Exploring the Potential of Endophytic Microorganisms and Nanoparticles for Enhanced Water Remediation
Abstract
1. Introduction
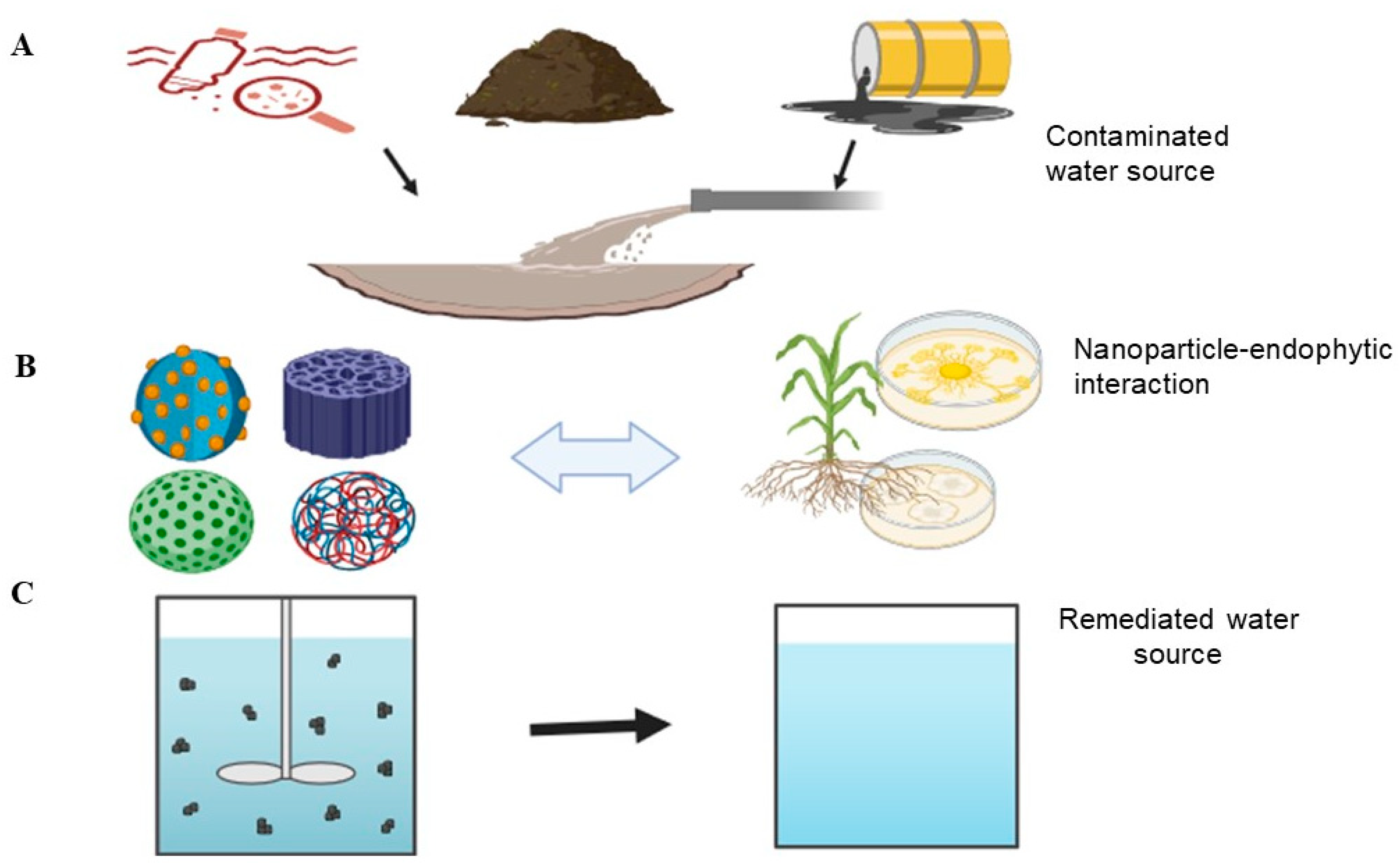
2. Novel Developments in Endophytes and Nanoparticles in Water Remediation
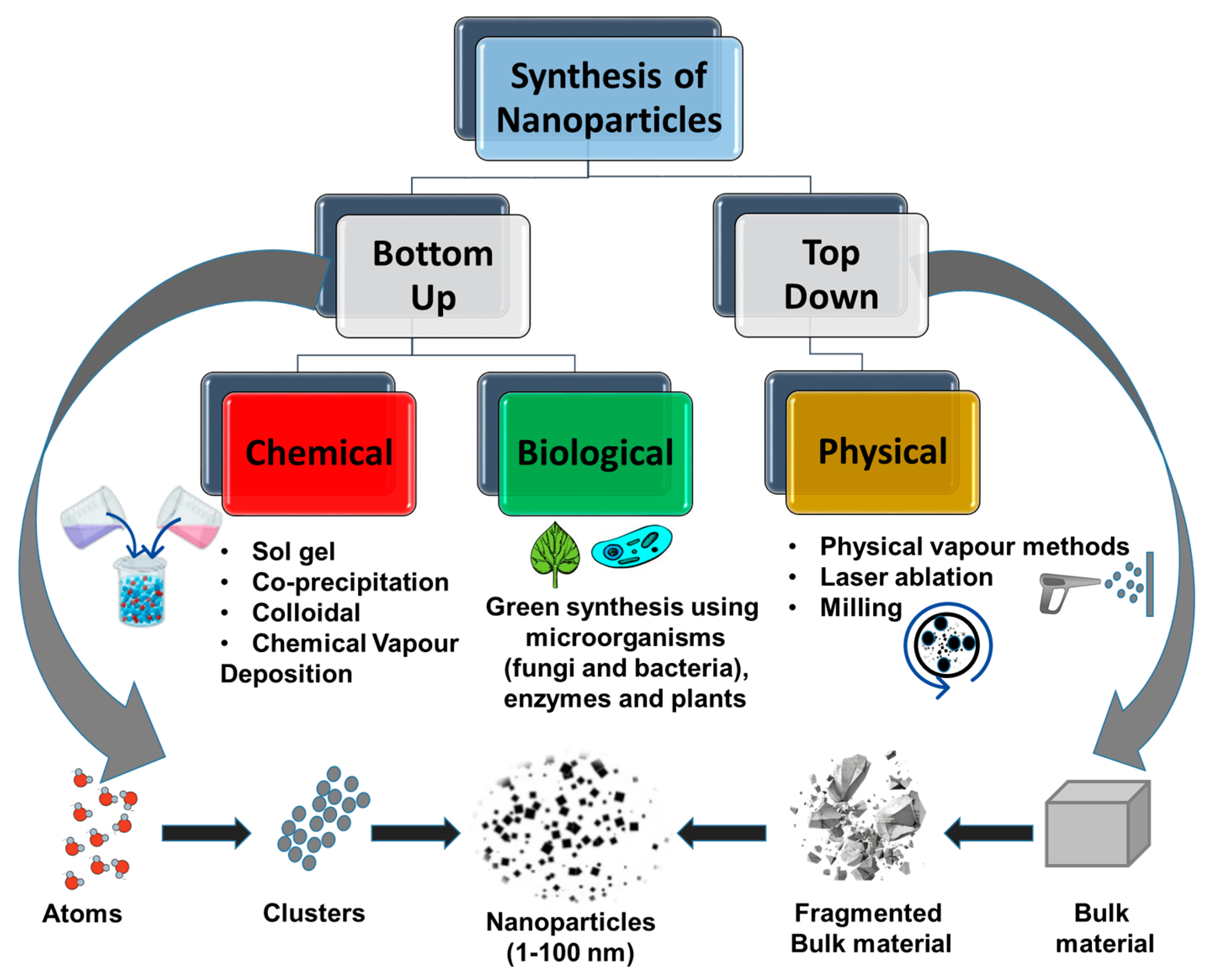
3. Synthetic Methods of Endophyte–Nanoparticle Composites
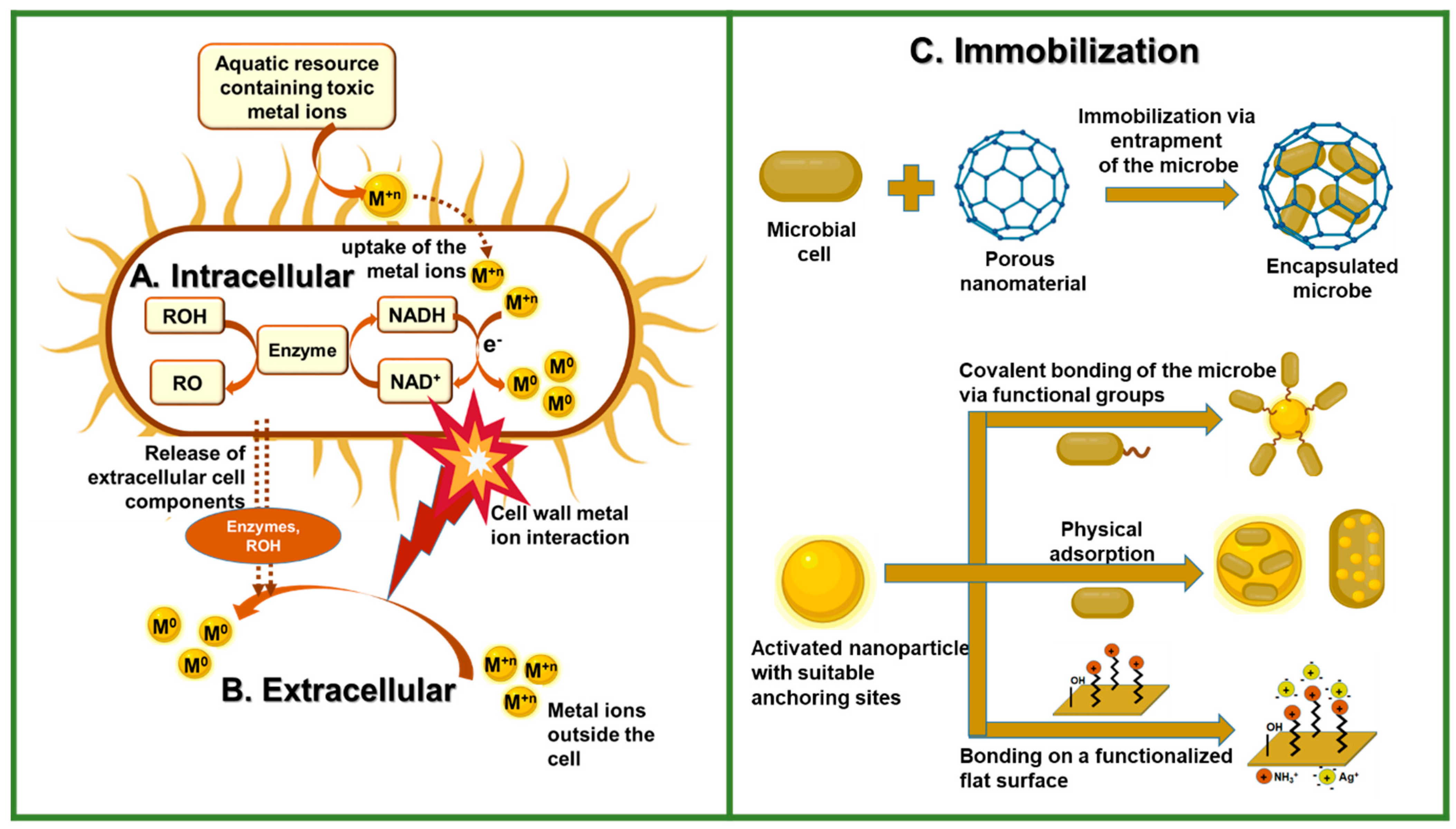
3.1. Biogenic Synthetic Method
3.2. Synthesis by Immobilization
3.3. Synthesis by Co-Cultivation
4. The Effect of Combining Endophytes and Nanoparticles for Water Remediation
5. Application of Endophytes and Nanoparticles in Water Remediation
6. Limitations and Risks of Using Endophytes and Nanoparticles in Water Remediation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeed, M.U.; Hussain, N.; Sumrin, A.; Shahbaz, A.; Noor, S.; Bilal, M.; Aleya, L.; Iqbal, H.M.N. Microbial Bioremediation Strategies with Wastewater Treatment Potentialities—A Review. Sci. Total Environ. 2022, 818, 151754. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; UNESCO: Paris, France, 2023; ISBN 978-92-3-100576-3. [Google Scholar]
- Del Prado-Audelo, M.L.; García Kerdan, I.; Escutia-Guadarrama, L.; Reyna-González, J.M.; Magaña, J.J.; Leyva-Gómez, G. Nanoremediation: Nanomaterials and Nanotechnologies for Environmental Cleanup. Front. Environ. Sci. 2021, 9, 793765. [Google Scholar] [CrossRef]
- Mnkandla, S.M.; Otomo, P.V. Effectiveness of Mycofiltration for Removal of Contaminants from Water: A Systematic Review Protocol. Environ. Evid. 2021, 10, 17. [Google Scholar] [CrossRef]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Chemosphere, M.I. Genetically Engineered Microorganisms for Environmental Remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef]
- Soldi, E.; Casey, C.; Murphy, B.R.; Hodkinson, T.R. Fungal Endophytes for Grass Based Bioremediation: An Endophytic Consortium Isolated from Agrostis Stolonifera Stimulates the Growth of Festuca Arundinacea in Lead Contaminated Soil. J. Fungi 2020, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Hussain, A.; Hamayun, M.; Rahman, H.; Iqbal, A.; Shah, M.; Irshad, M.; Qasim, M.; Islam, B. Bioremediation of Hexavalent Chromium by Endophytic Fungi; Safe and Improved Production of Lactuca sativa L. Chemosphere 2018, 211, 653–663. [Google Scholar]
- Herbst, A.; Patzelt, L.; Schoebe, S.; Schubert, H.; von Tümpling, W. Bioremediation Approach Using Charophytes—Preliminary Laboratory and Field Studies of Mine Drainage Water from the Mansfeld Region, Germany. Environ. Sci. Pollut. Res. 2019, 26, 34983–34992. [Google Scholar] [CrossRef]
- Cecchin, I.; Reddy, K.R.; Thomé, A.; Tessaro, E.F.; Schnaid, F. Nanobioremediation: Integration of Nanoparticles and Bioremediation for Sustainable Remediation of Chlorinated Organic Contaminants in Soils. Int. Biodeterior. Biodegrad. 2017, 119, 419–428. [Google Scholar] [CrossRef]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-Supported Metals and Metal Oxide Nanoparticles: Synthesis, Characterization, and Applications. J. Nanoparticle Res. 2012, 14, 715. [Google Scholar] [CrossRef]
- Tahir, M.B.; Sohaib, M.; Sagir, M.; Rafique, M. Role of Nanotechnology in Photocatalysis. Encycl. Smart Mater. 2022, 2, 578–589. [Google Scholar]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and Their Antimicrobial Properties against Pathogens Including Bacteria, Fungi, Parasites and Viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Mecha, A.C.; Chollom, M.N.; Babatunde, B.F.; Tetteh, E.K.; Rathilal, S. Versatile Silver-Nanoparticle-Impregnated Membranes for Water Treatment: A Review. Membranes 2023, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Tavker, N.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Alam, J.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M. Removal of Cadmium and Chromium by Mixture of Silver Nanoparticles and Nano-Fibrillated Cellulose Isolated from Waste Peels of Citrus Sinensis. Polymers 2021, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, J.; Sun, Y. Enhanced Immobilization of Lead, Cadmium, and Arsenic in Smelter-Contaminated Soil by Sulfidated Zero-Valent Iron. J. Hazard. Mater. 2023, 447, 130783. [Google Scholar] [CrossRef] [PubMed]
- Mogale, R.; Akpomie, K.G.; Conradie, J.; Langner, E.H.G. Dye Adsorption of Aluminium-and Zirconium-Based Metal Organic Frameworks with Azobenzene Dicarboxylate Linkers. J. Environ. Manag. 2022, 304, 114166. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Boubeta, C.; Simeonidis, K. Magnetic Nanoparticles for Water Purification. In Nanoscale Materials in Water Purification; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 521–552. [Google Scholar]
- He, D.; Zhang, X.; Hu, J. Methods for Separating Microplastics from Complex Solid Matrices: Comparative Analysis. J. Hazard. Mater. 2021, 409, 124640. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental Development of a New Protocol for Extraction and Characterization of Microplastics in Fish Tissues: First Observations in Commercial Species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption Mechanism of Cadmium on Microplastics and Their Desorption Behavior in Sediment and Gut Environments: The Roles of Water PH, Lead Ions, Natural Organic Matter and Phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of Microplastics from Water by Magnetic Nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef]
- Salazar Sandoval, S.; Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N.; Rojas-Romo, C.; González-Casanova, J.; Gómez, D.R.; Yutronic, N.; Urzúa, M.; et al. Nanomaterials for Potential Detection and Remediation: A Review of Their Analytical and Environmental Applications. Coatings 2023, 13, 2085. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- El Alouani, M.; Saufi, H.; Moutaoukil, G.; Alehyen, S.; Nematollahi, B.; Belmaghraoui, W.; Taibi, M. Application of Geopolymers for Treatment of Water Contaminated with Organic and Inorganic Pollutants: State-of-the-Art Review. J. Environ. Chem. Eng. 2021, 9, 105095. [Google Scholar] [CrossRef]
- Messaoudi, O.; Bendahou, M. Biological Synthesis of Nanoparticles Using Endophytic Microorganisms: Current Development. In Nanotechnology and the Environment; Sen, M., Ed.; Intech Open: London, UK, 2020; pp. 37–55. [Google Scholar] [CrossRef]
- Guo, K.; Han, F.X.; Kingery, W.; Sun, H.; Zhang, J. Development of Novel Nanomaterials for Remediation of Heavy Metals and Radionuclides in Contaminated Water. Nanotechnol. Environ. Eng. 2016, 1, 7. [Google Scholar] [CrossRef]
- Hernández-Hernández, K.A.; Illescas, J.; Díaz-Nava, M.D.C.; Muro-Urista, C.R.; Martínez-Gallegos, S.; Ortega-Aguilar, R.E. Polymer-Clay Nanocomposites and Composites: Structures, Characteristics, and Their Applications in the Removal of Organic Compounds of Environmental Interest. Med. Chem. 2016, 6, 201–210. [Google Scholar] [CrossRef]
- Baigorria, E.; Fraceto, L.F. Novel Nanostructured Materials Based on Polymer/Organic-Clay Composite Networks for the Removal of Carbendazim from Waters. J. Clean. Prod. 2022, 331, 129867. [Google Scholar] [CrossRef]
- Ban, Y.; Xiao, Z.; Wu, C.; Lv, Y.; Meng, F.; Wang, J.; Xu, Z. The Positive Effects of Inoculation Using Arbuscular Mycorrhizal Fungi and/or Dark Septate Endophytes on the Purification Efficiency of CuO-Nanoparticles-Polluted Wastewater in Constructed Wetland. J. Hazard. Mater. 2021, 416, 126095. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.D.; de Oliveira Junior, V.A.; dos Santos Ribeiro, M.A.; Polonio, J.C.; Rosini, B.; dos Santos Oliveira, J.A.; Bini, R.D.; Golias, H.C.; Fávaro-Polonio, C.Z.; Orlandelli, R.C.; et al. Synthesis, Characterization, and Reusability of Novel Nanobiocomposite of Endophytic Fungus Aspergillus Flavus and Magnetic Nanoparticles (Fe3O4) with Dye Bioremediation Potential. Chemosphere 2023, 340, 139956. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and Opportunities in the Bottom-up Mechanochemical Synthesis of Noble Metal Nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Tripathy, S.; Rodrigues, J.; Shimpi, N.G. Top-down and Bottom-up Approaches for Synthesis of Nanoparticles. In Materials Research Foundations; Cruz, J.N., Ed.; Materials Research Forum LLC: Millersville PA, USA, 2023; pp. 92–130. [Google Scholar]
- Daraio, C.; Jin, S. Synthesis and Patterning Methods for Nanostructures Useful for Biological Applications. In Nanotechnology for Biology and Medicine: At the Building Block Level; Silva, G.A., Parpura, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 27–44. [Google Scholar]
- Lizunova, A.; Mazharenko, A.; Masnaviev, B.; Khramov, E.; Efimov, A.; Ramanenka, A.; Shuklov, I.; Ivanov, V. Effects of Temperature on the Morphology and Optical Properties of Spark Discharge Germanium Nanoparticles. Materials 2020, 13, 4431. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnology 2018, 16, 84. [Google Scholar] [CrossRef]
- Priyadarshana, G.; Kottegoda, N.; Senaratne, A.; de Alwis, A.; Karunaratne, V. Synthesis of Magnetite Nanoparticles by Top-down Approach from a High Purity Ore. J. Nanomater. 2015, 2015, 317312. [Google Scholar] [CrossRef]
- Shuklov, I.A.; Toknova, V.F.; Lizunova, A.A.; Razumov, V.F. Controlled Aging of PbS Colloidal Quantum Dots under Mild Conditions. Mater. Today Chem. 2020, 18, 100357. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic Synthesis of Nanoparticles: A Review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Githala, C.K.; Raj, S.; Dhaka, A.; Mali, S.C.; Trivedi, R. Phyto-Fabrication of Silver Nanoparticles and Their Catalytic Dye Degradation and Antifungal Efficacy. Front. Chem. 2022, 10, 994721. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S. Bioremediation of Heavy Metals from Industrial Effluents by Endophytes and Their Metabolic Activity: Recent Advances. Bioresour. Technol. 2021, 339, 125589. [Google Scholar] [CrossRef] [PubMed]
- Jeeva, K.; Thiyagarajan, M.; Elangovan, V.; Geetha, N.; Venkatachalam, P. Caesalpinia Coriaria Leaf Extracts Mediated Biosynthesis of Metallic Silver Nanoparticles and Their Antibacterial Activity against Clinically Isolated Pathogens. Ind. Crops Prod. 2014, 52, 714–720. [Google Scholar] [CrossRef]
- Gürsoy, N.; Yilmaz Öztürk, B.; Dağ, İ. Synthesis of Intracellular and Extracellular Gold Nanoparticles with a Green Machine and Its Antifungal Activity. Turk. J. Biol. 2021, 45, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Hulkoti, N.; Taranath, T. Influence of Physico-Chemical Parameters on the Fabrication of Silver Nanoparticles Using Petrea volubilis L. Stem Broth and Its Anti-Microbial Efficacy. Int. J. Pharm. Sci. Drug Res. 2017, 9, 72–78. [Google Scholar] [CrossRef]
- Konishi, Y.; Tsukiyama, T.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S. Intracellular Recovery of Gold by Microbial Reduction of 4−Ions Using the Anaerobic Bacterium Shewanella algae. Hydrometallurgy 2006, 81, 24–29. [Google Scholar] [CrossRef]
- Alfryyan, N.; Kordy, M.G.M.; Abdel-Gabbar, M.; Soliman, H.A.; Shaban, M. Characterization of the Biosynthesized Intracellular and Extracellular Plasmonic Silver Nanoparticles Using Bacillus Cereus and Their Catalytic Reduction of Methylene Blue. Sci. Rep. 2022, 12, 12495. [Google Scholar] [CrossRef] [PubMed]
- Funari, R.; Ripa, R.; Söderström, B.; Skoglund, U.; Shen, A.Q. Detecting Gold Biomineralization by Delftia acidovorans Biofilms on a Quartz Crystal Microbalance. ACS Sens. 2019, 4, 3023–3033. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.R.; Ando, R.A.; Oller Nascimento, C.A.; Correa, B. Extra and Intracellular Synthesis of Nickel Oxide Nanoparticles Mediated by Dead Fungal Biomass. PLoS ONE 2015, 10, e0129799. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Kumar, V.; Kaushik, N.K.; Kumar, A.; Malik, V.; Singh, D.; Singh, B. Sporotrichum thermophile Culture Extract-Mediated Greener Synthesis of Silver Nanoparticles: Eco-Friendly Functional Group Transformation and Anti-Bacterial Study. Curr. Res. Green Sustain. Chem. 2020, 3, 100029. [Google Scholar] [CrossRef]
- Khan, M.; Al-Hamoud, K.; Liaqat, Z.; Shaik, M.R.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Mondeshki, M.; et al. Synthesis of Au, Ag, and Au-Ag Bimetallic Nanoparticles Using Pulicaria undulata Extract and Their Catalytic Activity for the Reduction of 4-Nitrophenol. Nanomaterials 2020, 10, 1885. [Google Scholar] [CrossRef]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of Microbes for Bioremediation of Crude Oil Polluted Environments: A Mini Review. Open Microbiol. J. 2015, 9, 48. [Google Scholar]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of Microbial Cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Arslan, M.; Malik, M.H.; Mohsin, M.; Iqbal, S.; Afzal, M. Treatment of the Textile Industry Effluent in a Pilot-Scale Vertical Flow Constructed Wetland System Augmented with Bacterial Endophytes. Sci. Total Environ. 2018, 645, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Riseh, R.; Moradi-Pour, M.; Mohammadinejad, R.; Thakur, V.K. Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms. Polymers 2021, 13, 1938. [Google Scholar] [CrossRef] [PubMed]
- Ramadevi, S.; Meenakshi, S. An Epitome on Encapsulation of Probiotics. Arch. Mater. Sci. Eng. 2022, 116, 34–41. [Google Scholar] [CrossRef]
- Krell, V.; Jakobs-Schoenwandt, D.; Persicke, M.; Patel, A. V Endogenous Arabitol and Mannitol Improve Shelf Life of Encapsulated Metarhizium brunneum. World J. Microbiol. Biotechnol. 2018, 34, 108. [Google Scholar] [CrossRef] [PubMed]
- Bepeyeva, A.; de Barros, J.M.S.; Albadran, H.; Kakimov, A.K.; Kakimova, Z.K.; Charalampopoulos, D.; Khutoryanskiy, V. V Encapsulation of Lactobacillus casei into Calcium Pectinate-Chitosan Beads for Enteric Delivery. J. Food Sci. 2017, 82, 2954–2959. [Google Scholar] [CrossRef]
- Sandoval-Castilla, O.; Lobato-Calleros, C.; García-Galindo, H.S.; Alvarez-Ramírez, J.; Vernon-Carter, E.J. Textural Properties of Alginate-Pectin Beads and Survivability of Entrapped Lb. casei in Simulated Gastrointestinal Conditions and in Yoghurt. Food Res. Int. 2010, 43, 111–117. [Google Scholar] [CrossRef]
- Lambrese, Y.S.; Illanes, C.O.; Ochoa, N.A. Advancing Bacterial Endophyte Encapsulation in Alginate for Sustainable Agriculture: Method Comparisons, Morphology and Viability Assessment. J. Clean. Prod. 2024, 457, 142473. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization Technologies and Support Materials Suitable in Alcohol Beverages Production: A Review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 219261. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green Synthesis of Gold Nanoparticles Using Brown Algae Cystoseira baccata: Its Activity in Colon Cancer Cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Manivasagan, P.; Kim, S.-K.; Kirthi, A.V.; Marimuthu, S.; Rahuman, A.A. Marine Algae-Mediated Synthesis of Gold Nanoparticles Using a Novel Ecklonia cava. Bioprocess Biosyst. Eng. 2014, 37, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.W.A.; Rehman, M.U.; Tauseef, M.; Islam, E.; Hayat, A.; Iqbal, S.; Arslan, M.; Afzal, M. Ciprofloxacin Removal from Aqueous Media Using Floating Treatment Wetlands Supported by Immobilized Bacteria. Sustainability 2022, 14, 14216. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Chubik, M.; Chubik, M.; Gromov, A.; Wei, G.; Han, W. Entrapment of Radioactive Uranium from Wastewater by Using Fungus-Fe3O4 Bio-Nanocomposites. RSC Adv. 2015, 5, 41611–41616. [Google Scholar] [CrossRef]
- Kegl, T.; Ban, I.; Lobnik, A.; Košak, A. Synthesis and Characterization of Novel γ-Fe2O3-NH4OH@SiO2(APTMS) Nanoparticles for Dysprosium Adsorption. J. Hazard. Mater. 2019, 378, 120764. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Al Saedi, M.H.; Bahkali, A.H.; Elgorgan, A.M.; Kharat, M.; Pai, K.; Pichtel, J.; Ahmad, A. A Au2S Nanoparticles: Fungal-Mediated Synthesis, Structural Characterization and Bioassay. Green Chem. Lett. Rev. 2022, 15, 59–68. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A.; Al-Sabri, A.; Almansob, A.; AlNAdhari, S. Anti-Oxidant, Anti-Fungal and Cytotoxic Effects of Silver Nanoparticles Synthesized Using Marine Fungus Cladosporium Halotolerans. Appl. Nanosci. 2023, 13, 623–631. [Google Scholar] [CrossRef]
- Rudrappa, M.; Kumar, R.S.; Nagaraja, S.K.; Hiremath, H.; Gunagambhire, P.V.; Almansour, A.I.; Perumal, K.; Nayaka, S. Myco-Nanofabrication of Silver Nanoparticles by Penicillium brasilianum NP5 and Their Antimicrobial, Photoprotective and Anticancer Effect on MDA-MB-231 Breast Cancer Cell Line. Antibiotics 2023, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Mamidyala, S.K. Extracellular Synthesis of Silver Nanoparticles Using Culture Supernatant of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2011, 84, 462–466. [Google Scholar] [CrossRef]
- Raza, S.; Ansari, A.; Siddiqui, N.N.; Ibrahim, F.; Abro, M.I.; Aman, A. Biosynthesis of Silver Nanoparticles for the Fabrication of Non Cytotoxic and Antibacterial Metallic Polymer Based Nanocomposite System. Sci. Rep. 2021, 11, 10500. [Google Scholar] [CrossRef]
- He, X.; Yang, D.-P.; Zhang, X.; Liu, M.; Kang, Z.; Lin, C.; Jia, N.; Luque, R. Waste Eggshell Membrane-Templated CuO-ZnO Nanocomposites with Enhanced Adsorption, Catalysis and Antibacterial Properties for Water Purification. Chem. Eng. J. 2019, 369, 621–633. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Hayder, G.; Yusuf, M.; Taheri, M.M.; Rezania, S.; Hasan, M.; Yadav, K.K.; Khorami, M.; Farajnezhad, M.; et al. Exploring the Potential of Layered Metal and Metal Oxide Nanomaterials for Sustainable Water and Wastewater Treatment: A Review of Their Antimicrobial Properties. Chemosphere 2023, 335, 139103. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.M.; Shukla, S. Devising and Exploiting Functionalities of Nanocomposites for Removal of Organic Pollutants and for Disinfection. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 2153–2178. [Google Scholar]
- Elbahnasawy, M.A.; Shehabeldine, A.M.; Khattab, A.M.; Amin, B.H.; Hashem, A.H. Green Biosynthesis of Silver Nanoparticles Using Novel Endophytic Rothia endophytica: Characterization and Anticandidal Activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102401. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Naveen, K.V.; Han, K.-S.; Zhang, X.; Jeong, M.S.; Wang, M.-H. Combination of Paraconiothyrium brasiliense Fabricated Titanium Dioxide Nanoparticle and Antibiotics Enhanced Antibacterial and Antibiofilm Properties: A Toxicity Evaluation. Environ. Res. 2022, 212, 113237. [Google Scholar] [CrossRef] [PubMed]
- Tabande, L.; Sepehri, M.; Yasrebi, J.; Zarei, M.; Ghasemi-Fasaei, R.; Khatabi, B. A comparison between the function of Serendipita indica and Sinorhizobium meliloti in modulating the toxicity of zinc oxide nanoparticles in alfalfa (Medicago sativa L.). Environ. Sci. Pollut. Res. 2022, 7, 2047–2060. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; White, J.C.; Adeel, M.; Jiang, R.; Zhao, Z.; Rao, Y.; Chen, G.; Rui, Y.; Xing, B. Carbon-Based Nanomaterials Alter the Composition of the Fungal Endophyte Community in Rice (Oryza sativa L.). Environ. Sci. Nano 2020, 7, 2047–2060. [Google Scholar] [CrossRef]
- Liosis, C.; Papadopoulou, A.; Karvelas, E.; Karakasidis, T.E.; Sarris, I.E. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials 2021, 14, 7500. [Google Scholar] [CrossRef]
- Sur, U.K. Biosynthesis of Metal Nanoparticles and Graphene. In Advanced Surface Engineering Materials; Tiwari, A., Wang, R., Wei, B., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 243–289. [Google Scholar]
- Ali, S.; Ali, H.; Siddique, M.; Gulab, H.; Haleem, M.A.; Ali, J. Exploring the Biosynthesized Gold Nanoparticles for Their Antibacterial Potential and Photocatalytic Degradation of the Toxic Water Wastes under Solar Light Illumination. J. Mol. Struct. 2020, 1215, 128259. [Google Scholar] [CrossRef]
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Wang, X.; Chen, L. Magnetic Copper-Based Metal Organic Framework as an Effective and Recyclable Adsorbent for Removal of Two Fluoroquinolone Antibiotics from Aqueous Solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef]
- Kaabo, H.E.; Saied, E.; Hassan, S.E.D.; Mahdy, H.M.; Sultan, M.H. Penicillium oxalicum-Mediated the Green Synthesis of Silica Nanoparticles: Characterization and Environmental Applications. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Gran, S.; Aziz, R.; Rafiq, M.T.; Abbasi, M.; Qayyum, A.; Elnaggar, A.Y.; Elganzory, H.H.; El-Bahy, Z.M.; Hussein, E.E. Development of Cerium Oxide/Corncob Nanocomposite: A Cost-Effective and Eco-Friendly Adsorbent for the Removal of Heavy Metals. Polymers 2021, 13, 4464. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, A.; Chaudhary, P.; Gangola, S. Chlorpyrifos Degradation Using Binary Fungal Strains Isolated from Industrial Waste Soil. Biologia 2021, 76, 3071–3080. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Albarico, F.P.J.B.; Pandey, A.; Chen, C.W.; Dong, C. Di Organic Wastes Bioremediation and Its Changing Prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Lal, S.; Maurya, S.K.; Bhattacherjee, A.K.; Chaudhary, P.; Gangola, S.; Rajan, S. Exploration of Klebsiella pneumoniae M6 for Paclobutrazol Degradation, Plant Growth Attributes, and Biocontrol Action under Subtropical Ecosystem. PLoS ONE 2021, 16, e0261338. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, M.; Kushveer, J.S.; Sarma, V. V A Worldwide List of Endophytic Fungi with Notes on Ecology and Diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- An, H.; Liu, Y.; Zhao, X.; Huang, Q.; Yuan, S.; Yang, X.; Dong, J. Characterization of Cadmium-Resistant Endophytic Fungi from Salix Variegata Franch. in Three Gorges Reservoir Region, China. Microbiol. Res. 2015, 176, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pietro-Souza, W.; de Campos Pereira, F.; Mello, I.S.; Stachack, F.F.F.; Terezo, A.J.; da Cunha, C.N.; White, J.F.; Li, H.; Soares, M.A. Mercury Resistance and Bioremediation Mediated by Endophytic Fungi. Chemosphere 2020, 240, 124874. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.C.; Ateba, C.N. Untapped Potentials of Endophytic Fungi: A Review of Novel Bioactive Compounds with Biological Applications. Microorganisms 2020, 8, 1934. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. Genomic Analysis of Bacillus cereus NWUAB01 and Its Heavy Metal Removal from Polluted Soil. Sci. Rep. 2020, 10, 19660. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of Environmental Wastes: The Role of Microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.J.; Liu, Y.; Liu, Z.; Zhang, A.N. Quantitative Contributions of the Major Sources of Heavy Metals in Soils to Ecosystem and Human Health Risks: A Case Study of Yulin, China. Ecotoxicol. Environ. Saf. 2018, 164, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Strong, P.J.; Burgess, J.E. Treatment Methods for Wine-Related and Distillery Wastewaters: A Review. Bioremediat. J. 2008, 12, 70–87. [Google Scholar] [CrossRef]
- Methneni, N.; Morales-González, J.A.; Jaziri, A.; Mansour, H.B.; Fernandez-Serrano, M. Persistent Organic and Inorganic Pollutants in the Effluents from the Textile Dyeing Industries: Ecotoxicology Appraisal via a Battery of Biotests. Environ. Res. 2021, 196, 110956. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.A.; Kumar, P.; Eid, E.M.; Singh, J.; Arya, A.K.; Goala, M.; Adelodun, B.; Abou Fayssal, S.; Kumar, V.; Širić, I. Risk Assessment of Heavy Metals Contamination in Soil and Two Rice (Oryza sativa L.) Varieties Irrigated with Paper Mill Effluent. Agriculture 2022, 12, 1864. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Physicochemical Properties, Heavy Metals, and Metal-Tolerant Bacteria Profiles of Abandoned Gold Mine Tailings in Krugersdorp, South Africa. Can. J. Soil Sci. 2020, 100, 217–233. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy Metal Immobilization Potential of Indigenous Bacteria Isolated from Gold Mine Tailings. Int. J. Environ. Res. 2020, 14, 71–86. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A Comprehensive Review on Green Synthesis of Nature-Inspired Metal Nanoparticles: Mechanism, Application and Toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Hulikere, M.M.; Joshi, C.G.; Danagoudar, A.; Poyya, J.; Kudva, A.K.; Dhananjaya, B.L. Biogenic Synthesis of Gold Nanoparticles by Marine Endophytic Fungus-Cladosporium cladosporioides Isolated from Seaweed and Evaluation of Their Antioxidant and Antimicrobial Properties. Process Biochem. 2017, 63, 137–144. [Google Scholar]
- Gnanaprakasam, P.; Jeena, S.E.; Premnath, D.; Selvaraju, T. Simple and Robust Green Synthesis of Au NPs on Reduced Graphene Oxide for the Simultaneous Detection of Toxic Heavy Metal Ions and Bioremediation Using Bacterium as the Scavenger. Electroanalysis 2016, 28, 1885–1893. [Google Scholar] [CrossRef]
- Verma, A.; Dua, R.; Singh, A.; Bishnoi, N.R. Biogenic Sulfides for Sequestration of Cr (VI), COD and Sulfate from Synthetic Wastewater. Water Sci. 2015, 29, 19–25. [Google Scholar] [CrossRef][Green Version]
- Bognár, S.; Putnik, P.; Merkulov, D.Š. Sustainable Green Nanotechnologies for Innovative Purifications of Water: Synthesis of the Nanoparticles from Renewable Sources. Nanomaterials 2022, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Hussain, C.M. Functionalization of Nanomaterials for Industrial Applications: Recent and Future Perspectives. In Handbook of Functionalized Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–14. ISBN 9780128167878. [Google Scholar]
- Kariim, I.; Abdulkareem, A.S.; Abubakre, O.K. Development and Characterization of MWCNTs from Activated Carbon as Adsorbent for Metronidazole and Levofloxacin Sorption from Pharmaceutical Wastewater: Kinetics, Isotherms and Thermodynamic Studies. Sci. Afr. 2020, 7, e00242. [Google Scholar] [CrossRef]
- Pourrahim, S.; Salem, A.; Salem, S.; Tavangar, R. Application of Solid Waste of Ductile Cast Iron Industry for Treatment of Wastewater Contaminated by Reactive Blue Dye via Appropriate Nano-Porous Magnesium Oxide. Environ. Pollut. 2020, 256, 113454. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, S.; Chatterjee, S.; Ghosh, S.; Tudu, P.; Gaine, T.; Bakshi, M.; Das, S.; Das, P.; Bhattacharyya, S.; Bandyopadhyay, S.; et al. Synergistic Approach towards the Sustainable Management of Heavy Metals in Wastewater Using Mycosynthesized Iron Oxide Nanoparticles: Biofabrication, Adsorptive Dynamics and Chemometric Modeling Study. J. Water Process Eng. 2020, 37, 101426. [Google Scholar] [CrossRef]
- Cheng, S.; Li, N.; Jiang, L.; Li, Y.; Xu, B.; Zhou, W. Biodegradation of Metal Complex Naphthol Green B and Formation of Iron--Sulfur Nanoparticles by Marine Bacterium Pseudoalteromonas sp. CF10-13. Bioresour. Technol. 2019, 273, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Shukla, P. Insights into the Resources Generation from Pulp and Paper Industry Wastes: Challenges, Perspectives and Innovations. Bioresour. Technol. 2020, 297, 122496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mathimani, T.; Rene, E.R.; Pugazhendhi, A. Application of Nanotechnology in Dark Fermentation for Enhanced Biohydrogen Production Using Inorganic Nanoparticles. Int. J. Hydrogen Energy 2019, 44, 13106–13113. [Google Scholar] [CrossRef]
- Elreedy, A.; Fujii, M.; Koyama, M.; Nakasaki, K.; Tawfik, A. Enhanced Fermentative Hydrogen Production from Industrial Wastewater Using Mixed Culture Bacteria Incorporated with Iron, Nickel, and Zinc-Based Nanoparticles. Water Res. 2019, 151, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Say, R.; Yilmaz, N.; Denizli, A. Removal of Heavy Metal Ions Using the Fungus Penicillium canescens. Adsorpt. Sci. Technol. 2003, 21, 643–650. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Lepre, L.F.; Ando, R.A.; Oller Do Nascimento, C.A.; Corrêa, B. Biosynthesis and Uptake of Copper Nanoparticles by Dead Biomass of Hypocrea lixii Isolated from the Metal Mine in the Brazilian Amazon Region. PLoS ONE 2013, 8, e80519. [Google Scholar] [CrossRef]
- Wróbel, M.; Śliwakowski, W.; Kowalczyk, P.; Kramkowski, K.; Dobrzyński, J. Bioremediation of Heavy Metals by the Genus Bacillus. Int. J. Environ. Res. Public Health 2023, 20, 4964. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, E.R. Cadmium (Heavy Metals) Bioremediation by Pseudomonas aeruginosa: A Minireview. Appl. Water Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Johnston, C.W.; Wyatt, M.A.; Li, X.; Ibrahim, A.; Shuster, J.; Southam, G.; Magarvey, N.A. Gold Biomineralization by a Metallophore from a Gold-Associated Microbe. Nat. Chem. Biol. 2013, 9, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, S.H.; Kandasamy, R. A Novel Single Step Synthesis and Surface Functionalization of Iron Oxide Magnetic Nanoparticles and Thereof for the Copper Removal from Pigment Industry Effluent. Sep. Purif. Technol. 2017, 188, 458–467. [Google Scholar]
- He, P.; Mao, T.; Wang, A.; Yin, Y.; Shen, J.; Chen, H.; Zhang, P. Enhanced Reductive Removal of Ciprofloxacin in Pharmaceutical Wastewater Using Biogenic Palladium Nanoparticles by Bubbling H2. RSC Adv. 2020, 10, 26067–26077. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Chen, X.; Xiong, D.; Liao, S.; Wang, G. Removal and Recovery of Toxic Silver Ion Using Deep-Sea Bacterial Generated Biogenic Manganese Oxides. PLoS ONE 2013, 8, e81627. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Saini, S.; Paul, E.; Sharma, C.; Mehtani, P. Endophytic Fungi Mediated Synthesis of Iron Nanoparticles: Characterization and Application in Methylene Blue Decolorization. Curr. Res. Green Sustain. Chem. 2021, 4, 100053. [Google Scholar] [CrossRef]
- Velez, P.; Gasca-Pineda, J.; Riquelme, M. Cultivable Fungi from Deep-Sea Oil Reserves in the Gulf of Mexico: Genetic Signatures in Response to Hydrocarbons. Mar. Environ. Res. 2020, 153, 104816. [Google Scholar] [CrossRef]
- Mahanty, S.; Bakshi, M.; Ghosh, S.; Gaine, T.; Chatterjee, S.; Bhattacharyya, S.; Das, S.; Das, P.; Chaudhuri, P. Mycosynthesis of Iron Oxide Nanoparticles Using Manglicolous Fungi Isolated from Indian Sundarbans and Its Application for the Treatment of Chromium Containing Solution: Synthesis, Adsorption Isotherm, Kinetics and Thermodynamics Study. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100276. [Google Scholar] [CrossRef]
- Peña-Castro, J.M.; Martínez-Jerónimo, F.; Esparza-García, F.; Cañizares-Villanueva, R.O. Heavy Metals Removal by the Microalga Scenedesmus incrassatulus in Continuous Cultures. Bioresour. Technol. 2004, 94, 219–222. [Google Scholar] [CrossRef]
- Guleri, S.; Singh, K.; Kaushik, R.; Dhankar, R.; Tiwari, A. Phycoremediation: A Novel and Synergistic Approach in Wastewater Remediation. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 98–106. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.M.; Javed, M.A.; Hassan, A.A.; Mohamed, M.M. Groundwater Remediation Using Zero-Valent Iron Nanoparticles (NZVI). Groundw. Sustain. Dev. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation Technologies for Heavy Metal Contaminated Groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Matlochová, A.; Plachá, D.; Rapantová, N. The Application of Nanoscale Materials in Groundwater Remediation. Pol. J. Environ. Stud. 2013, 22, 1401–1410. [Google Scholar]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Gunjyal, N.; Rani, S.; Asgari Lajayer, B.; Senapathi, V.; Astatkie, T. A Review of the Effects of Environmental Hazards on Humans, Their Remediation for Sustainable Development, and Risk Assessment. Environ. Monit. Assess. 2023, 195, 795. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Potter, P.M.; Navratilova, J.; Rogers, K.R.; Al-Abed, S.R. Transformation of Silver Nanoparticle Consumer Products during Simulated Usage and Disposal. Environ. Sci. Nano 2019, 6, 592–598. [Google Scholar] [CrossRef]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Engineered Nanoparticles in Aquatic Systems: Toxicity and Mechanism of Toxicity in Fish. Emerg. Contam. 2023, 9, 100212. [Google Scholar] [CrossRef]
- Babaei, M.; Tayemeh, M.B.; Jo, M.S.; Yu, I.J.; Johari, S.A. Trophic Transfer and Toxicity of Silver Nanoparticles along a Phytoplankton-Zooplankton-Fish Food Chain. Sci. Total Environ. 2022, 842, 156807. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Feng, S.; Zhang, X.; Xi, Y.; Xiang, X. Bioaccumulation and Biomagnification Effects of Nano-TiO2 in the Aquatic Food Chain. Ecotoxicology 2022, 31, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Leger, E.A.; Espeland, E.K. Perspective: Coevolution between Native and Invasive Plant Competitors: Implications for Invasive Species Management. Evol. Appl. 2010, 3, 169–178. [Google Scholar] [CrossRef]
- Medfu Tarekegn, M.; Zewdu Salilih, F.; Ishetu, A.I.; Yildiz, F. Microbes Used as a Tool for Bioremediation of Heavy Metal from the Environment. Cogent Food Agric. 2020, 6, 1783174. [Google Scholar] [CrossRef]
- Mollá, F.A.; Fito-López, C.; Alvaro, J.A.H.; Huertas-López, F. New Tools to Support the Risk Assessment Process of Nanomaterials in the Insurance Sector. Int. J. Environ. Res. Public Health 2021, 18, 6985. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.; Martí, V.; Darbra, R.M. Environmental Risk Assessment of Silver Nanoparticles in Aquatic Ecosystems Using Fuzzy Logic. Water 2022, 14, 1885. [Google Scholar] [CrossRef]
- Salari, S.; Sadeghi-Yarandi, M.; Golbabaei, F. An Integrated Approach to Occupational Health Risk Assessment of Manufacturing Nanomaterials Using Pythagorean Fuzzy AHP and Fuzzy Inference System. Sci. Rep. 2024, 14, 180. [Google Scholar] [CrossRef]
- Topuz, E.; van Gestel, C.A.M. An Approach for Environmental Risk Assessment of Engineered Nanomaterials Using Analytical Hierarchy Process (AHP) and Fuzzy Inference Rules. Environ. Int. 2016, 92–93, 334–347. [Google Scholar] [CrossRef]
- Odedele, T.O.; Obasi, S.C.; Ibrahim, H.D. Nanomaterials Characterization and Risk Assessment Using Fuzzy Support Vector Machines. Am. J. Comput. Sci. Eng. Surv. 2014, 2, 91–101. [Google Scholar]
- Dumont, E.; Johnson, A.C.; Keller, V.D.J.; Williams, R.J. Nano Silver and Nano Zinc-Oxide in Surface Waters—Exposure Estimation for Europe at High Spatial and Temporal Resolution. Environ. Pollut. 2015, 196, 341–349. [Google Scholar] [CrossRef] [PubMed]
| Endophytic Microorganism | Pollutant Remediated | Nanoparticle Type | References |
|---|---|---|---|
| Bacillus cereus | Methylene Blue dye | Silver | Alfryyan et al. [48] |
| Aspergillus tubingensis STSP 25 | Removal of Pb (II), Ni (II), Cu (II), Zn(II) | Iron oxide | Mahanty et al. [112] |
| Bacillus spp. | Cadmium, chromium, nickel, and copper | Silver Nanoparticles | Wróbel et al. [119] |
| Pseudomonas aeruginosa JP-11 | Removal of Cadmium, chromium, uranium, nickel and copper. | Cadmium Sulphide | Chellaiah et al. [120] |
| Delftia acidovorans | Toxic gold ions. | Gold | Johnston et al. [121] |
| Streptomyces thermolineatus | Removal of copper | Iron oxide magnetic | Suganthi S. et al. [122] |
| Escherichia coli | Removal of ciprofloxacin from hospital wastewater. | Biogenic Palladium | He et al. [123], Shah et al. [68] |
| Marinobacter sp. MnI-79 | Removal of Ag+ | Manganese oxide | Pei et al. [124] |
| Penicillium | Textile wastewater (methylene) | Iron | Mathur et al. [125] |
| Hypocrea lixii | Heavy metal mine. | Copper | Salvadori et al. [50,118] |
| Penicillium sp. | Deep sea hydrocarbon oil reserves. | Nickel oxide | Velez et al. [126] |
| Manglicolous fungi | Removal of Cr (VI) | Iron oxide | Mahanty et al. [127] |
| Scenedesmus incrassatulus | Removal of chromium (VI), cadmium (II) | Palladium | Pena-Castro et al. [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manganyi, M.C.; Dikobe, T.B.; Maseme, M.R. Exploring the Potential of Endophytic Microorganisms and Nanoparticles for Enhanced Water Remediation. Molecules 2024, 29, 2858. https://doi.org/10.3390/molecules29122858
Manganyi MC, Dikobe TB, Maseme MR. Exploring the Potential of Endophytic Microorganisms and Nanoparticles for Enhanced Water Remediation. Molecules. 2024; 29(12):2858. https://doi.org/10.3390/molecules29122858
Chicago/Turabian StyleManganyi, Madira Coutlyne, Tshegofatso Bridget Dikobe, and Mametsi Rahab Maseme. 2024. "Exploring the Potential of Endophytic Microorganisms and Nanoparticles for Enhanced Water Remediation" Molecules 29, no. 12: 2858. https://doi.org/10.3390/molecules29122858
APA StyleManganyi, M. C., Dikobe, T. B., & Maseme, M. R. (2024). Exploring the Potential of Endophytic Microorganisms and Nanoparticles for Enhanced Water Remediation. Molecules, 29(12), 2858. https://doi.org/10.3390/molecules29122858








