Exploring the Potential of Myrcia Genus Essential Oils: A Review of Biological Activities and Recent Advances
Abstract
1. Introduction
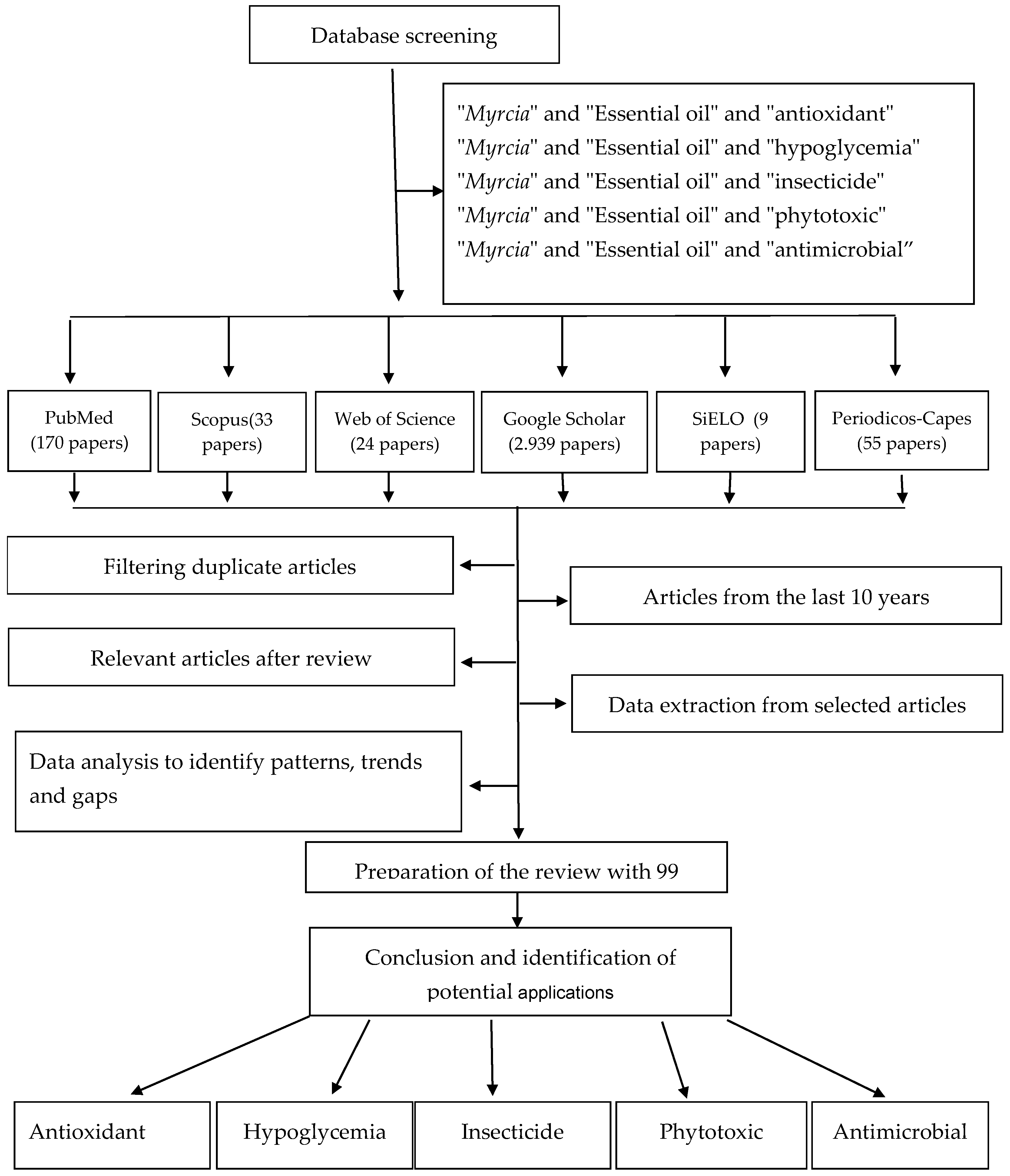
2. Methods
3. Botanical Characteristics
4. Chemical Composition of Essential Oils
| Species | Collection Site | Plant Part | Extraction Type | Major Components | References |
|---|---|---|---|---|---|
| Myrcia eximia (Specimen A) | Magalhães Barata, Pará, Brazil | Leaves | HD | hexanal (26.09%), (E)-Caryophyllene (20.3%), and (2E)-hexenal (6.63%). | [5] |
| M. eximia (Specimen B) | Magalhães Barata, Pará, Brazil | Leaves | SD | Caryophyllene oxide (22.16%), α-Copaene (10.98%), and 14-Hydroxy-9-epi-(E)-caryophyllene (7.84%) | [5] |
| M. guianensis | Rio Verde, Goiás, Brazil. | Flores | HD | methyl salicylate (11.13%), geraniol (8.03%), and eugenol (8.12%) | [51] |
| M. hatschbachii | Parana, Curitiba, Brazil | Leaves | HD | trans-calamenene (19.10%), (E)-caryophyllene (10.96%), and spathulenol (5.03%). | [42] |
| M. ovata | Japaratuba, in the state of Sergipe, Brazil | Leaves | HD | nerolic acid (50.56–60.63%), geraniol (73.64–77.07%) neral (18.21–28.39%), geranial (36.96–48.48%), and (E)-nerolidol (26.97–58.27%) | [43] |
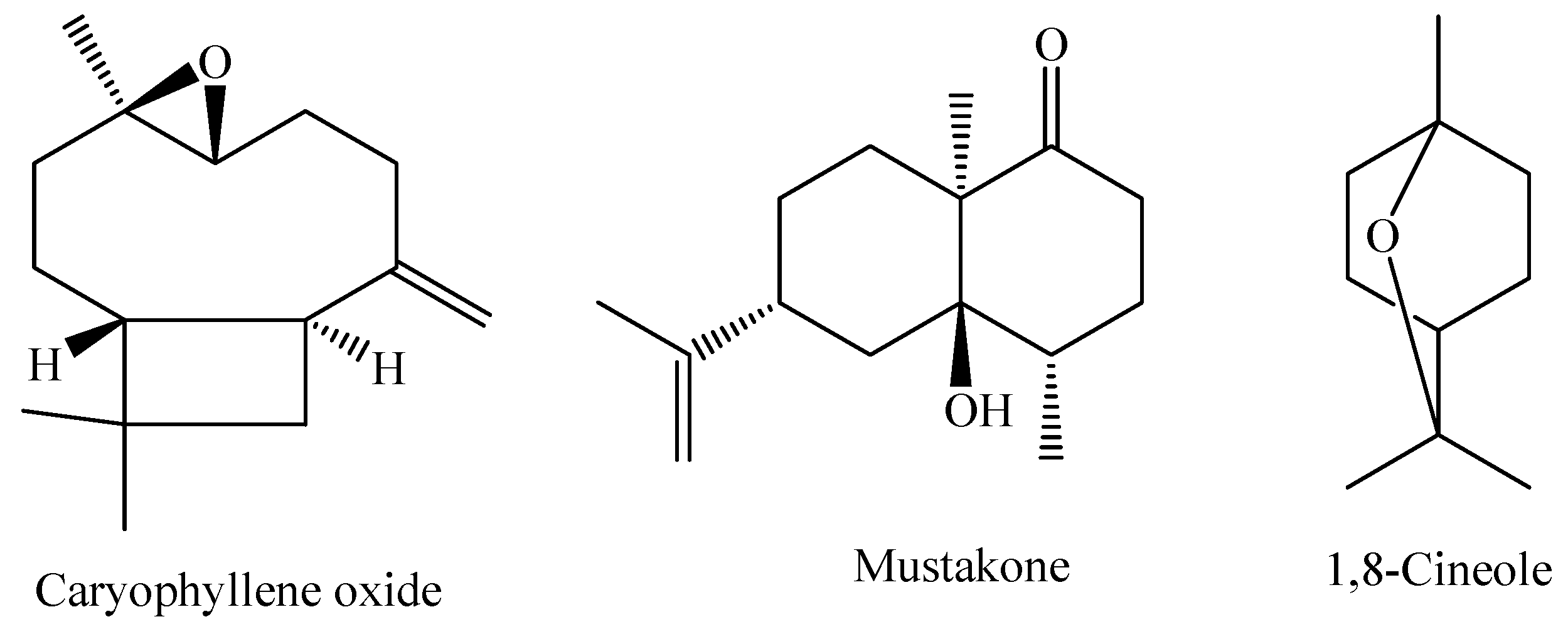
| M. lundiana chemotypes isopulegol (MLUN19) | Itabaiana, Sergipe, Brazil. | Freshly leaves | HD | Isopulegol (40.29%), Iso isopulegol (15.49%), and 1,8-cineol (14.16%) | [13] |
| M. lundiana chemotype citral (MLUN23) | Itabaiana, Sergipe, Brazil. | Freshly leaves | HD | Citral (23.43%), 1,8-cineol (16.43%), and Nerolic acid (16.42%) | [13] |
| M. mollis, | Gonzanamá, province of Loja, Equador | Leaves | SD | limonene (5.3–5.2%), 1,8-cineole (10.4–11.6%), and (Z)-caryophyllene (16.6–16.8%) | [39] |
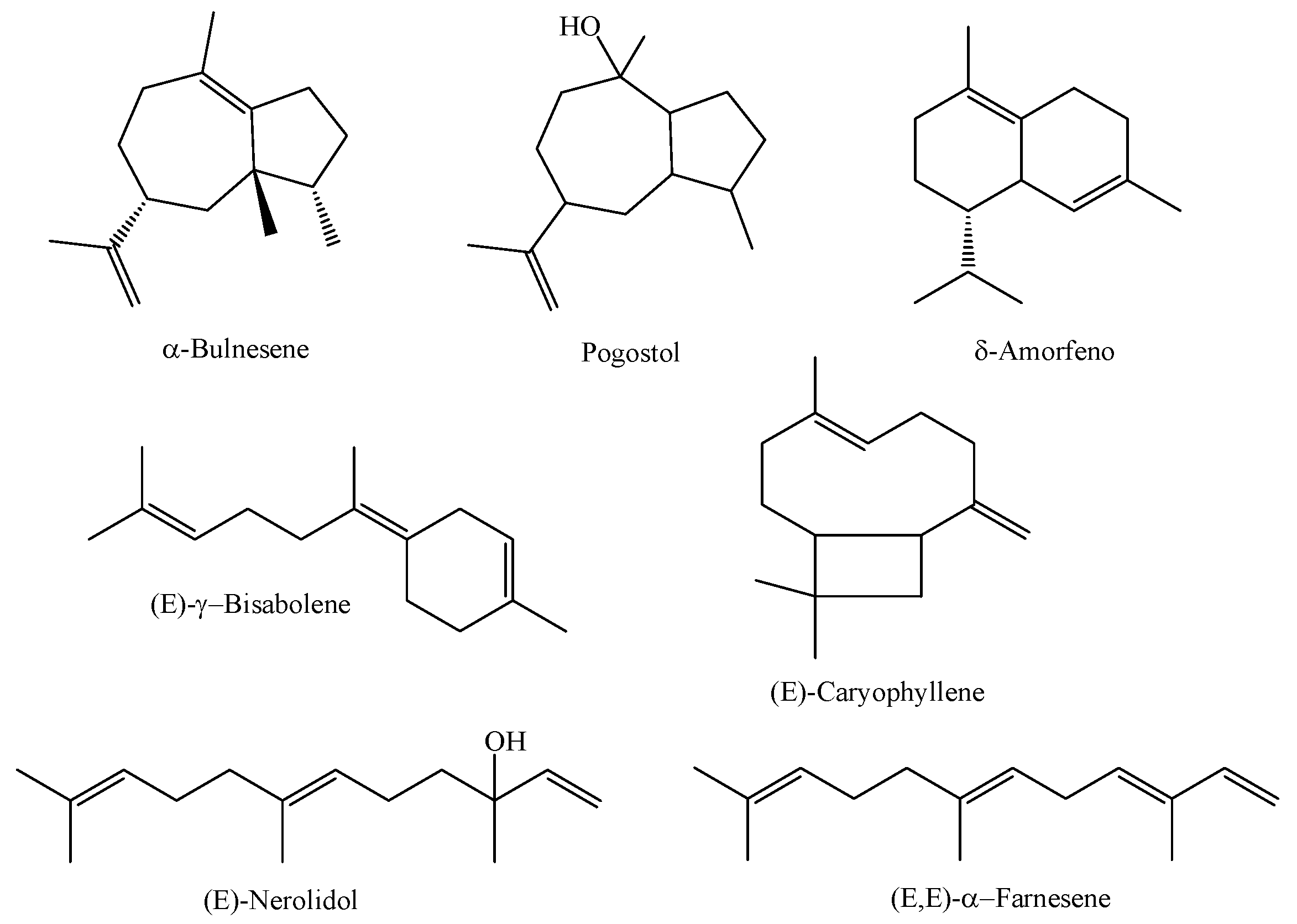
| M. multiflora (Specimen A) | Magalhães Barata, Pará, Brazil | Leaves | HD | α-bulnesene (26.79%), pogostol (21.27%), and δ-amorphene (6.76%) | [4] |
| M. multiflora (Specimen B) | Magalhães Barata, Pará, Brazil | Leaves | HD | (E)-nerolidol (44.4%), (E)-γ-bisabolene (10.64%), and (E,E)-α-farnesene (8.19%) | [4] |
| M. multiflora (Specimen C) | Magalhães Barata, Pará, Brazil | Leaves | HD | (E)-nerolidol (92.21%), (E,E)-α-farnesene (3.28%), and E-caryophyllene (1.11%) | [4] |
| M. oblongata | Cascavel, Paraná, Brazil. | Leaves | HD | caryophyllene oxide (22.03%), and trans-verbenol (11.94%). | [44] |
| M. ovata | Japaratuba, Sergipe, Brazil | Leaves | HD | Geranial (40.10%), Neral (28.39%), and Citronellal (9.19%) | [40] |
| Guaramiranga, Ceará, Brazil | Leaves | HD | Geranial (52.60%) and neral (37.14%) | [52] | |
| M. paivae | Peixe-Boi, Pará, Brazil | Leaves | HD | terpinolene (14.70%), α-phellandrene (14.69%), and γ-terpinene (9.64%) | [7] |
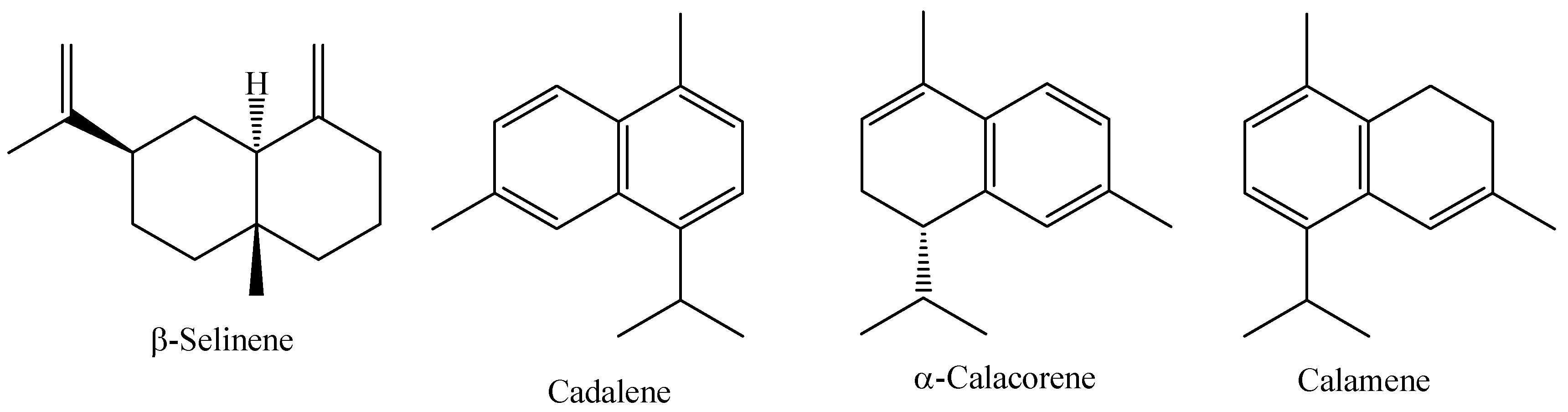
| M. sylvatica | Santarém, Pará, Brazil | Leaves | HD | β-selinene (9.96%), cadalene (9.36%), α-calacorene (9.17%) | [45] |
| Bujaru, Pará, Brazil | Leaves | HD | (Z)-α-trans-bergamotene(24.57%), α-sinensal (13.44%), and (Z)-α-bisabolene (8.33%) | [10] | |
| M. rostrata | Alagoinhas, Bahia, Brazil | Leaves | HD | carotol (17.68%), germacreno B (7.28%), and (E)-caryophyllene (6.45%) | [46] |
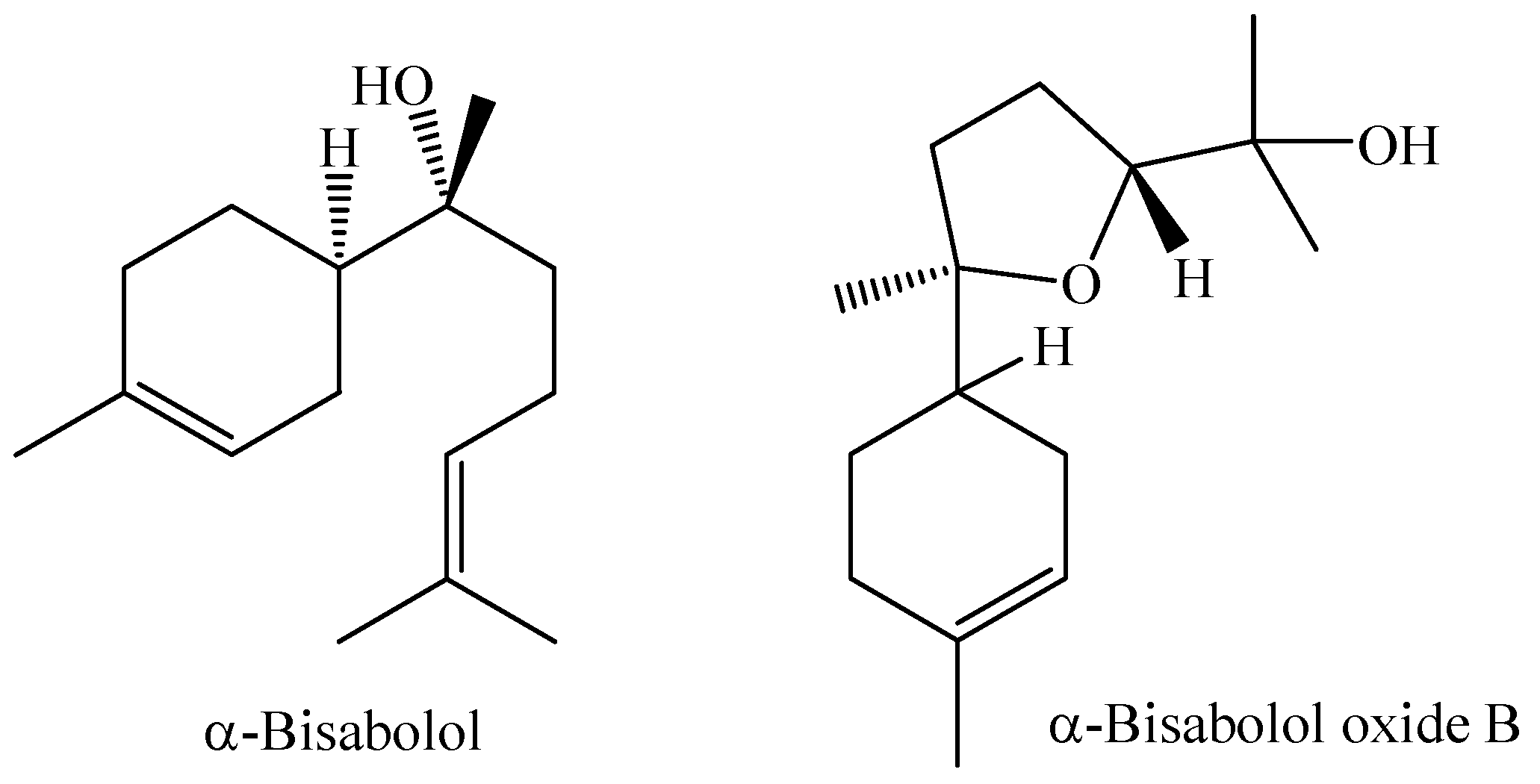
| M. splendens | Manaus, Amazonas, Brazil | Leaves | HD | trans-nerolidol (67.81%), α-bisabolol (17.51%), and β-caryophyllene (4.21% | [48] |
| Sergipe, Brazil | Fresh leaves | HD | bicyclogermacrene (15.4%), germacrene D (8.9%), and E-caryophyllene (10.1%) | [47] | |
| M. vittoriana | community in southeastern Brazil | Fresh leaves | HD | germacrene D (21.90%), germacrene B (17.30%), and bicyclogermacrene (11.90%) | [49] |
| M. tomentosa (specimen A) | Magalhães Barata, Pará, Brazil | Leaves | HD | γ-elemene (12.52%), germacrene D (11.45%), and (E)-caryophyllene (10.22%) | [3] |
| M. tomentosa (specimen B) | Magalhães Barata, Pará, Brazil | Leaves | HD | spathulenol (40.70%), α-zingiberene (9.58%), and γ-elemene (6.89%). | [3] |
| M. tomentosa | Belo Horizonte, Minas Gerais | Fresh leaves | SD | germacrene D (17.62%), (E)-caryophyllene (15.05%), and δ-cadinene (6.42%) | [50] |
5. Antioxidant Activity
6. Biological Activities
6.1. Antimicrobial (Fungi and Bacteria)
6.2. Phytotoxic Activity of Myrcia Genus
6.3. Insecticide
6.4. Hypoglycemic Potential of Myrcia Species
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, M.F.; Amorim, B.S.; Burton, G.P.; Fernandes, T.; Gaem, P.H.; Lourenço, A.R.L.; Lima, D.F.; Rosa, P.O.; Santos, L.L.D.; Staggemeier, V.G.; et al. Myrcia in Flora Do Brasil. Jardim Botânico Do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB10660 (accessed on 6 February 2024).
- dos Santos, C.; Galaverna, R.S.; Angolini, C.F.F.; Nunes, V.V.A.; de Almeida, L.F.R.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Duarte, R.M.T.; Duarte, M.C.T.; Eberlin, M.N. Antioxidative, Antiproliferative and Antimicrobial Activities of Phenolic Compounds from Three Myrcia Species. Molecules 2018, 23, 986. [Google Scholar] [CrossRef] [PubMed]
- de Franco, C.J.P.; Ferreira, O.O.; Antônio Barbosa de Moraes, Â.; Varela, E.L.P.; do Nascimento, L.D.; Percário, S.; de Oliveira, M.S.; de Andrade, E.H.A. Chemical Composition and Antioxidant Activity of Essential Oils from Eugenia patrisii Vahl, E. Punicifolia (Kunth) DC., and Myrcia tomentosa (Aubl.) DC., Leaf of Family Myrtaceae. Molecules 2021, 26, 3292. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.O.; da Silva, S.H.M.; de Oliveira, M.S.; de Andrade, E.H.A. Chemical Composition and Antifungal Activity of Myrcia multiflora and Eugenia florida Essential Oils. Molecules 2021, 26, 7259. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.O.; da Cruz, J.N.; de Franco, C.J.P.; Silva, S.G.; da Costa, W.A.; de Oliveira, M.S.; de Andrade, E.H.A. First Report on Yield and Chemical Composition of Essential Oil Extracted from Myrcia eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules 2020, 25, 783. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, Â.A.B.; de Jesus Pereira Franco, C.; Ferreira, O.O.; Varela, E.L.P.; do Nascimento, L.D.; Cascaes, M.M.; da Silva, D.R.P.; Percário, S.; de Oliveira, M.S.; de Aguiar Andrade, E.H. Myrcia paivae O.Berg (Myrtaceae) Essential Oil, First Study of the Chemical Composition and Antioxidant Potential. Molecules 2022, 27, 5460. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, L.M.S.; Riba, I.T.; Montanher, P.F.; Antonelo, F.A. Chemical Composition, Antioxidant and Antimicrobial Activities of Essential Oil Extracted from Myrceugenia euosma (O. Berg) D. Legrand (Myrtaceae). S. Afr. J. Bot. 2023, 159, 35–42. [Google Scholar] [CrossRef]
- Oliveira, E.S.C.; Acho, L.D.R.; Morales-Gamba, R.D.; do Rosário, A.S.; Barcellos, J.F.M.; Lima, E.S.; Machado, M.B. Hypoglycemic Effect of the Dry Leaf Extract of Myrcia multiflora in Streptozotocin-Induced Diabetic Mice. J. Ethnopharmacol. 2023, 307, 116241. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, Â.A.B.; Ferreira, O.O.; da Costa, L.S.; Almeida, L.Q.; Varela, E.L.P.; Cascaes, M.M.; de Jesus Pereira Franco, C.; Percário, S.; do Nascimento, L.D.; de Oliveira, M.S.; et al. Phytochemical Profile, Preliminary Toxicity, and Antioxidant Capacity of the Essential Oils of Myrciaria floribunda (H. West Ex Willd.) O. Berg. and Myrcia sylvatica (G. Mey) DC. (Myrtaceae). Antioxidants 2022, 11, 2076. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.C.S.; De Oliveira, J.C.S.; Da Câmara, C.A.G.; Castelar, I.; Carvalho, A.F.F.U.; Lima-Filho, J.V. Antibacterial and Cytotoxic Properties of Some Plant Crude Extracts Used in Northeastern Folk Medicine. Rev. Bras. Farmacogn. 2009, 19, 376–381. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Porte, A.; de Oliveira Godoy, R.L.; da Costa Souza, M.; Pacheco, S.; de Araujo Santiago, M.C.P.; Gouvêa, A.C.M.S.; da Silva de Mattos do Nascimento, L.; Borguini, R.G. Chemical Characterization of Myrciaria floribunda (H. West Ex Willd) Fruit. Food Chem. 2018, 248, 247–252. [Google Scholar] [CrossRef]
- Melo, C.R.; Blank, A.F.; Oliveira, B.M.S.; Santos, A.C.C.; Cristaldo, P.F.; Araújo, A.P.A.; Bacci, L. Formicidal Activity of Essential Oils of Myrcia lundiana Chemotypes on Acromyrmex balzani. Crop Prot. 2021, 139, 105343. [Google Scholar] [CrossRef]
- da Barbosa, D.C.S.; Holanda, V.N.; Ghosh, A.; Maia, R.T.; da Silva, W.V.; de Lima, V.L.M.; da Silva, M.V.; dos Santos Correia, M.T.; de Figueiredo, R.C.B.Q. Leishmanicidal and Cytotoxic Activity of Essential Oil from the Fruit Peel of Myrciaria floribunda (H. West Ex Willd.) O. Berg: Molecular Docking and Molecular Dynamics Simulations of Its Major Constituent onto Leishmania Enzyme Targets. J. Biomol. Struct. Dyn. 2022, 40, 13001–13016. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.J.; Amorim, B.S.; Lima, D.F.; Lima-Lourenço, A.R.; Nic Lughadha, E.M.; Proença, C.E.B.; Rosa, P.O.; Rosário, A.S.; Santos, L.L.; Santos, M.F.; et al. A New Infra-Generic Classification of the Species-Rich Neotropical Genus Myrcia s.l. Kew Bull. 2018, 73, 9. [Google Scholar] [CrossRef]
- Santos, M.F.; Amorim, B.S.; Burton, G.P.; Fernandes, T.; Gaem, P.H.; Lourenço, A.R.L.; Lima, D.F.; Rosa, P.O.; Santos, L.L.D.; Staggemeier, V.G.; et al. Myrcia in Flora do Brasil Research Institute Rio de Janeiro Botanical Garden. Available online: https://floradobrasil.jbrj.gov.br/FB10660 (accessed on 6 April 2024).
- Gaem, P.H.; Lucas, E.; Andrade, A.; Vicentini, A.; Mazine, F.F. A Taxonomic Account of Myrcia (Myrtaceae) at the Sites of the Biological Dynamics of Forest Fragments Project, Amazonas, Brazil. Rodriguésia 2022, 73, e00712020. [Google Scholar] [CrossRef]
- Do Rosário, A.S.; Baumgratz, J.F.A.; De Secco, R.S. Taxonomic Studies of Myrcia (Myrciinae, Myrtaceae) in Brazil: Morphological Novelties, Circumscriptions, and New Records for the Amazon. Iheringia Série Botânica 2017, 72, 165–172. [Google Scholar] [CrossRef]
- Faria, J.E.Q.; Lucas, E.J.; Sobral, M. Two New Species of Myrcia (Myrtaceae) from Brazil. Phytotaxa 2017, 319, 159–166. [Google Scholar] [CrossRef]
- Lucas, E.J.; Holst, B.; Sobral, M.; Mazine, F.F.; Nic Lughadha, E.M.; Barnes Proença, C.E.; Ribeiro da Costa, I.; Vasconcelos, T.N.C. A New Subtribal Classification of Tribe Myrteae (Myrtaceae). Syst. Bot. 2019, 44, 560–569. [Google Scholar] [CrossRef]
- Lucas, E.J.; Matsumoto, K.; Harris, S.A.; Nic Lughadha, E.M.; Benardini, B.; Chase, M.W. Phylogenetics, Morphology, and Evolution of the Large Genus Myrcia s.l. (Myrtaceae). Int. J. Plant Sci. 2011, 172, 915–934. [Google Scholar] [CrossRef]
- Vogado, N.O.; de Camargo, M.G.G.; Locosselli, G.M.; Morellato, L.P.C. Les Effets de Bord Sur La Phénologie de La Guamirim, Myrcia guianensis (Myrtaceae), Un Arbre de Cerrado. Trop. Conserv. Sci. 2016, 9, 291–312. [Google Scholar] [CrossRef]
- Scaravelli, F.S.; Gaem, P.H.; Valdemarin, K.S.; Lucas, E.; Mazine, F.F. Myrcia (Myrtaceae) in the Vale Natural Reserve, Linhares, Espírito Santo, Brazil. Rodriguésia 2022, 73, e02352020. [Google Scholar] [CrossRef]
- Gonçalves, E.G.; Lorenzini, H. Morfologia Vegetal: Organografia e Dicionário Ilustrado de Morfologia das Plantas Vasculares; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brasil, 2007; pp. 39–52. [Google Scholar]
- Pellis, V.F.; Ferreira, R.B.; Caddah, M.K. Sinopse de Myrtaceae Juss. No Monumento Natural Municipal Da Lagoa Do Peri, Florianópolis, SC, Brasil. Hoehnea 2021, 48, e202021. [Google Scholar] [CrossRef]
- do de Miranda, S.C.; De-Carvalho, P.S.; Ribon, A.A. Tópicos em Conservação e Manejo do Cerrado: Biodiversidade, Solos e Uso Sustentável; Editora Kelps: Goiânia, Brazil, 2019; ISBN 9788540026971. [Google Scholar]
- Stefanello, M.É.A.; Cervi, A.C.; Wisniewski, A.; Simionatto, E.L. Essential Oil Composition of Myrcia laruotteana Camb. J. Essent. Oil Res. 2007, 19, 466–467. [Google Scholar] [CrossRef]
- Alves, M.F.; de Castro Nizio, D.A.; Sampaio, T.S.; do Nascimento Junior, A.F.; de Andrade Brito, F.; de Melo, J.O.; de Fátima Arrigoni-Blank, M.; Gagliardi, P.R.; Machado, S.M.F. Myrcia Lundiana Kiaersk Native Populations Have Different Essential Oil Composition and Antifungal Activity against Lasiodiplodia theobromae. Ind. Crops Prod. 2016, 85, 266–273. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.C.; Souza-Silva, É.A.; Alvarenga, D.R.; Saboia, G.; Soares, G.L.G.; Zini, C.A.; Cavalleri, A.; Isaias, R.M.S. Structural and Chemical Profiles of Myrcia splendens (Myrtaceae) Leaves Under the Influence of the Galling Nexothrips Sp. (Thysanoptera). Front. Plant Sci. 2018, 9, 402567. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.D.O.T.; de Lucena, E.M.P.; Bonilla, O.H.; Edson-Chaves, B.; Freitas, M.A. Anatomia Ecológica Foliar de Myrcia guianensis (Aubl.) DC. Na Restinga Cearense. Ciência Florest. 2020, 30, 307–322. [Google Scholar] [CrossRef]
- da Silva, L.A.; Raposo, J.D.A.; Campos, L.P.G.; da Conceição, E.C.; de Oliveira, R.B.; Mourão, R.H.V. Atividade Antioxidante Do Óleo Essencial de Myrcia sylvatica (G. Mey.) DC. Por Diferentes Métodos de Análises Antioxidantes (ABTS, DPPH, FRAP, β-Caroteno/Ácido Linoleico). Rev. Fitos 2018, 12, 117–126. [Google Scholar] [CrossRef]
- Do Nascimento Silva, A.; Bomfim, H.F.; Magalhães, A.O.; Da Rocha, M.L.; Lucchese, A.M. Chemical Composition and Antinociceptive Activity of Essential Oil from Myrcia rostrata Dc. (Myrtaceae) in Animal Models. Quim. Nova 2018, 41, 982–988. [Google Scholar]
- Raposo, J.D.A.; Figueiredo, P.L.B.; Santana, R.L.; da Silva Junior, A.Q.; Suemitsu, C.; da Silva, R.; Mourão, R.H.V.; Maia, J.G.S. Seasonal and Circadian Study of the Essential Oil of Myrcia Sylvatica (G. Mey) DC., a Valuable Aromatic Species Occurring in the Lower Amazon River Region. Biochem. Syst. Ecol. 2018, 79, 21–29. [Google Scholar] [CrossRef]
- de Menezes Filho, A.C.P.; de Sousa, W.C.; de Castro, C.F.S. Composição Química, Físico-Química e Atividade Antifúngica Dos Óleos Essenciais Da Flor e Do Fruto de Myrcia guianensis (Aubl.) DC. Rev. Principia Divulg. Cient. Tecnol. IFPB 2020, 1, 92–104. [Google Scholar] [CrossRef]
- dos Santos, C.V.; Mallmann, A.P.; Toledo, A.G.; Bandeira, D.M.; da Costa, W.F.; Marins, D.M.Á.; Corrêa, J.M.; da Pinto, F.G.S. Composição Química, Atividade Antimicrobiana e Antioxidante Do Óleo Essencial de Folhas Myrcia palustris DC. (MYRTACEAE). Res. Soc. Dev. 2021, 10, e20510313303. [Google Scholar] [CrossRef]
- Cascaes, M.; Guilhon, G.; Andrade, E.; Zoghbi, M.; Santos, L. Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef] [PubMed]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a Renewable Source of Biologically Active Compounds. Pure Appl. Chem. 2017, 89, 1105–1117. [Google Scholar] [CrossRef]
- Montalván, M.; Peñafiel, M.A.; Ramírez, J.; Cumbicus, N.; Bec, N.; Larroque, C.; Bicchi, C.; Gilardoni, G. Chemical Composition, Enantiomeric Distribution, and Sensory Evaluation of the Essential Oils Distilled from the Ecuadorian Species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) Dc. (Myrtaceae). Plants 2019, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, I.C.; Santos Frazão, G.G.; Blank, A.F.; de Aquino Santana, L.C.L. Myrcia ovata Cambessedes Essential Oils: A Proposal for a Novel Natural Antimicrobial against Foodborne Bacteria. Microb. Pathog. 2016, 99, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and Their Derivatives-Natural Anticancer Compounds: An Update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.J.; Fabri, N.T.; de Souza, A.M.; da Fonseca, N.S.T.; Furusho, A.D.S.; Miguel, O.G.; de Dias, J.F.G.; Zanin, S.M.W.; Miguel, M.D. Chemical Composition, Phytotoxic Potential, Biological Activities and Antioxidant Properties of Myrcia hatschbachii D. Legrand Essential Oil. Braz. J. Pharm. Sci. 2020, 56, e18402. [Google Scholar] [CrossRef]
- Sampaio, T.S.; de Castro Nizio, D.A.; White, L.A.S.; Melo, J.d.O.; Almeida, C.S.; Alves, M.F.; Gagliardi, P.R.; de Fátima Arrigoni-Blank, M.; Wisniewski Junior, A.; Sobral, M.E.G.; et al. Chemical Diversity of a Wild Population of Myrcia Ovata Cambessedes and Antifungal Activity against Fusarium Solani. Ind. Crops Prod. 2016, 86, 196–209. [Google Scholar] [CrossRef]
- Santana, C.B.; de Souza, J.G.L.; Coracini, M.D.A.; Walerius, A.H.; Soares, V.D.; da Costa, W.F.; da Pinto, F.G.S. Chemical Composition of Essential Oil from Myrcia oblongata DC and Potencial Antimicrobial, Antioxidant and Acaricidal Activity against Dermanyssus gallinae (Degeer, 1778). Biosci. J. 2018, 34, 996–1009. [Google Scholar] [CrossRef]
- Saccol, E.M.H.; Toni, C.; Pês, T.S.; Ourique, G.M.; Gressler, L.T.; Silva, L.V.F.; Mourão, R.H.V.; Oliveira, R.B.; Baldisserotto, B.; Pavanato, M.A. Anaesthetic and Antioxidant Effects of Myrcia sylvatica (G. Mey.) DC. and Curcuma longa L. Essential Oils on Tambaqui (Colossoma macropomum). Aquac. Res. 2017, 48, 2012–2031. [Google Scholar] [CrossRef]
- Silva, A.; Bomfim, H.; Magalhães, A.; Rocha, M.; Lucchese, A. Composição Química e Atividade Antinociceptiva em Modelo Animal do Óleo Essencial de Myrcia rostrata DC. (MYRTACEAE). Quim. Nova 2018, 41, 982–988. [Google Scholar] [CrossRef]
- Montalvão, M.M.; Felix, F.B.; Propheta dos Santos, E.W.; Santos, J.F.; de Lucca Júnior, W.; Farias, A.S.; de Souza Ribeiro, A.; Cavaleiro, C.; Machado, S.M.F.; Scher, R.; et al. Cytotoxic Activity of Essential Oil from Leaves of Myrcia splendens against A549 Lung Cancer Cells. BMC Complement. Med. Ther. 2023, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.; Sacchetti, G.; Guerrini, A. Myrcia splendens (Sw.) DC. (Syn. M. Fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile. Molecules 2017, 22, 1163. [Google Scholar] [CrossRef]
- Vasconcelos, L.C.; Carrijo, T.T.; Venancio, A.N.; Alves, T.A.; Tuler, A.C.; Hollunder, R.K.; Garbin, M.L.; Menini, L.; Praca-Fontes, M.M. Phytochemical Screening and Phytocytotoxic Effects of the Tropical Myrcia vittoriana (Myrtaceae). An. Acad. Bras. Cienc. 2022, 94, e20210820. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.A.C.; Garcia, I.P.; Corrêa, E.J.A.; de Lima, L.H.F.; de Santos, H.L.; de Assis, R.M.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Larvicidal Susceptibility of Essential Oils from Cinnamodendron dinisii, Callistemon viminalis and Myrcia tomentosa against Culex quinquefasciatus (Say) (Diptera: Culicidae). S. Afr. J. Bot. 2023, 163, 95–104. [Google Scholar] [CrossRef]
- de Menezes Filho, A.C.P.; Sousa, W.C.; de Souza Castro, C.F.; de Souza, L.F. Chemical Composition of Essential Oil from Flowers of Myrcia guianensis (Aubl.) DC. Rev. Cuba. Plantas Med. 2019, 24, 1–12. [Google Scholar]
- Amorim Gomes, G.; Martins-Cardoso, K.; dos Santos, F.R.; Florencio, M.; Rosa, D.; Araujo Zuma, A.; Pinheiro Santiago, G.M.; Motta, M.C.M.; de Carvalho, M.G.; Fampa, P. Antileishmanial Activity of the Essential Oils of Myrcia ovata Cambess. and Eremanthus erythropappus (DC) McLeisch Leads to Parasite Mitochondrial Damage. Nat. Prod. Res. 2021, 35, 6117–6121. [Google Scholar] [CrossRef]
- Silva, L.; Sarrazin, S.; Oliveira, R.; Suemitsu, C.; Maia, J.; Mourão, R. Composition and Antimicrobial Activity of Leaf Essential Oils of Myrcia sylvatica (G. Mey.) DC. Eur. J. Med. Plants 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Rosa, C.S.; Veras, K.S.; Silva, P.R.; Lopes Neto, J.J.; Cardoso, H.L.M.; Alves, L.P.L.; Brito, M.C.A.; Amaral, F.M.M.; Maia, J.G.S.; Monteiro, O.S.; et al. Composição Química e Toxicidade Frente Aedes aegypti L. e Artemia salina Leach Do Óleo Essencial Das Folhas de Myrcia sylvatica (G. Mey.) DC. Rev. Bras. Plantas Med. 2016, 18, 19–26. [Google Scholar] [CrossRef]
- Santana, C.B.; Souza, J.G.L.; Toledo, A.G.; Alves, L.F.A.; Alves, D.S.; Corrêa, J.M.; Pinto, F.G.S. Antimicrobial and Insecticidal Effects of Essential Oil and Plant Extracts of Myrcia Oblongata DC in Pathogenic Bacteria and Alphitobius diaperinus. Braz. J. Biol. 2022, 82, e233425. [Google Scholar] [CrossRef] [PubMed]
- Antonelo, F.A.; Rodrigues, M.S.; Cruz, L.C.; Pagnoncelli, M.G.; da Cunha, M.A.A.; Bonatto, S.J.R.; Busso, C.; Wagner Júnior, A.; Montanher, P.F. Bioactive Compounds Derived from Brazilian Myrtaceae Species: Chemical Composition and Antioxidant, Antimicrobial and Cytotoxic Activities. Biocatal. Agric. Biotechnol. 2023, 48, 102629. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; da Costa, J.S.; Pinto, L.C.; Montenegro, R.C.; Setzer, W.N.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Antioxidant and Cytotoxic Activities of Myrtaceae Essential Oils Rich in Terpenoids From Brazil. Nat. Prod. Commun. 2021, 16, 1934578X21996156. [Google Scholar] [CrossRef]
- Ferreira Macedo, J.G.; Linhares Rangel, J.M.; de Oliveira Santos, M.; Camilo, C.J.; Martins da Costa, J.G.; Maria de Almeida Souza, M. Therapeutic Indications, Chemical Composition and Biological Activity of Native Brazilian Species from Psidium genus (Myrtaceae): A Review. J. Ethnopharmacol. 2021, 278, 114248. [Google Scholar] [CrossRef] [PubMed]
- Anjos da Silva, L.; Santos da Silva, R.; Rodrigues de Oliveira, M.; Guimarães, A.C.; Takeara, R. Chemical Composition and Biological Activities of Essential Oils from Myrtaceae Species Growing in Amazon: An Updated Review. J. Essent. Oil Res. 2023, 35, 103–116. [Google Scholar] [CrossRef]
- Cândido, C.S.; Portella, C.S.A.; Laranjeira, B.J.; da Silva, S.S.; Arriaga, A.M.C.; Santiago, G.M.P.; Gomes, G.A.; Almeida, P.C.; Carvalho, C.B.M. Effects of Myrcia ovata Cambess. Essential Oil on Planktonic Growth of Gastrointestinal Microorganisms and Biofilm Formation of Enterococcus Faecalis. Braz. J. Microbiol. 2010, 41, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in Foods by Using Essential Oils: A Review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cheng, Z. Research Progress on the Use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tan, J.; Yu, Y.; Dong, J.; Cao, L.; Yao, L.; Zhang, Y.; Yan, Z. Phytotoxic Effects and Potential Allelochemicals from Water Extracts of Paulownia tomentosa Flower Litter. Agronomy 2024, 14, 367. [Google Scholar] [CrossRef]
- Souza Filho, A.P.S.; Santos, R.A.; Santos, L.S.; Guilhon, G.M.P.; Santos, A.S.; Arruda, M.S.P.; Muller, A.H.; Arruda, A.C. Potencial Alelopático de Myrcia guianensis. Planta Daninha 2006, 24, 649–656. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M. Indirect Plant Defense against Insect Herbivores: A Review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.; Chomistek, N.; Dayment, H.; Goerzen, W.; Baines, D. Insecticidal Activity of Plant Powders against the Parasitoid, Pteromalus Venustus, and Its Host, the Alfalfa Leafcutting Bee. Insects 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.M.; Serrão, J.E.; dos Santos, M.M.; da Silva, R.S.; de Carvalho, A.G.; Pires, E.M.; Zanuncio, J.C.; Soares, M.A. Insecticidal Plants as Trade Opportunities and Use by Small Vegetable Producers: An Example Using Essential Oils to Control Diaphania hyalinata (Lepidoptera: Crambidae). An. Acad. Bras. Cienc. 2023, 95, e20191305. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.F.M.; Matos, A.P.; Volante, A.C.; Cunha, G.O.S.; Gualtieri, S.C.J. Insecticidal Activity from Leaves and Sesquiterpene Lactones of Tithonia diversifolia (Helms.) A. Gray (Asteraceae) on Spodoptera frugiperda (Lepidoptera: Noctuidae). S. Afr. J. Bot. 2022, 144, 377–379. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Rakotosaona, R.; Nzekoue, F.K.; Canale, A.; Nicoletti, M.; Maggi, F. Insecticidal and Mosquito Repellent Efficacy of the Essential Oils from Stem Bark and Wood of Hazomalania voyronii. J. Ethnopharmacol. 2020, 248, 112333. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, J.; Ma, S.; Gao, L.; Chen, C.; Ji, Z.; Hu, Z.; Shi, B.; Wu, W. Identification and Mechanism of Insecticidal Periplocosides from the Root Bark of Periploca sepium Bunge. Pest Manag. Sci. 2021, 77, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Kerdudo, A.; Ellong, E.N.; Burger, P.; Gonnot, V.; Boyer, L.; Chandre, F.; Adenet, S.; Rochefort, K.; Michel, T.; Fernandez, X. Chemical Composition, Antimicrobial and Insecticidal Activities of Flowers Essential Oils of Alpinia zerumbet (Pers.) B.L.Burtt & R.M.Sm. from Martinique Island. Chem. Biodivers. 2017, 14, e1600344. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Maggi, F.; Nkuimi Wandjou, J.G.; Yvette Fofie, N.G.B.; Koné-Bamba, D.; Sagratini, G.; Vittori, S.; Caprioli, G. Insecticidal Activity of the Essential Oil and Polar Extracts from Ocimum gratissimum Grown in Ivory Coast: Efficacy on Insect Pests and Vectors and Impact on Non-Target Species. Ind. Crops Prod. 2019, 132, 377–385. [Google Scholar] [CrossRef]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal Activity of Essential Oil from Cephalotaxus sinensis and Its Main Components against Various Agricultural Pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Guldbrandsen, N. Screening of Panamanian Plant Extracts for Pesticidal Properties, and HPLC-Based Identification of Active Compounds. Sci. Pharm. 2015, 83, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.L.; Mello, T.R.B.; Sousa, J.P.B.; Albernaz, L.C.; Magalhães, N.M.G.; Morais, L.S.; Francisco, L.R.; Leal, W.S.; Espindola, L.S. Brazilian Cerrado Biome Essential Oils to Control the Arbovirus Vectors Aedes aegypti and Culex quinquefasciatus. Ind. Crops Prod. 2022, 178, 114568. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and Incidence of Type 1 Diabetes in the World: A Systematic Review and Meta-Analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.G.; Vesa, C.M.; Bustea, C.; Purza, A.L.; Tit, D.M.; Brisc, M.C.; Radu, A.-F. Antioxidant and Hypoglycemic Potential of Essential Oils in Diabetes Mellitus and Its Complications. Int. J. Mol. Sci. 2023, 24, 16501. [Google Scholar] [CrossRef] [PubMed]
- Vareda, P.M.P.; Saldanha, L.L.; de Camaforte, N.A.P.; Violato, N.M.; Dokkedal, A.L.; Bosqueiro, J.R. Myrcia bella Leaf Extract Presents Hypoglycemic Activity via PI3k/Akt Insulin Signaling Pathway. Evid. Based Complement. Altern. Med. 2014, 2014, 543606. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Lemos Lima, R.; Kato, L.; Kongstad, K.T.; Staerk, D. Brazilian Insulin Plant as a Bifunctional Food: Dual High-Resolution PTP1B and α-Glucosidase Inhibition Profiling Combined with HPLC-HRMS-SPE-NMR for Identification of Antidiabetic Compounds in Myrcia rubella Cambess. J. Funct. Foods 2018, 45, 444–451. [Google Scholar] [CrossRef]
- da Costa, J.S.; da Freitas, J.J.S.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Variability in the Chemical Composition of Myrcia sylvatica (G. Mey) DC. Essential Oils Growing in the Brazilian Amazon. Molecules 2022, 27, 8975. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, N.; Melo, J.C.S.; Batista, L.F.; Paula-Souza, J.; Fronza, P.; Brandão, M.G.L. Edible Fruits from Brazilian Biodiversity: A Review on Their Sensorial Characteristics versus Bioactivity as Tool to Select Research. Food Res. Int. 2019, 119, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Nazareno, L.S.Q.; de Miranda, M.R.A.; Pinto, M.K.N.A.; Lopes, M.M.A.; Rufino, M.D.S.M. Non-Enzymatic and Enzymatic Antioxidant Components of the Mature Cambuí Metabolism. Acta Agron. 2019, 68, 16–21. [Google Scholar] [CrossRef]
- Matsuda, H.; Nishida, N.; Yoshikawa, M. Antidiabetic Principles of Natural Medicines. V. Aldose Reductase Inhibitors from Myrcia multiflora DC. (2): Structures of Myrciacitrins III, IV, and V. Chem. Pharm. Bull. 2002, 50, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Serpeloni, J.M.; Leal Specian, A.F.; Ribeiro, D.L.; Tuttis, K.; Vilegas, W.; Martínez-López, W.; Dokkedal, A.L.; Saldanha, L.L.; de Syllos Cólus, I.M.; Varanda, E.A. Antimutagenicity and Induction of Antioxidant Defense by Flavonoid Rich Extract of Myrcia bella Cambess. in Normal and Tumor Gastric Cells. J. Ethnopharmacol. 2015, 176, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Ejiofor, E.; Oyedemi, S.; Egedigwe-Ekeleme, C.; Obike, C.; Aguwamba, C.; Nweje-Anyalowu, P.; Ejiofor, M.; Okwuonu, O. Essential Oil Components with Antidiabetic and Anti-Obesity Properties: A Review of Mechanisms of Action and Toxicity. J. Essent. Oil Res. 2023, 35, 335–371. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Y. Antidiabetic Effects of Nerolidol through Promoting Insulin Receptor Signaling in High-Fat Diet and Low Dose Streptozotocin-Induced Type 2 Diabetic Rats. Hum. Exp. Toxicol. 2022, 41, 09603271221126487. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.H.; Sankaranarayanan, C. A-Caryophyllene, a Natural Sesquiterpene, Modulates Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Acta Histochem. 2014, 116, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, L.; Vilegas, W.; Dokkedal, A. Characterization of Flavonoids and Phenolic Acids in Myrcia bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS Combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, V.S.; de Pessoa, A.S.; Tokuhara, C.K.; Pagnan, A.L.; de Oliveira, G.S.N.; Liessa, M.R.S.; Inacio, K.K.; de Melo, F.P.d.S.R.; Dokkedal, A.L.; de Oliveira, R.C.; et al. Evaluation of Myrcia bella in Murine Osteosarcoma Cells: Effect of the Extract and Enriched Fractions of Tannins and Flavonoids. Nat. Prod. Res. 2022, 36, 5823–5827. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.; de Melo, N.C.; Ruiz, A.L.T.G.; Floglio, M.A. Antiproliferative Activity from Five Myrtaceae Essential Oils. Res. Soc. Dev. 2023, 12, e14612340536. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Riva, D.; Simionatto, E.L.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Salvador, M.J. Chemical Composition and Cytotoxic Activity of Essential Oil from Myrcia laruotteana Fruits. J. Essent. Oil Res. 2011, 23, 7–10. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and Biological Effects of Alpha-Bisabolol: An Updated Review of the Molecular Mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.S.; Guimarães, A.G.; Santana, M.T.; Siqueira, R.S.; Passos, L.O.; Machado, S.M.F.; de Ribeiro, A.S.; Sobral, M.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Phytochemical Screening, Antinociceptive and Anti-Inflammatory Effects of the Essential Oil of Myrcia pubiflora in Mice. Rev. Bras. Farmacogn. 2012, 22, 181–188. [Google Scholar] [CrossRef]
- dos Santos, R.H.G.; de Moura Silva, M.H.; de Oliveira Farias de Aguiar, J.C.R.; do Amaral Ferraz Navarro, D.M.; de Oliveira, A.F.M.; dos Santos Correia, M.T. Chemical Composition of the Essential Oil of Myrcia Loranthifolia (Myrtaceae) Leaves Grown in Atlantic Forest and Dry Forest of Brazil. Braz. J. Bot. 2023, 46, 845–852. [Google Scholar] [CrossRef]
- Seethalakshmi, S.; Sarumathi, R.; Sankaranarayanan, C. Effect of Alpha-Phellandrene on Glucose Uptake and Adipogenesis in Insulin Resistant 3T3-L1 Adipocytes: An in Vitro and in Silico Approach. Asian J. Biol. Life Sci. 2023, 12, 603. [Google Scholar] [CrossRef]
- Sheeba, S.; Nahida, T. Effect of Some Naturally Occurring Monoterpenes Viz D-Limonene, p-Cymene and Terpinolene on the Glycemic and Hepatic Function in a Rat Model of Type 2 Diabetes Mellitus. Pharmacogn. Res. 2022, 14, 446–453. [Google Scholar]
- Panigrahy, S.K.; Bhatt, R.; Kumar, A. Targeting Type II Diabetes with Plant Terpenes: The New and Promising Antidiabetic Therapeutics. Biologia 2021, 76, 241–254. [Google Scholar] [CrossRef]


| Myrcia Species | Major Components of EO | Method | (%) Inhibition | References |
|---|---|---|---|---|
| M. tomentosa | γ-elemene (12.52%), germacrene D (11.45%), and (E)-caryophyllene (10.22%) | Trolox equivalent antioxidant capacity (TEAC) by 1,1-diphenyl-2-picrylhydrazyl (DPPH) | 0.333 ± 0.247% (ABTS) and 208.5 ± 0.940% (DPPH) | [3] |
| M. hatschbachii D. Legrand | trans-calamenene (19.10%), (E)-caryophyllene (10.96%), and spathulenol (5.03%) | DPPH scavenging method; phosphomolybdenum complex formation assay | 9.14 ± 0.33% (DPPH) (DPPH) | [42] |
| M. floribunda | α-phellandrene (22.19%), 1,8-cineole (23.30%), terpinolene (22.23%), o-cymene (7.04%), and γ-terpinene (5.87%) | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and DPPH Assay | 53.27 ± 8.27% (ABTS) and 81.91 ± 3.46% (DPPH) | [10] |
| M. sylvatica | (Z)-α-trans-bergamotene (24.57%), α-sinensal (13.44%), (Z)-α-bisabolene (8.33%), α-trans-bergamotene (7.06%), and β-trans-bergamotene (5.07%) | ABTS and DPPH assay | 7.20 ± 0.72% (ABTS) and 80.55 ± 2.00% (DPPH) | [10] |
| M. paivae O.Berg | terpinolene (14.70%), α-phellandrene (14.69%), γ-terpinene (9.64%), sylvestrene (7.62%), α-thujene (6.46%), α-pinene (6.39%) | (ABTS) and (DPPH) assay | 0.886 ± 0.226% (ABTS) and 2.90 ± 0.083% (DPPH) | [7] |
| M. oblongata | not analyzed | free radical reduction method using DPPH radical | 90.18 | [55] |
| M. oblongata | α-pinene (36.81%), β-myrcene (13.99%), silvestrene (11.28%), and β-pinene (9.74%), | DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid)) radical scavenging methods and antioxidant power were assessed by Ferric reducing antioxidant power (FRAP) | 88.08 ± 1.3% (ABTS) and 55.74 ± 2.4% (DPPH) | [56] |
| M. splendens | trans-nerolidol (67.81%), α-bisabolol (17.51%), and β-caryophyllene (4.21%) | DPPH-HPTLC (1,1-diphenyl-2-picrylhydrazil—high performance thin layer chromatography) bioautographic assay and Spectrophotometric DPPH assay | 43,537.00 ± 15% (DPPH) | [48] |
| M. splendens | myrcene (7.9%), α-copaene (4.5%), E-caryophyllene (45.8%), α-humulene (5.0%), and germacrene B (5.9%) | DPPH Radical Scavenging Assay | 28.4 ± 7.1 (DPPH) | [57] |
| M. sylvatica | E-caryophyllene (10.0%), γ-elemene (12.5%), bicyclogermacrene (5.0%), and germacrene B (24.5%) | DPPH Radical Scavenging Assay | 18.5 ± 3.5 (DPPH) | [57] |
| Species | Main Constituents (%Area) | Activity Against | Reference |
|---|---|---|---|
| Nine plants of M. ovata Cambessedes | Geraniol (74.37 to 0.45%) Nerolic acid (73.97 to 6.33%) Geranial (40.10 to 0.10%) 1,8-Cineole (33.03 to 0.75%) Neral (28.39 to 0.11%) Isopulegol (27.50 to 2.30%) (E)-Nerolidol (20.24 to 0.29%) Linalool (19.61 to 0.53%) Citronellal (0 to 9.19%) Iso-isopulegol (0 to 10.29%) | Results of diameters of inhibition zone in mm Gram-positive bacteria Staphylococcus aureus (11–28.5) Bacillus cereus (10–25.5) Bacillus subtilis (13.5–30) Enterococcus faecalis (7.5–22) Gram-negative bacteria Pseudomonas aeruginosa (8–30) Serratia marcensces (6–27) Escherichia coli (8–24) Salmonella enteritidis (6–26.5) | [40] |
| M. hatschbachii D. Legrand | Trans-calamanene (19.10) (E)-Caryophyllene (10.96) Spathulenol (5.03) | Results of MIC in µg/mL Gram-positive bacteria Enterococcus faecalis (500) Staphylococcus aureus (1000) | [42] |
| M. multiflora | (A) α-Bulnesene (26.79%) Pogostol (21.27%) δ-Amorphene (6.76%) (B) (E)-Nerolidol (44.4%) (E)-γ-Bisabolene (10.64%) (E,E)-α-Farnesene (8.19%) (C) (E)-Nerolidol (92.21%) | Results of diameters of inhibition zone in mm Leveduras (A–B–C) Candida albicans (8–9–7) C. tropicalis (8–11–8) C. famata (6–10–8) C. krusei (9–10–8) C. auris (9–10–8) | [4] |
| M. oblongata DC | Uninformed | Results of MIC/MBC in µg/mL Gram-negative bacteria Salmonella Albany (3500/7000) S. braenderup (3500/7000) S. gafsa (1750/3500) S. heidelberg (3500/7000) S. idikan (1750/3500) S. Lexington (437.5/3500) S. livingstone (3500/3500) S. montevideo (3500/-) S. saintpaul (875/1750) S. senftenberg (3500/7000) | [55] |
| M. ovata | Geranial (50.4%) Neral (35.8%) 1,8-Cineole (4.8%) α-Terpineol (1.1%) | Results of diameter of inhibition zone in mm/MIC (%)/MBC (%) Gram-positive bacteria Enterococcus faecalis (36/0.031/0.031) Staphylococcus aureus (23/0.25/−0.5) Streptococcus pneumoniae (25/0.0625/0.25) Gram-negative bacteria Escherichia coli (21/1/1) Helicobacter pylori (0/0/0) Pseudomonas aeruginosa (16/>1/>1) Salmonella choleraesuis (26/0.5/0.5) Salmonella choleraesuis (26/0.5/0.5) Yeast Candida parapsilosis (30/0.031/-) | [60] |
| M. splendens (Sw.) DC. (sin. M. fallax (Rich.) DC.) | Trans-nerolidol (67.81%) α-Bisabolol (17.51%) | Results of MIC in µg/mL Gram-positive bacteria Clavibacter michiganensis subsp. nebraskensis (125) Enterococcus faecalis (2000) Listeria grayi (1000) Staphylococcus aureus (1000) Staphylococcus epidermidis (1000) Gram-negative bacteria Agrobacterium tumefaciens (500) Agrobacterium vitis (2000) Pseudomonas syringae pv. syringae (250) Escherichia coli (>2000) Pseudomonas aeruginosa (>2000) | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, E.d.J.B.; Bezerra, F.W.F.; da Silva, L.R.R.; da Silva, M.P.; Ferreira, O.O.; da Silva Martins, L.H.; de Jesus Chaves-Neto, A.M.; de Santana Botelho, A.; Kumar, R.; Bargali, P.; et al. Exploring the Potential of Myrcia Genus Essential Oils: A Review of Biological Activities and Recent Advances. Molecules 2024, 29, 2720. https://doi.org/10.3390/molecules29122720
dos Santos EdJB, Bezerra FWF, da Silva LRR, da Silva MP, Ferreira OO, da Silva Martins LH, de Jesus Chaves-Neto AM, de Santana Botelho A, Kumar R, Bargali P, et al. Exploring the Potential of Myrcia Genus Essential Oils: A Review of Biological Activities and Recent Advances. Molecules. 2024; 29(12):2720. https://doi.org/10.3390/molecules29122720
Chicago/Turabian Styledos Santos, Eliza de Jesus Barros, Fernanda Wariss Figueiredo Bezerra, Luiz Renan Ramos da Silva, Marcilene Paiva da Silva, Oberdan Oliveira Ferreira, Luiza Helena da Silva Martins, Antônio Maia de Jesus Chaves-Neto, Anderson de Santana Botelho, Ravendra Kumar, Pooja Bargali, and et al. 2024. "Exploring the Potential of Myrcia Genus Essential Oils: A Review of Biological Activities and Recent Advances" Molecules 29, no. 12: 2720. https://doi.org/10.3390/molecules29122720
APA Styledos Santos, E. d. J. B., Bezerra, F. W. F., da Silva, L. R. R., da Silva, M. P., Ferreira, O. O., da Silva Martins, L. H., de Jesus Chaves-Neto, A. M., de Santana Botelho, A., Kumar, R., Bargali, P., do Socorro de Souza Vilhena, K., de Aguiar Andrade, E. H., & de Oliveira, M. S. (2024). Exploring the Potential of Myrcia Genus Essential Oils: A Review of Biological Activities and Recent Advances. Molecules, 29(12), 2720. https://doi.org/10.3390/molecules29122720













