Abstract
Daucus capillifolius Gilli is a rare annual wild herb grown in Libya. It belongs to the Apiaceae family, which is one of the largest flowering plant families. Plants of this family are outstanding sources of various secondary metabolites with various biological activities. A UPLC-ESI-MS/MS analysis of different extracts of in vivo and in vitro tissues of Daucus capillifolius together with the fruit extract of the cultivated plant in both ionization modes was carried out for the first time in the current study. Our results reveal the tentative identification of eighty-seven compounds in the tested extracts, including thirty-two phenolic acids and their derivatives; thirty-seven flavonoid glycosides and aglycones of apigenin, luteolin, diosmetin, myricetin and quercetin, containing glucose, rhamnose, pentose and/or glucuronic acid molecules; seven anthocyanins; six tannins; three acetylenic compounds; and three nitrogenous compounds. The tentative identification of the above compounds was based on the comparison of their retention times and ESI-MS/MS fragmentation patterns with those previously reported in the literature. For this Apiaceae plant, our results confirm the presence of a wide array of secondary metabolites with reported biological activities. This study is among the first ones to shed light on the phytoconstituents of this rare plant.
1. Introduction
Recently, great attention has been paid to the chemical and biological investigation of native medicinal plants, which constitute a gold mine of phytoconstituents with exceptional biological activities and represent an essential source of novel bioactive drugs. The extraction and isolation of targeted, safe and potent antimicrobial natural drugs is becoming of vital importance to control the microbial resistance to reported chemically synthesized drugs and food deterioration resulting from fungal or bacterial infections. North Africa is still a very rich source of untapped medicinal plants that are undergoing extensive screening for novel natural drug discovery [1].
The Apiaceae family (previously Umbelliferae), commonly referred to as carrot or parsley family, comprises approximately 3780 species and 434 genera distributed in temperate zones. It includes various herbs and vegetables of variable medicinal and economical importance [2,3]. Notably, plants of this family are a rich source of specialized secondary metabolites (furanocoumarins, sesquiterpene lactones and sesquiterpene coumarins) [4] with various biological activities, such as antimicrobial, anti-cancer, and cyclooxygenase inhibitory activities [5]. Moreover, these plants are distinguished for their diverse uses, serving purposes in food, flavorings (spices and condiments), decorative applications and medicinal practices and contributing significantly to the food, fragrance, pharmaceutical, cosmetic and cosmeceutical industries [3,6,7,8,9,10,11].
Numerous societies, including the ancient Egyptian, Mexican, Indian and Chinese, were accustomed to Apiaceae plants. Plants of this family also contain a mixture of aliphatic C17 polyacetylenes, including falcarinol, the most bioactive polyacetylene present in this family. Falcarinol has cytotoxic activity against human gastric adenocarcinoma, as well as other possible beneficial effects, such as anti-inflammatory and anticoagulant properties. In addition, polyacetylenes are well-known antifungal compounds [2,12].
In addition, flavonoids and anthocyanins are commonly found in the Apiaceae family and exhibit a diverse range of biological activities. Flavonoids are renowned for their protective role in treating conditions such as carcinogenesis, inflammation, and atherosclerosis. They also exhibit diverse properties, including antiviral, antimicrobial, antihepatotoxic, antiosteoporotic, antiulcer, immunomodulatory, antiproliferative and high antioxidant capacity [13]. Recently, it was reported that Petroselinum crispum from Apiaceae showed significant antioxidant and estrogenic activities due to the presence of phytoestrogens like diosmetin and apigenin [14]. Similarly, anthocyanins demonstrate a diverse array of biological activities encompassing antioxidant, anti-inflammatory, antimicrobial and anti-carcinogenic effects.
Additionally, they play a role in enhancing vision, inducing apoptosis, providing neuroprotection, and influencing blood vessels and platelets, which may potentially reduce the risk of coronary heart disease [15]. Genus Daucus (Apiaceae) contains about 60 species distributed mainly in Africa, West Asia, and Europe, and a few species were found in Australia and North America [16,17]. It is represented in Libya by nine species, including D. durieua, D. muricatus, D. carota, D. guttatus, D. capillifolius, D. jordanicus, D. littorals, D. syrticus and D. sahariensis [18]
Chemically, Daucus is one of the richest sources of volatile oil, sterols and triterpenes, polyacetylenic compounds, flavonoids and sesquiterpene lactones. Biologically, the volatile oil of many species of this genus showed a wide range of important pharmacological activities, such as antioxidant, cytotoxicity, insecticidal, antimicrobial and anti-inflammatory activities [19]. Daucus capillifolius Gilli from Libya is a rare annual wild herb with an erect and smooth stem which reaches about 50 cm in length [18]
Based on the available literature, nothing has been reported on the phytochemical constituents of this plant, except our previous work, which investigated the micropropagation and callus culture of this endangered plant in addition to the GC-MS analysis of its essential-oil constituents [19]. The current study aimed to conduct a comparative phytochemical investigation of the methanolic extracts of in vivo (cultivated fruit) and in vitro tissues (calli grown on different media with various hormonal combinations) of this rare plant by using UPLC-ESI-MS/MS analysis in both ionization modes.
2. Results and Discussion
The methanolic extracts of in vivo (the fruit extract of the cultivated plant) and in vitro tissues of D. Capillifolius Gilli (Figure 1) were analyzed by using UPLC-ESI-MS/MS. The analysis revealed the tentative identification of 87 different phenolic and non-phenolic compounds (Figure 2).
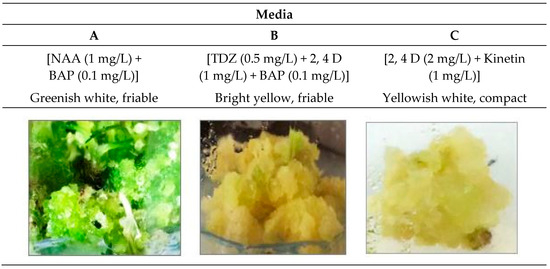
Figure 1.
Morphological characters of callus produced from leaf explants of D. capillifolius Gilli on different culture media (media A, B &C) after 40 days of cultivation.
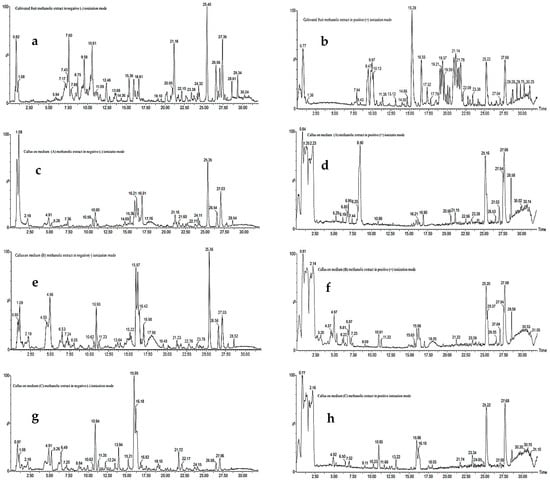
Figure 2.
LC-MS total ion chromatograms (TICs) of methanolic extracts of Daucus capillifolius Gilli cultivated fruits in negative ionization mode (a,c,e,g) and positive ionization mode (b,d,f,h).
2.1. Identification of Phenolic Compounds of Methanol Extracts of In Vivo and In Vitro Tissues of D. capillifoliusby UPLC–ESI-MS/MS
Eighty-one compounds were tentatively identified for the first time in the methanol extracts of in vivo and in vitro tissues of Daucus capillifolius fruits. The results are illustrated in Table 1, Table 2 and Table 3.
2.1.1. Identification of Phenolic Acids and Acid Derivatives in Methanol Extracts of In Vivo and In Vitro Tissues of D. capillifolius Gilli
As shown in Table 1, thirty-two phenolic acids and their derivatives were identified in methanol extracts of in vivo and in vitro tissues of D. capillifolius Gilli as follows.
Compound 1 was suggested to be malic acid due to the presence of the molecular ion peak at m/z of 133 [M + H]+ [20].
Compounds 2 and 25 showed the same pseudo-molecular ion peak at m/z of 279 [M-H]− and were suggested to be benzoic acid and coumaric acid derivatives, respectively. This suggestion was based on the presence of MS2 base peak fragment ions at m/z of 121.0 and 162.6, respectively [21].
Compound 3 exhibited a deprotonated molecular ion peak at m/z of 329. Based on the presence of one main fragment in the MS2 spectrum at m/z of 131.1 [pentose-H], which resulted from a neutral loss of the syringic acid molecule [M-H−198]−, it was identified as syringic acid pentoside, which has not been reported in Daucus species [22].
Compound 4 had a precursor ion at m/z of 327 [2M-H]− and a protonated molecular ion at m/z of 165. In negative ESI mode, the MS2 showed a base peak fragment ion at m/z of 163.7 for [M-H]−, so it was identified as coumaric acid [23]. Coumaric acid was previously isolated from the genus Daucus [24].
Compounds 5 and 7 had deprotonated molecular ions at m/z of 137 and an MS2 base fragment ion at m/z of 92.9. Based on the mass fragmentation and the low retention time, as well as previously published reports, these compounds were tentatively identified as hydroxybenzoic acid, which was previously detected in Daucus [25,26].
Compounds 6 and 9 were identified as gallic acid derivatives according to the LC-MS1 and MS2 data reported in Table 1. Similarly, compounds 28 and 31 were tentatively identified as benzoic acid and quinic acid derivatives, respectively [21].
Compound 8 had a molecular ion fragment at m/z of 179 [M-H]− and an MS2 base fragment ion at m/z of 135.2 [M-H-COOH] (Figure 3). Based on mass fragmentation, as well as previously reported data [25], compound 8 was identified as caffeic acid. It was previously isolated from the genus Daucus [24].
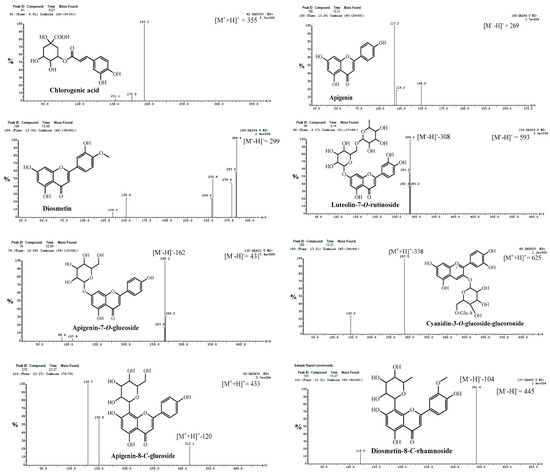
Figure 3.
Fragmentation pattern mass spectra of some identified phenolic compounds from methanolic extracts of cultivated fruits and calli grown on different media of Daucus capillifolius Gilliin in positive (+) and negative (−) ionization modes.
Compound 10 showed a deprotonated molecular ion peak at m/z of 279 [M-H]−. It was suggested to be a vanillic acid derivative based on the presence of the MS2 base peak fragment ion at m/z of 167.4 (Table 1) [22].
Compounds 11, 17 and 29 had deprotonated and protonated molecular ions at m/z of 353 and 355, respectively (Figure 3). They had MS2 fragment ions at m/z of 191.3 with a relative abundance of 100%. This fragmentation pattern was found to be consistent with previous findings on chlorogenic acid reported by [25]. Notably, chlorogenic acid has been detected in Daucus species, as mentioned by [26].
Compound 12 with a molecular ion compound at m/z of 329 [M−-H]− was tentatively identified as a cinnamic acid derivative. The HPLC-ESI-MS spectra of this compound showed an MS2 base peak fragment ion at m/z of 146.6 for the cinnamic acid moiety after the loss of 182 amu [22].

Table 1.
Phenolic compounds tentatively identified in in vivo and in vitro tissues of D. capillifolius Gilli by using UPLC-ESI-MS/MS analysis in positive (+) and negative (−) ionization modes.
Table 1.
Phenolic compounds tentatively identified in in vivo and in vitro tissues of D. capillifolius Gilli by using UPLC-ESI-MS/MS analysis in positive (+) and negative (−) ionization modes.
| No. | Rt (min) | Name | Parent Ion (m/z) | MS2 Fragments (m/z) | I | II | III | IV | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.87 | Malic acid | 133 | − | − | 4.92 | − | [20] | |
| 2 | 1.92 | Benzoic acid derivative | 279 | 121.0 (100%) | − | + | − | − | [21] |
| 3 | 1.96 | Syringic acid pentoside | 329 | 131.1 (100%) | + | − | + | + | [22] |
| 4 | 2.88 | Coumaric acid | 327/165 | 163.7 (100%) | + | − | − | − | [23,24] |
| 5 | 2.95 | Hydroxy benzoic acid isomer-1 | 137 | 121.0, 92.9 (100%) | − | + | − | − | [25,26] |
| 6 | 3.59 | gallic acid derivative | 293 | 171.0 (100%) | − | + | − | − | [21] |
| 7 | 4.91 | Hydroxy benzoic acid isomer-2 | 137 | 94 [M+H−44], 77 [M+H−44-OH] | − | 2.41 | 4.01 | 6.13 | [25,26] |
| 8 | 5.21 | Caffeic acid | 179 | 135.2 (100%) [M-H-COOH] | + | − | − | + | [24,25] |
| 9 | 5.41 | Gallic acid derivative | 259 | 169.0 | − | + | − | − | [21] |
| 10 | 5.82 | Vanillic acid derivative | 279 | 167.4 (100%) | + | − | − | − | [22] |
| 11 | 6.15 | Chlorogenic acid isomer-1 | 353/355 | 191.3 (100%),137.3 | + | − | − | + | [25,26] |
| 12 | 6.43 | Cinnamic acid derivative | 329 | 146.6 (100%) [M−182]− | + | − | − | − | [22] |
| 13 | 6.80 | Caffeic acid derivative | 371 | 178.9 (100%) | + | − | − | − | [24] |
| 14 | 7.59 | Quinic acid derivative | 271 | 191.0 100%) | − | + | − | − | [22] |
| 15 | 7.69 | Ferulic acid derivative | 273 | 192.7 (100%), 148.7 99 | + | − | − | − | [24] |
| 16 | 7.88 | Hydroxyl gallic acid | 185 | 1.99 | − | − | − | [27] | |
| 17 | 8.50 | Chlorogenic acid isomer 2 | 355 | − | 9.29 | − | − | [25,26] | |
| 18 | 8.22 | Caffeic acid derivative (malonyl rhamnoside) | 367 | 135.0 (100%) [M-H−232]− | + | − | − | + | [24] |
| 19 | 9.56 | Ellagic acid | 303 | 257.0, 229, 201.2, 164.9, 153.1, (100%)137.0, 123.0, 108.4 | + | − | − | − | [28] |
| 20 | 9.81 | Quinic acid | 193 | 119.0, 105.1, 91.0, 79 | + | − | − | − | [28,29] |
| 21 | 9.97 | Sinapic acid isomer 1 | 225 | 6.35 | − | − | − | [30] | |
| 22 | 10.13 | Sinapicacid isomer-2 | 225 | 2.21 | − | − | − | [30] | |
| 23 | 10.93 | Methyl gallate isomer 1 | 185 | 171, 125 | − | − | 1.81 | − | [20] |
| 24 | 10.91 | Methyl gallate isomer 2 | 185 | 171, 125 | − | − | − | 0.67 | [20] |
| 25 | 12.86 | Coumaric acid derivative | 279 | 162.6, 121.6 (100%) | − | + | − | − | [21] |
| 26 | 13.95 | Coumaric acid | 163 | − | − | 2.95 | − | [21] | |
| 27 | 15.29 | Hydroxy ferulic acid | 209 | 11.4 | − | − | − | [31] | |
| 28 | 15.92 | Benzoic acid derivative | 307 | 120.8 (100%) | + | − | − | + | [21] |
| 29 | 16.21 | Chlorogenic acid isomer-3 | 353 | − | − | 9.69 | − | [25,26] | |
| 30 | 16.55 | Benzoic acid methyl ester | 137 | 4.14 | − | − | − | [21] | |
| 31 | 21.44 | Quinic acid derivative | 371 | 191.0 (100%) | − | + | − | − | [21] |
| 32 | 22.31 | Coumaric acid glucuronide | 339 | 163.0 | − | 0.18 | − | − | [21] |

Table 2.
Tentatively identified flavonoid compounds from the in vivo and in vitro tissues of D. capillifolius Gilli by using UPLC-ESI-MS/MS analysis in positive (+) and negative (−) ionization modes. (+) present, (−) absent.
Table 2.
Tentatively identified flavonoid compounds from the in vivo and in vitro tissues of D. capillifolius Gilli by using UPLC-ESI-MS/MS analysis in positive (+) and negative (−) ionization modes. (+) present, (−) absent.
| No. | Rt (min) | Name | Parent Ion (m/z) | MS2 Fragments (m/z) | I | II | III | IV | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Flavonoid aglycones | |||||||||
| 33 | 13.56 | Diosmetin | 299/301 | -ve/284.9 (100%) [M-CH3], 255.6 [M−15-CO]; or +ve/286.1, 258.0 (100%), 168.4, 146.9, 135, 130.0 | + | − | − | − | [32] |
| 34 | 11.77 | Dimethoxyflavone | 281 | 149.3 (100%) [ring A], 132.0 [ring B] 149(A)/132(B) | + | − | − | − | [33] |
| 35 | 12.07 | Luteolin | 285 | 175.1, 132.8 (100%) | + | + | − | + | [34,35] |
| 36 | 13.21 | Apigenin | 269 | 148.9, 119.2, 117.2 (100%) | + | − | − | − | [34] |
| 37 | 16.20 | Galangin | 269/271 | 119.9 (100%), 152.9 (100%), 118.9 | + | + | − | − | [25,36] |
| 38 | 16.20 | 5,4′-dihydroxy-3,7-dimethoxyflavone | 313 | 283.0 [M-H−30], 254.8 (100%) [M-H−30−28] | + | + | − | − | [33] |
| 39 | 16.42 | Methyl apigenin (Acacetin) | 283 | 268.0 (100%) [M-H-CH3] | + | + | − | − | [33,37] |
| 40 | 16.51 | 5-hydroxy-3′,4′,7-trimethoxy-flavanone | 329 | 314, 299 | − | 2.51 | − | 3.11 | [37] |
| 41 | 17.32 | Isorhamnetin | 317 | 1.25 | − | − | − | [20] | |
| 42 | 22.92 | Dihydroxyflavone(chrysin) | 255 | 135.0, 119.0(100%) | − | + | − | − | [33] |
| Flavonoid-O-glycosides | |||||||||
| 43 | 1.76 | Diosmetin-7-O-glucuronopyranosyl-O-rhamnoside | 621 | 445.0 (100%) [M-H−176] | + | + | − | − | [38] |
| 44 | 6.75 | Myricetin-3-O-glucoside | 479 | 317.0, 183.7, 161.1, 159.0 | + | + | − | − | [39] |
| 45 | 6.80 | Myricetin-3-O- acetyl glucoside | 491 | 317.1 (100%) | − | + | + | [40] | |
| 46 | 8.15 | Quercetin-3-O-acetyl glucoside pentoside | 639 | 303.0 (100%) | + | − | − | − | [41] |
| 47 | 8.50 | Quercetin diglucoside | 625 | 301 | 1.08 | − | − | − | [20] |
| 48 | 8.75 | Quercetin glucoside | 463 | 301 | 1.83 | − | − | − | [20] |
| 49 | 9.14 | Luteolin-7-O-rutinoside | 593 | 285.2, 284.2 (100%) | + | − | − | − | [26,42] |
| 50 | 9.25 | Quercetin-O-rhamnoside | 447 | 301 | 0.89 | − | − | − | [20] |
| 51 | 9.43 | Apigenin-7-O-caffoeylhexoside | 593 | 269.4 (100%) | + | − | − | − | [43] |
| 52 | 9.43 | Diosmetin-O-coumaroyhexoside | 607 | 299.0 (100%), 163, 131.1 | + | − | − | + | [44] |
| 53 | 9.58 | Quercetin-3-O-galactoside | 463 | 300.6 (100%), 178.9 | 3.03 | − | − | − | [24] |
| 54 | 10.04 | Apigenin-7-O-glucoside | 431/433 | 268.0, 269.0 (100%), 108.0 | + | + | − | + | [45] |
| 55 | 10.14 | Luteolin-7-O-glucoside | 447 | 285.0 (100%) | + | + | − | − | [26,42] |
| 56 | 10.32 | Luteolin-7-O-glucuronoside | 461 | 284.5, 283.3 (100%) | + | + | − | + | [26,32] |
| 57 | 10.26 | Apigenin-7-O-glucoside | 431 | 270 | 0.77 | − | − | − | [45] |
| 58 | 10.40 | Quercetin-O- rhamnoside | 447 | 299.0 | 2.02 | − | − | [20] | |
| 59 | 12.85 | Diosmetin-O-rutinoside | 609 | 2.22 | − | − | − | [46] | |
| 60 | 13.27 | Diosmetin-7-O-hexoside | 461 | 299.3 (100%), 284.5 | + | − | − | + | [47] |
| 61 | 13.38 | Luteolin derivative | 567 | 285 (100%) | + | − | − | − | [34,35] |
| 62 | 15.36 | Luteolin acetyl glucoside | 489 | 285 | − | 1.12 | − | − | [34,35] |
| 63 | 19.44 | Diosmetin-7-O-rutinoside | 609 | 300.9, 206.3 (100%), 157.4 | − | − | − | − | [46] |
| 64 | 21.91 | Myricetin-3-O-rhamnoside | 463 | 316.6 (100%) | + | + | + | + | [20] |
| Flavonoid-C-glycosides | |||||||||
| 65 | 13.27 | Diosmetin-8-C-rhamnoside | 445 | 341 [M−104]− | + | − | − | + | [35,46] |
| 66 | 23.27 | Apigenin-8-C-hexoside | 433 | 313.1 [M+H−120] 150.6, 130.7 (100%) | − | + | + | + | [48] |
| 67 | 23.39 | Diosmetin-8-C-glucoside | 461 | 341.0 (M-H−120) (100%) | + | + | − | + | [46] |
| 68 | 24.49 | Diosmetin-8-C-glucoside-O-rhamnoside | 609 | 489.2 [M+H−120], 462.5 [M+H−146], 341.9 [aglycone+H+41] | − | + | + | − | [48] |

Table 3.
Tentatively identified anthocyanins, tannins, and acetylenic and nitrogenous compounds from the in vivo and in vitro tissues of D. capillifolius Gilli by using UPLC-ESI-MS/MS analysis in positive (+) and negative (−) ionization modes. (+) present, (−) absent.
Table 3.
Tentatively identified anthocyanins, tannins, and acetylenic and nitrogenous compounds from the in vivo and in vitro tissues of D. capillifolius Gilli by using UPLC-ESI-MS/MS analysis in positive (+) and negative (−) ionization modes. (+) present, (−) absent.
| No. | Rt (min) | Name | Parent Ion (m/z) | MS2 Fragments (m/z) | I | II | III | IV | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Anthocyanins | |||||||||
| 69 | 6.36 | Pelargonidin-3-O-glucuronosyl-O-glucoside | 610 | 271.3 | + | − | − | − | [49,50] |
| 70 | 10.51 | Cyanidin-3-O-glucoside | 449 | 287.0 | 0.80 | + | + | + | [51,52] |
| 71 | 10.60 | Cyanidin-3-O-glucoside | 449 | 287.0 | 5.78 | − | − | − | [51,52] |
| 72 | 12.90 | Cyanidin derivative | 620 | 287(100%) | + | − | + | + | [49] |
| 73 | 13.21 | Cyanidin-O-glucuronosyl-O-glucoside Or feruloyl-O-glucoside | 625 | 287.0 (100%) | + | − | − | + | [49,52] |
| 74 | 16.95 | Cyanidin derivative | 721 | 287.4 (100%) | + | + | + | + | [49,52,53] |
| 75 | 28.31 | Malvidin-3-O-glucoside-malonyl-glucoside | 741 | 331.4 [M−410] | + | + | − | + | [54] |
| Tannins | |||||||||
| 76 | 7.17 | Gallocatechin | 305 | 261, 119, 97.0 (100%) | 2.55 | − | − | − | [55] |
| 77 | 7.59 | Epigallocatechin | 305/307 | 261, 119, 97.0 (100%) | 5.31 | − | − | − | [55] |
| 78 | 7.68 | Epigallocotechin derivatives | 721 | 304.7 (100%) | + | − | − | − | [55] |
| 79 | 11.25 | Catechin-3-O-hexoside-pentoside | 585 | 294 [M−291] | − | − | + | − | [55] |
| 80 | 14.54 | Catechin-O-acetyl glucoside pentoside | 625 | 288.5 (100%) | + | − | − | − | [50] |
| 81 | 16.07 | Catechin | 291 | 174.9, 147.3, 137.3, 121, 106.9 | − | − | − | − | [50] |
| Acetylenic compounds | |||||||||
| 82 | 20.85 | 9-[Heptadeca-1-en-4,6,9-triyne-3,8-diol] | 281 | 57.3, 56.7, 43.2, 54.9, 66.7, 79.0, 81.1, 95.0, 109.2, 83.1, 122.8, 71.0(100%) | + | + | + | − | [12,56,57] |
| 83 | 23.45 | 11-[8-hydroxytetradeca-1-en-4,6,9-triyn-3-yl acetate] | 281 | 97.0, 56.9, 80.9, 69.2, 95.4 106.6, 107.2 146.8 55.0 (100%) | + | + | + | − | [12,56,57] |
| 84 | 28.27 | Falcaridiol-8-O-methyl ether | 297 | 167.3, 149.4, 134.9, 121.1, 106.8, 105, 97.0, 83.1 ,81.0, 69.1, 55.0 (100%), 42.8 | + | + | + | − | [12,56,57] |
| Nitrogenous compounds | |||||||||
| 85 | 1.35 | 3-Methyl-Indole | 132 | 76.0 | − | +11 | 18.55 | 17.6 | [58] |
| 86 | 1.77 | 4-(aminoethyl) benzoic acid | /166 or 120 | 119.9, 103.0 (100%), 93.0, 91.0, 76.9 | − | +12 | 22.67 | + | [59] |
| 87 | 2.23 | 4-(aminoethyl) benzoic acid isomer | 164/166 | 119.9, 103.0 (100%), 93.0, 91.0, 76.9 | − | +20.9 | 1.82 | 21.57 | [59] |
I: methanol extract of cultivated fruit; II: methanol extract of callus grown on medium A; III: methanol extract of callus grown on medium B; IV: methanol extract of callus grown on medium C. (+) present, (−) absent.
Compounds 13 and 18 were readily detected at m/z of 371 and 367 [M-H]−, respectively. Based on the MS2 data in Table 1, they were tentatively identified as caffeic acid derivatives, as they produced the MS2 base peak fragment ions at m/z of 178.9 [M-H−192]− and 135.0 [M-H−232]− (possibly malonyl rhamnoside), respectively. Several caffeic acid derivatives have been previously reported in the genus Daucus [41].
Compounds 14 had ESI-MS with a deprotonated molecular ion at m/z of 271, which fragmented in MS2 to produce a base peak fragment ion at m/z of 191.0 which was identified as quinic acid derivative [22].
Compound 15 (Rt7.69 min), a ferulic acid derivative, was determined with MS1 [M-H]− at m/z of 273 and an MS2 base fragment ion at m/z of 192.7. Several ferulic acid derivatives were detected in the genus Daucus [24].
Compound 16 with molecular ion peak at m/z of 185 [M-H]− was tentatively identified as hydroxy gallic acid [27].
Compound 19 showed a molecular ion peak at m/z of 303 [M+H]+ and was tentatively identified as ellagic acid. The MS2 showed typical fragmentation of ellagic acid at m/z of 257.0, 229.0, 201.2 and 164.9 and a base peak fragment at 153.1 [20,28]. Ellagic acid has gained a lot of interest due to its anti-inflammatory, antitumor antibacterial and liver protection effects [60].
Compound 20 had a protonated molecular ion fragment at m/z of 193 and gave an MS2 fragment at m/z of 119.0. This compound was identified as quinic acid [28]. Notably, it was previously reported in the genus Daucus [24].
Compounds 21 and 22 were identified as sinapic acid isomers 1 and 2, based on the presence of the molecular ion peak at m/z of 225 [30].
Compounds 23 and 24 were identified as isomers 1 and 2 of methyl gallic acid based on the molecular ion peak at m/z of 185 as reported by [20]. Meanwhile, compound 26 (Rt of 13.95) was identified as coumaric acid, supported by the presence of a molecular ion peak at m/z of 163 [M-H]− as reported by [21].
Compound 27 was tentatively identified as hydroxy ferulic acid according to [31] and was detected from the ion fragment at m/z of 209 [M-H]−. Further, compound 30 was identified as benzoic acid methyl ester after the detection of the ion fragment at m/z of 137 [M-H]− according to [21].
Finally, compound 32 was identified as coumaric acid glucuronide based on the presence of a molecular ion fragment at m/z of 339 [M-H]− and the fragment ion at 163 comprising coumaric acid after the loss of the glucuronic acid moiety [M-H−176]− [21].
2.1.2. Identification of Flavonoids
Flavonoids Aglycones
Ten flavonoid aglycones were identified in methanol extracts of in vivo and in vitro tissues, as well as in the fruit extract of the cultivated plant Daucus capillifolius, as described in the following.
Compound 33 (Rt of 13.56 min) was identified as diosmetin from the ESI-MS spectrum, which showed deprotonated and protonated molecular ions at m/z of 299 and 301, respectively. The ESI-MS/MS fragmentation pattern showed fragment ions at m/z of 284.9 (100%) [M+-H-CH3] and 255.6 [M+-CH3-CO], in addition to the fragment ions mentioned in Table 3, which are characteristic of fragmentation for diosmetin [32]. Flavones such as diosmetin and apigenin were reported in Petroselinum crispum, Apiaceae. It was used in menstrual disorders treatment due its phytoestrogen content [14]
Compound 34 produced a mass spectrum [M+-H] at m/z of 281. It was identified as 5,4-dimethoxyflavone through MS2 fragment ions at m/z of 149.3 (100%) and 132.0, which are characteristic for ring A and ring B, respectively, each with one methoxy group [33].
Compound 35 (Rt of 12.07 min) was identified as luteolin based on the [M+-H] at m/z of 285 and the MS2 fragmentation pattern presented in Table 2, as reported by [34]. It is worth noting that luteolin has been previously isolated from Daucus species [61].
Compounds 36 and 37 were identified as apigenin and galangin, respectively, from the fragmentation pattern in both positive and negative modes (Figure 4) of ESI-MS/MS, as shown in Table 2 [25,34,36].
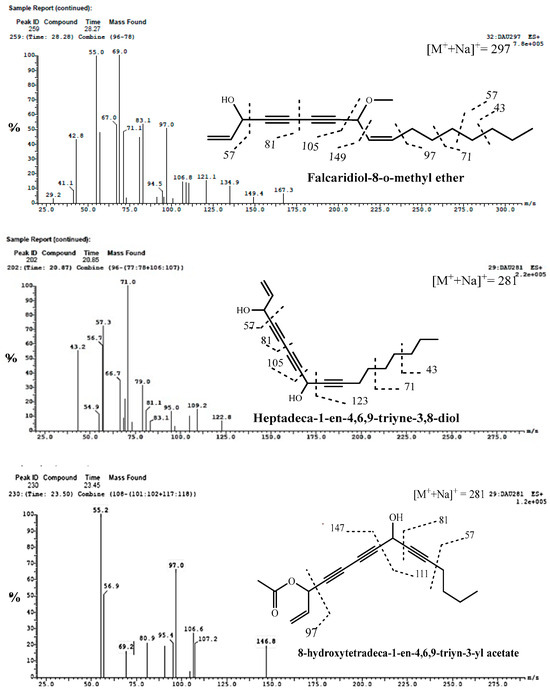
Figure 4.
Fragmentation pattern mass spectra of some identified acetylenic compounds from methanolic extracts of cultivated fruits and calli grown on different media of Daucus capillifolius Gilliin in positive (+) and negative (−) ionization modes.
Compound 38 was identified as 5,4′-dihydroxy-3,7-dimethoxyflavone according to the comparison with previous studies [33]. It had a molecular ion at m/z of 313 [M+-H]−, which produced MS2 fragment ions at m/z of 283.0 [M+−30 (OCH3)]− and 254.8 [M+-H−30−28 (OCH3+CO)] (100%) [33].
Regarding compound 39, its ESI-MS spectrum (Figure 5) showed a molecular ion compound at m/z of 283 [M−H]− with an MS2 fragment ion at m/z of 268.0 [M-H−15]−. Therefore, the compound was identified as apigenin-4′-methyl ether (acacetin) [33,37].
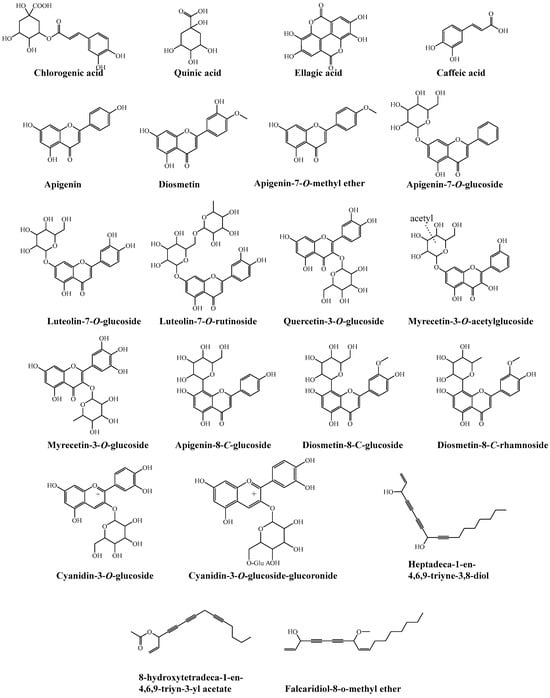
Figure 5.
Chemical structures of the tentatively identified compounds from methanolic extracts of cultivated fruits and calli grown on different media of D. capillifolius Gilli.
Compound 40 showed an [M+-H]− ion at m/z of 329, with the production of daughter ions at m/z of 314 and m/z of 299, indicating the loss of two methyl groups from the parent 329 ion. Therefore, it was tentatively identified as 5-hydroxy-3′,4′,7-trimethoxy-flavanone [33,37].
Compound 41 was tentatively identified as isorhamnetin, as the MS/MS spectrum of this compound showed the characteristic product ion at m/z of 317 [M+−H] [33,37].
Compound 42 (Rt of 22.92 min) was identified as a dihydroxy flavone from its ESI-MS spectrum with [M++H] at m/z of 255. MS2 fragmentation showed the presence of one hydroxyl group in both of rings A and B, where it showed a base compound fragment ion at m/z of 119.0 [M++H−136] and a fragment at m/z of 135.0 [33].
Identification of Flavonoid Glycosides
- Identification of O-glycosides
Twenty-two flavone or flavonol-O-glycosides were identified in methanol extracts of in vivo and in vitro tissues of D. capillifolius, as described below.
Compound 43 was identified asdiosmetin-7-O-glucuronopyranosyl-O-rhamnoside according to the ESI-MS data reported in Table 2, which show a pseudo-molecular ion at m/z of 621 [M+-H]− and an MS2 fragment ion at m/z of 445.0 [diosmetin-7-O-rhamnoside-H], which shows the loss of 176 amu [38].
Compound 44 exhibited a molecular ion compound at m/z of 479 [M-H]−. The fragment ions in MS2 at m/z of 317.0 [M-H−162]− showed the loss of a hexose moiety. Additionally, other fragment ions at m/z of 161.1 and 159.0 were observed. Based on these findings, this compound was identified as myricetin-3-O-glucoside, as depicted in Figure 5 [39].
Compound 45 presented a molecular ion at m/z of 491 [M-H]−. The MS data show a base fragment signal at m/z of 317.1 [M-H−204]−, indicating the loss of acetyl hexoside. From these results, compound 45 was tentatively identified as myricetin-3-O-acetylglucoside [40].
Compound 46 was identified as quercetin acetyl glucoside pentoside based on the [M+H]+ ion at m/z of 639 and at m/z of 303.0 (100%) [quercetin+H]+, which indicates a loss of acetylglucose and pentose moieties [204+132] [41].
Compounds 47, 48 and 50 exhibited pseudo-molecular ions at m/z of 625, 463 and 447, respectively. Through analysis of the MS/MS spectrum, these compounds displayed a characteristic product ion at m/z of 301, corresponding to quercetin. This product ion resulted from the loss of diglucoside [M+-H−324] for 47, a glucosyl [M+-H−162] for 48 and a rhamnosyl [M+-H−146] moiety for 50. As a result, these compounds were identified as quercetin diglucoside, quercetin-O-glucoside (Figure 5) and quercetin-O-rhamnoside [41].
Compounds 49 and 56 (Rt of 9.14 and 10.32 min) exhibited molecular ion peaks at m/z of 593 and 461 [M+-H]−, respectively, and MS2 fragment ions at m/z of 285.2 and 284.5 [luteolin-H]− after the neutral loss of rutinose [M+-H−308]− and glucuronide [M+-H−176]− moieties, respectively. The loss of 176 amu is characteristic of a glucuronic acid moiety [24]. Therefore, these compounds were identified to be luteolin-7-O-rutinoside (49) and luteolin-7-O-glucoronoide (56), respectively [42]. Luteolin-7-O-glucoronoide was previously isolated from the genus Daucus [26].
Compounds 51, 52 and 53 with Rt of 9.43 and 9.58 min gave deprotonated molecules for three compounds at m/z of 593, 609 and 463 [M+-H]−, respectively. The MS2 base fragment ions at m/z of 269.4, 299.0, and 300.6 for [Apigenin−H]−, [diosmetin−H]− and [quercetin−H]−, respectively, showed the neutral loss of caffoeylhexoside [M+−H−324]−, coumaroyl hexoside [M+−H−308]− and galactoside moieties [M−H−162]−, respectively. From the previous results and as shown in Table 2, these compounds were identified to be apigenin-7-O-caffoeyl hexoside (51) [43], diosmetin-7-O-coumaroyhexoside (52) [44] and quercetin-3-O-galactoside (53). Compound 53 was previously reported in Daucus species [24].
The ESI-mass spectra of compounds 54 and 55 (Figure 3) exhibited deprotonated molecules at m/z of 431 and 447 [M+−H]−, respectively, and MS2 base compound fragment ions at m/z of 269.0 [apigenin-H]− and 285.0 [luteolin-H]− due to the neutral loss of a glucose moiety [M-H−162]. These compounds were identified to be apigenin 7-O-glucoside and luteolin-7-O-glycoside, respectively [42]. Both compounds were previously isolated from the genus Daucus [26,61].
Compound 57 was identified as an isomer of compound 54 and identified as an apigenin 7-O-glucoside isomer. Similarly, compound 58 (Rt of 10.40 min) showed molecular ions at m/z of 447 and another fragment ion at m/z of 299, which corresponds to [M+-H] after the loss of the rhamnosyl moiety. It was identified as an isomer of compound 50 and identified as a quercetin-O-rhamnoside isomer.
Compounds 59 and 63 were proposed as isomers of diosmetin-O-rutinoside. They exhibited molecular ions at m/z of 609 [M+H]+. The product ion in the MS/MS spectrum was at m/z of 300.9 [M++H−308], showing loss of the rutinose moiety [46].
Compound 60 (Rt of 13.27 min) was tentatively identified as diosmetin-7-O-glucoside which was previously isolated from the genus Daucus [61]. This identification was based on the ESI-MS spectrum, which presented a molecular ion peak at m/z of 461 [M+-H]−, and the MS2 data that show a fragment ion at m/z of 299.3 [diosmetin-H], indicating a loss of the glucose moiety [47].
Compounds 61 and 62 had molecular ion fragments at 567 [M+-H] and 489 [M+-H], respectively. Upon analyzing the fragmentation pattern, MS2 fragment ions at m/z of 285.0 (100%) [M+-H−282]− were observed for 61, indicating a loss of 282 atomic mass units. For 62, the fragmentation pattern displayed 285 [M+-H−204]−, indicating the loss of an acetyl hexoside. Consequently, compound 61 was identified as a luteolin derivative, while compound 62 was identified as luteolin–7-O-acetyl hexoside [41]
Compound 64 exhibited a deprotonated molecular ion compound at m/z of 463 and was tentatively identified as myricetin-3-O-rhamnoside, as it gave the MS2 base compound fragment ion at m/z of 316.6, corresponding to the neutral loss of the rhamnose moiety (146 amu) [20].
Identification of C-glycosides
Four flavone C-glycosides (Table 2) were identified in methanol extracts of in vivo and in vitro tissues of D. capillifolius Gilli, as described below.
Compound 65 (Rt of 13.27 min) had the molecular ion peak in the ESI-MS spectrum at m/z of 445 [M+-H]− and an MS2 fragment ion at m/z of 341.0 [M−104]−, which showed the loss of 104 amu characteristic for the 8-C-rhamnoside of flavone [46,48]. Therefore, compound 65 was concluded to be diosmetin-8-C-rhamnoside (Figure 3). It is the first report of compound 65 in the genus Daucus. Diosmetin-di-C-rhamnoside was previously isolated from Daucus carota [35].
Compound 66 (Rt of 23.27 min) displayed a molecular ion compound at m/z of 433 [M+H]+ and an MS2 fragment ion at m/z of 313.1 [M+H−120] corresponding to the 0.3X ion. Another fragment ion signal at m/z of 150.6 and 130.7 (100%) was observed, which, along with the previous fragment, is characteristic for apigenin-8-C-glucoside [48].
Compound 67 (Rt of 23.39 min) presented a molecular ion at m/z of 461 [M+-H]−. The MS2 data show a fragment signal at m/z of 341.0, indicating the loss of 120 amu (0.3X ion). This fragment for [aglycone + 41] is a characteristic feature of mono C-glucoside flavonoid. Moreover, the absence of a fragment at [M+-H−18] indicates the presence of 8-C-glucoside instead of 6-C-glucoside [46]. Compound 67 was tentatively concluded to be diosmetin-8-C-glucoside (Figure 3) [46].
Compound 68 was suggested to be diosmetin-8-C-glucoside-O-rhamnoside. This was confirmed by the [M+H]+ ion at m/z of 609 and by the MS2 compound at m/z of 489.2, which indicates a loss of 120 amu (0.3X ion), a characteristic feature of a C-glucoside flavonoid. The fragment at m/z of 462.5 [M++H−146] and at m/z of 341.9 [aglycone+H+4] for mono C-glycoside flavonoid and the absence of a fragment at [M+-H−18] indicated the presence of 8-C-glycoside rather than 6-C glucoside [48].
2.1.3. Identification of Anthocyanins
Six anthocyanin compounds were identified in methanol extract of in vivo and in vitro tissues of D. capillifolius Gilli. They were identified as glycosides or acylated glycosides of cyanidin, pelargonidin and malvidin, as shown in Table 3 and described below.
Compound 69 exhibited a molecular ion peak [M+H]+ at m/z of 610, which on MS2 produced a fragment ion at m/z of 271.3 corresponding to pelargonidin aglycone [M++H −338] with the loss of glucose and glucuronide moieties [50]. From the previous results, compound 69 was identified as pelargonidin-3-O-glucuronosyl-O-glucoside. The position of the glucouronide moiety could not be identified.
Compounds 70 and 71 had a [M]+ at m/z of 449 (Figure 3), which on MS2 produced an ion at m/z of 287 (cyanidin, [M+−162]), with the loss of a glucose moiety. From the previous results, compounds 70 and 71 were identified as isomers of cyanidin-3-glucoside [51].
Compound 72 had [M++H] at m/z of 620 which on MS2 produced an ion at m/z of 287.0 (100%) (cyanidin, [M++H−332]). From the previous results, compound 72 was suggested to be a cyanidin derivative [49].
Compound 73 was suggested to be cyanidin-O-glucuronosyl-O-glucoside (Figure 3) or cyanidin-O-feruloylglucoside. This was confirmed by the [M+] ion at m/z of 625 and by the MS2 compound fragment at m/z of 287.0 (100%), indicating the loss of the glucuronosyl-O-glucoside moiety or feruloyl glucoside moiety [49,52].
Compound 74 was also suggested to be a cyanidin derivative. This was confirmed by the [M+] ion at m/z of 721 and by the MS2 compound ion at m/z of 287 (100%) [49,52].
Compound 75 (Rt of 28.31 min) was suggested to be malvidin-3-O-glucoside malonyl glucoside, which was confirmed by the [M+] ion at m/z of 741 and by the MS2 compound at m/z of 331.4 [M+−410] indicated the loss of glucoside malonylglucoside [54]. Anthocyanins of different cultivars of black carrot are relatively stable under low-acid conditions and could be used as natural food-coloring agents [24]
2.1.4. Identification of Tannins
Six flavanes compounds were identified in methanol extracts of in vivo and in vitro tissues of Daucus capillifolius as shown below.
Compounds 76, 77 and 78 exhibited characteristic features in their mass spectra. Compound 76 (gallocatechin) displayed [M+-H]− at m/z of 305 and MS2 fragments at m/z of 261.0, 119.0 and 97.0 (100%). Similarly, compound 77 (epigallocatechin) exhibited [M+-H]− at m/z of 305 and [M+H]+ at m/z of 307 and shared the same MS2 fragment ions as compound 76. On the other hand, compound 78, an epigallocatechin derivative, showed [M+-H]− at m/z of 721 and a specific MS2 fragment ion at m/z of 304.7 [55].
Compound 79 (Rt of 11.25 min) was suggested to be catechin-3-O-hexosidepentoside, which was confirmed by the [M+H]+ ion at m/z of 585 and by the MS2 compound at m/z of 294 [M+−291]− which indicates a loss of 291 amu [catechin+H] (Table 3) [55]. The fragment at m/z of 294 [162+132] is a characteristic feature of hexose and pentose moieties.
Compounds 80 and 81 displayed distinct molecular ion signals. Compound 80 exhibited an [M-H]− signal at m/z of 625, while compound 81 showed an [M+H]+ signal at m/z of 291. The fragmentation pattern for compound 80 revealed MS2 fragments at m/z of 288.5 (100%), indicating a loss of 336 atomic mass units. On the other hand, compound 81 displayed MS2 fragments at m/z of 174.9, 147.3, 137.3, 121.0 and 106.9, which are typical fragmentation patterns associated with catechin. As a result, compound 80 was identified as catechin-O-acetyl glucoside pentoside, while compound 81 was identified as catechin. These compounds are not common in daucus species but have been isolated and identified in green tea [50].
2.1.5. Identification of Acetylenic Compounds
Compounds 82 and 83 were tentatively identified as 9-[Heptadeca-1-en-4,6,9-triyne-3,8-diol] and 11-[8-hydroxytetradeca-1-en-4,6,9-triyn-3-yl acetate], respectively. From MS1 data, the compounds showed the same molecular ion peaks at m/z of 281 [M+Na]+ and characteristic fragmentation patterns as shown in Table 3 and Figure 4 at m/z of 81, 91, 97, 105, 123, 147, 149, 111, 71, 69, 71 and 57 [12,56,57].
Compound 84 showed predominantly sodiated ions and no [M++H] ions [57]. The ESI-MS spectrum (Figure 4) showed a molecular ion compound at m/z of 297 [M+Na]+ and MS2 fragments ions at m/z of 81, 105, 149 and 167, which are typical fragmentation patterns of falcarindiol-O-methyl ether. Consequently, it was identified as falcarindiol-8-O-methyl ether. Falcarinol, falcarindiol and falcarinone were previously reported in the genus Daucus [12,56,57].
In the extract obtained from the calli grown in media A and C, falcarindiol-8-O-methyl ether (84) was present at a higher concentration. Conversely, it was found at a lower concentration in medium B and cultivated fruits. On the other hand, compound 82 showed a higher concentration in the extract obtained from cultivated fruits compared with the calli grown on the three types of media, where it was present in very minimal amounts. Compound 83 was found in minor quantities in all the extracts.
2.1.6. Identification of Nitrogenous Compounds
Compound 85 (Rt of 1.35 min) exhibited protonated molecular ions [M++H]+ with an m/z value of 132 with a fragment ion at m/z of 76, which are characteristic of 3-methyl indole [58].
Compounds 86 and 87 (Rt of 1.77 and 2.23 min) were tentatively suggested to be 4-(aminoethyl) benzoic acid isomers based on the presence of a protonated molecular ion fragment at m/z of 166 [M+H]+ along with another fragment at m/z of 119.9 [M++H-COOH] and a base peak fragment at m/z of 103 [119.9-NH2]+, in addition to other characteristic fragments at m/z of 93.0, 91.0 and 76.9 [59]. Notably, this is the first report about the presence of nitrogenous compounds in this genus.
3. Materials and Methods
3.1. Plant Materials
The fruits of the cultivated Daucus capillifolius Gilli plant were collected in the fruiting stage in 2016 from the Farm of the Pharmacognosy Department, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt. The plant was kindly identified by the late Prof Dr. Hussein Abdel Basset, Professor of Taxonomy, Faculty of Science, Zagazig University. A voucher specimen (D.C 2016/12) was deposited at the herbarium in the Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University, Egypt. D. capillifolius fruits were air-dried and ground into coarse particles for use. Additionally, 50 g of 100-day-old calli grown on three different media, medium A [M&S + NAA (1 mg/L) + BAP (0.1 mg/L)], medium B [M&S + TDZ (0.5 mg/L) + 2,4 D (1 mg/L) + BAP (0.1 mg/L)] and medium C [M&S + 2, 4D (2 mg/L) + K (1 mg/L)], was prepared from the seedling explants of D. capillifolius fruits for the analysis.
3.1.1. Induction of Calli from In Vitro Germinated Seedlings
Callus was initiated from leaf explants of D. capillifolius seedlings as described by [19]. Excellent growth of calli with friable greenish white, friable bright yellow and compact yellowish white was obtained from calli grown on media A [M&S + NAA (1 mg/L) + BAP (0.1 mg/L)], B [M&S + TDZ (0.5 mg/L) + 2,4D (1 mg/L) + BAP (0.1 mg/L)] and C [M&S + 2,4 D (2 mg/L) + Kinetin (1 mg/L)], respectively (Figure 1).
3.1.2. Extract Preparation
Air-dried powdered fruits of D. capillifolius Gilli (200 g) were extracted by using methanol (HPLC analytical grade), filtered by using a membrane disk filter (0.2 µm) and then subjected to LC-ESI-MS analysis. A total of 50 g of 100-day-old non-organic calli grown separately on MS media with different hormonal compositions, including media A [M&S + NAA (1 mg/L) + BAP (0.1 mg/L)], B [M&S + TDZ (0.5 mg/L) + 2,4 D (1 mg/L) + BAP (0.1 mg/L)] and C [M&S + 2,4 D (2 mg/L) + Kinetin (1 mg/L)], was extracted with HPLC methanol (100 mL × 3). The extracts were collected and dried under vacuum by using a rotary evaporator at a temperature not exceeding 60 °C to give four extracts kept at 4 °C till analysis.
3.2. UPLC-ESI-MS/MS Analysis and Separation Method of D. capillifolius Extracts
UPLC-ESI-MS/MS (ultra-performance liquid chromatography–electrospray tandem mass spectrometry) in both ionization modes was carried out as described by [62] on an aXEVO-TQD triple-quadruple instrument (Waters Corporation, Milford, MA, USA) mass spectrometer (ACQUITY UPLC BEH C18 (1.7 μm, 2.1–50 mm) column; column flow rate of 0.2 mL/min). The solvent system consisted of (A) water and (B) methanol, both containing 0.1% formic acid (Ain Shams University, Cairo, Egypt). The gradient was programmed as follows: 0 min, 10% B; 5 min, 30% B; 15 min, 70% B; 22 min, 90% B; 25 min, 90% B; 26 min, 100% B; 29 min, 100% B; 32 min, 10% B. Finally, the initial conditions were held for 3 min as a re-equilibration step. The flow rate was 0.2 mL/min, and the sample at a concentration of 100 g/ml was prepared in HPLC-grade methanol, degassed, and filtered by using a 0.2 µm membrane disc filter before being subjected to LC-ESI-MS analysis. The injection volume was 10 µL. The parameters for analysis in negative ion mode were as follows: source temperature of 150 °C, cone voltage of 30 eV, capillary voltage of 3 kV, desolvation temperature of 440 °C, cone gas flow of 50 L/h and desolvation gas flow of 900 L/h. Mass spectra were detected in the ESI negative and/or positive ion modes between 50 m/z and 900 m/z. The peaks and spectra were processed by using MassLynx 4.1 software and tentatively identified by comparing their retention time (Rt) and mass spectrum with the reported data. A fragmentation collision energy of 40 eV was used.
4. Conclusions
Daucus capillifolius Gilli, grown in Libya, is an endangered plant. Its micropropagation and callus culture were successfully established in our previous work with GC-MS analysis of its essential oil. In the current study, we investigated its phytoconstituents for the first time by using UPLC-ESI-MS/MS analysis. Our results revealed that D. capillifolius fruit extract is a rich source of phenolic compounds, including simple phenolic acids, anthocyanidins, tannins, flavonoids, flavonoids -O- and -C-glycoside, and acetylenic compounds. Moreover, the extracts from the in vitro calli grown on media A, B and C with different hormonal combinations showed the accumulation of less phenolic acids, acid derivatives tannins, compared with the cultivated fruit extract. All the tested extracts exhibited the formation of acetylenic compounds, but only the extracts of the in vitro calli. showed the accumulation of nitrogenous compounds. Notably, only luteolin was detected in the extract of the in vitro calli grown on medium C, while calli grown on medium B did not show any flavonoidal aglycons. In summary, this variation in the accumulation of secondary metabolites based on the investigated hormonal combination requires further future studies to achieve the required amounts of secondary metabolites compared with the wild and cultivated D. capillifolius plant.
Author Contributions
Conceptualization, R.H.A., S.A. and W.H.B.H.; methodology, E.E.S., W.H.B.H., R.H.A., S.A. and S.I.E.; software, E.E.S., S.M.A.-M. and W.M.A.-M.; validation, E.E.S., S.M.A.-M., W.M.A.-M. and O.A.B.; formal analysis, W.H.B.H. and R.H.A. ; investigation, , R.H.A., S.A., S.M.A.-M. and W.H.B.H.; resources, S.I.E., E.E.S., S.M.A.-M., O.A.B. and M.P. ; data curation, W.M.A.-M., O.A.B. and M.P.; writing—original draft preparation, R.H.A., S.A. and W.H.B.H.; writing—review and editing R.H.A., S.A., S.M.A.-M., W.M.A.-M. and W.H.B.H.; visualization O.A.B. and M.P.; supervision, W.H.B.H. and S.I.E.; project administration, W.M.A.-M.; funding acquisition, W.M.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Researchers Supporting Project (number RSPD2024R1069), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this work are available in the article.
Acknowledgments
The authors acknowledge the Researchers Supporting Project number (RSPD2024R1069), King Saud University, Riyadh, Saudi Arabia for financial support. The authors thank the late Hussein Abdel Basset, Faculty of Science, Zagazig University for the plant identification.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hammami, S.; Elshamy, A.I.; Mokni, R.E.; Snene, A.; Iseki, K.; Dhaouadi, H.; Okamoto, Y.; Suenaga, M.; Noji, M.; Umeyama, A. Chemical constituents of the aerial parts of Daucus carota subsp. hispidus growing in Tunisia. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Wang, X.J.; Luo, Q.; Li, T.; Meng, P.H.; Pu, Y.T.; Liu, J.X.; Zhang, J.; Liu, H.; Tan, G.F.; Xiong, A.S. Origin, evolution, breeding, and omics of Apiaceae: A family of vegetables and medicinal plants. Hortic. Res. 2022, 9, uhac076. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an important source of antioxidants and their applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Cunha, A.C.; Fernandes-Ferreira, M. The potential of Apiaceae species as sources of singular phytochemicals and plant-based pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gunawardena, N.; Gamage, A.; Madhujith, T.; Merah, O. Apiaceae Family as a Valuable Source of Biocidal Components and their Potential Uses in Agriculture. Horticulturae 2022, 8, 614. [Google Scholar] [CrossRef]
- Ferrie, A.; Bethune, T.; Arganosa, G.; Waterer, D. Field evaluation of doubled haploid plants in the Apiaceae: Dill (Anethum graveolens L.), caraway (Carum carvi L.), and fennel (Foeniculum vulgare Mill.). Plant Cell Tissue Organ Cult. 2011, 104, 407–413. [Google Scholar] [CrossRef]
- Geoffriau, E.; Simon, P.W. Carrots and Related Apiaceae Crops; CABI: Wallingford, UK, 2020; Volume 33. [Google Scholar]
- Tamokou, J.; Mbaveng, A.; Kuete, V. Antimicrobial activities of African medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar]
- Kamte, S.L.N.; Ranjbarian, F.; Cianfaglione, K.; Sut, S.; Dall’Acqua, S.; Bruno, M.; Afshar, F.H.; Iannarelli, R.; Benelli, G.; Cappellacci, L. Identification of highly effective antitrypanosomal compounds in essential oils from the Apiaceae family. Ecotoxicol. Environ. Saf. 2018, 156, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Önder, A.; Cinar, A.S.; Sarialtin, S.Y.; Izgi, M.N.; Çoban, T. Evaluation of the antioxidant potency of Seseli L. species (Apiaceae). Turk. J. Pharm. Sci. 2020, 17, 197. [Google Scholar] [CrossRef]
- Saleem, F.; Eid, A.H.; Shetty, K. Potato–Herb Synergies as Food Designs for Hyperglycemia and Hypertension Management. In Functional Foods, Nutraceuticals, and Degenerative Disease Prevention; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 325–340. [Google Scholar]
- Christensen, L.P.; Kreutzmann, S. Determination of polyacetylenes in carrot roots (Daucus carota L.) by high-performance liquid chromatography coupled with diode array detection. J. Sep. Sci. 2007, 30, 483–490. [Google Scholar] [CrossRef]
- Plazonić, A.; Bucar, F.; Maleš, Ž.; Mornar, A.; Nigović, B.; Kujundžić, N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009, 14, 2466–2490. [Google Scholar] [CrossRef]
- Saeed, N.M.; Ramadan, L.A.; El-Sabbagh, W.A.; Said, M.A.; Abdel-Rahman, H.M.; Mekky, R.H. Exploring the anti-osteoporosis potential of Petroselinum crispum (Mill.) Fuss extract employing experimentally ovariectomized rat model and network pharmacology approach. Fitoterapia 2024, 175, 105971. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins and heart health. Ann. Ist. Super. Sanità 2007, 43, 369. [Google Scholar]
- Abd Alla, F.M.; Abdelshafeek, K.A.; El-soll, A.M.; ELsayed, W.M. Volatile oils, lipid constitutes and the antimicrobial activity of Daucus syrticus growing in Libya. J. Arab Soc. Med. Res. 2013, 8, 96–103. [Google Scholar]
- Jafri, S.; El-Gadi, A. Flora of Libya; Al Faatheh University, Faculty of Science Publication: Tripoli, Libya, 1985; Volume 118, p. 30. [Google Scholar]
- Jafri, S.M.; El-Gadi, A. Flora of Libya; Botany Department, Faculty of Science, Al Fatteh University: Tripoli, Libya, 1977. [Google Scholar]
- Hassan, W.; Abdelkader, M.; Senosy, E.; Eldahmy, S. In vitro Propagation and Essential Oils Composition with Cytotoxicity of Daucus capillifolius Gilli (Apiaceae). Int. J. Chemtech. Res. 2018, 11, 171–182. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L.(Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Tan, L.; Jin, Z.; Ge, Y.; Nadeem, H.; Cheng, Z.; Azeem, F.; Zhan, R. Comprehensive ESI-Q TRAP-MS/MS based characterization of metabolome of two mango (Mangifera indica L) cultivars from China. Sci. Rep. 2020, 10, 20017. [Google Scholar] [CrossRef]
- Mekky, R.H.; Abdel-Sattar, E.; Segura-Carretero, A.; Contreras, M.d.M. Phenolic compounds from sesame cake and antioxidant activity: A new insight for agri-food residues’ significance for sustainable development. Foods 2019, 8, 432. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. Eur. Food Res. Technol. 2004, 219, 479–486. [Google Scholar]
- Lin, Y.; Xu, W.; Huang, M.; Xu, W.; Li, H.; Ye, M.; Zhang, X.; Chu, K. Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in Yinhua Kanggan tablet by UPLC-QqQ-MS/MS. Molecules 2015, 20, 12209–12228. [Google Scholar] [CrossRef] [PubMed]
- Noui, A.; Boudiar, T.; Bakhouche, A.; del Mar Contreras, M.; Lozano-Sánchez, J.; Segura-Carretero, A.; Laouer, H.; Akkal, S. Chemical characterization of polyphenols from Daucus muricatus growing in Algeria by RP-UHPLC-ESI-QTOF-MS/MS. Nat. Prod. Res. 2018, 32, 982–986. [Google Scholar] [CrossRef]
- Sayed, S.; Alseekh, S.; Siems, K.; Fernie, A.R.; Luyten, W.; Schmitz-Linneweber, C.; Saul, N. Identification of a hydroxygallic acid derivative, zingibroside R1 and a sterol lipid as potential active ingredients of Cuscuta chinensis extract that has neuroprotective and antioxidant effects in aged Caenorhabditis Elegans. Nutrients 2022, 14, 4199. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Carle, R.; Schieber, A. Characterization of phenolic acids in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1331–1340. [Google Scholar] [PubMed]
- Thiyam, U.; Claudia, P.; Jan, U.; Alfred, B. De-oiled rapeseed and a protein isolate: Characterization of sinapic acid derivatives by HPLC–DAD and LC–MS. Eur. Food Res. Technol. 2009, 229, 825–831. [Google Scholar] [CrossRef]
- Misawa, N.; Hosoya, T.; Yoshida, S.; Sugimoto, O.; Yamada-Kato, T.; Kumazawa, S. 5-Hydroxyferulic acid methyl ester isolated from wasabi leaves inhibits 3T3-L1 adipocyte differentiation. Phytother. Res. 2018, 32, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Lech, K.; Witkoś, K.; Jarosz, M. HPLC–UV–ESI MS/MS identification of the color constituents of sawwort (Serratula tinctoria L.). Anal. Bioanal. Chem. 2014, 406, 3703–3708. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; He, Y.Q.; Wang, C.H.; Welbeck, E.W.; Bligh, S.A.; Wang, Z.T. Characterization of fifty-one flavonoids in a Chinese herbal prescription Longdan Xiegan Decoction by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry and photodiode array detection. Rapid Commun. Mass Spectrom. 2008, 22, 1767–1778. [Google Scholar] [CrossRef]
- Mena, P.; Sánchez-Salcedo, E.M.; Tassotti, M.; Martínez, J.J.; Hernández, F.; Del Rio, D. Phytochemical evaluation of eight white (Morus alba L.) and black (Morus nigra L.) mulberry clones grown in Spain based on UHPLC-ESI-MSn metabolomic profiles. Food Res. Int. 2016, 89, 1116–1122. [Google Scholar] [CrossRef]
- Shafik, N.; Shafek, R.; Michael, H. Antimicrobial activity of different extracts of Daucus carota canopy. Int. J. Pharm. 2015, 5, 352–356. [Google Scholar]
- Liu, R.; Li, H.; Wei, N.; Tan, Y. Simultaneous determination of two galangin metabolites from Alpinia Officinarum Hance in rat plasma by UF LC-MS/MS and its application in pharmacokinetics study. PeerJ 2021, 9, e11041. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Benites, J.; Areche, C.; Sepúlveda, B. Antioxidant capacities and analysis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.-Y.; Wang, S.-L.; Niu, X.-Y. Analysis of anthocyanins and flavonols in petals of 10 Rhododendron species from the Sygera Mountains in Southeast Tibet. Plant Physiol. Biochem. 2016, 104, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Wilson, T.; Luthria, D.; Freeman, M.R.; Scott, R.M.; Bilenker, D.; Shah, S.; Somasundaram, S.; Vorsa, N. LC-MS–MS characterisation of curry leaf flavonols and antioxidant activity. Food Chem. 2011, 127, 80–85. [Google Scholar] [CrossRef]
- Slimestad, R.; Francis, G.W.; Andersen, Ø.M. Directed search for plant constituents: A case study concerning flavonoids in Norway spruce. Euphytica 1999, 105, 119–123. [Google Scholar] [CrossRef]
- Desta, K.T.; Lee, W.S.; Lee, S.J.; Kim, Y.H.; Kim, G.S.; Lee, S.J.; Kim, S.T.; Abd El-Aty, A.; Warda, M.; Shin, H.C. Antioxidant activities and liquid chromatography with electrospray ionization tandem mass spectrometry characterization and quantification of the polyphenolic contents of Rumex nervosus Vahl leaves and stems. J. Sep. Sci. 2016, 39, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Inbaraj, B.S.; Chen, B.-H. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2011, 13, 260–285. [Google Scholar] [CrossRef]
- Švehlíková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert). Phytochem 2004, 65, 2323–2332. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Hassan, W.H.; Abdelaziz, S.; Amina, M.; Adel, R.; El-Sayed, M.A. UPLC-ESI-MS/MS profile and antioxidant, cytotoxic, antidiabetic, and antiobesity activities of the aqueous extracts of three different Hibiscus Species. J. Chem. 2020, 2020, 6749176. [Google Scholar] [CrossRef]
- Abdelhady, N.M.; Abdallah, G.M. HPLC/MS/MS study of phenolic compounds of Leucaena leucocephala legumes monitored with their in vitro antihyperglycemic activity. Eur. J. Med. Plants 2016, 17, 1–9. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three Citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC–DAD–ESI/MS analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Carle, R.; Schieber, A. Detection of peonidin and pelargonidin glycosides in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2407–2412. [Google Scholar] [PubMed]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Kajdžanoska, M.; Gjamovski, V.; Stefova, M. HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Maced. J. Chem. Chem. Eng. 2010, 29, 181–194. [Google Scholar] [CrossRef]
- Schwarz, M.; Wray, V.; Winterhalter, P. Isolation and identification of novel pyranoanthocyanins from black carrot (Daucus carota L.) juice. J. Agric. Food Chem. 2004, 52, 5095–5101. [Google Scholar] [CrossRef] [PubMed]
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar] [PubMed]
- Smeriglio, A.; Denaro, M.; Barreca, D.; D’Angelo, V.; Germanò, M.; Trombetta, D. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.). Fitoterapia 2018, 124, 49–57. [Google Scholar] [PubMed]
- Gates, P.J.; Lopes, N.P. Characterisation of flavonoid aglycones by negative ion chip-based nanospray tandem mass spectrometry. Int. J. Anal. Chem. 2012, 2012, 259217. [Google Scholar] [CrossRef]
- Kramer, M.; Mühleis, A.; Conrad, J.; Leitenberger, M.; Beifuss, U.; Carle, R.; Kammerer, D.R. Quantification of Polyacetylenes in Apiaceous Plants by High-Performance Liquid Chromatography Coupled with Diode Array Detection. Z. Naturforsch. C 2011, 66, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.K.; Brunton, N.P.; Koidis, A.; Rawson, A.; McLoughlin, P.; Griffiths, W.J. Characterisation of polyacetylenes isolated from carrot (Daucus carota) extracts by negative ion tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Jastrebova, J. Application of LC–MS for determination of indole and 3-methylindole in porcine adipose tissue. Chromatographia 2006, 64, 435–439. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 506066, 4-(2-Aminoethyl) benzoic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-_2-Aminoethyl_benzoic-acid (accessed on 18 February 2024).
- Xu, Q.; Li, S.; Tang, W.; Yan, J.; Wei, X.; Zhou, M.; Diao, H. The Effect of Ellagic Acid on Hepatic Lipid Metabolism and Antioxidant Activity in Mice. Front. Physiol. 2021, 12, 751501. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafeek, K.; ElMissiry, M.M.; Hussiny, H.A.; Elnasr, M. The flavonoids and anticomplement activity of two cruciferous plants growing in Egypt. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 223–227. [Google Scholar]
- Hassan, W.H.B.; Abdelaziz, S.; Al Yousef, H.M. Chemical Composition and Biological Activities of the Aqueous Fraction of Parkinsonea aculeata L. Growing in Saudi Arabia. Arab. J. Chem. 2019, 12, 377–387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).