Pleiotropic Effects of Direct Oral Anticoagulants in Chronic Heart Failure and Atrial Fibrillation: Machine Learning Analysis
Abstract
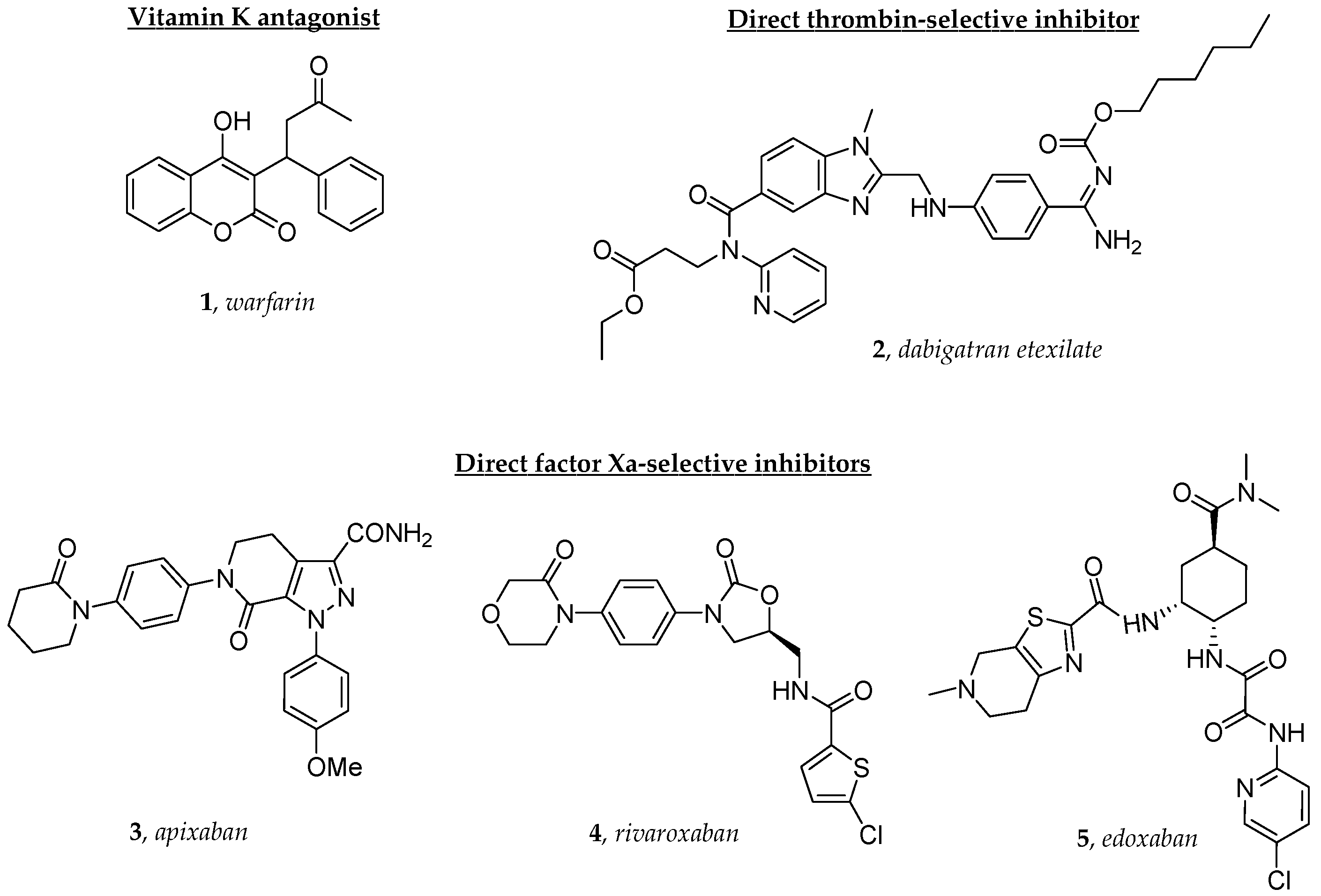
1. Introduction
2. Results
2.1. Patients’ Characteristics
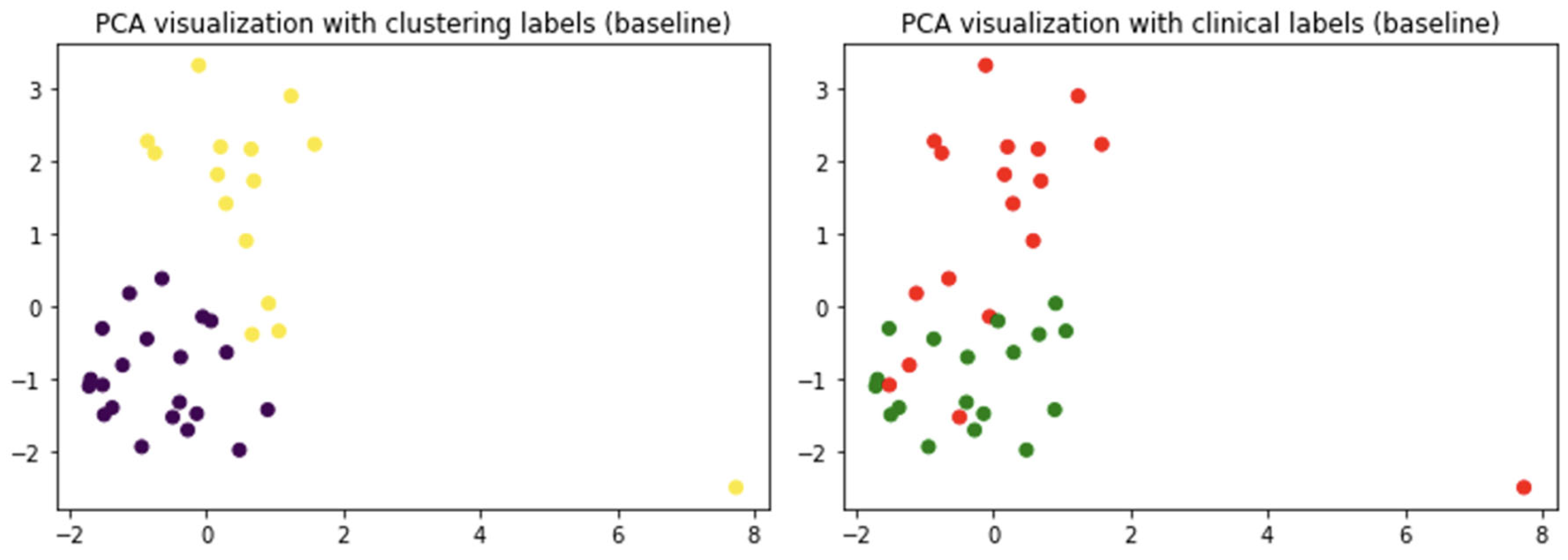
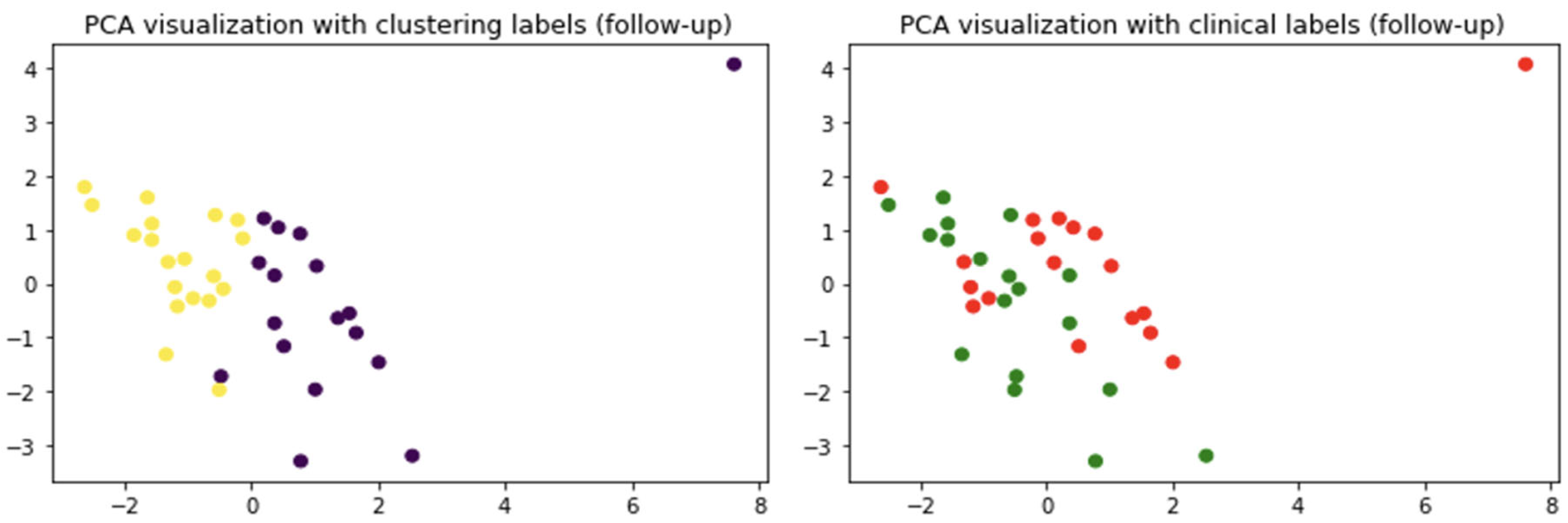
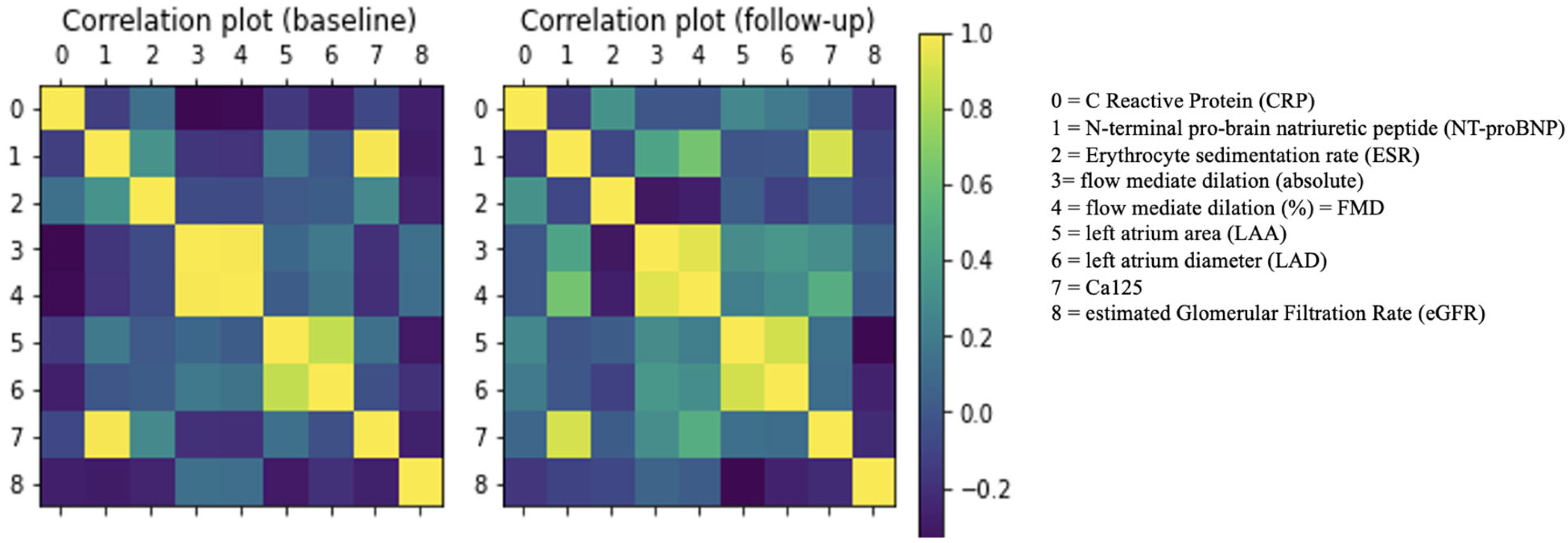
2.2. Do Clinical Cohorts Show Specific Patterns?
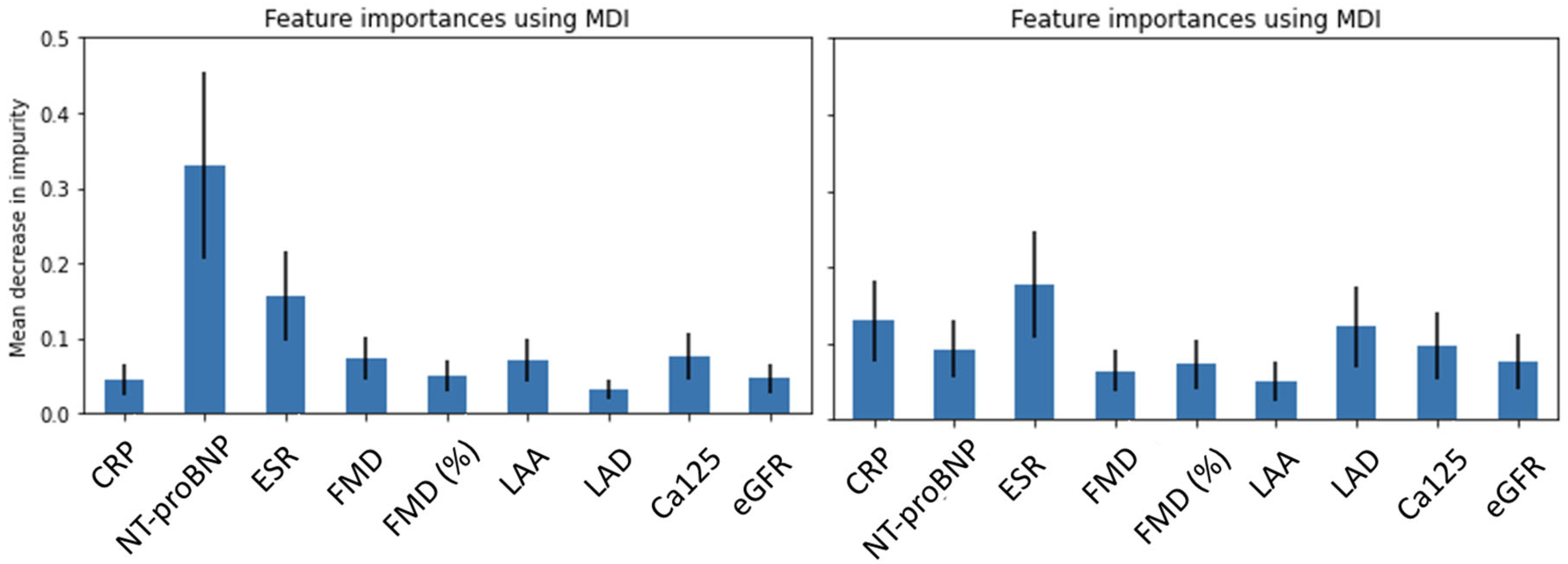
2.3. Outlining the Distinguishing Features of the Clinical Cohorts
3. Discussion
4. Data Sources and Methods
4.1. Data Sources
4.2. Method
4.2.1. Clustering Analyses
4.2.2. Random Forest Classification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, Y.N.V.; Borlaug, B.A.; Gersh, B.J. Management of Atrial Fibrillation across the Spectrum of Heart Failure with Preserved and Reduced Ejection Fraction. Circulation 2022, 146, 339–357. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Ezekowitz, J.A.; Lewis, B.S.; Gersh, B.J.; Van Diepen, S.; Amerena, J.; Bartunek, J.; Commerford, P.; Oh, B.-H.; Harjola, V.-P.; et al. Left Ventricular Systolic Dysfunction, Heart Failure, and the Risk of Stroke and Systemic Embolism in Patients with Atrial Fibrillation: Insights from the ARISTOTLE Trial. Circ. Heart Fail. 2013, 6, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, S.; Hellkamp, A.S.; Patel, M.R.; Becker, R.C.; Breithardt, G.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; Nessel, C.C.; Singer, D.E.; et al. Efficacy and Safety of Rivaroxaban in Patients with Heart Failure and Nonvalvular Atrial Fibrillation: Insights from ROCKET AF. Circ. Heart Fail. 2013, 6, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Apostolakis, S.; Lane, D.A.; Lip, G.Y.H. The Impact of Heart Failure and Left Ventricular Dysfunction in Predicting Stroke, Thromboembolism, and Mortality in Atrial Fibrillation Patients: A Systematic Review. Clin. Ther. 2014, 36, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Hori, M.; Tanahashi, N.; John Camm, A. Special Considerations for Therapeutic Choice of Non–Vitamin K Antagonist Oral Anticoagulants for Japanese Patients with Nonvalvular Atrial Fibrillation. Clin. Cardiol. 2017, 40, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the Efficacy and Safety of New Oral Anticoagulants with Warfarin in Patients with Atrial Fibrillation: A Meta-Analysis of Randomised Trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.M. Use of Novel Oral Anticoagulants for Patients with Atrial Fibrillation: Systematic Review and Clinical Implications. Heart Lung 2014, 43, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.A.; Warkentin, T.E. Bleeding Risk and the Management of Bleeding Complications in Patients Undergoing Anticoagulant Therapy: Focus on New Anticoagulant Agents. Blood 2008, 111, 4871–4879. [Google Scholar] [CrossRef]
- De Candia, M.; Lopopolo, G.; Altomare, C. Novel Factor Xa Inhibitors: A Patent Review. Expert Opin. Ther. Pat. 2009, 19, 1535–1580. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Laizure, S.C.; Parker, R.B.; Herring, V.L.; Hu, Z.-Y. Identification of Carboxylesterase-Dependent Dabigatran Etexilate Hydrolysis. Drug Metab. Dispos. 2014, 42, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Möckel, M.; Pudasaini, S.; Baberg, H.T.; Levenson, B.; Malzahn, J.; Mansky, T.; Michels, G.; Günster, C.; Jeschke, E. Oral Anticoagulation in Heart Failure Complicated by Atrial Fibrillation: A Nationwide Routine Data Study. Int. J. Cardiol. 2024, 395, 131434. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, A.; Sato, Y.; Sato, T.; Suzuki, S.; Oikawa, M.; Takeishi, Y. Better Clinical Outcome with Direct Oral Anticoagulants in Hospitalized Heart Failure Patients with Atrial Fibrillation. BMC Cardiovasc. Disord. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Motoki, H.; Fuchida, A.; Takeuchi, T.; Otagiri, K.; Kanai, M.; Kimura, K.; Minamisawa, M.; Yoshie, K.; Saigusa, T.; et al. Comparison of Prognostic Impact of Anticoagulants in Heart Failure Patients with Atrial Fibrillation and Renal Dysfunction: Direct Oral Anticoagulants versus Vitamin K Antagonists. Heart Vessel. 2022, 37, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Leopizzi, A.; Mallardi, A.; Ranieri, A.; Suriano, M.P.; D’Alessandro, D.; Tricarico, L.; Mazzeo, P.; Tucci, S.; Pastore, G.; et al. Switch to Direct Anticoagulants and Improved Endothelial Function in Patients with Chronic Heart Failure and Atrial Fibrillation. Thromb. Res. 2020, 195, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Danckwardt, S.; Hentze, M.W.; Kulozik, A.E. Pathologies at the Nexus of Blood Coagulation and Inflammation: Thrombin in Hemostasis, Cancer, and Beyond. J. Mol. Med. 2013, 91, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Targeting Factor Xa and Thrombin: Impact on Coagulation and Beyond. Thromb. Haemost. 2014, 111, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; Paradela-Dobarro, B.; Raposeiras-Roubín, S.; González-Juanatey, J.R. Protective, Repairing and Fibrinolytic Effects of Rivaroxaban on Vascular Endothelium. Brit. J. Clin. Pharma. 2018, 84, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Torramade-Moix, S.; Palomo, M.; Vera, M.; Jerez, D.; Moreno-Castaño, A.B.; Zafar, M.U.; Rovira, J.; Diekmann, F.; Garcia-Pagan, J.C.; Escolar, G.; et al. Apixaban Downregulates Endothelial Inflammatory and Prothrombotic Phenotype in an In Vitro Model of Endothelial Dysfunction in Uremia. Cardiovasc. Drugs Ther. 2021, 35, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Imano, H.; Kato, R.; Nomura, A.; Tamura, M.; Yamaguchi, Y.; Ijiri, Y.; Wu, H.; Nakano, T.; Okada, Y.; Yamaguchi, T.; et al. Rivaroxaban Attenuates Right Ventricular Remodeling in Rats with Pulmonary Arterial Hypertension. Biol. Pharm. Bull. 2021, 44, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Atzemian, N.; Kareli, D.; Ragia, G.; Manolopoulos, V.G. Distinct Pleiotropic Effects of Direct Oral Anticoagulants on Cultured Endothelial Cells: A Comprehensive Review. Front. Pharmacol. 2023, 14, 1244098. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Bea, F.; Preusch, M.; Wang, H.; Isermann, B.; Shahzad, K.; Katus, H.A.; Blessing, E. Evaluation of Plaque Stability of Advanced Atherosclerotic Lesions in Apo E-Deficient Mice after Treatment with the Oral Factor Xa Inhibitor Rivaroxaban. Mediat. Inflamm. 2011, 2011, 432080. [Google Scholar] [CrossRef]
- Funatsu, T.; Yamashita, A.; Kaku, S.; Iwatsuki, Y.; Asada, Y. Plasma Factor Xa Inhibition Can Predict Antithrombotic Effects of Oral Direct Factor Xa Inhibitors in Rabbit Atherothrombosis Models. Thromb. Haemost. 2012, 108, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Zolotoff, C.; Puech, C.; Roche, F.; Perek, N. Effects of Intermittent Hypoxia with Thrombin in an in Vitro Model of Human Brain Endothelial Cells and Their Impact on PAR-1/PAR-3 Cleavage. Sci. Rep. 2022, 12, 12305. [Google Scholar] [CrossRef]
- Gorzelak-Pabiś, P.; Broncel, M.; Pawlos, A.; Wojdan, K.; Gajewski, A.; Chałubiński, M.; Woźniak, E. Dabigatran: Its Protective Effect against Endothelial Cell Damage by Oxysterol. Biomed. Pharmacother. 2022, 147, 112679. [Google Scholar] [CrossRef]
- Paolillo, S.; Ruocco, G.; Filardi, P.P.; Palazzuoli, A.; Tocchetti, C.G.; Nodari, S.; Lombardi, C.; Metra, M.; Correale, M.; on behalf of “Right and Left Heart Failure Study Group” of the Italian Society of Cardiology. Direct Oral Anticoagulants across the Heart Failure Spectrum: The Precision Medicine Era. Heart Fail. Rev. 2022, 27, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhuisen, D.J.; Linssen, G.C.M.; Jaarsma, T.; Van Gilst, W.H.; Hoes, A.W.; Tijssen, J.G.P.; Paulus, W.J.; Voors, A.A.; Hillege, H.L. B-Type Natriuretic Peptide and Prognosis in Heart Failure Patients with Preserved and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2013, 61, 1498–1506. [Google Scholar] [CrossRef]
- Steiner, J.; Guglin, M. BNP or NTproBNP? A Clinician’s Perspective. Int. J. Cardiol. 2008, 129, 5–14. [Google Scholar] [CrossRef]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.B.; et al. Rapid Measurement of B-Type Natriuretic Peptide in the Emergency Diagnosis of Heart Failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef]
- Maisel, A.; Mueller, C.; Adams, K.; Anker, S.D.; Aspromonte, N.; Cleland, J.G.F.; Cohen-Solal, A.; Dahlstrom, U.; DeMaria, A.; Di Somma, S.; et al. State of the Art: Using Natriuretic Peptide Levels in Clinical Practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; McDonald, K.; De Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology Practical Guidance on the Use of Natriuretic Peptide Concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Nasab Mehrabi, E.; Toupchi-Khosroshahi, V.; Athari, S.S. Relationship of Atrial Fibrillation and N Terminal pro Brain Natriuretic Peptide in Heart Failure Patients. ESC Heart Fail. 2023, 10, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.; Brandon, L. Atrial Fibrillation, Thromboembolic Risk, and the Potential Role of the Natriuretic Peptides, a Focus on BNP and NT-proBNP—A Narrative Review. IJC Heart Vasc. 2022, 43, 101132. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Ma, L.-P.; Fu, M.L.X.; Svaninger, D.; Lundberg, P.-A.; Hammarsten, O. Inflammation Increases NT-proBNP and the NT-proBNP/BNP Ratio. Clin. Res. Cardiol. 2010, 99, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Fish-Trotter, H.; Ferguson, J.F.; Patel, N.; Arora, P.; Allen, N.B.; Bachmann, K.N.; Daniels, L.B.; Reilly, M.P.; Lima, J.A.C.; Wang, T.J.; et al. Inflammation and Circulating Natriuretic Peptide Levels. Circ. Heart Fail. 2020, 13, e006570. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-H. Comparison of Endothelial Vasodilator Function, Inflammatory Markers, and N-Terminal pro-Brain Natriuretic Peptide in Patients with or without Chronotropic Incompetence to Exercise Test. Heart 2006, 92, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, K.; Tay, W.T.; Tromp, J.; Ouwerkerk, W.; Teng, T.K.; Chandramouli, C.; Liew, O.W.; Chong, J.; Poppe, K.K.; Lund, M.; et al. Longitudinal NT-proBNP: Associations with Echocardiographic Changes and Outcomes in Heart Failure. J. Am. Heart Assoc. 2024, 13, e032254. [Google Scholar] [CrossRef] [PubMed]
- Almengló, C.; Mosquera-Garrote, N.; González-Peteiro, M.; González-Juanatey, J.R.; Álvarez, E. Edoxaban’s Contribution to Key Endothelial Cell Functions. Biochem. Pharmacol. 2020, 178, 114063. [Google Scholar] [CrossRef] [PubMed]
- Gorzelak-Pabiś, P.; Pawlos, A.; Broncel, M.; Wojdan, K.; Woźniak, E. Expression of Anti and Pro-inflammatory Genes in Human Endothelial Cells Activated by 25-hydroxycholesterol: A Comparison of Rivaroxaban and Dabigatran. Clin. Exp. Pharmacol. Physiol. 2022, 49, 805–812. [Google Scholar] [CrossRef]
- Ahmed, M.; Seraj, R.; Islam, S.M.S. The K-Means Algorithm: A Comprehensive Survey and Performance Evaluation. Electronics 2020, 9, 1295. [Google Scholar] [CrossRef]
- Breiman, L. Random Forest. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]


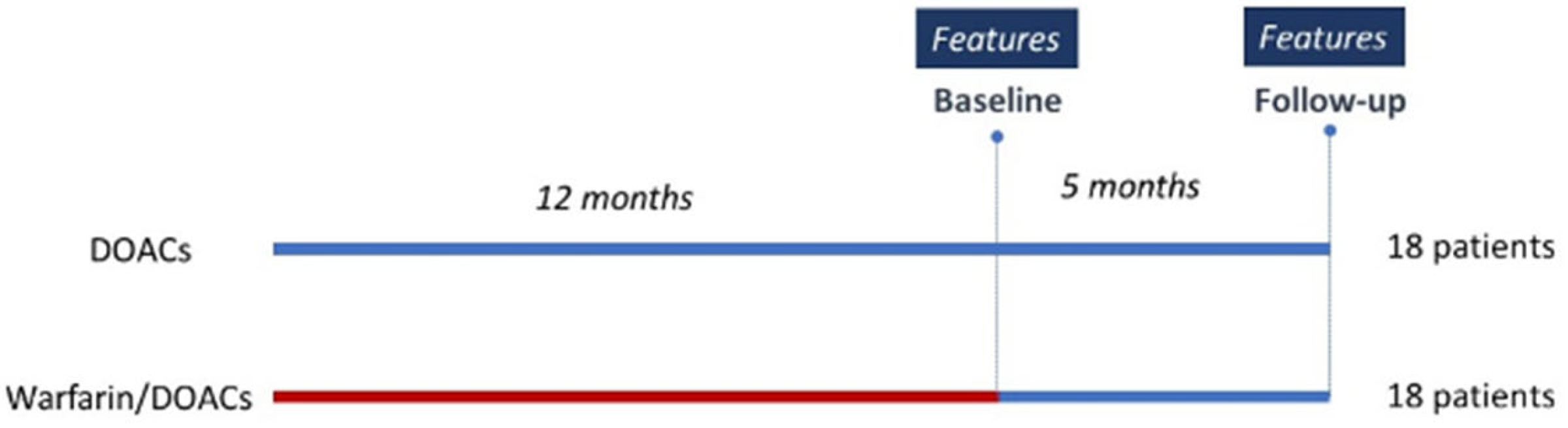
| Clinical Features | Medications | % | |
|---|---|---|---|
| Age (years) | 70.6 ± 1.5 | ACEi/ARB/ARNI | 92.8 |
| Male (%) | 64.3 | Beta-blockers | 78.5 |
| Body weight (kg) | 74 ± 1.5 | MRA | 40.5 |
| SBP (mmHg) | 110 ± 2.0 | Diuretics | 54.7 |
| DBP (mmHg) | 66 ± 1.7 | Ivabradine | 2.4 |
| Heart rate (bpm) | 64 ± 1.1 | Digoxin | 9.7 |
| LVEF (%) | 47 ± 1.8 | Amiodarone | 31.7 |
| Clinical Significance | Feature | Value |
|---|---|---|
| Index of inflammation | ||
| state of systemic inflammation | CRP (mg/L) | 1.7 ± 0.1 |
| ESR (mm/h) | 30.0 ± 1.9 | |
| Index of clinical status | ||
| congestion/fluid overload | NTproBNP (pg/mL) | 652 ± 190 |
| Ca125 (U/mL) | 34.0 ± 11.6 | |
| kidney function | eGFR (mL/min) | 62.8 ± 3.3 |
| Index of endothelial function | ||
| vasodilation in response to increased blood flow | FMD (%) | 11.8 ± 1.78 |
| Index of cardiac remodeling | ||
| left atrial size | LAD (mm) | 44.6 ± 1.0 |
| LAA (mm2) | 24.0 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mele, M.; Mele, A.; Imbrici, P.; Samarelli, F.; Purgatorio, R.; Dinoi, G.; Correale, M.; Nicolotti, O.; De Luca, A.; Brunetti, N.D.; et al. Pleiotropic Effects of Direct Oral Anticoagulants in Chronic Heart Failure and Atrial Fibrillation: Machine Learning Analysis. Molecules 2024, 29, 2651. https://doi.org/10.3390/molecules29112651
Mele M, Mele A, Imbrici P, Samarelli F, Purgatorio R, Dinoi G, Correale M, Nicolotti O, De Luca A, Brunetti ND, et al. Pleiotropic Effects of Direct Oral Anticoagulants in Chronic Heart Failure and Atrial Fibrillation: Machine Learning Analysis. Molecules. 2024; 29(11):2651. https://doi.org/10.3390/molecules29112651
Chicago/Turabian StyleMele, Marco, Antonietta Mele, Paola Imbrici, Francesco Samarelli, Rosa Purgatorio, Giorgia Dinoi, Michele Correale, Orazio Nicolotti, Annamaria De Luca, Natale Daniele Brunetti, and et al. 2024. "Pleiotropic Effects of Direct Oral Anticoagulants in Chronic Heart Failure and Atrial Fibrillation: Machine Learning Analysis" Molecules 29, no. 11: 2651. https://doi.org/10.3390/molecules29112651
APA StyleMele, M., Mele, A., Imbrici, P., Samarelli, F., Purgatorio, R., Dinoi, G., Correale, M., Nicolotti, O., De Luca, A., Brunetti, N. D., Liantonio, A., & Amoroso, N. (2024). Pleiotropic Effects of Direct Oral Anticoagulants in Chronic Heart Failure and Atrial Fibrillation: Machine Learning Analysis. Molecules, 29(11), 2651. https://doi.org/10.3390/molecules29112651











