Investigation of Combustion and NO/SO2 Emission Characteristics during the Co-Combustion Process of Torrefied Biomass and Lignite
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. TG Analysis
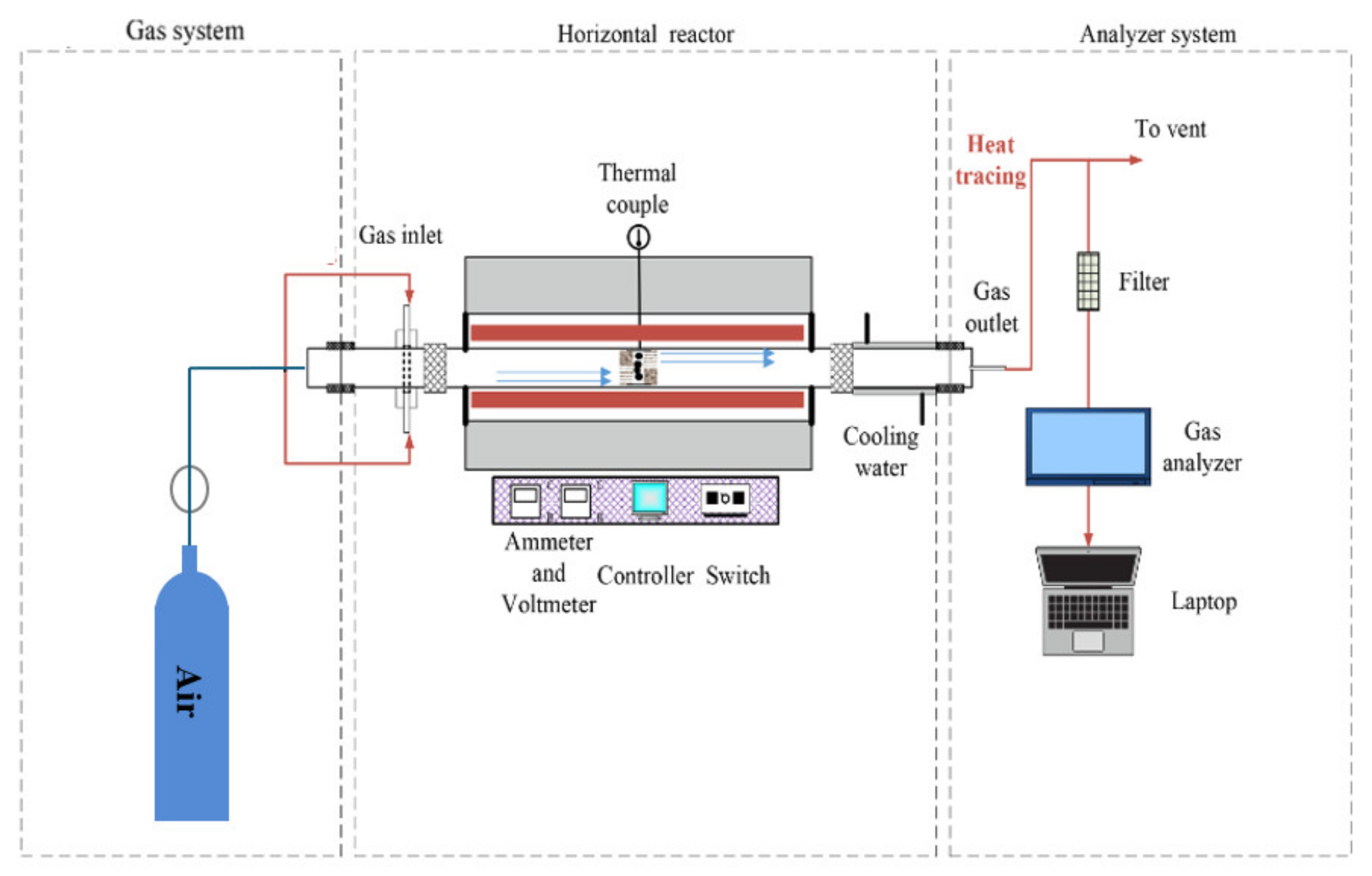
2.3. Fixed-Bed Combustion Experiment System and Conditions
2.4. Analy of Physical and Chemical Properties
2.5. Kinetics Analysis
3. Results and Discussion
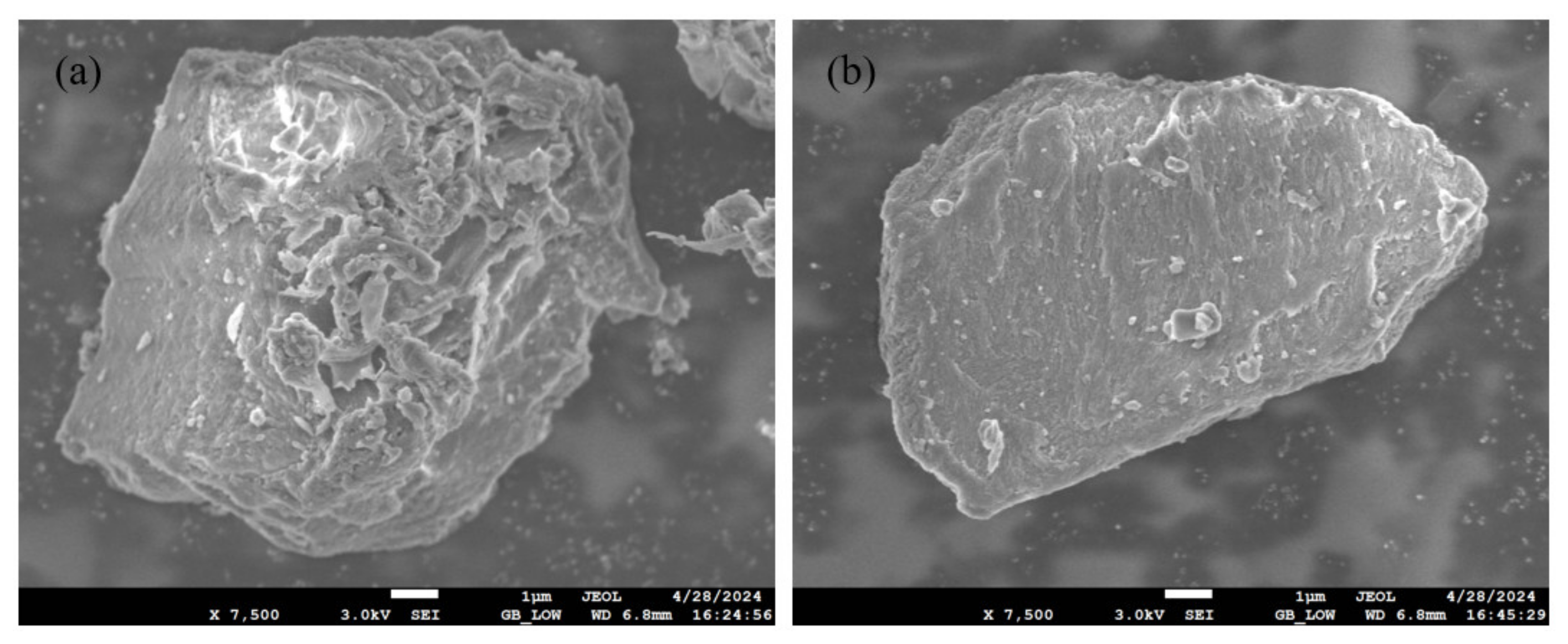
3.1. Physicochemical Characteristics of Samples
3.2. The Thermochemical Results of Individual Fuel
3.3. Combustion Characteristics of Fuel Blends
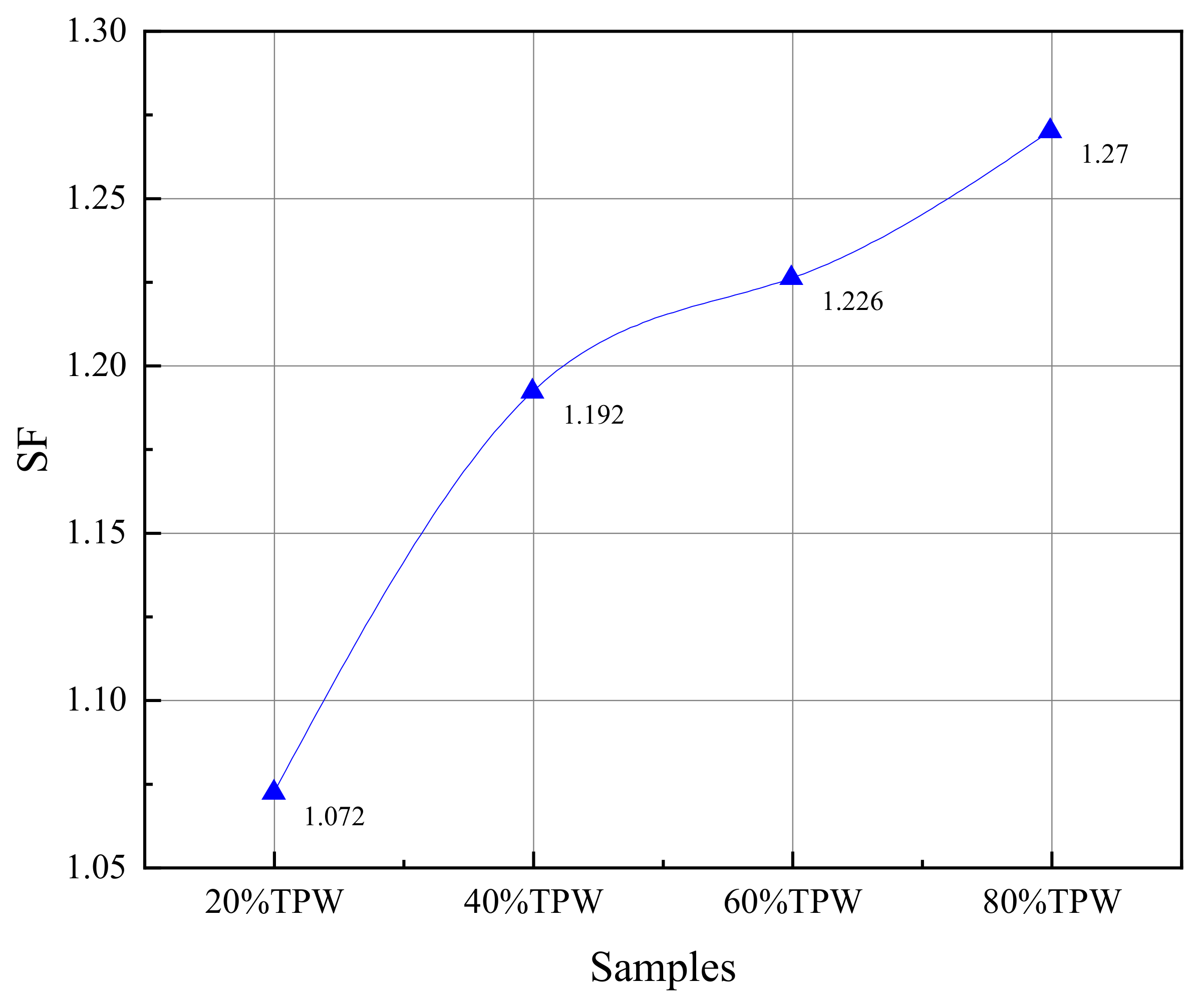
3.4. Synergistic Effects of Co-Combustion Reaction
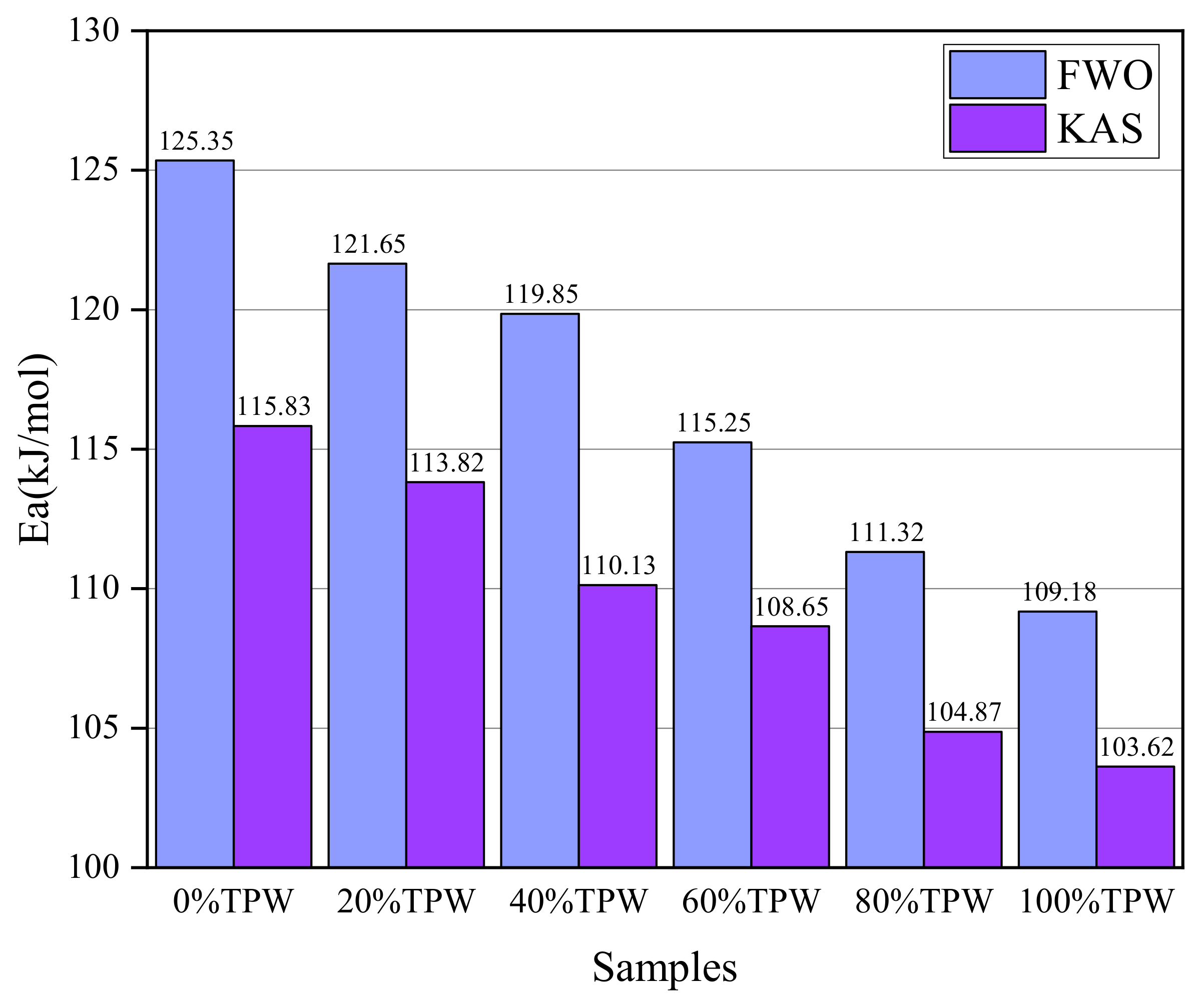
3.5. Kinetics Analysis
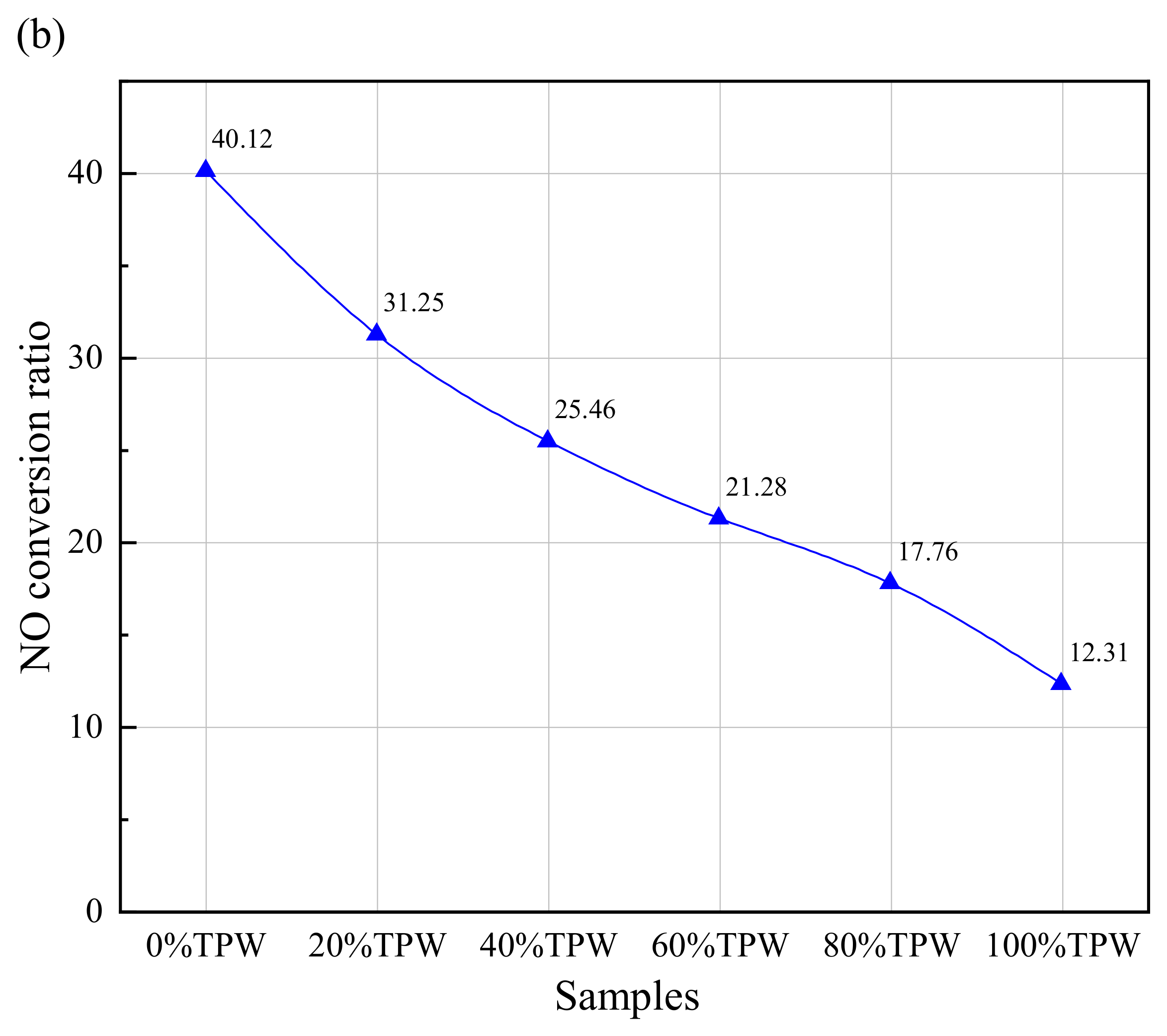
3.6. NO Emission Character
3.7. SO2 Emission Character
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| L | lignite |
| TPW | torrefied pine wood |
| FWO | Flynn–Wall–Ozawa |
| KAS | Kissinger–Akahira–Sunose |
| Ti | ignition temperature |
| Tb | burnout temperature |
| S | comprehensive combustion index |
| HHV | high heating value |
| TGA | non-isothermal thermogravimetric analysis |
| AAEMs | alkali and alkaline earth metals |
References
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Qi, J.; Fan, C.; Wu, H.; Li, S. Structure Evolution of Lignite Char in Step Pyrolysis and Its Combustion Reactivity. Fuel 2022, 315, 123256. [Google Scholar] [CrossRef]
- Yao, J. Biomass energy: Only through diversified development can we go further. China Energy News, 11 May 2023. [Google Scholar] [CrossRef]
- Parvej, A.M.; Rahman, M.A.; Reza, K.M.A. The Combined Effect of Solar Assisted Torrefaction and Pyrolysis on the Production of Valuable Chemicals Obtained from Water Hyacinth Biomass. Clean. Waste Syst. 2022, 3, 100027. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, S.; Zhao, Y.; Wang, Z.; Ma, J.; Xu, L.; Yang, J.; Shen, B. Investigation on the Fuel Quality and Hydrophobicity of Upgraded Rice Husk Derived from Various Inert and Oxidative Torrefaction Conditions. Renew. Energy 2022, 189, 1234–1248. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Ma, J.; Kong, W.; Yuan, P.; Sun, R.; Shen, B. Analysis of Functionality Distribution and Microstructural Characteristics of Upgraded Rice Husk after Undergoing Non-Oxidative and Oxidative Torrefaction. Fuel 2022, 310, 122477. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Xiong, L.; Xu, L.; Yang, J.; Shen, B. Study on the regulation mechanism of torrefaction pretreat-ment on fuel quality and pyrolysis characteristics of rice husk. J. Fuel Chem. Technol. 2023, 51, 320–329. [Google Scholar] [CrossRef]
- Kim, H.; Yu, S.; Ra, H.; Yoon, S.; Ryu, C. Prediction of Pyrolysis Kinetics for Torrefied Biomass Based on Raw Biomass Properties and Torrefaction Severity. Energy 2023, 278, 127759. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goricanec, D.; Krope, J.; Urbancl, D. Torrefaction Pretreatment of Lignocellulosic Biomass for Sustainable Solid Biofuel Production. Energy 2022, 240, 122483. [Google Scholar] [CrossRef]
- Lu, H.; Gong, Y.; Areeprasert, C.; Ding, L.; Guo, Q.; Chen, W.-H.; Yu, G. Integration of Biomass Torrefaction and Gasification Based on Biomass Classification: A Review. Energy Technol. 2021, 9, 2001108. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, S.; Zhang, Z.; Zhang, Y.; Li, J.; Ahmed, S.; Yan, B.; Chen, G.; Li, N. Flue Gas Torrefaction of Distilled Spirit Lees and the Effects on the Combustion and Nitrogen Oxide Emission. Bioresour. Technol. 2021, 342, 125975. [Google Scholar] [CrossRef]
- Munir, S.; Nimmo, W.; Gibbs, B.M. The Effect of Air Staged, Co-Combustion of Pulverised Coal and Biomass Blends on NOx Emissions and Combustion Efficiency. Fuel 2011, 90, 126–135. [Google Scholar] [CrossRef]
- Liu, Y.; Rokni, E.; Yang, R.; Ren, X.; Sun, R.; Levendis, Y.A. Torrefaction of Corn Straw in Oxygen and Carbon Dioxide Containing Gases: Mass/Energy Yields and Evolution of Gaseous Species. Fuel 2021, 285, 119044. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A State-of-the-Art Review of Biomass Torrefaction, Densification and Applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Gao, M.; Cheng, C.; Miao, Z.; Wan, K.; He, Q. Physicochemical Properties, Combustion Kinetics and Thermodynamics of Oxidized Lignite. Energy 2023, 268, 126657. [Google Scholar] [CrossRef]
- Gürel, B.; Kurtuluş, K.; Yurdakul, S.; Karaca Dolgun, G.; Akman, R.; Önür, M.E.; Varol, M.; Keçebaş, A.; Gürbüz, H. Combustion of Chicken Manure and Turkish Lignite Mixtures in a Circulating Fluidized Bed. Renew. Sustain. Energy Rev. 2024, 189, 113960. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, R.; Shi, B.; Shi, M.; Zhang, L.; Liu, D. The Study of Co-Combustion Characteristics of Coal and Duckweed by Single Particle and TGA Methods. Powder Technol. 2023, 421, 118410. [Google Scholar] [CrossRef]
- Guo, F.; He, Y.; Hassanpour, A.; Gardy, J.; Zhong, Z. Thermogravimetric Analysis on the Co-Combustion of Biomass Pellets with Lignite and Bituminous Coal. Energy 2020, 197, 117147. [Google Scholar] [CrossRef]
- Wang, S.; Zou, C.; Lou, C.; Yang, H.; Pu, Y.; Luo, J.; Peng, C.; Wang, C.; Li, Z. Influence of the Synergistic Effects between Coal and Hemicellulose/Cellulose/Lignin on the Co-Combustion of Coal and Lignocellulosic Biomass. Fuel 2022, 311, 122585. [Google Scholar] [CrossRef]
- Yu, D.; Chen, M.; Wei, Y.; Niu, S.; Xue, F. An Assessment on Co-Combustion Characteristics of Chinese Lignite and Eucalyptus Bark with TG–MS Technique. Powder Technol. 2016, 294, 463–471. [Google Scholar] [CrossRef]
- Xinjie, L.; Singh, S.; Yang, H.; Wu, C.; Zhang, S. A Thermogravimetric Assessment of the Tri-Combustion Process for Coal, Biomass and Polyethylene. Fuel 2021, 287, 119355. [Google Scholar] [CrossRef]
- Yi, B.; Chen, M.; Gao, Y.; Cao, C.; Wei, Q.; Zhang, Z.; Li, L. Investigation on the Co-Combustion Characteristics of Multiple Biomass and Coal under O2/CO2 Condition and the Interaction between Different Biomass. J. Environ. Manag. 2023, 325, 116498. [Google Scholar] [CrossRef]
- Zhang, Y.; Zahid, I.; Danial, A.; Minaret, J.; Cao, Y.; Dutta, A. Hydrothermal Carbonization of Miscanthus: Processing, Properties, and Synergistic Co-Combustion with Lignite. Energy 2021, 225, 120200. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, S.; Luo, M.; Cai, J. Combustion Characteristics and Synergistic Effects during Co-Combustion of Lignite and Lignocellulosic Components under Oxy-Fuel Condition. Fuel 2022, 310, 122399. [Google Scholar] [CrossRef]
- Lu, J.-J.; Chen, W.-H. Investigation on the Ignition and Burnout Temperatures of Bamboo and Sugarcane Bagasse by Thermogravimetric Analysis. Appl. Energy 2015, 160, 49–57. [Google Scholar] [CrossRef]
- Liang, W.; Jiang, C.; Wang, G.; Ning, X.; Zhang, J.; Guo, X.; Xu, R.; Wang, P.; Ye, L.; Li, J.; et al. Research on the Co-Combustion Characteristics and Kinetics of Agricultural Waste Hydrochar and Anthracite. Renew. Energy 2022, 194, 1119–1130. [Google Scholar] [CrossRef]
- Mo, W.; Wu, Z.; He, X.; Qiang, W.; Wei, B.; Wei, X.; Wu, Y.; Fan, X.; Ma, F. Functional Group Characteristics and Pyrolysis/Combustion Performance of Fly Ashes from Karamay Oily Sludge Based on FT-IR and TG-DTG Analyses. Fuel 2021, 296, 120669. [Google Scholar] [CrossRef]
- Fatehi, H.; Bai, X.-S. Structural Evolution of Biomass Char and Its Effect on the Gasification Rate. Appl. Energy 2017, 185, 998–1006. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass Torrefaction: An Overview on Process Parameters, Economic and Environmental Aspects and Recent Advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Mundike, J.; Collard, F.-X.; Görgens, J.F. Co-Combustion Characteristics of Coal with Invasive Alien Plant Chars Prepared by Torrefaction or Slow Pyrolysis. Fuel 2018, 225, 62–70. [Google Scholar] [CrossRef]
- Wang, P.; Wang, G.; Zhang, J.; Lee, J.-Y.; Li, Y.; Wang, C. Co-Combustion Characteristics and Kinetic Study of Anthracite Coal and Palm Kernel Shell Char. Appl. Therm. Eng. 2018, 143, 736–745. [Google Scholar] [CrossRef]
- Jia, W.; Guo, Y.; Guo, F.; Li, H.; Li, Y.; Zhang, Y.; Wu, J.; Si, C. Co-Combustion of Carbon-Rich Fraction from Coal Gasification Fine Slag and Biochar: Gas Emission, Ash Sintering, Heavy Metals Evolutions and Environmental Risk Evaluation. Chem. Eng. J. 2023, 471, 144312. [Google Scholar] [CrossRef]
- Oladejo, J.M.; Adegbite, S.; Pang, C.H.; Liu, H.; Parvez, A.M.; Wu, T. A Novel Index for the Study of Synergistic Effects during the Co-Processing of Coal and Biomass. Appl. Energy 2017, 188, 215–225. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Huang, J.; Feng, Y.; Yellezuome, D.; Zhao, R.; Chen, T.; Wu, J. Synergistic Effect and Volatile Emission Characteristics during Co-Combustion of Biomass and Low-Rank Coal. Energy 2024, 289, 130015. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, L.; Guo, B.; Shen, X.; Zheng, X.; Xiang, J.; Jin, Y. Investigation of Interaction Mechanisms during Co-Combustion of Sewage Sludge and Coal Slime: Combustion Characteristics and NO/SO2 Emission Behavior. Sci. Total Environ. 2022, 851, 158166. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, L.; Guo, B.; Wang, B.; Zhang, L.; Zheng, X.; Xiang, J.; Jin, Y. N Migration and Transformation during the Co-Combustion of Sewage Sludge and Coal Slime. Waste Manag. 2022, 145, 83–91. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, W.; Liang, S.; Pan, X. Study on the Co-Combustion Behavior of Semi-Coke and Typical Biomass: Combustion, NO Emission and Ash Characteristics Analysis. Fuel 2024, 358, 130068. [Google Scholar] [CrossRef]
- Hashemi, H.; Hansen, S.; Toftegaard, M.B.; Pedersen, K.H.; Jensen, A.D.; Dam-Johansen, K.; Glarborg, P. A Model for Nitrogen Chemistry in Oxy-Fuel Combustion of Pulverized Coal. Energy Fuels 2011, 25, 4280–4289. [Google Scholar] [CrossRef]
- Ma, M.; Liang, Y.; Xu, D.; Sun, S.; Zhao, J.; Wang, S. Gas Emission Characteristics of Sewage Sludge Co-Combustion with Coal: Effect of Oxygen Atmosphere and Feedstock Mixing Ratio. Fuel 2022, 322, 124102. [Google Scholar] [CrossRef]
- Gao, C.; Meng, J.; Liu, Z.; Hu, Y.; Wang, J.; Xu, J.; Wang, H.; Chen, R. Effects of Water Hyacinth and Anthracite Co-Combustion under Different Conditions on SO2 and NOX Emission. Chem. Eng. Process.-Process Intensif. 2023, 183, 109254. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Ling, P.; An, X.; Han, H.; Chen, Y.; Jiang, L.; Wang, Y.; Su, S.; Hu, S.; et al. A Study on the Release Characteristics and Formation Mechanism of SO2 during Co-Combustion of Sewage Sludge and Coal Slime. Fuel 2023, 333, 126511. [Google Scholar] [CrossRef]
- Yan, J.; Wu, Y.; Zhang, L.; Huang, S.; Lei, Z.; Li, Z.; Zhang, W.; Ren, S.; Wang, Z.; Shui, H. Synergistic Retention of Heavy Metals and In-Situ Reduction of NO and SO2 by Co-Combustion of Sewage Sludge and Coal Gangue: A Promising Approach for Contaminant Management and Emission Reduction. Fuel Process. Technol. 2023, 252, 107984. [Google Scholar] [CrossRef]





| Samples | Ultimate Analysis (daf) (wt.%) | Proximate Analysis (ar)(wt.%) | HHV(MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O a | N | S | FC | Ash | Volatile | Mar | ||
| TPW | 61.33 | 4.92 | 32.76 | 0.98 | 0.01 | 33.21 | 30.86 | 33.27 | 2.66 | 23.16 |
| L | 80.67 | 3.95 | 14.17 | 0.56 | 0.65 | 45.22 | 18.9 | 33.37 | 2.51 | 31.01 |
| Sample | Surface AreaBET (m2/g) | Pore Volume (cm2/g) | Pore DiameterBJH (nm) |
|---|---|---|---|
| TPW | 16.462 | 0.026 | 11.724 |
| L | 7.265 | 0.024 | 9.693 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhu, W.; Li, Z.; Xu, L.; Zhu, S.; Tian, J.; Wang, Z.; Shen, B. Investigation of Combustion and NO/SO2 Emission Characteristics during the Co-Combustion Process of Torrefied Biomass and Lignite. Molecules 2024, 29, 2728. https://doi.org/10.3390/molecules29122728
Yang X, Zhu W, Li Z, Xu L, Zhu S, Tian J, Wang Z, Shen B. Investigation of Combustion and NO/SO2 Emission Characteristics during the Co-Combustion Process of Torrefied Biomass and Lignite. Molecules. 2024; 29(12):2728. https://doi.org/10.3390/molecules29122728
Chicago/Turabian StyleYang, Xu, Wenkun Zhu, Zhaoming Li, Li Xu, Shujun Zhu, Jilin Tian, Zhuozhi Wang, and Boxiong Shen. 2024. "Investigation of Combustion and NO/SO2 Emission Characteristics during the Co-Combustion Process of Torrefied Biomass and Lignite" Molecules 29, no. 12: 2728. https://doi.org/10.3390/molecules29122728
APA StyleYang, X., Zhu, W., Li, Z., Xu, L., Zhu, S., Tian, J., Wang, Z., & Shen, B. (2024). Investigation of Combustion and NO/SO2 Emission Characteristics during the Co-Combustion Process of Torrefied Biomass and Lignite. Molecules, 29(12), 2728. https://doi.org/10.3390/molecules29122728







