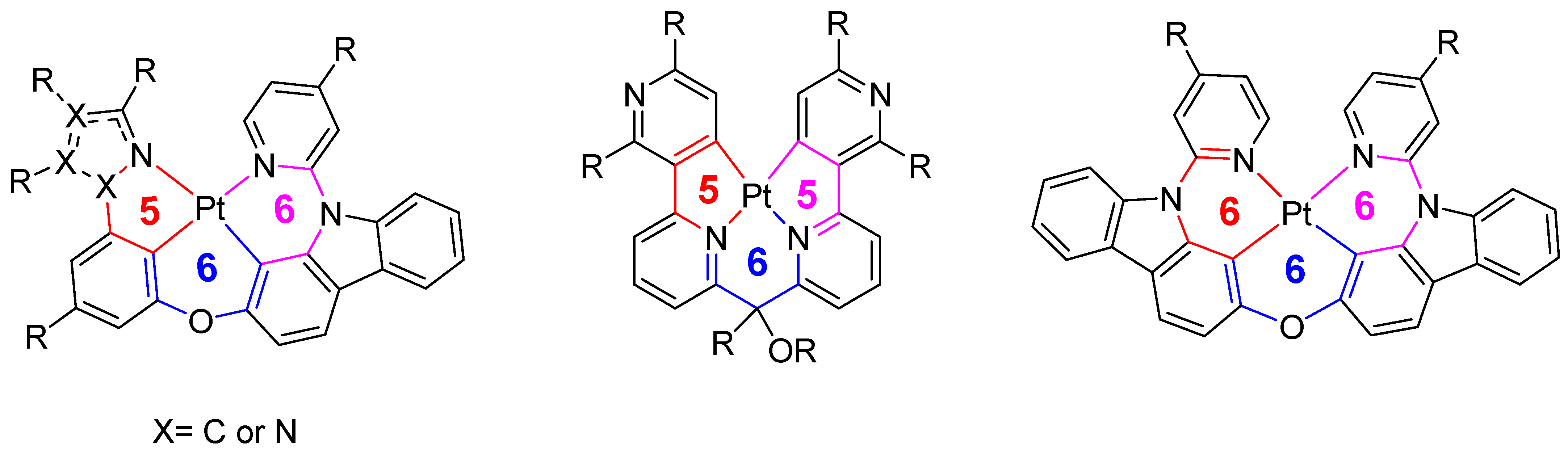
1. Introduction
2. Results and Discussion
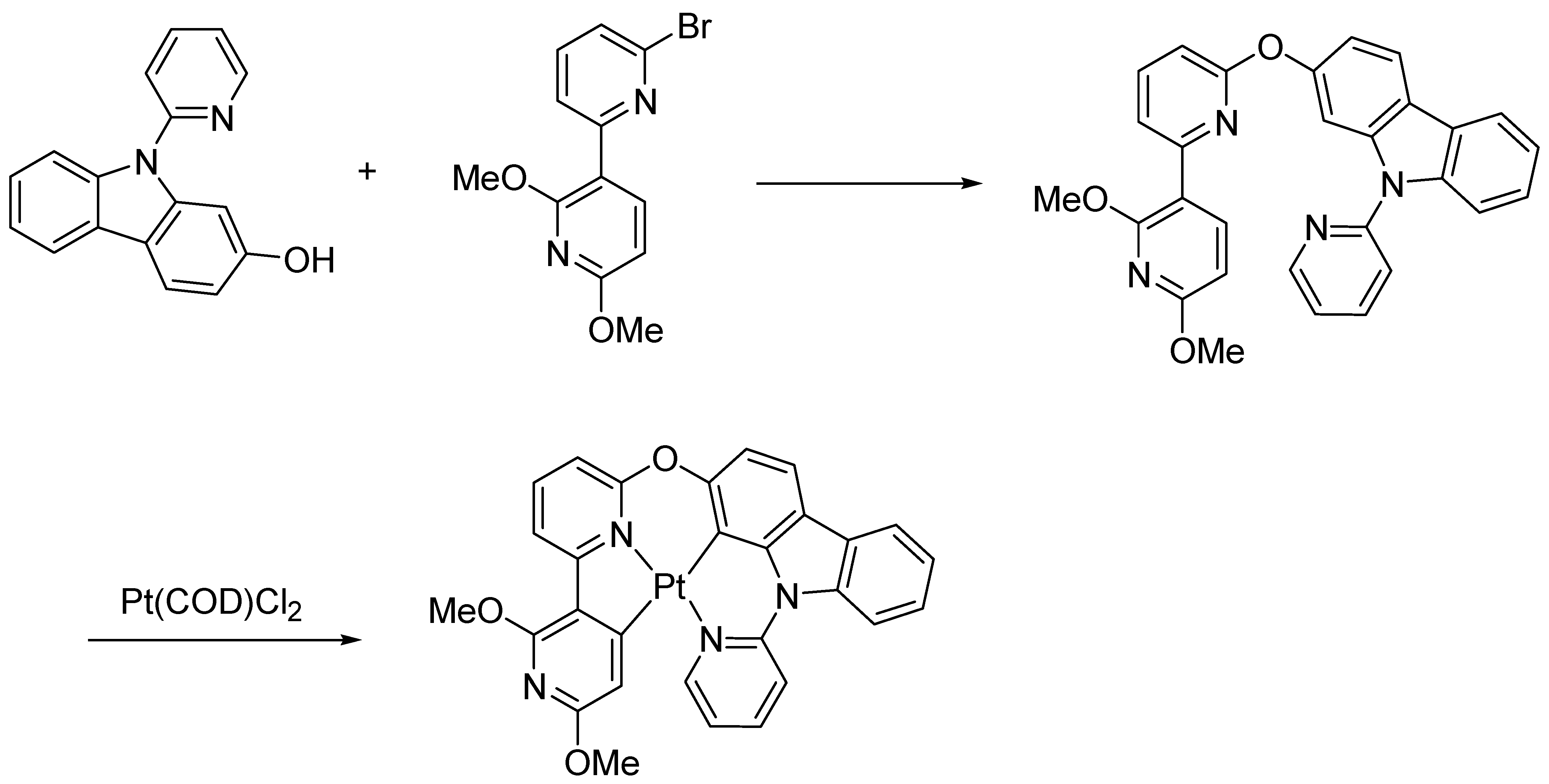
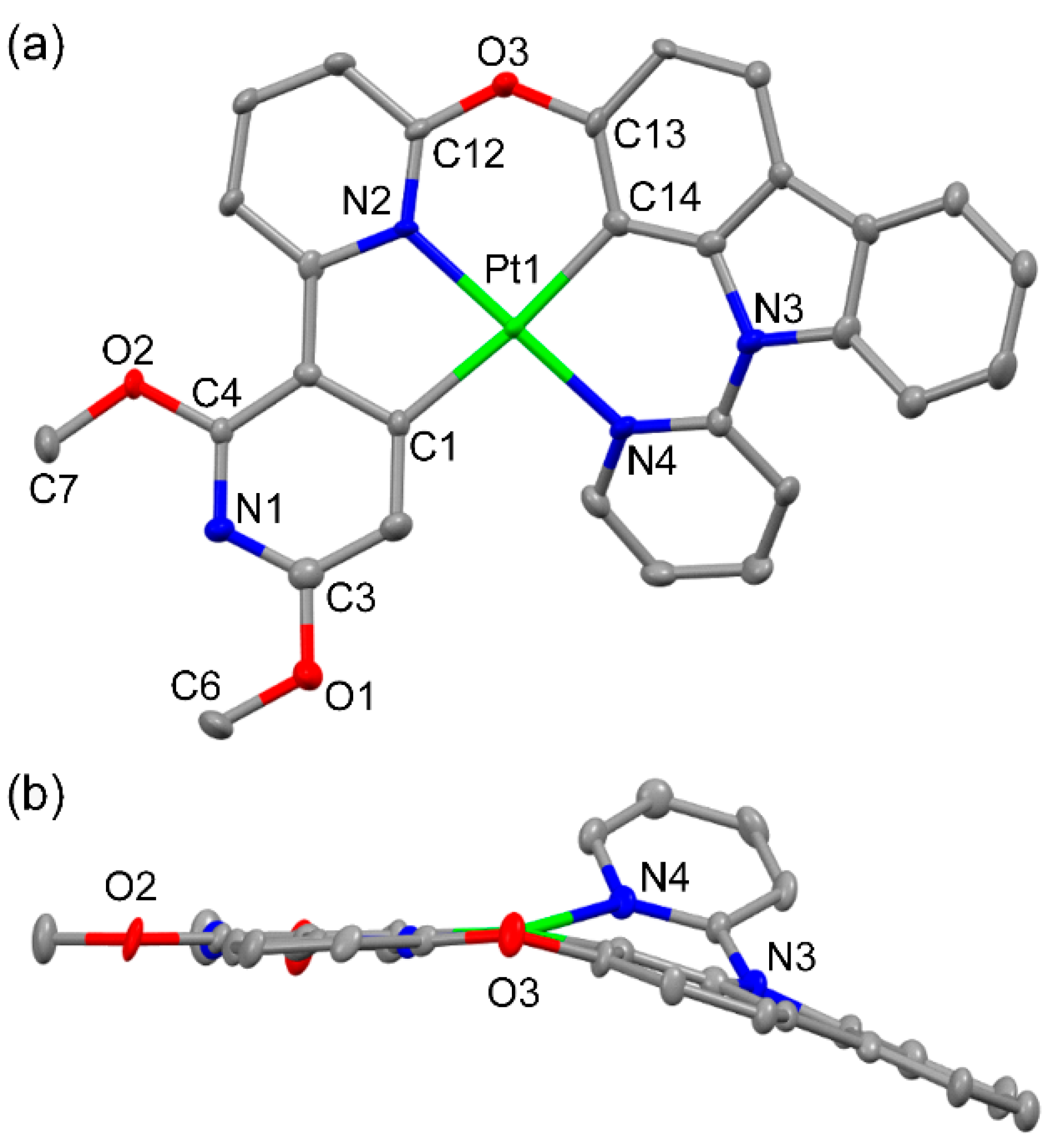
2.1. Synthesis and Crystal Structure
2.2. Time Dependent-Density Functional Theory (TD-DFT) Calculation
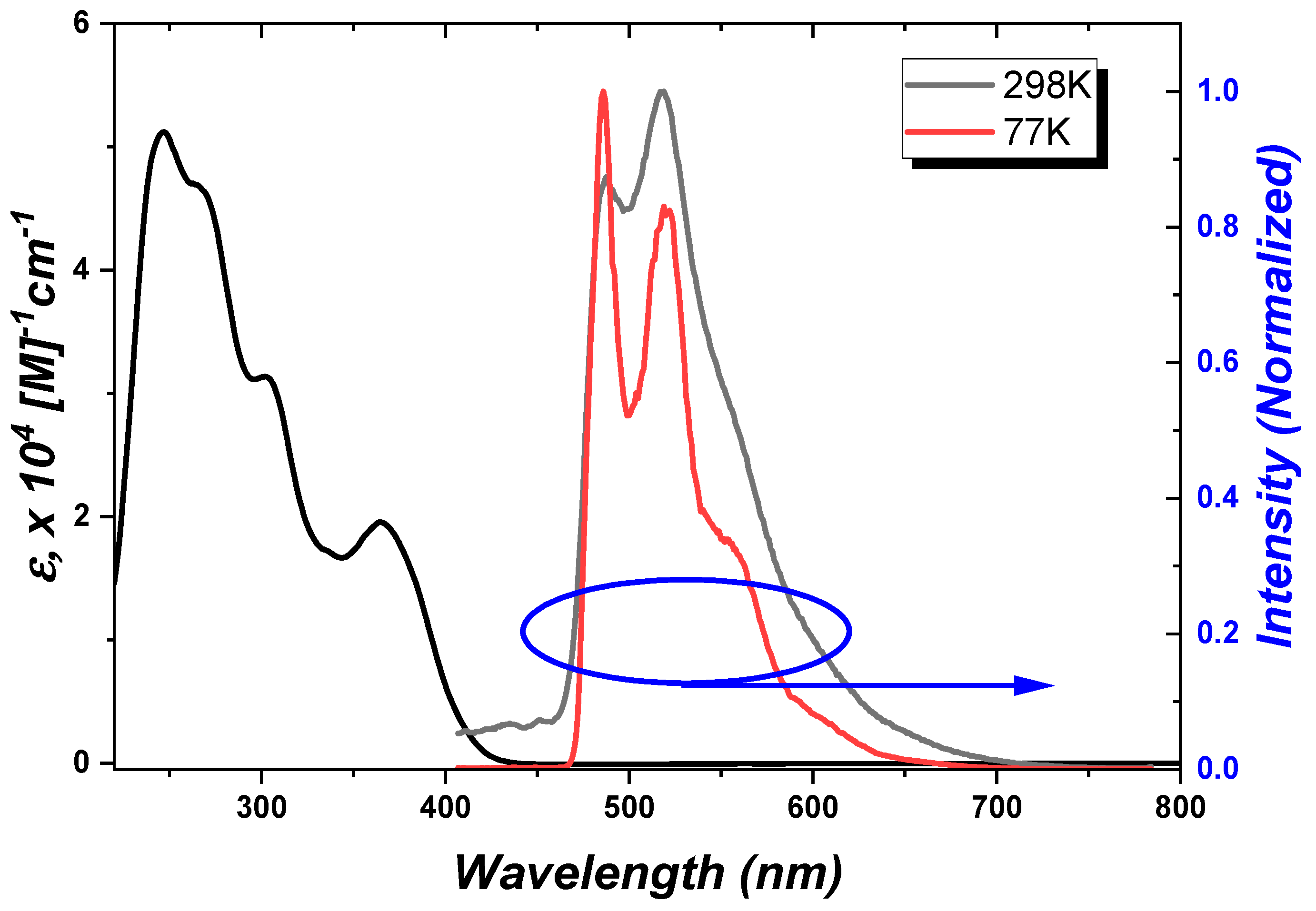
2.3. Photophysical Properties
2.4. PHOLEDs Performances
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.3. Device Fabrication and EL Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.-M.; Cheong, K.; Jiang, J.; Jeon, S.O.; Hong, W.P.; Lee, J.Y. Tetradentate Pt complexes for organic light-emitting diodes. Trends Chem. 2023, 5, 267–278. [Google Scholar] [CrossRef]
- Turner, E.; Bakken, N.; Li, J. Cyclometalated platinum complexes with luminescent quantum yields approaching 100%. Inorg. Chem. 2013, 52, 7344–7351. [Google Scholar] [CrossRef]
- Fleetham, T.; Li, G.; Wen, L.; Li, J. Efficient “pure” blue OLEDs employing tetradentate Pt complexes with a narrow spectral bandwidth. Adv. Mater. 2014, 26, 7116–7121. [Google Scholar] [CrossRef]
- Li, G.; Fleetham, T.; Turner, E.; Hang, X.-C.; Li, J. Highly efficient and stable narrow-band phosphorescent emitters for OLED applications. Adv. Opt. Mater. 2015, 3, 390–397. [Google Scholar] [CrossRef]
- Li, G.; Klimes, K.; Fleetham, T.; Zhu, Z.-Q.; Li, J. Stable and efficient sky-blue organic light emitting diodes employing a tetradentate platinum complex. Appl. Phy. Lett. 2017, 110, 113301. [Google Scholar] [CrossRef]
- Zhao, D.; Tang, X.; Liu, X.-Y.; Fan, J.; Liao, L.-S. Highly luminescent platinum(II) complexes based on pyrazolo[1,5-f]phenanthridine-containing ligands. Org. Electron. 2017, 50, 473–479. [Google Scholar] [CrossRef]
- Ecton, J.; Fleetham, T.; Hang, X.; Li, J. Highly efficient blue-green OLEDs from tetradentate cyclometalated platinum complexes. SID Int. Symp. Dig. Tec. 2013, 44, 152–155. [Google Scholar] [CrossRef]
- Li, G.; Ecton, J.; O’Brien, B.; Li, J. Efficient and stable organic light emitting devices from a tetradentate cyclometalated platinum complex. Org. Electron. 2014, 15, 1862–1867. [Google Scholar] [CrossRef]
- Zhu, Z.-Q.; Klimes, K.; Holloway, S.; Li, J. Efficient cyclometalated platinum(II) complexes with superior operational stability. Adv. Mater. 2017, 29, 605002. [Google Scholar]
- Liu, W.; Zhou, L.; Jin, L.Y.; Cheng, G. Improved Efficiency Roll-Off and Operational Lifetime of Organic Light-Emitting Diodes with a Tetradentate Platinum(II) Complex by Using an n-Doped Electron-Transporting Layer. Molecules 2021, 26, 1835. [Google Scholar] [CrossRef]
- Cheng, G.; Kui, S.C.F.; Ang, W.H.; Ko, M.-Y.; Chow, P.-K.; Kwong, C.-L.; Kwok, C.-C.; Ma, C.; Guan, X.; Low, K.-H. Structurally robust phosphorescent [Pt(OˆNˆCˆN)] emitters for high performance organic light-emitting devices with power efficiency up to 126 lm W−1 and external quantum efficiency over 20%. Chem. Sci. 2014, 5, 4819–4830. [Google Scholar] [CrossRef]
- Wu, J.; Ameri, L.; Cao, L.; Li, J. Efficient excimer-based white OLEDs with reduced efficiency roll-off. Appl. Phys. Lett. 2021, 118, 073301. [Google Scholar] [CrossRef]
- Li, G.; Ameri, L.; Fleetham, T.; Zhu, Z.-Q.; Li, J. Stable and efficient blue and green organic light emitting diodes employing tetradentate Pt(II) complexes. Appl. Phys. Lett. 2020, 117, 253301. [Google Scholar] [CrossRef]
- Klimes, K.; Zhu, Z.Q.; Li, J. Efficient Blue Phosphorescent OLEDs with Improved Stability and Color Purity through Judicious Triplet Exciton Management. Adv. Funct. Mater. 2019, 29, 1903068. [Google Scholar] [CrossRef]
- Ryu, C.H.; Jo, U.; Shin, I.; Kim, M.; Cheong, K.; Bin, J.-K.; Lee, J.Y.; Lee, K.M. Enhancing the Luminescence Efficiency of Blue Phosphorescent Organic Light-Emitting Diodes: Constructing Platinum(II) Complexes with a Functionalized Tetradentate Ligand. Adv. Opt. Mater. 2024, 12, 2303109. [Google Scholar] [CrossRef]
- Li, G.; She, Y. Tetradentate Cyclometalated Platinum(II) Complexes for Efficient and Stable Organic Light-Emitting Diodes. In Light-Emitting Diode—An Outlook On the Empirical Features and Its Recent Technological Advancements; InTechOpen: London, UK, 2018; pp. 77–102. [Google Scholar]
- Ryu, C.H.; Kim, S.C.; Kim, M.; Yi, S.; Lee, J.Y.; Lee, K.M. Novel Tetradentate Platinum(II) Complexes and Their Use in Blue Phosphorescent Organic Light-Emitting Diodes. Adv. Opt. Mater. 2022, 10, 2201799. [Google Scholar] [CrossRef]
- Lee, C.; Zaen, R.; Park, K.-M.; Lee, K.H.; Lee, J.Y.; Kang, Y. Blue phosphorescent platinum complexes based on tetradentate bipyridine ligands and their application to organic light-emitting diodes (OLEDs). Organometallics 2018, 37, 4639–4647. [Google Scholar] [CrossRef]
- Fleetham, T.; Huang, L.; Klimes, K.; Brooks, J.; Li, J. Tetradentate Pt(II) complexes with 6-membered chelate rings: A new route for stable and efficient blue organic light emitting diodes. Chem. Mater. 2016, 28, 3276–3282. [Google Scholar] [CrossRef]
- Xia, B.-H.; Ma, Y.-S.; Bai, F.-Q. Density Functional Calculation and Evaluation of the Spectroscopic Properties and Luminescent Material Application Potential of the N-Heterocyclic Platinum(II) Tetracarbene Complexes. Molecules 2024, 29, 524. [Google Scholar] [CrossRef]
- Feng, T.-T.; Bai, F.-Q.; Xie, L.-M.; Tang, Y.; Zhang, H.-X. Theoretical study and design of highly efficient platinum(II) complexes bearing tetradentate ligands for OLED. RSC Adv. 2016, 6, 11648–11656. [Google Scholar] [CrossRef]
- Li, G.; Wolfe, A.; Brooks, J.; Zhu, Z.Q.; Li, J. Modifying emission spectral bandwidth of phosphorescent platinum(II) complexes through synthetic control. Inorg. Chem. 2017, 56, 8244–8256. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Xu, K.; Fang, X.; Yang, Y.-F.; Lou, W.; Hu, Y.; Zhang, Q.; Li, G. Tetradentate platinum(II) and palladium(II) complexes containing fused 6/6/6 or 6/6/5 metallocycles with azacarbazolylcarbazole-based ligands. Inorg. Chem. 2021, 60, 12972–12983. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, S.C.; Lee, J.Y.; Kang, Y. Blue phosphorescent platinum(II) complexes based on tetradentate ligand with rigid and highly distorted linkage for efficient organic light-emitting diodes. Dye. Pigment. 2022, 207, 110770. [Google Scholar] [CrossRef]
- Park, H.J.; Jang, J.-H.; Lee, J.-H.; Hwang, D.-H. Highly efficient deep-blue phosphorescent OLEDs based on a trimethylsilyl-substituted tetradentate Pt(II) complex. ACS Appl. Mater. Interfaces 2022, 14, 34901–34908. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-S.; Lee, D.Y.; Park, K.H.; Kwon, S.-K.; Kim, Y.-H.; Kim, J.-J. Control of the horizontal dipole ratio and emission color of deep blue tetradentate Pt(II) complexes using aliphatic spacer groups. Chem. Eng. J. 2022, 450, 137836–137843. [Google Scholar] [CrossRef]
- Sun, J.; Ahn, H.; Kang, S.; Ko, S.-B.; Song, D.; Um, H.A.; Kim, S.; Lee, Y.; Jeon, P.; Hwang, S.-H. Exceptionally Stable Blue Phosphorescent Organic Light-Emitting Diodes. Nat. Photon. 2022, 16, 212–218. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Kim, S.C.; Lee, J.Y.; Kang, Y. Strategic molecular engineering of blue phosphorescent Pt (II) compounds for the development of high-efficiency single-doped white OLEDs. Dye. Pigment. 2023, 220, 111717. [Google Scholar] [CrossRef]
- Kang, J.; Zaen, R.; Lee, J.H.; Hwang, H.; Park, K.-M.; Kim, S.C.; Lee, J.Y.; Kang, Y. Effect of ancillary ligand on the photoluminescent and electroluminescent properties of blue Ir(III) complexes bearing main bipyridine ligand. Chem. Eng. J. 2022, 431, 134249. [Google Scholar] [CrossRef]
- Kang, J.; Zaen, R.; Park, K.-M.; Lee, K.H.; Lee, J.Y.; Kang, Y. Cyclometalated platinum(II) β-diketonate complexes with extremely high external quantum efficiency for white organic light-emitting diodes. Adv. Opt. Mater. 2021, 9, 2101233. [Google Scholar] [CrossRef]
- Yan, J.; Wang, S.F.; Hsu, C.-H.; Shi, E.H.-C.; Wu, C.-C.; Chou, P.-T.; Yiu, S.-M.; Chi, Y.; You, C.; Peng, I.C.; et al. Engineering of Cyano Functionalized Benzo[d]imidazol-2-ylidene Ir(III) Phosphors for Blue Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2023, 15, 21333–21343. [Google Scholar] [CrossRef]
- Evariste, S.; Sandroni, M.; Rees, T.W.; Roldán-Carmona, C.; Gil-Escrig, L.; Bolink, H.J.; Baranoff, E.; Zysman-Colman, E. Fluorine-Free Blue-Green Emitters for Light-emitting Electrochemical Cells. J. Mater. Chem. C 2014, 2, 5793–5804. [Google Scholar] [CrossRef]
- Bai, R.; Meng, X.; Wang, X.; He, L. Blue-Emitting Iridium(III) Complexes for Light-Emitting Electrochemical Cells: Advances, Challenges, and Future Prospects. Adv. Funct. Mater. 2020, 30, 1907169. [Google Scholar] [CrossRef]
- Reddy, M.L.P.; Bejoymohandas, K.S. Evolution of 2,3′-bipyridine class of cyclometalating ligands as efficient phosphorescent iridium(III) emitters for applications inorganic light emitting diodes. J. Photochem. Photobiol. C Photochem. Rev. 2016, 29, 29–47. [Google Scholar] [CrossRef]
- Duan, T.; Chang, T.K.; Chi, Y.; Wang, J.Y.; Chen, Z.N.; Hung, W.Y.; Chen, C.H.; Lee, G.H. Blue-Emitting Heteroleptic Ir(III) Phosphors with Functional 2,3′-Bipyridine or 2-(Pyrimidin-5-yl)Pyridine Cyclometalates. Dalton Trans. 2015, 44, 14613–14624. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.; Curchod, B.F.E.; Scopelliti, R.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Baranoff, E. Structure-property relationships based on Hammett constants in cyclometalated Iridium(III) complexes: Their application to the design of a fluorine-free FIrPic-like emitter. Dalton Trans. 2014, 43, 5667–5679. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Xu, K.; Zhang, C.; Chen, J.; Chu, Q.; Yang, Y.F.; She, Y. Perimidocarbene-Based Tetradentate Platinum(II) Complexes with an Unexpectedly Negligible 3MLCT Character. Inorg. Chem. 2024, 63, 6435–6444. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qian, C.; Qin, K.; Li, H.; Shi, X.; Lu, Z.; Ma, H.; Qin, T.; Hang, X.-C.; Huang, W. Ultrapure Blue Phosphorescent Organic Light-Emitting Diodes Employing a Twisted Pt(II) Complex. ACS Appl. Mater. Interfaces 2021, 13, 52833–52839. [Google Scholar] [CrossRef]
- Wu, S.H.; Shao, J.Y.; Zhao, Z.; Ma, J.; Yang, R.; Chen, N.; Tang, J.H.; Bian, Z.; Zhong, Y.W. Ligand Engineering toward Deep Blue Emission in Nonplanar Terdentate Platinum(II) Complexes. Organometallics 2021, 40, 156–165. [Google Scholar] [CrossRef]
- Guo, Z.A.; Xian, J.Y.; Rong, L.R.; Qin, H.; Jie, Z. Theoretical study of metal ion impact on geometric and electronic properties of terbutaline compounds. Monatsh. Chem. 2019, 150, 1355–1364. [Google Scholar] [CrossRef]
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the Origin and Nature of Internal Methyl Rotation Barriers: An Information-Theoretic Approach Study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
- Wu, J.; Yu, D.; Liu, S.; Rong, C.; Zhong, A.; Chattaraj, P.K.; Liu, S. Is It Possible To Determine Oxidation States for Atoms in Molecules Using Density-Based Quantities? An Information-Theoretic Approach and Conceptual Density Functional Theory Study. J. Phys. Chem. A 2019, 123, 6751–6760. [Google Scholar] [CrossRef]
- Ma, H.; Shen, K.; Wu, Y.; Xia, F.; Yu, F.; Sun, Z.; Qian, C.; Peng, Q.; Zhang, H.-H.; You, C.; et al. High-Color-Purity and Efficient Solution-Processable Blue Phosphorescent Light-Emitting Diodes with Pt(II) Complexes Featuring 3ππ* Transitions. Mater. Chem. Front. 2019, 3, 2448–2454. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chan, S.C.; Chan, M.C.W.; Hou, Y.J.; Zhu, N.; Che, C.M.; Liu, Y.; Wang, Y. Structural, photophysical, and electrophosphorescent properties of platinum(II) complexes supported by tetradentate N2O2 chelates. Chem. Eur. J. 2003, 9, 1263–1272. [Google Scholar] [CrossRef]
- Lees, A.J. The Luminescence Rigidochromic Effect Exhibited by Organometallic Complexes: Rationale and Applications. Comments Inorg. Chem. 1995, 17, 319–346. [Google Scholar] [CrossRef]
- Hang, X.C.; Fleetham, T.; Turner, E.; Brooks, J.; Li, J. Highly efficient blue-emitting cyclometalated platinum(II) complexes by judicious molecular design. Angew. Chem. Int. Ed. 2013, 52, 6753–6756. [Google Scholar] [CrossRef]
- Yonemoto, D.T.; Papa, C.M.; Mongin, C.; Castellano, F.N. Thermally Activated Delayed Photoluminescence: Deterministic Control of Excited-State Decay. J. Am. Chem. Soc. 2020, 142, 10883–10893. [Google Scholar] [CrossRef]
- Rao, Y.L.; Schoenmakers, D.; Chang, Y.L.; Lu, J.S.; Lu, Z.H.; Kang, Y.; Wang, S. Bluish-Green BMes2-Functionalized PtII Complexes for High Efficiency PhOLEDs: Impact of the BMes2 Location on Emission Color. Chem. Eur. J. 2012, 18, 11306–11316. [Google Scholar] [CrossRef]
- Kang, Y.; Chang, Y.L.; Lu, J.S.; Ko, S.B.; Rao, Y.; Varlan, M.; Lu, Z.H.; Wang, S. Highly efficient blue phosphorescent and electroluminescent Ir(III) compounds. J. Mater. Chem. C 2013, 1, 441–443. [Google Scholar] [CrossRef]
- Moon, S.H.; Park, K.-M.; Kim, J.; Kang, Y. Crystal structure and luminescence properties of 2-[(2′,6′-dimethoxy-2,3′-bipyridin-6-yl) oxy]-9-(pyridin-2-yl)-9H-carbazole. Acta Cryst. 2019, E75, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, X.; Fang, K.; Li, J.; She, Y. CuCl-Catalyzed Hydroxylation of N-Heteroarylcarbazole Bromide: Approach for the Preparation of N-Heteroarylcarbazolyl Phenols and Its Application in the Synthesis of Phosphorescent Emitters. J. Org. Chem. 2017, 82, 8634–8644. [Google Scholar] [CrossRef]
- Li, G.; Chen, Q.; Zheng, J.; Wang, Q.; Zhan, F.; Lou, W.; Yang, Y.-F.; She, Y. Metal-assisted delayed fluorescent Pd(II) complexes and phosphorescent Pt(II) complex based on 1,2,4 triazolo 4,3-a pyridine-containing ligands: Synthesis, characterization, electrochemistry, photophysical studies, and application. Inorg. Chem. 2019, 58, 14349–14360. [Google Scholar] [CrossRef] [PubMed]



| Doping [wt%] | Von a [V] | Vd b [V] | J b [mA cm−2] | CIE b | EQEmax [%] | CEmax [cd A−1] | PEmax [lm W−1] | |
|---|---|---|---|---|---|---|---|---|
| x | y | |||||||
| 3 | 3.50 | 5.41 | 16.4 | 0.244 | 0.516 | 4.6 | 13.5 | 11.9 |
| 5 | 3.51 | 5.44 | 14.3 | 0.247 | 0.533 | 5.2 | 15.5 | 13.4 |
| 10 | 3.52 | 5.71 | 18.8 | 0.254 | 0.541 | 4.3 | 13.0 | 10.9 |
| 20 | 3.52 | 5.79 | 22.0 | 0.267 | 0.551 | 3.6 | 11.1 | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Ryu, C.-H.; Hong, M.; Lee, K.M.; Jo, U.; Kang, Y. Blue Phosphorescent Pt(II) Compound Based on Tetradentate Carbazole/2,3′-Bipyridine Ligand and Its Application in Organic Light-Emitting Diodes. Molecules 2024, 29, 2929. https://doi.org/10.3390/molecules29122929
Kim H, Ryu C-H, Hong M, Lee KM, Jo U, Kang Y. Blue Phosphorescent Pt(II) Compound Based on Tetradentate Carbazole/2,3′-Bipyridine Ligand and Its Application in Organic Light-Emitting Diodes. Molecules. 2024; 29(12):2929. https://doi.org/10.3390/molecules29122929
Chicago/Turabian StyleKim, Hakjo, Chan-Hee Ryu, Miso Hong, Kang Mun Lee, Unhyeok Jo, and Youngjin Kang. 2024. "Blue Phosphorescent Pt(II) Compound Based on Tetradentate Carbazole/2,3′-Bipyridine Ligand and Its Application in Organic Light-Emitting Diodes" Molecules 29, no. 12: 2929. https://doi.org/10.3390/molecules29122929
APA StyleKim, H., Ryu, C.-H., Hong, M., Lee, K. M., Jo, U., & Kang, Y. (2024). Blue Phosphorescent Pt(II) Compound Based on Tetradentate Carbazole/2,3′-Bipyridine Ligand and Its Application in Organic Light-Emitting Diodes. Molecules, 29(12), 2929. https://doi.org/10.3390/molecules29122929






