Molybdenum-Modified Titanium Dioxide Nanotube Arrays as an Efficient Electrode for the Electroreduction of Nitrate to Ammonia
Abstract
1. Introduction
2. Results and Discussion
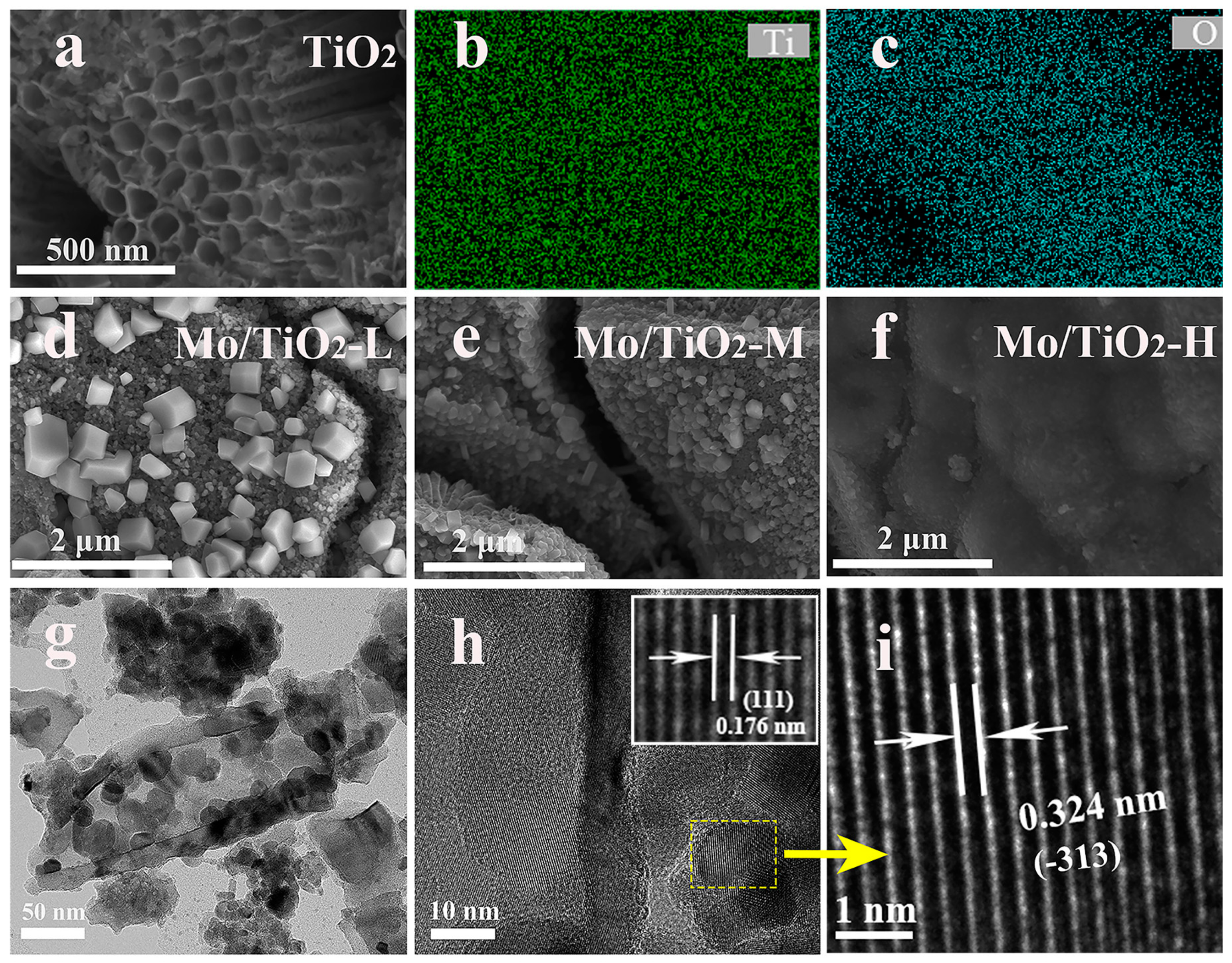
2.1. Morphological and Structural Analysis of Catalysts
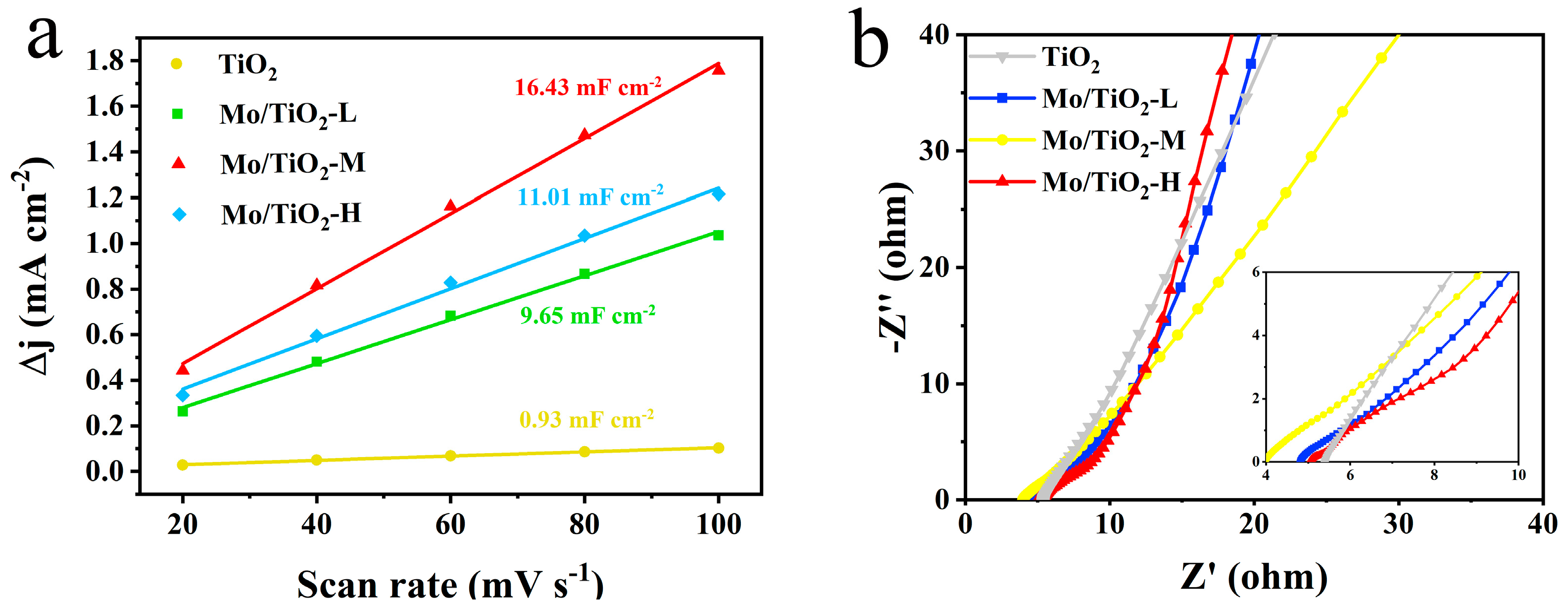
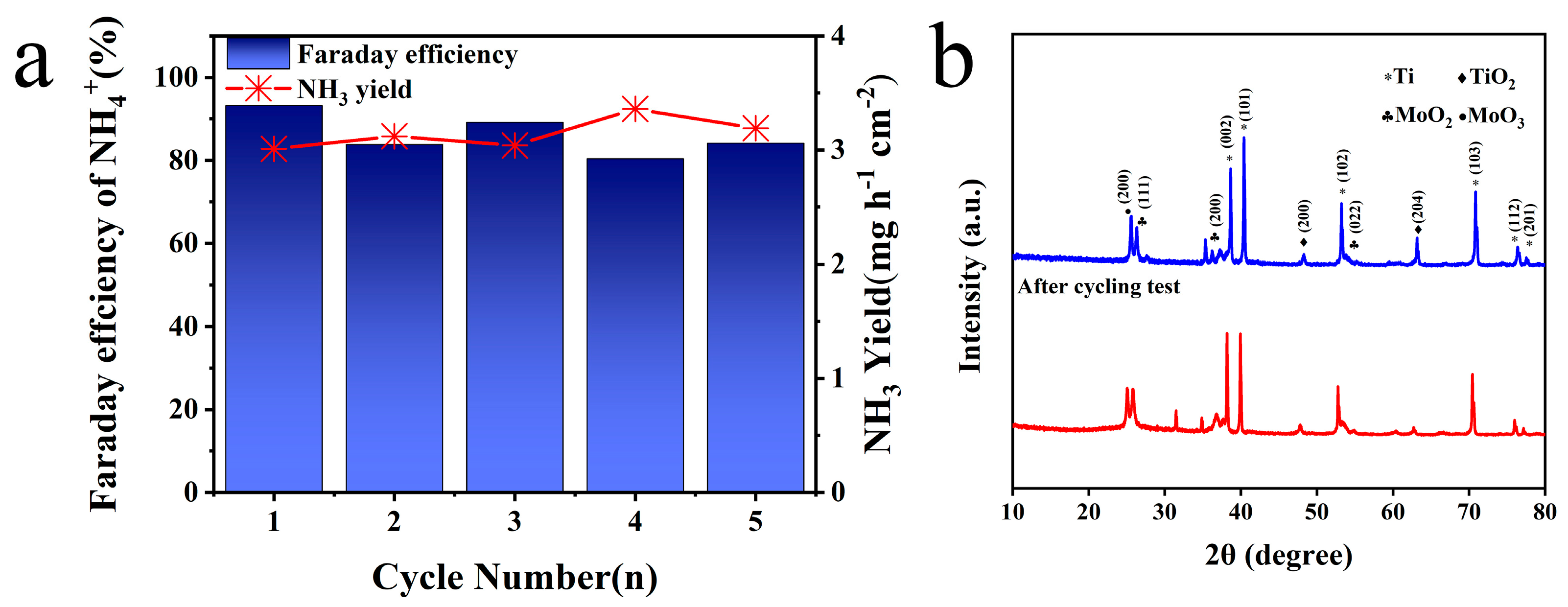
2.2. Electrocatalytic Performance of Electrodes for Mo/TiO2
3. Experimental Methods
3.1. Materials
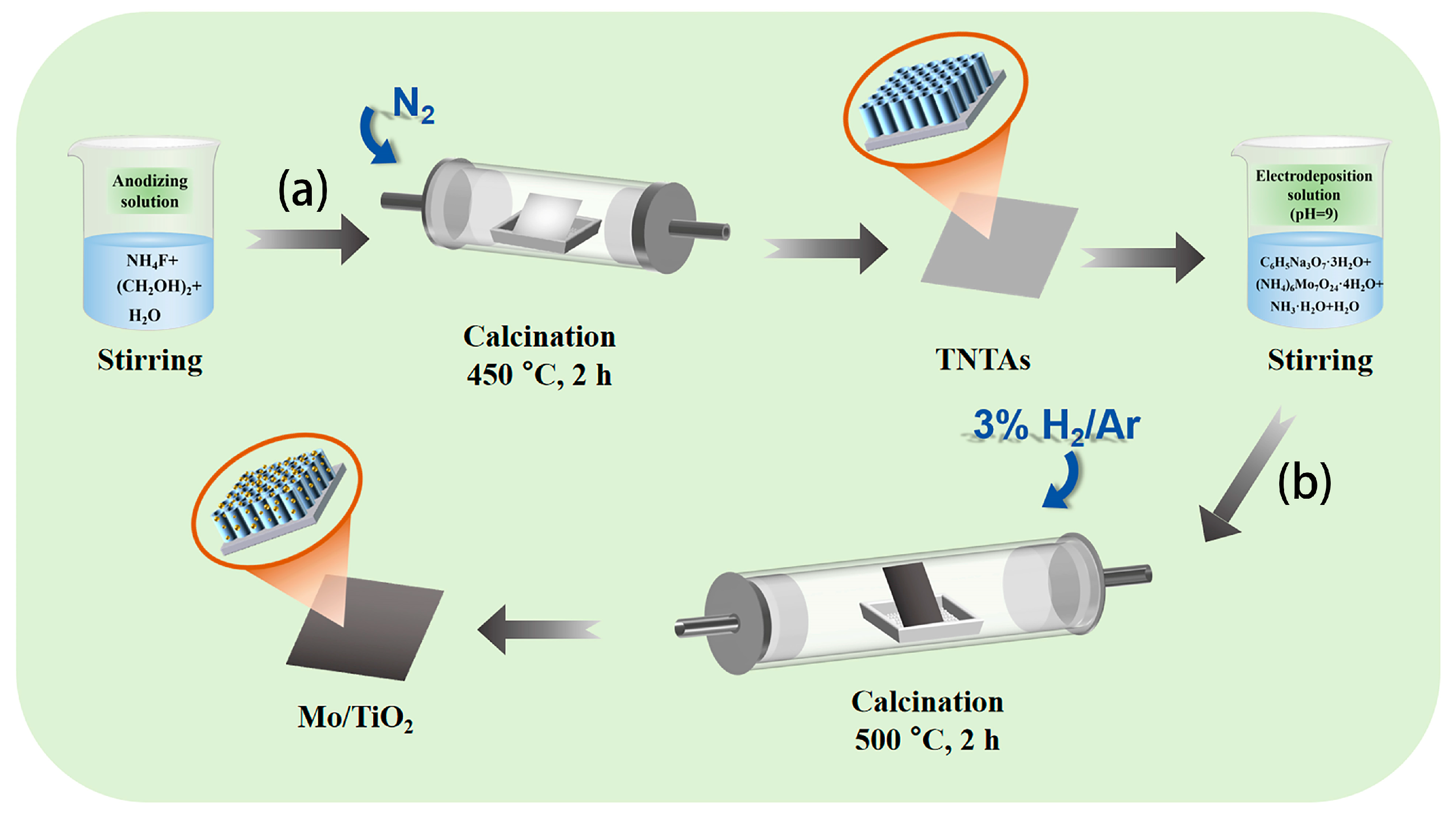
3.2. Preparation of Electrode Material
3.2.1. Pre-Treatment of Ti
3.2.2. Preparation of TNTAs
3.2.3. Preparation of Mo/TiO2 Electrode
3.3. Characterization
3.4. Electrochemical Measurement
3.5. Determination of Ion Concentration
3.5.1. Nitrite-N Detection
3.5.2. NH3-N Detection
3.6. Product Calculation (Yield and Faraday Efficiency)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, X.; Pedersen, J.B.; Zhou, Y.; Saccoccio, M.; Li, S.; Sažinas, R.; Li, K.; Andersen, S.Z.; Xu, A.; Deissler, N.H.; et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 2023, 379, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Fu, X.; Pedersen, J.B.; Saccoccio, M.; Andersen, S.Z.; Enemark-Rasmussen, K.; Kempen, P.J.; Damsgaard, C.D.; Xu, A.; et al. Long-term continuous ammonia electrosynthesis. Nature 2024, 629, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Torrente-Murciano, L. The potential of green ammonia for agricultural and economic development in Sierra Leone. One Earth 2021, 4, 104–113. [Google Scholar] [CrossRef]
- Ashida, Y.; Arashiba, K.; Nakajima, K.; Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 2019, 568, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, T.; Schuster, M.E.; Senyshyn, A.; Behrens, M.; Schlögl, R. The Haber-Bosch process revisited: On the real structure and stability of “ammonia iron” under working conditions. Angew. Chem. Int. Ed. 2013, 52, 12723–12726. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.J.; Shinagawa, T.; Pérez-Ramírez, J. Electrocatalytic reduction of nitrogen: From Haber-Bosch to ammonia artificial leaf. Chem 2019, 5, 263–283. [Google Scholar] [CrossRef]
- Kitano, M.; Kanbara, S.; Inoue, Y.; Kuganathan, N.; Sushko, P.V.; Yokoyama, T.; Hara, M.; Hosono, H. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat. Commun. 2015, 6, 6731. [Google Scholar] [CrossRef]
- van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Ren, J.; Yao, Y.; Yuan, Z. Fabrication strategies of porous precious-metal-free bifunctional electrocatalysts for overall water splitting: Recent advances. Green Energy Environ. 2021, 6, 620–643. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; He, G.; Zhang, T.C.; Wang, Y.; Yuan, S. Direct electrocatalytic reduction of As(III) on CuSn alloy electrode: A green and sustainable strategy to recover elemental arsenic from arsenic wastewater. Ind. Eng. Chem. Res. 2024, 63, 8509–8523. [Google Scholar] [CrossRef]
- Batoo, K.M.; Kamona, S.M.H.; Al-Majdi, K.; Rasen, F.A.; Altimari, U.S.; Hussain, S.; Al-khalidi, A.; Abdulkadhim, A.H.; Kareem, A.T.; Alawadi, A.; et al. Metal doped nanostructures as catalysts of nitrogen reduction to ammonia. Silicon 2024, 16, 1421–1431. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, K.; Pan, Q.; Xu, Y.; Liu, Q.; Cui, G.; Guo, X.; Sun, X. Boron-doped TiO2 for efficient electrocatalytic N2 fixation to NH3 at ambient conditions. ACS Sustain. Chem. Eng. 2019, 7, 117–122. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, T.; Ji, X.; Yuan, S. Ti-doped iron phosphide nanoarrays grown on carbon cloth as a self-supported electrode for enhanced electrocatalytic nitrogen reduction. Nanoscale 2023, 15, 16219–16226. [Google Scholar] [CrossRef]
- Fang, J.; Fan, J.; Liu, S.; Sun, S.; Lou, Y. Copper-based electrocatalysts for nitrate reduction to ammonia. Materials 2023, 16, 4000. [Google Scholar] [CrossRef]
- Zeng, Y.; Priest, C.; Wang, G.; Wu, G. Restoring the nitrogen cycle by electrochemical reduction of nitrate: Progress and prospects. Small Methods 2020, 4, 2000672. [Google Scholar] [CrossRef]
- Modi, A.; Kasher, R. Nitrate removal from contaminated groundwater by micellar-enhanced ultrafiltration using a polyacrylonitrile membrane with a hydrogel-stabilized ZIF-L layer. Water Res. 2024, 254, 121384. [Google Scholar] [CrossRef]
- Su, J.; Ruzybayev, I.; Shah, I.; Huang, C. The electrochemical reduction of nitrate over micro-architectured metal electrodes with stainless steel scaffold. Appl. Catal. B 2016, 180, 199–209. [Google Scholar] [CrossRef]
- Chazelas, E.; Pierre, F.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaesse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; Srour, B.; et al. Nitrites and nitrates from food additives and natural sources and cancer risk: Results from the NutriNet-Santé cohort. Int. J. Epidemiol. 2022, 51, 1106–1119. [Google Scholar] [CrossRef]
- de Groot, M.T.; Koper, M.T.M. The influence of nitrate concentration and acidity on the electrocatalytic reduction of nitrate on platinum. J. Electroanal. Chem. 2004, 562, 81–94. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Du, F.; Yang, L.; Huang, S.; Gao, J.; Li, C.; Guo, C. A core–shell copper oxides-cobalt oxides heterostructure nanowire arrays for nitrate reduction to ammonia with high yield rate. Green Energy Environ. 2023, 8, 1619–1629. [Google Scholar] [CrossRef]
- Liu, H.; Park, J.; Chen, Y.; Qiu, Y.; Cheng, Y.; Srivastava, K.; Gu, S.; Shanks, B.H.; Roling, L.T.; Li, W. Electrocatalytic nitrate reduction on oxide-derived silver with tunable selectivity to nitrite and ammonia. ACS Catal. 2021, 11, 8431–8442. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, Q.; Zhang, J.; An, X.; Liu, Q.; Xie, L.; Yao, W.; Sun, X.; Kong, Q. Electrodeposited Ni-Mo-P nanoparticles on TiO2 nanoribbon array for electrocatalytic ammonia synthesis by reducing nitrite. Mater. Today Phys. 2023, 36, 101162. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Li, L.; Li, Z.; Zhang, W.; Xue, Z.; Liu, D.; Meng, X.; Li, C.; Sunarso, J.; et al. Electrocatalytic ammonia synthesis on Fe@MXene catalyst as cathode of intermediate-temperature proton-conducting solid oxide cell. Int. J. Hydrog. Energy 2023, 48, 17677–17688. [Google Scholar] [CrossRef]
- Liu, D.; Qiao, L.; Peng, S.; Bai, H.; Liu, C.; Ip, W.F.; Lo, K.H.; Liu, H.; Ng, K.W.; Wang, S.; et al. Recent advances in electrocatalysts for efficient nitrate reduction to ammonia. Adv. Funct. Mater. 2023, 33, 2303480. [Google Scholar] [CrossRef]
- Murphy, E.; Liu, Y.; Matanovic, I.; Guo, S.; Tieu, P.; Huang, Y.; Ly, A.; Das, S.; Zenyuk, I.; Pan, X.; et al. Highly durable and selective Fe- and Mo-based atomically dispersed electrocatalysts for nitrate reduction to ammonia via distinct and dynergized NO2– Pathways. ACS Catal. 2022, 12, 6651–6662. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Q.; An, X.; Liu, Q.; Xie, L.; Zhang, J.; Yao, W.; Liu, X.; Sun, S.; Sun, X.; et al. Ambient ammonia production via electrocatalytic nitrite reduction over MoO2 nanoparticles self-supported on molybdenum plate. Colloid Surfaces A 2023, 657, 130549. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, X.; Liu, Y.; Yang, S.; Li, H.; Wang, Q.; Shang, D.; Du, F.; Yuan, A.; Yang, F. Interfacial MoO2 nanograins assembled over graphitic carbon nanofibers boosting efficient electrocatalytic reduction of nitrate to ammonia. J. Environ. Chem. Eng. 2024, 12, 111871. [Google Scholar] [CrossRef]
- Yan, J.; Liu, P.; Li, J.; Huang, H.; Song, W. Effect of valence state on electrochemical nitrate reduction to ammonia in molybdenum catalysts. Chem. Eng. J. 2023, 459, 141601. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, L.; Yilmaz, M.; Zhang, T.; Wang, Y.; Yuan, S. MgFe2O4-loaded N-doped biochar derived from waste cooked rice for efficient low-temperature desulfurization of H2S. Fuel 2023, 339, 127385. [Google Scholar] [CrossRef]
- Liao, Y.; Shang, Z.; Ju, G.; Wang, D.; Yang, Q.; Wang, Y.; Yuan, S. Biomass derived N-doped porous carbon made from reed straw for an enhanced supercapacitor. Molecules 2023, 28, 4633. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, H.; Zhang, T.; Zhang, T.; Wang, Y.; Yuan, S. A biomimetic beetle-like membrane with superoleophilic SiO2-induced oil coalescence on superhydrophilic CuC2O4 nanosheet arrays for effective O/W emulsion separation. J. Hazard. Mater. 2023, 451, 131142. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhou, M.; Li, H.; Ding, Z.; Zhang, D.; Lv, Y. Electrocatalytic ammonia synthesis catalyzed by mesoporous nickel oxide nanosheets loaded with Pt nanoparticles. Chin. J. Catal. 2022, 43, 1371–1378. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xiao, J.; Tian, X.; Yuan, S. Enhancing electrochemical performance of ultrasmall Fe2O3-embedded carbon nanotubes via combusting-induced high-valence dopants. J. Mater. Sci. Technol. 2023, 134, 142–150. [Google Scholar] [CrossRef]
- Miao, X.; Shen, J.; Ji, W.; Zhang, T.C.; Liang, Y.; Yuan, S. Boosting electrochemical oxidation of As (III) on Fe-doped RuO2/PEDOT/SnO2 nanocomposite anode: Fabrication, performance and mechanism. J. Mater. Sci. Technol. 2024, 1180, 243–258. [Google Scholar] [CrossRef]
- Ji, W.; Wang, Y.; Zhang, T.; Ouyang, L.; Yuan, S. Heterostructure Cu2O@TiO2 nanotube array coated titanium anode for efficient photoelectrocatalytic oxidation of As(III) in aqueous solution. Ind. Eng. Chem. Res. 2021, 60, 17545–17555. [Google Scholar] [CrossRef]
- Ji, W.; Xiong, Y.; Wang, Y.; Zhang, T.C.; Yuan, S. Multilayered TNAs/SnO2/PPy/β-PbO2 anode achieving boosted electrocatalytic oxidation of As(III). J. Hazard. Mater. 2022, 430, 128449. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Hossen, M.A.; Tahir, M.; Aziz, A.A. A comprehensive review on anodic TiO2 nanotube arrays (TNTAs) and their composite photocatalysts for environmental and energy applications: Fundamentals, recent advances and applications. Coord. Chem. Rev. 2024, 499, 215495. [Google Scholar] [CrossRef]
- Ji, W.; Li, W.; Wang, Y.; Zhang, T.C.; Yuan, S. Fe-MOFs/graphene oxide-derived magnetic nanocomposite for enhanced adsorption of As (V) in aqueous solution. Sep. Purif. Technol. 2024, 334, 126003. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Pimlott, J.L.; Down, M.P.; Rowley-Neale, S.J.; Banks, C.E. MoO2 nanowire electrochemically decorated graphene additively manufactured supercapacitor platforms. Adv. Energy Mater. 2021, 11, 2100433. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Zhang, T.; Ouyang, L.; Yuan, S. Single-step preparation of ultrasmall iron oxide-embedded carbon nanotubes on carbon cloth with excellent superhydrophilicity and enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 2021, 13, 45670–45678. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Zheng, X.; Tian, X.; Yuan, S. Enhancing energy storage via confining sulfite anions onto iron oxide/poly(3,4-ethylenedioxythiophene) heterointerface. ACS Appl. Mater. Interfaces 2023, 15, 59413–59421. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, H.; Duan, Q.; Liu, Y.; Wang, Y.; Yuan, S. Phosphorus-functionalized low-crystallinity transition-metal oxide nanorod arrays grown on carbon cloth for high-performance asymmetric supercapacitors. Colloids Surf. A 2022, 654, 130189. [Google Scholar] [CrossRef]
- Ji, W.; Li, W.; Zhang, T.; Wang, Y.; Yuan, S. Constructing dimensionally stable TiO2 nanotube arrays/SnO2/RuO2 anode via successive electrodeposition for efficient electrocatalytic oxidation of As (III). Sep. Purif. Technol. 2023, 312, 123370. [Google Scholar] [CrossRef]
- Zheng, J.; Lyu, Y.; Wang, R.; Xie, C.; Zhou, H.; Jiang, S.P.; Wang, S. Crystalline TiO2 protective layer with graded oxygen defects for efficient and stable silicon-based photocathode. Nat. Commun. 2018, 9, 3572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, J.; Zheng, X.; Zhang, W.; Lu, S.; Duan, F.; Zhu, H.; Du, M. Solid-phase synthesis of ultra-small CuMo solid solution alloy for efficient electroreduction CO2-to-C2+ production. Chem. Commun. 2023, 59, 5221–5224. [Google Scholar] [CrossRef]
- Thomas, L.; Bahri, M.; Ersen, O.; Lefkir, Y.; Cardenas, L.; Villar-Garcia, I.J.; Virginia Pérez, D.; Llorca, J.; Perret, N.; Checa, R.; et al. Ultradispersed Mo/TiO2 catalysts for CO2 hydrogenation to methanol. Green Chem. 2021, 23, 7259–7268. [Google Scholar]
- Hammer, B.; Nørskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Yang, X.; Ying, J. Stabilization and compressive strain effect of AuCu core on Pt shell for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 8976–8981. [Google Scholar] [CrossRef]
- Lačnjevac, U.; Vasilić, R.; Dobrota, A.; Đurđić, S.; Tomanec, O.; Zbořil, R.; Mohajernia, S.; Nhat Truong, N.; Skorodumova, N.; Manojlović, D.; et al. High-performance hydrogen evolution electrocatalysis using proton-intercalated TiO2 nanotube arrays as interactive supports for Ir nanoparticles. J. Mater. Chem. A 2020, 8, 22773–22790. [Google Scholar] [CrossRef]
- Lačnjevac, U.; Vasilić, R.; Tokarski, T.; Cios, G.; Żabiński, P.; Elezović, N.; Krstajić, N. Deposition of Pd nanoparticles on the walls of cathodically hydrogenated TiO2 nanotube arrays via galvanic displacement: A novel route to produce exceptionally active and durable composite electrocatalysts for cost-effective hydrogen evolution. Nano Energy 2018, 47, 527–538. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, T.; Sow, C.H.; Shen, Z.X.; Lim, C.T.; Subba Rao, G.V.; Chowdari, B.V.R. α-Fe2O3 nanoflakes as an anode material for Li-ion batteries. Adv. Funct. Mater. 2007, 17, 2792–2799. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, H.; Mu, X.; Chen, J.; Zhang, P.; Wang, Y.; He, Y.; Zhang, Z.; Pan, X.; Xie, E. Importance of polypyrrole in constructing 3D hierarchical carbon nanotube@MnO2 perfect core-shell nanostructures for high-performance flexible supercapacitors. Nanoscale 2015, 7, 14697. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yuan, Y.; Jiang, H.; Shi, R.; Liang, D.; Lu, M.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nature energy 2020, 5, 605–613. [Google Scholar]
- Liu, L.; Xiao, T.; Fu, H.; Chen, Z.; Qu, X.; Zheng, S. Construction and identification of highly active single-atom Fe1-NC catalytic site for electrocatalytic nitrate reduction. Appl. Catal. B 2023, 323, 122181. [Google Scholar]
- Wang, Z.; Xia, S.; Deng, X.; Baryshnikov, G.; Kuklin, A.; Ågren, H.; Zhang, H. Platinum group nanoparticles doped BCN matrix: Efficient catalysts for the electrocatalytic reduction of nitrate to ammonia. J. Colloid Interface Sci. 2024, 664, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yu, Y.; Zhao, Z.; Mushtaq, M.A.; Ji, Q.; Yasin, G.; Rehman, L.N.U.; Liu, X.; Cai, X.; Tsiakaras, P.; et al. N, O trans-coordinating silver single-atom catalyst for robust and efficient ammonia electrosynthesis from nitrate. Appl. Catal. B 2023, 331, 122687. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Wang, Z.; Huang, L.; Li, J.; Li, F.; Wicks, J.; Luo, M.; Nam, D.-H.; Tan, C.-S.; et al. Enhanced Nitrate-to-Ammonia Activity on Copper–Nickel Alloys via Tuning of Intermediate Adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef]
- Liu, H.; Lang, X.; Zhu, C.; Timoshenko, J.; Rüscher, M.; Bai, L.; Guijarro, N.; Yin, H.; Peng, Y.; Li, J.; et al. Efficient Electrochemical Nitrate Reduction to Ammonia with Copper-Supported Rhodium Cluster and Single-Atom Catalysts. Angew. Chem. Int. Ed. 2022, 61, 23. [Google Scholar]
- Wang, Y.; Liu, X.; Chen, Q.; Zhang, T.C.; Ouyang, L.; Yuan, S. Simultaneous photocatalytic oxidation and adsorption for efficient As(III) removal by magnetic BiOI/γ-Fe2O3 core–shell nanoparticles. Mater. Today Chem. 2022, 24, 100823. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, S.; Wang, H.; He, J.; Zhang, R.; Qi, Y.; Lu, H. Electrochemical synthesis of trimetallic nickel-iron-copper nanoparticles via potential-cycling for high current density anion exchange membrane water-splitting applications. J. Energy Chem. 2024, 89, 535–542. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Hu, W.; Ma, T.; Pu, Y.; Wang, S.; Wang, Y.; Yuan, S. Molybdenum-Modified Titanium Dioxide Nanotube Arrays as an Efficient Electrode for the Electroreduction of Nitrate to Ammonia. Molecules 2024, 29, 2782. https://doi.org/10.3390/molecules29122782
Chen H, Hu W, Ma T, Pu Y, Wang S, Wang Y, Yuan S. Molybdenum-Modified Titanium Dioxide Nanotube Arrays as an Efficient Electrode for the Electroreduction of Nitrate to Ammonia. Molecules. 2024; 29(12):2782. https://doi.org/10.3390/molecules29122782
Chicago/Turabian StyleChen, Huixi, Wenqi Hu, Tingting Ma, Yixuan Pu, Senhao Wang, Yuan Wang, and Shaojun Yuan. 2024. "Molybdenum-Modified Titanium Dioxide Nanotube Arrays as an Efficient Electrode for the Electroreduction of Nitrate to Ammonia" Molecules 29, no. 12: 2782. https://doi.org/10.3390/molecules29122782
APA StyleChen, H., Hu, W., Ma, T., Pu, Y., Wang, S., Wang, Y., & Yuan, S. (2024). Molybdenum-Modified Titanium Dioxide Nanotube Arrays as an Efficient Electrode for the Electroreduction of Nitrate to Ammonia. Molecules, 29(12), 2782. https://doi.org/10.3390/molecules29122782







