Insight into the Discriminative Efficiencies and Mechanisms of Peroxy Activation via Fe/Cu Bimetallic Catalysts for Wastewater Purification
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Materials
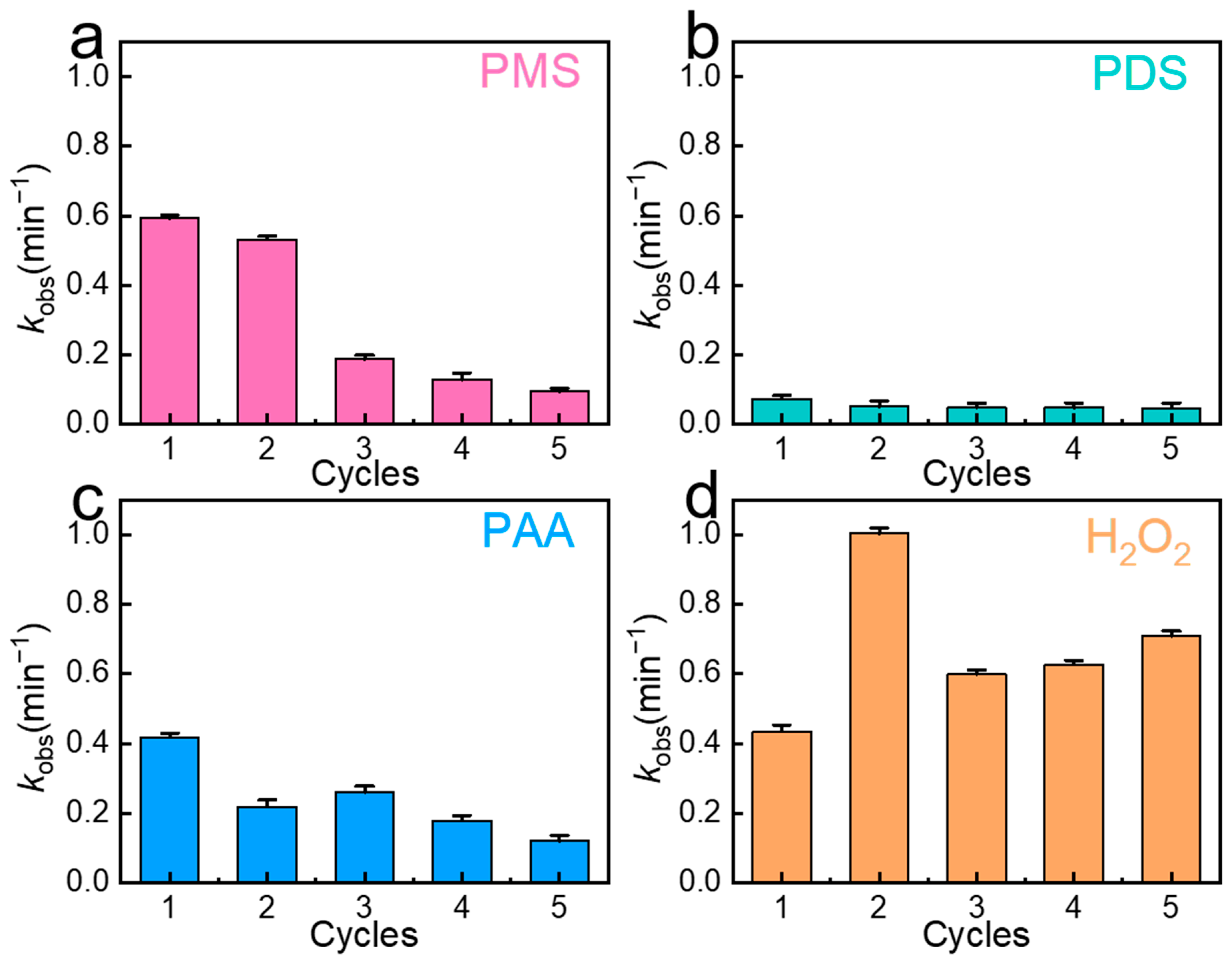
2.2. Catalytic Properties and Reproducibility of Materials
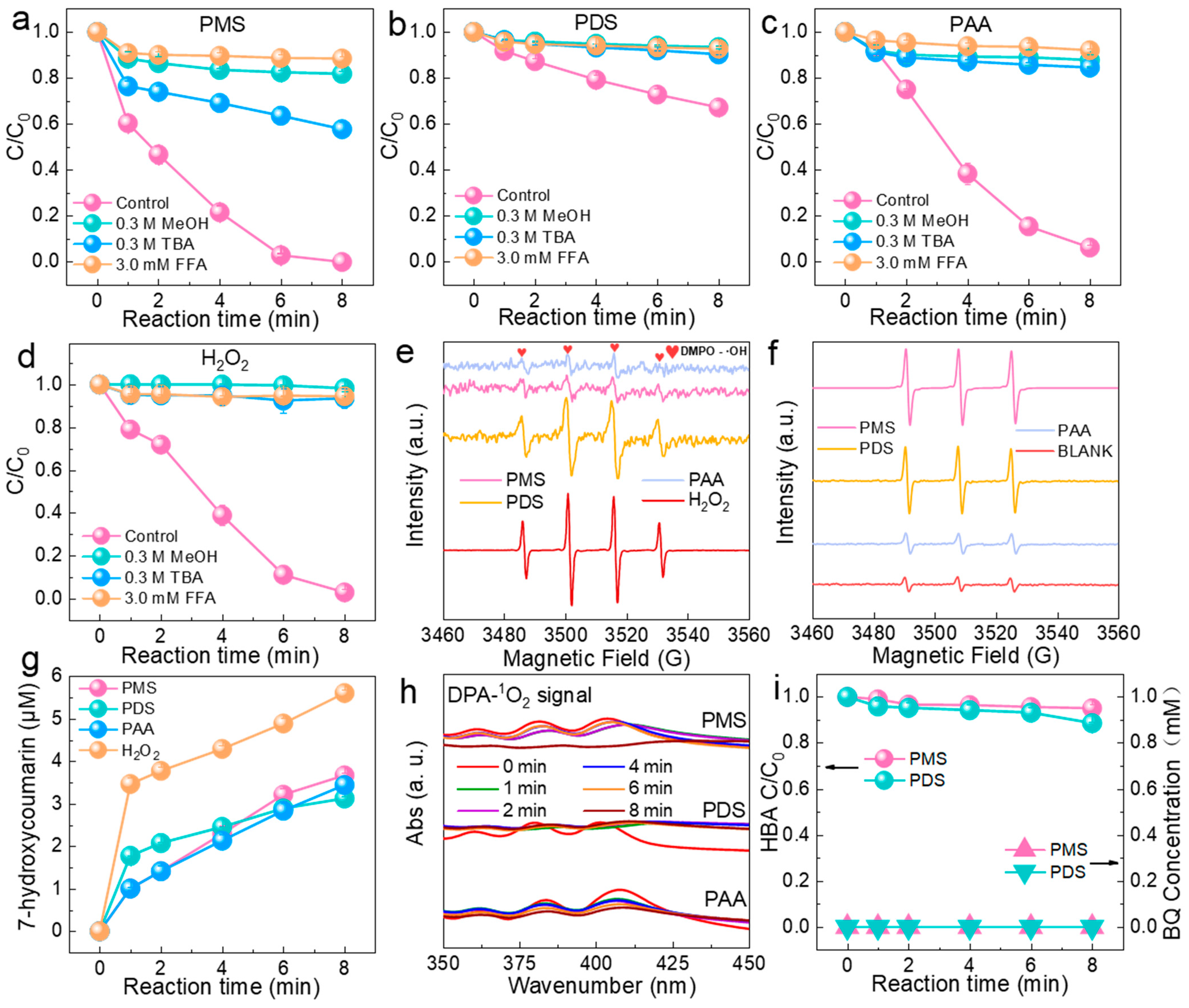
2.3. Identification of Reactive Oxygen Species (ROS)
2.4. Effects of Co-Existing Substances
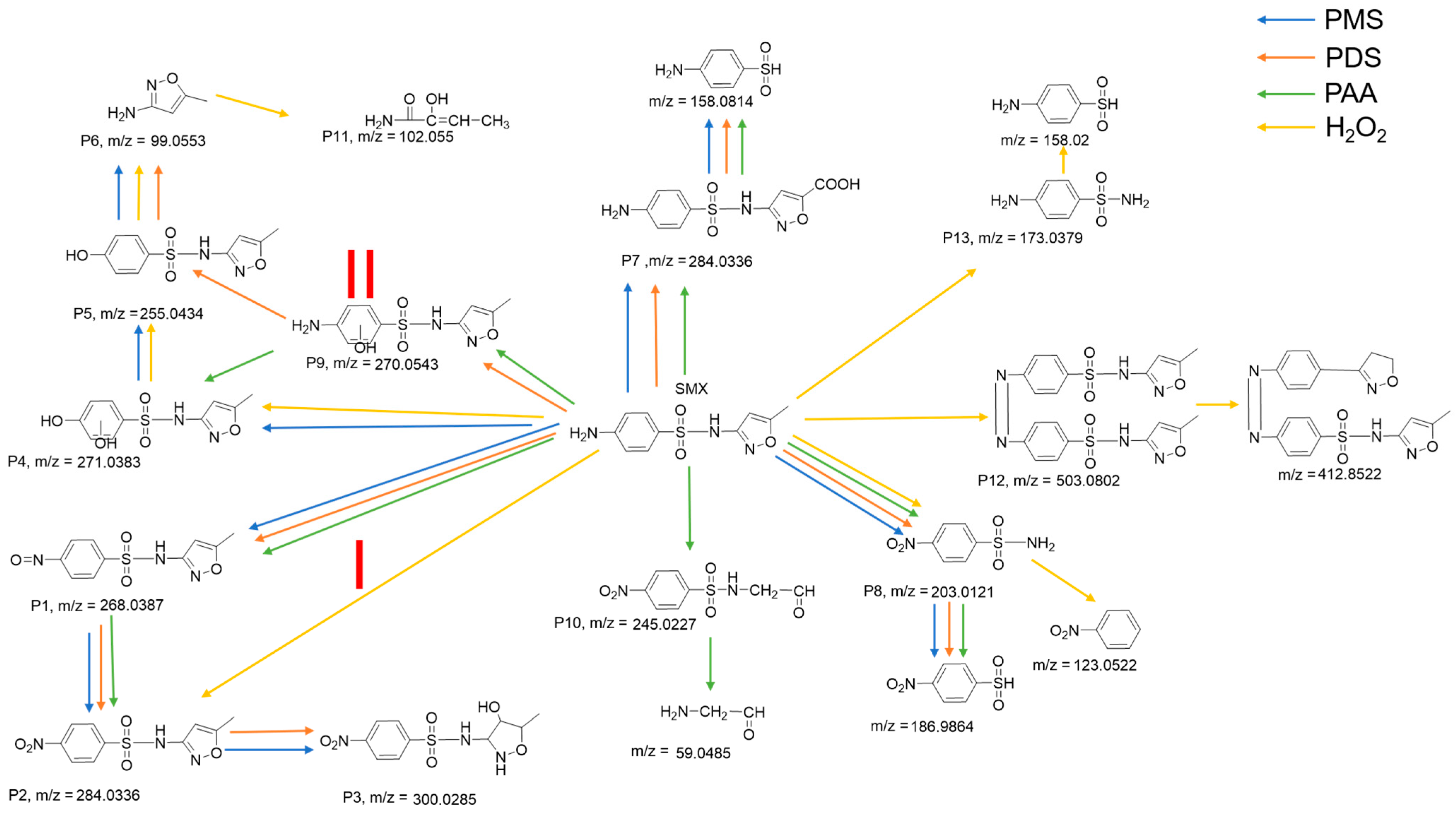
2.5. Possible Degradation Pathway
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Experimental Procedure
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eryildiz, B.; Yavuzturk Gul, B.; Koyuncu, I. A sustainable approach for the removal methods and analytical determination methods of antiviral drugs from water/wastewater: A review. J. Water Process Eng. 2022, 49, 103036. [Google Scholar] [CrossRef] [PubMed]
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and personal care products in waters: Occurrence, toxicity, and risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Krasucka, P.; Rombel, A.; Yang, X.J.; Rakowska, M.; Xing, B.; Oleszczuk, P. Adsorption and desorption of antiviral drugs (ritonavir and lopinavir) on sewage sludges as a potential environmental risk. J. Hazard. Mater. 2022, 425, 127901. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, D.; Li, J.; Wu, F.; Brigante, M.; Mailhot, G. Rapid oxidation of paracetamol by Cobalt(II) catalyzed sulfite at alkaline pH. Catal. Today 2018, 313, 155–160. [Google Scholar] [CrossRef]
- Xie, P.; Guo, Y.; Chen, Y.; Wang, Z.; Shang, R.; Wang, S.; Ding, J.; Wan, Y.; Jiang, W.; Ma, J. Application of a novel advanced oxidation process using sulfite and zero-valent iron in treatment of organic pollutants. Chem. Eng. J. 2017, 314, 240–248. [Google Scholar] [CrossRef]
- Zhou, S.; Li, H.; Wu, Z.; Li, S.; Cao, Z.; Ma, B.; Zou, Y.; Zhang, N.; Liu, Z.; Wang, Y.; et al. The addition of nano zero-valent iron during compost maturation effectively removes intracellular and extracellular antibiotic resistance genes by reducing the abundance of potential host bacteria. Bioresour. Technol. 2023, 384, 129350. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Jassby, D.; Zhang, Y.; Keller, A.A.; Adeleye, A.S. Comparison of the colloidal stability, mobility, and performance of nanoscale zerovalent iron and sulfidated derivatives. J. Hazard. Mater. 2020, 396, 122691. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, L.F.; Torrey, J.D.; Amaro, R.L.; Shaw, J.M. Kinetics of zero valent iron nanoparticle oxidation in oxygenated water. Environ. Sci. Technol. 2012, 46, 12913–12920. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Jiang, Z.; Zhang, C.; Deng, J.; Hou, K.; Cheng, Y.; Zhang, L.; Zeng, G. Removal of tetracycline by Fe/Ni bimetallic nanoparticles in aqueous solution. J. Colloid. Interface Sci. 2018, 513, 117–125. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. One-step green synthesis of bimetallic Fe/Pd nanoparticles used to degrade Orange II. J. Hazard. Mater. 2016, 303, 145–153. [Google Scholar] [CrossRef]
- Fu, C.; Yi, X.; Liu, Y.; Zhou, H. Cu(2+) activated persulfate for sulfamethazine degradation. Chemosphere 2020, 257, 127294. [Google Scholar] [CrossRef]
- Xu, W.; Huang, D.; Wang, G.; Li, S.; Du, L.; Zhou, W.; Huang, H. Enhanced sulfamethazine degradation via peroxydisulfate activation by biochar-supported nano zero-valent iron-copper: The key role of Fe(Ⅳ) and electron transfer induced by doped Cu. J. Clean. Prod. 2024, 434, 140133. [Google Scholar] [CrossRef]
- Qin, W.; Fang, G.; Wang, Y.; Zhou, D. Mechanistic understanding of polychlorinated biphenyls degradation by peroxymonosulfate activated with CuFe2O4 nanoparticles: Key role of superoxide radicals. Chem. Eng. J. 2018, 348, 526–534. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Dong, H.; Pang, Z.; Zhao, M.; Huang, D.; Dong, J.; Li, L. Highly efficient activation of peracetic acid via zero-valent iron-copper bimetallic nanoparticles (nZVIC) for the oxidation of sulfamethazine in aqueous solution under neutral condition. Appl. Catal. B Environ. 2024, 340, 123183. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Yan, D.; Wang, L.; Liu, X. Efficient Reduction of Bromate by Iodide-Assisted UV/Sulfite Process. Catalysts 2018, 8, 652. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yang, S.Y.; Wang, M.X.; Guan, Y.H.; Ma, J. Fast degradation of atrazine by nZVI-Cu(0)/PMS: Re-evaluation and quantification of reactive species, generation pathways, and application feasibility. Water Res. 2023, 243, 120311. [Google Scholar] [CrossRef]
- Song, W.; Zhou, Y.; Wang, Z.; Li, J.; Zhang, X.; Fu, C.; Du, X.; Wang, Z.; Qiu, W. Accelerate sulfamethoxazole degradation and detoxification by persulfate mediated with Fe(2+)&dithionite: Experiments and DFT calculation. J. Hazard. Mater. 2022, 436, 129254. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.R.; He, C.S.; Xie, Z.H.; Li, L.L.; Xiong, Z.K.; Zhang, H.; Zhou, P.; Jiang, F.; Mu, Y.; Lai, B. Efficient activation of PAA by FeS for fast removal of pharmaceuticals: The dual role of sulfur species in regulating the reactive oxidized species. Water Res. 2022, 217, 118402. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ge, L.; Peng, X.; Wang, W.; Zhang, W. Enhanced degradation of sulfamethoxazole by a novel Fenton-like system with significantly reduced consumption of H(2)O(2) activated by g-C(3)N(4)/MgO composite. Water Res. 2021, 190, 116777. [Google Scholar] [CrossRef]
- Zhang, C.; Brown, P.J.B.; Miles, R.J.; White, T.A.; Grant, D.G.; Stalla, D.; Hu, Z. Inhibition of regrowth of planktonic and biofilm bacteria after peracetic acid disinfection. Water Res. 2019, 149, 640–649. [Google Scholar] [CrossRef]
- Dominguez Henao, L.; Turolla, A.; Antonelli, M. Disinfection by-products formation and ecotoxicological effects of effluents treated with peracetic acid: A review. Chemosphere 2018, 213, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Pan, D.; Cao, C.; Wang, Q.; Chen, F.-X. Diastereoselective and Enantioselective Epoxidation of Acyclic β-Trifluoromethyl-β,β-Disubstituted Enones by Hydrogen Peroxide with a Pentafluorinated Quinidine-Derived Phase-Transfer Catalyst. Adv. Synth. Catal. 2013, 355, 1917–1923. [Google Scholar] [CrossRef]
- Maggi, R.; Martra, G.; Piscopo, C.G.; Alberto, G.; Sartori, G. Oxidation of alkenes to 1,2-diols: FT-IR and UV studies of silica-supported sulfonic acid catalysts and their interaction with H2O and H2O2. J. Catal. 2012, 294, 19–28. [Google Scholar] [CrossRef]
- De Faveri, G.; Ilyashenko, G.; Watkinson, M. Recent advances in catalytic asymmetric epoxidation using the environmentally benign oxidant hydrogen peroxide and its derivatives. Chem. Soc. Rev. 2011, 40, 1722–1760. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hu, Y.; Xiao, J.; Huang, Y.; Wang, M.; Yang, H.; Zou, J.; Yuan, B.; Ma, J. Enhanced diclofenac elimination in Fe(II)/peracetic acid process by promoting Fe(III)/Fe(II) cycle with ABTS as electron shuttle. Chem. Eng. J. 2021, 420. [Google Scholar] [CrossRef]
- Cai, H.; Liu, X.; Zou, J.; Xiao, J.; Yuan, B.; Li, F.; Cheng, Q. Multi-wavelength spectrophotometric determination of hydrogen peroxide in water with peroxidase-catalyzed oxidation of ABTS. Chemosphere 2018, 193, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pu, X.; Yuan, Y.; Xiang, Y.; Zhang, Y.; Xiong, Z.; Yao, G.; Lai, B. An old story with new insight into the structural transformation and radical production of micron-scale zero-valent iron on successive reactivities. Chin. Chem. Lett. 2020, 31, 2634–2640. [Google Scholar] [CrossRef]
- Lyu, J.; Ge, M.; Hu, Z.-w.; Guo, C. One-pot synthesis of magnetic CuO/Fe2O3/CuFe2O4 nanocomposite to activate persulfate for levofloxacin removal: Investigation of efficiency, mechanism and degradation route. Chem. Eng. J. 2020, 389, 124456. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, C.-S.; Tu, Y.-J.; Huang, Y.-H.; Zhang, H. Heterogeneous Degradation of Organic Pollutants by Persulfate Activated by CuO-Fe3O4: Mechanism, Stability, and Effects of pH and Bicarbonate Ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron—A SEM, TEM and XPS study. Mater. Res. Bull. 2010, 45, 1361–1367. [Google Scholar] [CrossRef]
- Kamil, M.P.; Kaseem, M.; Ko, Y.G. Soft plasma electrolysis with complex ions for optimizing electrochemical performance. Sci. Rep. 2017, 7, 44458. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Dong, H.; Li, Y.; Xiao, J.; Xiang, S.; Hou, X.; Chu, D. Degradation of sulfamethazine in water by sulfite activated with zero-valent Fe-Cu bimetallic nanoparticles. J. Hazard. Mater. 2022, 431, 128601. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, Z.; Shi, H.; Wu, Z.; Huang, B.; Zhang, H.; Zhou, P.; Pan, Z.; Liu, W.; Lai, B. Switching the reaction mechanisms and pollutant degradation routes through active center size-dependent Fenton-like catalysis. Appl. Catal. B Environ. 2023, 329. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Duan, X.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S. N-Doped Graphene from Metal–Organic Frameworks for Catalytic Oxidation of p-Hydroxylbenzoic Acid: N-Functionality and Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 2693–2701. [Google Scholar] [CrossRef]
- De-Nasri, S.J.; Nagarajan, S.; Robertson, P.K.J.; Ranade, V.V. Quantification of hydroxyl radicals in photocatalysis and acoustic cavitation: Utility of coumarin as a chemical probe. Chem. Eng. J. 2021, 420, 127560. [Google Scholar] [CrossRef]
- Shao, B.; Dong, H.; Sun, B.; Guan, X. Role of Ferrate(IV) and Ferrate(V) in Activating Ferrate(VI) by Calcium Sulfite for Enhanced Oxidation of Organic Contaminants. Environ. Sci. Technol. 2018, 53, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Hai, H.; Xing, X.; Li, S.; Xia, S.; Xia, J. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Sci. Total Environ. 2020, 738, 139909. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Xie, Z.; Shi, Y.; Zhang, H.; Luo, M.; Yu, S.-Y.; Zhao, J.; Liu, Y.; Zhou, P.; Xiong, Z.; Lai, B. Enhanced Removal of Sulfamethoxazole by a Fe(VI)/Redox Mediator System: Insights into the Key Role of Fe(V). ACS EST Eng. 2023, 3, 1728–1737. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Fan, L.; Xiong, Z.; Lai, B. Insight into the Discriminative Efficiencies and Mechanisms of Peroxy Activation via Fe/Cu Bimetallic Catalysts for Wastewater Purification. Molecules 2024, 29, 2868. https://doi.org/10.3390/molecules29122868
Xu T, Fan L, Xiong Z, Lai B. Insight into the Discriminative Efficiencies and Mechanisms of Peroxy Activation via Fe/Cu Bimetallic Catalysts for Wastewater Purification. Molecules. 2024; 29(12):2868. https://doi.org/10.3390/molecules29122868
Chicago/Turabian StyleXu, Tingjin, Lu Fan, Zhaokun Xiong, and Bo Lai. 2024. "Insight into the Discriminative Efficiencies and Mechanisms of Peroxy Activation via Fe/Cu Bimetallic Catalysts for Wastewater Purification" Molecules 29, no. 12: 2868. https://doi.org/10.3390/molecules29122868
APA StyleXu, T., Fan, L., Xiong, Z., & Lai, B. (2024). Insight into the Discriminative Efficiencies and Mechanisms of Peroxy Activation via Fe/Cu Bimetallic Catalysts for Wastewater Purification. Molecules, 29(12), 2868. https://doi.org/10.3390/molecules29122868






