Imidazopyridine Family: Versatile and Promising Heterocyclic Skeletons for Different Applications
Abstract
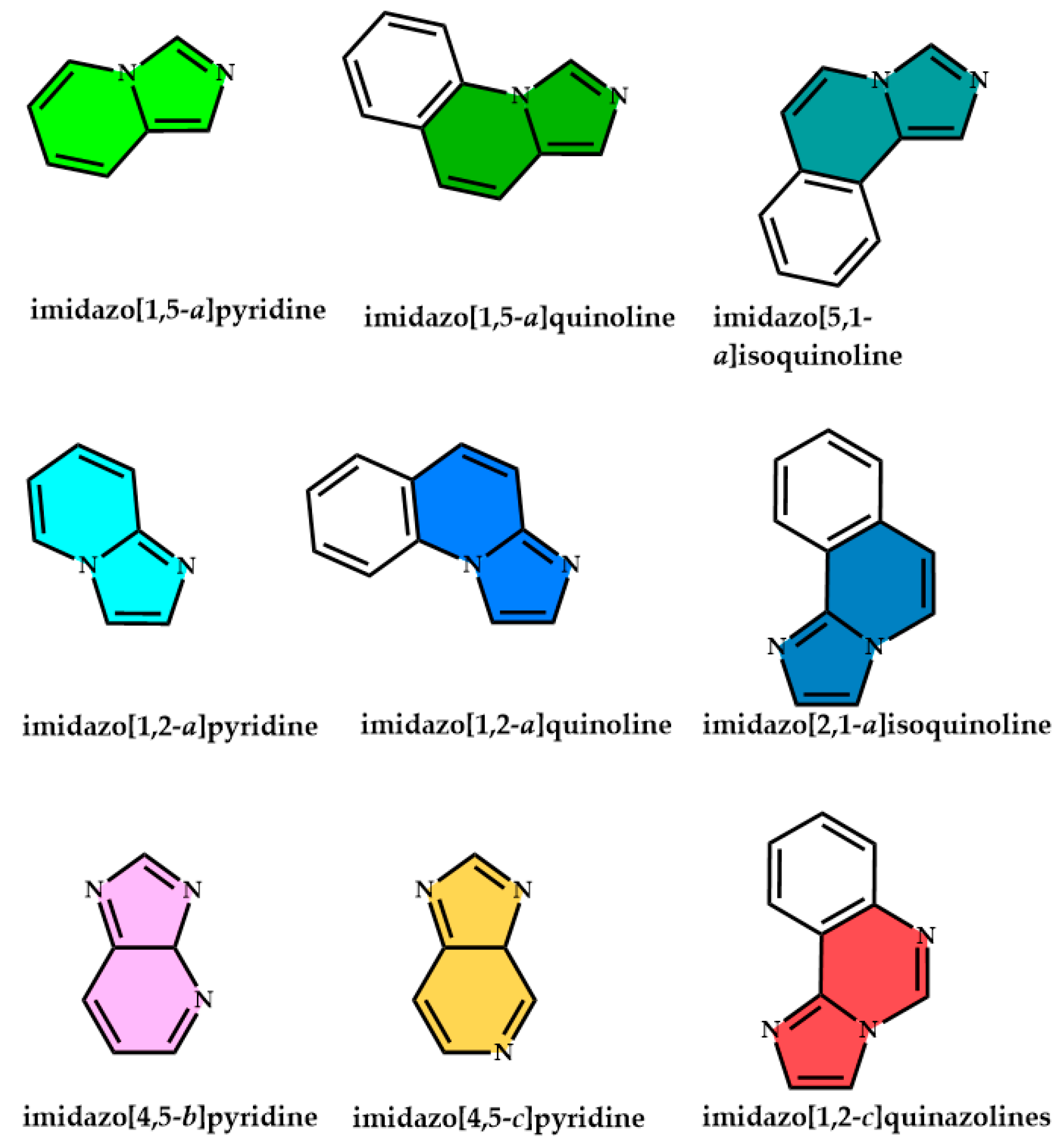
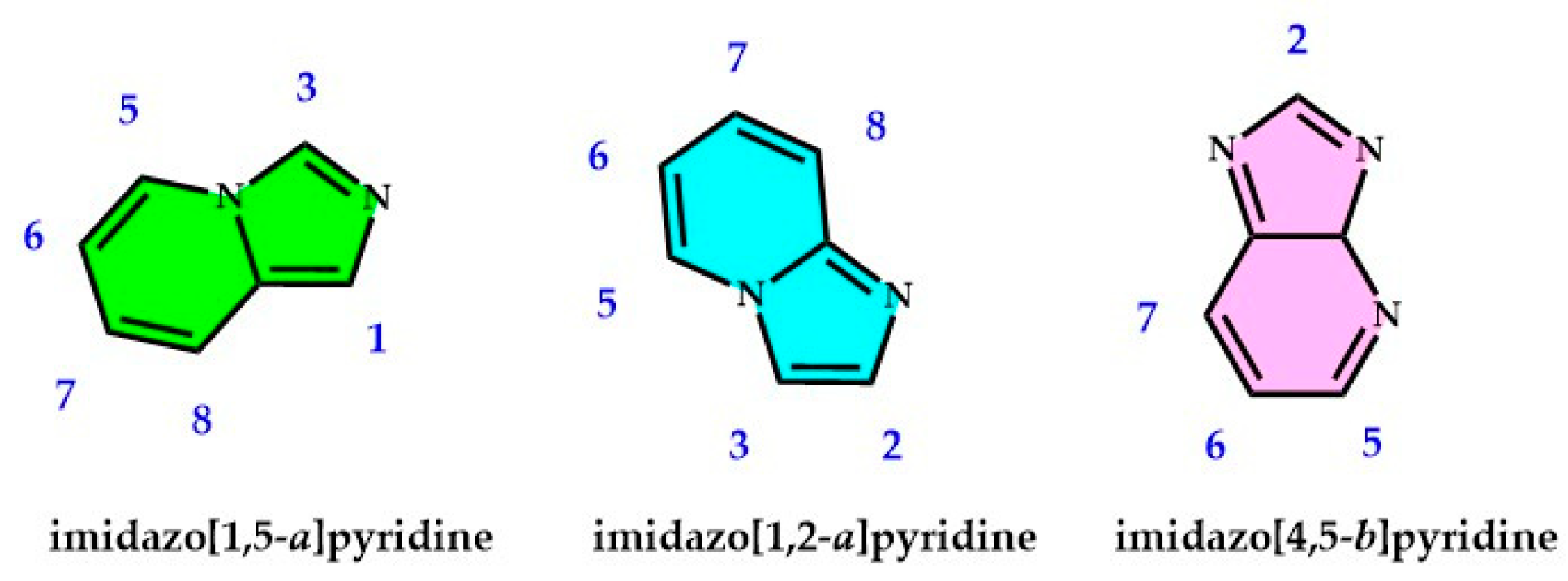
1. Introduction
- Imidazo[4,5-c]pyridines display a bioisosteric resemblance to the purine nucleus, manifesting similar structural and electronic properties. This structural affinity facilitates imidazopyridine’s facile interaction with macromolecules such as DNA, RNA, and specific proteins. Moreover, imidazo[4,5-c]pyridines exhibit notable cytotoxic activity by selectively inhibiting kinase activity [29,30,31].
2. Studies and Applications of Imidazopyridine Derivatives
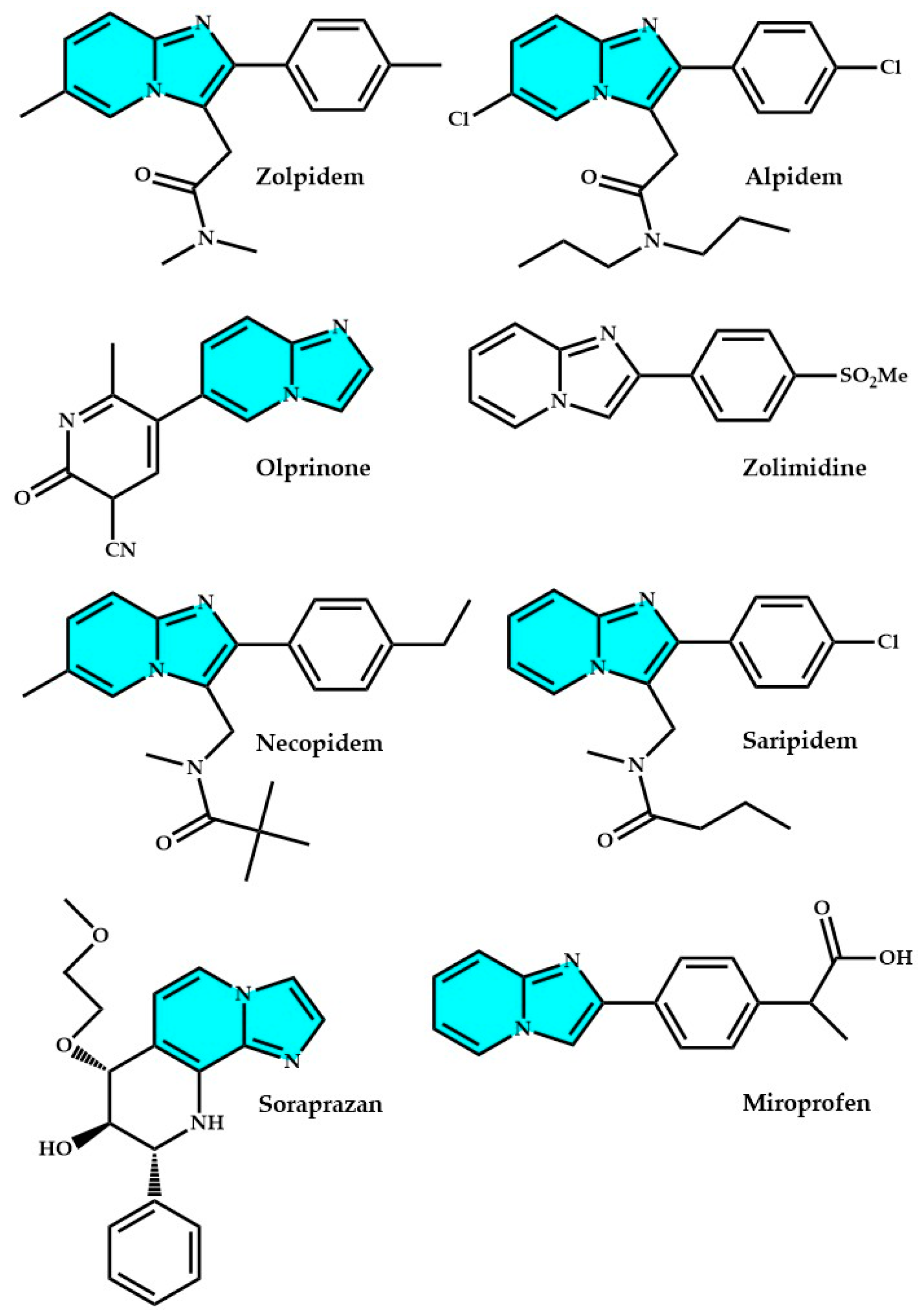
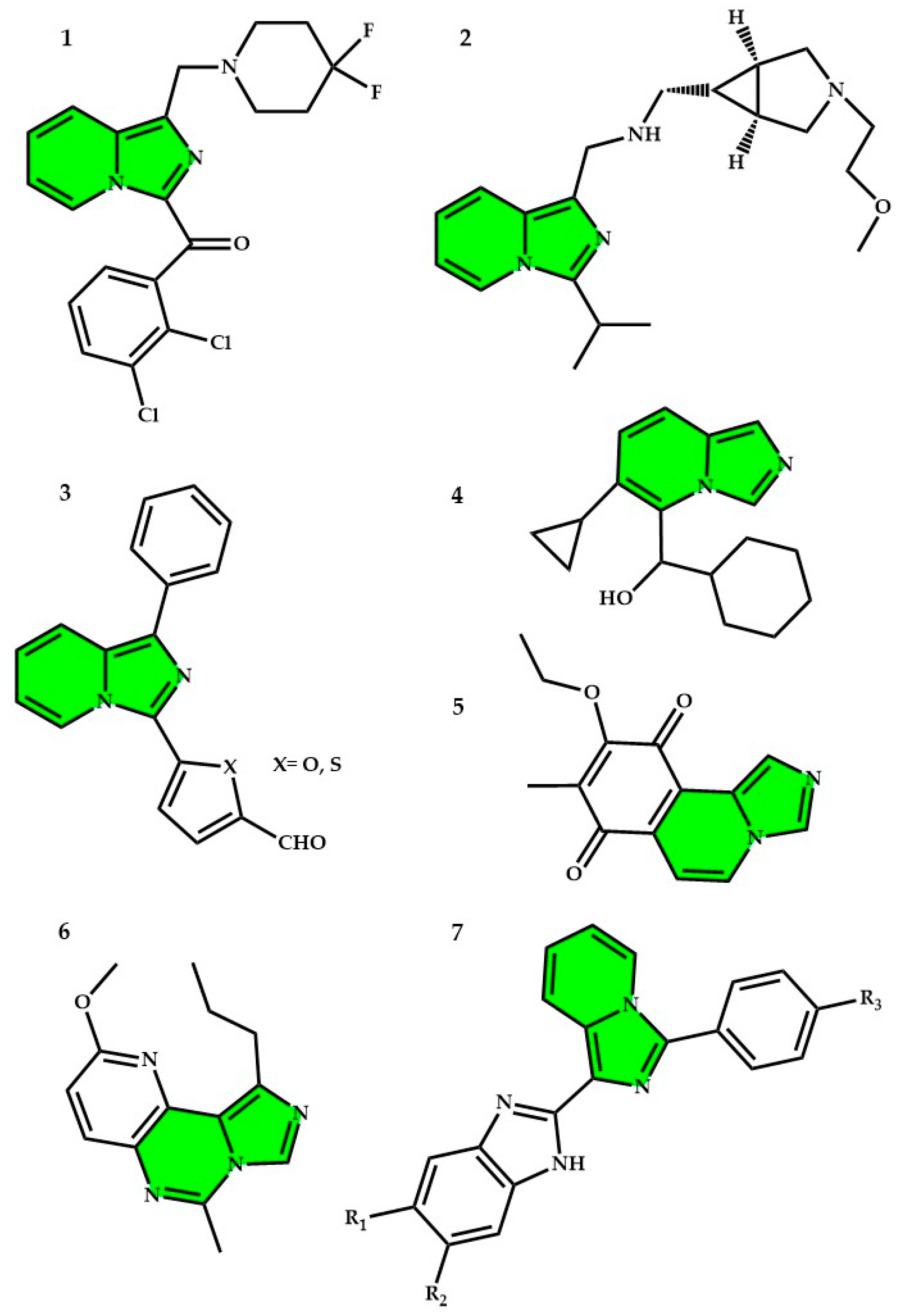
2.1. Imidazopyridines in Medicine
- Imidazo[1,2-b]pyridazines represent an important class of heterocyclic nuclei that provide various bioactive molecules. Among these, the successful kinase inhibitor ponatinib has sparked renewed interest in exploring new imidazo[1,2-b]pyridazine derivatives for their potential therapeutic applications in medicine, including anticancer agents, diagnostic tools for neuropathic diseases, thymic enhancers, antiparasitic, antibacterial, and antiviral agents, anti-inflammatory agents, and treatments for circadian rhythm sleep disorders [116,117,118,119].
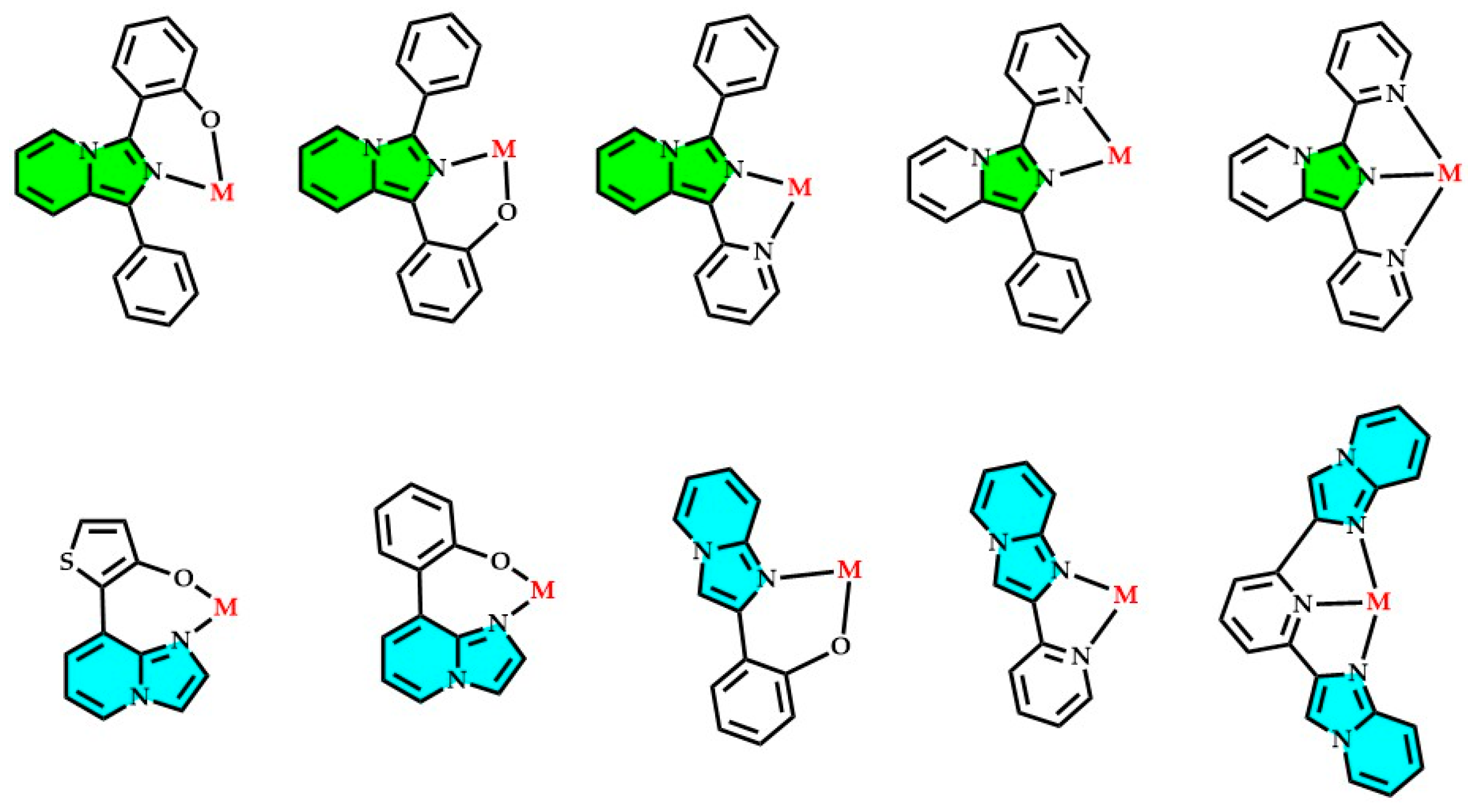
2.2. Imidazopyridines as Ligands toward Different Metal Ions
2.3. Imidazopyridines Applications in On–Off-Type Sensors
2.4. Imidazopyridines as Luminescent Probes in Energy Conversion Technology
2.5. Imidazopyridines in Fluorescent and Confocal Microscopy
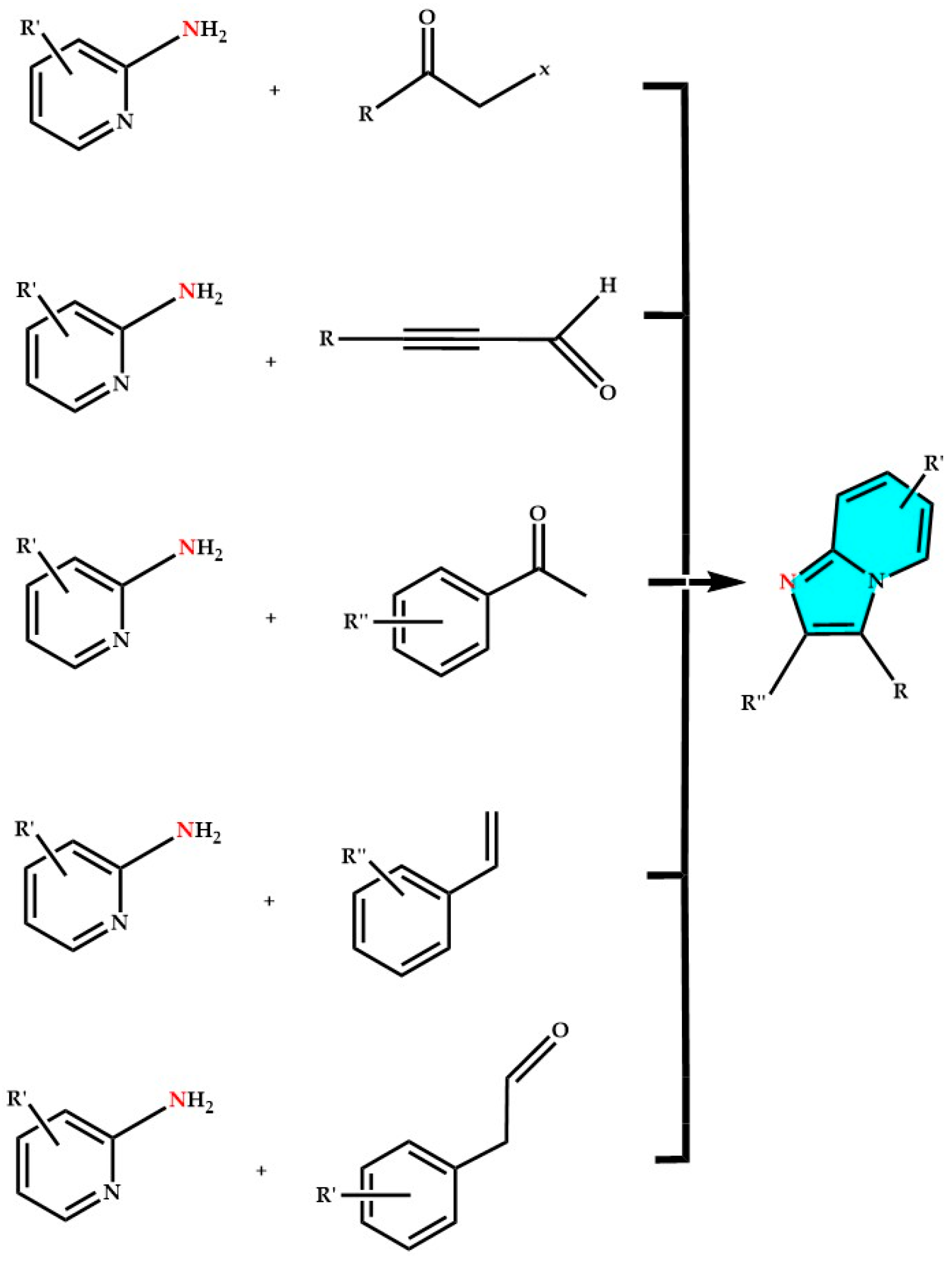
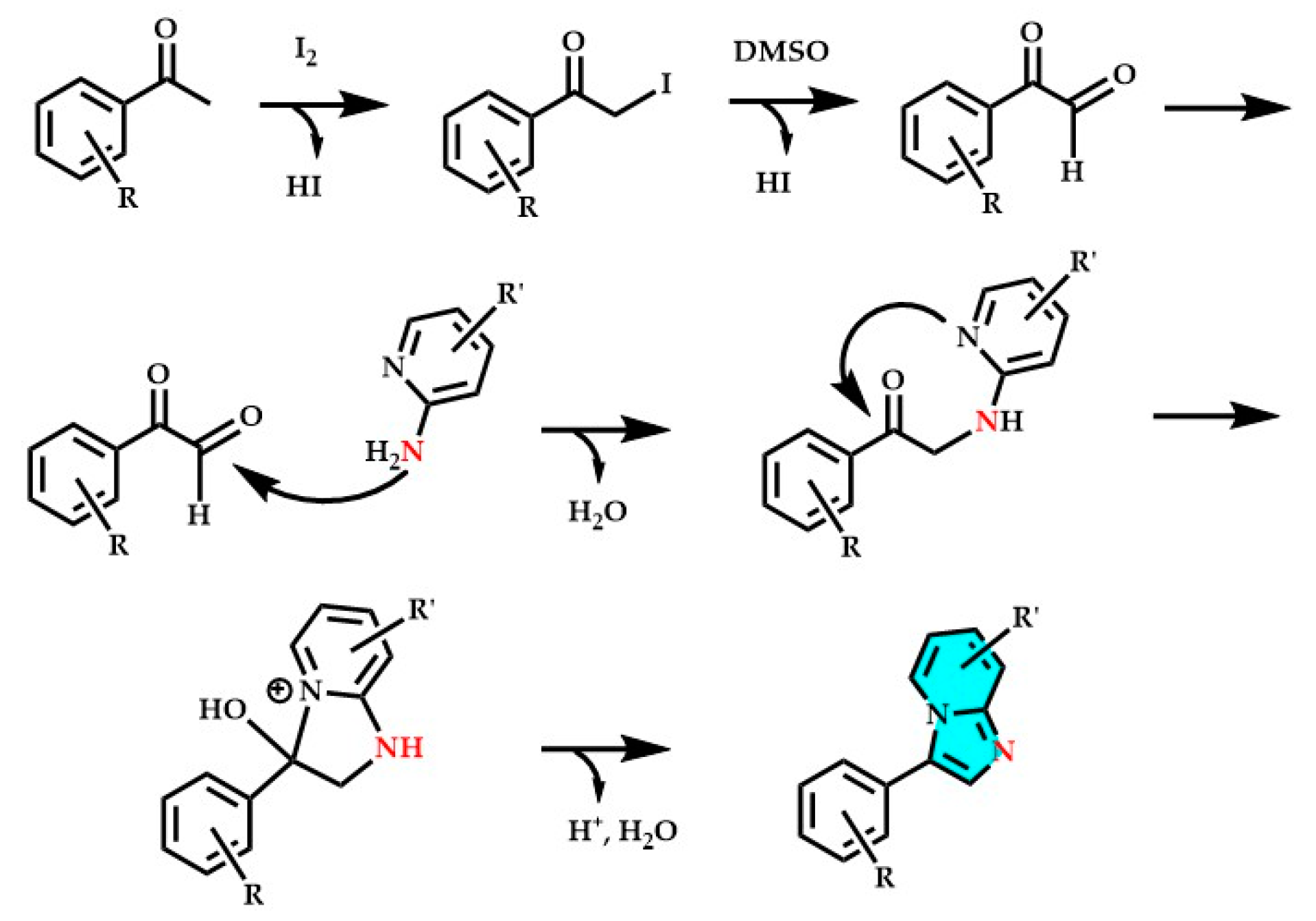
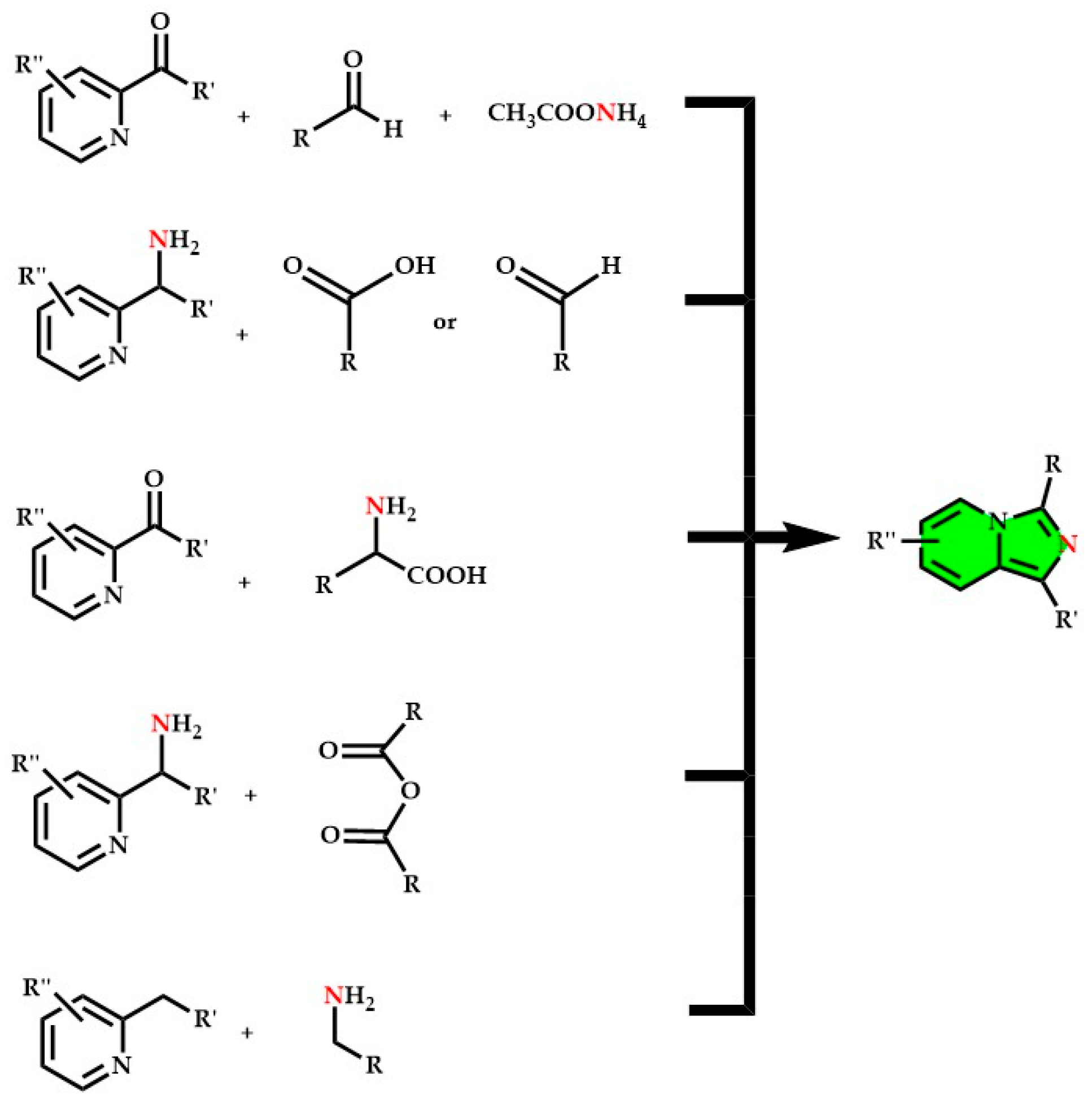
3. Imidazopyridines: Different Synthetic Approaches
- Substituted picolylamines (2-(Aminomethyl)pyridin) with carboxylic acids in strongly acidic and dehydrating conditions [9];
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanapalli, B.K.R.; Ashames, A.; Sigalapalli, D.K.; Shaik, A.B.; Bhandare, R.R.; Yele, V. Synthetic Imidazopyridine-Based Derivatives as Potential Inhibitors against Multi-Drug Resistant Bacterial Infections: A Review. Antibiotics 2022, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, R.; Zhang, D.; Wu, R.; Liu, T.; Wu, Z.; Zhang, J.; Hu, D. Synthesis, Insecticidal Activity, and Mode of Action of Novel Imidazopyridine Mesoionic Derivatives Containing an Amido Group. Pest Manag. Sci. 2022, 78, 4983–4993. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Ghosh, S.; Hajra, A. Electrochemical Functionalization of Imidazopyridine and Indazole: An Overview. Adv. Synth. Catal. 2021, 363, 5047–5071. [Google Scholar] [CrossRef]
- Camats, M.; Favier, I.; Mallet-Ladeira, S.; Pla, D.; Gómez, M. Understanding Cu(II)-Based Systems for C(Sp3)–H Bond Functionalization: Insights into the Synthesis of Aza-Heterocycles. Org. Biomol. Chem. 2022, 20, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chaudhran, P.A.; Sharma, A. Progress in the Development of Imidazopyridine-Based Fluorescent Probes for Diverse Applications. Crit. Rev. Anal. Chem. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Rabezzana, R. Imidazo[1,5-a]Pyridine Derivatives: Useful, Luminescent and Versatile Scaffolds for Different Applications. New J. Chem. 2021, 45, 5737–5743. [Google Scholar] [CrossRef]
- Pericherla, K.; Kaswan, P.; Pandey, K.; Kumar, A. Recent Developments in the Synthesis of Imidazo[1,2-a]Pyridines. Synthesis 2015, 47, 887–912. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Wu, Y.; Liu, W.; Wang, Y.; Li, Y. Syntheses and Optical Properties of BODIPY Derivatives Based on Imidazo[1,5-a]Pyridine. Chem. Lett. 2015, 44, 645–647. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Powell, J.R. Syntheses of 1,3-Disubstituted Imidazo[1,5-a]Pyridines. Tetrahedron Lett. 1986, 27, 3713–3714. [Google Scholar] [CrossRef]
- Murai, T.; Nagaya, E.; Shibahara, F.; Maruyama, T.; Nakazawa, H. Rhodium(I) and Iridium(I) Imidazo[1,5-a]Pyridine-1-Ylalkylalkoxy Complexes: Synthesis, Characterization and Application as Catalysts for Hydrosilylation of Alkynes. J. Organomet. Chem. 2015, 794, 76–80. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Fresta, E.; Casamassa, E.; Giordano, M.; Barolo, C.; Viscardi, G. Strategies to Increase the Quantum Yield: Luminescent Methoxylated Imidazo[1,5-a]Pyridines. Dye. Pigment. 2021, 192, 109455. [Google Scholar] [CrossRef]
- Volpi, G. Luminescent Imidazo[1,5-a]Pyridine Scaffold: Synthetic Heterocyclization Strategies-Overview and Promising Applications. Asian J. Org. Chem. 2022, 11, e202200171. [Google Scholar] [CrossRef]
- Colombo, G.; Attilio Ardizzoia, G.; Brenna, S. Imidazo[1,5-a]Pyridine-Based Derivatives as Highly Fluorescent Dyes. Inorganica Chim. Acta 2022, 535, 120849. [Google Scholar] [CrossRef]
- Mohbiya, D.R.; Sekar, N. Tuning ‘Stokes Shift’ and ICT Character by Varying the Donor Group in Imidazo[1,5 a]Pyridines: A Combined Optical, DFT, TD-DFT and NLO Approach. ChemistrySelect 2018, 3, 1635–1644. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Priola, E.; Magistris, C.; Chierotti, M.R.; Barolo, C. Halogenated Imidazo[1,5-a]Pyridines: Chemical Structure and Optical Properties of a Promising Luminescent Scaffold. Dye. Pigment. 2019, 171, 107713. [Google Scholar] [CrossRef]
- Vanda, D.; Zajdel, P.; Soural, M. Imidazopyridine-Based Selective and Multifunctional Ligands of Biological Targets Associated with Psychiatric and Neurodegenerative Diseases. Eur. J. Med. Chem. 2019, 181, 111569. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, R.; Moghimi-Rad, P.; Bule, M.H.; Souri, E.; Nadri, H.; Mahdavi, M.; Ghobadian, R.; Amini, M. New Imidazo[1,2-a]Pyridin-2-Yl Derivatives as AChE, BChE, and LOX Inhibitors; Design, Synthesis, and Biological Evaluation. Lett. Drug Des. Discov. 2023, 20, 1784–1798. [Google Scholar] [CrossRef]
- Samanta, S.; Kumar, S.; Aratikatla, K.E.; Ghorpade, S.R.; Singh, V. Recent Developments of Imidazo[1,2-a]Pyridine Analogues as Antituberculosis Agents. RSC Med. Chem. 2023, 14, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, E.; Shibahara, F.; Murai, T. 1-Alkynyl- and 1-Alkenyl-3-Arylimidazo[1,5-a]Pyridines: Synthesis, Photophysical Properties, and Observation of a Linear Correlation between the Fluorescent Wavelength and Hammett Substituent Constants. J. Org. Chem. 2011, 76, 6146–6158. [Google Scholar] [CrossRef]
- Murai, T.; Nagaya, E.; Miyahara, K.; Shibahara, F.; Maruyama, T. Synthesis and Characterization of Boron Complexes of Imidazo[1,5-a]Pyridylalkyl Alcohols. Chem. Lett. 2013, 42, 828–830. [Google Scholar] [CrossRef]
- Shibahara, F.; Kitagawa, A.; Yamaguchi, E.; Murai, T. Synthesis of 2-Azaindolizines by Using an Iodine-Mediated Oxidative Desulfurization Promoted Cyclization of N-2-Pyridylmethyl Thioamides and an Investigation of Their Photophysical Properties. Org. Lett. 2006, 8, 5621–5624. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, F.; Mizuno, T.; Shibata, Y.; Murai, T. Transfer Semihydrogenation of Alkynes Catalyzed by Imidazo[1,5-a]Pyrid-3-Ylidene–Pd Complexes: Positive Effects of Electronic and Steric Features on N-Heterocyclic Carbene Ligands. Bull. Chem. Soc. Jpn. 2020, 93, 332–337. [Google Scholar] [CrossRef]
- Pavlinac, I.B.; Zlatić, K.; Persoons, L.; Daelemans, D.; Banjanac, M.; Radovanović, V.; Butković, K.; Kralj, M.; Hranjec, M. Biological Activity of Amidino-Substituted Imidazo [4,5-b]Pyridines. Molecules 2023, 28, 34. [Google Scholar] [CrossRef] [PubMed]
- Boček, I.; Hranjec, M.; Vianello, R. Imidazo[4,5-b]Pyridine Derived Iminocoumarins as Potential pH Probes: Synthesis, Spectroscopic and Computational Studies of Their Protonation Equilibria. J. Mol. Liq. 2022, 355, 118982. [Google Scholar] [CrossRef]
- Boček, I.; Hok, L.; Persoons, L.; Daelemans, D.; Vianello, R.; Hranjec, M. Imidazo[4,5-b]Pyridine Derived Tubulin Polymerization Inhibitors: Design, Synthesis, Biological Activity in Vitro and Computational Analysis. Bioorg. Chem. 2022, 127, 106032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Suo, Q.; Li, Q.; Hu, J.; Zhu, Y.; Gao, Y.; Wang, Y. Multiresponsive Chemosensors Based on Ferrocenylimidazo[4,5-b]Pyridines: Solvent-Dependent Selective Dual Sensing of Hg2+ and Pb2+. Tetrahedron 2022, 120, 132878. [Google Scholar] [CrossRef]
- Zhang, B.; Suo, Q.; Li, Q.; Zhu, Y.; Gao, X.; Lv, L.; Gao, Y.; Jia, H.; Wang, Y. New Sulfur-Containing Ferrocenylimidazo[4,5-b]Pyridines: Multiresponsive Hg2+ Ion Sensing and Structure-Sensing Correlation. ChemistrySelect 2022, 7, e202103565. [Google Scholar] [CrossRef]
- Boček, I.; Starčević, K.; Novak Jovanović, I.; Vianello, R.; Hranjec, M. Novel Imidazo[4,5-b]Pyridine Derived Acrylonitriles: A Combined Experimental and Computational Study of Their Antioxidative Potential. J. Mol. Liq. 2021, 342, 117527. [Google Scholar] [CrossRef]
- Jarmoni, K.; Misbahi, K.; Ferrières, V. Imidazo[4,5-b]Pyridines: From Kinase Inhibitors to More Diversified Biological Properties. Curr. Med. Chem. 2024, 31, 515–528. [Google Scholar] [CrossRef]
- Krause, M.; Foks, H.; Gobis, K. Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]Pyridine and Imidazo[4,5-c]Pyridine Derivatives. Molecules 2017, 22, 399. [Google Scholar] [CrossRef]
- Rejinthala, S.; Endoori, S.; Thumma, V.; Mondal, T. New Imidazo[4,5-c]Pyridine-Piperidine Hybrids as Potential Anti-Cancer Agents and In-Silico Studies. ChemistrySelect 2024, 9, e202303299. [Google Scholar] [CrossRef]
- Mahata, S.; Dey, S.; Mandal, B.B.; Manivannan, V. 3-(2-Hydroxyphenyl)Imidazo[5, 1-a]Isoquinoline as Cu(II) Sensor, Its Cu(II) Complex for Selective Detection of CN− Ion and Biological Compatibility. J. Photochem. Photobiol. A Chem. 2022, 427, 113795. [Google Scholar] [CrossRef]
- Khalili, G. A Diastereoselective Synthesis of (Z)-3-[(Aryl)(Hydroxyimino)Methyl]-2-Cyclohexyl-1-(Cyclohexylamino)Imidazo[5,1-a]Isoquinolinium Chlorides from Isoquinoline, Chlorooximes, and Cyclohexyl Isocyanide. Monatshefte Chem. 2016, 147, 429–433. [Google Scholar] [CrossRef]
- Jafarpour, F.; Ashtiani, P.T. A Novel One-Step Synthesis of Imidazo[5,1-a]Isoquinolines via a Tandem Pd-Catalyzed Alkylation−Direct Arylation Sequence. J. Org. Chem. 2009, 74, 1364–1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Cui, H.-L. Asymmetric Synthesis of Imidazo[2,1-a]Isoquinolin-3-Ones with Dihydroisoquinolines and N-Substituted Amino Acids. Asian J. Org. Chem. 2022, 11, e202100761. [Google Scholar] [CrossRef]
- Meena, N.; Dhiman, S.; Rangan, K.; Kumar, A. Cobalt-Catalyzed Tandem One-Pot Synthesis of Polysubstituted Imidazo[1,5-a]Pyridines and Imidazo[1,5-a]Isoquinolines. Org. Biomol. Chem. 2022, 20, 4215–4223. [Google Scholar] [CrossRef]
- Cappelli, A.; Anzini, M.; Castriconi, F.; Grisci, G.; Paolino, M.; Braile, C.; Valenti, S.; Giuliani, G.; Vomero, S.; Di Capua, A.; et al. Design, Synthesis, and Biological Evaluation of Imidazo[1,5-a]Quinoline as Highly Potent Ligands of Central Benzodiazepine Receptors. J. Med. Chem. 2016, 59, 3353–3372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Chen, Y.; Xie, H.; Ding, C.; Tan, J.; Xu, K. Electrochemical Synthesis of Selenocyanated Imidazo[1,5-a]Quinolines under Metal Catalyst- and Chemical Oxidant-Free Conditions. Chin. Chem. Lett. 2020, 31, 1576–1579. [Google Scholar] [CrossRef]
- Albrecht, G.; Geis, C.; Herr, J.M.; Ruhl, J.; Göttlich, R.; Schlettwein, D. Electroluminescence and Contact Formation of 1-(Pyridin-2-Yl)-3-(Quinolin-2-Yl)Imidazo[1,5-a]Quinoline Thin Films. Org. Electron. 2019, 65, 321–326. [Google Scholar] [CrossRef]
- Albrecht, G.; Rössiger, C.; Herr, J.M.; Locke, H.; Yanagi, H.; Göttlich, R.; Schlettwein, D. Optimization of the Substitution Pattern of 1,3-Disubstituted Imidazo[1,5-a]Pyridines and -Quinolines for Electro-Optical Applications. Phys. Status Solidi B 2020, 257, 1900677. [Google Scholar] [CrossRef]
- Reddy, V.P.; Iwasaki, T.; Kambe, N. Synthesis of Imidazo and Benzimidazo[2,1-a]Isoquinolines by Rhodium-Catalyzed Intramolecular Double C–H Bond Activation. Org. Biomol. Chem. 2013, 11, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Volpi, G.; Garino, C.; Cardano, F.; Barolo, C.; Viscardi, G.; Fin, A. New Fluorescent Derivatives from Papaverine: Two Mechanisms to Increase the Quantum Yield. Dye. Pigment. 2023, 218, 111482. [Google Scholar] [CrossRef]
- Panda, J.; Raiguru, B.P.; Mishra, M.; Mohapatra, S.; Nayak, S. Recent Advances in the Synthesis of Imidazo[1,2-a]Pyridines: A Brief Review. ChemistrySelect 2022, 7, e202103987. [Google Scholar] [CrossRef]
- Yan, Z.; Wan, C.; Yang, Y.; Zha, Z.; Wang, Z. The Synthesis of Imidazo[1,5-a]Quinolines via a Decarboxylative Cyclization under Metal-Free Conditions. RSC Adv. 2018, 8, 23058–23065. [Google Scholar] [CrossRef]
- Kumar Bagdi, A.; Santra, S.; Monir, K.; Hajra, A. Synthesis of Imidazo[1,2-a]Pyridines: A Decade Update. Chem. Commun. 2015, 51, 1555–1575. [Google Scholar] [CrossRef]
- Ramana Reddy, M.; Darapaneni, C.M.; Patil, R.D.; Kumari, H. Recent Synthetic Methodologies for Imidazo[1,5-a]Pyridines and Related Heterocycles. Org. Biomol. Chem. 2022, 20, 3440–3468. [Google Scholar] [CrossRef]
- Ge, Y.; Xing, X.; Liu, A.; Ji, R.; Shen, S.; Cao, X. A Novel Imidazo[1,5-a]Pyridine-Rhodamine FRET System as an Efficient Ratiometric Fluorescent Probe for Hg2+ in Living Cells. Dye. Pigment. 2017, 146, 136–142. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, W.; Qin, Y.; Zhang, J.; Lu, N.; Liu, W.; Li, H.; Wang, Y.; Li, Y. Bidentate BODIPY-Appended 2-Pyridylimidazo[1,2-a]Pyridine Ligand and Fabrication of Luminescent Transition Metal Complexes. Polyhedron 2018, 148, 22–31. [Google Scholar] [CrossRef]
- Wu, W.H.; Zhao, J.Z.; Guo, H.M.; Sun, J.F.; Ji, S.M.; Wang, Z.L. Long-Lived Room-Temperature Near-IR Phosphorescence of BODIPY in a Visible-Light-Harvesting N C N PtII-Acetylide Complex with a Directly Metalated BODIPY Chromophore. Chem.-Eur. J. 2012, 18, 1961–1968. [Google Scholar] [CrossRef]
- Anupriya; Thomas, K.R.J.; Nagar, M.R.; Shahnawaz; Jou, J.-H. Imidazo[1,2-a]Pyridine Based Deep-Blue Emitter: Effect of Donor on the Optoelectronic Properties. J. Mater. Sci. Mater. Electron. 2021, 32, 26838–26850. [Google Scholar] [CrossRef]
- Zheng, X.-H.; Zhao, J.-W.; Chen, X.; Cai, R.; Yang, G.-X.; Zhu, J.-J.; Tang, S.-S.; Lin, Z.-H.; Tao, S.-L.; Tong, Q.-X. Imidazo[1,2-a]Pyridine as an Electron Acceptor to Construct High-Performance Deep-Blue Organic Light-Emitting Diodes with Negligible Efficiency Roll-Off. Chem.—Eur. J. 2020, 26, 8588–8596. [Google Scholar] [CrossRef]
- Girase, J.D.; Kajjam, A.B.; Dubey, D.K.; Kesavan, K.K.; Jou, J.-H.; Vaidyanathan, S. Unipolar 1-Phenylimidazo[1,5-a]Pyridine: A New Class of Ultra-Bright Sky-Blue Emitters for Solution-Processed Organic Light Emitting Diodes. New J. Chem. 2022, 46, 16717–16729. [Google Scholar] [CrossRef]
- Herr, J.M.; Rössiger, C.; Albrecht, G.; Yanagi, H.; Göttlich, R. Solvent-Free Microwave-Assisted Synthesis of Imidazo[1,5-a]Pyridine and –Quinoline Derivatives. Synth. Commun. 2019, 49, 2931–2940. [Google Scholar] [CrossRef]
- Albrecht, G.; Herr, J.M.; Steinbach, M.; Yanagi, H.; Göttlich, R.; Schlettwein, D. Synthesis, Optical Characterization and Thin Film Preparation of 1-(Pyridin-2-Yl)-3-(Quinolin-2-Yl)Imidazo[1,5-a]Quinoline. Dye. Pigment. 2018, 158, 334–341. [Google Scholar] [CrossRef]
- Weber, M.D.; Garino, C.; Volpi, G.; Casamassa, E.; Milanesio, M.; Barolo, C.; Costa, R.D. Origin of a Counterintuitive Yellow Light-Emitting Electrochemical Cell Based on a Blue-Emitting Heteroleptic Copper(I) Complex. Dalton Trans. 2016, 45, 8984–8993. [Google Scholar] [CrossRef]
- Fresta, E.; Volpi, G.; Garino, C.; Barolo, C.; Costa, R.D. Contextualizing Yellow Light-Emitting Electrochemical Cells Based on a Blue-Emitting Imidazo-Pyridine Emitter. Polyhedron 2018, 140, 129–137. [Google Scholar] [CrossRef]
- Fresta, E.; Volpi, G.; Milanesio, M.; Garino, C.; Barolo, C.; Costa, R.D. Novel Ligand and Device Designs for Stable Light-Emitting Electrochemical Cells Based on Heteroleptic Copper(I) Complexes. Inorg. Chem. 2018, 57, 10469–10479. [Google Scholar] [CrossRef]
- Yagishita, F.; Nii, C.; Tezuka, Y.; Tabata, A.; Nagamune, H.; Uemura, N.; Yoshida, Y.; Mino, T.; Sakamoto, M.; Kawamura, Y. Fluorescent N-Heteroarenes Having Large Stokes Shift and Water Solubility Suitable for Bioimaging. Asian J. Org. Chem. 2018, 7, 1614–1619. [Google Scholar] [CrossRef]
- Wang, L.; Han, X.; Qu, G.; Su, L.; Zhao, B.; Miao, J. A pH Probe Inhibits Senescence in Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 343. [Google Scholar] [CrossRef]
- Fukaminato, T. Single-Molecule Fluorescence Photoswitching: Design and Synthesis of Photoswitchable Fluorescent Molecules. J. Photochem. Photobiol. C-Photochem. Rev. 2011, 12, 177–208. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Zhou, Z.; Wang, S.; Xu, Y.; Gu, Y.; Zha, X. A New Fluorescent Probe for Quick and Highly Selective Detection of Hydrogen Sulfide and Its Application in Living Cells. New J. Chem. 2018, 42, 13884–13888. [Google Scholar] [CrossRef]
- Volpi, G.; Lace, B.; Garino, C.; Priola, E.; Artuso, E.; Cerreia Vioglio, P.; Barolo, C.; Fin, A.; Genre, A.; Prandi, C. New Substituted Imidazo[1,5-a]Pyridine and Imidazo[5,1-a]Isoquinoline Derivatives and Their Application in Fluorescence Cell Imaging. Dye. Pigment. 2018, 157, 298–304. [Google Scholar] [CrossRef]
- Kumari, N.; Singh, S.; Baral, M.; Kanungo, B.K. Schiff Bases: A Versatile Fluorescence Probe in Sensing Cations. J. Fluoresc. 2023, 33, 859–893. [Google Scholar] [CrossRef]
- Nirogi, R.; Mohammed, A.R.; Shinde, A.K.; Bogaraju, N.; Gagginapalli, S.R.; Ravella, S.R.; Kota, L.; Bhyrapuneni, G.; Muddana, N.R.; Benade, V.; et al. Synthesis and SAR of Imidazo[1,5-a]Pyridine Derivatives as 5-HT4 Receptor Partial Agonists for the Treatment of Cognitive Disorders Associated with Alzheimer’s Disease. Eur. J. Med. Chem. 2015, 103, 289–301. [Google Scholar] [CrossRef]
- Nicholson, A.N.; Pascoe, P.A. Hypnotic Activity of an Imidazo-Pyridine (Zolpidem). Br. J. Clin. Pharmacol. 1986, 21, 205–211. [Google Scholar] [CrossRef]
- Thakare, P.P.; Dakhane, S.; Shikh, A.N.; Modak, M.; Patil, A.; Bobade, V.D.; Mhaske, P.C. Design, Synthesis, Antimicrobial and Ergosterol Inhibition Activity of New 4-(Imidazo[1,2-a]Pyridin-2-Yl)Quinoline Derivatives. Polycycl. Aromat. Compd. 2022, 42, 5282–5299. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, G.; Huang, G. Design, Synthesis and Biological Evaluation of Imidazo[1,2-a]Pyridine Analogues or Derivatives as Anti-helmintic Drug. Chem. Biol. Drug Des. 2019, 93, 503–510. [Google Scholar] [CrossRef]
- Roby, O.; Kadiri, F.Z.; Loukhmi, Z.; Moutaouakil, M.; Tighadouini, S.; Saddik, R.; Aboulmouhajir, A. Synthesis of new set of imidazo[1,2-a]pyridine-schiff bases derivatives as potential antimicrobial agents: Experimental and theoretical approaches. J. Mol. Struct. 2023, 1292, 136186. [Google Scholar] [CrossRef]
- Chakravarty, H.; Ojha, D.; Konreddy, A.K.; Bal, C.; Chandra, N.S.; Sharon, A.; Chattopadhyay, D. Synthesis of Multi Ring-Fused Imidazo [1,2-a]Isoquinoline-Based Fluorescent Scaffold as Anti-Herpetic Agent. Antivir. Chem. Chemother. 2015, 24, 127–135. [Google Scholar] [CrossRef]
- Mannem, G.R.; Navudu, R.; Dubasi, N.; Mohammed, M.A.; Bollikolla, H.B. Design, and Synthesis of Aryl Derivatives of Imidazopyridine-Thiadiazoles as Possible Anticancer Agents. ChemistrySelect 2022, 7, e202200455. [Google Scholar] [CrossRef]
- Ge, Y.Q.; Li, F.R.; Zhang, Y.J.; Bi, Y.S.; Cao, X.Q.; Duan, G.Y.; Wang, J.W.; Liu, Z.L. Synthesis, Crystal Structure, Optical Properties and Antibacterial Evaluation of Novel Imidazo [1, 5-a] Pyridine Derivatives Bearing a Hydrazone Moiety. Luminescence 2014, 29, 293–300. [Google Scholar] [CrossRef]
- Guo, L.; Ding, Y.; Wang, H.; Liu, Y.; Qiang, Q.; Luo, Q.; Song, F.; Li, C. Imidazo[1,2-a]Pyridine Derivatives Synthesis from Lignin β-O-4 Segments via a One-Pot Multicomponent Reaction. iScience 2023, 26, 106834. [Google Scholar] [CrossRef]
- Chitrakar, R.; Rawat, D.; Sistla, R.; Vadithe, L.N.; Subbarayappa, A. Design, Synthesis and Anticancer Activity of Sulfenylated Imidazo-Fused Heterocycles. Bioorg. Med. Chem. Lett. 2021, 49, 128307. [Google Scholar] [CrossRef]
- Fauber, B.P.; Gobbi, A.; Robarge, K.; Zhou, A.; Barnard, A.; Cao, J.; Deng, Y.; Eidenschenk, C.; Everett, C.; Ganguli, A.; et al. Discovery of Imidazo[1,5-a]Pyridines and -Pyrimidines as Potent and Selective RORc Inverse Agonists. Bioorg. Med. Chem. Lett. 2015, 25, 2907–2912. [Google Scholar] [CrossRef]
- Roy, M.; Chakravarthi, B.V.S.K.; Jayabaskaran, C.; Karande, A.A.; Chakravarty, A.R. Impact of Metal Binding on the Antitumor Activity and Cellular Imaging of a Metal Chelator Cationic Imidazopyridine Derivative. Dalton Trans. 2011, 40, 4855–4864. [Google Scholar] [CrossRef]
- Spallarossa, A.; Rapetti, F.; Signorello, M.G.; Rosano, C.; Iervasi, E.; Ponassi, M.; Brullo, C. New Insights on Pharmacological Activity of Imidazo-Pyrazole Scaffold. ChemMedChem 2023, 18, e202300252. [Google Scholar] [CrossRef]
- Lončar, B.; Perin, N.; Mioč, M.; Boček, I.; Grgić, L.; Kralj, M.; Tomić, S.; Stojković, M.R.; Hranjec, M. Novel Amino Substituted Tetracyclic Imidazo[4,5-b]Pyridine Derivatives: Design, Synthesis, Antiproliferative Activity and DNA/RNA Binding Study. Eur. J. Med. Chem. 2021, 217, 113342. [Google Scholar] [CrossRef]
- Hranjec, M.; Lučić, B.; Ratkaj, I.; Pavelić, S.K.; Piantanida, I.; Pavelić, K.; Karminski-Zamola, G. Novel Imidazo[4,5-b]Pyridine and Triaza-Benzo[c]Fluorene Derivatives: Synthesis, Antiproliferative Activity and DNA Binding Studies. Eur. J. Med. Chem. 2011, 46, 2748–2758. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Rashwan, H.; Jamil, W.; Ali, S.; Kashif, S.M.; Rahim, F.; Salar, U.; Khan, K.M. Synthesis of 6-Chloro-2-Aryl-1H-Imidazo[4,5-b]Pyridine Derivatives: Antidiabetic, Antioxidant, β-Glucuronidase Inhibiton and Their Molecular Docking Studies. Bioorg. Chem. 2016, 65, 48–56. [Google Scholar] [CrossRef]
- Sedic, M.; Poznic, M.; Gehrig, P.; Scott, M.; Schlapbach, R.; Hranjec, M.; Karminski-Zamola, G.; Pavelic, K.; Pavelic, S.K. Differential Antiproliferative Mechanisms of Novel Derivative of Benzimidazo[1,2- α ]Quinoline in Colon Cancer Cells Depending on Their P53 Status. Mol. Cancer Ther. 2008, 7, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Kurteva, V. Recent Progress in Metal-Free Direct Synthesis of Imidazo[1,2-a]Pyridines. ACS Omega 2021, 6, 35173–35185. [Google Scholar] [CrossRef] [PubMed]
- Tali, J.A.; Kumar, G.; Sharma, B.K.; Rasool, Y.; Sharma, Y.; Shankar, R. Synthesis and Site Selective C–H Functionalization of Imidazo-[1,2-a]Pyridines. Org. Biomol. Chem. 2023, 21, 7267–7289. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Kaur Bhatia, R.; Kaur, R.; Kumar, S.; Kumar Jain, U.; Singh, H.; Batra, S.; Kaushik, D.; Kishore Deb, P. Imidazo[1,2-a]Pyridine Scaffold as Prospective Therapeutic Agents. Curr. Top. Med. Chem. 2017, 17, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Enguehard-Gueiffier, C.; Gueiffier, A. Recent Progress in the Pharmacology of Imidazo[1,2-a]Pyridines. Mini Rev. Med. Chem. 2007, 7, 888–899. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kumadaki, I.; Kobayashi, E. 1,3-Dipolar Cycloaddition Reaction of Trifluoroacetonitrile with Heterocyclic Ylides. Heterocycles 1981, 15, 1223. [Google Scholar] [CrossRef]
- Trotter, B.W.; Nanda, K.K.; Burgey, C.S.; Potteiger, C.M.; Deng, J.Z.; Green, A.I.; Hartnett, J.C.; Kett, N.R.; Wu, Z.; Henze, D.A.; et al. Imidazopyridine CB2 Agonists: Optimization of CB2/CB1 Selectivity and Implications for in Vivo Analgesic Efficacy. Bioorg. Med. Chem. Lett. 2011, 21, 2354–2358. [Google Scholar] [CrossRef]
- Hatano, K.; Sekimata, K.; Yamada, T.; Abe, J.; Ito, K.; Ogawa, M.; Magata, Y.; Toyohara, J.; Ishiwata, K.; Biggio, G.; et al. Radiosynthesis and in Vivo Evaluation of Two Imidazopyridineacetamides, [11C]CB184 and [11C]CB190, as a PET Tracer for 18 kDa Translocator Protein: Direct Comparison with [11C](R)-PK11195. Ann. Nucl. Med. 2015, 29, 325–335. [Google Scholar] [CrossRef]
- Chaudhari, V.; Bagwe-Parab, S.; Buttar, H.S.; Gupta, S.; Vora, A.; Kaur, G. Challenges and Opportunities of Metal Chelation Therapy in Trace Metals Overload-Induced Alzheimer’s Disease. Neurotox. Res. 2023, 41, 270–287. [Google Scholar] [CrossRef]
- El-Sayed, W.M.; Hussin, W.A.; Al-Faiyz, Y.S.; Ismail, M.A. The Position of Imidazopyridine and Metabolic Activation Are Pivotal Factors in the Antimutagenic Activity of Novel Imidazo[1,2-a]Pyridine Derivatives. Eur. J. Pharmacol. 2013, 715, 212–218. [Google Scholar] [CrossRef]
- Rival, Y.; Grassy, G.; Michel, G. Synthesis and Antibacterial Activity of Some Imidazo(1,2-a)Pyrimidine Derivatives. Chem. Pharm. Bull. 1992, 40, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A.; Lyons, A.S.; Muniyan, S.; D’Cunha, N.; Robinson, T.; Hoelting, K.; Dwyer, J.G.; Bu, X.R.; Batra, S.K.; Lin, M.-F. Novel Imidazopyridine Derivatives Possess Anti-Tumor Effect on Human Castration-Resistant Prostate Cancer Cells. PLoS ONE 2015, 10, e0131811. [Google Scholar] [CrossRef]
- Ahmadi, N.; Khoramjouy, M.; Azami Movahed, M.; Amidi, S.; Faizi, M.; Zarghi, A. Design, Synthesis, In Vitro and In Vivo Evaluation of New Imidazo[1,2-a]Pyridine Derivatives as Cyclooxygenase-2 Inhibitors. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2024, 24, 504–513. [Google Scholar] [CrossRef]
- Goel, R.; Luxami, V.; Paul, K. Imidazo[1,2-a]Pyridines: Promising Drug Candidate for Antitumor Therapy. Curr. Top. Med. Chem. 2016, 16, 3590–3616. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Bando, K.; Ashino, H.; Taguchi, K.; Shiraishi, H.; Fujimoto, O.; Kitamura, C.; Matsushima, S.; Fujinaga, M.; Zhang, M.-R.; et al. Synthesis and Biological Evaluation of Novel Radioiodinated Imidazopyridine Derivatives for Amyloid-β Imaging in Alzheimer’s Disease. Bioorg. Med. Chem. 2014, 22, 4189–4197. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Solozobova, V.; Kuznik, N.C.; Jung, N.; Gräßle, S.; Gourain, V.; Heneka, Y.M.; Cramer von Clausbruch, C.A.; Fuhr, O.; Munuganti, R.S.N.; et al. Identification of an Imidazopyridine-Based Compound as an Oral Selective Estrogen Receptor Degrader for Breast Cancer Therapy. Cancer Res. Commun. 2023, 3, 1378–1396. [Google Scholar] [CrossRef]
- Sucu, B.O. Biological Evaluation of Imidazopyridine Derivatives as Potential Anticancer Agents against Breast Cancer Cells. Med. Chem. Res. 2022, 31, 2231–2242. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Song, G.; Yang, D.; Huang, J.; Yao, X.; Qin, H.; Chen, Z.; Xu, Z.; Xu, C. A Novel Imidazopyridine Derivative Exerts Anticancer Activity by Inducing Mitochondrial Pathway-Mediated Apoptosis. BioMed Res. Int. 2020, 2020, e4929053. [Google Scholar] [CrossRef]
- Peytam, F.; Emamgholipour, Z.; Mousavi, A.; Moradi, M.; Foroumadi, R.; Firoozpour, L.; Divsalar, F.; Safavi, M.; Foroumadi, A. Imidazopyridine-Based Kinase Inhibitors as Potential Anticancer Agents: A Review. Bioorg. Chem. 2023, 140, 106831. [Google Scholar] [CrossRef]
- Soumyashree, D.K.; Reddy, D.S.; Nagarajaiah, H.; Naik, L.; Savanur, H.M.; Shilpa, C.D.; Sunitha Kumari, M.; Shanavaz, H.; Padmashali, B. Imidazopyridine Chalcones as Potent Anticancer Agents: Synthesis, Single-Crystal X-ray, Docking, DFT and SAR Studies. Arch. Pharm. 2023, 356, 2300106. [Google Scholar] [CrossRef]
- Zeng, K.; Ye, J.; Meng, X.; Dechert, S.; Simon, M.; Gong, S.; Mata, R.A.; Zhang, K. Anomeric Stereoauxiliary Cleavage of the C-N Bond of D-glucosamine for the Preparation of Imidazo[1,5-α]Pyridines. Chem.—A Eur. J. 2022, 28, e202200648. [Google Scholar] [CrossRef] [PubMed]
- Davey, D.; Erhardt, P.W.; Lumma, W.C.; Wiggins, J.; Sullivan, M.; Pang, D.; Cantor, E. Cardiotonic Agents. 1. Novel 8-Aryl-Substituted Imidazo[1,2-a] and [1,5-a]Pyridines and Imidazo[1,5-a]Pyridinones as Potential Positive Inotropic Agents. J. Med. Chem. 1987, 30, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mason, R.; VanDerveer, D.; Feng, K.; Bu, X.R. Convenient Preparation of a Novel Class of Imidazo[1,5-a]Pyridines: Decisive Role by Ammonium Acetate in Chemoselectivity. J. Org. Chem. 2003, 68, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.F.; Browne, L.J.; Campbell, T.; Gemenden, C.; Goldstein, R.; Gude, C.; Wasley, J.W.F. Imidazo[1,5-a]Pyridines—A New Class of Thromboxane-A2 Synthetase Inhibitors. J. Med. Chem. 1985, 28, 164–170. [Google Scholar] [CrossRef]
- Renno, G.; Cardano, F.; Volpi, G.; Barolo, C.; Viscardi, G.; Fin, A. Imidazo[1,5-a]Pyridine-Based Fluorescent Probes: A Photophysical Investigation in Liposome Models. Molecules 2022, 27, 3856. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, F.; Tanigawa, J.; Nii, C.; Tabata, A.; Nagamune, H.; Takanari, H.; Imada, Y.; Kawamura, Y. Fluorescent Imidazo[1,5-a]Pyridinium Salt for a Potential Cancer Therapy Agent. ACS Med. Chem. Lett. 2019, 10, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Fuse, S.; Ohuchi, T.; Asawa, Y.; Sato, S.; Nakamura, H. Development of 1-Aryl-3-Furanyl/Thienyl-Imidazopyridine Templates for Inhibitors against Hypoxia Inducible Factor (HIF)-1 Transcriptional Activity. Bioorg. Med. Chem. Lett. 2016, 26, 5887–5890. [Google Scholar] [CrossRef] [PubMed]
- Browne, L.J.; Gude, C.; Rodriguez, H.; Steele, R.E.; Bhatnager, A. Fadrozole Hydrochloride—A Potent, Selective, Nonsteroidal Inhibitor of Aromatase for the Treatment of Estrogen-Dependent Disease. J. Med. Chem. 1991, 34, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Perin, N.; Martin-Kleiner, I.; Nhili, R.; Laine, W.; David-Cordonnier, M.-H.; Vugrek, O.; Karminski-Zamola, G.; Kralj, M.; Hranjec, M. Biological Activity and DNA Binding Studies of 2-Substituted Benzimidazo[1,2-a]Quinolines Bearing Different Amino Side Chains. Med. Chem. Commun. 2013, 4, 1537. [Google Scholar] [CrossRef]
- Hranjec, M.; Kralj, M.; Piantanida, I.; Sedić, M.; Šuman, L.; Pavelić, K.; Karminski-Zamola, G. Novel Cyano- and Amidino-Substituted Derivatives of Styryl-2-Benzimidazoles and Benzimidazo[1,2-a]Quinolines. Synthesis, Photochemical Synthesis, DNA Binding, and Antitumor Evaluation, Part 3. J. Med. Chem. 2007, 50, 5696–5711. [Google Scholar] [CrossRef]
- Pässler, U.; Knölker, H.-J. The Pyrrolo[2,1-a]Isoquinoline Alkaloids. In The Alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 70, pp. 79–151. ISBN 978-0-12-391426-2. [Google Scholar]
- Newhouse, B.J.; Wenglowsky, S.; Grina, J.; Laird, E.R.; Voegtli, W.C.; Ren, L.; Ahrendt, K.; Buckmelter, A.; Gloor, S.L.; Klopfenstein, N.; et al. Imidazo[4,5-b]Pyridine Inhibitors of B-Raf Kinase. Bioorg. Med. Chem. Lett. 2013, 23, 5896–5899. [Google Scholar] [CrossRef] [PubMed]
- Krajčovičová, S.; Jorda, R.; Vanda, D.; Soural, M.; Kryštof, V. 1,4,6-Trisubstituted Imidazo[4,5-c]Pyridines as Inhibitors of Bruton’s Tyrosine Kinase. Eur. J. Med. Chem. 2021, 211, 113094. [Google Scholar] [CrossRef] [PubMed]
- Puerstinger, G.; Paeshuyse, J.; De Clercq, E.; Neyts, J. Antiviral 2,5-Disubstituted Imidazo[4,5-c]Pyridines: From Anti-Pestivirus to Anti-Hepatitis C Virus Activity. Bioorg. Med. Chem. Lett. 2007, 17, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.; Suresha Kumara, T.H.; Nagendrappa, G.; Sowmya, H.B.V.; Jasinski, J.P.; Millikan, S.P.; Chandrika, N.; More, S.S.; Harish, B.G. New Polyfunctional Imidazo[4,5-C]Pyridine Motifs: Synthesis, Crystal Studies, Docking Studies and Antimicrobial Evaluation. Eur. J. Med. Chem. 2014, 77, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Vera, G.; Delaye, P.-O.; Enguehard-Gueiffier, C. Imidazo[1,2-b]Pyridazine as Privileged Scaffold in Medicinal Chemistry: An Extensive Review. Eur. J. Med. Chem. 2021, 226, 113867. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.H.; Sorrell, F.J.; Bennett, J.M.; Fedorov, O.; Hanley, M.T.; Godoi, P.H.; Ruela de Sousa, R.; Robinson, S.; Navratilova, I.H.; Elkins, J.M.; et al. Imidazo[1,2-b]Pyridazines as Inhibitors of DYRK Kinases. Eur. J. Med. Chem. 2024, 269, 116292. [Google Scholar] [CrossRef] [PubMed]
- Akwata, D.; Kempen, A.L.; Lamptey, J.; Dayal, N.; Brauer, N.R.; Sintim, H.O. Discovery of Imidazo[1,2-b]Pyridazine-Containing TAK1 Kinase Inhibitors with Excellent Activities against Multiple Myeloma. RSC Med. Chem. 2024, 15, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xu, Y.; Yu, X.; Chen, Y.; Zhao, W.; Xie, Z.; Zhu, X.; Xu, H.; Yang, Y.; Zhang, P. Discovery of Imidazo[1,2-b]Pyridazine Macrocyclic Derivatives as Novel ALK Inhibitors Capable of Combating Multiple Resistant Mutants. Bioorg. Med. Chem. Lett. 2023, 89, 129309. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, F.; Kozai, N.; Nii, C.; Tezuka, Y.; Uemura, N.; Yoshida, Y.; Mino, T.; Sakamoto, M.; Kawamura, Y. Synthesis of Dimeric Imidazo[1, 5-a]Pyridines and Their Photophysical Properties. ChemistrySelect 2017, 2, 10694–10698. [Google Scholar] [CrossRef]
- Roseblade, S.J.; Ros, A.; Monge, D.; Alcarazo, M.; Alvarez, E.; Lassaletta, J.M.; Fernandez, R. Imidazo[1,5-a]Pyridin-3-Ylidene/Thioether Mixed C/S Ligands and Complexes Thereof. Organometallics 2007, 26, 2570–2578. [Google Scholar] [CrossRef]
- Yagishita, F.; Hoshi, K.; Mukai, S.; Kinouchi, T.; Katayama, T.; Yoshida, Y.; Minagawa, K.; Furube, A.; Imada, Y. Effect of Phenolic Substituent Position in Boron Complexes of Imidazo[1,5-a]Pyridine. Asian J. Org. Chem. 2022, 11, e202200040. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Conterosito, E.; Barolo, C.; Gobetto, R.; Viscardi, G. Facile Synthesis of Novel Blue Light and Large Stoke Shift Emitting Tetradentate Polyazines Based on Imidazo[1,5-a]Pyridine. Dye. Pigment. 2016, 128, 96–100. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Priola, E.; Diana, E.; Gobetto, R.; Buscaino, R.; Viscardi, G.; Barolo, C. Facile Synthesis of Novel Blue Light and Large Stoke Shift Emitting Tetradentate Polyazines Based on Imidazo[1,5-a]Pyridine—Part 2. Dye. Pigment. 2017, 143, 284–290. [Google Scholar] [CrossRef]
- Cerrato, V.; Volpi, G.; Priola, E.; Giordana, A.; Garino, C.; Rabezzana, R.; Diana, E. Mono-, Bis-, and Tris-Chelate Zn(II) Complexes with Imidazo[1,5-a]Pyridine: Luminescence and Structural Dependence. Molecules 2023, 28, 3703. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dyers, L.; Mason, R.; Amoyaw, P.; Bu, X.R. Highly Efficient and Direct Heterocyclization of Dipyridyl Ketone to N,N-Bidentate Ligands. J. Org. Chem. 2005, 70, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Herr, J.M.; Rössiger, C.; Locke, H.; Wilhelm, M.; Becker, J.; Heimbrodt, W.; Schlettwein, D.; Göttlich, R. Synthesis, Optical and Theoretical Characterization of Heteroleptic Iridium(III) Imidazo[1,5-a]Pyridine and -Quinoline Complexes. Dye. Pigment. 2020, 180, 108512. [Google Scholar] [CrossRef]
- Wei, Q.; Wei, M.-J.; Ou, Y.-J.; Zhang, J.-Y.; Huang, X.; Cai, Y.-P.; Si, L.-P. Formation and Conversion of Six Temperature-Dependent Fluorescent ZnII-Complexes Containing Two in Situ Formed N-Rich Heterocyclic Ligands. RSC Adv. 2017, 7, 6994–7002. [Google Scholar] [CrossRef]
- Priola, E.; Volpi, G.; Rabezzana, R.; Borfecchia, E.; Garino, C.; Benzi, P.; Martini, A.; Operti, L.; Diana, E. Bridging Solution and Solid-State Chemistry of Dicyanoaurate: The Case Study of Zn–Au Nucleation Units. Inorg. Chem. 2020, 59, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Zago, S.; Rabezzana, R.; Diana, E.; Priola, E.; Garino, C.; Gobetto, R. N-Based Polydentate Ligands and Corresponding Zn(II) Complexes: A Structural and Spectroscopic Study. Inorganics 2023, 11, 435. [Google Scholar] [CrossRef]
- Priola, E.; Conterosito, E.; Giordana, A.; Volpi, G.; Garino, C.; Andreo, L.; Diana, E.; Barolo, C.; Milanesio, M. Polymorphism and Solid State Peculiarities in Imidazo[1,5-a]Pyridine Core Deriving Compounds: An Analysis of Energetic and Structural Driving Forces. J. Mol. Struct. 2022, 1253, 132175. [Google Scholar] [CrossRef]
- Álvarez, C.M.; Álvarez-Miguel, L.; García-Rodríguez, R.; Martín-Álvarez, J.M.; Miguel, D. 3-(Pyridin-2-Yl)Imidazo[1,5-a]Pyridine (Pyridylindolizine) as Ligand in Complexes of Transition and Main-Group Metals: N,N-Chelating Ligands. Eur. J. Inorg. Chem. 2015, 2015, 4921–4934. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Brenna, S.; Durini, S.; Therrien, B. Synthesis and Characterization of Luminescent Zinc(II) Complexes with a N,N-Bidentate 1-Pyridylimidazo[1,5-a]Pyridine Ligand. Polyhedron 2015, 90, 214–220. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Ghiotti, D.; Therrien, B.; Brenna, S. Homoleptic Complexes of Divalent Metals Bearing N,O-Bidentate Imidazo[1,5-a]Pyridine Ligands: Synthesis, X-Ray Characterization and Catalytic Activity in the Heck Reaction. Inorganica Chim. Acta 2018, 471, 384–390. [Google Scholar] [CrossRef]
- Durini, S.; Ardizzoia, G.A.; Therrien, B.; Brenna, S. Tuning the Fluorescence Emission in Mononuclear Heteroleptic Trigonal Silver(I) Complexes. New J. Chem. 2017, 41, 3006–3014. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Brenna, S.; Durini, S.; Therrien, B.; Veronelli, M. Synthesis, Structure, and Photophysical Properties of Blue-Emitting Zinc(II) Complexes with 3-Aryl-Substituted 1-Pyridylimidazo[1,5-a]Pyridine Ligands: Blue-Emitting Zinc(II) Complexes. Eur. J. Inorg. Chem. 2014, 2014, 4310–4319. [Google Scholar] [CrossRef]
- Colombo, G.; Romeo, A.; Ardizzoia, G.A.; Furrer, J.; Therrien, B.; Brenna, S. Boron Difluoride Functionalized (Tetrahydroimidazo[1,5-a]Pyridin-3-Yl)Phenols: Highly Fluorescent Blue Emissive Materials. Dye. Pigment. 2020, 182, 108636. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Colombo, G.; Therrien, B.; Brenna, S. Tuning the Fluorescence Emission and HOMO-LUMO Band Gap in Homoleptic Zinc(II) Complexes with N,O-Bidentate (Imidazo[1,5-a]Pyrid-3-Yl)Phenols. Eur. J. Inorg. Chem. 2019, 2019, 1825–1831. [Google Scholar] [CrossRef]
- Colombo, G.; Attilio Ardizzoia, G.; Furrer, J.; Therrien, B.; Brenna, S. Driving the Emission Towards Blue by Controlling the HOMO-LUMO Energy Gap in BF2-Functionalized 2-(Imidazo[1,5-a]Pyridin-3-yl)Phenols. Chem. Eur. J. 2021, 27, 12380–12387. [Google Scholar] [CrossRef]
- Evans, R.C.; Douglas, P.; Winscom, C.J. Coordination Complexes Exhibiting Room-Temperature Phosphorescence: Evaluation of Their Suitability as Triplet Emitters in Organic Light Emitting Diodes. Coord. Chem. Rev. 2006, 250, 2093–2126. [Google Scholar] [CrossRef]
- Volpi, G.; Priola, E.; Garino, C.; Daolio, A.; Rabezzana, R.; Benzi, P.; Giordana, A.; Diana, E.; Gobetto, R. Blue Fluorescent Zinc(II) Complexes Based on Tunable Imidazo[1,5-a]Pyridines. Inorganica Chim. Acta 2020, 509, 119662. [Google Scholar] [CrossRef]
- Kundu, N.; Abtab, S.M.T.; Kundu, S.; Endo, A.; Teat, S.J.; Chaudhury, M. Triple-Stranded Helicates of Zinc(II) and Cadmium(II) Involving a New Redox-Active Multiring Nitrogenous Heterocyclic Ligand: Synthesis, Structure, and Electrochemical and Photophysical Properties. Inorg. Chem. 2012, 51, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, M.; Chierotti, M.R.; Gobetto, R.; Gomila, R.M.; Marzaroli, V.; Priola, E.; Volpi, G.; Zago, S.; Frontera, A.; Garino, C. One-Dimensional and Two-Dimensional Zn(II) Coordination Polymers with Ditopic Imidazo[1,5-a]Pyridine: A Structural and Computational Study. Molecules 2024, 29, 653. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Hidalgo, F.J. 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) Formation and Fate: An Example of the Coordinate Contribution of Lipid Oxidation and Maillard Reaction to the Production and Elimination of Processing-Related Food Toxicants. RSC Adv. 2015, 5, 9709–9721. [Google Scholar] [CrossRef]
- Kim, J.; Hahm, H.; Ryu, J.Y.; Byun, S.; Park, D.-A.; Lee, S.H.; Lim, H.; Lee, J.; Hong, S. Pyridine-Chelated Imidazo[1,5-a]Pyridine N-Heterocyclic Carbene Nickel(II) Complexes for Acrylate Synthesis from Ethylene and CO2. Catalysts 2020, 10, 758. [Google Scholar] [CrossRef]
- Swamy, C.A.P.; Varenikov, A.; Ruiter, G. de Chiral Imidazo[1,5-a]Pyridine–Oxazolines: A Versatile Family of NHC Ligands for the Highly Enantioselective Hydrosilylation of Ketones. Organometallics 2020, 39, 247–257. [Google Scholar] [CrossRef]
- Fresta, E.; Costa, R.D. Beyond Traditional Light-Emitting Electrochemical Cells—A Review of New Device Designs and Emitters. J. Mater. Chem. C 2017, 5, 5643–5675. [Google Scholar] [CrossRef]
- Cavinato, L.M.; Volpi, G.; Fresta, E.; Garino, C.; Fin, A.; Barolo, C. Microwave-Assisted Synthesis, Optical and Theoretical Characterization of Novel 2-(Imidazo[1,5-a]Pyridine-1-Yl)Pyridinium Salts. Chemistry 2021, 3, 714–727. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Yang, X.; Zhang, Q.; Li, Y.; Yao, J. Fe II, Co II and Ni II Complexes Based on 1-Chloro-3-(Pyridin-2-Yl)Imidazo[1,5-a]Pyridine: Synthesis, Structures, Single-Molecule Magnetic and Electrocatalytic Properties. New J. Chem. 2022, 46, 21780–21787. [Google Scholar] [CrossRef]
- Gao, Q.; Chen, Y.; Liu, Y.; Li, C.; Gao, D.; Wu, B.; Li, H.; Li, Y.; Liu, W.; Li, W. A Series of ZnII and CoII Complexes Based on 2-(Imidazo[1,5-a]Pyridin-3-Yl)Phenol: Syntheses, Structures, and Luminescent and Magnetic Properties. J. Coord. Chem. 2014, 67, 1673–1692. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dhar, S.; Nethaji, M.; Chakravarty, R.A. Ternary Iron(II) Complex with an Emissive Imidazopyridine Arm from Schiff Base Cyclizations and Its Oxidative DNA Cleavage Activity. Dalton Trans. 2005, 2, 349–353. [Google Scholar] [CrossRef]
- Santra, S.; Bagdi, A.K.; Majee, A.; Hajra, A. Iron(III)-Catalyzed Cascade Reaction between Nitroolefins and 2-Aminopyridines: Synthesis of Imidazo[1,2-a]Pyridines and Easy Access towards Zolimidine. Adv. Synth. Catal. 2013, 355, 1065–1070. [Google Scholar] [CrossRef]
- Prakasham, A.P.; Gangwar, M.K.; Ghosh, P. β-Enaminone Synthesis from 1,3-Dicarbonyl Compounds and Aliphatic and Aromatic Amines Catalyzed by Iron Complexes of Fused Bicyclic Imidazo[1,5-a]Pyridine Derived N-Heterocyclic Carbenes. Eur. J. Inorg. Chem. 2019, 2019, 295–313. [Google Scholar] [CrossRef]
- Wu, J.-J.; Cao, M.-L.; Ye, B.-H. Spontaneous Chiral Resolution of Mer-[CoII(N,N,O-L3)2] Enantiomers Mediated by π–π Interactions. Chem. Commun. 2010, 46, 3687–3689. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Garino, C.; Salassa, L.; Fiedler, J.; Hardcastle, K.I.; Gobetto, R.; Nervi, C. Cationic Heteroleptic Cyclometalated Iridium Complexes with 1-Pyridylimidazo[1,5-Alpha]Pyridine Ligands: Exploitation of an Efficient Intersystem Crossing. Chem.-Eur. J. 2009, 15, 6415–6427. [Google Scholar] [CrossRef] [PubMed]
- Salassa, L.; Garino, C.; Albertino, A.; Volpi, G.; Nervi, C.; Gobetto, R.; Hardcastle, K.I. Computational and Spectroscopic Studies of New Rhenium(I) Complexes Containing Pyridylimidazo[1,5-a]Pyridine Ligands: Charge Transfer and Dual Emission by Fine-Tuning of Excited States. Organometallics 2008, 27, 1427–1435. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Gobetto, R.; Nervi, C. Dipyridylmethane Ethers as Ligands for Luminescent Ir Complexes. Molecules 2021, 26, 7161. [Google Scholar] [CrossRef] [PubMed]
- Garino, C.; Ruiu, T.; Salassa, L.; Albertino, A.; Volpi, G.; Nervi, C.; Gobetto, R.; Hardcastle, K.I. Spectroscopic and Computational Study on New Blue Emitting ReL(CO)(3)Cl Complexes Containing Pyridylimidazo[1,5-a]Pyridine Ligands. Eur. J. Inorg. Chem. 2008, 23, 3587–3591. [Google Scholar] [CrossRef]
- Takizawa, S.; Nishida, J.; Tsuzuki, T.; Tokito, S.; Yamashita, Y. Phosphorescent Iridium Complexes Based on 2-Phenylimidazo[1,2-a]Pyridine Ligands: Tuning of Emission Color toward the Blue Region and Application to Polymer Light-Emitting Devices. Inorg. Chem. 2007, 46, 4308–4319. [Google Scholar] [CrossRef]
- Blanco-Rodríguez, A.M.; Kvapilova, H.; Sykora, J.; Towrie, M.; Nervi, C.; Volpi, G.; Zalis, S.; Vlcek, A. Photophysics of Singlet and Triplet Intraligand Excited States in [ReCl(CO)3(1-(2-Pyridyl)-Imidazo[1,5-α]Pyridine)] Complexes. J. Am. Chem. Soc. 2014, 136, 5963–5973. [Google Scholar] [CrossRef]
- Guckian, A.L.; Doering, M.; Ciesielski, M.; Walter, O.; Hjelm, J.; O’Boyle, N.M.; Henry, W.; Browne, W.R.; McGarvey, J.J.; Vos, J.G. Assessment of Intercomponent Interaction in Phenylene Bridged Dinuclear Ruthenium(II) and Osmium(II) Polypyridyl Complexes. Dalton Trans. 2004, 23, 3943–3949. [Google Scholar] [CrossRef]
- Sandroni, M.; Volpi, G.; Fiedler, J.; Buscaino, R.; Viscardi, G.; Milone, L.; Gobetto, R.; Nervi, C. Iridium and Ruthenium Complexes Covalently Bonded to Carbon Surfaces by Means of Electrochemical Oxidation of Aromatic Amines. Catal. Today 2010, 158, 22–28. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Chen, Z.; Liu, Y.; Hu, H.; Chen, W.; Liu, W.; Li, Y.; Lei, T.; Cao, Y.; et al. Metal-Mediated Controllable Creation of Secondary, Tertiary, and Quaternary Carbon Centers: A Powerful Strategy for the Synthesis of Iron, Cobalt, and Copper Complexes with in Situ Generated Substituted 1-Pyridineimidazo[1,5-a]Pyridine Ligands. Inorg. Chem. 2012, 51, 9705–9713. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Niu, J.-L.; Yang, M.-Z.; Li, Z.; Wu, L.-Y.; Hao, X.-Q.; Song, M.-P. New Type of 2,6-Bis(Imidazo[1,2-a]Pyridin-2-Yl)Pyridine-Based Ruthenium Complexes: Active Catalysts for Transfer Hydrogenation of Ketones. Organometallics 2015, 34, 1170–1176. [Google Scholar] [CrossRef]
- Giuso, V.; Jouaiti, E.; Cebrián, C.; Parant-Aury, S.; Kyritsakas, N.; Gourlaouen, C.; Mauro, M. Symmetry-Broken Charge-Transfer Excited State in Homoleptic Zinc(II) Imidazo[1,2-a]Pyridine Complexes. ChemPhotoChem 2023, 7, e202300092. [Google Scholar] [CrossRef]
- Divya, D.; Mala, R.; Nandhagopal, M.; Narayanasamy, M.; Thennarasu, S. Coordination of Distal Carboxylate Anion Alters Metal Ion Specific Binding in Imidazo[1,2-a]Pyridine Congeners. J. Fluoresc. 2023, 33, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, Ş.; Badoğlu, S. FT-IR Spectra, Vibrational Assignments, and Density Functional Calculations of Imidazo[1,2-a]Pyridine Molecule and Its Zn(II) Halide Complexes. Struct. Chem. 2009, 20, 423–434. [Google Scholar] [CrossRef]
- Song, G.-J.; Bai, S.-Y.; Dai, X.; Cao, X.-Q.; Zhao, B.-X. A Ratiometric Lysosomal pH Probe Based on the Imidazo[1,5-a]Pyridine-Rhodamine FRET and ICT System. RSC Adv. 2016, 6, 41317–41322. [Google Scholar] [CrossRef]
- Alharbi, K.H. A Review on Organic Colorimetric and Fluorescent Chemosensors for the Detection of Zn(II) Ions. Crit. Rev. Anal. Chem. 2022, 53, 1472–1488. [Google Scholar] [CrossRef]
- Ge, Y.; Ji, R.; Shen, S.; Cao, X.; Li, F. A Ratiometric Fluorescent Probe for Sensing Cu2+ Based on New Imidazo[1,5-a]Pyridine Fluorescent Dye. Sens. Actuators B Chem. 2017, 245, 875–881. [Google Scholar] [CrossRef]
- Bej, S.; Das, R.; Mondal, A.; Saha, R.; Sarkar, K.; Banerjee, P. Knoevenagel Condensation Triggered Synthesis of Dual-Channel Oxene Based Chemosensor: Discriminative Spectrophotometric Recognition of F−, CN– and HSO4− with Breast Cancer Cell Imaging, Real Sample Analysis and Molecular Keypad Lock Applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 273, 120989. [Google Scholar] [CrossRef]
- Hou, P.; Sun, J.; Wang, H.; Liu, L.; Zou, L.; Chen, S. TCF-Imidazo[1,5-α]Pyridine: A Potential Robust Ratiometric Fluorescent Probe for Glutathione Detection with High Selectivity. Sens. Actuators B Chem. 2020, 304, 127244. [Google Scholar] [CrossRef]
- Priyanga, S.; Khamrang, T.; Velusamy, M.; Karthi, S.; Ashokkumar, B.; Mayilmurugan, R. Coordination Geometry-Induced Optical Imaging of L-Cysteine in Cancer Cells Using Imidazopyridine-Based Copper(II) Complexes. Dalton Trans. 2019, 48, 1489–1503. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.; Hou, P. A Novel Imidazo[1,5-α]Pyridine-Based Fluorescent Probe with a Large Stokes Shift for Imaging Hydrogen Sulfide. Sens. Actuators B Chem. 2018, 256, 1086–1092. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Zhou, Z.; Luo, Y.; Wang, S.; Yuan, S.; Gu, Y.; Xu, Y.; Zha, X. A New Lysosome-Targetable Fluorescent Probe with a Large Stokes Shift for Detection of Endogenous Hydrogen Polysulfides in Living Cells. Anal. Chim. Acta 2019, 1056, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Chen, L.-L.; Zhu, X.-H.; Yuan, Z.-H.; Duan, Y.-T.; Yang, Y.-S.; Wang, B.-Z.; Wang, X.-M.; Zhu, H.-L. An Imidazo[1,5-α]Pyridine-Derivated Fluorescence Sensor for Rapid and Selective Detection of Sulfite. Talanta 2020, 217, 121087. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, A.; Ji, R.; Xu, Z.; Dong, J.; Ge, Y. A FRET-Based Probe for Detection of the Endogenous SO2 in Cells. Dye. Pigment. 2019, 165, 212–216. [Google Scholar] [CrossRef]
- Strianese, M.; Brenna, S.; Ardizzoia, G.A.; Guarnieri, D.; Lamberti, M.; D’Auria, I.; Pellecchia, C. Imidazo-Pyridine-Based Zinc(II) Complexes as Fluorescent Hydrogen Sulfide Probes. Dalton Trans. 2021, 50, 17075–17085. [Google Scholar] [CrossRef]
- Chen, S.; Hou, P.; Sun, J.; Wang, H.; Liu, L. Imidazo[1,5-α]Pyridine-Based Fluorescent Probe with a Large Stokes Shift for Specific Recognition of Sulfite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117508. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Liu, C.; Zhang, P.; Qin, K.; Ge, Y. An Imidazo[1,5-a]Pyridine Benzopyrylium-Based NIR Fluorescent Probe with Ultra-Large Stokes Shifts for Monitoring SO2. Molecules 2023, 28, 515. [Google Scholar] [CrossRef]
- Shen, S.-L.; Huang, X.-Q.; Zhang, Y.-Y.; Zhu, Y.; Hou, C.; Ge, Y.-Q.; Cao, X.-Q. Ratiometric Fluorescent Probe for the Detection of HOCl in Lysosomes Based on FRET Strategy. Sens. Actuators B Chem. 2018, 263, 252–257. [Google Scholar] [CrossRef]
- Song, G.-J.; Ma, H.-L.; Luo, J.; Cao, X.-Q.; Zhao, B.-X. A New Ratiometric Fluorescent Probe for Sensing HOC1 Based on TBET in Real Time. Dye. Pigment. 2018, 148, 206–211. [Google Scholar] [CrossRef]
- Shen, S.-L.; Zhang, X.-F.; Ge, Y.-Q.; Zhu, Y.; Cao, X.-Q. A Novel Ratiometric Fluorescent Probe for the Detection of HOCl Based on FRET Strategy. Sens. Actuator B Chem. 2018, 254, 736–741. [Google Scholar] [CrossRef]
- Ciupa, A.; Mahon, F.M.; Bank, P.A.D.; Caggiano, L. Simple Pyrazoline and Pyrazole “Turn on” Fluorescent Sensors Selective for Cd2+ and Zn2+ in MeCN. Org. Biomol. Chem. 2012, 10, 8753–8757. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Thakur, N.; Singh, A.; Shukla, P.; Kumar Maikhuri, V.; Garg, N.; Prasad, A.; Pandey, R. Development of a Fused Imidazo[1,2-a]Pyridine Based Fluorescent Probe for Fe3+ and Hg2+ in Aqueous Media and HeLa Cells. RSC Adv. 2019, 9, 29856–29863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Zhang, Q.; Li, D.; Li, Y. A One-Dimensional Cadmium Coordination Polymer: Synthesis, Structure, and Application as Luminescent Sensor for Cu2+ and CrO42−/Cr2O72− Ions. Eur. J. Inorg. Chem. 2021, 2021, 1349–1357. [Google Scholar] [CrossRef]
- Hutt, J.T.; Jo, J.; Olasz, A.; Chen, C.-H.; Lee, D.; Aron, Z.D. Fluorescence Switching of Imidazo[1,5-a]Pyridinium Ions: pH-Sensors with Dual Emission Pathways. Org. Lett. 2012, 14, 3162–3165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, G.-J.; Cao, X.-J.; Liu, J.-T.; Chen, M.-Y.; Cao, X.-Q.; Zhao, B.-X. A New Fluorescent pH Probe for Acidic Conditions. RSC Adv. 2015, 5, 89827–89832. [Google Scholar] [CrossRef]
- Shen, S.-L.; Zhang, X.-F.; Ge, Y.-Q.; Zhu, Y.; Cao, X.-Q. A Mitochondria-Targeting Ratiometric Fluorescent Probe for the Detection of Hypochlorite Based on the FRET Strategy. RSC Adv. 2017, 7, 55296–55300. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Alswaidan, I.A.; Rhyman, L.; Ramasami, P.; Sekar, N. Effect of Methoxy Group on NLOphoric Properties of Fluorescent 7-Arylstyryl-2-Methoxyphenylimidazo[1,2-a]Pyridine—Solvatochromic and Computational Method. J. Mol. Struct. 2018, 1173, 349–365. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Ramasami, P.; Sekar, N. Substituent Effects on Linear and Nonlinear Optical Properties of Fluorescent (E)-2-(4-Halophenyl)-7-Arlstyrylimidazo[1,2-A] Pyridine: Spectroscopic and Computational Methods. Physical Sciences Reviews 2019, 4, 20180032. [Google Scholar] [CrossRef]
- Volpi, G.; Galliano, S.; Buscaino, R.; Viscardi, G.; Barolo, C. Fluorescent Trifluoromethylated Imidazo[1,5-a]Pyridines and Their Application in Luminescent down-Shifting Conversion. J. Lumin. 2022, 242, 118529. [Google Scholar] [CrossRef]
- Volpi, G.; Magnano, G.; Benesperi, I.; Saccone, D.; Priola, E.; Gianotti, V.; Milanesio, M.; Conterosito, E.; Barolo, C.; Viscardi, G. One Pot Synthesis of Low Cost Emitters with Large Stokes’ Shift. Dye. Pigment. 2017, 137, 152–164. [Google Scholar] [CrossRef]
- Rössiger, C.; Oel, T.; Schweitzer, P.; Vasylets, O.; Kirchner, M.; Abdullahu, A.; Schlettwein, D.; Göttlich, R. Synthesis and Optical and Theoretical Characterization of Imidazo[5,1-a]Isoquinolines and Imidazo[1,5-a]Quinolines. Eur. J. Org. Chem. 2024, e202400298. [Google Scholar] [CrossRef]
- Kulhanek, N.; Martin, N.; Göttlich, R. Highly Versatile Preparation of Imidazo[1,5-a]Quinolines and Characterization of Their Photoluminescent Properties. Eur. J. Org. Chem. 2024, 27, e202301007. [Google Scholar] [CrossRef]
- Nitha, P.R.; Soman, S.; John, J. Indole Fused Heterocycles as Sensitizers in Dye-Sensitized Solar Cells: An Overview. Mater. Adv. 2021, 2, 6136–6168. [Google Scholar] [CrossRef]
- Giordano, M.; Volpi, G.; Bonomo, M.; Mariani, P.; Garino, C.; Viscardi, G. Methoxy-Substituted Copper Complexes as Possible Redox Mediators in Dye-Sensitized Solar Cells. New J. Chem. 2021, 45, 15303–15311. [Google Scholar] [CrossRef]
- Prostota, Y.; Kachkovsky, O.D.; Reis, L.V.; Santos, P.F. New Unsymmetrical Squaraine Dyes Derived from Imidazo[1,5-a]Pyridine. Dye. Pigment. 2013, 96, 554–562. [Google Scholar] [CrossRef]
- Irfan, A.; Chaudhry, A.R.; Al-Sehemi, A.G.; Assiri, M.A.; Hussain, A. Charge Carrier and Optoelectronic Properties of Phenylimidazo[1,5-a]Pyridine-Containing Small Molecules at Molecular and Solid-State Bulk Scales. Comput. Mater. Sci. 2019, 170, 109179. [Google Scholar] [CrossRef]
- Colombo, G.; Cinco, A.; Ardizzoia, G.A.; Brenna, S. Long-Alkyl Chain Functionalized Imidazo[1,5-a]Pyridine Derivatives as Blue Emissive Dyes. Colorants 2023, 2, 179–193. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, X.; Ran, P.; Xia, Q.; Yang, C.; Ma, J.; Fu, Y. A Novel Luminescence-Functionalized Metal-Organic Framework Nanoflowers Electrochemiluminesence Sensor via “on-off” System. Biosens. Bioelectron. 2017, 91, 436–440. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Kwon, N.; Ma, H.; Yoon, J. Activatable Fluorescent Probes for in Situ Imaging of Enzymes. Chem. Soc. Rev. 2022, 51, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Mohana Roopan, S.; Patil, S.M.; Palaniraja, J. Recent Synthetic Scenario on Imidazo[1,2-a]Pyridines Chemical Intermediate. Res. Chem. Intermed. 2016, 42, 2749–2790. [Google Scholar] [CrossRef]
- Ravi, C.; Adimurthy, S. Synthesis of Imidazo[1,2-a]Pyridines: C-H Functionalization in the Direction of C-S Bond Formation. Chem. Rec. 2017, 17, 1019–1038. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Laru, S.; Hajra, A. Ortho C−H Functionalization of 2-Arylimidazo[1,2-a]Pyridines. Chem. Rec. 2022, 22, e202100240. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.D.; Ramage, G.R. Heterocyclic Systems Related to Pyrrocoline. Part I. 2: 3a-Diazaindene. J. Chem. Soc. 1955; 2834–2837. [Google Scholar] [CrossRef]
- Palazzo, G.; Picconi, G. Derivatives if imidazol (1,5-a) pyridin-3(2H)-one. Farm. Sci. 1975, 30, 197–207. [Google Scholar]
- Volpi, G.; Garino, C.; Breuza, E.; Gobetto, R.; Nervi, C. Dipyridylketone as a Versatile Ligand Precursor for New Cationic Heteroleptic Cyclometalated Iridium Complexes. Dalton Trans. 2011, 41, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Garino, C.; Nervi, C. Exploring Synthetic Pathways to Cationic Heteroleptic Cyclometalated Iridium Complexes Derived from Dipyridylketone. Dalton Trans. 2012, 41, 7098–7108. [Google Scholar] [CrossRef] [PubMed]
- Andreo, L.; Volpi, G.; Rossi, F.; Benzi, P.; Diana, E. Two-Step Synthesis of a New Twenty-Membered Macrocycle: Spectroscopic Characterization and Theoretical Calculations. ChemistrySelect 2022, 7, e202202564. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Priola, E.; Barolo, C. A New Auspicious Scaffold for Small Dyes and Fluorophores. Dye. Pigment. 2022, 197, 109849. [Google Scholar] [CrossRef]
- Rahmati, A.; Zahra, K. One-Pot Three-Component Synthesis of Imidazo[1,5-a]Pyridines. Int. J. Org. Chem. 2011, 01, 15–19. [Google Scholar] [CrossRef]
- Crawforth, J.M.; Paoletti, M. A One-Pot Synthesis of Imidazo[1,5-a]Pyridines. Tetrahedron Lett. 2009, 50, 4916–4918. [Google Scholar] [CrossRef]
- Siddiqui, S.; Potewar, T.; Lahoti, R.; Srinivasan, K. Ionic Liquid Promoted Facile One-Pot Synthesis of 1-Pyridylimidazo[1,5-a]Pyridines from Dipyridylketone and Aryl Aldehydes. Synthesis 2006, 2006, 2849–2854. [Google Scholar] [CrossRef]
- Aksenov, D.A.; Arutiunov, N.A.; Maliuga, V.V.; Aksenov, A.V.; Rubin, M. Synthesis of Imidazo[1,5-a]Pyridines via Cyclocondensation of 2-(Aminomethyl)Pyridines with Electrophilically Activated Nitroalkanes. Beilstein J. Org. Chem. 2020, 16, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Fulwa, V.K.; Sahu, R.; Jena, H.S.; Manivannan, V. Novel Synthesis of 2,4-Bis(2-Pyridyl)-5-(Pyridyl)Imidazoles and Formation of N-(3-(Pyridyl)Imidazo[1,5-a]Pyridine)Picolinamidines: Nitrogen-Rich Ligands. Tetrahedron Lett. 2009, 50, 6264–6267. [Google Scholar] [CrossRef]
- Volpi, G.; Magistris, C.; Garino, C. FLUO-SPICES: Natural Aldehydes Extraction and One-Pot Reaction to Prepare and Characterize New Interesting Fluorophores. Educ. Chem. Eng. 2018, 24, 1–6. [Google Scholar] [CrossRef]
- Volpi, G.; Magistris, C.; Garino, C. Natural Aldehyde Extraction and Direct Preparation of New Blue Light-Emitting Imidazo[1,5-a]Pyridine Fluorophores. Nat. Prod. Res. 2018, 32, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Mohan, D.C.; Adimurthy, S. Copper-Catalyzed Denitrogenative Transannulation Reaction of Pyridotriazoles: Synthesis of Imidazo[1,5-a]Pyridines with Amines and Amino Acids. Org. Lett. 2016, 18, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Gómez, A.; Gutiérrez-Hernández, A.I.; Alvarado-Castillo, M.A.; Toscano, R.A.; Ortega-Alfaro, M.C.; López-Cortés, J.G. Selenoamides as Powerful Scaffold to Build Imidazo[1,5-a]Pyridines Using a Grinding Protocol. J. Organomet. Chem. 2020, 919, 121315. [Google Scholar] [CrossRef]
- Wang, L.-S.; Zhou, Y.; Lei, S.-G.; Yu, X.-X.; Huang, C.; Wu, Y.-D.; Wu, A.-X. Iodine-Mediated Multicomponent Cascade Cyclization and Sulfenylation/Selenation: Synthesis of Imidazo[2,1-a]Isoquinoline Derivatives. Org. Lett. 2022, 24, 4449–4453. [Google Scholar] [CrossRef]
- Sahoo, S.; Pal, S. Rapid Access to Benzimidazo[1,2-a]Quinoline-Fused Isoxazoles via Pd(II)-Catalyzed Intramolecular Cross Dehydrogenative Coupling: Synthetic Versatility and Photophysical Studies. J. Org. Chem. 2021, 86, 4081–4097. [Google Scholar] [CrossRef]
- Yamaguchi, E.; Shibahara, F.; Murai, T. Direct Sequential C3 and C1 Arylation Reaction of Imidazo[1,5-a]Pyridine Catalyzed by a 1,10-Phenanthroline–Palladium Complex. Chem. Lett. 2011, 40, 939–940. [Google Scholar] [CrossRef]
- Santra, S.; Mitra, S.; Bagdi, A.K.; Majee, A.; Hajra, A. Iron(III)-Catalyzed Three-Component Domino Strategy for the Synthesis of Imidazo[1,2-a]Pyridines. Tetrahedron Lett. 2014, 55, 5151–5155. [Google Scholar] [CrossRef]
- Albano, S.; Olivo, G.; Mandolini, L.; Massera, C.; Ugozzoli, F.; Di Stefano, S. Formation of Imidazo[1,5-a]Pyridine Derivatives Due to the Action of Fe2+ on Dynamic Libraries of Imines. J. Org. Chem. 2017, 82, 3820–3825. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, X.; Zheng, Q.; Li, Z.; Wu, J.; Gao, G. Thioether-Assisted Cu-Catalyzed C5–H Arylation of Imidazo[1,5-a]Pyridines. Org. Lett. 2022, 24, 3834–3838. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, W.; Wang, Z.; Yu, L.; Xu, K. Copper-Catalyzed Oxidative Amination of Sp(3) C-H Bonds under Air: Synthesis of 1,3-Diarylated Imidazo[1,5-Alpha]Pyridines. J. Org. Chem. 2015, 80, 2431–2435. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y.; Ye, Y.; Zou, Y.; Jiang, H.; Zeng, W. Cu(I)-Catalyzed Transannulation of N-Heteroaryl Aldehydes or Ketones with Alkylamines via C(Sp3)–H Amination. Org. Lett. 2014, 16, 6232–6235. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.F.; Saraiva, M.T.; Debia, N.P.; Silva, L.; Chaves, O.A.; Stieler, R.; Iglesias, B.A.; Rodembusch, F.S.; Lüdtke, D.S. Imidazo[1,2-a]Pyridine-Based Hybrids. Copper-Catalyzed Cycloaddition Synthesis, Photophysics, Docking, and Interaction Studies with Biomacromolecules. Dye. Pigment. 2023, 214, 111212. [Google Scholar] [CrossRef]
- Bluhm, M.E.; Ciesielski, M.; Görls, H.; Döring, M. Copper-Catalyzed Oxidative Heterocyclization by Atmospheric Oxygen. Angew. Chem. Int. Ed. 2002, 41, 2962–2965. [Google Scholar] [CrossRef]
- Bluhm, M.E.; Ciesielski, M.; Görls, H.; Walter, O.; Döring, M. Complexes of Schiff Bases and Intermediates in the Copper-Catalyzed Oxidative Heterocyclization by Atmospheric Oxygen§. Inorg. Chem. 2003, 42, 8878–8885. [Google Scholar] [CrossRef]
- Bluhm, M.E.; Folli, C.; Pufky, D.; Kröger, M.; Walter, O.; Döring, M. 3-Aminoiminoacrylate, 3-Aminoacrylate, and 3-Amidoiminomalonate Complexes as Catalysts for the Dimerization of Olefins. Organometallics 2005, 24, 4139–4152. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Xin, L.; Liu, W.; Wang, Z.; Xu, K. Synthesis of 1,3-Disubstituted Imidazo[1,5-a]Pyridines from Amino Acids via Catalytic Decarboxylative Intramolecular Cyclization. J. Org. Chem. 2016, 81, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Dao, P.D.Q.; Lim, H.-J.; Cho, C.S. Recyclable Magnetic Cu-MOF-74-Catalyzed C(Sp2)-N Coupling and Cyclization under Microwave Irradiation: Synthesis of Imidazo[1,2-c]Quinazolines and Their Analogues. ACS Omega 2023, 8, 16218–16227. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Yan, Z.; Zhou, Z.; Hu, K.; Wang, J.; Li, Z.; Zha, Z.; Wang, Z. Electrocatalytic Intermolecular C(Sp3)–H/N–H Coupling of Methyl N-Heteroaromatics with Amines and Amino Acids: Access to Imidazo-Fused N-Heterocycles. Org. Lett. 2018, 20, 6359–6363. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Mohan, D.C.; Adimurthy, S. Lewis Acid-Catalyzed Denitrogenative Transannulation of Pyridotriazoles with Nitriles: Synthesis of Imidazopyridines. J. Org. Chem. 2016, 81, 9461–9469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zha, M.; Zhou, F.; Chen, X.; Wang, B.; Liu, G.; Qian, L. CBr 4 Mediated [4 + 1] Dehydrocyclization for the Synthesis of Functionalized Imidazo[1,5-a]Heterocycles from Pyridin-2-Ylmethanamines and Aldehydes. ACS Omega 2021, 6, 20303–20308. [Google Scholar] [CrossRef] [PubMed]
- Bori, J.; Manivannan, V. A New Synthetic Route for Synthesis of 3-substituted Imidazo[1,5-a]Pyridines. J. Heterocycl. Chem. 2022, 59, 1073–1078. [Google Scholar] [CrossRef]
- Grigg, R.; Kennewell, P.; Savic, V.; Sridharan, V. X=Y-Zh Systems as Potential 1,3-Dipoles Part 38. 1,5-Electrocyclization of Vinyl-Azomethine and Iminyl-Azomethine Ylides—2-Azaindolizines and Pyrrolo-Dihydroisoquinolines. Tetrahedron 1992, 48, 10423–10430. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Zha, Z.; Wang, Z. Mild Metal-Free Sequential Dual Oxidative Amination of C(Sp3)–H Bonds: Efficient Synthesis of Imidazo[1,5-a]Pyridines. Org. Lett. 2013, 15, 2274–2277. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Hou, J.; Liu, J.; Yu, W.; Chang, J. Synthesis of Imidazo[1,5-a]Pyridines via I 2 -Mediated Sp 3 C–H Amination. Org. Biomol. Chem. 2018, 16, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, F.; Sugiura, R.; Yamaguchi, E.; Kitagawa, A.; Murai, T. Synthesis of Fluorescent 1,3-Diarylated Imidazo[1,5-a]Pyridines: Oxidative Condensation−Cyclization of Aryl-2-Pyridylmethylamines and Aldehydes with Elemental Sulfur as an Oxidant. J. Org. Chem. 2009, 74, 3566–3568. [Google Scholar] [CrossRef]
- Qin, M.; Tian, Y.; Guo, X.; Yuan, X.; Yang, X.; Chen, B. I2/TBPB Mediated Oxidative Reaction to Construct of Imidazo[1,5-α]Pyridines under Metal-Free Conditions. Asian J. Org. Chem. 2018, 7, 1591–1594. [Google Scholar] [CrossRef]
- Cui, T.; Zhan, Y.; Dai, C.; Lin, J.; Liu, P.; Sun, P. Electrochemical Oxidative Regioselective C–H Cyanation of Imidazo[1,2-a]Pyridines. J. Org. Chem. 2021, 86, 15897–15905. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.B.; Coelho, F.L.; Rodembusch, F.S.; Schwab, R.S.; Schneider, J.M.F.M.; da Silveira Rampon, D.; Schneider, P.H. Straightforward Synthesis of Photoactive Chalcogen Functionalized Benzimidazo[1,2-a]Quinolines. New J. Chem. 2019, 43, 11596–11603. [Google Scholar] [CrossRef]
- Qian, P.; Yan, Z.; Zhou, Z.; Hu, K.; Wang, J.; Li, Z.; Zha, Z.; Wang, Z. Electrocatalytic Tandem Synthesis of 1,3-Disubstituted Imidazo[1,5-a]Quinolines via Sequential Dual Oxidative C(Sp3)–H Amination in Aqueous Medium. J. Org. Chem. 2019, 84, 3148–3157. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, Y.-M.; Park, B.-R.; Kim, J.-N. Synthesis of Imidazo[1,5-α]Quinolines and Imidazo[5,1-α]Isoquinolines via the In-Mediated Allylation of Reissert Compounds. Bull. Korean Chem. Soc. 2010, 31, 3031–3034. [Google Scholar] [CrossRef]
- Pelletier, G.; Charette, A.B. Triflic Anhydride Mediated Synthesis of Imidazo[1,5-a]Azines. Org. Lett. 2013, 15, 2290–2293. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Glover, E.E.; Vaughan, K.D. The N-Amination and Subsequent Oxidation by Bromine of Imidazo[1,5-a]Pyridines. J. Chem. Soc. Perkin Trans. 1 1976, 16, 1722–1724. [Google Scholar] [CrossRef]
- Fulwa, V.K.; Manivannan, V. Synthesis of Some 1,3-Disubstituted Imidazo[1,5-a]Pyridines Using 2-Cyanopyridine. Tetrahedron Lett. 2012, 53, 2420–2423. [Google Scholar] [CrossRef]
- Moulin, A.; Garcia, S.; Martinez, J.; Fehrentz, J.-A. Synthesis of Substituted Imidazo[1,5-a]Pyridines Starting from N-2-Pyridylmethylamides Using Lawesson’s Reagent and Mercury(II) Acetate. Synthesis 2007, 17, 2667–2673. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Nguyen, O.T.K.; Truong, T.; Phan, N.T.S. Synthesis of Imidazo[1,5-a]Pyridines via Oxidative Amination of the C(Sp(3))-H Bond under Air Using Metal-Organic Framework Cu-MOF-74 as an Efficient Heterogeneous Catalyst. RSC Adv. 2016, 6, 36039–36049. [Google Scholar] [CrossRef]
- Zimmer, H.; Glasgow, D.G.; McClanahan, M.; Novinson, T. Imidazo[5,1-a]Isochinoline. Tetrahedron Lett. 1968, 9, 2805–2807. [Google Scholar] [CrossRef]
- Bori, J.; Behera, N.; Mahata, S.; Manivannan, V. Synthesis of Imidazo[5,1-a]Isoquinoline and Its 3-Substituted Analogues Including the Fluorescent 3-(1-Isoquinolinyl)Imidazo[5,1-a]Isoquinoline. ChemistrySelect 2017, 2, 11727–11731. [Google Scholar] [CrossRef]
- Middleton, R.W.; Wibberley, D.G. Synthesis of Imidazo[4,5-b]- and [4,5-c]Pyridines. J. Heterocycl. Chem. 1980, 17, 1757–1760. [Google Scholar] [CrossRef]
- Altaib, M.; Doganc, F.; Kaşkatepe, B.; Göker, H. Synthesis of Some New 2-(Substituted-Phenyl)Imidazo[4,5-c] and [4,5-b]Pyridine Derivatives and Their Antimicrobial Activities. Mol. Divers. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpi, G.; Laurenti, E.; Rabezzana, R. Imidazopyridine Family: Versatile and Promising Heterocyclic Skeletons for Different Applications. Molecules 2024, 29, 2668. https://doi.org/10.3390/molecules29112668
Volpi G, Laurenti E, Rabezzana R. Imidazopyridine Family: Versatile and Promising Heterocyclic Skeletons for Different Applications. Molecules. 2024; 29(11):2668. https://doi.org/10.3390/molecules29112668
Chicago/Turabian StyleVolpi, Giorgio, Enzo Laurenti, and Roberto Rabezzana. 2024. "Imidazopyridine Family: Versatile and Promising Heterocyclic Skeletons for Different Applications" Molecules 29, no. 11: 2668. https://doi.org/10.3390/molecules29112668
APA StyleVolpi, G., Laurenti, E., & Rabezzana, R. (2024). Imidazopyridine Family: Versatile and Promising Heterocyclic Skeletons for Different Applications. Molecules, 29(11), 2668. https://doi.org/10.3390/molecules29112668







