Ti3C2Tx Coated with TiO2 Nanosheets for the Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid
Abstract
1. Introduction
2. Result and Discussion
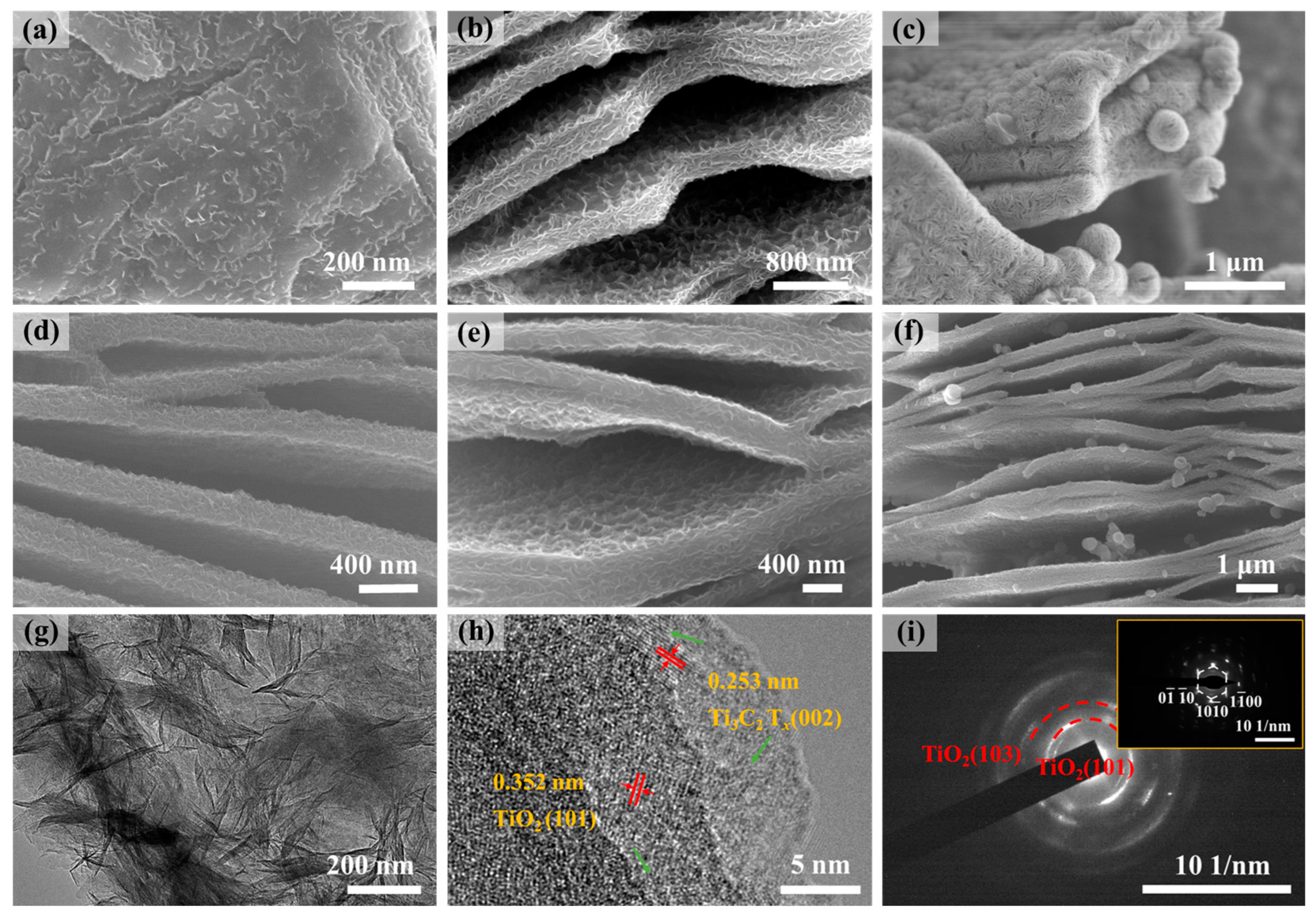
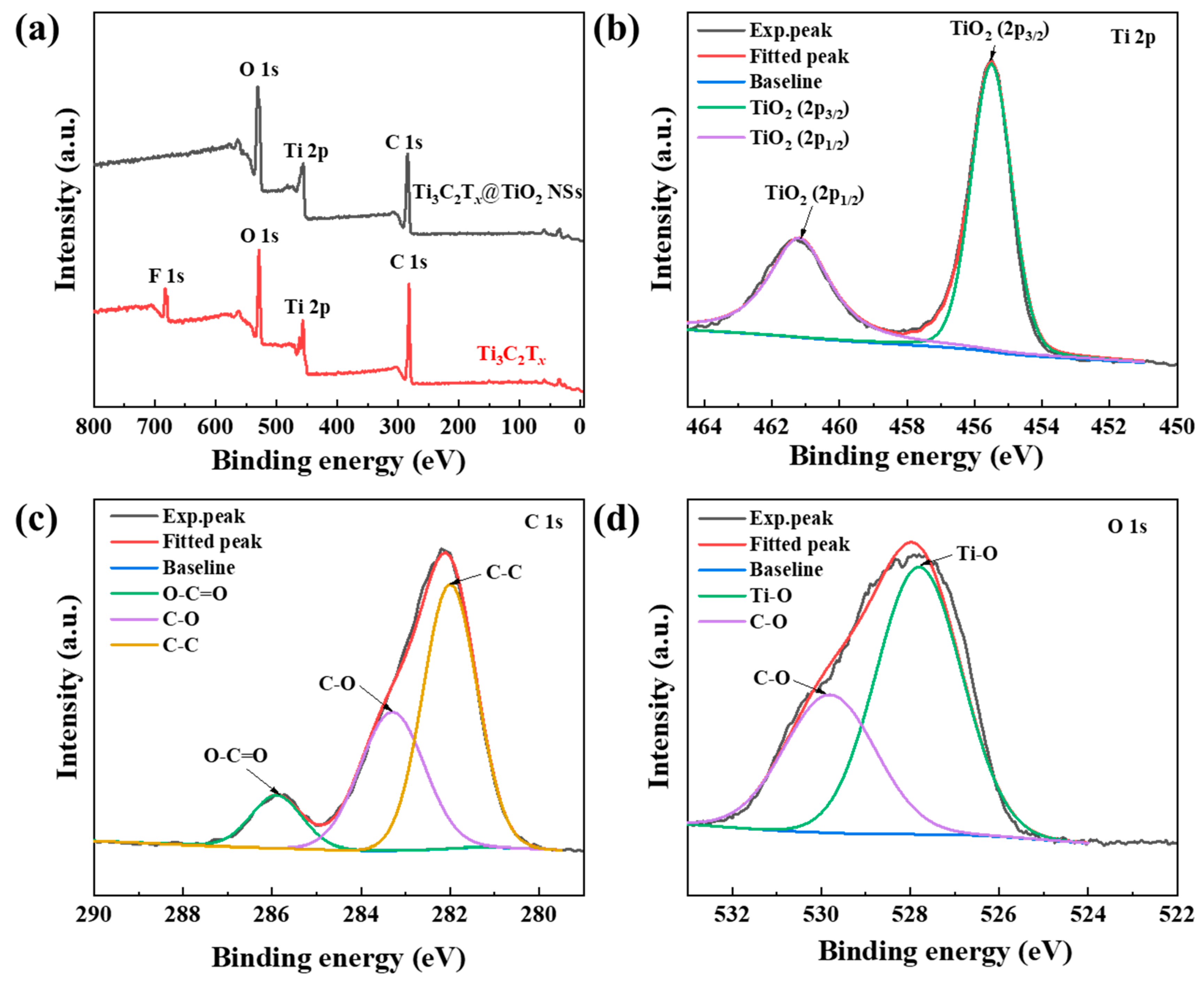
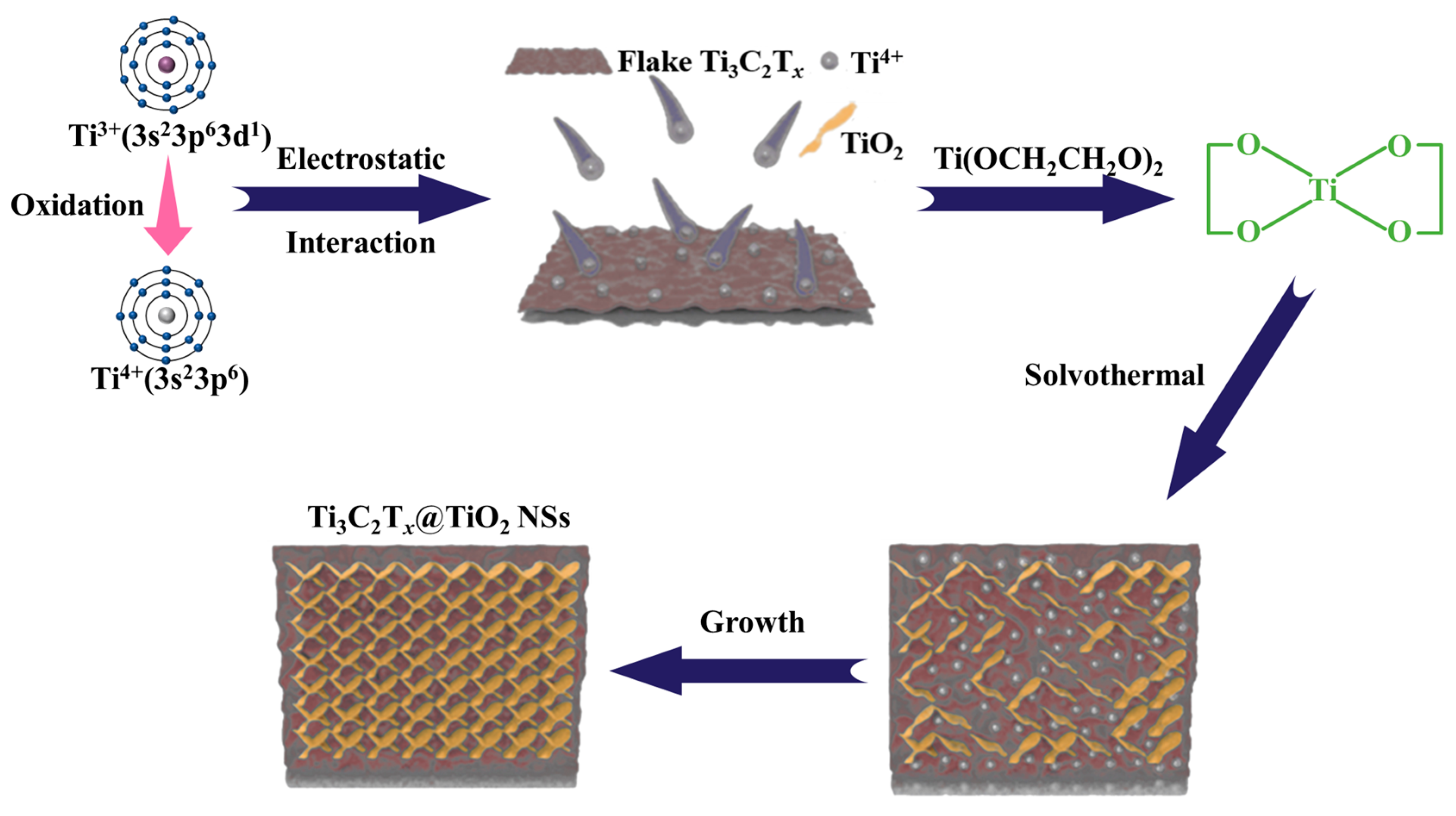
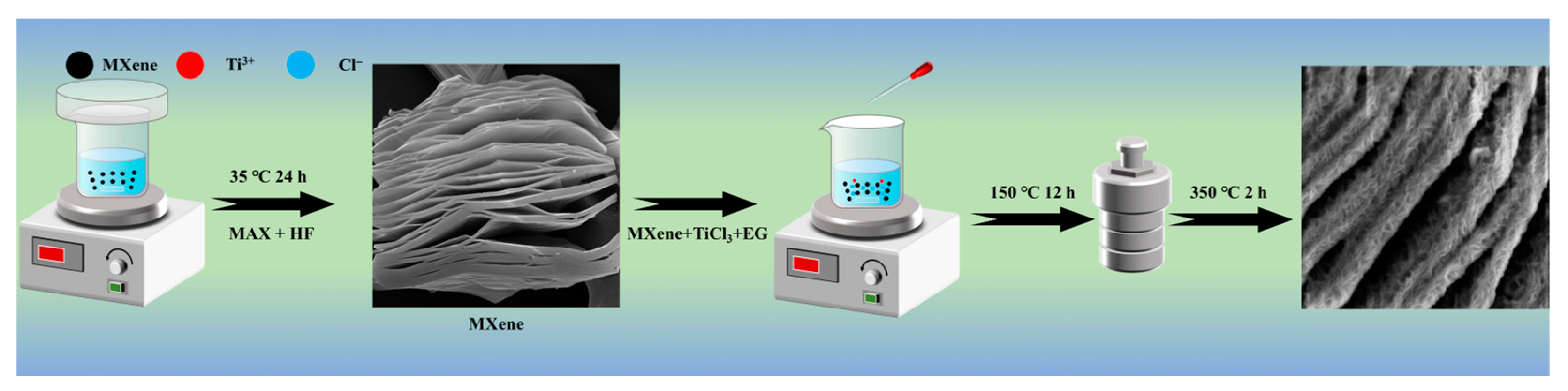
2.1. Microstructural Characterizations of Ti3C2Tx@TiO2 NSs Samples
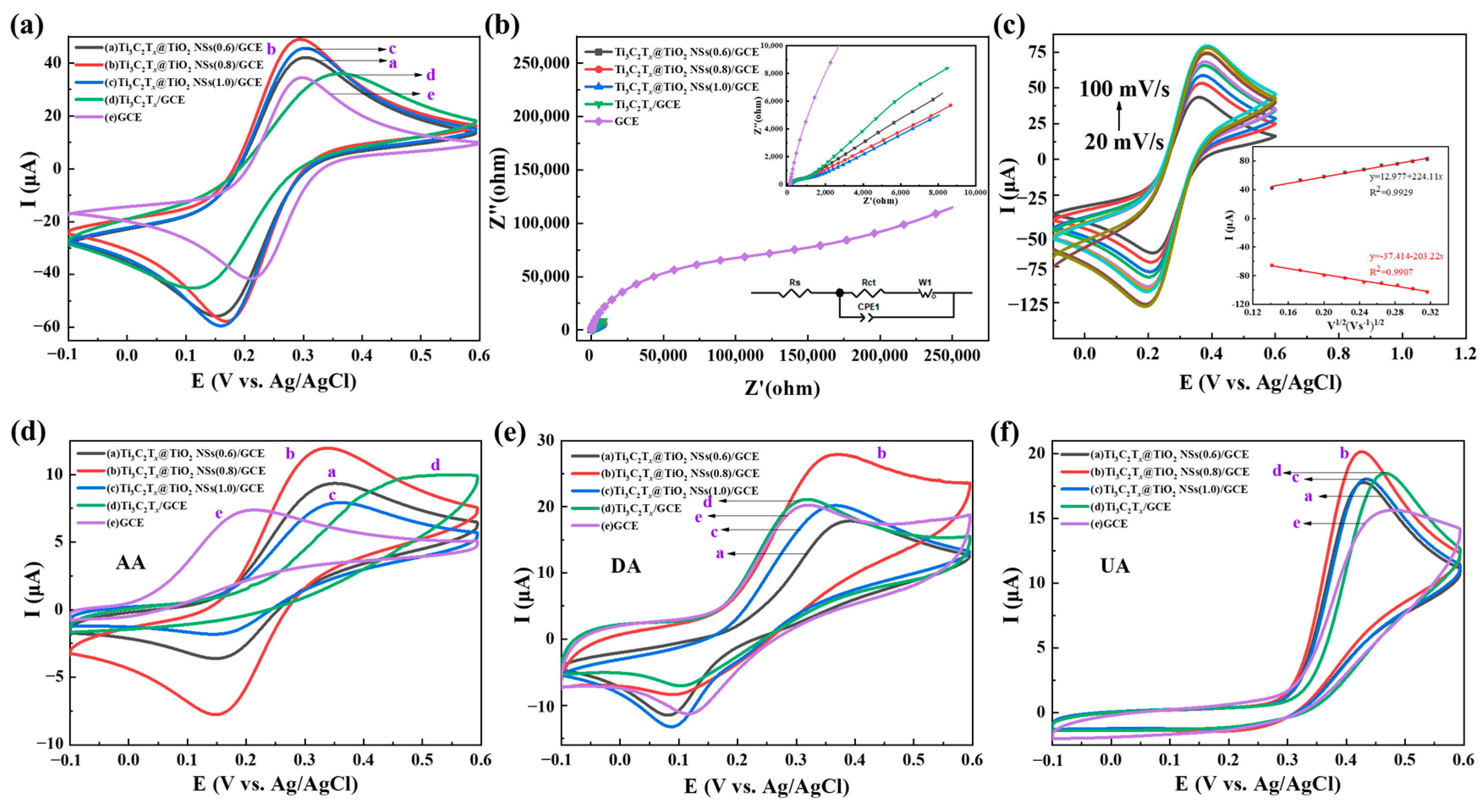
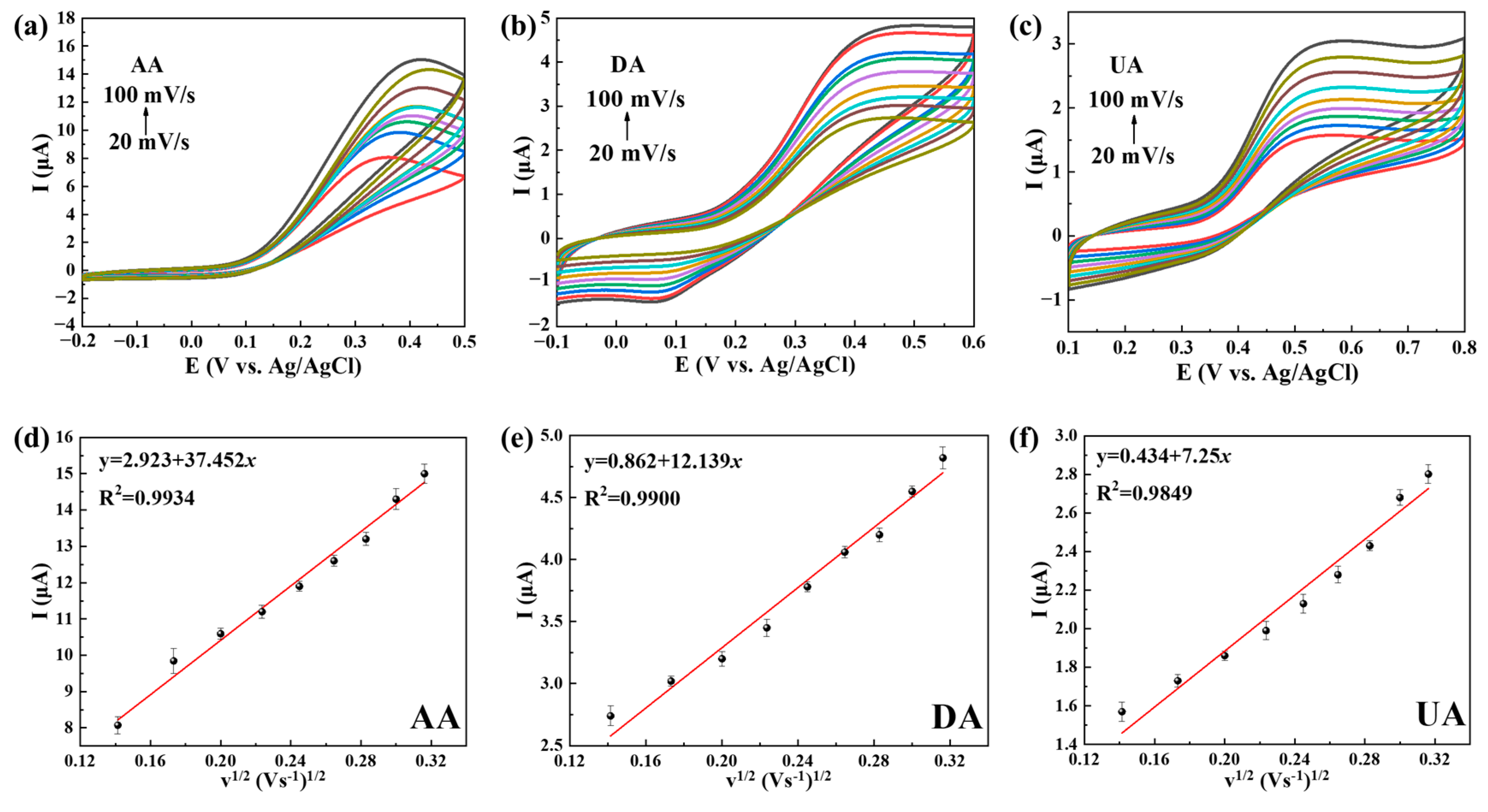
2.2. Electrochemical Behaviors of Ti3C2Tx@TiO2 NSs
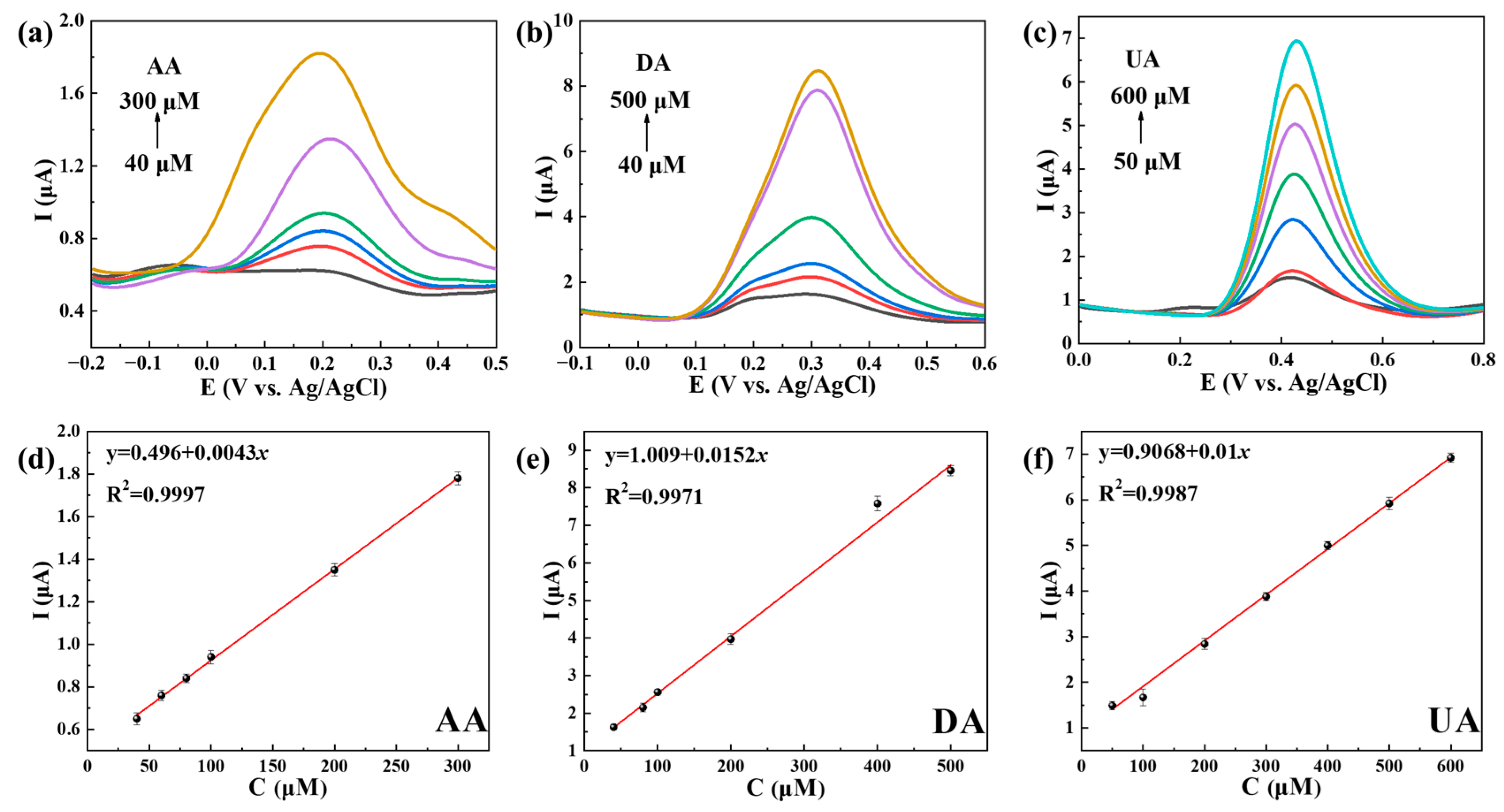
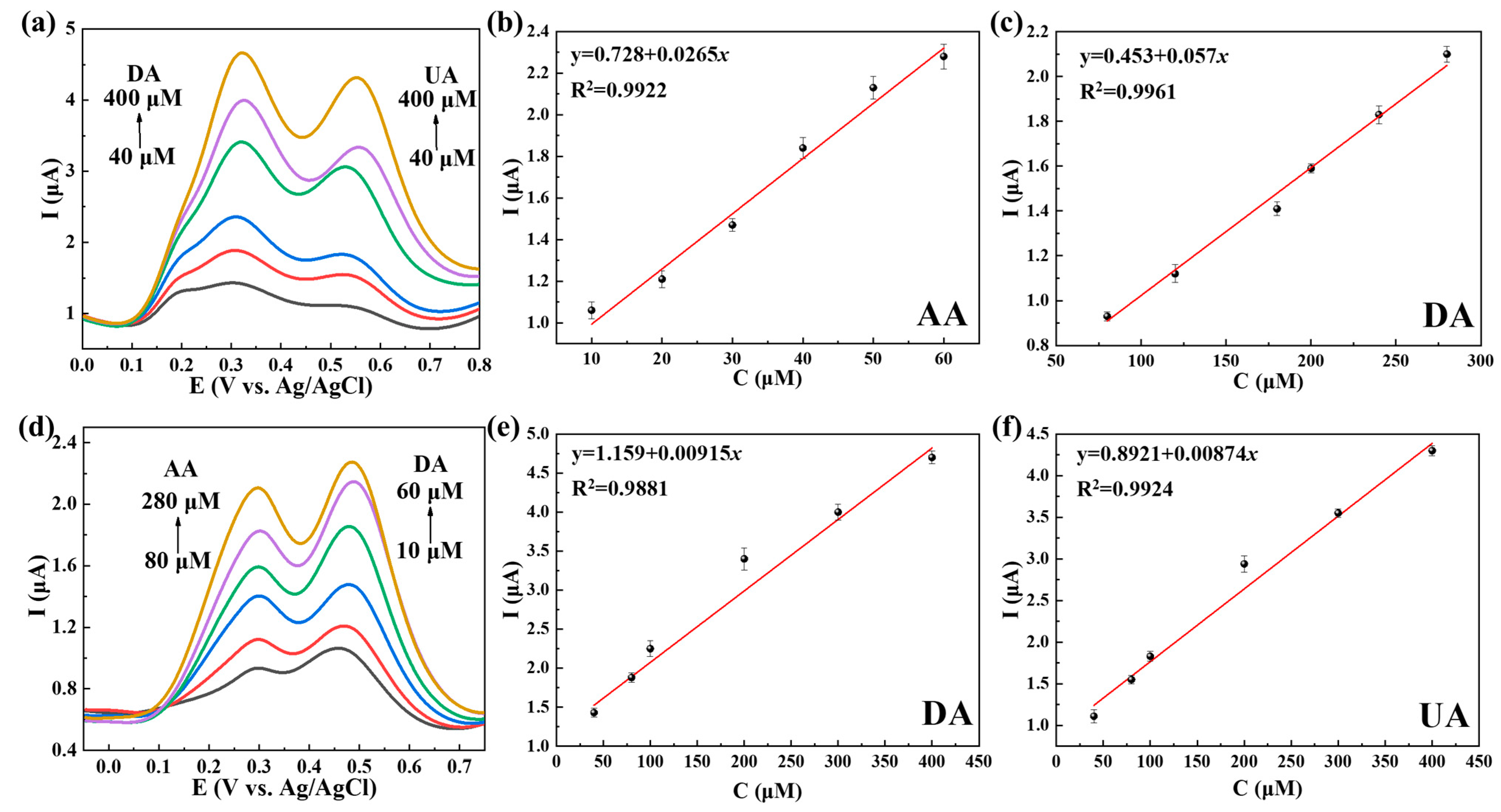
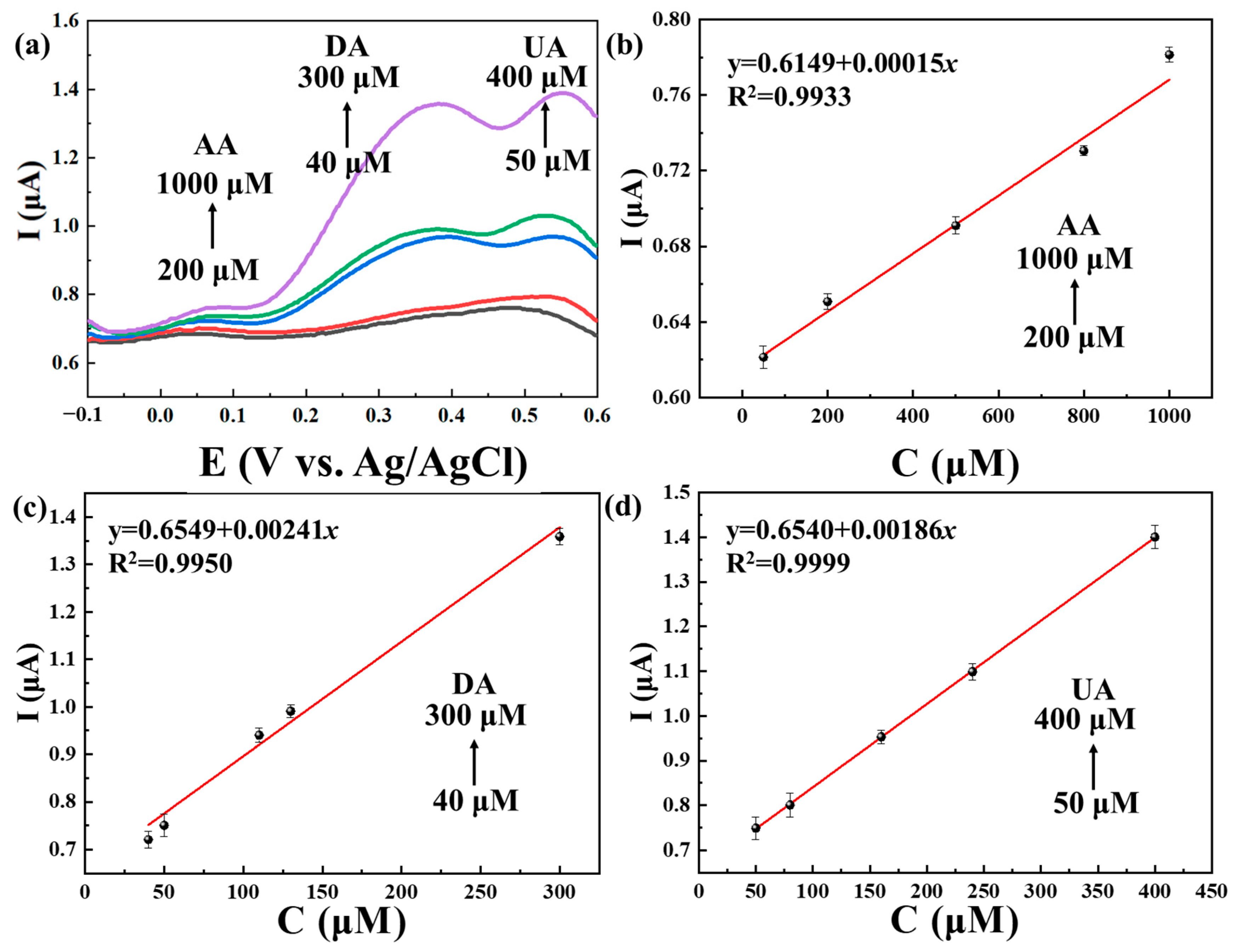
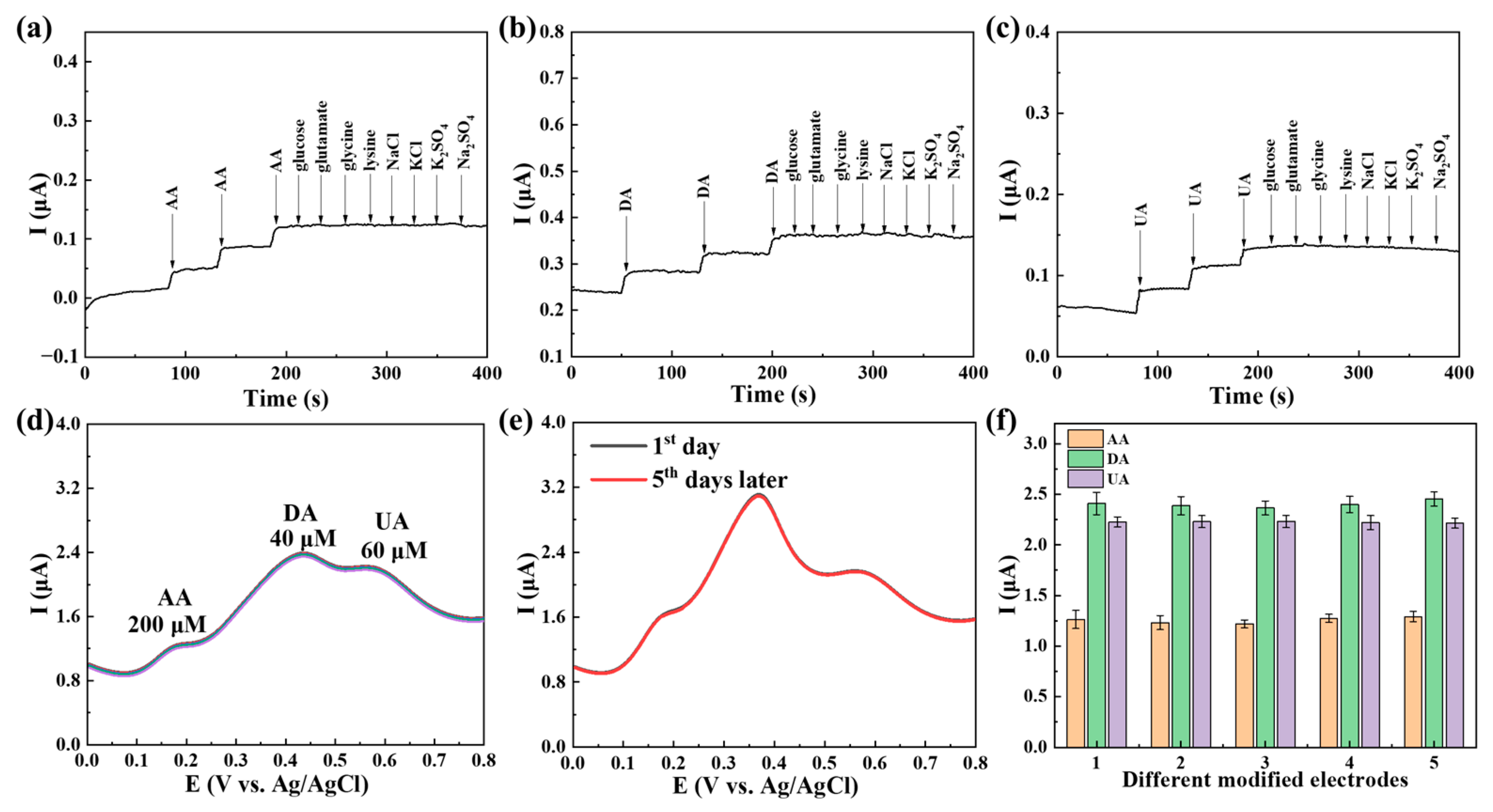
2.3. The Use of Ti3C2Tx@TiO2 NSs/GCE for Separate and Simultaneous Detection of AA, DA and UA
| Electrode | Linear Range (μM) | LOD (μM) | Refs. | ||||
|---|---|---|---|---|---|---|---|
| AA | DA | UA | AA | DA | UA | ||
| FGP | 10–1800 | 1–300 | 5–800 | 4.2 | 0.42 | 2.2 | [46] |
| P-4-ABA/GCE | 20–800 | 5–100 | 1–80 | 5.0 | 1.0 | 0.5 | [47] |
| HNP-PtTi | 200–1000 | 4–500 | 100–1000 | 24.2 | 3.2 | 5.3 | [48] |
| p-TA/nano-Au/GCE | 2.1–50.1 | 0.6–340 | 1.6–110 | 14.8 | 0.05 | 0.1 | [49] |
| Fe3O4/Co3O4/mc@g-C3N4/GCE | 500–8000 | 1–70 | 5–100 | 12.55 | 0.21 | 0.18 | [50] |
| SPGNE | 4–4500 | 0.5–2000 | 0.8–2500 | 0.95 | 0.12 | 0.20 | [8] |
| PG/GCE | 9–2314 | 5–710 | 6–1330 | 6.45 | 2.00 | 4.82 | [51] |
| Pt@g-C3N4/N-CNTs/GCE | 2–2000 | 5–100 | 1–110 | 29.44 | 0.21 | 2.99 | [52] |
| Co/MoSe2/PPy@CNF | 30–3212 | 1.2–536 | 10–1071 | 6.32 | 0.45 | 0.81 | [37] |
| Ti3C2Tx/TiO2 NSs | 200–1000 | 40–300 | 50–400 | 2.9 | 0.19 | 0.25 | This work |
2.4. Reproducibility, Interference Immunity Testing and Stability Analysis
3. Experimental Section
3.1. Chemicals
3.2. Apparatus
3.3. Synthesis of Ti3C2Tx@TiO2 NSs
3.4. Fabrication of the Ti3C2Tx@TiO2 NSs Electrode
3.5. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Huang, H.; Chu, X.; Xie, Y.; Xiong, D.; Yan, C.; Zhao, H.; Zhang, H.; Yang, W. Unraveling and regulating self-discharge behavior of Ti3C2Tx MXene-based supercapacitors. ACS Nano 2020, 14, 4916–4924. [Google Scholar] [CrossRef]
- Xie, H.; Li, P.; Shao, J.; Huang, H.; Chen, Y.; Jiang, Z.; Chu, P.K.; Yu, X.F. Electrostatic self-assembly of Ti3C2Tx MXene and gold nanorods as an efficient surface-enhanced raman scattering platform for reliable and high-sensitivity determination of organic pollutants. ACS Sens. 2019, 4, 2303–2310. [Google Scholar] [CrossRef]
- Song, D.; Jiang, X.; Li, Y.; Lu, X.; Luan, S.; Wang, Y.; Li, Y.; Gao, F. Metal-organic frameworks-derived MnO2/Mn3O4 microcuboids with hierarchically ordered nanosheets and Ti3C2 MXene/Au NPs composites for electrochemical pesticide detection. J. Hazard. Mater. 2019, 373, 367–376. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Li, Y.; Wang, D.; Ma, H.; Ren, H.; Shi, Y.; Han, Y.; Ye, B.C. Electrochemical sensing platform based on the biomass-derived microporous carbons for simultaneous determination of ascorbic acid, dopamine, and uric acid. Biosens. Bioelectron. 2018, 121, 96–103. [Google Scholar] [CrossRef]
- Sabatier, M.; Rytz, A.; Husny, J.; Dubascoux, S.; Nicolas, M.; Dave, A.; Singh, H.; Bodis, M.; Glahn, R.P. Impact of ascorbic acid on the In vitro iron bioavailability of a casein-based iron fortificant. Nutrients 2020, 12, 2776. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Kai, S.; Wang, H.Y.; Yang, J.; Wu, F.G.; Chen, Z. Highly sensitive and selective detection of dopamine using one-pot synthesized highly photoluminescent silicon nanoparticles. Anal. Chem. 2015, 87, 3360–3365. [Google Scholar] [CrossRef]
- Ping, J.; Wu, J.; Wang, Y.; Ying, Y. Simultaneous determination of ascorbic acid, dopamine and uric acid using high-performance screen-printed graphene electrode. Biosens. Bioelectron. 2012, 34, 70–76. [Google Scholar] [CrossRef]
- Shang, N.G.; Papakonstantinou, P.; McMullan, M.; Chu, M.; Stamboulis, A.; Potenza, A.; Dhesi, S.S.; Marchetto, H. Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv. Funct. Mater. 2008, 18, 3506–3514. [Google Scholar] [CrossRef]
- Habibi, B.; Pournaghi-Azar, M.H. Simultaneous determination of ascorbic acid, dopamine and uric acid by use of a MWCNT modified carbon-ceramic electrode and differential pulse voltammetry. Electrochim. Acta 2010, 55, 5492–5498. [Google Scholar] [CrossRef]
- You, Q.; Guo, Z.; Zhang, R.; Chang, Z.; Ge, M.; Mei, Q.; Dong, W.F. Simultaneous recognition of dopamine and uric acid in the presence of ascorbic acid via an intercalated MXene/PPy nanocomposite. Sensors 2021, 21, 3069. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; Wang, E.; Yang, T.; Wang, H.; Hou, X. Ti3C2Tx (MXene)/Pt nanoparticle electrode for the accurate detection of DA coexisting with AA and UA. Dalton Trans. 2022, 51, 4549–4559. [Google Scholar] [CrossRef]
- Song, F.; Li, G.; Zhu, Y.; Wu, Z.; Xie, X.; Zhang, N. Rising from the horizon: Three-dimensional functional architectures assembled with MXene nanosheets. J. Mater. Chem. A 2020, 8, 18538–18559. [Google Scholar] [CrossRef]
- Schultz, T.; Frey, N.C.; Hantanasirisakul, K.; Park, S.; May, S.J.; Shenoy, V.B.; Gogotsi, Y.; Koch, N. Surface termination dependent work function and electronic properties of Ti3C2Tx MXene. Chem. Mater. 2019, 31, 6590–6597. [Google Scholar] [CrossRef]
- Rizwan, K.; Rahdar, A.; Bilal, M.; Iqbal, H.M.N. MXene-based electrochemical and biosensing platforms to detect toxic elements and pesticides pollutants from environmental matrices. Chemosphere 2022, 291, 132820. [Google Scholar] [CrossRef]
- Huang, H.; Dong, C.; Feng, W.; Wang, Y.; Huang, B.; Chen, Y. Biomedical engineering of two-dimensional MXenes. Adv. Drug Deliv. Rev. 2022, 184, 114178. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, B.; Ding, A.; Weng, B.; Chen, J. Synthesis of MXene/DNA/Pd/Pt nanocomposite for sensitive detection of dopamine. J. Electroanal. Chem. 2018, 816, 189–194. [Google Scholar] [CrossRef]
- Amara, U.; Mehran, M.T.; Sarfaraz, B.; Mahmood, K.; Hayat, A.; Nasir, M.; Riaz, S.; Nawaz, M.H. Perylene diimide/MXene-modified graphitic pencil electrode-based electrochemical sensor for dopamine detection. Mikrochim. Acta 2021, 188, 230. [Google Scholar] [CrossRef]
- Zhou, R.; Tu, B.; Xia, D.; He, H.; Cai, Z.; Gao, N.; Chang, G.; He, Y. High-performance Pt/Ti3C2Tx MXene based graphene electrochemical transistor for selective detection of dopamine. Anal. Chim. Acta 2022, 1201, 339653. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Yang, J.; Han, B.; Nie, R.; Wang, J.; Wang, J.; Jing, H. In-situ grown nanocrystal TiO2 on 2D Ti3C2 nanosheets for artificial photosynthesis of chemical fuels. Nano Energy 2018, 51, 442–450. [Google Scholar] [CrossRef]
- Yang, H.; Cao, G.; Huang, Y.; Lin, Y.; Zheng, F.; Lin, L.; Liu, F.; Li, S. Nitrogen-doped carbon@TiO2 double-shelled hollow spheres as an electrochemical sensor for simultaneous determination of dopamine and paracetamol in human serum and saliva. J. Pharm. Anal. 2022, 12, 436–445. [Google Scholar] [CrossRef]
- da Silva, E.P.; Araujo, M.D.S.; Kunita, M.H.; Matos, R.; Medeiros, R.A. Electrochemical sensor based on multi-walled carbon nanotubes and N-doped TiO2 nanoparticles for voltametric simultaneous determination of benserazide and levodopa. Molecules 2022, 27, 8614. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Zhuang, Y.; Jing, W.; Ye, H.; Cui, Z. Assembly of 2D MXene nanosheets and TiO2 nanoparticles for fabricating mesoporous TiO2-MXene membranes. J. Membr. Sci. 2018, 564, 35–43. [Google Scholar] [CrossRef]
- Ono, K.; Kimura, K.; Kato, T.; Hayashi, K.; Rajapakse, R.M.G.; Shimomura, M. Epitaxial growth of a homogeneous anatase TiO2 thin film on LaAlO3 (0 0 1) using a solvothermal method with anticorrosive ligands. Chem. Eng. J. 2023, 451, 138893. [Google Scholar] [CrossRef]
- Liu, L.; Du, Y.-E.; Niu, X.; Li, W.; Li, J.; Yang, X.; Feng, Q. Synthesis, transformation mechanism and photocatalytic properties of various morphologies anatase TiO2 nanocrystals derived from tetratitanate nanobelts. ChemistrySelect 2018, 3, 9953–9959. [Google Scholar] [CrossRef]
- Li, Q.; Huo, C.; Yi, K.; Zhou, L.; Su, L.; Hou, X. Preparation of flake hexagonal BN and its application in electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2018, 260, 346–356. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/polymer membranes: Synthesis, properties, and emerging applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Han, G.; Du, C.; Deng, Q.; Gao, Y.; Yin, G.; Song, Y. Pt decorated Ti3C2 MXene for enhanced methanol oxidation reaction. Ceram. Int. 2019, 45, 2411–2417. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Wang, L.; Wu, W.; Lv, W.; Zhu, J. Room temperature oxidation of Ti3C2MXene for supercapacitor electrodes. J. Electrochem. Soc. 2017, 164, A3933–A3942. [Google Scholar] [CrossRef]
- O’Regan, L.K.B. Preparation of TiO, (anatase) films on electrodes by anodic oxidative hydrolysis of TiCl3. J. Electroanal. Chem. 1993, 346, 291–307. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ishikawa, Y.; Nishida, M.; Ii, S. A new electrochemical method to prepare mesoporous titanium(IV) oxide photocatalyst fixed on alumite substrate. J. Phys. Chem. B 2000, 104, 4204–4209. [Google Scholar] [CrossRef]
- Froschl, T.; Hormann, U.; Kubiak, P.; Kucerova, G.; Pfanzelt, M.; Weiss, C.K.; Behm, R.J.; Husing, N.; Kaiser, U.; Landfester, K.; et al. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Gao, S.; Li, M.; Xu, S.; Li, C.; Guo, W.; Qu, C.; Yang, B. MgO nanobelt-modified graphene-tantalum wire electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2015, 168, 191–198. [Google Scholar] [CrossRef]
- Sudhakara, S.M.; Kotresh, H.M.N.; Devendrachari, M.C.; Khan, F. Synthesis and electrochemical investigation of tetra amino cobalt (II) phthalocyanine functionalized polyaniline nanofiber for the selective detection of dopamine. Electroanalysis 2020, 32, 1807–1817. [Google Scholar] [CrossRef]
- Demirkan, B.; Bozkurt, S.; Cellat, K.; Arikan, K.; Yilmaz, M.; Savk, A.; Calimli, M.H.; Nas, M.S.; Atalar, M.N.; Alma, M.H.; et al. Palladium supported on polypyrrole/reduced graphene oxide nanoparticles for simultaneous biosensing application of ascorbic acid, dopamine, and uric acid. Sci. Rep. 2020, 10, 2946. [Google Scholar] [CrossRef]
- Cogal, G.C.; Cogal, S.; Machata, P.; Oksuz, A.U.; Omastová, M. Electrospun cobalt-doped 2D-MoSe2/polypyrrole hybrid-based carbon nanofibers as electrochemical sensing platforms. Microchimica. Acta 2024, 191, 75. [Google Scholar] [CrossRef]
- Yan, J.; Liu, S.; Zhang, Z.; He, G.; Zhou, P.; Liang, H.; Tian, L.; Zhou, X.; Jiang, H. Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid based on graphene anchored with Pd-Pt nanoparticles. Colloids Surf. B 2013, 111, 392–397. [Google Scholar] [CrossRef]
- Peng, C.; Yang, X.; Li, Y.; Yu, H.; Wang, H.; Peng, F. Hybrids of two-dimensional Ti3C2 and TiO2 exposing 001 facets toward enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2016, 8, 6051–6060. [Google Scholar] [CrossRef]
- Li, H.; Zhou, K.; Cao, J.; Wei, Q.; Lin, C.-T.; Pei, S.E.; Ma, L.; Hu, N.; Guo, Y.; Deng, Z.; et al. A novel modification to boron-doped diamond electrode for enhanced, selective detection of dopamine in human serum. Carbon 2021, 171, 16–28. [Google Scholar] [CrossRef]
- Blom, L.B.; Morrison, G.M.; Roux, M.S.; Mills, G.; Greenwood, R. Metal diffusion properties of a Nafion-coated porous membrane in an aquatic passive sampler system. J. Environ. Monit. 2003, 5, 404–409. [Google Scholar] [CrossRef]
- Tan, C.K.; Loh, K.P.; John, T.T. Direct amperometric detection of glucose on a multiple-branching carbon nanotube forest. Analyst 2008, 133, 448–451. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, K.; Zhang, H.; Mao, L. Layer-by-layer assembled carbon nanotubes for selective determination of dopamine in the presence of ascorbic acid. Biosens. Bioelectron. 2005, 20, 1270–1276. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Chen, W.; Wu, P. Electrocatalytic oxidation and determination of dopamine in the presence of ascorbic acid and uric acid at a poly (p-nitrobenzenazo resorcinol) modified glassy carbon electrode. Sens. Actuators B Chem. 2007, 122, 309–314. [Google Scholar] [CrossRef]
- Alwarappan, S.; Liu, G.; Li, C.Z. Simultaneous detection of dopamine, ascorbic acid, and uric acid at electrochemically pretreated carbon nanotube biosensors. Nanomedicine 2010, 6, 52–57. [Google Scholar] [CrossRef]
- Dung, N.Q.; Toan, T.Q.; Chuyen, P.H.; Nang, L.V.; Dang, N.V.; Hien, T.N.; Anh, L.P.; Thanh, D.V. Straightforward method for the electrochemical identification of dopamine in the presence of uric acid and ascorbic acid. Meas. Sci. Technol. 2024, 35, 055114. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, X.; Ji, X.; Lin, R.; Lin, W. Simultaneous determination of ascorbic acid, dopamine and uric acid using poly(4-aminobutyric acid) modified glassy carbon electrode. Sens. Actuators B Chem. 2013, 178, 359–365. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, R.; Chai, Y.; Zhang, Y.; Hu, F.; Zhang, M. Au-nanoclusters incorporated 3-amino-5-mercapto-1,2,4-triazole film modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2011, 30, 315–319. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Y.; Wang, Z.-W.; Song, Y.; Wang, M.; Zhang, Z.; Liu, C.-S. Bimetallic-organic framework derived porous Co3O4/Fe3O4/C-loaded g-C3N4 nanocomposites as non-enzymic electrocatalysis oxidization toward ascorbic acid, dopamine acid, and uric acid. Appl. Surf. Sci. 2018, 441, 694–707. [Google Scholar] [CrossRef]
- Qi, S.; Zhao, B.; Tang, H.; Jiang, X. Determination of ascorbic acid, dopamine, and uric acid by a novel electrochemical sensor based on pristine graphene. Electrochim. Acta 2015, 161, 395–402. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, L.; Peng, J.; Hou, X.; Du, H. Highly sensitive and simultaneous detection of ascorbic acid, dopamine, and uric acid using Pt@g-C3N4/N-CNTs nanocomposites. iScience 2024, 27, 109241. [Google Scholar] [CrossRef]
- Patel, B.R.; Imran, S.; Ye, W.; Weng, H.; Noroozifar, M.; Kerman, K. Simultaneous voltammetric detection of six biomolecules using a nanocomposite of titanium dioxide nanorods with multi-walled carbon nanotubes. Electrochim. Acta 2020, 362, 137094. [Google Scholar] [CrossRef]
- Sun, L.; Li, H.; Li, M.; Li, P.; Li, C.; Yangb, B. Simultaneous determination of small biomolecules and nitrite using an Au/TiO2/Carbon nanotube composite-modified electrode. J. Electrochem. Soc. 2016, 163, 567–572. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.X.; Wang, S.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, D.; Yang, T.; Wang, K.; Wang, H.; Wang, E.; Chou, K.-C.; Hou, X. Ti3C2Tx Coated with TiO2 Nanosheets for the Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Molecules 2024, 29, 2915. https://doi.org/10.3390/molecules29122915
Jia D, Yang T, Wang K, Wang H, Wang E, Chou K-C, Hou X. Ti3C2Tx Coated with TiO2 Nanosheets for the Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Molecules. 2024; 29(12):2915. https://doi.org/10.3390/molecules29122915
Chicago/Turabian StyleJia, Dengzhou, Tao Yang, Kang Wang, Hongyang Wang, Enhui Wang, Kuo-Chih Chou, and Xinmei Hou. 2024. "Ti3C2Tx Coated with TiO2 Nanosheets for the Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid" Molecules 29, no. 12: 2915. https://doi.org/10.3390/molecules29122915
APA StyleJia, D., Yang, T., Wang, K., Wang, H., Wang, E., Chou, K.-C., & Hou, X. (2024). Ti3C2Tx Coated with TiO2 Nanosheets for the Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Molecules, 29(12), 2915. https://doi.org/10.3390/molecules29122915








