Synthesis of a New Class of β-Carbonyl Selenides Functionalized with Ester Groups with Antioxidant and Anticancer Properties—Part II
Abstract
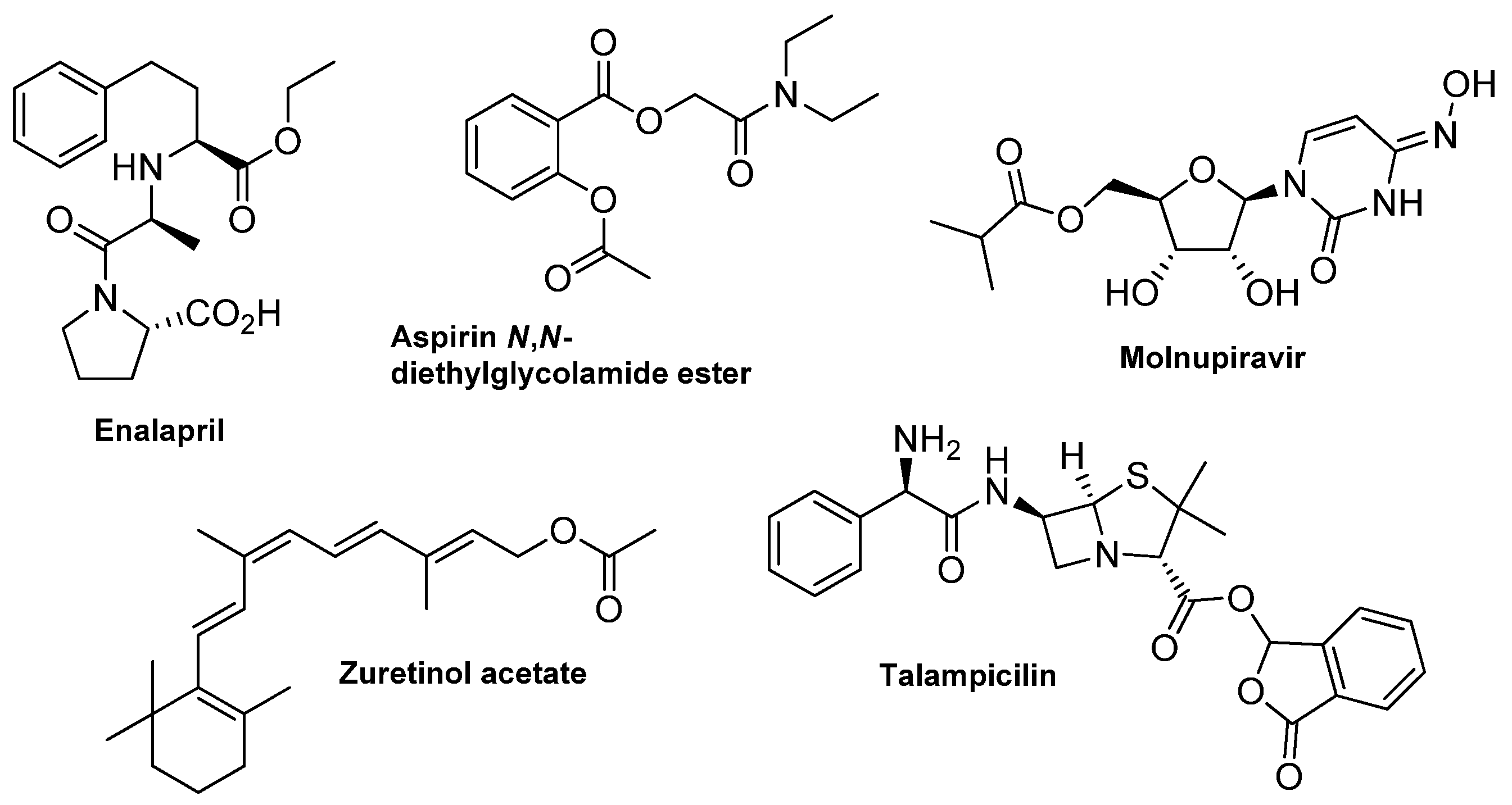
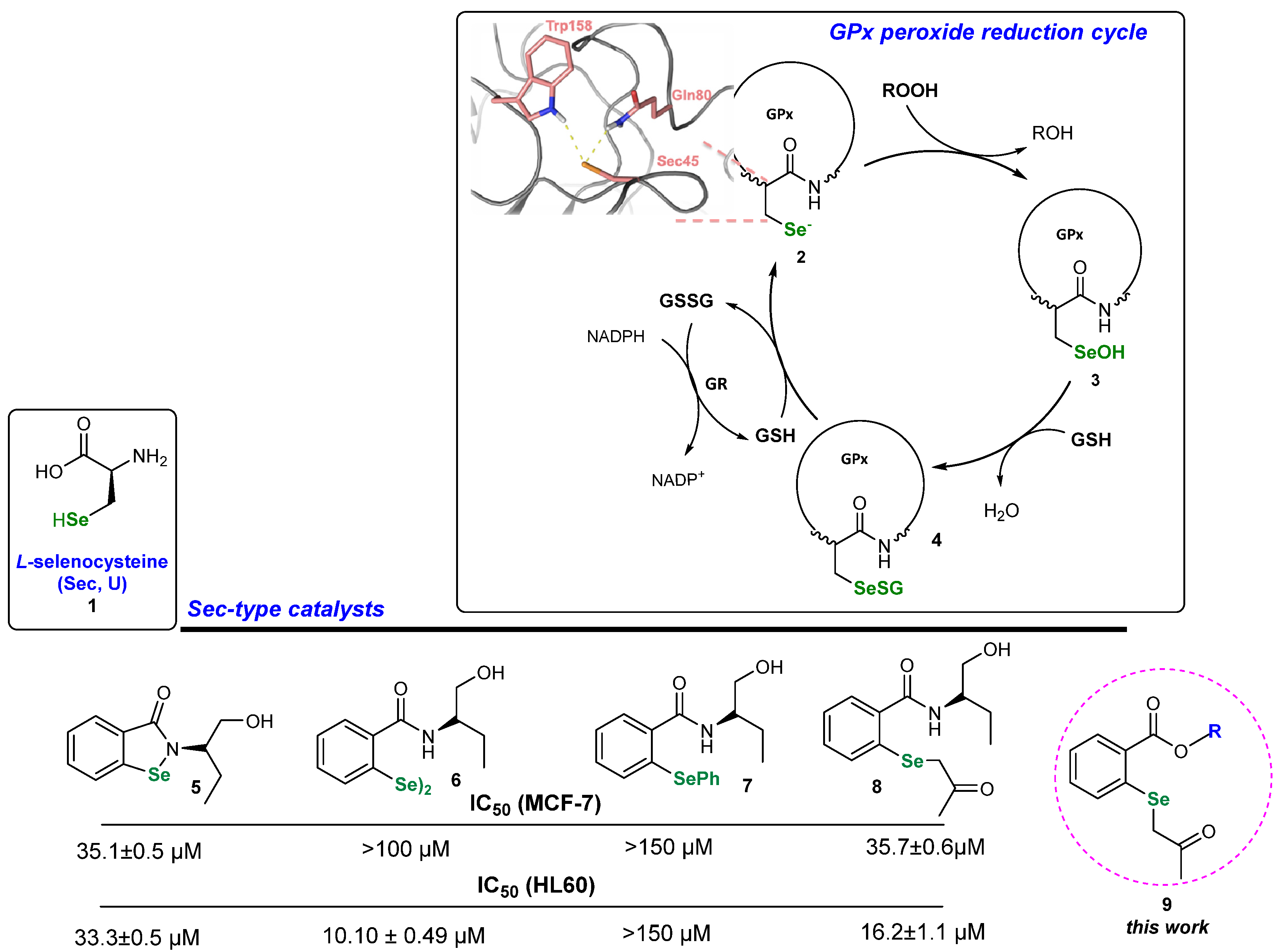
1. Introduction
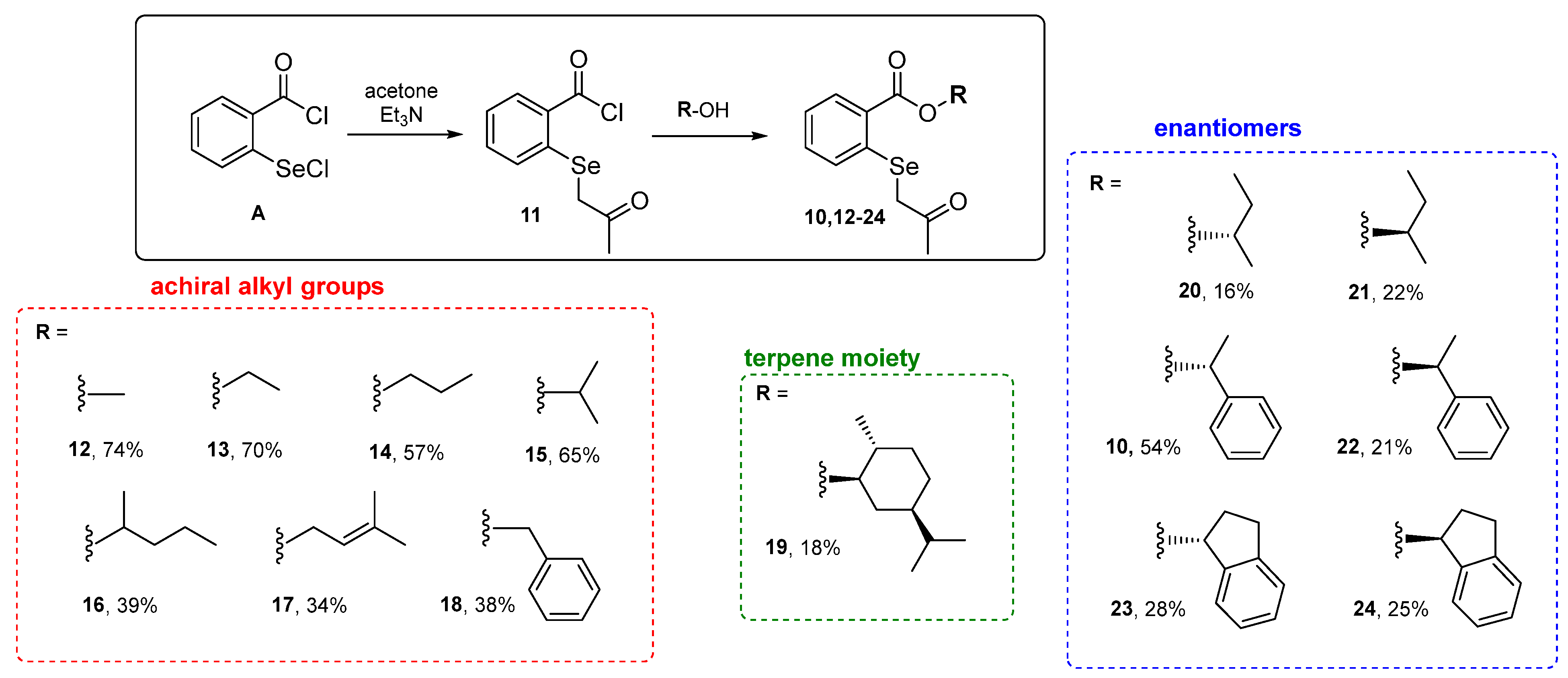
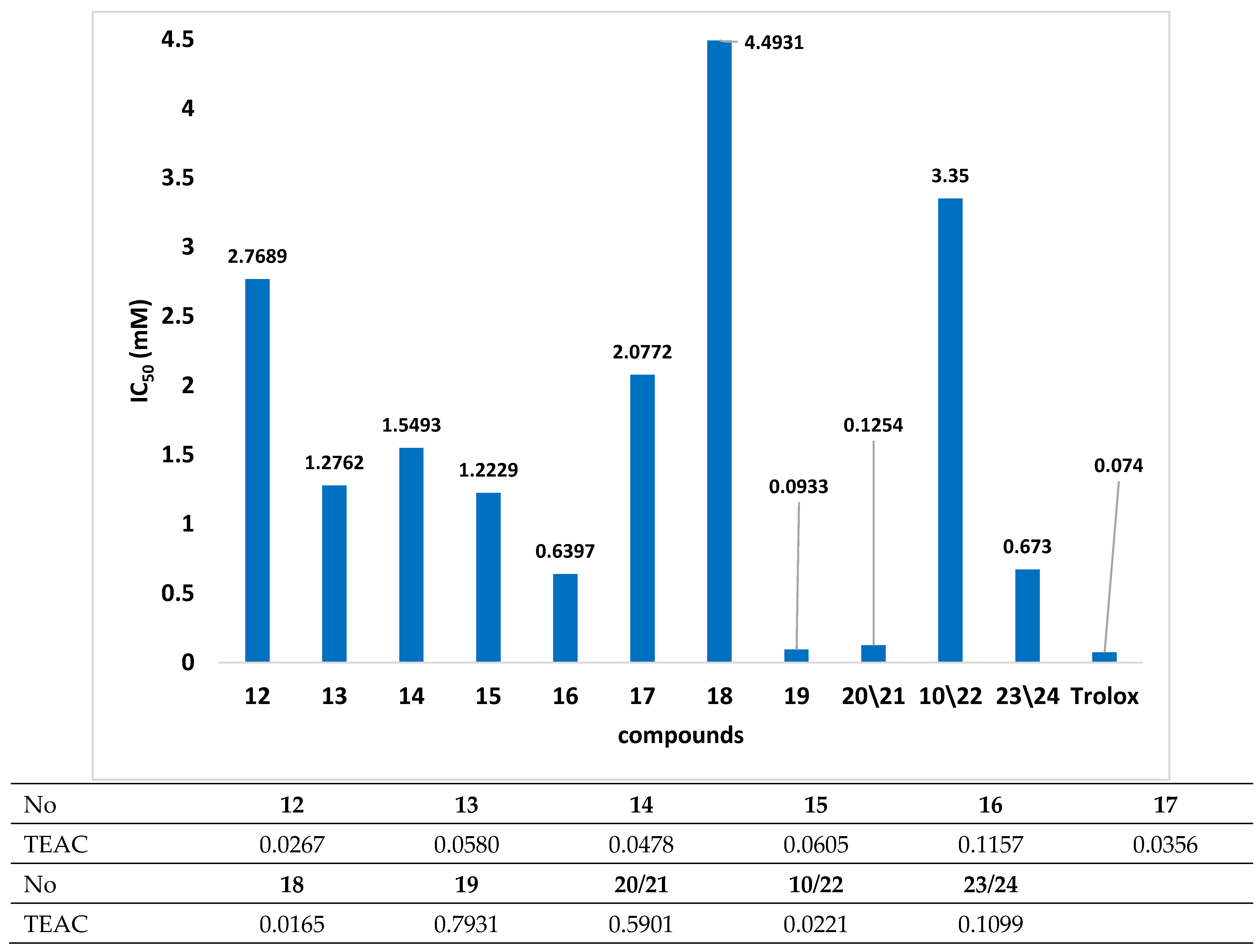
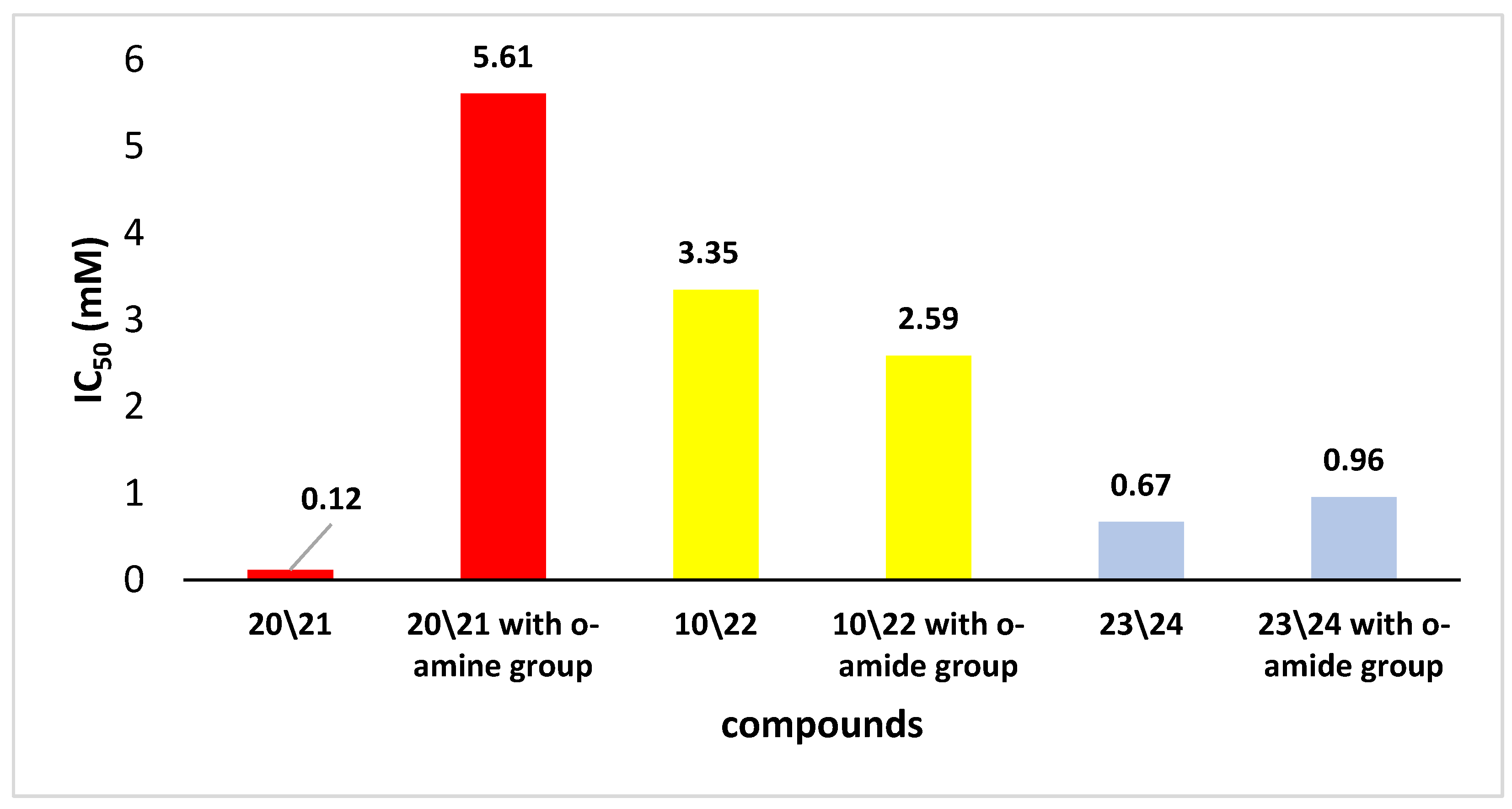
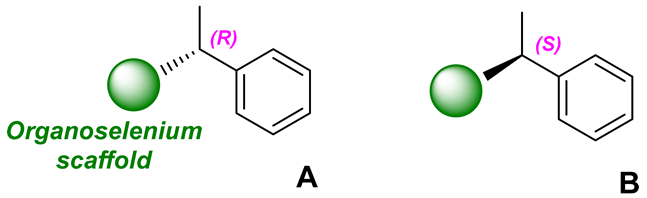
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedure and Analysis Data
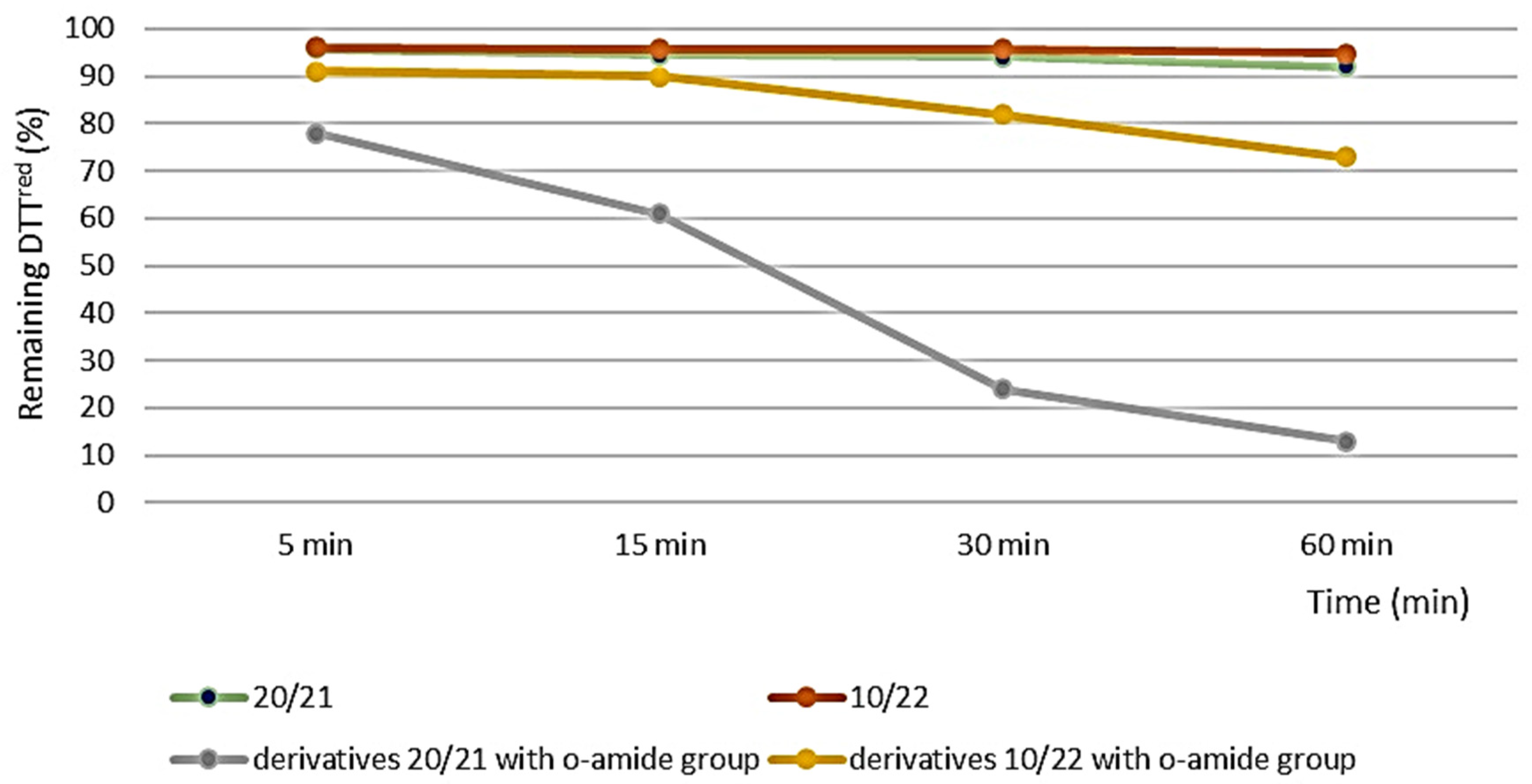
3.3. Antioxidant Activity Evaluation
3.3.1. DTT Activity Assay
3.3.2. The 2,2-di(4-tert-Octyl phenyl)-1-picrylhydrazyl) (DPPH) Test
3.4. MTT Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baek, Y.; Lee, J.; Son, J.; Lee, T.; Sobhan, A.; Lee, J.; Koo, S.-M.; Shin, W.H.; Oh, J.-M.; Park, C. Enzymatic Synthesis of Formate Ester through Immobilized Lipase and Its Reuse. Polymers 2020, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined Bead Milling and Enzymatic Hydrolysis for Efficient Fractionation of Lipids, Proteins, and Carbohydrates of Chlorella Vulgaris Microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Lal, M.A. Secondary Metabolites. In Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2023; pp. 765–808. [Google Scholar]
- Tsakos, M.; Schaffert, E.S.; Clement, L.L.; Villadsen, N.L.; Poulsen, T.B. Ester Coupling Reactions—An Enduring Challenge in the Chemical Synthesis of Bioactive Natural Products. Nat. Prod. Rep. 2015, 32, 605–632. [Google Scholar] [CrossRef]
- Takahashi, M.; Hirota, I.; Nakano, T.; Kotani, T.; Takani, D.; Shiratori, K.; Choi, Y.; Haba, M.; Hosokawa, M. Effects of Steric Hindrance and Electron Density of Ester Prodrugs on Controlling the Metabolic Activation by Human Carboxylesterase. Drug Metab. Pharmacokinet. 2021, 38, 100391. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T.; Yokoi, T. The Emerging Role of Human Esterases. Drug Metab. Pharmacokinet. 2012, 27, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Casey Laizure, S.; Herring, V.; Hu, Z.; Witbrodt, K.; Parker, R.B. The Role of Human Carboxylesterases in Drug Metabolism: Have We Overlooked Their Importance? Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Ohura, K. Evaluation of the Oral Absorption of Ester-Type Prodrugs. Yakugaku Zasshi 2020, 140, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.M.; Raunio, H.; Rautio, J. Prodrugs—From Serendipity to Rational Design. Pharmacol. Rev. 2011, 63, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Nunoya, K.; Nakamura, Y.; Bi, J.; Mukunoki, A.; Takeo, T.; Nakagata, N.; Hitoshi, M.; Yamaura, Y.; Imawaka, H.; et al. Species Difference in Hydrolysis of an Ester-Type Prodrug of Levodopa in Human and Animal Plasma: Different Contributions of Alpha-1 Acid Glycoprotein. Mol. Pharm. 2021, 18, 1985–1991. [Google Scholar] [CrossRef]
- Lavis, L.D. Ester Bonds in Prodrugs. ACS Chem. Biol. 2008, 3, 203–206. [Google Scholar] [CrossRef]
- Noronha, G.; Paul, P.; Katz, B.; Teuscher, N. PK Model with Concentration-Dependent Clearance for Zuretinol Acetate, an Oral Agent in Development for Treatment of Inherited Retinal Dystrophy Caused by LRAT or RPE65 Mutations. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4942. [Google Scholar]
- dos Santos Fernandes, G.F.; Prokopczyk, I.M.; Chin, C.M.; dos Santos, J.L. The Progress of Prodrugs in Drug Solubility. Recent Adv. Prodrugs 2020, 165, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Mamgain, R.; Kostic, M.; Singh, F.V. Synthesis and Antioxidant Properties of Organoselenium Compounds. Curr. Med. Chem. 2023, 30, 2421–2448. [Google Scholar] [CrossRef] [PubMed]
- Başeğmez, M. An Overview of the Antioxidant and Anti-Inflammatory Activity of Selenium. In Selenium and Human Health; IntechOpen: London, UK, 2023. [Google Scholar]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, D.; Mugesh, G. Introduction of a Catalytic Triad Increases the Glutathione Peroxidase-like Activity of Diaryl Diselenides. Org. Biomol. Chem. 2015, 13, 9072–9082. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, M.A.; Nelson, S.J.; O’Leary, C.; Self, W.T. Exploring the Selenium-over-Sulfur Substrate Specificity and Kinetics of a Bacterial Selenocysteine Lyase. Biochimie 2021, 182, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Chotana, G.A.; Faisal, A.; Zaib Saleem, R.S. Chemical Synthesis of Selenium-Containing Peptides. Mini-Rev. Med. Chem. 2023, 23, 1090–1117. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, E.; Doğan, S. Glutathione Peroxidase in Health and Diseases. In Glutathione System and Oxidative Stress in Health and Disease; IntechOpen: London, UK, 2020. [Google Scholar]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Długosz-Pokorska, A.; Janecka, A.; Wojtczak, A.; Ścianowski, J. Attachment of Chiral Functional Groups to Modify the Activity of New GPx Mimetics. Materials 2022, 15, 2068. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Gach-Janczak, K.; Janecka, A.; Ścianowski, J. Facile Synthesis of Chiral Phenylselenides as Novel Antioxidants and Cytotoxic Agents. RSC Adv. 2023, 13, 14698–14702. [Google Scholar] [CrossRef]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Długosz-Pokorska, A.; Gach-Janczak, K.; Ścianowski, J. Synthesis of New Chiral β-Carbonyl Selenides with Antioxidant and Anticancer Activity Evaluation—Part I. Materials 2024, 17, 899. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-Like Catalytic Activities. Eur. J. Org. Chem. 2010, 2010, 440–445. [Google Scholar] [CrossRef]
- Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Długosz-Pokorska, A.; Gach-Janczak, K.; Ścianowski, J. The Influence of Long Carbon Chains on the Antioxidant and Anticancer Properties of N-Substituted Benzisoselenazolones and Corresponding Diselenides. Pharmaceuticals 2023, 16, 1560. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model Studies on the Antioxidant Activity of Common Terpenoid Constituents of Essential Oils by Means of the 2,2-Diphenyl-1-Picrylhydrazyl Method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Cieśla, Ł.M.; Waksmundzka-Hajnos, M. Approach to Determination a Structure—Antioxidant Activity Relationship of Selected Common Terpenoids Evaluated by ABTS •+ Radical Cation Assay. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Obieziurska, M.; Pacuła, A.J.; Długosz-Pokorska, A.; Krzemiński, M.; Janecka, A.; Ścianowski, J. Bioselectivity Induced by Chirality of New Terpenyl Organoselenium Compounds. Materials 2019, 12, 3579. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.-J.; Shao, X.-T.; Wang, S.; Lu, G.-H.; Xu, T.; Zhou, J.-Y. Sesquiterpene Lactone Parthenolide Markedly Enhances Sensitivity of Human A549 Cells to Low-Dose Oxaliplatin via Inhibition of NF-ΚB Activation and Induction of Apoptosis. Planta Med. 2010, 76, 258–264. [Google Scholar] [CrossRef]
- Marchetti, P.; Galla, D.A.P.; Russo, F.P.; Ricevuto, E.; Flati, V.; Porzio, G.; Ficorella, C.; Cifone, M.G. Apoptosis Induced by Oxaliplatin in Human Colon Cancer HCT15 Cell Line. Anticancer Res. 2004, 24, 219–226. [Google Scholar]
- Oliveira, M.d.S.; Barbosa, M.I.F.; de Souza, T.B.; Moreira, D.R.M.; Martins, F.T.; Villarreal, W.; Machado, R.P.; Doriguetto, A.C.; Soares, M.B.P.; Bezerra, D.P. A Novel Platinum Complex Containing a Piplartine Derivative Exhibits Enhanced Cytotoxicity, Causes Oxidative Stress and Triggers Apoptotic Cell Death by ERK/P38 Pathway in Human Acute Promyelocytic Leukemia HL-60 Cells. Redox Biol. 2019, 20, 182–194. [Google Scholar] [CrossRef]
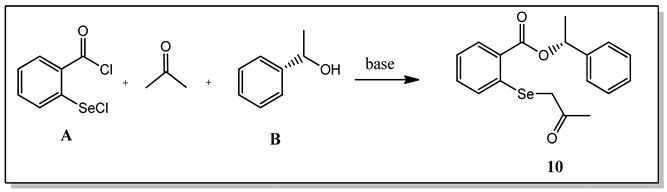 | ||||
|---|---|---|---|---|
| Entry | Solvent (Solubilization of A) | Base | Conditions and Order of Addition | Yield (%) |
| 1 | Acetone | NaHCO3 | 1. (acetone + NaHCO3, rt, 30 min) + A, rt, 1 h 2. B, rt, 15 h | 16 |
| 2 | Acetone | Et3N | 1. (acetone + Et3N, rt, 30 min) + A, rt, 1 h 2. B, rt, 15 h | 54 |
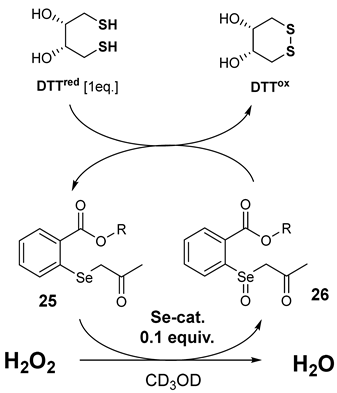 | ||||
|---|---|---|---|---|
| Remaining DTTred (%) | ||||
| Catalyst (0.1 Equiv.) | 5 min | 15 min | 30 min | 60 min |
| 12 | 95.3 ± 0.2 | 95.2 ± 0.3 | 94.8 ± 0.4 | 94.4 ± 0.4 |
| 13 | 96.7 ± 0.1 | 96.5 ± 0.2 | 95.0 ± 0.1 | 94.9 ± 0.4 |
| 14 | 92.8 ± 0.1 | 88.5 ± 0.3 | 82.0 ± 1.9 | 75.0 ± 1.1 |
| 15 | 93.6 ± 0.2 | 93.0 ± 0.1 | 92.9 ± 0.2 | 92.6 ± 0.2 |
| 16 | 95.1 ± 0.1 | 94.9 ± 0.1 | 94.7 ± 0.1 | 94.4 ± 0.2 |
| 17 | 90.8 ± 0.2 | 90.6 ± 0.3 | 90.3 ± 0.3 | 89.2 ± 0.2 |
| 18 | 93.5 ± 0.1 | 91.5 ± 0.2 | 89.2 ± 0.3 | 85.8 ± 0.7 |
| 19 | 95.1 ± 0.1 | 94.9 ± 0.1 | 94.6 ± 0.1 | 94.3 ± 0.2 |
| 20/21 | 95.8 ± 0.1 | 94.4 ± 0.2 | 94.0 ± 1.5 | 92.1 ± 2.7 |
| 10/22 | 96.1 ± 0.1 | 95.7 ± 0.1 | 95.7 ± 0.3 | 94.9 ± 0.4 |
| 23/24 | 94.4 ± 0.9 | 93.9 ± 0.8 | 93.6 ± 0.7 | 92.8 ± 0.4 |
| Ebselen | 75 | 64 | 58 | 52 |
| Compound | IC50 (µM) ± SEM | |
|---|---|---|
| MCF-7 | HL-60 | |
| 12 | 51.1 ± 0.6 | 97.2 ± 0.5 |
| 13 | 129.5 ± 1.2 | 147.0 ± 5.0 |
| 14 | 166.0 ± 3.0 | 97.2 ± 0.1 |
| 15 | 273.0 ± 3.0 | 105.0 ± 1.0 |
| 16 | 121.0 ± 0.4 | 84.0 ± 2.0 |
| 17 | 194.0 ± 5.0 | 103.0 ± 2.0 |
| 18 | 133.0 ± 2.0 | 110.0 ± 5.0 |
| 19 | 121.0 ± 10.0 | 243.0 ± 8.0 |
| 20 | 313.0 ± 10.0 | 147.5 ± 2.0 |
| 21 | 243.0 ± 10.0 | 89.4 ± 0.9 |
| 10 | 176.0 ± 3.0 | 126.0 ± 2.5 |
| 22 | 107.0 ± 0.8 | 94.2 ± 3.0 |
| 23 | 123.0 ± 0.4 | 94.4 ± 3.0 |
| 24 | 136.0 ± 3.0 | 94.2 ± 0.7 |
| Oxaliplatin | 35 [30,31] | 0.8 [32] |
 | ||||
|---|---|---|---|---|
| IC50 (µM) ± SEM | ||||
| A | B | |||
| Se-derivative | MCF-7 | HL-60 | MCF-7 | HL-60 |
| β-carbonyl selenide with o-ester group | 176.0 ± 3.0 | 126.0 ± 2.5 | 107.0 ± 0.8 | 94.2 ± 3.0 |
| β-carbonyl selenide with o-amide group | 235.0 ± 1.0 | 303.0 ± 3.0 | 237.0 ± 11.0 | 23.5 ± 1.4 |
| Phenyl selenide | >150 | >150 | >150 | >150 |
| Benzisoselenazolone | 32.8 ± 2.8 | 16.1 ± 0.0 | 38.8 ± 0.8 | 16.8 ± 0.4 |
| Diselenide | >100 | >100 | >100 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Długosz-Pokorska, A.; Gach-Janczak, K.; Ścianowski, J. Synthesis of a New Class of β-Carbonyl Selenides Functionalized with Ester Groups with Antioxidant and Anticancer Properties—Part II. Molecules 2024, 29, 2866. https://doi.org/10.3390/molecules29122866
Laskowska A, Pacuła-Miszewska AJ, Obieziurska-Fabisiak M, Jastrzębska A, Długosz-Pokorska A, Gach-Janczak K, Ścianowski J. Synthesis of a New Class of β-Carbonyl Selenides Functionalized with Ester Groups with Antioxidant and Anticancer Properties—Part II. Molecules. 2024; 29(12):2866. https://doi.org/10.3390/molecules29122866
Chicago/Turabian StyleLaskowska, Anna, Agata J. Pacuła-Miszewska, Magdalena Obieziurska-Fabisiak, Aneta Jastrzębska, Angelika Długosz-Pokorska, Katarzyna Gach-Janczak, and Jacek Ścianowski. 2024. "Synthesis of a New Class of β-Carbonyl Selenides Functionalized with Ester Groups with Antioxidant and Anticancer Properties—Part II" Molecules 29, no. 12: 2866. https://doi.org/10.3390/molecules29122866
APA StyleLaskowska, A., Pacuła-Miszewska, A. J., Obieziurska-Fabisiak, M., Jastrzębska, A., Długosz-Pokorska, A., Gach-Janczak, K., & Ścianowski, J. (2024). Synthesis of a New Class of β-Carbonyl Selenides Functionalized with Ester Groups with Antioxidant and Anticancer Properties—Part II. Molecules, 29(12), 2866. https://doi.org/10.3390/molecules29122866









