The Key Role of Amino Acids in Pollen Quality and Honey Bee Physiology—A Review
Abstract
1. Introduction
2. Composition of Bee Pollen
3. Recognition of the Nutritional Value of Pollen by Worker Bees
4. Physiology of Protein Digestion
5. The Key Role of Protein and Amino Acids in the Metabolism of Apis mellifera Workers
| Amino Acids | Role in the Honey Bee Metabolism | Literature |
|---|---|---|
| Tryptophan | A precursor of serotonin, a neuromodulator and a hormone whose level in the brain increases with age | [85] |
| Methionine | The major substitute and active methyl donor for DNA methylation, which is an epigenetic driver of caste differentiation | [89] |
| Arginine | A substrate used by the enzyme nitric oxide synthase to produce NO, participates in the immune response during injury | [38] |
| Leucine | Affects many TOR signaling pathways and genes; in insects, as in other animals, it may be associated with the activity of many enzymes such as alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatine kinase (CK) | [90] |
| Phenylalanine | Has a strong phagostimulatory effect | [91] |
| Tyrosine | Participates in in the formation of sclerotin, the matrix in which chitin fibers are embedded | [91] |
| Histidine | A precursor to histamine | [92] |
| Cysteine | A limited resource in most insects and is necessary for the production of glutathione; an antioxidant that neutralizes the oxygen forms produced as a result of the reaction and supports immune functions | [93] |
| Proline | Takes part in physiological changes in temperature, preventing overcooling; proline increases cold tolerance; participates in energy metabolism during flight (energy boost for flight); increases the survival rate and weight of brood larvae | [87,94,95] |
| Glutamic acid | An important neurotransmitter regulating the processes of learning and memory | [95,96] |
| Lysine | This amino acid is directly involved in the synthesis of nitric oxide, a known neurotransmitter affecting memory | [85] |
6. The Phenomenon of Hunger and Low Protein Diversity in a Bee Colony Conditioned by a Mono Diet and Invasive Plants
7. Conclusions and Further Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barraud, A.; Barascou, L.; Lefebvre, V.; Sene, D.; Le Conte, Y.; Alaux, C.; Grillenzoni, F.V.; Corvucci, F.; Serra, G.; Costa, C.; et al. Variations in Nutritional Requirements Across Bee Species. Front. Sustain. Food Syst. 2022, 6, 824750. [Google Scholar] [CrossRef]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional Physiology and Ecology of Honey Bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef]
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antúnez, K. Impact of Nutritional Stress on Honeybee Gut Microbiota, Immunity, and Nosema ceranae Infection. Microb. Ecol. 2020, 80, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, J.M.; Chakrabarti, P.; Sagili, R.R. Honey Bee Nutrition. Vet. Clin. N. Am.-Food Anim. Pract. 2021, 37, 505–519. [Google Scholar] [CrossRef]
- Ebrahimi, Y.; Ramírez-Coronel, A.A.; Al-Dhalimy, A.M.B.; Alfilm, R.H.C.; Al-Hassan, M.; Obaid, R.F.; Alameri, A.A.; Rastiani, F.; Khaledian, Y.; Shokri, S. Effects of honey and bee venom on human health. Casp. J. Environ. Sci. 2023, 21, 245–249. [Google Scholar]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Fat body—Multifunctional insect tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Kuszewska, K.; Chobotow, J.; Wójcik, Ł.; Paleolog, J.; Woyciechowski, M. Segmentation of the subcuticular fat body in Apis mellifera females with different reproductive potentials. Sci. Rep. 2021, 11, 13887. [Google Scholar] [CrossRef]
- Basualdo, M.; Barragán, S.; Vanagas, L.; García, C.; Solana, H.; Rodríguez, E.; Bedascarrasbure, E. Conversion of high and low pollen protein diets into protein in worker honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2013, 106, 1553–1558. [Google Scholar] [CrossRef]
- Paray, B.A.; Kumari, I.; Hajam, Y.A.; Sharma, B.; Kumar, R.; Albeshr, M.F.; Farah, M.A.; Khan, J.M. Honeybee nutrition and pollen substitutes: A review. Saudi J. Biol. Sci. 2021, 28, 1167–1176. [Google Scholar] [CrossRef]
- Ghosh, S.; Jeon, H.; Jung, C. Foraging behaviour and preference of pollen sources by honey bee (Apis mellifera) relative to protein contents. J. Ecol. Environ. 2020, 44, 4. [Google Scholar] [CrossRef]
- Avni, D.; Hendriksma, H.P.; Dag, A.; Uni, Z.; Shafir, S. Nutritional aspects of honey bee-collected pollen and constraints on colony development in the eastern Mediterranean. J. Insect Physiol. 2014, 69, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bezerra da Silva Santos, K.C.; Frost, E.; Samnegård, U.; Saunders, M.E.; Rader, R. Pollen collection by honey bee hives in almond orchards indicate diverse diets. Basic Appl. Ecol. 2022, 64, 68–78. [Google Scholar] [CrossRef]
- Ricigliano, V.A. Honey Bee Proteome Responses to Plant and Cyanobacteria (blue-green algae) Diets. ACS Food Sci. Technol. 2021, 1, 17–26. [Google Scholar] [CrossRef]
- Raderschall, C.A.; Bommarco, R.; Lindström, S.A.M.; Lundin, O. Landscape crop diversity and semi-natural habitat affect crop pollinators, pollination benefit and yield. Agric. Ecosyst. Environ. 2021, 306, 107189. [Google Scholar] [CrossRef]
- Filipiak, Z.M.; Denisow, B.; Stawiarz, E.; Filipiak, M. Unravelling the dependence of a wild bee on floral diversity and composition using a feeding experiment. Sci. Total Environ. 2022, 820, 153326. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.; Jones, L.; Brennan, G.; Creer, S.; de Vere, N. Seasonal progression and differences in major floral resource use by bees and hoverflies in a diverse horticultural and agricultural landscape revealed by DNA metabarcoding. J. Appl. Ecol. 2022, 59, 1484–1495. [Google Scholar] [CrossRef]
- Filipiak, M. A better understanding of bee nutritional ecology is needed to optimize conservation strategies for wild bees—The application of ecological stoichiometry. Insects 2018, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.E.D.; Barbosa, C.D.; Lourenço, A.P. Pollen nutrition in honey bees (Apis mellifera): Impact on adult health. Apidologie 2016, 47, 15–25. [Google Scholar] [CrossRef]
- Quinlan, G.; Milbrath, M.; Otto, C.; Smart, A.; Iwanowicz, D.; Cornman, R.S.; Isaacs, R. Honey bee foraged pollen reveals temporal changes in pollen protein content and changes in forager choice for abundant versus high protein flowers. Agric. Ecosyst. Environ. 2021, 322, 107645. [Google Scholar] [CrossRef]
- Bryś, M.S.; Skowronek, P.; Strachecka, A. Pollen diet—Properties and impact on a bee colony. Insects 2021, 12, 798. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Da Silva Das Neves, S.; Human, H.; Pirk, C.W.W. Digestibility and nutritional value of fresh and stored pollen for honey bees (Apis mellifera scutellata). J. Insect Physiol. 2018, 107, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Somerville, D.C.; Nicol, H.I. Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity. Aust. J. Exp. Agric. 2006, 46, 141–149. [Google Scholar] [CrossRef]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, utilization, and genetic improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Taha, E.K.A.; Al-Kahtani, S.; Taha, R. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.S.; Wu, T.H.; Huang, M.Y.; Wang, D.Y.; Wu, M.C. Nutritive value of 11 bee pollen samples from major floral sources in taiwan. Foods 2021, 10, 2229. [Google Scholar] [CrossRef]
- Vergun, O.M.; Grygorieva, O.; National, M.M.G.; Garden, B.; Brindza, J. Nutritional composition of Phacelia tanacetifolia Benth. bee pollen and inflorescences. Agrobiodivers. Improv. Nutr. Health Life Qual. 2023, 2023, 95–104. [Google Scholar] [CrossRef]
- Jachuła, J.; Denisow, B.; Strzałkowska-Abramek, M. Does an invader have a bright side? Floral reward in two Solidago species. J. Apic. Res. 2020, 59, 599–608. [Google Scholar] [CrossRef]
- McAulay, M.K.; Forrest, J.R.K. How do sunflower pollen mixtures affect survival of queenless microcolonies of bumblebees (Bombus impatiens)? Arthropod. Plant. Interact. 2019, 13, 517–529. [Google Scholar] [CrossRef]
- Somerville, D.C.; Nicol, H.I. Mineral content of honeybee-collected pollen from southern New South Wales. Aust. J. Exp. Agric. 2002, 42, 1131–1136. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Sedláčková, V.H.; Grygorieva, O.; Šramková, K.F.; Shelepova, O.; Goncharovska, I.; Mňahončáková, E. The Chemical Composition Of Pollen, Staminate Catkins, And Honey Of Castanea Sativa Mill. Potravin. Slovak J. Food Sci. 2021, 15, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Kostryco, M.; Chwil, M. Structure of anther epidermis and endothecium, production of pollen, and content of selected nutrients in pollen grains from six Rubus idaeus L. Cultivars. Agronomy 2021, 11, 1723. [Google Scholar] [CrossRef]
- Omar, E.; Abd-Ella, A.A.; Khodairy, M.M.; Moosbeckhofer, R.; Crailsheim, K.; Brodschneider, R. Influence of different pollen diets on the development of hypopharyngeal glands and size of acid gland sacs in caged honey bees (Apis mellifera). Apidologie 2017, 48, 425–436. [Google Scholar] [CrossRef]
- Agarwal, A.; Nair, P.K.K. Short communication free and protein-bound amino acids of pollen of Acacia Auriculaeformis (Mimosaceae). Grana 1989, 28, 155–157. [Google Scholar] [CrossRef]
- Vanderplanck, M.; Moerman, R.; Rasmont, P.; Lognay, G.; Wathelet, B.; Wattiez, R.; Michez, D. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS ONE 2014, 9, e86209. [Google Scholar] [CrossRef] [PubMed]
- Liolios, V.; Tananaki, C.; Dimou, M.; Kanelis, D.; Goras, G.; Karazafiris, E.; Thrasyvoulou, A. Clasificación del polen de las plantas melíferas en función de su aportación de proteínas para las abejas de la miel. J. Apic. Res. 2015, 54, 582–592. [Google Scholar] [CrossRef]
- Negri, P.; Ramirez, L.; Quintana, S.; Szawarski, N.; Maggi, M.; Conte, Y.L.; Lamattina, L.; Eguaras, M. Dietary supplementation of honey bee larvae with arginine and abscisic acid enhances nitric oxide and granulocyte immune responses after trauma. Insects 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Wilson, K. Honeybee nutrition is linked to landscape composition. Ecol. Evol. 2014, 4, 4195–4206. [Google Scholar] [CrossRef]
- Simanonok, M.P.; Otto, C.R.V.; Smart, M.D. Do the Quality and Quantity of Honey Bee-Collected Pollen Vary Across an Agricultural Land-Use Gradient? Environ. Entomol. 2020, 49, 189–196. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Gage, S.L.; Corby-Harris, V.; Carroll, M.; Chambers, M.; Graham, H.; Watkins deJong, E.; Hidalgo, G.; Calle, S.; Azzouz-Olden, F.; et al. Connecting the nutrient composition of seasonal pollens with changing nutritional needs of honey bee (Apis mellifera L.) colonies. J. Insect Physiol. 2018, 109, 114–124. [Google Scholar] [CrossRef]
- Jeannerod, L.; Carlier, A.; Schatz, B.; Daise, C.; Richel, A.; Agnan, Y.; Baude, M.; Jacquemart, A.L. Some bee-pollinated plants provide nutritionally incomplete pollen amino acid resources to their pollinators. PLoS ONE 2022, 17, e0269992. [Google Scholar] [CrossRef]
- de Groot, A.P. Amino acid requirements for growth of the honeybee (Apis mellifica L.). Experientia 1952, 8, 192–194. [Google Scholar] [CrossRef]
- Somerville, D.C. Nutritional Value of Bee Collected Pollens; Rural Industries Research and Development Corporation, 2001; pp. 1–166. Available online: https://books.google.pl/books/about/Nutritional_Value_of_Bee_Collected_Polle.html?id=D1A7vgAACAAJ&redir_esc=y (accessed on 28 April 2024).
- Szczęsna, T. Protein content and amino acid composition of bee-collected pollen from selected botanical origins. J. Apic. Sci. 2006, 50, 81–90. [Google Scholar]
- Bayram, N.E.; Gercek, Y.C.; Çelik, S.; Mayda, N.; Kostić, A.; Dramićanin, A.M.; Özkök, A. Phenolic and free amino acid profiles of bee bread and bee pollen with the same botanical origin—Similarities and differences. Arab. J. Chem. 2021, 14, 103004. [Google Scholar] [CrossRef]
- Degrandi-Hoffman, G.; Eckholm, B.; Huang, M. Methods for comparing nutrients in beebread made by Africanized and European honey bees and the effects on hemolymph protein titers. J. Vis. Exp. 2015, 2015, e52448. [Google Scholar] [CrossRef] [PubMed]
- Alshallash, K.S.; Abolaban, G.; Elhamamsy, S.M.; Zaghlool, A.; Nasr, A.; Nagib, A.; El-Hakim, A.F.A.; Zahra, A.A.; Hamdy, A.E.; Taha, I.M. Bee Pollen as a Functional Product—Chemical Constituents and Nutritional Properties. J. Ecol. Eng. 2023, 24, 173–183. [Google Scholar] [CrossRef]
- Behmer, S.T.; David Nes, W. Insect Sterol Nutrition and Physiology: A Global Overview. Adv. Insect Physiol. 2003, 31, 1–72. [Google Scholar]
- London-Shafir, I.; Shafir, S.; Eisikowitch, D. Amygdalin in almond nectar and pollen—Facts and possible roles. Plant Syst. Evol. 2003, 238, 87–95. [Google Scholar] [CrossRef]
- Tauber, J.P.; Tozkar, C.Ö.; Schwarz, R.S.; Lopez, D.; Irwin, R.E.; Adler, L.S.; Evans, J.D. Colony-level effects of amygdalin on honeybees and their microbes. Insects 2020, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Motta, E.V.S.; Gage, A.; Smith, T.E.; Blake, K.J.; Kwong, W.K.; Riddington, I.M.; Moran, N.A. Host-microbiome metabolism of a plant toxin in bees. eLife 2022, 11, e82595. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pólit, C.; Gonzalez-Pastor, R.; Heredia-Moya, J.; Carrera-Pacheco, S.E.; Castillo-Solis, F.; Vallejo-Imbaquingo, R.; Barba-Ostria, C.; Guamán, L.P. Chemical Properties and Biological Activity of Bee Pollen. Molecules 2023, 28, 7768. [Google Scholar] [CrossRef]
- Elsayeh, W.A.; Cook, C.; Wright, G.A. B-Vitamins Influence the Consumption of Macronutrients in Honey Bees. Front. Sustain. Food Syst. 2022, 6, 804002. [Google Scholar] [CrossRef]
- Ghouizi, A.E.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Somerville, D. Fat Bees Skinny Bees—A Manual on Honey Bee Nutrition for Beekeepers; RIRDC: Kingston, ON, Canada, 2005. [Google Scholar]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, Z.M.; Filipiak, M. The scarcity of specific nutrients in wild bee larval food negatively influences certain life history traits. Biology 2020, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Layek, U.; Manna, S.S.; Karmakar, P. Pollen foraging behaviour of honey bee (Apis mellifera L.) in southern West Bengal, India. Palynology 2020, 44, 114–126. [Google Scholar] [CrossRef]
- Raffiudin, R.; Dyahastuti, M.; Nugraha, R.; Sayusti, T.; Djuita, N.R.; Suwananda, E.; Allvioningrum, V.; Mardhony, R.; Biagioni, S.; Setyaningsih, C.A.; et al. The effect of land cover on the foraging behavior and pollen in the honey of the giant bee Apis dorsata in Sumatra. Front. Bee Sci. 2024, 2, 1366287. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Snyder, L.; Meador, C.; Ayotte, T. Honey bee (Apis mellifera) nurses do not consume pollens based on their nutritional quality. PLoS ONE 2018, 13, e0191050. [Google Scholar] [CrossRef] [PubMed]
- Beekman, M.; Preece, K.; Schaerf, T.M. Dancing for their supper: Do honeybees adjust their recruitment dance in response to the protein content of pollen? Insectes Soc. 2016, 63, 117–126. [Google Scholar] [CrossRef]
- Roulston, T.; Cane, J. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 2002, 16, 49–65. [Google Scholar] [CrossRef]
- Pernal, S.F.; Currie, R.W. The influence of pollen quality on foraging behavior in honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 2001, 51, 53–68. [Google Scholar] [CrossRef]
- Fewell, J.H.; Winston, M.L. Colony state and regulation of pollen foraging in the honey bee, Apis mellifera L. Behav. Ecol. Sociobiol. 1992, 30, 387–393. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N. The same, but different: Pollen foraging in honeybee and bumblebee colonies. Apidologie 2012, 43, 449–464. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Patch, H.M.; Biddinger, D.J.; Coccia, M.; Crone, M.K.; Fiely, M.; Francis, J.S.; Hines, H.M.; Hodges, M.; et al. Pollen protein: Lipid macronutrient ratios may guide broad patterns of bee species floral preferences. Insects 2020, 11, 132. [Google Scholar] [CrossRef]
- Leponiemi, M.; Freitak, D.; Moreno-Torres, M.; Pferschy-Wenzig, E.M.; Becker-Scarpitta, A.; Tiusanen, M.; Vesterinen, E.J.; Wirta, H. Honeybees’ foraging choices for nectar and pollen revealed by DNA metabarcoding. Sci. Rep. 2023, 13, 14753. [Google Scholar] [CrossRef]
- Zayed, A. Monoculture is good if you are a squash bee. Proc. Natl. Acad. Sci. USA 2023, 120, 10–11. [Google Scholar] [CrossRef] [PubMed]
- McKinstry, M.; Prado-Irwin, S.R.; Adames, T.R.; Snow, J.W. Retained metabolic activity in honey bee collected pollen has implications for pollen digestion and effects on honey bee health. Apidologie 2020, 51, 212–225. [Google Scholar] [CrossRef]
- McNally, J.B.; Mccaughey, W.F.; Standifer, L.N.; Todd, F.E. Partition of Excreted Nitrogen From Honey Bees Fed Various Proteins. J. Nutr. 1965, 85, 113–116. [Google Scholar] [CrossRef]
- Nation, J.L. Insect Physiology and Biochemistry; CRC Press, Cop: Boca Raton, FL, USA, 2002. [Google Scholar]
- Li, X.; Zhou, Y.; Wu, K. Biological Characteristics and Energy Metabolism of Migrating Insects. Metabolites 2023, 13, 439. [Google Scholar] [CrossRef]
- Crailsheim, K.; Leonhard, B. Amino acids in honeybee worker haemolymph. Amino Acids 1997, 13, 141–153. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Fitz, W.; Copeland, D.C.; Mott, B.M.; Maes, P.; Floyd, A.S.; Dockstader, A.; Anderson, K.E. The impact of pollen consumption on honey bee (Apis mellifera) digestive physiology and carbohydrate metabolism. Arch. Insect Biochem. Physiol. 2017, 96, e21406. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Ding, G.; Jia, G.; Feng, M.; Huang, J. Hemolymph Metabolism Analysis of Honey Bee. Insects 2023, 14, 37. [Google Scholar] [CrossRef]
- Teulier, L.; Weber, J.M.; Crevier, J.; Darveau, C.A. Proline as a fuel for insect flight: Enhancing carbohydrate oxidation in hymenopterans. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, L.; Hang, X.; Yang, W.; Liu, F.; Wang, Y.; Ma, L.; Hang, X.; Yang, W.; Liu, F. Digestion of protein of two pollen types in China by the honeybee (Apis mellifera L.) To cite this version: HAL Id: Hal-01234760 Digestion of protein of two pollen types in China by the honeybee (Apis mellifera L.). Apidologie 2015, 45, 590–600. [Google Scholar] [CrossRef]
- Strachecka, A.; Walerowicz, M. Anatomia i Fizjologia Pszczoły Miodnej; Kobiałka, T., Ed.; BEE & HONEY: Klecza Dolna, Poland, 2022; pp. 168–181. [Google Scholar]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef]
- Dias, J.M.V.A.; Morais, M.M.; Francoy, T.M.; Pereira, R.A.; Turcatto, A.P.; De Jong, D. Fermentation of a Pollen Substitute Diet with Beebread Microorganisms Increases Diet Consumption and Hemolymph Protein Levels of Honey Bees (Hymenoptera, Apidae). Sociobiology 2018, 65, 760–765. [Google Scholar] [CrossRef]
- Danihlík, J.; Škrabišová, M.; Lenobel, R.; Šebela, M.; Omar, E.; Petřivalský, M.; Crailsheim, K.; Brodschneider, R. Does the pollen diet influence the production and expression of antimicrobial peptides in individual honey bees? Insects 2018, 9, 79. [Google Scholar] [CrossRef]
- Saisavoey, T.; Sangtanoo, P.; Chanchao, C.; Reamtong, O.; Karnchanatat, A. Identification of novel anti-inflammatory peptides from bee pollen (Apis mellifera) hydrolysate in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Apic. Res. 2021, 60, 280–289. [Google Scholar] [CrossRef]
- Dufour, C.; Fournier, V.; Giovenazzo, P. Diversity and nutritional value of pollen harvested by honey bee (Hymenoptera: Apidae) colonies during lowbush blueberry and cranberry (Ericaceae) pollination. Can. Entomol. 2020, 152, 622–645. [Google Scholar] [CrossRef]
- Gage, S.L.; Calle, S.; Jacobson, N.; Carroll, M.; DeGrandi-Hoffman, G. Pollen Alters Amino Acid Levels in the Honey Bee Brain and This Relationship Changes With Age and Parasitic Stress. Front. Neurosci. 2020, 14, 231. [Google Scholar] [CrossRef]
- Bursell, E. The Role of Proline in Energy Metabolism. In Energy Metabolism in Insects; Downer, R.G.H., Ed.; Springer: Boston, MA, USA, 1981; pp. 135–154. [Google Scholar] [CrossRef]
- Mollaei, M.; Hoseini, S.A.; Karimi, M.; Hekmat, Z. Short communication. Impact of the amino acid proline on the cold hardiness of honey bee, Apis mellifera L. Span. J. Agric. Res. 2013, 11, 714–717. [Google Scholar] [CrossRef]
- Huang, Z. Honey bee nutrition. Am. Bee J. 2010, 150, 773–776. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Zhang, W.; Liu, Z.; Xu, B.; Wang, H. Methionine as a methyl donor regulates caste differentiation in the European honey bee (Apis mellifera). Insect Sci. 2021, 28, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Y.; Zhang, W.; Wang, H.; Liu, Z.; Xu, B. Alterations in protein and amino acid metabolism in honeybees (Apis mellifera) fed different L-leucine diets during the larval stage. J. Asia. Pac. Entomol. 2016, 19, 769–774. [Google Scholar] [CrossRef]
- Inouye, D.W.; Waller, G.D. Responses of Honey Bees (Apis mellifera) to Amino Acid Solutions Mimicking Floral Nectars. Ecology 2016, 65, 618–625. [Google Scholar] [CrossRef]
- Ghosh, S.; Meyer-Rochow, V.B.; Jung, C. Honey bees and their brood: A potentially valuable resource of food, worthy of greater appreciation and scientific attention. J. Ecol. Environ. 2021, 45, 31. [Google Scholar] [CrossRef]
- McMillan, L.E.; Miller, D.W.; Adamo, S.A. Eating when ill is risky: Immune defense impairs food detoxification in the caterpillar Manduca sexta. J. Exp. Biol. 2018, 221, jeb173336. [Google Scholar] [CrossRef]
- Micheu, S.; Crailsheim, K.; Leonhard, B. Importance of proline and other amino acids during honeybee flight (Apis mellifera carnica POLLMANN). Amino Acids 2000, 18, 157–175. [Google Scholar] [CrossRef]
- Noor-ul-Ane, M.; Jung, C. Effect of non-essential amino acids (proline and glutamic acid) and sugar polyol (sorbitol) on brood of honey bees. Front. Ecol. Evol. 2022, 10, 1009670. [Google Scholar] [CrossRef]
- Locatelli, F.; Bundrock, G.; Müller, U. Focal and temporal release of glutamate in the mushroom bodies improves olfactory memory in Apis mellifera. J. Neurosci. 2005, 25, 11614–11618. [Google Scholar] [CrossRef] [PubMed]
- Schmickl, T.; Crailsheim, K. Cannibalism and early capping: Strategy of honeybee colonies in times of experimental pollen shortages. J. Comp. Physiol.-A Sens. Neural Behav. Physiol. 2001, 187, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Schmickl, T.; Crailsheim, K. How honeybees (Apis mellifera L.) change their broodcare behaviour in response to non-foraging conditions and poor pollen conditions. Behav. Ecol. Sociobiol. 2002, 51, 415–425. [Google Scholar] [CrossRef]
- Jachuła, J.; Denisow, B.; Wrzesień, M.; Ziółkowska, E. The need for weeds: Man-made, non-cropped habitats complement crops and natural habitats in providing honey bees and bumble bees with pollen resources. Sci. Total Environ. 2022, 840, 156551. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.J.; Mommer, L.; Barry, K.; van Ruijven, J. Stress gradients and biodiversity: Monoculture vulnerability drives stronger biodiversity effects during drought years. Ecology 2021, 102, e03193. [Google Scholar] [CrossRef]
- Bednarska, A.J.; Mikołajczyk, Ł.; Ziółkowska, E.; Kocjan, K.; Wnęk, A.; Mokkapati, J.S.; Teper, D.; Kaczyński, P.; Łozowicka, B.; Śliwińska, R.; et al. Effects of agricultural landscape structure, insecticide residues, and pollen diversity on the life-history traits of the red mason bee Osmia bicornis. Sci. Total Environ. 2022, 809, 151142. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Ghramh, H.A. Pollen source preferences and pollination efficacy of honey bee, Apis mellifera (Apidae: Hymenoptera) on Brassica napus crop. J. King Saud Univ.-Sci. 2021, 33, 101487. [Google Scholar] [CrossRef]
- Evans, D.E.; Taylor, P.E.; Singh, M.B.; Knox, R.B. Quantitative analysis of lipids and protein from the pollen of Brassica napus L. Plant Sci. 1991, 73, 117–126. [Google Scholar] [CrossRef]
- Salisbury, A.; Armitage, J.; Bostock, H.; Perry, J.; Tatchell, M.; Thompson, K. Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): Should we plant native or exotic species? J. Appl. Ecol. 2015, 52, 1156–1164. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M. Integration of alien plants into a native flower-pollinator visitation web. Proc. R. Soc. B Biol. Sci. 2002, 269, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- Drossart, M.; Michez, D.; Vanderplanck, M. Invasive plants as potential food resource for native pollinators: A case study with two invasive species and a generalist bumble bee. Sci. Rep. 2017, 7, 16242. [Google Scholar] [CrossRef] [PubMed]
- Denisow, B. Pollen Production of Selected Ruderal Plant Species in the Lublin Area; WUP Wydawnictwo Uniwersytetu Przyrodniczego: Lublin, Poland, 2011; pp. 1–86. [Google Scholar]
- Kabuce, N.; Environment, L.; Agency, M.; Priede, A.; Agency, N.C. NOBANIS—Invasive Alien Species Fact Sheet Solidago canadensis. 2012; pp. 1–10. Available online: http://proborshevik.ru/wp-content/uploads/2010/12/Kabuce,%20Priede_2010.pdf (accessed on 28 April 2024).
- Moroń, D.; Marjańska, E.; Skórka, P.; Lenda, M.; Woyciechowski, M. Invader–pollinator paradox: Invasive goldenrods benefit from large size pollinators. Divers. Distrib. 2021, 27, 632–641. [Google Scholar] [CrossRef]
- Lenda, M.; Skórka, P.; Kuszewska, K.; Moroń, D.; Bełcik, M.; Baczek Kwinta, R.; Janowiak, F.; Duncan, D.H.; Vesk, P.A.; Possingham, H.P.; et al. Misinformation, internet honey trading and beekeepers drive a plant invasion. Ecol. Lett. 2021, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Haydak, M.H. Value of Foods Other Than Pollen in Nutrition of the Honeybee. J. Econ. Entomol. 1936, 29, 870–877. [Google Scholar] [CrossRef]
- Kumari, I.; Kumar, R. Pollen substitute diet for Apis mellifera: Consumption and effects on colony parameters in sub-tropical himalaya. Indian J. Agric. Res. 2020, 54, 147–153. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.K.; Al-Khaibari, A.M.; Omar, M.O. Consumption rate of some proteinic diets affecting hypopharyngeal glands development in honeybee workers. Saudi J. Biol. Sci. 2011, 18, 73–77. [Google Scholar] [CrossRef]
- Darvishzadeh, A.; Nehzati, G.; Nowzari, J.; Hosseininaveh, V.; Nozari, J. Effect of proline as a nutrient on hypopharyngeal glands during development of Apis mellifera (Hymenoptera: Apidae). Arthropods 2015, 4, 137–143. [Google Scholar]
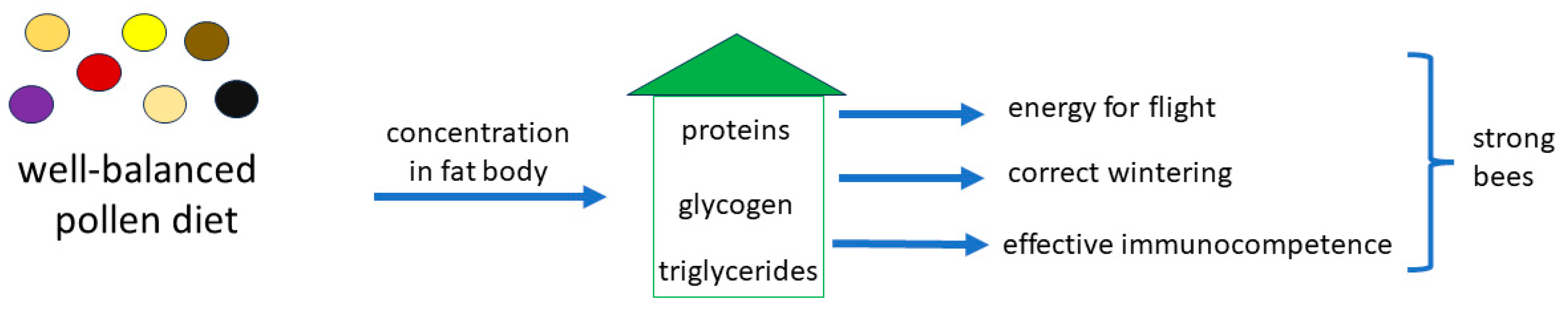
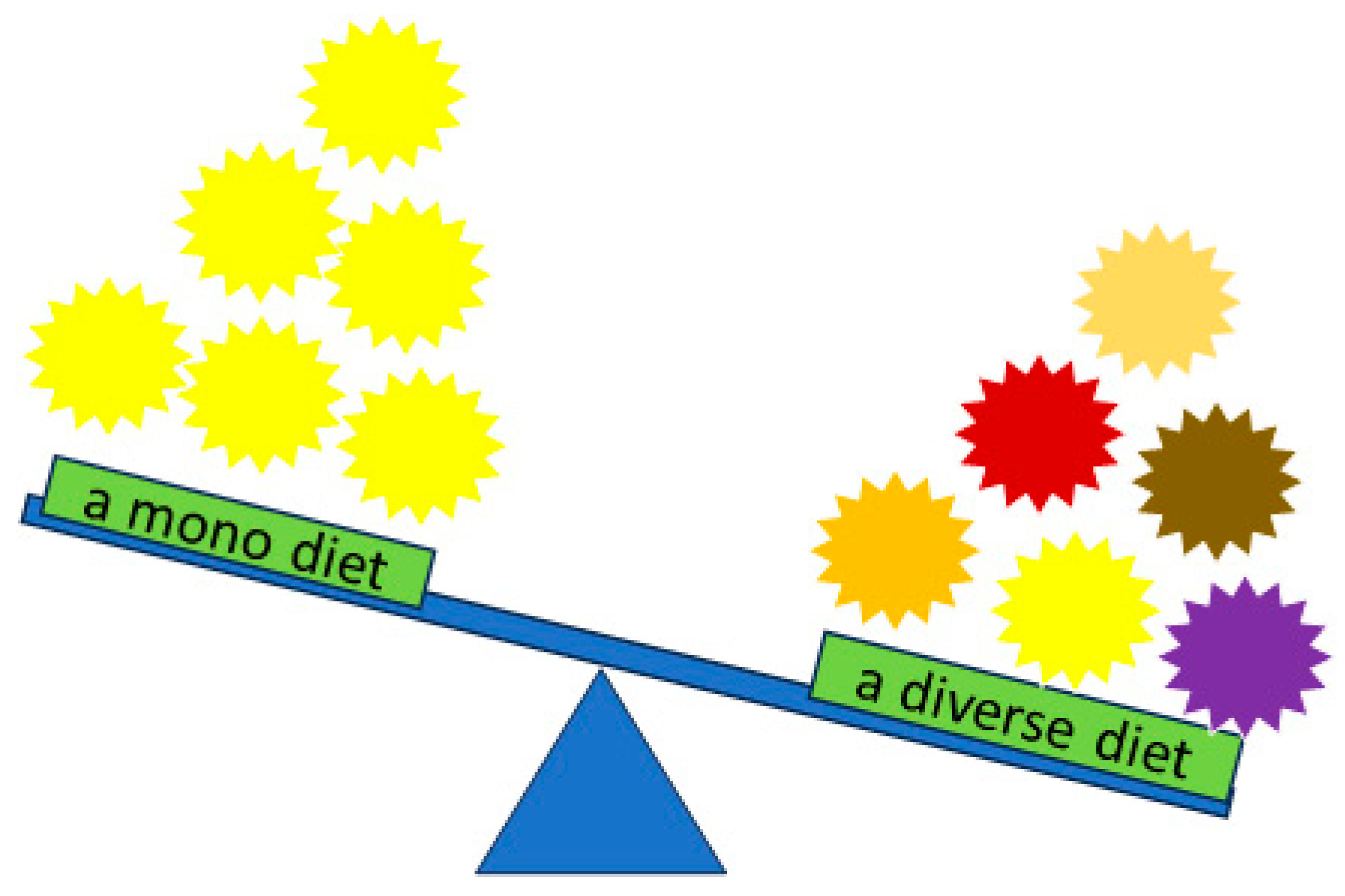
| Taxon | Total Protein Content [%] | Dominant Amino Acid Composition | Literature |
|---|---|---|---|
| Brassica napus | from 22 to 27 | Aspartic acid, Glutamic acid, Lysine, Leucine | [22,23,24,25,26] |
| Phacelia tanacetifolia | 27.44 | Glutamic acid, Proline, Aspartic acid, Leucine, Lysine, Valine | [27] |
| Solidago gigantea; Solidago canadensis | >20 | No literature data available | [28] |
| Fagopyrum | 11.4 | Glutamic acid, Proline, Aspartic acid, Leucine, Tryptophan, Lysine, Valine, Alanine, Arginine | [22,25] |
| Medicago sativa | 20.23 | Valine, Leucine, Izoleucine Phenylalanine, Proline | [24] |
| Phoenix dactylifera | 19.77 | Methionine, Histidine, Glycine, Alanine | [24] |
| Vicia faba | from 22 to 24 | Proline, Aspartic acid, Glutamic acid, Arginine, Leucine, Tryptophan | [29,30] |
| Helianthus annus | 15.19 | Leucine, Valine, Lysine, Histidine, Aspartic acid, Arginine, Tryptophan, Glutamic acid | [24,25,29] |
| Zea mays | 14.9 | Proline, Aspartic acid, Lysine, Alanine, Arginine, Tryptophan | [22,26] |
| Eucalyptus bridgesiana: | 23.1 | Proline, Glutamic acid, Aspartic acid, Leucine | [22] |
| Echium plantagineum | 37.4 | Aspartic acid, Glutamic acid, Leucine, Lysine | [22] |
| Salix discolour | 21.9 | Glutamic acid, Aspartic acid, Leucine, Lysine | [22] |
| Castanea sativa | 21.6 | Proline, Aspartic acid, Glutamic acid | [31,32] |
| Rubus sp. | 22 | Leucine, Lysine, Valine, Phenylalanine, Threonine, Izoleucine | [31,33] |
| Sinapis | No literature data available | Aspartic acid, Glutamic acid, Proline, Lysine | [34] |
| Acacia sp. | 21.8 | Aspartic acid, Glutamic, Glycine | [35] |
| Calluna vulgaris | 17 | Glutamic, Aspartic acid, Glycine | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryś, M.S.; Strachecka, A. The Key Role of Amino Acids in Pollen Quality and Honey Bee Physiology—A Review. Molecules 2024, 29, 2605. https://doi.org/10.3390/molecules29112605
Bryś MS, Strachecka A. The Key Role of Amino Acids in Pollen Quality and Honey Bee Physiology—A Review. Molecules. 2024; 29(11):2605. https://doi.org/10.3390/molecules29112605
Chicago/Turabian StyleBryś, Maciej Sylwester, and Aneta Strachecka. 2024. "The Key Role of Amino Acids in Pollen Quality and Honey Bee Physiology—A Review" Molecules 29, no. 11: 2605. https://doi.org/10.3390/molecules29112605
APA StyleBryś, M. S., & Strachecka, A. (2024). The Key Role of Amino Acids in Pollen Quality and Honey Bee Physiology—A Review. Molecules, 29(11), 2605. https://doi.org/10.3390/molecules29112605









