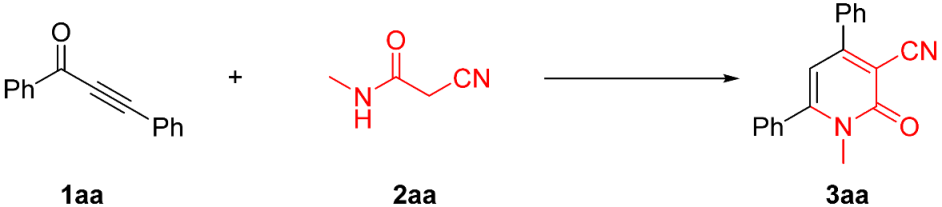
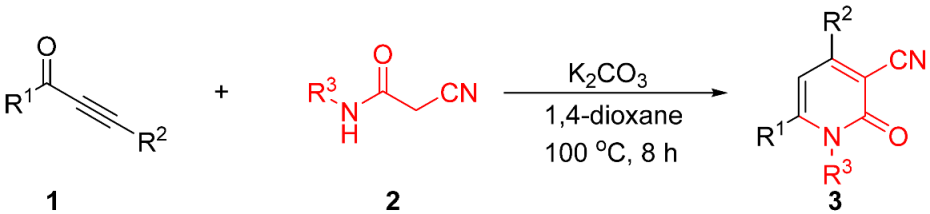
Abstract
A straightforward and efficient methodology has been developed for the synthesis of 3-cyano-2-pyridones via the C–C and C–N bond formation processes. A total of 51 diverse 3-cyano-2-pyridone derivatives were obtained in moderate to excellent yields. This reaction featured advantages such as a metal-free process, wide functional group tolerance, simple operation, and mild conditions. A plausible mechanism for the reaction was proposed. 3-cyano-2-pyridones as ricinine analogues for insecticidal properties were evaluated, and the compound 3ci (LC50 = 2.206 mg/mL) showed the best insecticidal property.
1. Introduction
2-pyridone is considered a privileged heteroaromatic skeleton endowed with pharmaceutical properties and biological activities [1,2,3,4,5,6,7,8,9,10]. They are also widely applicable in organic synthesis, materials science, and polymer chemistry, which are used as valuable building blocks for the synthesis of nitrogen-containing heterocycles like pyridines, quinolizidines, β-lactams, piperidines, and indolizidine alkaloids [11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Owing to their multifarious and prominent properties, 2-pyridones often display promising therapeutical areas such as antifungal, anti-HIV, antibacterial, antiepileptic, anti-inflammatory, and antitumoral activities [25,26,27,28,29,30,31,32,33,34,35,36,37]. As shown in Figure 1A, very typical commercial drugs based on the 2-pyridone scaffold include ciclopirox [38], pifenidone [39], gimeracil [40], and ricinine [41,42,43]. Due to their importance and utility in chemical and pharmaceutical applications, extensive effort has been devoted to the development of efficient methods for the synthesis of diversely functionalized 2-pyridones.
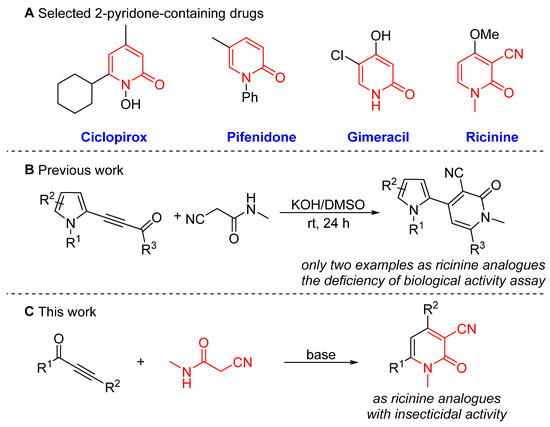
Figure 1.
(A) Selected 2-pyridone-containing drugs. (B) Previous report of the synthesis of 2-pyridones. (C) Our synthesis route to 3-cyano-2-pyridones.
Typically, transition metals such as ruthenium, nickel, and rhodium have been exploited in the cycloaddition of 1,6-diynes with isocyanates towards 2-pyridones [44,45,46,47]. Complementarily, several other methods involving FeCl3-catalyzed intramolecular cascade reactions of acetoacetanilide with 3-formyl chromone [48], Ni-catalyzed reactions of alkynes via azazirconacycles [49], gold-catalyzed cycloisomerization of N-alkenyl alkynylamides [50], and indium-catalyzed domino reactions of 3-formylchromones with N-methyl-1-(methylthio)-2-nitroethenamine [51] have also been developed. However, the cytotoxicity of trace transition metals has limited their utilization in the fields of pharmaceutical application. Moreover, a literature survey implied that other existing approaches to 2-pyridones focus on ring-closing metathesis of α-amino acrylamides [52], t-BuOK-mediated condensation of enones with cyanoacetamides [53], CsF-catalyzed reaction of α,β-unsaturated diester chromones with aromatic amines [54], tandem Blaise reaction of nitriles with propiolates [55], heteropolyacid-catalyzed reaction of amines and acetylenic esters [56], intramolecular ketene trapping of enamine-dioxinones [57], nucleophilic addition of malonic esters with alkynyl imines [58], addition reaction of 2-(phenylsulfinyl)acetamide and α,β-unsaturated ketones [59]. Nevertheless, those reported methods for the synthesis of 2-pyridones suffer from considerable disadvantages, like expensive catalysts [44,45,46,47] and contamination of heavy metals [56], which have limited their potential application in the life science and pharmaceutical industries. Consequently, the development of efficient and environmentally benign procedures for the synthesis of 2-pyridones continues to be of great significance.
Ricinine [60] has been reported to exhibit pharmacological activities such as antifertility, antiimplantation, antinociceptive, anticancer, antidiabetic, and insecticidal activities (Figure 1A). On the other hand, ynones have emerged as valuable building blocks for the construction of complex heterocyclic rings, such as indole, pyrrole, pyridine, triazole, quinoline, thiazole, pyrimidine, and so on [61,62,63,64,65,66,67,68,69,70,71,72,73]. Recently, Trofimov and co-workers [74] reported the condensation of acylethynylpyrroles and 2-cyano-N-methylacetamide to form N-methyl-3-cyano-2-pyridones (Figure 1B). However, considerable disadvantages like the relatively poor functional scopes (only 2 examples of N-methyl-3-cyano-2-pyridone as ricinine analogues) and the deficiency of biological activity assays still exist in this process. Inspired by the aforementioned works and previous reports [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76], our primary aim is to develop an easy and efficacious synthetic strategy for the expeditious assembly of 3-cyano-2-pyridones from the cycloaddition of ynones and 2-cyanoacetamide (Figure 1C). Simultaneously, we strive to identify 3-cyano-2-pyridones that serve as ricinine analogues and display potent insecticidal properties.
2. Results and Discussion
2.1. Optimization of the Reaction Conditions
In an initial effort, we began our investigation with the cascade cycloaddition of 1,3-diphenylprop-2-yn-1-one 1aa and 2-cyano-N-methylacetamide 2aa in 1,4-dioxane in the presence of K2CO3 as a base at 100 °C for 8 h (Table 1). To our delight, the preliminary result showed the desired product 3aa was obtained with an 81% yield (entry 1). The yield was decreased when the transition-metal-free reaction was conducted with Na2CO3 or Cs2CO3 instead of K2CO3 (entries 2–3). Moreover, various kinds of bases were screened, whereas no reaction or trace transformation was observed, neither strong inorganic bases such as NaOH, NaH, EtONa, LiOtBu, NaOtBu, and KOtBu (entries 4–9) nor organic bases including Et3N, DBU, and DABCO (entries 10–12) involving the transformation. Further investigation showed that this reaction was highly solvent-dependent (entries 13–19). When the reaction was carried out in DMSO, DMF, and NMP, an acceptable yield of 3aa was detected (entries 13–15). However, other solvents, such as CH3CN, toluene, THF, and EtOH, afforded only a trace amount of the desired product 3aa (entries 16–19). Finally, decreasing the amount of K2CO3 lowered the yield (entry 20), while no obvious deviation was observed when the loading of K2CO3 was increased (entries 21–22). On the basis of the above results, the optimal conditions for this transformation were obtained as follows: K2CO3 (1.2 equiv.) in 1,4-dioxane at 100 °C for 8 h.

Table 1.
Optimization of the reaction conditions a.
2.2. Scope of the Reaction
With the optimum conditions in hand, the generality and scope of this metral-free cascade reaction of ynones and 2-cyanoacetamides were explored, and the results are shown in Table 2. Firstly, we studied the scope of alkynones with substituents in Ar–R1 (entries 2–20). The reaction shows preference for electron-rich alkynones (Me, OMe) (entries 2–6), which provided the corresponding products in relatively higher yields than their counterparts bearing electron-withdrawing groups (F, Cl, Br, I, CF3) (entries 7–20). Compared to the yield of 3ab and the yield of 3ad (87% vs. 73%), we found that the steric hindrance influenced this reaction. Particularly, a 2-naphthyl-substituted substrate could also be subjected to the reaction, and the corresponding product, 3au, was obtained with a 90% yield. Moreover, both alkyl- and heterocyclyl involving-alkynones were also suitable candidates for this transformation, and the corresponding products, 3av, 3aw, and 3ax were obtained in 83%, 76%, and 84% yields, respectively.

Table 2.
Chemical structures and synthesis yields of 3-cyano-2-pyridones a.
As for regioisomeric substrates, alkynones with substituents in Ar–R2 were also investigated (entries 25–34). Similar to alkynones with functional groups in Ar–R1, the cycloaddition reactions proceeded smoothly to give the corresponding 3-cyano-2-pyridones in satisfactory yields. Notably, both electronic effect (entries 25–29 vs. entries 30–34) and steric hindrance influence (3bg vs. 3bh) existed in this reaction. As outlined in Table 2, electron-donating and para-substituted alkynones were good substrates, leading to the target products in moderate to excellent yields (entries 25–29). Interestingly, alkynones with cyclopropyl, n-hexyl, and 2-thienyl groups were also tolerated in this procedure and provided 3-cyano-2-pyridones in satisfactory yield (entries 35–37).
Additionally, alkynones with substituents in Ar–R1 and Ar–R2 were also evaluated under the standard reaction conditions. The corresponding 3-cyano-2-pyridones were obtained in moderate to excellent yields regardless of the electron-donating or electron-withdrawing groups at benzene rings (entries 38–45). Encouragingly, alkynones bearing two electron-rich functional groups provided excellent yields because of the strong electron-donating effect (entries 38–40). However, with ortho-functional group substrates, the yields of corresponding products decreased, maybe due to the steric hindrance (entries 41–42 and 44–45). Furthermore, alkynones possessing a naphthyl and cyclopropyl ring were carried out in this cascade reaction system and furnished the desired products 3ci, 3cj, and 3ck in 88%, 80%, and 60% yields, respectively (entries 46–48). Finally, 2-cyanoacetamides with functional groups phenyl and benzyl were tested and provided the products in moderate yield (entries 49–50), while 2-cyanoacetamide involved in the transformation obtained the 3-cyano-2-pyridone in excellent yield (entry 51).
2.3. Gram-Scale of the Reaction
To demonstrate the reliability and practicality of this present synthetic protocol, a gram-scale preparation was performed using starting materials 1aa, where the desired product, 3aa, was isolated in an 83% yield (Scheme 1).
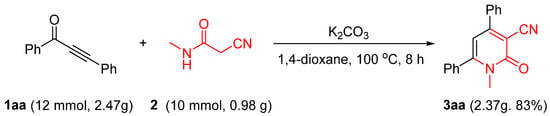
Scheme 1.
Gram-scale of 3aa.
2.4. Plausible Mechanism of the Reaction
On the basis of the aforementioned observations and previous reports [74], the mechanistic pathways of this cascade methodology were proposed and presented in Scheme 2. Initially, 2-cyano-N-methylacetamide 2aa undergoes a K2CO3-initiated deprotonation of the CH2 group to give carbanion A, and then the nucleophilic addition of carbanion A to the triple bond of ynone 1aa produces carbanion B. Subsequently, the carbanion B is quenched by a proton of the medium to generate intermediate C. Finally, an intramolecular cycloaddition of the intermediate C results in the formation of the cyclization product 3aa.
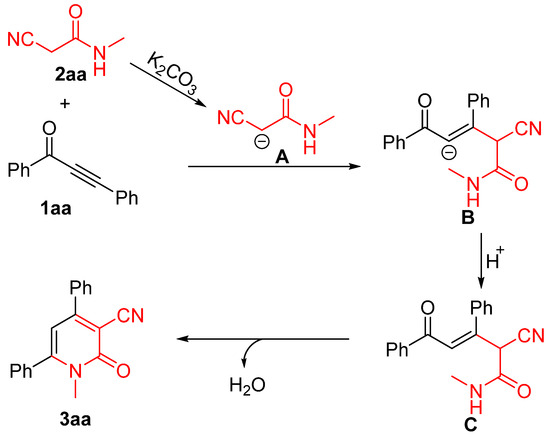
Scheme 2.
Plausible mechanism.
2.5. Study of the Insecticidal Properties of 3-Cyano-2-Pyridones
A literature survey implied that ricinine is well known as a compound with a high level of biological activity and has been proven valuable due to its insecticidal properties [77]. Based on the structure of ricinine, we have prepared several 3-cyano-2-pyridones with novel scaffolds as ricinine analogues to evaluate their insecticidal activity (see the experimental details in Supplementary Materials). Fortunately, in the trials of killing mealworm, we observed that the compounds 3au (LC50 = 3.245 mg/mL) and 3bc (LC50 = 3.579 mg/mL) exhibited powerful pesticidal activity using ricinine as a positive control. These results implied that 2-naphthyl and para-n-butyl benzene are good functional groups in the 3-cyano-2-pyridone framework as insecticides. Subsequently, the suitable functional candidate 3ci (LC50 = 2.206 mg/mL) bearing 2-naphthyl and para-n-butyl benzene groups was obtained, and the insecticidal activity was evaluated. Upon comparing the LC50 values in Figure 2, we found that the compound 3ci was the best candidate drug with insecticidal properties.
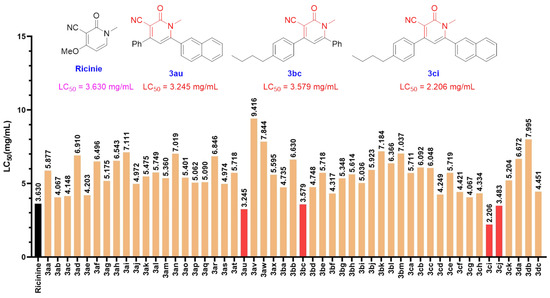
Figure 2.
The insecticidal properties of 3-cyano-2-pyridones.
3. Experimental Section
3.1. General Methods and Materials for the Synthesis of 3-Cyano-2-Pyridones 3
Unless otherwise stated, all reagents were used directly without further purification. Silica gel was purchased from Qing Dao Hai Yang Chemical Industry Co. (Qinqdao, China). All melting points were determined on a Beijing Science Instrument Dianguang Instrument Factory XT4B (Beijing, China) melting point apparatus and uncorrected. 1H and 13C NMR spectra were measured on a 400 MHz JEOL JNM-ECZ400S/L1 spectrometer (Jeol Ltd., Tokyo, Japan), (1H 400 MHz, 13C 100 MHz), using CDCl3 as the solvent with tetramethylsilane (TMS) as the internal standard at room temperature. HRMS-ESI spectra were equipped with an ESI source and a TOF detector. PE is petroleum ether (60–90 °C).
A Typical Procedure for the Synthesis of 1-methyl-2-oxo-4,6-diphenyl-1,2-dihydropyridine-3-carbonitrile (3aa).
A suspension of 1,3-diphenylprop-2-yn-1-one 1aa (0.6 mmol, 123.6 mg), 2-cyano-N-methylacetamide 2aa (0.5 mmol, 49.0 mg), and K2CO3 (0.6 mmol, 82.8 mg) in 1,4-dioxane (2 mL) was stirred at 100 °C for 8 h. After 2aa was exhausted completely (monitored by TLC), it was cooled down to room temperature and saturated aqueous brine (20 mL) was added. The mixture was stirred for 10 min and then extracted by EtOAc (3 × 10 mL). The combined organic layers were dried over Na2SO4. Removal of the solvent gave a residue, which was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v) to afford 3aa as a yellow solid, 116 mg, 81% yield; mp 148–151 °C. 1H NMR (400 MHz, chloroform-d) δ 7.63 (d, J = 3.96 Hz, 2H), 7.53–7.47 (m, 6H), 7.40 (d, J = 3.88 Hz, 2H), 6.32 (s, 1H), 3.47 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ 161.7, 158.2, 154.0, 135.5, 134.1, 130.5, 130.3, 129.1 (2C), 128.9 (2C), 128.01 (2C), 127.97 (2C), 116.0, 109.1, 100.1, 35.0.
For 3aa (gram-scale), a mixture of 1,3-diphenylprop-2-yn-1-one 1aa (12.0 mmol, 2.47 g), 2-cyano-N-methylacetamide 2aa (10 mmol, 0.98 g), and K2CO3 (12.0 mmol, 1.66 g) in 1,4-dioxane (15 mL) was stirred at 100 °C for 8 h. After completion of the reaction (monitored by TLC), it was cooled down to room temperature, and the resulting solution was poured into water (100 mL), then extracted with EtOAc (3 × 50 mL). The combined EtOAc extracts were dried over Na2SO4 and concentrated. Then, the solvent was evaporated, and the residue was purified by chromatography (silica gel, PE/EtOAc = 3/1 v/v) to give 3aa in 83% yield (2.37 g).
A similar procedure was used for the preparation of products 3ab–3ck.
1-methyl-2-oxo-4-phenyl-6-(p-tolyl)-1,2-dihydropyridine-3-carbonitrile (3ab).
The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 130 mg, 87% yield; yellow soild; mp 131–134 °C. 1H NMR (400 MHz, CDCl3) δ 7.55 (s, 2H), 7.38 (s, 3H), 7.26 (d, J = 12.9 Hz, 4H), 6.23 (s, 1H), 3.41 (s, 3H), 2.35 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.9, 158.2, 154.6, 140.8, 135.8, 131.4, 130.6, 129.8 (2C), 129.0 (2C), 128.2 (2C), 128.1 (2C), 116.3, 109.3, 99.7, 35.2, 21.5. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H17N2O+ 301.1335; found, 301.1342.
1-methyl-2-oxo-4-phenyl-6-(m-tolyl)-1,2-dihydropyridine-3-carbonitrile (3ac). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 122 mg, 81% yield; yellow soild; mp 157–160 °C. 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 2H), 7.47 (s, 3H), 7.38 (t, J = 7.5 Hz, 1H), 7.31 (d, J = 7.9 Hz, 1H), 7.17 (s, 2H), 6.29 (s, 1H), 3.45 (s, 3H), 2.41 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.8, 158.2, 154.3, 139.1, 135.6, 134.1, 131.0, 130.6, 128.9, 128.9 (2C), 128.5, 128.0 (2C), 125.0, 116.0, 109.1, 100.1, 35.1, 21.4. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H17N2O+ 301.1335; found, 301.1342.
1-methyl-2-oxo-4-phenyl-6-(o-tolyl)-1,2-dihydropyridine-3-carbonitrile (3ad). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 109 mg, 73% yield; yellow soild; mp 170–173 °C. 1H NMR (400 MHz, CDCl3) δ 7.63 (s, 2H), 7.47 (s, 3H), 7.41 (t, J = 7.5 Hz, 1H), 7.32 (d, J = 7.5 Hz, 2H), 7.19 (d, J = 8.0 Hz, 1H), 6.26 (s, 1H), 3.30 (s, 3H), 2.19 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.6, 158.3, 153.7, 135.5, 135.4, 133.8, 130.8, 130.6, 130.3, 128.9 (2C), 128.1 (2C), 128.0, 126.6, 115.9, 108.6, 100.2, 33.7, 19.3. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H17N2O+ 301.1335; found, 301.1345.
6-(4-methoxyphenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3ae). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 136 mg, 86% yield; yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 2H), 7.47 (s, 3H), 7.31 (s, 2H), 7.01 (s, 2H), 6.27 (s, 1H), 3.86 (s, 3H), 3.47 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.9, 161.1, 158.1, 154.1, 135.7, 130.5, 129.6 (2C), 128.9 (2C), 128.0 (2C), 126.4, 116.1, 114.4 (2C), 109.2, 99.8, 55.5, 35.1. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H17N2O2+ 317.1285; found, 317.1297.
6-(2-methoxyphenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3af). The compound was purified by column chromatography (silica gel, PE/EtOAc = 1/1 v/v): 119 mg, 75% yield; yellow soild; mp 173–176 °C. 1H NMR (400 MHz, CDCl3) δ 7.64 (s, 2H), 7.46 (s, 4H), 7.25 (s, 1H), 7.05 (d, J = 2.7 Hz, 2H), 6.27 (s, 1H), 3.83 (s, 3H), 3.37 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.8, 158.3, 156.4, 152.3, 135.9, 132.3, 130.6, 129.8, 129.0 (2C), 128.3 (2C), 123.3, 121.3, 116.4, 111.2, 109.4, 100.2, 55.7, 34.0. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H17N2O2+ 317.1285; found, 317.1289.
6-(4-fluorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3ag). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 114 mg, 75% yield; brown soild; mp 147–150 °C. 1H NMR (400 MHz, CDCl3) δ 7.59 (s, 2H), 7.46 (s, 3H), 7.40 (s, 2H), 7.20 (d, J = 7.7 Hz, 2H), 6.28 (s, 1H), 3.43 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.9 (d, J = 250.9 Hz), 161.6, 158.2, 152.9, 135.4, 130.6, 130.3 (d, J = 46.0 Hz, 2C), 128.9 (2C), 128.0 (2C), 116.5 (d, J = 22.0 Hz, 2C), 115.9, 109.3, 100.4, 35.0. 19F NMR (376 MHz, CDCl3) δ −109.13 (s, 1F). HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C19H13FN2NaO+ 327.0904; found, 327.0906.
6-(3-fluorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3ah). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 111 mg, 73% yield; brown soild; mp 128–131 °C. 1H NMR (400 MHz, CDCl3) δ 7.59 (s, 2H), 7.47 (s, 4H), 7.21 (d, J = 9.3 Hz, 2H), 7.14 (d, J = 8.8 Hz, 1H), 6.30 (s, 1H), 3.45 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.1 (d, J = 248.7 Hz), 161.8, 158.5, 152.7, 136.2 (d, J = 7.4 Hz), 135.6, 131.4 (d, J = 8.2 Hz), 131.0, 129.2 (2C), 128.3 (2C), 124.3, 117.8 (d, J = 20.8 Hz), 116.1, 115.8 (d, J = 22.9 Hz), 109.4, 101.0, 35.3. 19F NMR (376 MHz, CDCl3) δ −110.32 (s, 1F). HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C19H13FN2NaO+ 327.0904; found, 327.0912.
6-(2-fluorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3ai). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 97 mg, 64% yield; brown soild; mp 161–164 °C. 1H NMR (400 MHz, CDCl3) δ 7.62 (s, 2H), 7.53 (s, 1H), 7.47 (s, 3H), 7.34 (d, J = 5.7 Hz, 2H), 7.24 (s, 1H), 6.33 (s, 1H), 3.42 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.4, 160.2 (d, J = 248.9 Hz), 158.2, 148.6, 135.4, 132.8 (d, J = 8.1 Hz), 130.7, 130.1, 128.9 (2C), 128.1 (2C), 125.2, 122.1 (d, J = 15.5 Hz), 116.4 (d, J = 20.9 Hz), 115.8, 109.7, 101.1, 34.2. 19F NMR (376 MHz, CDCl3) δ −112.26 (s, 1F). HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C19H13FN2NaO+ 327.0904; found, 327.0907.
6-(4-chlorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3aj). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 122 mg, 76% yield; brown soild; mp 171–174 °C. 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 7.9 Hz, 2H), 7.49 (s, 1H), 7.47 (s, 1H), 7.44 (s, 2H), 7.42 (d, J = 1.7 Hz, 1H), 7.37 (s, 1H), 7.36 (s, 1H), 6.27 (s, 1H), 3.42 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.7, 158.3, 153.0, 136.8, 135.5, 132.6, 130.8, 129.7 (2C), 129.6(2C), 129.1 (2C), 128.2 (2C), 116.1, 109.4, 100.5, 35.1. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C19H13ClN2NaO+ 343.0609; found, 343.0616.
6-(3-chlorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3ak). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 119 mg, 74% yield; brown soild; mp 162–165 °C. 1H NMR (400 MHz, CDCl3) δ 7.60 (s, 2H), 7.48 (s, 4H), 7.39 (s, 1H), 7.30 (s, 1H), 7.24 (s, 1H), 6.29 (s, 1H), 3.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.4, 158.3, 152.2, 135.7, 135.3, 135.2, 130.7, 130.5, 130.5, 129.0 (2C), 128.1, 128.0 (2C), 126.2, 115.8, 109.2, 100.8, 35.0. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C19H13ClN2NaO+ 343.0609; found, 327.0610.
6-(2-chlorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3al). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 108 mg, 67% yield; brown soild; mp 151–154 °C. 1H NMR (400 MHz, CDCl3) δ 7.64 (s, 2H), 7.54 (d, J = 7.9 Hz, 1H), 7.48 (s, 4H), 7.43 (d, J = 7.4 Hz, 1H), 7.34 (d, J = 7.8 Hz, 1H), 6.28 (s, 1H), 3.37 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.5, 158.5, 151.1, 135.5, 133.3, 132.9, 131.9, 130.8, 130.30, 130.0, 129.1 (2C), 128.3 (2C), 127.8, 116.0, 109.1, 101.2, 33.8. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H14ClN2O+ 321.0789; found, 321.0786.
6-(4-bromophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3am). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 137 mg, 76% yield; brown soild; mp 176–179 °C. 1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 8.6 Hz, 2H), 7.57 (d, J = 8.0 Hz, 2H), 7.43 (d, J = 7.7 Hz, 3H), 7.30 (d, J = 8.6 Hz, 2H), 6.27 (s, 1H), 3.42 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.7, 158.3, 153.0, 135.5, 133.1, 132.5 (2C), 130.8, 129.9 (2C), 129.1 (2C), 128.2 (2C), 125.0, 116.1, 109.3, 100.5, 35.1. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H14BrN2O+ 365.0284; found, 365.0287.
6-(2-bromophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3an). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 113 mg, 62% yield; yellow soild; mp 187–190 °C. 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 6.5 Hz, 1H), 7.61 (s, 2H), 7.45 (s, 4H), 7.37 (d, J = 7.9 Hz, 2H), 6.25 (s, 1H), 3.33 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.4, 158.5, 152.4, 135.5, 135.3, 133.4, 132.0, 130.8, 130.0, 129.0 (2C), 128.4, 128.3 (2C), 122.3, 116.0, 109.1, 101.0, 33.9. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H14BrN2O+ 365.0284; found, 365.0267.
1-methyl-2-oxo-4-phenyl-6-(4-(trifluoromethyl)phenyl)-1,2-dihydropyridine-3-carbonitrile (3ao). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 115 mg, 65% yield; yellow soild; mp 166–169 °C. 1H NMR (400 MHz, CDCl3) δ 7.78 (s, 2H), 7.58 (s, 4H), 7.47 (s, 3H), 6.30 (s, 1H), 3.43 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.4, 158.3, 152.2, 137.5, 135.2, 132.6 (d, J = 32.7 Hz), 130.8, 129.0 (2C), 128.7 (2C), 128.0 (2C), 126.2, 126.2, 124.8, 115.7, 109.2, 101.0, 35.0. 19F NMR (376 MHz, CDCl3) δ −62.80 (s, 3F). HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H14F3N2O+ 355.1053; found, 355.1069.
6-(4-fluoro-2-methylphenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3ap). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 112 mg, 70% yield; yellow soild; mp 187–190 °C. 1H NMR (400 MHz, CDCl3) δ 7.60 (s, 2H), 7.44 (s, 3H), 7.22 (s, 1H), 7.02 (s, 2H), 6.22 (s, 1H), 3.28 (s, 3H), 2.20 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.9 (d, J = 249.5 Hz), 161.8, 158.5, 153.0, 138.8 (d, J = 8.4 Hz), 135.6, 130.9, 130.4 (d, J = 22.7 Hz), 129.1 (2C), 128.3 (2C), 118.0 (d, J = 22.0 Hz), 116.1, 114.1 (d, J = 22.0 Hz), 109.2, 109.2, 100.7, 34.0, 19.8. 19F NMR (376 MHz, CDCl3) δ −110.18 (s, 1F). HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C20H15FN2NaO+ 341.1061; found, 341.1061.
6-(2,4-dichlorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrilee (3aq). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 98 mg, 55% yield; yellow liquid; 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 7.9 Hz, 2H), 7.54 (s, 1H), 7.45 (s, 2H), 7.43 (s, 1H), 7.41 (s, 1H), 7.36 (d, J = 8.3 Hz, 1H), 6.25 (s, 1H), 3.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.3, 158.4, 150.0, 137.5, 135.3, 133.8, 131.7, 131.1, 130.9, 130.2, 129.1 (2C), 128.3, 128.2 (2C), 115.9, 109.3, 101.4, 33.8. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H13Cl2N2O+ 355.0399; found, 355.0397.
6-(2,3-dichlorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3ar). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 103 mg, 58% yield; brown soild; mp 204–207 °C. 1H NMR (400 MHz, CDCl3) δ 7.59 (s, 3H), 7.44 (s, 3H), 7.35 (s, 1H), 7.27 (s, 1H), 6.23 (s, 1H), 3.31 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.2, 158.5, 150.3, 135.3, 135.2, 134.4, 132.5, 131.46, 130.9, 129.1 (2C), 128.5, 128.2 (3C), 115.8, 108.9, 101.5, 33.8. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C19H12Cl2N2NaO+ 377.0219; found, 377.0222.
6-(2-chloro-4-fluorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3as). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 95 mg, 56% yield; brown soild; mp 151–154 °C. 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 2H), 7.46 (s, 3H), 7.38 (s, 1H), 7.29 (d, J = 5.4 Hz, 1H), 7.15 (s, 1H), 6.26 (s, 1H), 3.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.7 (d, J = 274.1 Hz), 161.2, 158.3, 149.9, 135.2, 134.2 (d, J = 10.2 Hz), 131.4 (d, J = 9.1 Hz), 130.8, 129.5, 129.4, 128.9 (2C), 128.1 (2C), 118.0 (d, J = 25.0 Hz), 115.7, 115.3 (d, J = 21.5 Hz), 109.3, 101.3, 33.7. 19F NMR (376 MHz, CDCl3) δ −106.66 (s, 1F). HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H13ClFN2O+ 339.0695; found, 339.0694.
6-(2,4-difluorophenyl)-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3at). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 92 mg, 57% yield; yellow soild; mp 136–139 °C. 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 1H), 7.59 (s, 1H), 7.45 (d, J = 7.3 Hz, 3H), 7.41 (s, 1H), 7.08–7.03 (m, 1H), 7.01–6.96 (m, 1H), 6.30 (s, 1H), 3.41 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 166.0 (d, J = 11.5 Hz), 163.4 (d, J = 11.6 Hz), 161.5, 161.0 (d, J = 12.2 Hz), 158.4, 147.8, 135.5, 131.7 (d, J = 9.6 Hz), 131.0, 129.2 (2C), 128.3 (2C), 118.7 (d, J = 15.8 Hz), 116.0, 113.1 (d, J = 21.6 Hz), 110.2, 105.5 (d, J = 50.4 Hz), 105.2, 101.5, 34.4. 19F NMR (376 MHz, CDCl3) δ −104.13 (s, 1F), −107.54 (s, 1F). HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H13F2N2O+ 323.0990; found, 323.0997.
1-methyl-6-(naphthalen-2-yl)-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3au). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 152 mg, 90% yield; yellow soild; mp 191–194 °C. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.5 Hz, 1H), 7.90 (s, 3H), 7.66–7.63 (m, 2H), 7.62–7.58 (m, 2H), 7.48 (s, 3H), 7.46–7.43 (m, 1H), 6.41 (s, 1H), 3.50 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.7, 158.2, 154.1, 135.6, 133.5, 132.7, 131.4, 130.6, 128.9, 128.9 (2C), 128.3, 128.0 (3C), 127.9, 127.9, 127.4, 124.6, 116.0, 109.4, 100.2, 35.2. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C23H17N2O+ 337.1335; found, 337.1347.
6-cyclopropyl-1-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3av). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 104 mg, 83% yield; yellow soild; mp 154–157 °C. 1H NMR (400 MHz, CDCl3) δ 7.52 (s, 2H), 7.45 (s, 3H), 6.09 (s, 1H), 3.74 (s, 3H), 1.89 (s, 1H), 1.16 (s, 2H), 0.87 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 161.7, 158.4, 155.7, 135.9, 130.3, 128.8 (2C), 127.9 (2C), 116.1, 105.7, 99.0, 31.6, 15.0, 7.6 (2C). HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C16H14N2NaO+ 273.0998; found, 273.1005.
1,6-dimethyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carbonitrile (3aw). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 170 mg, 76% yield; yellow soild; mp 164–167 °C. 1H NMR (400 MHz, CDCl3) δ 7.56 (s, 2H), 7.47 (s, 3H), 6.23 (s, 1H), 3.58 (s, 3H), 2.46 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.7, 158.1, 151.6, 135.6, 130.4, 128.8 (2C), 127.9 (2C), 116.1, 108.1, 98.7, 31.8, 21.6.
1-methyl-2-oxo-4-phenyl-6-(thiophen-2-yl)-1,2-dihydropyridine-3-carbonitrile (3ax). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 123 mg, 84% yield; yellow soild; mp 144–147 °C. 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 2H), 7.55 (d, J = 3.9 Hz, 1H), 7.48 (d, J = 2.7 Hz, 3H), 7.29 (d, J = 3.7 Hz, 1H), 7.16 (d, J = 1.4 Hz, 1H), 6.46 (s, 1H), 3.62 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.6, 157.8, 147.0, 135.4, 134.1, 130.6, 130.0, 129.2, 128.9, 128.0 (2C), 127.9 (2C), 115.9, 110.4, 100.6, 35.0. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C17H12N2NaOS+ 315.0563; found, 315.0567.
1-methyl-2-oxo-6-phenyl-4-(p-tolyl)-1,2-dihydropyridine-3-carbonitrile (3ba). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 132 mg, 88% yield; white soild; mp 129–132 °C. 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 5H), 7.37 (s, 2H), 7.26 (s, 2H), 6.28 (s, 1H), 3.42 (s, 3H), 2.37 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.8, 158.1, 153.8, 141.0, 134.2, 132.6, 130.3, 129.6 (2C), 129.1 (2C), 128.0 (4C), 116.2, 109.1, 99.8, 35.9, 21.4.
1-methyl-2-oxo-6-phenyl-4-(m-tolyl)-1,2-dihydropyridine-3-carbonitrile (3bb). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 126 mg, 84% yield; yellow soild; mp 159–162 °C. 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 3H), 7.39 (s, 4H), 7.32 (s, 1H), 7.27 (s, 1H), 6.28 (s, 1H), 3.44 (s, 3H), 2.36 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.9, 158.5, 154.1, 138.8, 135.7, 134.3, 131.4, 130.4, 129.2 (2C), 128.9, 128.7, 128.2 (2C), 125.3, 116.2, 109.3, 100.2, 35.1, 21.5. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H17N2O+ 301.1335; found, 301.134.
4-(4-butylphenyl)-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3bc). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 146 mg, 85% yield; white liquid. 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 8.2 Hz, 2H), 7.51 (s, 3H), 7.38 (s, 2H), 7.27 (d, J = 8.3 Hz, 2H), 6.29 (s, 1H), 3.44 (s, 3H), 2.63 (s, 2H), 1.59 (s, 2H), 1.35 (s, 2H), 0.90 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.8, 158.2, 153.8, 146.0, 134.3, 132.8, 130.3, 129.1 (2C), 129.0 (2C), 128.0 (2C), 128.0 (2C), 116.2, 109.1, 99.8, 35.5, 35.0, 33.3, 22.3, 13.9. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C23H23N2O+ 343.1805; found, 343.1818.
4-(4-methoxyphenyl)-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3bd). The compound was purified by column chromatography (silica gel, PE/EtOAc = 1/1 v/v): 151 mg, 95% yield; yellow soild; mp 139–142 °C. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 8.9 Hz, 2H), 7.47 (s, 3H), 7.38 (s, 2H), 6.90 (d, J = 8.9 Hz, 2H), 6.26 (s, 1H), 3.77 (s, 3H), 3.38 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 162.0, 161.6, 157.6, 154.0, 134.4, 130.4, 130.0 (2C), 129.2 (2C), 128.2 (2C), 127.7, 116.7, 114.4 (2C), 109.2, 99.1, 55.5, 35.0. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H17N2O2+ 317.1285; found, 317.1293.
4-(3-methoxyphenyl)-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3be). The compound was purified by column chromatography (silica gel, PE/EtOAc = 1/1 v/v): 146 mg, 92% yield; yellow soild; mp 149–152 °C. 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 3H), 7.39 (s, 2H), 7.33 (d, J = 5.8 Hz, 1H), 7.16 (d, J = 7.8 Hz, 1H), 7.11 (s, 1H), 6.99 (s, 1H), 6.30 (s, 1H), 3.79 (s, 3H), 3.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.8, 159.8, 158.2, 154.3, 136.9, 135.0, 130.5, 130.1, 129.2 (2C), 128.2 (2C), 120.5, 116.6, 116.1, 113.4, 109.3, 100.2, 55.6, 35.2. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C20H17N2O2+ 317.1285; found, 317.1292.
4-(4-fluorophenyl)-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3bf). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 113 mg, 74% yield; brown soild; mp 172–175 °C. 1H NMR (400 MHz, CDCl3) δ 7.62 (s, 2H), 7.52 (s, 3H), 7.38 (s, 2H), 7.14 (d, J = 7.6 Hz, 2H), 6.26 (s, 1H), 3.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.3, 162.8, 161.6, 157.1, 154.3, 134.1, 131.6, 130.4 (2C), 130.3 (d, J = 8.7 Hz, 2C), 129.1 (2C), 128.0 (2C), 116.2 (d, J = 21.8 Hz), 108.9, 100.1, 35.1. 19F NMR (376 MHz, CDCl3) δ −109.15 (s, 1F).
4-(4-chlorophenyl)-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3bg). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 121 mg, 75% yield; brown soild; mp 165–168 °C. 1H NMR (400 MHz, CDCl3) δ 7.56 (s, 1H), 7.53 (s, 1H), 7.51 (d, J = 2.3 Hz, 3H), 7.42 (s, 1H), 7.40 (s, 3H), 6.26 (s, 1H), 3.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.6, 157.0, 154.6, 137.0, 134.2, 134.1, 130.6, 129.6 (2C), 129.3 (2C), 129.3 (2C), 128.1 (2C), 116.0, 108.9, 100.1, 35.2. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C19H13ClN2NaO+ 343.0609; found, 343.0615.
4-(2-chlorophenyl)-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3bh). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 97 mg, 60% yield; brown soild; mp 172–175 °C. 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 2.4 Hz, 2H), 7.48 (s, 3H), 7.38 (s, 2H), 7.37 (s, 2H), 6.22 (s, 1H), 3.47 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.4, 156.7, 154.1, 134.9, 134.1, 131.8, 131.2, 130.6, 130.4, 130.0, 129.2 (2C), 128.2 (2C), 127.3, 115.2, 110.1, 102.8, 35.3.
4-(4-bromophenyl)-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3bi). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 143 mg, 78% yield; white liquid. 1H NMR (400 MHz, CDCl3) δ 7.58 (s, 2H), 7.51 (s, 5H), 7.38 (s, 2H), 6.25 (s, 1H), 3.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.5, 156.9, 154.5, 134.4, 134.0, 132.2 (2C), 130.4, 129.6 (2C), 129.1 (2C), 127.9 (2C), 125.2, 115.8, 108.7, 100.0, 35.1. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H14BrN2O+ 365.0284; found, 365.0292.
Methyl 4-(3-cyano-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridin-4-yl)benzoate (3bj). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 121 mg, 70% yield; orange soild; mp 121–124 °C. 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 8.6 Hz, 2H), 7.67 (d, J = 8.5 Hz, 2H), 7.52 (s, 3H), 7.39 (s, 2H), 6.29 (s, 1H), 3.92 (s, 3H), 3.46 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 166.2, 161.4, 157.1, 154.5, 139.8, 134.0, 131.8, 130.5, 130.1 (2C), 129.2 (2C), 128.2 (2C), 128.0 (2C), 115.6, 108.7, 100.5, 52.4, 35.2. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C21H17N2O3+ 345.1234; found, 345.1240.
4-cyclopropyl-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3bk). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 109 mg, 87% yield; white soild; mp 167–170 °C. 1H NMR (400 MHz, CDCl3) δ 7.45 (s, 3H), 7.28 (s, 2H), 5.49 (s, 1H), 3.28 (s, 3H), 2.21 (s, 1H), 1.20 (s, 2H), 0.88 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 164.2, 161.0, 154.2, 134.5, 130.3, 129.1 (2C), 128.1 (2C), 115.9, 102.8, 101.4, 34.7, 15.0, 11.4 (2C). HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C16H14N2NaO+ 273.0998; found, 273.1001.
4-hexyl-1-methyl-2-oxo-6-phenyl-1,2-dihydropyridine-3-carbonitrile (3bl). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 121 mg, 82% yield; yellow soild; mp 115–118 °C. 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 2.3 Hz, 3H), 7.32 (d, J = 3.1 Hz, 2H), 6.08 (s, 1H), 3.35 (s, 3H), 2.70–2.65 (m, 2H), 1.65–1.57 (m, 2H), 1.36 (s, 2H), 1.27 (d, J = 3.3 Hz, 2H), 1.26 (s, 2H), 0.86–0.81 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 162.5, 161.5, 153.9, 134.3, 130.3, 129.2 (2C), 128.1 (2C), 115.4, 109.2, 102.1, 35.0, 34.9, 31.5, 29.4, 29.0, 22.5, 14.1. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C19H22N2NaO+ 317.1624; found, 317.1627.
1-methyl-2-oxo-6-phenyl-4-(thiophen-2-yl)-1,2-dihydropyridine-3-carbonitrile (3bm). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 117 mg, 80% yield; orange soild; mp 151–154 °C. 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 3.0 Hz, 1H), 7.52 (d, J = 2.1 Hz, 2H), 7.51 (d, J = 1.3 Hz, 1H), 7.49 (s, 1H), 7.42 (d, J = 5.1 Hz, 1H), 7.39 (d, J = 2.2 Hz, 1H), 7.38 (s, 1H), 6.36 (s, 1H), 3.42 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.9, 154.0, 151.3, 135.9, 134.2, 130.3, 129.1 (2C), 128.24, 128.0 (2C), 127.0, 126.9, 116.6, 108.3, 98.6, 38.3. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C17H12N2NaOS+ 315.0563; found, 315.0571.
4-(4-methoxyphenyl)-1-methyl-2-oxo-6-(p-tolyl)-1,2-dihydropyridine-3-carbonitrile (3ca). The compound was purified by column chromatography (silica gel, PE/EtOAc = 1/1 v/v): 159 mg, 96% yield; orange soild; mp 155–158 °C. 1H NMR (400 MHz, CDCl3) δ 7.58 (s, 2H), 7.28 (s, 4H), 6.93 (s, 2H), 6.26 (s, 1H), 3.80 (s, 3H), 3.41 (s, 3H), 2.40 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 162.1, 161.6, 157.7, 154.1, 140.7, 131.6, 129.9 (2C), 129.8 (2C), 128.1 (2C), 127.9, 116.7, 114.4 (2C), 109.1, 99.1, 55.5, 35.1, 21.5. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C21H19N2O2+ 331.1441; found, 331.1447.
4-(4-butylphenyl)-1-methyl-2-oxo-6-(p-tolyl)-1,2-dihydropyridine-3-carbonitrile (3cb). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 161 mg, 90% yield; yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.55 (s, 1H), 7.53 (s, 1H), 7.29 (s, 2H), 7.27 (s, 2H), 7.25 (s, 2H), 6.28 (s, 1H), 3.45 (s, 3H), 2.63 (t, J = 7.8 Hz, 2H), 2.42 (s, 3H), 1.64–1.55 (m, 2H), 1.38–1.31 (m, 2H), 0.91 (d, J = 14.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 162.1, 158.2, 154.2, 146.1, 140.8, 133.0, 131.6, 129.8 (2C), 129.1 (2C), 128.2 (2C), 128.1 (2C), 116.4, 109.3, 99.7, 35.6, 35.1, 33.4, 22.4, 21.5, 14.0. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C24H25N2O+ 357.1961; found, 357.1973.
4,6-bis(4-methoxyphenyl)-1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (3cc). The compound was purified by column chromatography (silica gel, PE/EtOAc = 1/1 v/v): 170 mg, 98% yield; white soild; mp 162–165 °C. 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 2H), 7.24 (s, 2H), 6.91 (s, 2H), 6.85 (s, 2H), 6.19 (s, 1H), 3.77 (s, 3H), 3.74 (s, 3H), 3.36 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 162.2, 161.5, 161.1, 157.5, 153.9, 129.8 (2C), 129.7 (2C), 127.8, 126.6, 116.7, 114.5 (2C), 114.3 (2C), 109.2, 98.7, 55.6, 55.5, 35.1.
4-(2-chlorophenyl)-6-(2-fluorophenyl)-1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (3cd). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 107 mg, 64% yield; yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.54 (s, 1H), 7.47 (d, J = 7.4 Hz, 1H), 7.37 (d, J = 5.8 Hz, 4H), 7.30 (d, J = 7.4 Hz, 1H), 7.22 (d, J = 8.5 Hz, 1H), 6.25 (s, 1H), 3.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.1, 160.4 (d, J = 249.1 Hz), 156.8, 148.8, 134.8, 133.2 (d, J = 8.2 Hz), 131.9, 131.4, 130.5, 130.4, 130.2, 127.5, 125.4, 122.1 (d, J = 15.4 Hz), 116.7 (d, J = 20.6 Hz), 115.1, 110.8, 103.9, 34.6. 19F NMR (376 MHz, CDCl3) δ −112.17 (s, 1F). HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H13ClFN2O+ 339.0695; found, 339.0708.
6-(2-bromophenyl)-4-(4-methoxyphenyl)-1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (3ce). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 135 mg, 68% yield; yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 6.6 Hz, 2H), 7.47 (s, 1H), 7.38 (s, 1H), 7.33 (s, 1H), 6.97 (d, J = 8.6 Hz, 2H), 6.25 (s, 1H), 3.83 (s, 3H), 3.33 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.7, 161.5, 157.8, 151.8, 135.4, 133.3, 131.7, 129.9 (2C), 129.8, 128.1, 127.5, 122.4, 116.3, 114.3 (2C), 108.7, 100.1, 55.4, 33.62. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C20H15BrN2NaO2+ 417.0209; found, 417.0212.
6-(4-cyanophenyl)-4-(4-methoxyphenyl)-1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (3cf). The compound was purified by column chromatography (silica gel, PE/EtOAc = 1/1 v/v): 92 mg, 70% yield; brown soild; mp 226–229 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.01 (s, 1H), 7.99 (s, 1H), 7.80 (s, 1H), 7.78 (s, 1H), 7.68 (s, 1H), 7.66 (s, 1H), 7.07 (s, 1H), 7.05 (s, 1H), 6.46 (s, 1H), 3.79 (s, 3H), 3.25 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 161.8, 161.5, 157.7, 152.9, 138.9, 133.2 (2C), 130.6 (2C), 130.2 (2C), 127.7, 118.8, 117.3, 114.9 (2C), 113.3, 109.2, 98.7, 56.0, 35.2.
4-(2-chlorophenyl)-6-(2,4-difluorophenyl)-1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (3cg). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 95 mg, 53% yield; yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.46 (s, 1H), 7.37 (s, 4H), 7.01 (s, 1H), 6.98 (s, 1H), 6.23 (s, 1H), 3.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.6 (t, J = 253.0 Hz, J = 488.0 Hz), 160.7, 156.5, 147.3, 134.4, 131.6, 131.2 (2C), 130.3, 129.9, 127.2, 118.2 (d, J = 15.5 Hz), 114.7, 112.8 (d, J = 21.5 Hz), 110.7, 105.2 (d, J = 49.9 Hz), 105.0, 104.0, 34.2. 19F NMR (376 MHz, CDCl3) δ −104.04 (s, 1F), −107.46 (s, 1F). HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H12ClF2N2O+ 357.0601; found, 357.0614.
4-(2-chlorophenyl)-6-(2,3-dichlorophenyl)-1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (3ch). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 115 mg, 59% yield; yellow soild; mp 178–181 °C. 1H NMR (400 MHz, CDCl3) δ 7.62 (s, 1H), 7.44 (s, 1H), 7.37 (s, 2H), 7.35 (s, 2H), 7.29 (s, 1H), 6.19 (s, 1H), 3.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 160.8, 157.0, 150.2, 134.9, 134.5, 134.4, 132.6, 131.8, 131.4 (2C), 130.4, 130.0, 128.6, 128.2, 127.4, 114.9, 110.0, 104.1, 33.9. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C19H12Cl3N2O+ 389.0010; found, 389.0018.
4-(4-butylphenyl)-1-methyl-6-(naphthalen-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile (3ci). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 145 mg, 88% yield; yellow soild; mp 144–147 °C. 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.6 Hz, 1H), 7.91 (s, 3H), 7.59 (d, J = 1.5 Hz, 1H), 7.59 (s, 2H), 7.57–7.56 (m, 1H), 7.45 (d, J = 10.1 Hz, 1H), 7.26 (d, J = 8.3 Hz, 2H), 6.41 (s, 1H), 3.48 (s, 3H), 2.63 (t, J = 7.7 Hz, 2H), 1.59 (t, J = 8.4 Hz, 2H), 1.38–1.31 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 162.0, 158.3, 154.1, 146.1, 133.7, 133.0, 132.9, 131.7, 129.1, 129.1 (2C), 128.5, 128.2 (3C), 128.1, 128.0, 127.5, 124.8, 116.5, 109.6, 99.9, 35.6, 35.2, 33.4, 22.4, 14.0. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C27H25N2O+ 393.1961; found, 393.1964.
4-(2-chlorophenyl)-1-methyl-6-(naphthalen-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile (3cj). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 149 mg, 80% yield; orange liquid. 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.6 Hz, 1H), 7.90 (s, 3H), 7.59 (d, J = 3.1 Hz, 2H), 7.48 (s, 1H), 7.45 (s, 1H), 7.41 (s, 1H), 7.38 (d, J = 3.0 Hz, 2H), 6.35 (s, 1H), 3.53 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.4, 156.8, 154.2, 134.9, 133.7, 132.9, 131.8, 131.4, 131.3, 130.4, 130.1, 129.1, 128.5, 128.3, 128.1 (2C), 127.6, 127.4, 124.8, 115.2, 110.4, 103.0, 35.5. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C23H16ClN2O+ 371.0946; found, 371.0950.
6-(2-bromophenyl)-4-cyclopropyl-1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (3ck). The compound was purified by column chromatography (silica gel, PE/EtOAc = 2/1 v/v): 99 mg, 60% yield; yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.65 (s, 1H), 7.44 (s, 1H), 7.36 (s, 1H), 7.24 (s, 1H), 5.46 (s, 1H), 3.23 (s, 3H), 2.28 (s, 1H), 1.26 (s, 2H), 0.89 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 164.4, 160.5, 152.1, 135.4, 133.3, 131.6, 129.7, 128.1, 122.3, 115.6, 102.4, 33.3, 15.0, 11.4, 11.3. HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C16H14BrN2O+ 329.0284; found, 329.0283.
2-oxo-1,4-diphenyl-6-(m-tolyl)-1,2-dihydropyridine-3-carbonitrile (3da). The compound was purified by column chromatography (silica gel, PE/EtOAc = 6/1 v/v): 100 mg, 53% yield; white soild; mp204–207 °C. 1H NMR (400 MHz, CDCl3) δ 7.70 (s, 2H), 7.50 (s, 3H), 7.26 (s, 3H), 7.10 (s, 2H), 7.03 (s, 2H), 6.94 (s, 1H), 6.88 (s, 1H), 6.43 (s, 1H), 2.19 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.3, 159.2, 153.7, 138.1, 137.3, 135.7, 134.1, 130.7, 130.2, 129.3, 129.0(2C), 128.9(2C), 128.6(3C), 128.1(2C), 128.0, 125.8, 115.7, 109.3, 101.1, 21.1. HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C25H18N2NaO+ 385.1311; found, 385.1320.
1-benzyl-2-oxo-4-phenyl-6-(m-tolyl)-1,2-dihydropyridine-3-carbonitrile (3db). The compound was purified by column chromatography (silica gel, PE/EtOAc = 10/1 v/v): 125 mg, 69% yield; white liquid. 1H NMR (400 MHz, CDCl3) δ 7.65 (s, 2H), 7.46 (s, 3H), 7.26 (s, 2H), 7.21 (s, 3H), 6.98 (s, 1H), 6.93 (s, 2H), 6.89 (s, 1H), 6.27 (s, 1H), 5.20 (s, 2H), 2.27 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.8, 158.6, 154.6, 138.7, 136.3, 135.6, 134.0, 131.0, 130.8, 129.1, 129.1 (2C), 128.7, 128.6 (2C), 128.2 (2C), 127.7, 127.5 (2C), 125.3, 116.2, 109.6, 100.9, 49.7, 21.4.
2-oxo-4-phenyl-6-(m-tolyl)-1,2-dihydropyridine-3-carbonitrile (3dc). The compound was purified by column chromatography (silica gel, PE/EtOAc = 3/1 v/v): 129mg, 90% yield; white solid; mp 169–172 °C. 1H NMR (400 MHz, CDCl3) δ 7.86 (s, 1H), 7.70 (s, 3H), 7.54 (s, 3H), 7.45 (s, 1H), 7.39 (s, 1H), 6.76 (s, 1H), 2.54 (s, 3H), 1.26 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 164.1, 161.0, 151.0, 139.9, 136.1, 132.7, 131.5, 130.6, 129.3, 129.0 (2C), 128.1 (2C), 127.9, 124.3, 115.7, 106.5, 99.7, 21.4.
3.2. Insecticidal Properties Study Methods
The experimental details are presented in the Supplementary Materials.
4. Conclusions
In summary, we have developed an efficient and straightforward methodology to construct functional 3-cyano-2-pyridones. The C–C and C–N bond formation were the key procedures of this transformation. Our study presents the utilization of these pyridones for insecticidal properties, and compound 3ci was selected as the best insecticide. With advantages such as a metal-free process, wide functional group tolerance, simple operation, and mild conditions, further chemical modification and biological exploration of these types of compounds are underway in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29122792/s1, Copies of NMR spectra for compounds 3aa–3dc (page 2). Insecticidal properties study methods (page 58).
Author Contributions
Conceptualization, Y.L. and X.W.; synthetic work and data curation, Y.T. and N.W.; biological experiments and interpretation of results, Y.T., J.X. and X.Z.; chemical characterization, Y.T. and X.W.; project administration and supervision, X.W.; validation, X.W.; writing—original draft, Y.T. and X.W.; writing—review and editing, N.W. and X.W.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Key Research and Development Program of Hainan Province (No. ZDYF2022SHFZ037), Hainan Provincial Association for Science and Technology Youth Science and Technology Talent Innovation Program (no. QCQTXM202214), the National Natural Science Foundation of China (no. 82360838), Fundamental Research Funds of Hainan Medical University (No. XRC180009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Misra, R.; Pandey, R.C.; Silverton, J.V. Fredericamycin A, an Antitumor Antibiotic of a Novel Skeletal Type. J. Am. Chem. Soc. 1982, 104, 4478–4479. [Google Scholar] [CrossRef]
- Bryan, M.C.; Burdick, D.J.; Chan, B.K.; Chen, Y.; Clausen, S.; Dotson, J.; Eigenbrot, C.; Elliott, R.; Hanan, E.J.; Heald, R.; et al. Pyridones as Highly Selective, Noncovalent Inhibitors of T790M Double Mutants of EGFR. ACS Med. Chem. Lett. 2016, 7, 100–104. [Google Scholar] [CrossRef]
- Fioravanti, R.; Stazi, G.; Zwergel, C.; Valente, S.; Mai, A. Six Years (2012–2018) of Researches on Catalytic Ezh2 Inhibitors: The Boom of the 2-Pyridone Compounds. Chem. Rec. 2018, 18, 1818–1832. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Brown, E.L.; Maldonado, F.C.; Fuhrman, S.A.; Zalman, L.S.; Tuntland, T.; Lee, C.A.; Patick, A.K.; et al. Structure–Based Design, Synthesis, and Biological Evaluation of Irreversible Human Rhinovirus 3C Protease Inhibitors. 6. Structure-Activity Studies of Orally Bioavailable, 2-Pyridone-Containing Peptidomimetics. J. Med. Chem. 2002, 45, 1607–1623. [Google Scholar] [CrossRef]
- Hibi, S.; Ueno, K.; Nagato, S.; Kawano, K.; Ito, K.; Norimine, Y.; Takenaka, O.; Hanada, T.; Yonaga, M. Discovery of 2-(2-Oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile (Perampanel): A Novel, Noncompetitive α-Amino-3-hydroxy-5-methyl-4-isoxazolepropanoic Acid (AMPA) Receptor Antagonist. J. Med. Chem. 2012, 55, 10584–10600. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Morrell, A.; Dexheimer, T.S.; Scher, E.S.; Pommier, Y.; Cushman, M. Design, Synthesis, and Biological Evaluation of 14-Substituted Aromathecins as Topoisomerase I Inhibitors. J. Med. Chem. 2008, 51, 4609–4619. [Google Scholar] [CrossRef]
- Du, W. Towards new anticancer drugs: A decade of advances in synthesis of camptothecins and related alkaloids. Tetrahedron 2003, 59, 8649–8687. [Google Scholar] [CrossRef]
- Ravinder, M.; Mahendar, B.; Mattapally, S.; Hamsini, K.V.; Reddy, T.N.; Rohit, C.; Srinivas, K.; Banerjee, S.K.; Rao, V.J. Synthesis and evaluation of novel 2-pyridone derivatives as inhibitors of phosphodiesterase3 (PDE3): A target for heart failure and platelet aggregation. Bioorg. Med. Chem. Lett. 2012, 22, 6010–6015. [Google Scholar] [CrossRef]
- Pfefferkorn, J.A.; Lou, J.H.; Minich, M.L.; Filipski, K.J.; He, M.Y.; Zhou, R.; Ahmed, S.; Benbow, J.; Perez, A.G.; Tu, M.; et al. Pyridones as glucokinase activators: Identification of a unique metabolic liability of the 4-sulfonyl-2-pyridone heterocycle. Bioorg. Med. Chem. Lett. 2009, 19, 3247–3252. [Google Scholar] [CrossRef]
- Chen, J.; Lu, M.M.; Liu, B.; Chen, Z.; Li, Q.B.; Tao, L.J.; Hu, G.Y. Synthesis and structure-activity relationship of 5-substituent-2(1H)-pyridone derivatives as anti-fibrosis agents. Bioorg. Med. Chem. Lett. 2012, 22, 2300–2302. [Google Scholar] [CrossRef]
- Mizutani, S.; Komori, K.; Taniguchi, T.; Monde, K.; Kuramochi, K.; Tsubaki, K. A Bioinspired Synthesis of (±)-Rubrobramide, (±)-Flavipucine, and (±)-Isoflavipucine. Angew. Chem. Int. Ed. 2016, 55, 9553–9556. [Google Scholar] [CrossRef]
- Wech, F.; Hasenbeck, M.; Gellrich, U. Semihydrogenation of Alkynes Catalyzed by a Pyridone Borane Complex: Frustrated Lewis Pair Reactivity and Boron–Ligand Cooperation in Concert. Chem.–Eur. J. 2020, 26, 13445–13450. [Google Scholar] [CrossRef]
- Kumar, V.; Keshavayya, J.; Matada, M.N.; Srinivasa, S.M.; Rangappa, S. Synthesis, Characterization and Biological Potency of Butyl-Pyridone Based Azo Dyes. ChemistrySelect 2020, 5, 5460–5464. [Google Scholar] [CrossRef]
- Brown, T.R.; Lange, J.P.; Mortimer, M.J.; Berry, J.F. New Oxypyridinate Paddlewheel Ligands for Alkane-Soluble, Sterically-Protected Ru2(II,III) and Ru2(II,II) Complexes. Inorg. Chem. 2018, 57, 10331–10340. [Google Scholar] [CrossRef]
- Fotiadou, A.D.; Zografos, A.L. Accessing the Structural Diversity of Pyridone Alkaloids: Concise Total Synthesis of Rac–Citridone A. Org. Lett. 2011, 13, 4592–4595. [Google Scholar] [CrossRef]
- Nakao, Y.; Idei, H.; Kanyiva, K.S.; Hiyama, T. Direct Alkenylation and Alkylation of Pyridone Derivatives by Ni/AlMe3 Catalysis. J. Am. Chem. Soc. 2009, 131, 15996–15997. [Google Scholar] [CrossRef]
- Phakdeeyothin, K.; Yotphan, S. Metal-free regioselective direct thiolation of 2-pyridones. Org. Biomol. Chem. 2019, 17, 6432–6440. [Google Scholar] [CrossRef]
- Maity, S.; Das, D.; Sarkar, S.; Samanta, R. Direct Pd(II)-Catalyzed Site-Selective C5-Arylation of 2-Pyridone Using Aryl Iodides. Org. Lett. 2018, 20, 5167–5171. [Google Scholar] [CrossRef]
- Tao, S.Q.; Xiao, J.; Li, Y.D.; Sun, F.X.; Du, Y.F. PhICl2/NH4SCN-Mediated Oxidative Regioselective Thiocyanation of Pyridin-2(1H)-ones. Chin. J. Chem. 2021, 39, 2536–2546. [Google Scholar] [CrossRef]
- Kittikool, T.; Phakdeeyothin, K.; Chantarojsiri, T.; Yotphan, S. Manganese-Promoted Regioselective Direct C3-Phosphinoylation of 2-Pyridones. Eur. J. Org. Chem. 2021, 21, 3071–3078. [Google Scholar] [CrossRef]
- Kamali, M.; Ebrahimi, A. One-pot multicomponent green synthesis of novel series of 4-hydroxy-2-pyridone-fused spiropyrans catalyzed by acetic acid. Synthetic Commun. 2023, 53, 1439–1450. [Google Scholar] [CrossRef]
- Li, J.; Tan, H.R.; An, Y.L.; Shao, Z.Y.; Zhao, S.Y. Synthesis and DABCO-induced demethylation of 3-cyano-4-methoxy-2-pyridone derivatives. J. Heterocyclic Chem. 2019, 57, 486–496. [Google Scholar] [CrossRef]
- Burger, B.S.; Cherioux, F.; Monnier-Jobe, K.; Laude, B.; Maillotte, H. 2-Pyridones as a New Photochemically Stable Structural Design for the Off-Resonant Optical Kerr Effect. Adv. Funct. Mater. 2022, 12, 339–347. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Abbas, H.K.; Jacob, M.R.; Herath, W.H.M.W.; Nanayakkara, N.P.D. N-Methyl-4-hydroxy-2-pyridinone Analogues from Fusarium oxysporum. J. Nat. Prod. 2006, 69, 439–442. [Google Scholar] [CrossRef]
- Breinholt, J.; Ludvigsen, S.; Rassing, B.R.; Rosendahl, C.N. Oxysporidinone: A Novel, Antifungal N-Methyl-4-hydroxy-2-pyridone from Fusarium oxysporum. J. Nat. Prod. 1997, 60, 33–35. [Google Scholar] [CrossRef]
- Singh, S.B.; Liu, W.G.; Li, X.H.; Chen, T.; Shafiee, A.; Card, D.; Abruzzo, G.; Flattery, A.; Gill, C.; Thompson, J.R.; et al. Antifungal Spectrum, In Vivo Efficacy, and Structure—Activity Relationship of Ilicicolin H. ACS Med. Chem. Lett. 2012, 3, 814–817. [Google Scholar] [CrossRef]
- Storck, P.; Aubertin, A.M.; Grierson, D.S. Tosylation/mesylation of 4-hydroxy-3-nitro-2-pyridinones as an activation step in the construction of dihydropyrido[3,4-b] benzo[f][1,4]thiazepin-1-one based anti-HIV agents. Tetrahedron Lett. 2005, 46, 2919–2922. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V. New bis(pyridyl)methane derivatives from 4-hydroxy-2-pyridones: Synthesis and antitumoral activity. Eur. J. Med. Chem. 2003, 38, 37–47. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V. Synthesis and antitumour activity of 4-hydroxy-2-pyridone derivatives. Eur. J. Med. Chem. 2000, 35, 545–552. [Google Scholar] [CrossRef]
- Jessen, H.J.; Gademann, K. 4-Hydroxy-2-pyridone alkaloids: Structures and synthetic approaches. Nat. Prod. Rep. 2010, 27, 1168–1185. [Google Scholar] [CrossRef]
- Tohda, C.; Kuboyama, T.; Komatsu, K. Search for Natural Products Related to Regeneration of the Neuronal Network. Neurosignals 2005, 14, 34–35. [Google Scholar] [CrossRef]
- Wilson, R.M.; Danishefsky, S.J. Applications of Total Synthesis to Problems in Neurodegeneration: Fascinating Chemistry along the Way. Acc. Chem. Res. 2006, 39, 539–549. [Google Scholar] [CrossRef]
- Metifiot, M.; Johnson, B.; Smith, S.; Zhao, X.Z.; Marchand, C.; Burke, T.; Hughes, S.; Pommier, Y. MK-0536 Inhibits HIV-1 Integrases Resistant to Raltegravir. Antimicrob. Agents Ch. 2011, 55, 127–133. [Google Scholar] [CrossRef]
- Manfroni, G.; Meschini, F.; Barreca, M.L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; et al. Pyridobenzothiazole derivatives as new chemotype targeting the HCV NS5B polymerase. Bioorg. Med. Chem. 2012, 20, 866–876. [Google Scholar] [CrossRef]
- Yan, T.Y.; Ding, W.J.; Liu, H.W.; Wang, P.M.; Zheng, D.Q.; Xu, J.Z. New pyridone alkaloids from marine-derived fungus Penicillium sp. Tetrahedron Lett. 2020, 61, 151843–151848. [Google Scholar] [CrossRef]
- Gonçalves, D.S.; Melo, S.M.S.; Jacomini, A.P.; Silva, M.J.V.; Pianoski, K.E.; Ames, F.Q.; Aguiar, R.P.; Oliveira, A.F.; Volpato, H.; Bidóia, D.L.; et al. Synthesis of novel 3,5,6-trisubstituted 2-pyridone derivatives and evaluation for their anti-inflammatory activity. Bioorg. Med. Chem. 2020, 28, 115549–115590. [Google Scholar] [CrossRef]
- Xie, W.L.; Wu, Y.Q.; Zhang, J.G.; Qi, H.M.; Zhang, Y.H.; Zhu, N.; Liu, R.Z.; Zhang, H.L. Design, synthesis and biological evaluations of novel pyridonethiazole hybrid molecules as antitumor agents. Eur. J. Med. Chem. 2018, 145, 35–51. [Google Scholar] [CrossRef]
- Jue, S.G.; Dawson, G.W.; Brogden, R.N. Ciclopirox olamine 1% cream a preliminary review of its antimicrobial activity and therapeutic use. Drugs 1985, 29, 330–341. [Google Scholar] [CrossRef]
- Kim, E.S.; Keating, G.M. Pirfenidone: A review of its use in idiopathic pulmonary fibrosis. Drugs 2015, 75, 219–230. [Google Scholar] [CrossRef]
- Yang, Z.H.; Ren, J.; Yi, L.J.; Zheng, J.H.; Wei, H. Tegafur gimeracil oter combined with oxaliplatin for advanced colorectal cancer. Eur. Rev. Med. Pharmaco. 2015, 19, 3391–3396. [Google Scholar]
- Hamelin, E.I.; Johnson, R.C.; Osterloh, J.D.; Howard, D.J.; Thomas, J.D. Evaluation of Ricinine, a Ricin Biomarker, from a Non-Lethal Castor Bean Ingestion. J. Anal. Toxicol. 2012, 36, 660–662. [Google Scholar] [CrossRef]
- Rana, M.; Dhamija, H.; Prashar, B.; Sharma, S. Ricinus communis L.–A Review. Int. J. Pharm Tech Res. 2012, 4, 1706–1711. [Google Scholar]
- Moshiri, M.; Hamid, F.; Etemad, L. Ricin Toxicity: Clinical and Molecular Aspects. Rep. Biochem. Mol. Biol. 2016, 4, 60–66. [Google Scholar]
- Duong, H.A.; Cross, M.J.; Louie, J. Nickel-Catalyzed Cycloaddition of Alkynes and Isocyanates. J. Am. Chem. Soc. 2004, 126, 11438–11439. [Google Scholar] [CrossRef]
- Tanaka, K.; Wada, A.; Noguchi, K. Rhodium-Catalyzed Chemo-, Regio-, and Enantioselective [2+2+2] Cycloaddition of Alkynes with Isocyanates. Org. Lett. 2005, 7, 4737–4739. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kinpara, K.; Saigoku, T.; Takagishi, H.; Okuda, S.; Nishiyama, H.; Itoh, K. Cp*RuCl-Catalyzed [2+2+2] Cycloadditions of r,ω-Diynes with Electron-Deficient Carbon-Heteroatom Multiple Bonds Leading to Heterocycles. J. Am. Chem. Soc. 2005, 127, 605–613. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takagishi, H.; Itoh, K. Ruthenium(II)-Catalyzed Cycloaddition of 1,6-Diynes with Isocyanates Leading to Bicyclic Pyridones. Org. Lett. 2001, 3, 2117–2119. [Google Scholar] [CrossRef]
- Sengupta, T.; Gayen, K.S.; Pandit, P.; Maiti, D.K. FeCl3.6H2O-Catalyzed Intermolecular-Cascade Cyclization of Acetoacetanilide: Aldehyde-Tuned Synthesis to Valuable 2-Pyridone Analogues. Chem. Eur. J. 2012, 18, 1905–1909. [Google Scholar] [CrossRef]
- Takahashi, T.; Tsai, F.Y.; Li, Y.Z.; Wang, H.; Kondo, Y.; Yamanaka, M.; Nakajima, K.; Kotora, M. Selective Preparation of Pyridines, Pyridones, and Iminopyridines from Two Different Alkynes via Azazirconacycles. J. Am. Chem. Soc. 2002, 124, 5059–5067. [Google Scholar] [CrossRef]
- Imase, H.; Noguchi, K.; Hirano, M.; Tanaka, K. Convergent and Rapid Assembly of Substituted 2-Pyridones through Formation of N-Alkenyl Alkynylamides Followed by Gold-Catalyzed Cycloisomerization. Org. Lett. 2008, 10, 3563–3566. [Google Scholar] [CrossRef]
- Poomathi, N.; Perumal, P.T.; Ramakrishna, S. An efficient and eco-friendly synthesis of 2-pyridones and functionalized azaxanthone frameworks via indium triflate catalyzed domino reaction. Green Chem. 2017, 19, 2524–2529. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, H.J.; Nan, F.J. Construction of a 3-Amino-2-pyridone Library by Ring-Closing Metathesis of r-Amino Acrylamide. J. Comb. Chem. 2004, 6, 684–687. [Google Scholar] [CrossRef]
- Carles, L.; Narkunan, K.; Penlou, S.B.; Rousset, L.; Bouchu, D.; Ciufolini, M.A. 2-Pyridones from Cyanoacetamides and Enecarbonyl Compounds: Application to the Synthesis of Nothapodytine B. J. Org. Chem. 2002, 67, 4304–4308. [Google Scholar] [CrossRef]
- Pintiala, C.; Lawson, A.M.; Comesse, S.; Daïch, A. A versatile domino process for the synthesis of substituted 3-aminomethylene-chromanones and 2-pyridones catalyzed by CsF. Tetrahedron Lett. 2013, 54, 2853–2857. [Google Scholar] [CrossRef]
- Chun, Y.S.; Ryu, K.Y.; Ko, Y.O.; Hong, J.Y.; Hong, J.K.; Shin, H.; Lee, S.G. One-Pot Synthesis of 2-Pyridones via Chemo-and Regioselective Tandem Blaise Reaction of Nitriles with Propiolates. J. Org. Chem. 2009, 74, 7556–7558. [Google Scholar] [CrossRef]
- Samzadeh-Kermani, A. Heteropolyacid-Catalyzed One-Pot Synthesis of 2-Pyridone Derivatives. Synlett 2016, 27, 461–464. [Google Scholar] [CrossRef]
- Patel, B.H.; Mason, A.M.; Barrett, A.G.M. Synthesis of 6-Substituted-4-Hydroxy-2-pyridinones via Intramolecular Ketene Trapping of Functionalized Enamine-Dioxinones. Org. Lett. 2011, 13, 5156–5159. [Google Scholar] [CrossRef]
- Hachiya, I.; Ogura, K.; Shimizu, M. Novel 2-Pyridone Synthesis via Nucleophilic Addition of Malonic Esters to Alkynyl Imines. Org. Lett. 2002, 4, 2755–2757. [Google Scholar] [CrossRef]
- Fujii, M.; Nishimura, T.; Koshiba, T.; Yokoshima, S.; Fukuyama, T. 2-Pyridone Synthesis Using 2-(Phenylsulfinyl)acetamide. Org. Lett. 2013, 15, 232–234. [Google Scholar] [CrossRef]
- Kumar, M. A review on phytochemical constituents and pharmacological activities of ricinus communis L. Plant. Int. J. Pharmco. Phytochem. Res. 2017, 4, 466–472. [Google Scholar] [CrossRef]
- Bernini, R.; Fabrizi, G.; Sferrazza, A.; Cacchi, S. Copper-Catalyzed C–C Bond Formation through C–H Functionalization: Synthesis of Multisubstituted Indoles from N-Aryl Enaminones. Angew. Chem. Int. Ed. 2009, 48, 8078–8081. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, G.; Cui, X. “One pot” regiospecific synthesis of polysubstituted pyrroles from benzylamines and ynones under metal free conditions. Chem. Commun. 2013, 49, 10641–10643. [Google Scholar] [CrossRef]
- Cheng, G.; Xue, L.; Weng, Y.; Cui, X. Transition-Metal-Free Cascade Approach toward 2-Alkoxy/2-Sulfenylpyridines and Dihydrofuro[2,3-b]pyridines by Trapping In Situ Generated 1,4-Oxazepine. J. Org. Chem. 2017, 82, 9515–9524. [Google Scholar] [CrossRef]
- Cheng, G.; Weng, Y.; Yang, X.; Cui, X. Base-Promoted N-Pyridylation of Heteroarenes Using N-Propargyl Enaminones as Equivalents of Pyridine Scaffolds. Org. Lett. 2015, 17, 3790–3793. [Google Scholar] [CrossRef]
- Cheng, G.; Zeng, X.; Shen, J.; Wang, X.; Cui, X. A Metal-Free Multicomponent Cascade Reaction for the Regiospecific Synthesis of 1,5-Disubstituted 1,2,3-Triazoles. Angew. Chem. Int. Ed. 2013, 52, 13265–13268. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, X.; Wang, W.; Zhong, X.; Tan, Y.; Wang, Y.; Zhang, J.; Li, Y.; Wang, X. Regitz Diazo Transfer Reaction for the Synthesis of 1,4,5-Trisubstituted 1,2,3-Triazoles and Subsequent Regiospecific Construction of 1,4-Disubstituted 1,2,3-Triazoles via C–C Bond Cleavage. J. Org. Chem. 2021, 86, 4071–4080. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Wang, W.; Zeng, T.; Wang, Y.; Tan, Y.; Liu, D.; Wang, X.; Li, Y. Base-Promoted Regiospecific Synthesis of Fully Substituted 1,2,3-Triazoles and 1,5-Disubstituted 1,2,3-Triazoles. Asian J. Org. Chem. 2020, 9, 2176–2183. [Google Scholar] [CrossRef]
- Xia, X.F.; He, W.; Wang, D. Metal-Free Oxidative Annulation/Cyclization of 1,6-Enynes for the Synthesis of 4-Carbonylquinolines. Adv. Synth. Catal. 2019, 361, 2959–2964. [Google Scholar] [CrossRef]
- Xia, X.F.; Zhang, L.L.; Song, X.R.; Liu, X.Y.; Liang, Y.M. Copper-Catalyzed Oxidative Cyclization of Enynes for the Synthesis of 4-Carbonyl-quinolines with O2. Org. Lett. 2012, 14, 2480–2483. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, Y.; An, Z.; Qi, Z.; Yan, R. Iron-Catalyzed Synthesis of Substituted Thiazoles from Enamines and Elemental Sulfur through C−S Bond Formation. Adv. Synth. Catal. 2018, 360, 4236–4240. [Google Scholar] [CrossRef]
- Liu, J.; Ba, D.; Lv, W.; Chen, Y.; Zhao, Z.; Cheng, G. Base-Promoted Michael Addition/Smiles Rearrangement/N-Arylation Cascade: One-Step Synthesis of 1,2,3-Trisubstituted 4-Quinolones from Ynones and Sulfonamides. Adv. Synth. Catal. 2020, 362, 213–223. [Google Scholar] [CrossRef]
- Cui, X.; Ma, J.; Zeng, T.; Xu, J.; Li, Y.; Wang, X. Metal-free cascade synthesis of unsymmetrical 2-aminopyrimidines from imidazolate enaminones. RSC Adv. 2021, 11, 24247–24253. [Google Scholar] [CrossRef]
- Ma, J.; Tan, Y.; Tang, Y.; Cui, X.; Xu, J.; Li, Y.; Wang, X. Base-Promoted Cascade C–N and C–C Formation: An Approach to Pyrido[1,2-a] pyrimidinones from Ynones and 2-Methylpyrimidin-4-ols. Asian J. Org. Chem. 2022, 11, e202200451. [Google Scholar] [CrossRef]
- Saliy, I.V.; Gotsko, M.D.; Sobenina, L.N.; Ushakov, I.A.; Trofimov, B.A. Bio-inspired Functionalized Pyrrole-Pyridone Ensembles: Synthesis on the Platform of Acylethynylpyrroles. Synthesis 2020, 52, 2698–2704. [Google Scholar]
- Al Nakib, T.; Meegan, M.J. A novel synthetic route to 2,3,4-trisubstituted 6-phenyl-pyridines. J. Chem. Res. Synop. 1988, 5, 146–147. [Google Scholar]
- Kuthan, J.; Nesvadba, P.; Popl, M.; Fähnrich, J. Some 3-cyano-4, 6-diaryl-2-pyridones with luminiscent properties. Collect. Czech. Chem. Commun. 1979, 44, 2409–2416. [Google Scholar] [CrossRef]
- Sharma, S.; Vasudevan, P.; Madan, M. Insecticidal value of castor (Ricinus communis) against termites. Int. Biodeter. 1990, 27, 249–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).