Halogenated Boroxine K2[B3O3F4OH] Modulates Metabolic Phenotype and Autophagy in Human Bladder Carcinoma 5637 Cell Line
Abstract
1. Introduction
2. Results
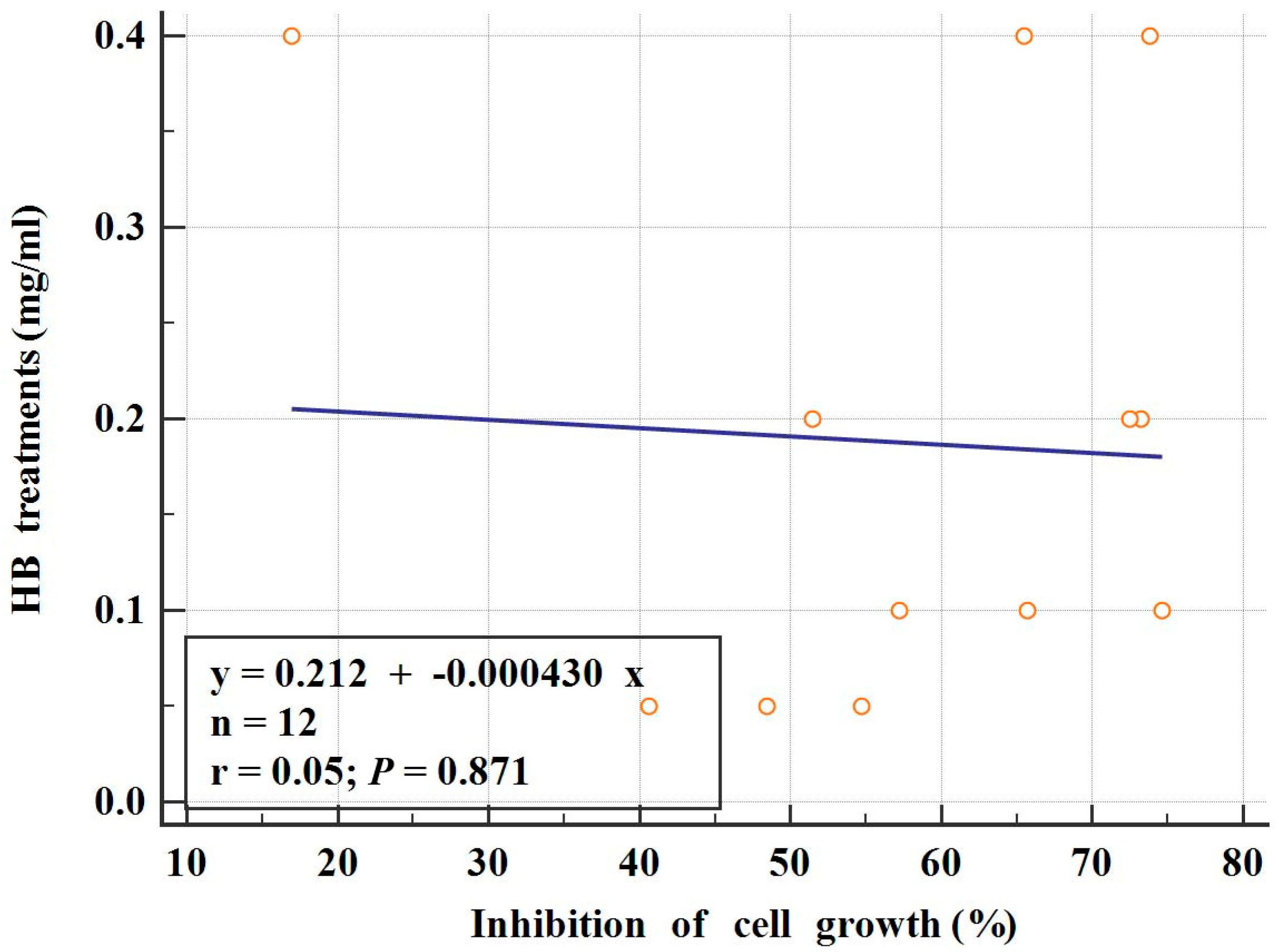
2.1. Cytotoxic Effects of HB on BC Cells
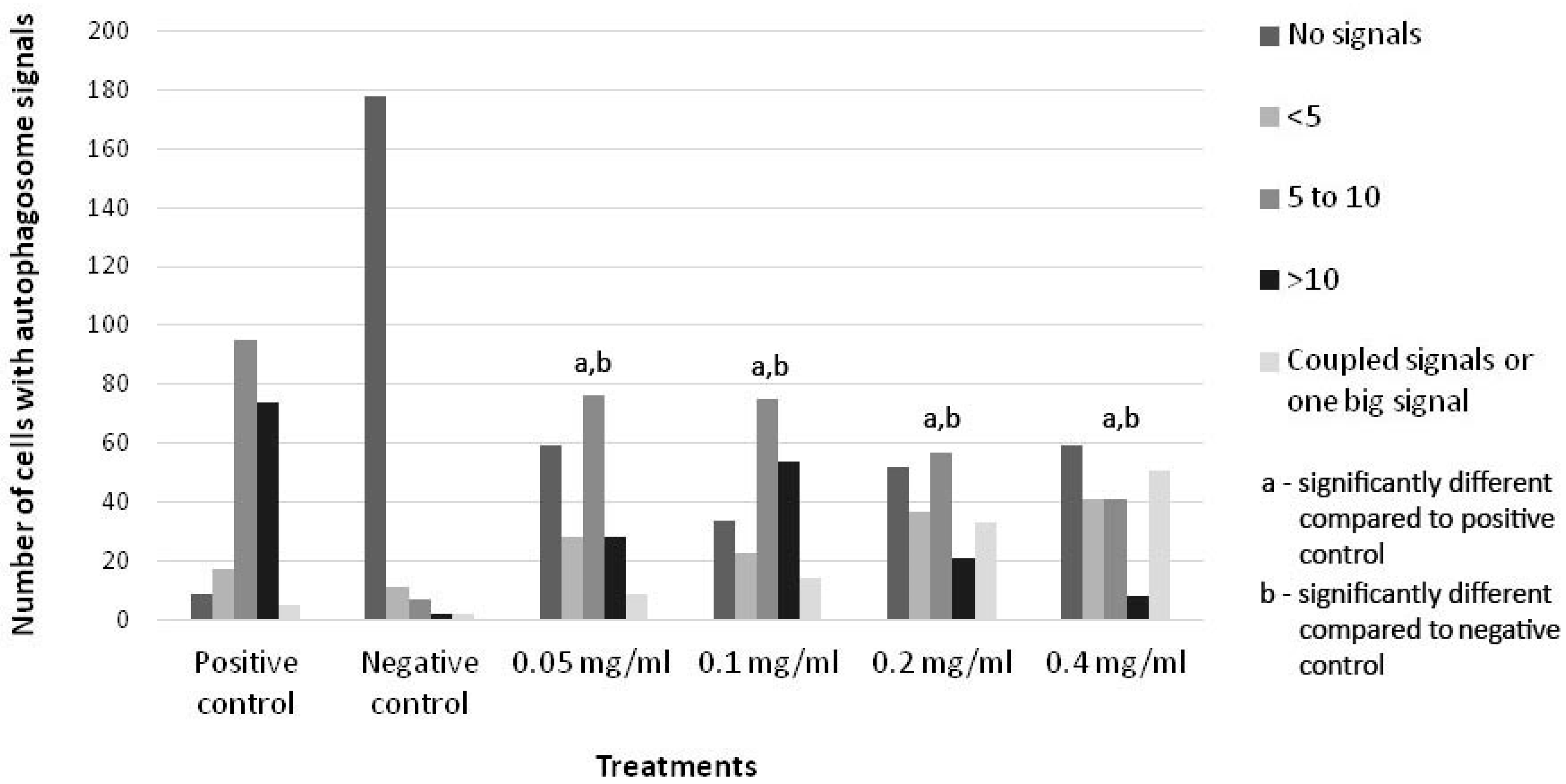
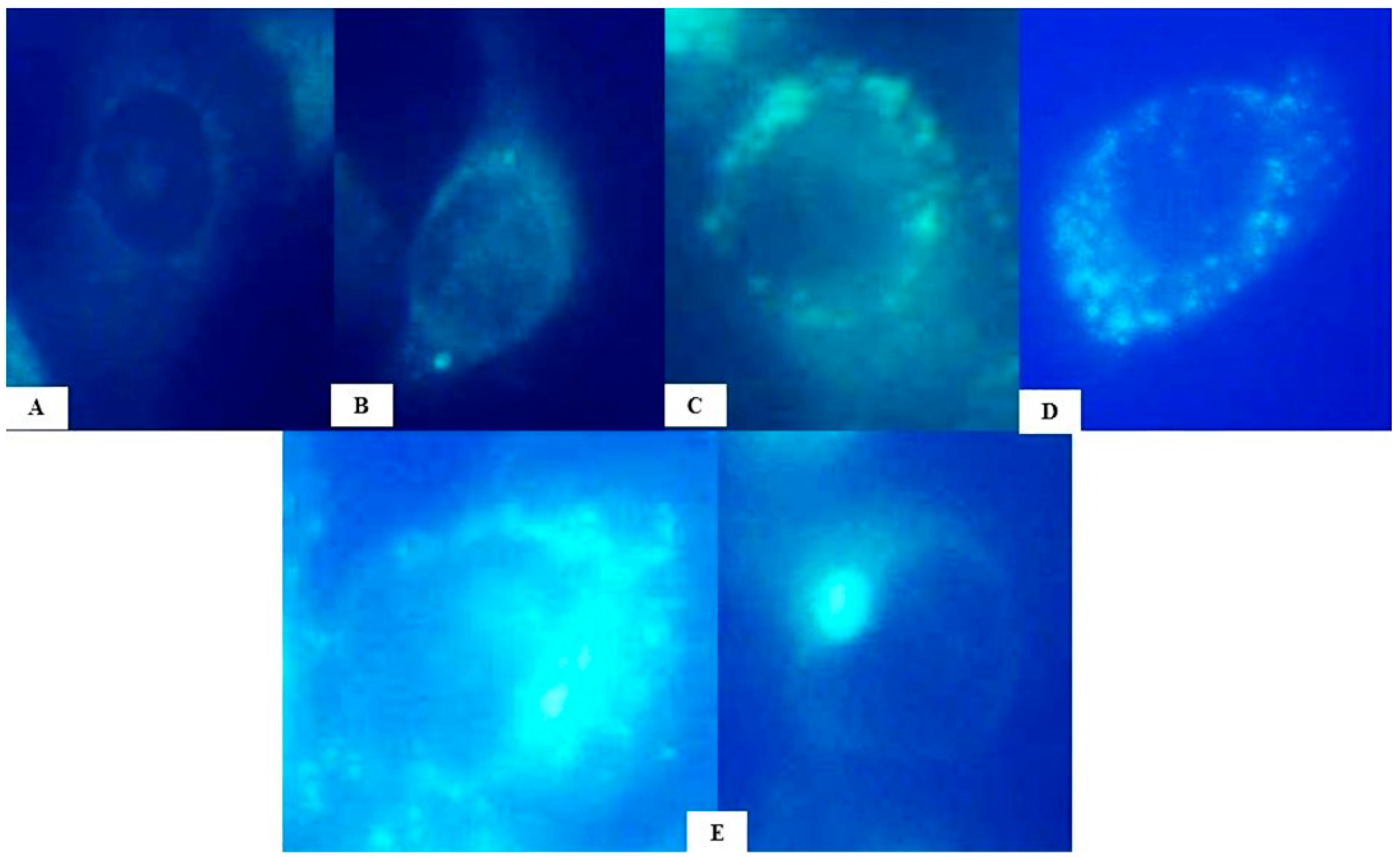
2.2. Evaluation of Autophagy Induction by HB
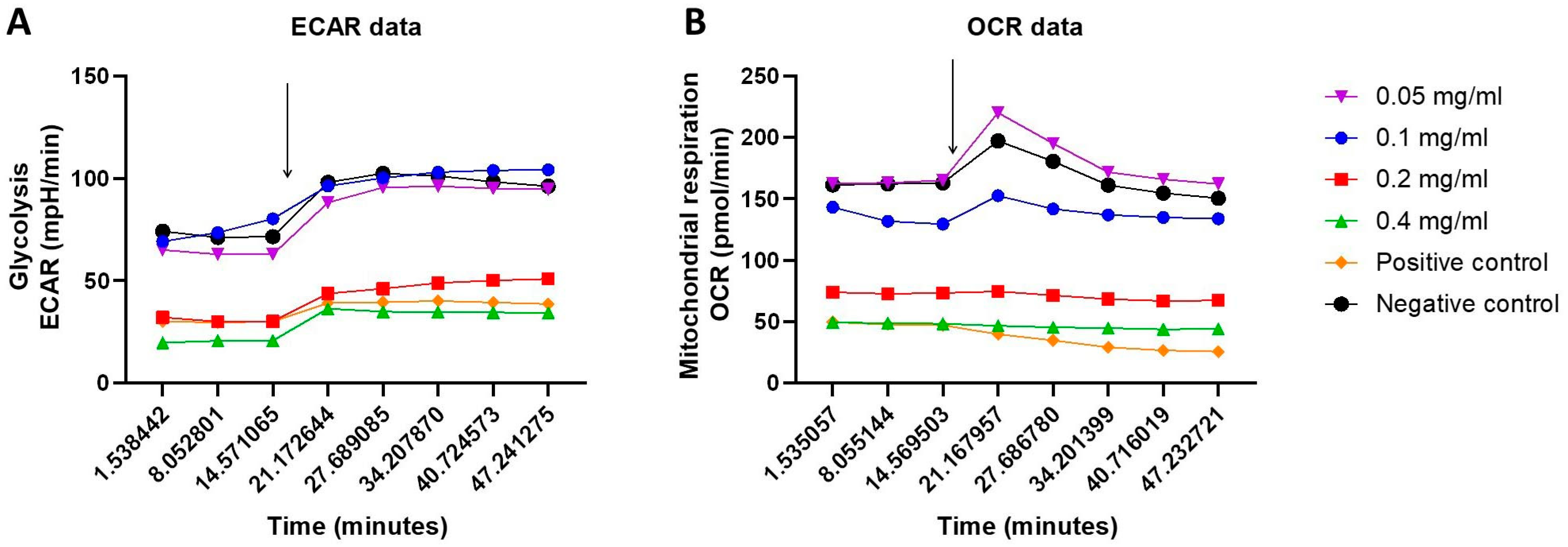
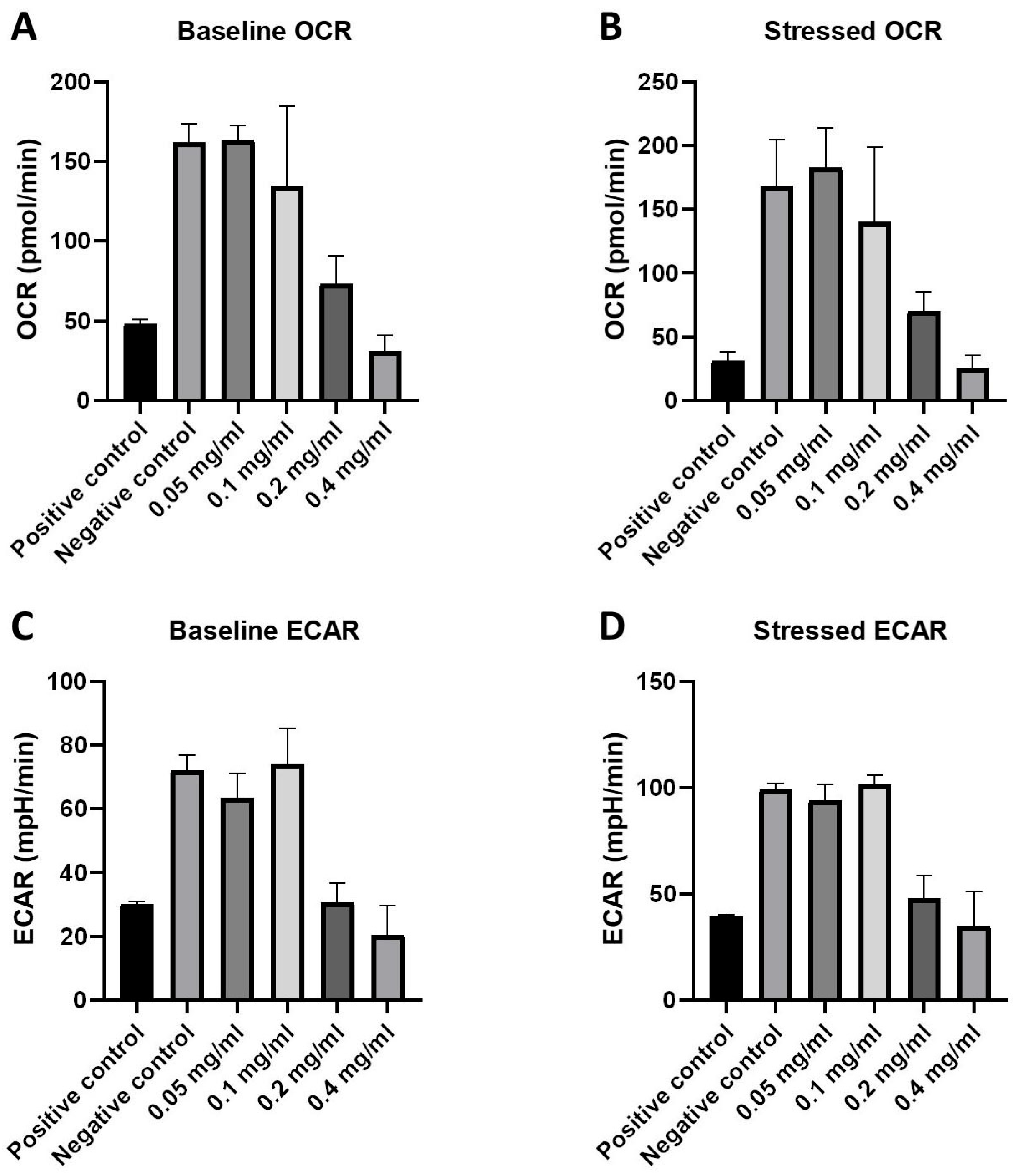
2.3. Cellular Energy Phenotype and the Investigation of Metabolic Switching: OCR and ECAR Rates in Response to HB Treatment—Nts
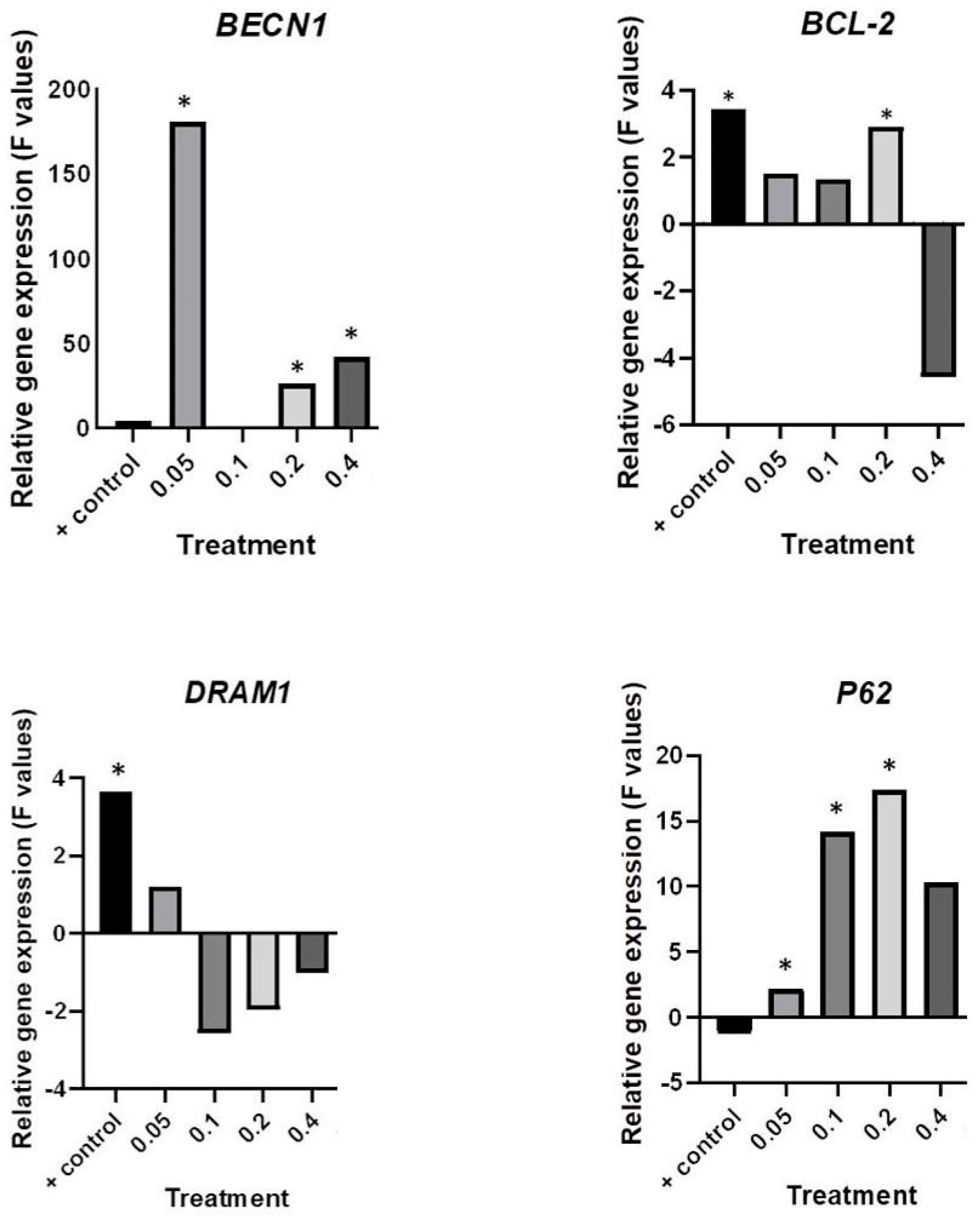
2.4. Deregulation of Autophagy-Associate Genes by HB
3. Discussion
4. Materials and Methods
4.1. Dipotassium Trioxohydroxytetrafluorotriborate, K2[B3O3F4OH], Halogenated Boroxine (HB)—Synthesis and Solution Preparation
4.2. Cell Culture Treatment
4.3. Alamar Blue Assay
4.4. Autophagy Detection
4.5. Oxygen Consumption Rate and Extracellular Acidification Rate
4.6. q-PCR and Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Guo, J.Y.; Chen, H.Y.; Mathew, R.; Fan, J.; Strohecker, A.M.; Karsli-Uzunbas, G.; Kamphorst, J.J.; Chen, G.; Lemons, J.M.; Karantza, V.; et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumourigenesis. Genes Dev. 2011, 25, 460–470. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.F.; Wen, S.I.; Yang, S.C.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Hwang, T.I. Inhibition of High Basal Level of Autophagy Induces Apoptosis in Human Bladder Cancer Cells. J. Urol. 2016, 195, 1126–1135. [Google Scholar] [CrossRef]
- Xie, X.; White, E.P.; Mehnert, J.M. Coordinate autophagy and mTOR pathway inhibition enhances cell death in melanoma. PLoS ONE 2013, 8, e55096. [Google Scholar] [CrossRef]
- Ma, X.H.; Piao, S.; Wang, D.; McAfee, Q.W.; Nathanson, K.L.; Lum, J.J.; Li, L.Z.; Amaravadi, R.K. Measurements of tumour cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin. Cancer Res. 2011, 17, 3478–3489. [Google Scholar] [CrossRef]
- Goldsmith, J.; Levine, B.; Debnath, J. Autophagy and cancer metabolism. Methods Enzymol. 2014, 542, 25–57. [Google Scholar]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Nishiyama, H.; Habuchi, T.; Watanabe, J.; Teramukai, S.; Tada, H.; Ono, Y.; Ohshima, S.; Fujimoto, K.; Hirao, Y.; Fukushima, M.; et al. Clinical outcome of a large-scale multi-institutional retrospective study for locally advanced bladder cancer: A survey including 1131 patients treated during 1990–2000 in Japan. Eur. Urol. 2004, 45, 176–181. [Google Scholar] [CrossRef]
- Gazzaniga, P.; Silvestri, I.; Gradilone, A.; Scarpa, S.; Morrone, S.; Gandini, O.; Gianni, W.; Frati, L.; Agliano, A.M. Gemcitabine-induced apoptosis in 5637 cell line: An in-vitro model for high-risk superficial bladder cancer. Anticancer Drugs 2007, 18, 179–185. [Google Scholar] [CrossRef]
- Rieger, K.M.; Little, A.F.; Swart, J.M.; Kastrinakis, W.V.; Fitzgerald, J.M.; Hess, D.T.; Libertino, J.A.; Summerhayes, I.C. Human bladder carcinoma cell lines as indicators of oncogenic change relevant to urothelial neoplastic progression. Br. J. Cancer 1995, 72, 683–690. [Google Scholar] [CrossRef]
- Vousden, K.H.; Ryan, K.M. p53 and metabolism. Nat. Rev. Cancer 2009, 9, 691–700. [Google Scholar] [CrossRef]
- Bensaad, K.; Tsuruta, A.; Selak, M.A.; Vidal, M.N.C.; Nakano, K.; Bartrons, R.; Gottlieb, E.; Vousden, K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006, 126, 107–120. [Google Scholar] [CrossRef]
- Whyard, T.; Waltzer, W.C.; Waltzer, D.; Romanov, V. Metabolic alterations in bladder cancer: Applications for cancer imaging. Exp. Cell Res. 2016, 341, 77–83. [Google Scholar] [CrossRef]
- Zhu, H.B.; Yang, K.; Xie, Y.Q.; Lin, Y.W.; Mao, Q.Q.; Xie, L.P. Silencing of mutant p53 by siRNA induces cell cycle arrest and apoptosis in human bladder cancer cells. World J. Surg. Oncol. 2013, 11, 22. [Google Scholar] [CrossRef]
- Lin, J.F.; Lin, Y.C.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Hwang, T.I. Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des. Devel. Ther. 2017, 11, 1517–1533. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Lin, T. Emerging strategies for the improvement of chemotherapy in bladder cancer: Current knowledge and future perspectives. J. Adv. Res. 2022, 39, 187–202. [Google Scholar] [CrossRef]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaborators Group. Adjuvant Chemotherapy for Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis of Individual Participant Data from Randomised Controlled Trials. Eur. Urol. 2022, 81, 50–61. [Google Scholar] [CrossRef]
- Hall, D.G. Boronic Acids; John Wiley and Sons: New York, NY, USA, 2005. [Google Scholar]
- Galić, B. Boroxine Composition for Removal of Skin Changes. U.S. Patent US8278289, 2 October 2012. [Google Scholar]
- Galić, B. Removal of Skin Changes. European Patent EP1996514, 31 July 2013. [Google Scholar]
- Haverić, S.; Haverić, A.; Bajrović, K.; Galić, B.; Maksimović, M. Effects of dipotassium trioxohydroxytetrafluorotriborate (K2(B3O3F4OH)) on genetic material and inhibition of cell division in human cell cultures. Drug Chem. Toxicol. 2011, 34, 250–254. [Google Scholar] [CrossRef]
- Hadžić, M.; Haverić, S.; Haverić, A.; Galić, B. Inhibitory effects of delphinidin and luteolin on genotoxicity induced by K2(B3O3F4OH) in human lymphocytes in vitro. Biologia 2015, 70, 553–558. [Google Scholar] [CrossRef]
- Haverić, S.; Hadžić, M.; Haverić, A.; Mijanović, M.; Hadžiselimović, R.; Galić, B. Genotoxicity Evaluation of Dipotassium-Trioxohydroxytetrafluorotriborate, K2(B3O3F4OH), in Human Lymphocyte Cultures and Mice Reticulocytes. Braz. Arch. Biol. Technol. 2016, 59, e16160195. [Google Scholar] [CrossRef][Green Version]
- Pojskić, L.; Haverić, S.; Lojo-Kadrić, N.; Hadžić, M.; Haverić, A.; Galić, Z.; Galić, B.; Vullo, D.; Supuran, C.T.; Miloš, M. Effects of dipotassium-trioxohydroxytetrafluorotriborate, K2(B3O3F4OH), on cell viability and gene expression of common human cancer drug targets in a melanoma cell line. J. Enzym. Inhib. Med. Chem. 2016, 31, 999–1004. [Google Scholar] [CrossRef][Green Version]
- Hadžić, M.; Haverić, S.; Haverić, A.; Lojo-Kadrić, N.; Galić, B.; Ramić, J.; Pojskić, L. Bioflavonoids protect cells against halogenated boroxine-induced genotoxic damage by upregulation of hTERT expression. Z. Naturforsch. C J. Biosci. 2019, 74, 125–129. [Google Scholar] [CrossRef]
- Ostojić, J.; Herenda, S.; Bešić, Z.; Miloš, M.; Galić, B. Advantages of an Electrochemical Method Compared to the Spectrophotometric Kinetic Study of Peroxidase Inhibition by Boroxine Derivative. Molecules 2017, 22, 1120. [Google Scholar] [CrossRef]
- Islamović, S.; Galić, B.; Miloš, M. A study of the inhibition of catalase by dipotassium trioxohydroxytetrafluorotriborate K2[B3O3F4OH]. J. Enzym. Inhib. Med. Chem. 2014, 29, 744–748. [Google Scholar] [CrossRef]
- Herenda, S.; Ostojić, J.; Hasković, E.; Hasković, D.; Miloš, M.; Galić, B. Electrochemical Investigation of the Influence of K2[B3O3F4OH] on the Activity of Immobilized Superoxide Dismutase. Int. J. Electrochem. Sci. 2018, 13, 3279–3287. [Google Scholar] [CrossRef]
- Vullo, D.; Miloš, M.; Galić, B.; Scozzafava, A.; Supuran, C.T. Dipotassium trioxohydroxytetrafluorotriborate K2[B3O3F4OH], is a potent inhibitor of human carbonic anhydrases. J. Enzyme Inhib. Med. Chem. 2015, 30, 341–344. [Google Scholar] [CrossRef]
- Elez-Burnjaković, N.; Pojskić, L.; Haverić, A.; Lojo-Kadrić, N.; Omanović, M.H.; Ramić, J.; Smajlović, A.; Maksimović, M.; Haverić, S. New in vitro findings about halogenated boroxine cytotoxicity and deregulation of cell death-related genes in GR-M melanoma cells. Arh. Hig. Rada Toksikol. 2023, 74, 16–21. [Google Scholar] [CrossRef]
- Ivanković, S.; Stojković, R.; Maksimović, M.; Galić, B.; Miloš, M. Impact of calcium ion on cytotoxic effect of the boroxine derivative, K2(B3O3F4OH). J. Enzym. Inhib. Med. Chem. 2016, 31, 70–74. [Google Scholar] [CrossRef]
- Ivanković, S.; Stojković, R.; Galić, Z.; Galić, B.; Ostojić, J.; Marasović, M.; Miloš, M. In vitro and in vivo antitumour activity of the halogenated boroxine dipotassium-trioxohydroxytetrafluorotriborate (K2(B3O3F4OH)). J. Enzym. Inhib. Med. Chem. 2015, 30, 354–359. [Google Scholar] [CrossRef][Green Version]
- Haverić, A.; Durmić-Pašić, A.; Alić, A.; Mujezinović, I.; Smajlović, A.; Ostojić, J.; Ahatović, A.; Hadžić, M.; Prašović, S.; Haverić, S.; et al. Biochemical and histomorphological findings in Swiss Wistar rats treated with potential boron-containing therapeutic—K2(B3O3F4OH). J. Trace Elem. Med. Biol. 2020, 62, 126642. [Google Scholar] [CrossRef]
- Haverić, A.; Haverić, S.; Hadžić, M.; Ezić, J.; Ćetković, T.; Galić, B. Moderate Toxicity of Potential Boron-containing Therapeutic, Dipotassium-trioxohydroxytetrafl uorotriborate—K2(B3O3F4OH) in Rats and Mice. Braz. J. Pharm. Sci. 2023, 59, e21384. [Google Scholar] [CrossRef]
- Liu, J.; Phan, E.; Saračević, O.; Burnett, M.; Lojo-Kadrić, N.; Haverić, A.; Galić, B. In vitro study of the anti-proliferative effects of dipotassium-trioxohydroxytetra fluorotriborate on the H520 non-small cell lung cancer cell line. Genet. Appl. 2017, 1, 2–7. [Google Scholar]
- Gwangwa, M.V.; Joubert, A.M.; Visagie, M.H. Crosstalk between the Warburg effect, redox regulation and autophagy induction in tumourigenesis. Cell Mol. Biol. Lett. 2018, 23, 20. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Sweep, F.C.; Geurts-Moespot, A.; Bussink, J.; Span, P.N. Therapeutic targeting of autophagy in cancer. Part I: Molecular pathways controlling autophagy. Semin. Cancer Biol. 2015, 31, 89–98. [Google Scholar] [CrossRef]
- Jia, D.; Park, J.H.; Jung, K.H.; Levine, H.; Kaipparettu, B.A. Elucidating the Metabolic Plasticity of Cancer: Mitochondrial Reprogramming and Hybrid Metabolic States. Cells 2018, 7, 21. [Google Scholar] [CrossRef]
- Zhang, X.D.; Qi, L.; Wu, J.C.; Qin, Z.H. DRAM1 Regulates Autophagy Flux through Lysosomes. PLoS ONE 2013, 8, e63245. [Google Scholar] [CrossRef]
- Galavotti, S.; Bartesaghi, S.; Faccenda, D.; Shaked-Rabi, M.; Sanzone, S.; McEvoy, A.; Dinsdale, D.; Condorelli, F.; Brandner, S.; Campanella, M.; et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene 2013, 32, 699–712. [Google Scholar] [CrossRef]
- Vats, S.; Manjithaya, R. A reversible autophagy inhibitor blocks autophagosome-lysosome fusion by preventing Stx17 loading onto autophagosomes. Mol. Biol. Cell 2019, 30, 2283–2295. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Zhou, C.; Ding, J.; Wu, Y. Resveratrol induces apoptosis of bladder cancer cells via miR21 regulation of the Akt/Bcl2 signaling pathway. Mol. Med. Rep. 2014, 9, 1467–1473. [Google Scholar] [CrossRef]
- Hadžić, M.; Pojskić, L.; Lojo-Kadrić, N.; Haverić, A.; Ramić, J.; Galić, B.; Haverić, S. Novel boron-containing compound, halogenated boroxine, induces selective cytotoxicity through apoptosis triggering in UT-7 leukemia. J. Biochem. Mol. Toxicol. 2022, 36, e23005. [Google Scholar] [CrossRef]
- Kucera, R.; Treskova, I.; Vrzalova, J.; Svobodova, S.; Topolcan, O.; Fuchsova, R.; Rousarova, M.; Treska, V.; Kydlicek, T. Evaluation of IGF1 serum levels in malignant melanoma and healthy subjects. Anticancer. Res. 2014, 34, 5217–5220. [Google Scholar]
- Weroha, S.J.; Haluska, P. IGF-1 receptor inhibitors in clinical trials-early lessons. J. Mammary Gland. Biol. Neoplasia 2008, 13, 471–483. [Google Scholar] [CrossRef]
- Shen, H.M.; Codogno, P. Autophagic cell death: Loch Ness monster or endangered species? Autophagy 2011, 7, 457–465. [Google Scholar] [CrossRef]
- Mehrpour, M.; Esclatine, A.; Beau, I.; Codogno, P. Autophagy in health and disease. 1. Regulation and significance of autophagy: An overview. Am. J. Physiol. Cell Physiol. 2010, 298, 776–785. [Google Scholar] [CrossRef]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Fullgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F.; et al. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef]
- Sawa, C.; Yofu, S.; Kiriyama, K.; Sutoh, K.; Saito, T.; Kishi, S.; Gunji, M.; Inoue, Y.; Sugi, M.; Shioda, S.; et al. High concentration of extracellular nucleotides suppresses cell growth via delayed cell cycle progression in cancer and noncancer cell lines. Heliyon 2021, 7, e08318. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]


| Gene | Reference Sequence (Fasta Acc Number) | Primers |
|---|---|---|
| BECN1 | NM_003766.5 | Forward: CTCCCGAGGTGAAGAGCATC |
| Reverse: GGGGGATGAATCTGCGAGAG | ||
| P62/SQSTM | NM_003900.5 | Forward: CCGTGAAGGCCTACCTTCTG |
| Reverse: TCCTCGTCACTGGAAAAGGC | ||
| BCL-2 | NM_000633.3 | Forward: GGGGTCATGTGTGTGGAGAG |
| Reverse: GAAATCAAACAGAGGCCGCA | ||
| DRAM1 | NM_018370.3 | Forward: TTGGTGCAGCCACGATGTAT |
| Reverse: ACACCACAGACAAAGGCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elez-Burnjaković, N.; Pojskić, L.; Haverić, A.; Lojo-Kadrić, N.; Hadžić Omanović, M.; Smajlović, A.; Kalaydjiev, S.; Maksimović, M.; Joksimović, B.; Haverić, S. Halogenated Boroxine K2[B3O3F4OH] Modulates Metabolic Phenotype and Autophagy in Human Bladder Carcinoma 5637 Cell Line. Molecules 2024, 29, 2919. https://doi.org/10.3390/molecules29122919
Elez-Burnjaković N, Pojskić L, Haverić A, Lojo-Kadrić N, Hadžić Omanović M, Smajlović A, Kalaydjiev S, Maksimović M, Joksimović B, Haverić S. Halogenated Boroxine K2[B3O3F4OH] Modulates Metabolic Phenotype and Autophagy in Human Bladder Carcinoma 5637 Cell Line. Molecules. 2024; 29(12):2919. https://doi.org/10.3390/molecules29122919
Chicago/Turabian StyleElez-Burnjaković, Nikolina, Lejla Pojskić, Anja Haverić, Naida Lojo-Kadrić, Maida Hadžić Omanović, Ajla Smajlović, Svetoslav Kalaydjiev, Milka Maksimović, Bojan Joksimović, and Sanin Haverić. 2024. "Halogenated Boroxine K2[B3O3F4OH] Modulates Metabolic Phenotype and Autophagy in Human Bladder Carcinoma 5637 Cell Line" Molecules 29, no. 12: 2919. https://doi.org/10.3390/molecules29122919
APA StyleElez-Burnjaković, N., Pojskić, L., Haverić, A., Lojo-Kadrić, N., Hadžić Omanović, M., Smajlović, A., Kalaydjiev, S., Maksimović, M., Joksimović, B., & Haverić, S. (2024). Halogenated Boroxine K2[B3O3F4OH] Modulates Metabolic Phenotype and Autophagy in Human Bladder Carcinoma 5637 Cell Line. Molecules, 29(12), 2919. https://doi.org/10.3390/molecules29122919









