Thermal Hazard Analysis of Two Non-Ideal Explosives Based on Ammonium Perchlorate/Ammonium Nitrate and Aluminium Powder
Abstract
1. Introduction
2. Experiment
2.1. Reagents and Instruments
2.2. Determination of Experimental Formulation
2.3. Test Sample Preparation and Thermal Analysis Experiment
3. Thermokinetic Analysis Methods
3.1. The Ozawa Method Based on Integral Calculus
3.2. The Kissinger Method Based on Differential Calculus
4. Experimental Results and Discussion
4.1. Results of the Thermal Analysis Experiment of the Ammonium Perchlorate-Aluminium System
4.2. Thermokinetic Parameters of the Ammonium Perchlorate-Aluminium System
4.3. Results of the Thermal Analysis Experiment of the Ammonium Nitrate-Aluminium System
4.4. Thermokinetic Parameters of the Ammonium Nitrate-Aluminium System
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaturvedi, S.; Dave, P.N. Solid propellants: AP/HTPB composite propellants. Arab. J. Chem. 2019, 12, 2061–2068. [Google Scholar] [CrossRef]
- Lysien, K.; Stolarczyk, A.; Jarosz, T. Solid propellant formulations: A review of recent progress and utilized components. Materials 2021, 14, 6657. [Google Scholar] [CrossRef] [PubMed]
- Kohga, M. Burning characteristics and thermochemical behavior of AP/HTPB composite propellant using coarse and fine AP particles. Propellants Explos. Pyrotech. 2011, 36, 57–64. [Google Scholar] [CrossRef]
- Due-Hansen, M.E.; Dullum, O. Review and analysis of the explosion accident in Drevja, Norway: A consequence of fire in a mobile explosives manufacturing unit (MEMU) carrying precursors for the on-site production of bulk explosives. Saf. Sci. 2017, 96, 33–40. [Google Scholar] [CrossRef]
- Burdea, F.I.; Moraru, R.I.; Burdea, C.M. Risk analysis, evaluation and management tool development for civil explosives deposits. Pol. J. Manag. Stud. 2021, 24, 67–87. [Google Scholar] [CrossRef]
- Sivapirakasam, S.P.; Mohamed, M.N.; Surianarayanan, M.; Sridhar, V.P. Evaluation of thermal hazards and thermo-kinetic parameters of a matchhead composition by DSC and ARC. Thermochim. Acta 2013, 557, 13–19. [Google Scholar] [CrossRef]
- Radeanu, C.; Vasilescu, G.; Jitea, C.; Radermacher, L.; Ilcea, G. Analysis of dangerous situations generated by explosive materials in non-compliant operations performed on industrial locations intended for their storage. In Proceedings of the MATEC Web of Conferences, Purwokerto, Indonesia, 12 October 2022; EDP Sciences: Les Ulis, France, 2022; Volume 373, p. 00046. [Google Scholar]
- Dong, L.Y.; Wang, Z.J.; Xiao, Y.C.; Tang, X.Z.; Zhang, X.J.; Fan, C.Y. Sympathetic detonation reaction behavior of a fuze explosive train. Combust. Explos. Shock Waves 2021, 57, 607–619. [Google Scholar] [CrossRef]
- Pakkirisamy, S.V.; Mahadevan, S.; Paramashivan, S.S.; Mandal, A.B. Adiabatic thermokinetics and process safety of pyrotechnic mixtures: Atom bomb, Chinese, and palm leaf crackers. J. Therm. Anal. Calorim. 2012, 109, 1387–1395. [Google Scholar] [CrossRef]
- Burdea, M.C.; Moraru, R.I.; Burdea, F.I.; Dregan, C.V. Methodology for Analysis, Assessment and Classification of Major-Accident Hazards (Explosion) in the Case of Explosives Depots. In Proceedings of the International Conference Knowledge-Based Organization, Verona, Italy, 7–9 September 2022; Volume 28, pp. 19–24. [Google Scholar]
- Matečić Mušanić, S.; Fiamengo Houra, I.; SućeSkA, M. Applicability of non-isothermal DSC and Ozawa method for studying kinetics of double base propellant decomposition. Cent. Eur. J. Energetic Mater. 2010, 7, 233–251. [Google Scholar]
- Rus, D.; Vasilescu, G.; Kovacs, A.; Gheorghiosu, E.; Jitea, I.C. Improving the group decision by optimization of risk management for specific activities with explosives for civil use. Qual. Access Success 2017, 18, 41. [Google Scholar]
- Burdea, M.C.; Moraru, R.I.; Burdea, F.I.; Dregan, C.V. Comparative Analysis of the Risk of Explosion Generated By Civil Explosives in Industrial Places for Storage of Explosive Materials. In Proceedings of the International Conference Knowledge-Based Organization, Verona, Italy, 7–9 September 2022; Volume 28, pp. 12–18. [Google Scholar]
- Niu, H.; Chen, S.; Shu, Q.; Li, L.; Jin, S. Preparation, characterization and thermal risk evaluation of dihydroxylammonium 5, 5′-bistetrazole-1, 1′-diolate based polymer bonded explosive. J. Hazard. Mater. 2017, 338, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Ravanbod, M.; Pouretedal, H.R.; Amini, M.K.; Ebadpour, R. Kinetic study of the thermal decomposition of potassium chlorate using the non-isothermal TG/DSC technique. Cent. Eur. J. Energetic Mater. 2016, 13, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chang, Y. A predictive method for the heat of explosion of non-ideal aluminized explosives. Cent. Eur. J. Energetic Mater. 2013, 10, 541–554. [Google Scholar]
- Cummock, N.R.; Mares, J.O., Jr.; Gunduz, I.E.; Son, S.F. Relating a small-scale shock sensitivity experiment to large-scale failure diameter in an aluminized ammonium nitrate non-ideal explosive. Combust. Flame 2018, 194, 271–277. [Google Scholar] [CrossRef]
- Hotta, M.; Tone, T.; Koga, N. Effects of particle size on the kinetics of physico-geometrical consecutive reactions in solid–gas systems: Thermal decomposition of potassium hydrogen carbonate. J. Phys. Chem. C 2021, 125, 22023–22035. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Okazaki, T.; Sakai, Y.; Iwasaki, S.; Koga, N. Kinetic analysis of the multistep thermal decomposition of Maya blue-type pigments to evaluate thermal stability. J. Therm. Anal. Calorim. 2020, 142, 1073–1085. [Google Scholar] [CrossRef]
- Green, S.P.; Wheelhouse, K.M.; Payne, A.D.; Hallett, J.P.; Miller, P.W.; Bull, J.A. On the use of differential scanning calorimetry for thermal hazard assessment of new chemistry: Avoiding explosive mistakes. Angew. Chem. Int. Ed. Engl. 2020, 59, 15798–15802. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharia, S.K.; Weeks, B.L.; Chen, C.-C. Melting behavior and heat of fusion of compounds that undergo simultaneous melting and decomposition: An investigation with HMX. J. Chem. Eng. Data 2017, 62, 967–972. [Google Scholar] [CrossRef]
- Burnham, A.K.; Stanford, V.L.; Vyazovkin, S.; Kahl, E.M. Effect of pressure on TATB and LX-17 thermal decomposition. Thermochim. Acta 2021, 699, 178908. [Google Scholar] [CrossRef]
- Gorn, M.V.; Monogarov, K.A.; Dalinger, I.L.; Melnikov, I.N.; Kiselev, V.G.; Muravyev, N.V. Pressure DSC for energetic materials. Part 2. Switching between evaporation and thermal decomposition of 3,5-dinitropyrazole. Thermochim. Acta 2020, 690, 178697. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Meerov, D.B.; Monogarov, K.A.; Melnikov, I.N.; Kosareva, E.K.; Fershtat, L.L.; Sheremetev, A.B.; Dalinger, I.L.; Fomenkov, I.V.; Pivkina, A.N. Sensitivity of energetic materials: Evidence of thermodynamic factor on a large array of CHNOFCL compounds. Chem. Eng. J. 2021, 421, 129804. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Monogarov, K.A.; Melnikov, I.N.; Pivkina, A.N.; Kiselev, V.G. Learning to fly: Thermochemistry of energetic materials by modified thermogravimetric analysis and highly accurate quantum chemical calculations. Phys. Chem. Chem. Phys. 2021, 23, 15522–15542. [Google Scholar] [CrossRef]
- Yang, F.; Liao, L.; Zhao, C.; Tian, Y. Combined kinetic analysis of overlapping multistep thermal decomposition of 5-nitro-2,4,6-triaminopyrimidine-1,3-di-N-oxide (ICM-102). Thermochim. Acta 2020, 690, 178663. [Google Scholar] [CrossRef]
- Lobanova, M.; Aleshkevich, V.; Yablokova, M.; Morozov, O.; Babkin, A.; Kepman, A.; Avdeev, V.; Bulgakov, B. Kinetics of the oxidative aging of phthalonitrile resins and their effects on the mechanical properties of thermosets. Thermochim. Acta 2023, 724, 179492. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S. The effect of process and structural parameters on the stability, thermo-mechanical and thermal degradation of polymers with hydrocarbon skeleton containing PE, PP, PS, PVC, NR, PBR and SBR. J. Therm. Anal. Calorim. 2021, 143, 2867–2882. [Google Scholar] [CrossRef]
- Mazhar, H.; Shehzad, F.; Hong, S.-G.; Al-Harthi, M.A. Thermal degradation kinetics analysis of ethylene propylene copolymer and EP-1-hexene terpolymer. Polymers 2022, 14, 634. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Perejón, A.; Arcenegui-Troya, J.; Pérez-Maqueda, L.A. Predictions of polymer thermal degradation: Relevance of selecting the proper kinetic model. J. Therm. Anal. Calorim. 2022, 147, 2335–2341. [Google Scholar] [CrossRef]
- Janković, B.; Manić, N. Kinetic analysis and reaction mechanism of p-alkoxybenzyl alcohol ([4 (hydroxymethyl)phenoxymethyl]polystyrene) resin pyrolysis: Revealing new information on thermal stability. Polym. Degrad. Stab. 2021, 189, 109606. [Google Scholar] [CrossRef]
- Dwyer, D.B.; Gallego, N.C.; Niedziela, J.L.; Kapsimalis, R.J.; Duckworth, D.C. Product specific thermal degradation kinetics of bisphenol F epoxy in inert and oxidative atmospheres using evolved gas analysis–mass spectrometry. J. Anal. Appl. Pyrolysis 2022, 165, 105563. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L., Jr.; Ornaghi, F.G.; Amico, S.C.; Zattera, A.J. Degradation kinetics and lifetime prediction for polystyrene/nanocellulose nanocomposites. J. Therm. Anal. Calorim. 2022, 147, 879–890. [Google Scholar] [CrossRef]
- Nardella, F.; Mattonai, M.; Ribechini, E. Evolved gas analysis-mass spectrometry and isoconversional methods for the estimation of component-specific kinetic data in wood pyrolysis. J. Anal. Appl. Pyrolysis 2020, 145, 104725. [Google Scholar] [CrossRef]
- Purnomo, D.M.; Richter, F.; Bonner, M.; Vaidyanathan, R.; Rein, G. Role of optimisation method on kinetic inverse modelling of biomass pyrolysis at the microscale. Fuel 2020, 262, 116251. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Pérez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee recommendations for analysis of thermal decomposition kinetics. Thermochim. Acta 2023, 719, 179384. [Google Scholar] [CrossRef]
- Várhegyi, G.; Wang, L.; Skreiberg, Ø. Non-isothermal kinetics: Best-fitting empirical models instead of model free methods. J. Therm. Anal. Calorim. 2020, 142, 1043–1054. [Google Scholar] [CrossRef]
- Roduit, B.; Hartmann, M.; Folly, P.; Sarbach, A.; Baltensperger, R. Prediction of thermal stability of materials by modified kinetic and model selection approaches based on limited amount of experimental points. Thermochim. Acta 2014, 579, 31–39. [Google Scholar] [CrossRef]
- Song, M.Y.; Kwak, Y.J. Simple Ways to Obtain Activation Energy for Hydride Decomposition by Applying Data from a Volumetric Method to the Kissinger Equation. Mater. Sci. 2023, 29, 305–309. [Google Scholar] [CrossRef]
- Hui, M.; Yu-Cun, L.; Tao, C.; Tuo-Ping, H.; Jia-Hu, G.; Yan-Wu, Y.; Jun-Ming, Y.; Jian-Hua, W.; Ning, Q.; Liang, Z. Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry. e-Polymers 2017, 17, 89–94. [Google Scholar] [CrossRef]
- Arumsari, A.G.; Hernowo, P.; Wibowo, J.; Fadlulloh, M.Y.; Setiawan, R. Study of Reaction Kinetics by Flynn Wall Ozawa Method on Sawdust Pyrolysis Process. Indones. J. Appl. Chem. 2022, 24, 9–14. [Google Scholar]
- Bafna, S. On the use of Ozawa/Flynn/Wall method to determine aging activation energy for elastomers. J. Elastomers Plast. 2022, 54, 56–66. [Google Scholar] [CrossRef]
- Yanwu, Y.; Hui, M.; Jiahu, G.; Jingwei, M.; Suming, J.; Guimin, C.; Zihui, W. Thermal analysis of a new heat-resistant energetic material. Sci. Adv. Mater. 2022, 14, 147–154. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger method in kinetics of materials: Things to beware and be aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Kwak, Y.J. Three methods for application of data from a volumetric method to the Kissinger equation to obtain activation energy. Micromachines 2022, 13, 1809. [Google Scholar] [CrossRef]
- Chang, P.-J.; Mogi, T.; Dobashi, R. An investigation on the dust explosion of micron and nano scale aluminium particles. J. Loss Prev. Process Ind. 2021, 70, 104437. [Google Scholar] [CrossRef]
- Li, X.; Pei, H.; Zhang, X.; Zheng, X. Effect of aluminum particle size on the performance of aluminized explosives. Propellants Explos. Pyrotech. 2020, 45, 807–813. [Google Scholar] [CrossRef]
- Li-Na, Y.; Cai-Ling, W.; Zhi-Xin, D.; Hai-Qing, W.; Xiao-Qiang, D.; Sheng-Xiang, Z. Influence of nano-aluminum on the explosion heat and thermal stability of DNTF pressed mixed explosives. Initiat. Pyrotech. 2014, 4, 47–49. [Google Scholar]
- Wang, H.; Cheng, Y.; Zhu, S.; Li, Z.; Shen, Z. Effects of content and particle size of TiH2 powders on the energy output rules of RDX composite explosives. Def. Technol. 2024, 32, 297–308. [Google Scholar] [CrossRef]
- Wu, X.L.; Xu, S.; Pang, A.M.; Cao, W.G.; Liu, D.B.; Zhu, X.Y.; Xu, F.Y.; Wang, X. Hazard evaluation of ignition sensitivity and explosion severity for three typical MH2 (M = Mg, Ti, Zr) of energetic materials. Def. Technol. 2021, 17, 1262–1268. [Google Scholar] [CrossRef]


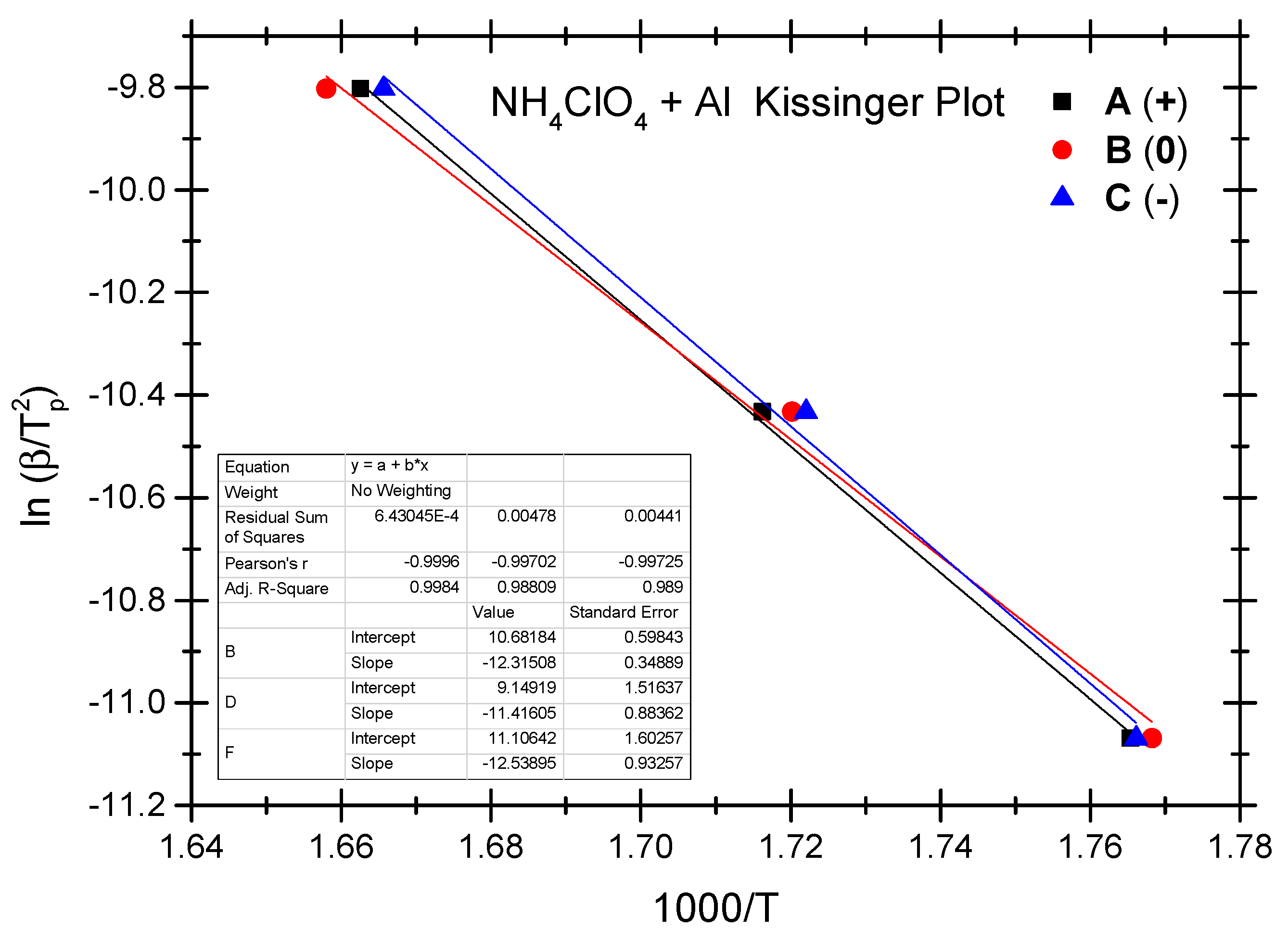
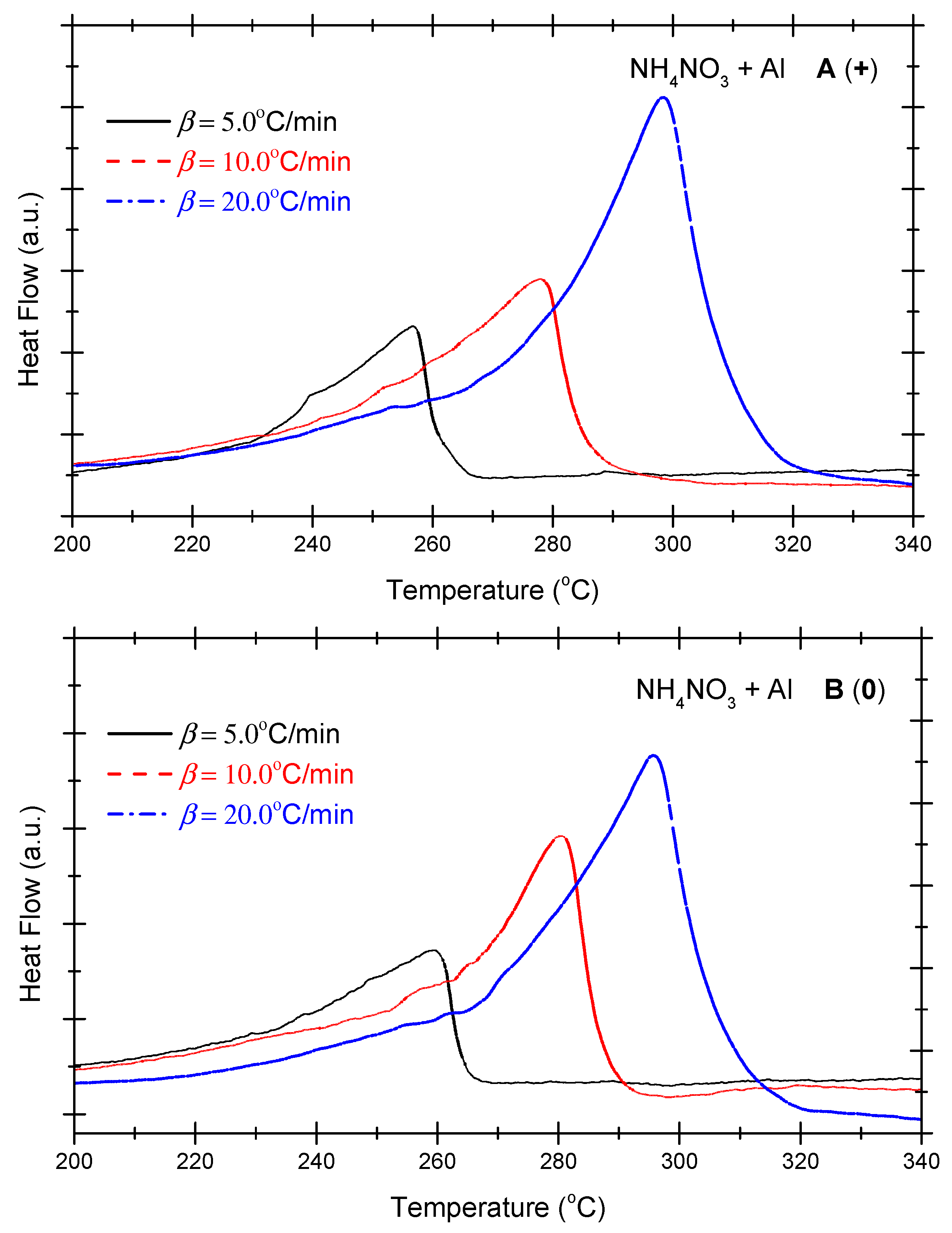

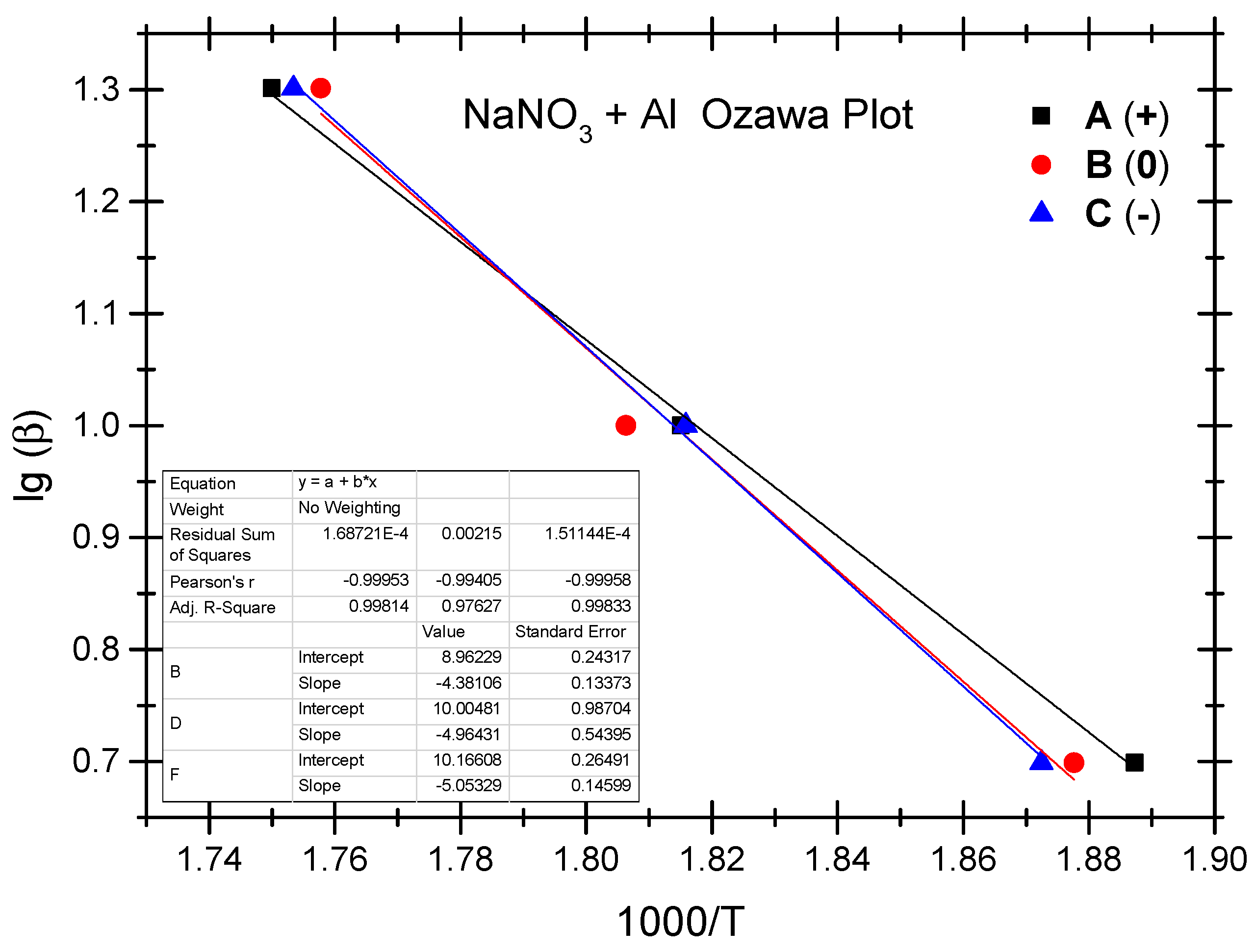
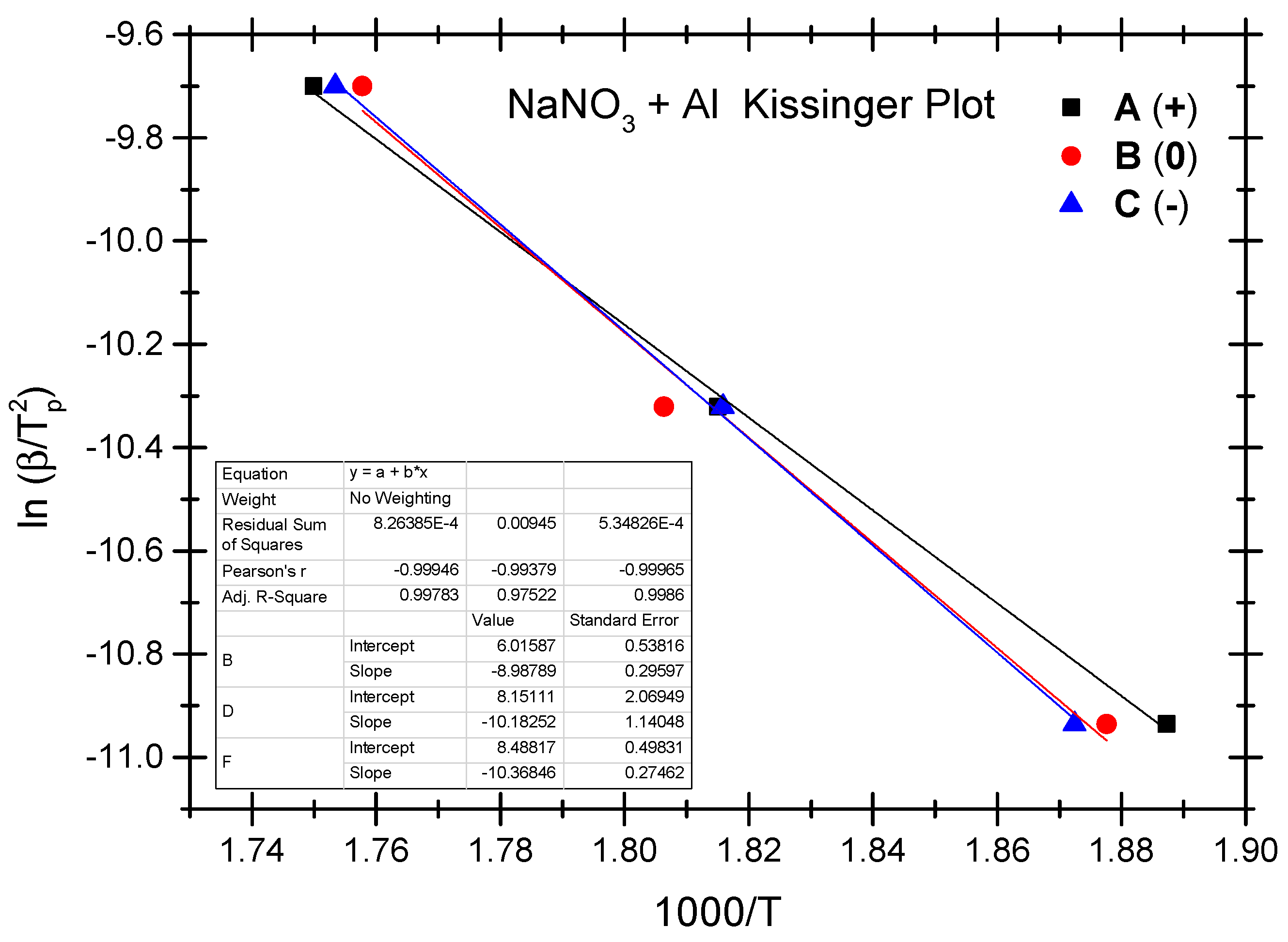
| No. | Test Sample | Positive OB A (+) | Zero OB B (0) | Negative OB C (−) |
|---|---|---|---|---|
| 1 | NH4ClO4 + Al | 1:0.33 | 1:0.37 | 1:0.43 |
| 2 | NH4NO3 + Al | 1:0.19 | 1:0.22 | 1:0.25 |
| β (°C/min) | TP (°C) | ||
|---|---|---|---|
| A (+) | B (0) | C (−) | |
| 5.0 | 293.3 | 292.4 | 293.1 |
| 10.0 | 309.5 | 308.3 | 307.5 |
| 20.0 | 328.3 | 330.0 | 327.2 |
| OB | Ozawa Method | Kissinger Method | ||||
|---|---|---|---|---|---|---|
| Ea (kJ/mol) | R2 | Ek (kJ/mol) | R2 | Ak | ||
| A (+) | 0.0325 | 106.6 | 0.9987 | 102.4 | 0.9984 | 5.36 × 105 |
| B (0) | 0.0079 | 98.8 | 0.9891 | 94.9 | 0.9881 | 1.07 × 105 |
| C (−) | −0.0290 | 108.5 | 0.9899 | 104.2 | 0.9890 | 8.35 × 105 |
| β (°C/min) | TP (°C) | ||
|---|---|---|---|
| A (+) | B (0) | C (−) | |
| 5.0 | 256.7 | 259.4 | 260.9 |
| 10.0 | 277.8 | 280.5 | 277.6 |
| 20.0 | 298.3 | 295.8 | 297.2 |
| OB | Ozawa Method | Kissinger Method | ||||
|---|---|---|---|---|---|---|
| Ea (kJ/mol) | R2 | Ek (kJ/mol) | R2 | Ak | ||
| A (+) | 0.0256 | 79.8 | 0.9981 | 74.7 | 0.9978 | 3.68 × 103 |
| B (0) | 0.0035 | 90.4 | 0.9763 | 84.7 | 0.9752 | 3.53 × 104 |
| C (−) | −0.0180 | 92.0 | 0.9983 | 86.2 | 0.9986 | 5.04 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Chen, X.; Yu, Y.; Dong, J.; Zhang, J.; Meng, J.; Xin, C.; Wang, Z. Thermal Hazard Analysis of Two Non-Ideal Explosives Based on Ammonium Perchlorate/Ammonium Nitrate and Aluminium Powder. Molecules 2024, 29, 2680. https://doi.org/10.3390/molecules29112680
Guo J, Chen X, Yu Y, Dong J, Zhang J, Meng J, Xin C, Wang Z. Thermal Hazard Analysis of Two Non-Ideal Explosives Based on Ammonium Perchlorate/Ammonium Nitrate and Aluminium Powder. Molecules. 2024; 29(11):2680. https://doi.org/10.3390/molecules29112680
Chicago/Turabian StyleGuo, Jiahu, Xiaoping Chen, Yanwu Yu, Jianhui Dong, Jun Zhang, Jingwei Meng, Chenglai Xin, and Zhigang Wang. 2024. "Thermal Hazard Analysis of Two Non-Ideal Explosives Based on Ammonium Perchlorate/Ammonium Nitrate and Aluminium Powder" Molecules 29, no. 11: 2680. https://doi.org/10.3390/molecules29112680
APA StyleGuo, J., Chen, X., Yu, Y., Dong, J., Zhang, J., Meng, J., Xin, C., & Wang, Z. (2024). Thermal Hazard Analysis of Two Non-Ideal Explosives Based on Ammonium Perchlorate/Ammonium Nitrate and Aluminium Powder. Molecules, 29(11), 2680. https://doi.org/10.3390/molecules29112680






