Abstract
To better assess the practical value and avoid potential risks of the traditionally medicinal and edible basidiomycete Schizophyllum commune, which may arise from undescribed metabolites, a combination of elicitors was introduced for the first time to discover products from cryptic and low-expressed gene clusters under laboratory cultivation. Treating S. commune NJFU21 with the combination of five elicitors led to the upregulated production of a class of unusual linear diterpene-derived variants, including eleven new ones (1–11), along with three known ones (12–14). The structures and stereochemistry were determined by 1D and 2D NMR, HRESIMS, ECD, OR and VCD calculations. Notably, the elongation terminus of all the diterpenes was decorated by an unusual butenedioic acid moiety. Compound 1 was a rare monocyclic diterpene, while 2–6 possessed a tetrahydrofuran moiety. The truncated metabolites 4, 5 and 13 belong to the trinorditerpenes. All the diterpenes displayed approximately 70% scavenging of hydroxyl radicals at 50 μM and null cytotoxic activity at 10 μM. In addition, compound 1 exhibited potent antifungal activity against the plant pathogenic fungi Colletotrichum camelliae, with MIC values of 8 μg/mL. Our findings indicated that this class of diterpenes could provide valuable protectants for cosmetic ingredients and the lead compounds for agricultural fungicide development.
1. Introduction
The basidiomycete Schizophyllum commune has generated considerable attention as a kind of valuable medicinal and edible mushroom [1,2,3]. The species S. commune is well-known for being rich in highly nutritious supplements, such as a wealth of amino acids and essential trace elements for the human body, high-quality protein, etc., and thus it is unusually regarded as a delicious dish [4]. S. commune has also shown great potential for drug development, particularly in the area of macromolecular exopolysaccharide substances [1]. A notable example is schizophyllan, an exopolysaccharide used as a biological-response modifier in combination with chemo and radiation therapy [4].
In 2023, the crude extract of S. commune was approved as a cosmetic ingredient for use as a skin moisturizer and protectant [5], wherein the exopolysaccharide plays a crucial role, by the China National Regulatory Commission. Unlike macromolecules in higher fungi, the potential applications of small molecules remain a mystery [6,7,8]. Currently, according to available bioinformatics data, this extremophilic fungus is known to encode at least 19 biosynthetic gene clusters, including those related to terpenes, non-ribosomal peptide synthetases (NRPSs), etc. [9]. To date, only four classes of small molecules, including terpenoids [10], alkaloids [4,11], aromatics [12] and polyketides [13], have been identified. It is evident that most chemotypes of small molecules, encoded by gene clusters that are cryptic and low-expressed, have not yet been characterized. Given the multiple values in drug development, and the uses of this organism as an edible mushroom and a raw material for cosmetics, it is necessary to explore the yet-undiscovered small molecules. It is also important to comprehensively assess the in-depth potentials and risks associated with the safety and new applications of higher fungi.
A variety of methods have been applied to activate natural products, including One Strain Many Compounds (OSMACs) [14,15], co-culture [16], chemical epigenetic regulation [17,18] and heterologous expression [19] methods, and so on. The High-Throughput Elicitors Screening (HITES) strategy is another highly effective approach for activating silent gene clusters. It offers wide applicability and represents a cutting-edge method for activating microbial secondary metabolites. Initially applied primarily to actinomycetes and bacteria, the use of HITES in fungi was first reported by Professor Mohammod in 2022 [20]. During the elicitor screening process, single-factor experiments are typically employed, which tend to be time-consuming and not conducive to high-throughput screening. The utilization of combined elicitors might offer a higher probability of activating new substances. This approach also has the potential to enhance efficiency when screening a large number of microorganisms. However, the application of a combinatorial elicitor strategy in microbial screening has not yet been reported.
Herein, we developed a combination of elicitors to significantly enhance the likelihood of discovering previously undescribed small molecules in fungi. This approach was applied to activate and up-regulate the production of small molecules in S. commune NJFU21. As a result, a series of linear diterpene-derived variants 1–14 (Figure 1), were successfully produced in higher quantities. These small molecules enable us to more thoroughly investigate the practical value and assess the potential risks associated with the small molecules from S. commune, as well as their biological functions.
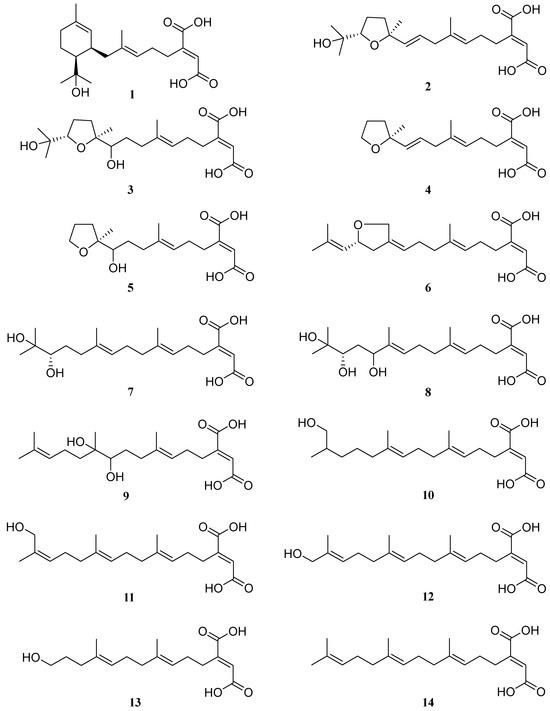
Figure 1.
The structures of compounds 1–14.
2. Results and Discussion
2.1. Selection and Screening
Previous studies have provided sufficient evidence that a single elicitor could activate the expression of metabolites of a cryptic or extremely low-expressed gene cluster. Thus, it is logically hypothesized that elicitors regulating gene expression in various manners could synergistically affect each other; the combination strategy of elicitors was able to activate and up-regulate the metabolite production as much as possible, once.
To verify the hypothesis, five function-verified elicitors–Sodium Laurate, Aniline, p-Trifluoromethylaniline, Vorinostat (SAHA) and 5-Azacytidine (5-Aza) were selected and eight concentration gradients were set to acquire the suitable work concentration of each elicitor and the universal applicability for most fungi. The mycelium growth and changes in secondary metabolite profiles were considered as indication signs. The change in secondary metabolite profile was analyzed by HPLC. As results, 0.1 mM of sodium laurate, aniline, and p-trifluoromethylaniline, respectively, and 0.075 mM of SAHA and 0.05 mM of 5-Azacytidine were determined as the suitable work concentration at which each elicitor does not affect the growth of fungi. When the combination of the five elicitors with individual concentrations was added into the culture of S. commune NJFU21, a series of new peaks with similar UV absorption appeared by HPLC analysis, indicating a class of secondary metabolites which was unregulated (Figure 2).
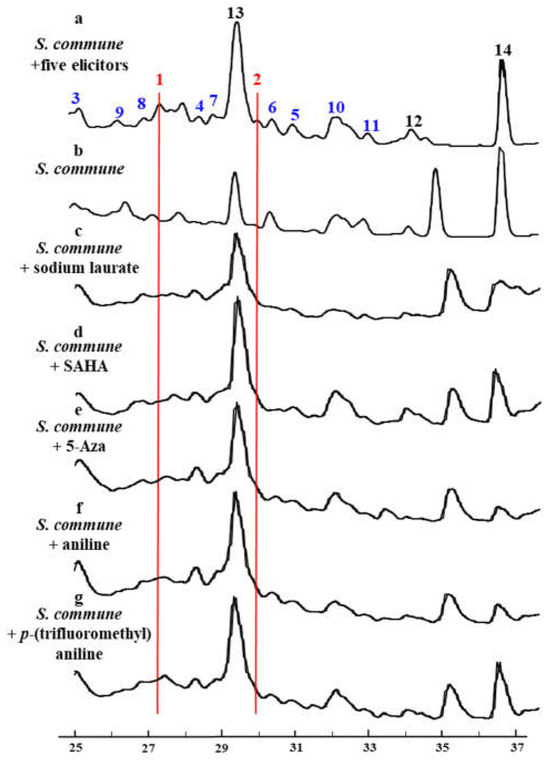
Figure 2.
Changes in secondary metabolite profiles of S. commune NJFU21 analyzed by HPLC. a: Five elicitors were concurrently introduced into S. commune NJFU21 strain. b–g: Representing the individual S. commune NJFU21 and the elicitors added independently, respectively.
2.2. Structural Elucidation
Schizostatin B (1) was obtained as white powder. The molecular formula was determined as C20H30O5 based on the HR-ESI-MS ions at m/z, 351.2174 [M + H]+ (calcd for C20H31O5, 351.2166), indicating six degrees of unsaturation. Absorption bands at 3405 and 1647 cm–1 in the IR spectrum suggested the existence of hydroxy and carboxylic acid groups. Careful inspection of 1D NMR spectra (Table 1 and Table 2) suggested 1 possessed four methyls, five sp3 methylenes, five methines (two sp3 ones and three olefinic ones), six non-protonated carbons, including an oxygenated tertiary carbon at 73.4 (C-15), three olefinic carbons at 148.6 (C-3), 135.9 (C-7), 133.6 (C-11), and two carbonyl carbons at 170.1 (C-1), 169.0 (C-20), indicative of the fact that 1 is a monocyclic product.

Table 1.
1H NMR (600 MHz) Spectroscopic Data for compounds 1–3 and 6–11 in CD3OD (δ in ppm, J in Hz).

Table 2.
13C NMR (150 MHz) Spectroscopic Data for compounds 1–3 and 6–11 in CD3OD (δ in ppm).
Based on the 1H-1H COSY spectrum, three spin systems of H2-4/H2-5/H-6, H-10/H-9/H2-8, H-14/H2-13/H2-12 were observed. The key HMBC correlations for H3-19 (δC/H 16.4/1.63) to C-6 (δC 125.6), C-7 (δC 135.9) and C-8 (δC 42.5) unambiguously established the connection between the fragment of H2-4/H2-5/H-6 and the fragment of H2-8/H-9/H-10 by a double-bond carbon of C-7 (Figure 3). Subsequently, a trisubstituted cyclohexene was established by the HMBC correlations for H3-18 (δC/H 23.7/1.62) to C-10 (δC 127.4), C-11 (δC 133.6) and C-12 (δC 32.9), in which the connectivity of C-10 and C-12 was determined through a double-bond carbon of C-11. The gem-dimethyl group was positioned to the oxygenated tertiary carbon C-15 (δC 73.4), and the latter carbon was attached to the C-14 of cyclohexene, evidenced by the HMBC correlations from H3-16 (δC/H 29.2/1.25)/17 (δC/H 27.7/1.23) to C-14 (δC 49.5), C-15 (δC 73.4) and H-14 (δC/H 49.5/1.55) to C-15 (δC 73.4). On the other end, the presence of the butenedioic acid moiety was confirmed by a combination of unsaturated-degree consideration and the key HMBC correlations of H-2 (δC/H 128.4/6.72) with C-1 (δC 170.1) and C-20 (δC 169.0), which was further attached to C-4, supported by the HMBC correlations from H-4 (δC/H 28.7/2.79) to C-3 (δC 148.6), C-2 (δC 128.3), C-1 (δC 170.1), as well as from H2-5 (δC/H 28.6/2.19) to C-3 (δC 148.6) (Figure 3). Accordingly, the planar structure of 1 was a monocyclic diterpene with an unusual butenedioic acid moiety.
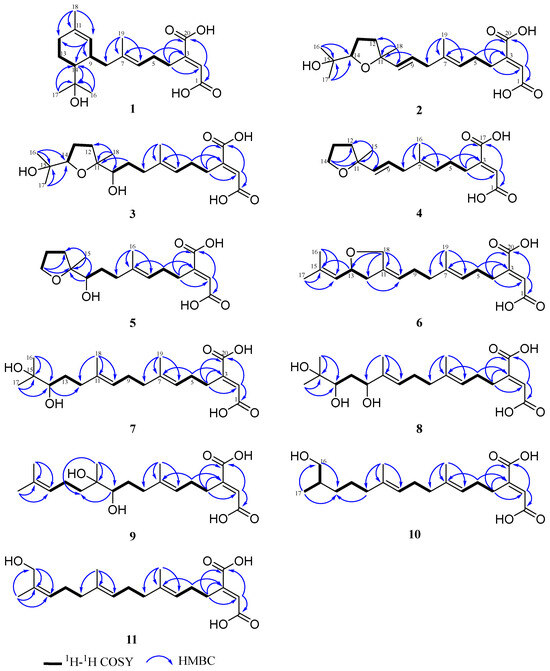
Figure 3.
Key 1H-1H COSY, HMBC correlations of compounds 1–11.
The relative stereochemistry of 1 was determined by the NOESY spectrum. The vital correlations from H-6 to H2-8 and from H3-19 to H2-5 indicated the trans configuration of the double bond at C-6 (Figure 4). However, the NOESY correlations of H2-8 with H3-16, and H-9 (δH 2.43) with H3-16 (δH 1.25) were observed, which failed to assign the relative configuration between C-9 and C-14 in the cyclohexene of 1. To establish the relative configurations of C-9 and C-14, 13C NMR calculations were performed at the B3LYP/6-311+G (d, p) level. As a result, (9R*, 14S*)−1 shows a 100% probability in contrast to (9S*, 14S*)−1 (Figures S9 and S10).
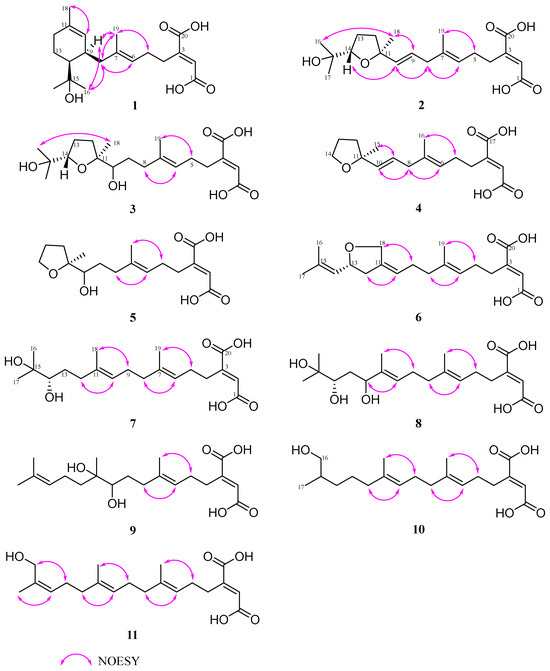
Figure 4.
Key NOESY correlations of compounds 1–11.
Schizostatin C (2) was obtained as white powder, and the molecular formula was deduced as C20H30O6 from HR-ESI-MS ion peak at m/z 367.2108 [M + H]+ (calcd for C20H31O6, 367.2115), corresponding to six degrees of unsaturation. Detailed inspection of the 1D NMR data of 2 (Table 1 and Table 2) showed they were similar to those of 1, suggesting 2 was also a diterpene compound. When 1D and 2D NMR data were analyzed, a distinct difference was found in the fragment of C-9 to C-14 between 2 and 1. The presence of the three spin systems in 2 (Figure 3) was confirmed by the 1H-1H COSY correlations of H2-4/H2-5/H-6, H2-8/H-9/H-10 and H2-12/H2-13/H-14. Both spin systems were connected to an oxygenated tertiary carbon at C-11 (δC 84.2) by a C-C single bond of C-10-C-11 and C-11-C-12, respectively, as evidenced by key HMBC correlations for H3-18 (δC/H 27.4/1.30) to C-10 (δC 138.1), C-11 (δC 84.2) and C-12 (δC 38.5). A gem-dimethyl group was also confirmed to be localized at an oxygenated tertiary carbon C-15 (δC 72.7) and the group is attached to an oxygenated methine carbon of C-14, evidenced by HMBC correlations from H3-16 (δC/H 25.7/1.15)/17 (δC/H 25.8/1.17) to C-14 (δC 86.6), C-15 (δC 72.7). Considering one degree of unsaturation remaining, and the extreme downfield chemical shifts of two oxygenated carbons at δC 84.2 (C-11) and δC 86.6 (C-14) (Figure 3), a tetrahydrofuran moiety was deduced to establish the planar structure. Thus, compound 2 was a tetrahydrofuran-containing monocyclic diterpene.
The E-configuration of double bonds at C-6/C-7 and C-9/C-10 was easily assigned through significant spatial NOE correlations observed between H3-19/H2-5, H-6/H2-8, H2-8/H-10 and H-9/H3-18. The key NOESY correlations between H-10/H-14 and H3-18/H3-16 allowed us to determine the relative configuration of tetrahydrofuran as 11S*, 14S* (Figure 4).
To determine the absolute configuration of 1 and 2, the ECD calculations on the optimized conformation of (2E, 6E, 9R, 14S)−1 and (2E, 6E, 9E, 11R, 14R)−2 obtained at the B3LYP/6-31+G(d) level were performed. The overall pattern of the experimental ECD spectrum was in reasonable agreement with the calculated spectrum of (2E, 6E, 9R, 14S)−1a and (2E, 6E, 9E, 11S, 14S)−2b (Figure 5), thereby confirming the absolute configuration of (2E, 6E, 9R, 14S)−1 and (2E, 6E, 9E, 11S, 14S)−2, respectively.
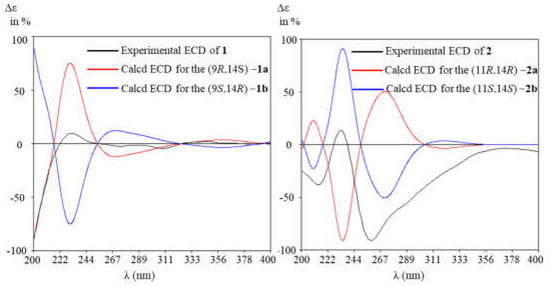
Figure 5.
Experimental and calculated ECD spectra of 1 and 2 (in MeOH).
Schizostatin K (3) was isolated as yellow oil and the molecular formula was determined as C20H32O7 by the HR-ESI-MS ions at m/z 385.2220 [M + H]+ (calcd for C20H33O7, 385.2219), indicating five degrees of unsaturation. Comparison of NMR data of 3 and those of 2 revealed that the skeleton outline of 3 is very similar to that of 2. The only difference was that the double bond at C-9/C-10 which disappeared in 2 was replaced by the OH-10 group at C-10 in 3, supported by COSY correlations of H2-8/H2-9/H-10 and HMBC correlations of H2-9 (δC/H 77.1/1.34, 1.71) with C-11 (δC 86.8) and H3-18 (δC/H 23.0/1.13) with C-10 (δC 77.1). The relative configuration of tetrahydrofuran moiety was deduced by the NOESY correlation of H3-18/H3-16. Considering the common biosynthetic origin, the absolute configuration of both C-14 and C-11 in 3 was proposed as S, which is consistent with those of 2.
Schizostatin E (4) was obtained as white powder, and was found to be C17H24O5, based on positive HR-ESI-MS at m/z 309.1691 [M + H]+, indicating it was a truncated diterpene, like compound 13. The unsaturation degree of compound 4 is 6. Analysis of the 1D NMR data of 4 (Table 3) with 2 revealed they shared a similar framework, and the tetrahydrofuran unit was also present, which was evidenced by the key HMBC correlations from H2-14 (δC/H 68.4/3.85) to C-11 (δC 83.9) and C-12 (δC 38.6). The major difference between the NMR spectra of 4 and those of 2 was that the gem-dimethyl group at C-15-C-17 which appeared in 2 disappeared in 4, evidenced by the presence of oxygenated methylene carbon, rather than oxygenated methine carbon, as well as 2D data. The C-6/C-7 and C-10/C-11 double bonds could be assigned as E-configuration on the basis of NOESY correlations of H3-16/H2-5, H-6/H2-8, H2-8/H-10 and H-9/H3-15 (Figure 4).

Table 3.
1H (600 MHz) and 13C (150 MHz) NMR Data for compounds 4, 5 in CD3OD (δ in ppm, J in Hz).
To assign the absolute configuration of 4, the VCD calculations were performed. Conformational search for molecule 4 was carried out using the MMFF94 force field by the MOE 2019.01 software (Chemical Computing Group ULC). DFT calculations were used to optimize the conformers at the B3LYP/6-31G(d) and B3LYP/6-311+G(d) levels, respectively. We calculated IR and VCD spectra for (11R)−4 and (11S)−4. The VCD calculations for 4 were carried out at the B3LYP/6-311+G(d) level in the gas phase. Boltzmann statistics were used for final simulations of the VCD for these molecules. The VCD calculation simulation shows that the signals at 1247~1412 cm−1 are related to the vibrations of the calculated chiral carbon positions, and a comparison of the calculated values of the VCD signals for the R and S configurations at 1247~1412 cm−1 with the experimental values at 1213~1300 cm−1 showed that the configuration S is more consistent with the experimental values [21,22] (Figure 6). Thus, the absolute configuration of 4 was determined as 2E, 6E, 10E, 11S.
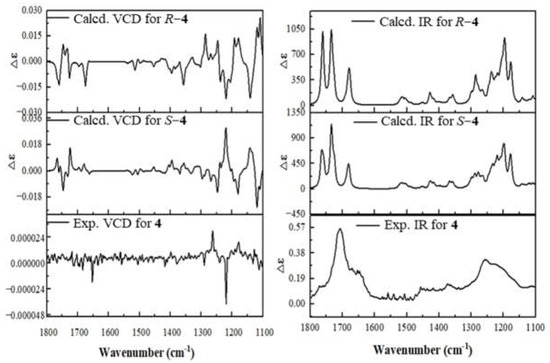
Figure 6.
The VCD (left) and IR (right) spectra of compound 4 (measures in DMSO) with those calculated for 11R−4, 11S−4.
Schizostatin F (5) was obtained as yellow oil. The molecular formula was determined as C17H26O6 based on the HR-ESI-MS ions at m/z 327.1800 ([M + H]+, calcd for C17H27O6, 327.1802). Detailed analyses of its 1H and 13C NMR (Table 1 and Table 2) and 2D NMR spectra revealed the structure of 5 was almost identical to that of 4. The only difference between 5 and 4 is that the double-bond group of C-9/C-10 in 4 disappeared and was replaced by the OH-10 group at C-10 in 5. The total E-geometry of the double bond of C-6/C-7 was confirmed by key NOESY correlations of H3-19/H2-5 and H-6/H2-8 (Figure 4). Thus, 5 was also a truncated and tetrahydrofuran-containing monocyclic diterpene. However, the Mosher reaction was not carried out, so that the absolute configuration of OH-10 was not determined. Considering the common biosynthesis between 5 and 2, the absolute configuration of C-11 in 5 was proposed to be S.
Schizostatin G (6) was obtained as yellow oil. The molecular formula of C20H28O5 was determined according to the HR-ESI-MS ions at m/z 349.2006 [M + H]+ (calcd for C20H29O5, 349.2009). Detailed inspection of 1D NMR (Table 1 and Table 2) revealed the structure of 6 was similar to that of 14. The main difference between NMR data 6 and 14 is that the methyl group of 14 is replaced with oxygenated methylene carbon at the C-18 position, and the sp3 methylene group at the C-13 position is replaced by oxygenated methine carbon. The HMBC correlations (Figure 3) from H2-18 (δC/H 69.0/4.38, 4.22) to C-12 (δC 40.2), C-11 (δC 139.7), C-10 (δC 121.3), C-13 (δC 77.5) and from H-13 (δC/H 77.5/4.57) to C-15 (δC 138.0), H2-12 (δC/H 40.2/2.59, 2.20) to C-10 (δC 121.3), along with the COSY correlations between H2-12, H-13 and H-14 allowed us to determine the presence of a tetrahydrofuran in compound 6. The NOESY correlations of H2-18/H2-9 and H-10/H2-12 provided the evidence for Z-configuration of C-10/C-11. The optical rotation calculations were carried out to ascertain the exact configuration of C-13 at the B3LYP/6-31+G(d) level. Comparing the calculated optical rotation of R-6 (+234.90) with the experimental data of 6-(+4.21), the absolute configuration of C-13 was assigned as R. Thus, the absolute configuration of 6 was determined as 2E, 10Z, 13R.
All the compounds 7–11 were observed to contain twenty carbons and are noncyclic, as deduced from their HRESIMS and 1D NMR spectra (Table 1 and Table 2), indicative of the fact that they possessed an unabridged linear diterpene-type skeleton. The detailed interpretation of 2D NMR spectra of 7–11 established the identical fragments from C-1 to C-9, including the existence of the butenedioic acid moiety, and the E-configuration of the double bond of C-6/C-7 (Figure 4). The structures of 7–11 should be oxidized variants of schizostatin A (14), a potent squalene synthase inhibitor which was also co-isolated from the fungus.
Schizostatin H (7) and schizostatin I (8) were obtained as yellow oil and were found to be C20H32O6 and C20H32O7, based on positive HR-ESI-MS at m/z 351.2173 [M-H2O + H]+ and 385.2219 [M + H]+ (calcd for C20H31O5, 351.2166 for 7; 385.2221 for 8), respectively. A careful comparison of 1D NMR data between 7 and 15 showed an almost identical planar structure (Table 1 and Table 2), except for the replacement of the double bond of C-14/C-15 in 15 with the pinacol group in 7, evidenced by the key HMBC correlations from H3-17 (δC/H 25.0/1.12)/ H3-16 (δC/H 25.6/1.15) to C-15 (δC 73.8) and C-14 (δC 79.1), as well as the COSY correlations of H2-12/H2-13/H-14 (Figure 3). The structure of schizostatin I (8) was determined to be very similar to that of 7, except for the loss of the signal corresponding to the methylene carbon at C-12, and replacement with an oxygenated methine carbon in 8. It was confirmed by correlations of H-12/H2-13/H-14 in the COSY spectrum and of Me-18 (δC/H 11.0/1.61) with C-10 (δC 128.2), C-11 (δC 137.0) and C-12 (δC 78.0) in the HMBC spectrum. The absolute configuration of C-14 in both 7 and 8 was proposed as S, which was deduced by the biosynthetically related product 2.
The molecular formula of C20H32O6 of schizostatin J (9) was same as that of Schizostatin H (7). Comparison of NMR data between 9 and 7 indicated that the structure of 9 is similar to that of 7. The difference is that the pinacol group was positioned at C-10 and C-11 and the double bond at C-14/C-15 remained, supported by the related COSY and HMBC correlations. Due to the limited amount, the absolute configuration of C-10 and C-11 was unresolved.
Schizostatin K (10) was obtained as yellow oil with the molecular formula of C20H32O5 from the HR-ESI-MS (m/z, 353.2325 [M + H]+, calcd for C20H33O5, 353.2322). According to 1D and 2D NMR, compound 10 is structurally very similar to compound 14, except for the loss of the signal of the double bond at C-14/C-15, and replacement with two sp3 carbons, evidenced by COSY correlations of H-12 to H-17 and the key HMBC correlation from H2-12 (δC/H 40.8/2.08), H2-16 (δC/H 68.5/3.30, 3.41) and H3-17 (δC/H 17.1/0.89) to C-14 (δC 33.9) (Figure 3). Moreover, a methyl group was oxidized to a hydroxymethyl group, which was determined via the COSY correlation of H-16/H-15/H-17, and the HMBC correlation of H3-17 (δC/H 17.1/0.89) with C-16 (δC 68.5). Due to limited stockage and lack of an adjacent chromophore group at C-15 (δC 36.8), the absolute configuration of C-15 was unresolved.
The molecular formula of C20H30O5 of schizostatin L (11) was the same as that of 12. Comparison of NMR data between 11 and 12 indicated that the structure of 11 was almost identical to that of 12, with the difference in chemical shifts of C-16 (δC/H 61.4/4.05 for 11 vs. δC/H 69.0/3.9 for 12) and C-17 (δC/H 21.5/1.75 for 11 vs. δC/H 13.7/1.6 for 12). The strong NOESY correlations of H3-17/H-14 and H2-16/H2-13 allowed us to assign the Z-configuration of C-14/C-15.
In addition, three known compounds, (2E)-2-[(3E, 7E, 11E)-13-Hydroxy-4,8,12-trimethyl-3,7,11-tridecatrien-1-yl]-2-butenedioic acid (12), (2E)-2-[(3E,7E)-11-Hydroxy-4,8-dimethyl-3,7-undecadien-1-yl]-2-butenedioic acid (13) [22], and schizostatin A [23] (14), were also co-isolated and were determined based on comparisons of NMR data with previously reported data.
Compounds 1–14 were tested for cytotoxic, antimicrobial and OH scavenging activities. None of them displayed any cytotoxic activity at 10 µM. Compound 1 showed potent antifungal activity against plant pathogenic fungi Colletotrichum camelliae with MIC values of 8 μg/mL, while others did not exhibit any antifungal activity at a concentration of 64 μg/mL.
At a concentration of 50 μM, the inhibition rates of compounds 1–14 ranged from 42.3% to 76.0%. For comparison, the inhibition rate of the positive control, VC, was 81.69%, which is shown in Table 4. The hydroxyl radical scavenging data suggested that compounds 1–14 are beneficial for the interest in preservation of foodstuffs, drug products and cosmetics.

Table 4.
Hydroxyl radical scavenging activities of compounds 1–14.
3. Materials and Methods
3.1. General Experimental Procedures
NMR spectra (in ppm, J in Hz) were measured using a Bruker AVANCE Neo 600 MHz spectrometer (Bruker BioSpin, Fällanden, Switzerland), with CD3OD and TMS as the internal standard. HR-ESI-MS spectra were acquired with a Q-Exactive Orbitrap tandem mass spectrometer (Q-Exactive Orbitrap-MS) (Thermo Scientific, Bremen, Germany). Electric Circular Dichroism (ECD) spectra were obtained using a Jasco J1500 spectrometer (Jasco Inc., Tokyo, Japan). A JASCO P-1020 digital polarimeter (JASCO Corporation, Tokyo, Japan) was used to obtain optical rotations. IR spectra were acquired using Fourier Transform Infrared Spectrometer IEC/EN 60825-1 (Thermo Fisher Scientific, Madison, WI, USA). High-performance liquid chromatography (HPLC) 1260 system (Agilent, Santa Clara, CA, USA), and a chromatographic column (xbridge-C18, Thermo Fisher Scientific, Waltham, MA, USA) with a pipe diameter of 5 μm, 4.6 mm × 250 mm, were used to analyze the samples at room temperature. An ODS column (PRC-ODS EC0704, 50 mm × 250 mm, 15 μm, 8 mL/min, Shimadzu, Kyoto, Japan) served as the stationary phase in preparative HPLC and a C18 column (FWXB12S05-2510, 10 mm × 250 mm, 5 µm, 4 mL/min, Thermo Fisher Scientific, Waltham, MA, USA) was used for the stationary phases in semi-preparative HPLC. The 200–300-mesh silica gel (Nuotai Biotechnology Co., Ltd, Shan Xi, China), ODS (50 μm, YMC, Kyoto, Japan), and TOYOPEARL HW-40F (TOSOH, Tokyo, Japan) were employed for column chromatography (CC). The solvents for CC were of analytical grade.
3.2. Fungi Materials
The strain NJFU21 was isolated from the larval intestines of the cutworm, which was collected from the Zi Jin Mountain in Nanjing, Jiangsu province, China, in 2020. The voucher specimen has been deposited in our laboratory collection. The fungus was identified as Schizophyllum commune through sequence-based rDNA ITS region analysis, as evidence by its GenBank accession number OP761877 (Supporting Information).
3.3. The Screening of Elicitors
Five elicitors with various regulatory mechanisms were selected in the study, including sodium laurate, SAHA, 5-Aza, p-(trifluoromethyl)aniline and aniline. Each elicitor was screened individually to determine a suitable concentration for S. commune NJFU21, acting as a control. Following this, the selected elicitors were combined and added into the fermentation of S. commune NJFU21 in the suitable concentration, individually. HPLC analysis was conducted to analyze and compare the changes in the metabolites of S. commune NJFU21 among themselves.
3.4. Fermentation and Extraction
S. commune NJFU21 was cultured on potato dextrose agar at 28 °C for 7 days. Fresh mycelium blocks (3 mm × 3 mm, 1 block per flask) were then added to seventy 1000 mL Erlenmeyer flasks, each containing 80.0 g of rice, 120 mL of H2O, and a combination of five selected elicitors: 0.1 mM (Sodium Laurate, Aniline and p-Trifluoromethylaniline), 0.075 mM (SAHA), and 0.05 mM (5-Aza). After 30 days of cultivation, the secondary metabolites were extracted with an equal volume of ethyl acetate, three times. The ethyl acetate extract was then concentrated under reduced pressure to yield 60.4 g of crude extract.
The crude extract was separated by CC with gradient elution of CH2Cl2–MeOH to obtain eight fractions (Fr.1–Fr.8). The targeted fractions Fr. 6 (7.38 g) and Fr. 7 (6.50 g) were further separated and purified by silica gel ODS with gradient elution of H2O–MeOH, to give Fr. 6-1 to Fr. 6-12 and Fr. 7-1 to Fr. 7-12, respectively. Subsequently, Fr. 6-2 (533.8 mg) and Fr. 7-1 (356.8 mg) were subjected to further purification using silica gel HW-40F and 100% MeOH (1.5 L) to yield targeted fractions Fr. 6-2-10 (316.7 mg), Fr. 6-2-15 (14, 125.6 mg) and Fr. 7-1-8 (106.3 mg).
Fr. 6-2-10 was separated by preparative HPLC (MeOH–H2O, 55–85%, 8 mL/min) to obtain six subfractions (Fr. 6-2-10-1–Fr. 6-2-10-6), followed by the purifying of Fr. 6-2-10-1–Fr. 6-2-10-5 by semi-preparative HPLC (35:65 MeCN–H2O, 4 mL/min) to afford compounds 2 (1.9 mg, tR = 31 min), 4 (5.6 mg, tR = 33 min), 13 (4.4 mg, tR = 30 min), 1 (3.5 mg, tR = 43 min), 7 (2.9 mg, tR = 28 min) and 9 (2.4 mg, tR = 31 min). Fr. 6-2-10-6 was purified by semi-preparative HPLC (42:58, MeCN–H2O, 4 mL/min) to afford compounds 12 (3.5 mg, tR = 41 min), 11 (1.3 mg, tR = 49 min), 5 (1.7 mg, tR = 43 min) and 6 (1.9 mg, tR = 56 min). Likewise, Fr. 7-1-8 was further purified by preparative HPLC eluting with a linear gradient (MeOH–H2O, 55–85%), giving three subfractions (Fr. 7-1-8-1–Fr. 7-1-8-3), followed by the purifying of Fr. 7-1-8-1–Fr. 7-1-8-3 by semi-preparative HPLC (23:77 MeCN–H2O, 4 mL/min) to obtain compounds 8 (2.1 mg, tR = 39 min), 3 (3.5 mg, tR = 45 min), and 10 (2.4 mg, tR = 57 min).
Schizostatin B (1): white powder (MeOH); [α] 25D + 14.86(c 0.35, MeOH); UV (CH3CN) λmax (log ε) 210 (1.67) nm; ECD (0.5 mM, MeOH) λmax (Δ ε) 219 (−3.05) nm, 240 (−0.5) nm, 240 (-0.5) nm; IR (KBr) νmax: 3405, 1647, 1454, 1053, 1032, 1018 and 656 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HR-ESI-MS: [M + H]+ m/z, 351.2174 (calcd, 351.2166).
Schizostatin C (2): yellow oil (MeOH); [α] 25D + 29.47(c 0.19, MeOH); λmax (log ε) 202 (2.12) nm; ECD (0.6 mM, MeOH) λmax (Δ ε) 233 (0.48) nm; IR (KBr) νmax: 3389, 1648, 1453, 1032 and 657 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HR-ESI-MS: [M + H]+ m/z, 367.2108 (calcd, 367.2115).
Schizostatin D (3): yellow oil (MeOH); [α] 25D 30(c 0.24, MeOH); λmax (log ε) 200 (2.52) nm; IR (KBr) νmax: 3405, 2518, 2075, 1647, 1020 and 641 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESI-MS: [M + H]+ m/z, 385.2220 (calcd, 385.2219).
Schizostatin E (4): white powder (MeOH); [α] 25D + 29.47(c 0.1, MeOH); λmax (log ε) 200 (2.13) nm; IR (KBr) νmax: 3386, 1650, 1454, 1413, 1032 and 657 cm−1; 1H and 13C NMR data, see Table 3; HRESI-MS: [M + H]+ m/z, 309.1691 (calcd, 309.1696).
Schizostatin F (5): yellow oil (MeOH); [α] 25D 1.9(c 0.21, MeOH); λmax (log ε) 194 (3.10) nm; IR (KBr) νmax: 3447, 1636, and 537 cm−1; 1H and 13C NMR data, see Table 3; HRESI-MS: [M + H]+ m/z, 327.1800 (calcd, 327.1802).
Schizostatin G (6): yellow oil (MeOH); [α] 25D 4.21(c 0.19, MeOH); λmax (log ε) 200 (1.63) nm; IR (KBr) νmax: 3405, 2949, 2843, 1648, 1053, 1033, 1019 and 656 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESI-MS: [M + H]+ m/z, 349.2006 (calcd, 349.2009).
Schizostatin H (7): yellow oil (MeOH); [α] 25D −16.55(c 0.29, MeOH); λmax (log ε) 200 (1.01) nm; IR (KBr) νmax: 3440, 1640, 1054, 1033, 1015 and 599 cm−1; for 1H and 13C NMR data, see Table 1 and Table 2; HRESI-MS: [M-H2O + H]+ m/z, 351.2173 (calcd, 351.2166).
Schizostatin I (8): yellow oil (MeOH); [α] 25D 3.43(c 0.35, MeOH); λmax (log ε) 200 (4.49) nm; IR (KBr) νmax: 3452, 1640, 1054, 1033, 1015 and 591 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESI-MS: [M + H]+ m/z, 385.2219 (calcd, 385.2221).
Schizostatin J (9): yellow oil (MeOH); [α] 25D −7.03(c 0.74, MeOH); λmax (log ε) 200 (2.14) nm; IR (KBr) νmax: 3451, 1636, 1054, 1033, 1015 and 576 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESI-MS: [M-H2O + H]+ m/z, 351.2159 (calcd, 351.2166).
3.5. Nuclear Magnetic Resonance (NMR) Calculation Assay
Conformational searches were employed by Spartan’14 (Wavefunction Inc., Irvine, CA, USA), based on the MMFF94. All conformers were optimized with DFT calculations at the B3LYP/6-31+G(d) level by using the Gaussian 09 program. NMR calculation of 1 was performed at B3LYP/6-31+G (d)//B3LYP/6-311+G (d, p) levels and further checked by DP4+ probability [24].
3.6. Electric Circular Dichroism (ECD) Calculation Assay
Conformational searches were run employing the ‘systematic’ procedure implemented in Spartan’14, based on the MMFF (Merck Molecular Force Field). All conformers were further optimized with Density functional theory (DFT) calculations at the B3LYP/6-31+G(d) level by using the Gaussian 09 program [25].
3.7. Regarding the Optical Rotation (OR) Calculation Assay
For OR computation, all conformers of compound 6 were first optimized at the B3LYP/6-31+G(d) in MeOH (PCM). DFT at the B3LYP/6-31+G(d) level in Gaussian 09 was used to theoretically calculate the OR in MeOH for each conformer of compound 6 [26].
3.8. Vibrational Circular Dichroism (VCD) Calculation Assay
The conformational search for the molecule R-compound-4 and S-compound-4 was carried out using the MMFF94 force field by the MOE 2019.01 software (Chemical Computing Group ULC). A total of 129 stable conformers for R-compound-4 and 140 stable conformers for S-compound-4 were recorded with relative energy within a 5 kcal/mol energy window. DFT calculations were used to optimize the conformers at the B3LYP/6-31G(d) and B3LYP/6-311+G(d) levels, respectively. The VCD calculations for the stable conformers were performed by Gaussian 09 (Gaussian Inc., Wallingford, CT, USA) software. VCD calculations for R-compound-4 and S-compound-4 were carried out at the B3LYP/6-311+G(d) level in the gas phase. Boltzmann statistics were used for final simulations of the VCD for these molecules. The VCD spectrum obtained from R-compound-4 and S-compound-4 both have a half-peak width of 0.16 and a wavelength range of 1100 to 1800 [27].
3.9. Assay of Antimicrobial Activity
The major plant pathogenic fungi C. camelliae was used to evaluate the antifungal activity of each compound, with carbendazim serving as the benchmark control at a concentration of 64 μg/mL in DMSO. The compounds were dissolved in DMSO to generate 128 mg/mL stock solutions. A total of 1 × 105 cells/mL of C. camelliae were inoculated into each well of a 96-well plate [28]. Subsequently, the stock solutions were then serially diluted with PDB liquid medium to afford working concentrations of 128 to 2 μg/mL. Following a 48 h incubation at 28 °C, we determined the minimum inhibitory concentration (MIC) based on the growth outcomes in the 96-well plates. Carbendazim was used as a positive control.
3.10. Hydroxyl Radical Scavenging Assay
The salicylate technique was utilized to assess the hydroxyl radical scavenging capacity [29,30]. The hydroxyl radical scavenging test was performed in 96-well microplates. Twenty-five microliters of samples (200 μM) was mixed with 25 μL of FeSO4·7H2O (9 mM) and 25 μL of ethanolic salicylic acid (9 mM), which were thoroughly mixed using a vortex mixer. Subsequently, 25 μL of H2O2 (8.8 mM) was added to the reaction mixture and then incubated at 37 °C for 30 min. Vitamin C was prepared as positive control and the absorbance was measured at 510 nm.
The hydroxyl radical scavenging percentage was calculated using the following equation:
Scavenging activity(%) = [OD(control) − (OD(sample) − OD(blank))]/OD(control) × 100%
4. Conclusions
In conclusion, with the aim of assessing the practical value and potential risk of the traditionally medicinal and edible higher fungus Schizophyllum commune, induced by natural undescribed small molecules, we implemented a combination strategy of elicitors to characterize products of cryptic and extremely low-expressed gene clusters. The resulting combination of five elicitors with different concentrations and induction mechanism was selected and added into fermentation medium. As a result, we were able to significantly upregulate a class of linear diterpene-derived metabolites. In total, fifteen linear diterpene-derived variants were isolated and identified, including eleven new ones and three known ones. All the isolated terpenes contain an unusual butenedioic acid moiety in the elongation terminus. Compound 1 was a rare monocyclic diterpene, while 2–6 possessed a tetrahydrofuran moiety. The compounds 4, 5 and 13 are considered as trinorditerpene-type metabolites. In contrast to polycyclic terpene products, the linear terpene-derived products are unusual in nature. Recently, the Zou group [31] had been characterized as a globin-like enzyme, TutaA, in the Schizophyllum commune, which is responsible for truncating schizostain A (14) to form trinorditerpene product 2-butenedioic acid (13), indicating that the production of the new truncated compounds 4 and 5 is probably involved in a similar formation mechanism.
All the diterpene compounds displayed the ability for the scavenging of hydroxyl radicals and showed null cytotoxic activity at 10 µM. Considering that the crude extract of Schizophyllum commune has been approved as a cosmetic ingredient in China, the diterpenes would be beneficial protectants for a cosmetic ingredient. In addition, compound 1 showed potent antifungal activity, which highlights the potential of 1 as a lead compound for a novel agricultural fungicide development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29112608/s1, The NMR, HRESIMS, IR and VCD data for 1–14.
Author Contributions
Y.W.: data curation, formal analysis, investigation, writing—original draft; F.C.: methodology, software, data curation, writing—review and editing; L.Z.: methodology, software, data curation, investigation; H.L.: methodology, supervision, writing—review and editing; H.G.: data curation, formal analysis; G.C.: data curation; C.N.: formal analysis; P.Z.: methodology, validation; D.L.: resources, supervision; S.L.: data curation; Y.J.: resources, supervision, review and editing; G.W.: funding acquisition, methodology, project administration, resources, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful for the financial support provided by the Start-up Research Fund from Nanjing Forestry University, China (Grant No. 163030196), the Student Practice Innovation and Training Program of Nanjing Forestry University (Grant Nos. 2021NFUSPITP0035, 2022NFUSPITP0061, and 2023NFUSPITP0063), and the National Natural Science Foundation of China (Grant No. 22207030).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article or Supplementary Material.
Acknowledgments
The authors thank the Ocean University of China for the data calculations.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Du, B.; Yang, Y.; Bian, Z.; Xu, B. Characterization and Anti-Inflammatory Potential of an Exopolysaccharide from Submerged Mycelial Culture of Schizophyllum commune. Front. Pharmacol. 2017, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; Schiavenin, C.; Mendonça, S.; de Siqueira, F.G.; Salvador, M.; Camassola, M. Chemical features and antioxidant profile by Schizophyllum commune produced on different agroindustrial wastes and byproducts of biodiesel production. Food. Chem. 2020, 329, 127089. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Yang, Y.; Bian, Z.; Xu, B. Molecular weight and helix conformation determine intestinal anti-inflammatory effects of exopolysaccharide from Schizophyllum commune. Carbohyd. Polym. 2017, 172, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Frydenvang, K.; Liu, H.; Zhai, L.; Chen, M.; Olsen, C.E.; Christensen, S.B. Iminolactones from Schizophyllum commune. J. Nat. Prod. 2015, 78, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- National Medical Products Administration. 2023. Available online: https://www.nmpa.gov.cn (accessed on 27 September 2023).
- Gao, J.M. New biologically active metabolites from Chinese higher fungi. Curr. Org. Chem. 2006, 10, 849–871. [Google Scholar]
- Tang, H.Y.; Yin, X.; Zhang, C.C.; Jia, Q.; Gao, J.M. Structure diversity, synthesis, and biological activity of cyathane diterpenoids in higher fungi. Curr. Med. Chem. 2015, 22, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, C.C.; Yin, X.; Wei, J.; Gao, J.M.; Striatoids, A.-F. Cyathane Diterpenoids with Neurotrophic Activity from Cultures of the Fungus Cyathus striatus. J. Nat. Prod. 2015, 78, 783–788. [Google Scholar] [CrossRef]
- Ohm, R.A.; de Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; de Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef]
- Kogen, H.; Tago, K.; Kaneko, S.; Hamano, K.; Onodera, K.; Haruyama, H.; Minagawa, K.; Kinoshita, T.; Ishikawa, T.; Tanimoto, T.; et al. Schizostatin, a novel squalene synthase inhibitor produced by the mushroom, Schizophyllum commune. II. Structure elucidation and total synthesis. J. Antibiot. 1996, 49, 624–630. [Google Scholar] [CrossRef]
- Chunyu, W.X.; Ding, Z.G.; Zhao, J.Y.; Wang, Y.X.; Han, X.L.; Li, M.G.; Wen, M.L. Two new diketopiperazines from the tin mine tailings-derived fungus Schizophyllum commune YIM DT 10058. Nat. Prod. Res. 2017, 31, 1566–1572. [Google Scholar] [CrossRef]
- Chen, G.G.; Zhu, Q.F.; Long, X.M.; Lu, Q.; Li, K.Y.; Chen, Q.; Zhou, M.; Liao, G.-S.; Xu, G.B. Antibacterial activities of the chemical constituents of Schizophyllum commune MST7-3 collected from coal area. Nat. Prod. Res. 2022, 36, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Yang, X.Q.; Sun, J.; Wang, T.; Cui, H.R.; Yang, Y.B. New Metabolites, Antifeedant, Insecticidal Activities, and Reciprocal Relationship Between Insect and Fungus from Endophyte Schizophyllum commune. Chem. Biodivers. 2022, 19, e202200130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Zou, G.; Wang, G.; Kang, W.; Yuan, J.; She, Z. Cytotoxic Bromine- and Iodine-Containing Cytochalasins Produced by the Mangrove Endophytic Fungus Phomopsis sp. QYM-13 Using the OSMAC Approach. J. Nat. Prod. 2022, 85, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.Q.; Tang, H.Y.; Li, X.J.; Zhang, L.; Xiao, J.; Gao, Q.Y.; Zhang, A.L.; Gao, J.M. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain-many compounds method. J. Agric. Food. Chem. 2013, 61, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.-Y.; Ji, C.-h.; Lee, D.; Shim, S.H.; Joo, H.-S.; Kang, H.S. Acidonemycins A–C, Glycosylated Angucyclines with Antivirulence Activity Produced by the Acidic Culture of Streptomyces indonesiensis. J. Nat. Prod. 2023, 86, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhou, H.; Zhang, P.; Wang, X.; Li, W.; Zhang, W.; Liu, X.; Liu, H.-W.; Keller, N.P.; An, Z.; et al. Polyketide Production of Pestaloficiols and Macrodiolide Ficiolides Revealed by Manipulations of Epigenetic Regulators in an Endophytic Fungus. Org. Lett. 2016, 18, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Zhang, Q.; Gao, Y.Q.; Zhang, A.L.; Gao, J.M. Miniolins A–C, novel isomeric furanones induced by epigenetic manipulation of Penicillium minioluteum. RSC. Advances. 2015, 5, 2185–2190. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhong, Y.; Yu, Y.; Shi, D.F.; Huang, H.Y.; Tang, X.L.; Wang, Y.H.; Chen, G.D.; Zhang, H.P.; Liu, C.L.; et al. 4-Hydroxy Pyridones from Heterologous Expression and Cultivation of the Native Host. J. Nat. Prod. 2020, 83, 3338–3346. [Google Scholar] [CrossRef]
- Lee, S.R.; Seyedsayamdost, M.R. Induction of Diverse Cryptic Fungal Metabolites by Steroids and Channel Blockers. Angew Chem. Int. Ed. Engl. 2022, 61, e202204519. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Merten, C.; Firsova, D.; Fearnhead, H.; Tasdemir, D. Oxygenated Acyclic Diterpenes with Anticancer Activity from the Irish Brown Seaweed Bifurcaria bifurcata. Mar. Drugs 2020, 18, 581. [Google Scholar] [CrossRef]
- Yun, B.S.; Lee, I.K.; Woo, E.E.; Kim, Y.J.; Lee, J.Y.; Wo, S.K.; Jin, K.S.; Seon, K.J.; Ja, S.S. Antimicrobial Composition Including Extract of Schizophyllum commune Strain Culture and Preparing Method Thereof. KR Patent 20190050037-A, 1 August 2018. [Google Scholar]
- Tanimoto, T.; Tsujita, Y.; Hamano, K.; Haruyama, H.; Kinoshita, T.; Hosoya, T.; Kaneko, S.; Tago, K.; Kogen, H. Schizostatin, a potent squalene synthase inhibitor from Schizophyllum commune: Isolation, structure elucidation, and total synthesis. Tetrahedron. Lett. 1995, 36, 6301–6304. [Google Scholar] [CrossRef]
- Anjum, K.; Huang, X.; Zhou, L.; Zhu, T.; Che, Q.; Zhang, G.; Li, D. New cyclic dipeptide discovered from deep-sea derived Aspergillus sp. HDN20-1401. Nat. Prod. Res. 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Xing, L.; Sun, C.; Liang, S.; Liu, T.; Zhang, X.; Zhu, T.; Pfeifer, B.A.; Che, Q.; Zhang, G.; et al. Monacycliones G-K and ent-Gephyromycin A, Angucycline Derivatives from the Marine-Derived Streptomyces sp. HDN15129. J. Nat. Prod. 2020, 83, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Mándi, A.; Kurtán, T. Applications of OR/ECD/VCD to the structure elucidation of natural products. Nat. Prod. Rep. 2019, 36, 889–918. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Merten, C.; Kaiser, M.; Tasdemir, D. Bifurcatriol, a New Antiprotozoal Acyclic Diterpene from the Brown Alga Bifurcaria bifurcata. Mar. Drugs 2017, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Alishir, A.; Zhang, S.; Zhang, Y.; Choi, S.; Pang, C.; Bae, H.Y.; Jung, W.H.; Kim, K.H. Identification of Obscurolide-Type Metabolites and Antifungal Metabolites from the Termite-Associated Streptomyces neopeptinius BYF101. J. Nat. Prod. 2023, 86, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, J. A comparative study of caffeic acid and a novel caffeic acid conjugate SMND-309 on antioxidant properties in vitro. Lwt. Food. Sci. Technol. 2012, 46, 239–244. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.D.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food. Chem. 2008, 106, 888–895. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Zou, Y. Genome mining of fungal globin-like enzymes for catalyzing the synthesis of linear terpenes. Chin. J. Nat. Med. 2022, 20, 795–800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).