Exploring Phenolic Compounds Extraction from Saffron (C. sativus) Floral By-Products Using Ultrasound-Assisted Extraction, Deep Eutectic Solvent Extraction, and Subcritical Water Extraction
Abstract
1. Introduction
2. Results and Discussion
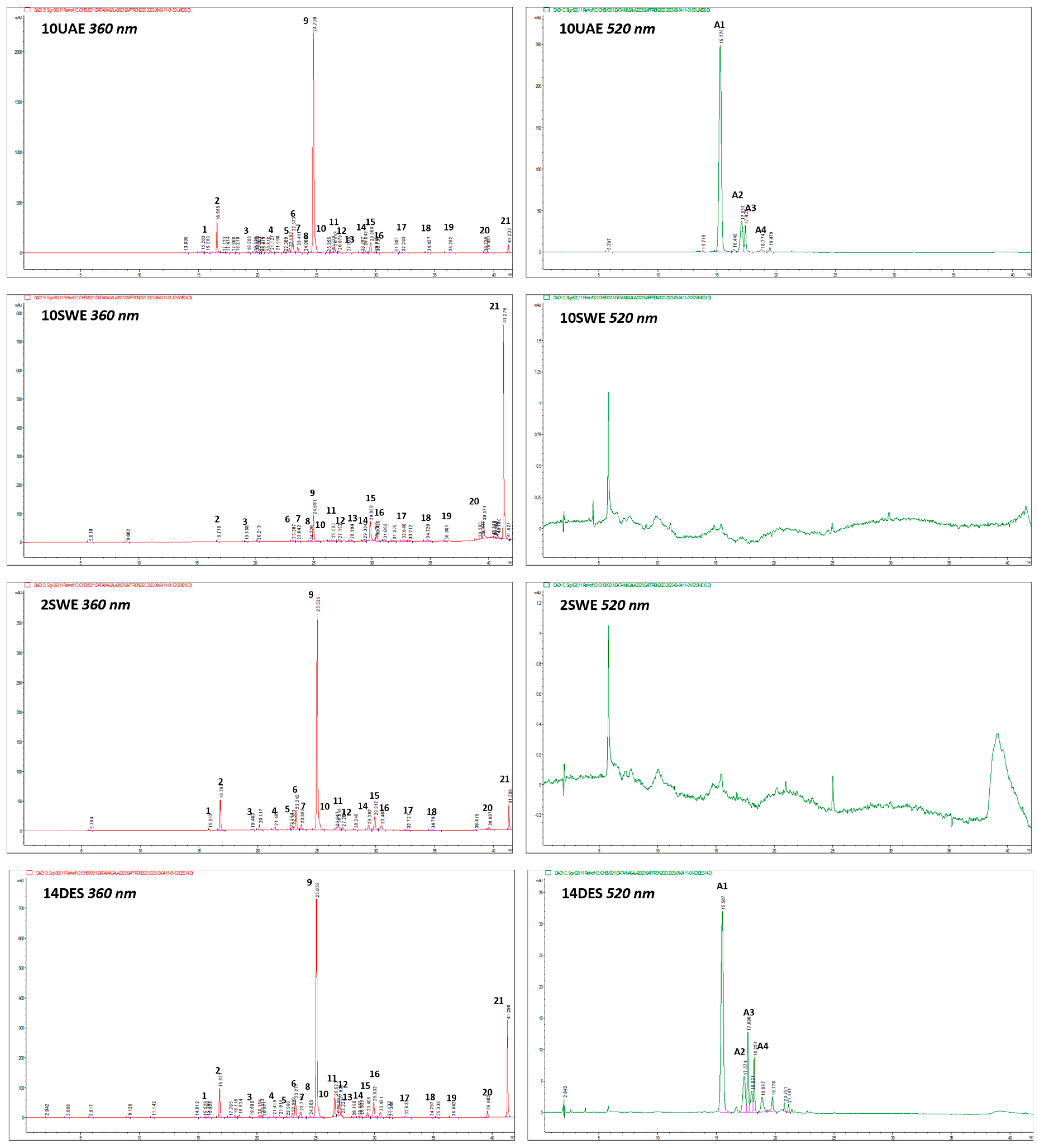
2.1. Qualitative Determination of Phenolic Compounds in C. sativus Extracts
| # n° | Rt min | Identity | Molecular Formula | [M-H]− m/z | MS/MS * m/z | Δppm | [M]+/[M+H]+ m/z | MS/MS * m/z | Δppm | References | Level |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.69 | Kaempferol sophoroside-glucoside | C33H40O21 | 771.1999 | 609.1441(80)/285.0382(80)/284.0300(100)/283.0243(100) | 1.05 | 773.2142 | 287.0553(100) | 0.52 | [6,43,44] | 2 |
| 2 | 16.56 | Kaempferol tri-O-glucoside | C33H40O21 | 771.2004 | 609.1447(63)/284.0847(33)/283.0236(20)/285.0354(100) | −0.97 | 773.2014 | 287.0546(100) | 0.55 | [6,43,44] | 2 |
| 3 | 19.40 | Kaempferol acetyl tri-O-glucoside | C35H42O22 | 813.2088 | 651.1588(29)/285.0396(53)/284.0322(100)/283.0240(73) | −0.79 | 815.2234 | 287.0542(100) | −0.82 | [43] | 2 |
| 4 | 21.32 | Myricetin glucosyl-glucoside | C27H30O18 | 641.1354 | 316.0215(100) | −0.86 | 643.1504 | 319.0450(100) | −0.2 | [43] | 2 |
| 5 | 22.69 | Quercetin di-O-glucoside | C27H30O17 | 625.1415 | 301.0335(32)/300.0271(100)/271.0299(19) | 0.49 | 627.1565 | 303.0499(100) | 1.27 | [6,43,44] | 2 |
| 6 | 22.97 | Quercetin sophoroside | C27H30O17 | 625.1409 | 463.0375(78)/300.0261(69)/301.0346(100) | −0.21 | 627.1568 | 303.0500(100) | 1.56 | [6,43,44] | 2 |
| 7 | 23.41 | Kaempferol di-O-glucoside | C27H30O16 | 609.1465 | 285.0391(32)/284.0323(100) | 0.66 | 611.1606 | 287.0550(100) | −0.11 | [43] | 2 |
| 8 | 24.01 | Isorhamnetin di-O-glucoside | C28H32O17 | 639.1568 | 313.0339(82) | −0.11 | 641.1716 | 317.0658(100) | 0.52 | [6,43,44] | 2 |
| 9 | 24.73 | Kaempferol 3-O-sophoroside | C27H30O16 | 609.1463 | 285.0386(33)/284.0325(100)/255.0291(14) | 0.33 | 611.1615 | 287.0555(100) | 1.37 | [6,43,44] | 1 |
| 10 | 24.80 | Isorhamnetin sophoroside | C28H32O17 | 639.156 | 315.0495(43)/314.0422(100)/299.0171(41)/300.0261(14) | −0.81 | 641.1710 | 317.0659(100) | −0.26 | [43] | 2 |
| 11 | 25.99 | Quercetin 3-O-glucoside | C21H20O12 | 463.0878 | 301.0322(33)/300.0263(100) | −1.3 | 465.1030 | 303.0501(100)/85.0287(10) | 0.49 | [6,43] | 1 |
| 12 | 26.32 | Kaempferol glucoside rhamnose | C27H30O15 | 593.1508 | 284.0312(100) | −0.8 | 595.1656 | 287.0551(100) | −0.34 | [43] | 2 |
| 13 | 26.51 | Isorhamnetin (rhamnosyl)-glucoside | C28H32O16 | 623.1618 | 314.0424(100) | −0.16 | 625.1768 | 317.0658(100) | 0.55 | [43] | 2 |
| 14 | 29.04 | Kaempferol 3-O-glucoside | C21H20O11 | 447.0935 | 285.0386(53)/284.0332(100)/255.0305(33) | −0.5 | 449.2088 | 287.0543(100) | −0.12 | [6,43,44] | 1 |
| 15 | 29.57 | Kaempferol acetyl di-O-glucoside | C29H32O17 | 651.1574 | 285.0385(27)/284.0322(100)/255.0285(18) | 0.81 | 653.1725 | 287.0551(100) | 1.63 | [43] | 2 |
| 16 | 29.99 | Isorhamnetin 3-O-glucoside | C22H22O12 | 477.1036 | 314.0405(100)/315.0467(80)/271.0240(56) | −0.82 | 479.1191 | 317.0656(100) | 1.32 | [43] | 1 |
| 17 | 32.26 | Kaempferol acetyl glucoside | C23H22O12 | 489.1034 | 285.0356(38)/284.0296(100) | −0.7 | 491.1190 | 287.0549(100) | 0.8 | [43] | 2 |
| 18 | 34.43 | Quercetin | C15H10O7 | 301.0346 | 178.0970(61)/151.0012(100)/177.0557(51) | −2.88 | 303.0501 | 303.0502(100)/165.0165(13) | 1.05 | [6] | 1 |
| 19 | 36.25 | Quercetin coumaroyl-glucoside | C30H26O14 | 609.124 | 463.0971(25)/301.0343(67)/300.0261(100) | −1.65 | 611.1398 | 147.0435(100)/303.049(16) | 1.00 | [43] | 2 |
| 20 | 39.45 | Isorhamnetin coumaroyl-glucoside | C31H28O14 | 623.1395 | 315.0508(100)/314.0446(64)/258.0551(22) | −1.73 | 625.1547 | 147.0427(100) | −0.65 | [43] | 2 |
| 21 | 41.23 | Kaempferol | C15H10O6 | 285.0399 | 285.0399 (100) | −1.98 | 287.0552 | 287.0553(100) | 0.45 | [6,43] | 1 |
| A1 | 15.05 | Delphinidin 3,5-di-O-glucoside | C27H31O17 | - | - | − | 627.1564 | 465.1039 | 0.6 | [6,43] | 1 |
| A2 | 17.37 | Petunidin 3,5-di-O-glucoside | C28H33O17 | - | - | − | 641.1717 | 465.1044 | −0.9 | [6,43] | 1 |
| A3 | 17.77 | Delphinidin 3-O-glucoside | C21H21O12 | - | - | − | 465.1015 | 303.0092 | −3.2 | [6,43] | 1 |
| A4 | 18.22 | Petunidin 3-O-glucoside | C22H23O12 | - | - | − | 479.1108 | 317.2589 | −1.9 | [6,43] | 1 |
2.2. Quantitative Determination of Phenolic Compounds in C. sativus Extracts and Influence of Extraction Technique on Selected Phenolic Compounds’ Content
2.2.1. Ultrasound-Assisted Extraction (UAE) with Sonotrode
2.2.2. Subcritical Water Extraction (SWE)
2.2.3. Deep Eutectic Solvents Extraction (DESE)
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction Techniques
3.3.1. Ultrasound-Assisted Extraction (UAE) with Sonotrode
3.3.2. Subcritical Water Extraction (SWE)
3.3.3. Extraction with Deep Eutectic Solvents (DESs)
3.4. High-Resolution HPLC-ESI-QToF-MS/MS and HPLC-DAD Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahnoushi, N.; Abolhassani, L.; Kavakebi, V.; Reed, M.; Saghaian, S. Chapter 21—Economic analysis of saffron production. In Saffron: Science, Technology and Health; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2020; pp. 337–356. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Clemente-Villalba, J.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.-J. Novel insight into the volatile profile and antioxidant properties of Crocus sativus L. flowers. Antioxidants 2022, 11, 1650. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pérez-Llamas, F.; Pastor, J.J.; Frutos, M.J. Underutilized Crocus Sativus L. flowers: A hidden source of sustainable high value-added ingredients. Plant Foods Hum. Nutr. 2023, 78, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Goupy, P.; Vian, M.A.; Chemat, F.; Caris-Veyrat, C. Identification and quantification of flavonols, anthocyanins and lutein diesters in tepals of Crocus sativus by ultra-performance liquid chromatography coupled to diode array and ion trap mass spectrometry detections. Ind. Crops Prod. 2013, 44, 496–510. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Rosa, A.; Montoro, P.; Fenu, M.A.; Pizza, C. Antioxidant activity, cytotoxic activity and metabolic profiling of juices obtained from saffron (Crocus sativus L.) floral by-products. Food Chem. 2016, 199, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vidal, Y.; Zamilpa, A.; Jiménez-Ferrer, E.; Jiménez-Aparicio, A.R.; Camacho-Díaz, B.H.; Trejo-Tapia, G.; Tapia-Maruri, D.; Monterrosas-Brisson, N.; Herrera-Ruiz, M. A mixture of kaempferol-3-O-sambubioside and kaempferol-3-O-sophoroside from Malvaviscus arboreus prevents ethanol-induced gastric inflammation, oxidative stress, and histologic changes. Plants 2022, 11, 2951. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, J. Anti-inflammatory effects of flavonoids and phenylethanoid glycosides from Hosta plantaginea flowers in LPS-stimulated RAW 264.7 macrophages through inhibition of the NF-κB signaling pathway. BMC Complement. Med. Ther. 2022, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Luo, J.; Hu, D.; Yang, S.; Zhang, A.; Qiu, Y.; Ma, X.; Wang, J.; Hou, J.; Bai, J. Total flavonoids of Crocus sativus petals release tert-butyl hydroperoxide-induced oxidative stress in BRL-3A cells. Oxid. Med. Cell. Longev. 2021, 2021, 5453047. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Hernández, A.-B.; Vicente-López, J.-J.; Pérez-Llamas, F.; Candela-Castillo, M.-E.; García-Conesa, M.-T.; Frutos, M.-J.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Antioxidant activity, total phenolic and flavonoid contents in floral saffron bio-residues. Processes 2023, 11, 1400. [Google Scholar] [CrossRef]
- Wali, A.F.; Abou Alchamat, H.A.; Hariri, H.K.; Hariri, B.K.; Menezes, G.A.; Zehra, U.; Rehman, M.U.; Parvaiz, A. Antioxidant, antimicrobial, antidiabetic and cytotoxic activity of Crocus sativus L. petals. Appl. Sci. 2020, 10, 1519. [Google Scholar] [CrossRef]
- Khazaei, K.M.; Jafari, S.M.; Ghorbani, M.; Hemmati Kakhki, A.; Sarfarazi, M. Optimization of anthocyanin extraction from saffron petals with response surface methodology. Food Anal. Methods 2016, 9, 1993–2001. [Google Scholar] [CrossRef]
- Righi, V.; Parenti, F.; Tugnoli, V.; Schenetti, L.; Mucci, A. Crocus sativus petals: Waste or valuable resource? The answer of high-resolution and high-resolution magic angle spinning nuclear magnetic resonance. J. Agric. Food Chem. 2015, 63, 8439–8444. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Santana-Méridas, O.; Polissiou, M.; Vioque, J.; Astraka, K.; Alaiz, M.; Herraiz-Peñalver, D.; Tarantilis, P.A.; Girón-Calle, J. Polyphenol composition and in vitro antiproliferative effect of corm, tepal and leaf from Crocus sativus L. on human colon adenocarcinoma cells (Caco-2). J. Funct. Foods 2016, 24, 18–25. [Google Scholar] [CrossRef]
- Orabona, C.; Orecchini, E.; Volpi, C.; Bacaloni, F.; Panfili, E.; Pagano, C.; Perioli, L.; Belladonna, M.L. Crocus sativus L. petal extract inhibits inflammation and osteoclastogenesis in RAW 264.7 cell model. Pharmaceutics 2022, 14, 1290. [Google Scholar] [CrossRef]
- Jadouali, S.M.; Atifi, H.; Mamouni, R.; Majourhat, K.; Bouzoubaâ, Z.; Laknifli, A.; Faouzi, A. Chemical characterization and antioxidant compounds of flower parts of Moroccan Crocus sativus L. J. Saudi Soc. Agric. Sci. 2019, 18, 476–480. [Google Scholar] [CrossRef]
- Álvarez, A.; Terreros, S.; Cocero, M.J.; Mato, R.B. Microwave pretreatment for the extraction of anthocyanins from saffron flowers: Assessment of product quality. Antioxidants 2021, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Bernad, D.; Baixinho, J.P.; Fernández, N.; Frutos, M.J. Evaluation of microwave-assisted extraction as a potential green technology for the isolation of bioactive compounds from saffron (Crocus sativus L.) floral by-products. Foods 2022, 11, 2335. [Google Scholar] [CrossRef]
- Gil, K.A.; Tuberoso, C.I.G. Crucial challenges in the development of green extraction technologies to obtain antioxidant bioactive compounds from agro-industrial by-products. Chem. Biochem. Eng. Q. 2021, 35, 105–138. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Mahmoodi Sourestani, M.; Kiss, T.; Horváth, A.; Tóth, B.; Ayanmanesh, M.; Khamushi, A.; Csupor, D. Comprehensive chemotaxonomic analysis of saffron crocus tepal and stamen samples, as raw materials with potential antidepressant activity. J. Pharm. Biomed. Anal. 2020, 184, 113183. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Apreutesei, O.T.; Trifan, A.; Puitel, A.C.; Suteu, D. Valorization of bioactive compounds from residual saffron biomass (Crocus sativus L.) to obtain high value added dermato-cosmetic products. BioResources 2022, 17, 4730–4744. [Google Scholar] [CrossRef]
- Stelluti, S.; Caser, M.; Demasi, S.; Scariot, V. Sustainable processing of floral bio-residues of saffron (Crocus sativus L.) for valuable biorefinery products. Plants 2021, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Gahruie, H.; Parastouei, K.; Mokhtarian, M.; Rostami, H.; Niakousari, M.; Mohsenpour, Z. Application of innovative processing methods for the extraction of bioactive compounds from saffron (Crocus sativus) petals. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100264. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Extraction kinetic modelling of total polyphenols and total anthocyanins from saffron floral bio-residues: Comparison of extraction methods. Food Chem. 2018, 258, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, A.; Vassilev, K.; Nenov, N.; Passon, M.; Shikov, V.; Schieber, A.; Mihalev, K. Combining enzymatic and subcritical water treatments for green extraction of polyphenolic co-pigments from saffron tepals. Waste Biomass Valorization 2023, 15, 207–217. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraie, Z.; Niazmand, R.; Najafi, M.N. Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box-Behnken design and principal component analysis. Innov. Food Sci. Emerg. Technol. 2016, 36, 234–244. [Google Scholar] [CrossRef]
- Pappas, V.M.; Athanasiadis, V.; Palaiogiannis, D.; Poulianiti, K.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Pressurized liquid extraction of polyphenols and anthocyanins from saffron processing waste with aqueous organic acid solutions: Comparison with stirred-tank and ultrasound-assisted techniques. Sustainability 2021, 13, 12578. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- SShikov, A.N.; Obluchinskaya, E.D.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Pozharitskaya, O.N. The impact of natural deep eutectic solvents and extraction method on the co-extraction of trace metals from Fucus vesiculosus. Mar. Drugs 2022, 20, 324. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Pitterou, I.; Tzani, A.; Detsi, A.; Frutos, M.J. Novel chitosan/alginate hydrogels as carriers of ohenolic-enriched extracts from saffron floral by-products using natural deep eutectic solvents as green extraction media. Food Sci. 2023, 6, 100469. [Google Scholar] [CrossRef] [PubMed]
- Sitthisak, C.; Nisoa, M.; Chunglok, W.; Prasopthum, A.; Phaisan, S.; Putalun, W.; Kanchanapoom, T.; Juengwatanatrakul, T.; Yusakul, G. Efficient extraction of quassinoids and alkaloids from Eurycoma longifolia Jack roots using natural deep eutectic solvents and microwave-assisted extraction. Microchem. J. 2024, 196, 109676. [Google Scholar] [CrossRef]
- Petrochenko, A.A.; Orlova, A.; Frolova, N.; Serebryakov, E.B.; Soboleva, A.; Flisyuk, E.V.; Frolov, A.; Shikov, A.N. Natural deep eutectic solvents for the extraction of triterpene saponins from Aralia elata var. mandshurica (Rupr. & Maxim.). J. Wen. Molecules 2023, 28, 3614. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zeng, J.; Tang, H.; Cheng, Y.; Tan, J.; Li, T.; Li, X.; He, J.; Zhang, Y. Effect of deep eutectic solvent extraction on Auricularia auricula polysaccharide solubilization and antioxidant potential. Sustain. Chem. Pharm. 2023, 34, 101166. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of extraction of phlorotannins from the arctic Fucus vesiculosus using natural deep eutectic solvents and their HPLC profiling with tandem high-resolution mass spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef]
- Jokić, S.; Aladić, K.; Šubarić, D. Subcritical water extraction laboratory plant design and application. Annu. Croat. Acad. Eng. 2018, 21, 247–258. [Google Scholar]
- Kovač, M.J.; Jokić, S.; Jerković, I.; Molnar, M. Optimization of deep eutectic solvent extraction of phenolic acids and tannins from Alchemilla vulgaris L. Plants 2022, 11, 474. [Google Scholar] [CrossRef]
- Gil, K.A.; Jokić, S.; Cikoš, A.-M.; Banožić, M.; Jakovljević Kovač, M.; Fais, A.; Tuberoso, C.I.G. Comparison of different green extraction techniques used for the extraction of targeted flavonoids from edible feijoa (Acca sellowiana (O.Berg) Burret) flowers. Plants 2023, 12, 1461. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS Data in metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Nothias, L.F.; Ludwig, M.; Fleischauer, M.; Gentry, E.C.; Witting, M.; Dorrestein, P.C.; Dührkop, K.; Böcker, S. High-confidence structural annotation of metabolites absent from spectral libraries. Nat. Biotechnol. 2022, 40, 411–421. [Google Scholar] [CrossRef]
- KNApSAcK Core System. Available online: http://www.knapsackfamily.com/knapsack_core/top.php (accessed on 14 September 2023).
- Chen, N.; Xiang, J.; Liu, Y.; Li, H.; Yang, B. Preparation and characterization of antioxidant flavonoid-enriched extract from saffron by-product: A combination of ultrasound-assisted extraction and macroporous resin purification. Chem. Papers 2021, 75, 2749–2763. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Sánchez, A.M.; Martínez-Tomé, M.; Winterhalter, P.; Alonso, G.L. Flavonoid determination in the quality control of floral bioresidues from Crocus sativus L. J. Agric. Food Chem. 2014, 62, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, V.; Kajdžanoska, M.; Gjamovski, V.; Stefova, M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Rodríguez-Saona, L.E.; Griffin, D.; Wrolstad, R.E. Electrospray and tandem mass spectroscopy as tools for anthocyanin characterization. J. Agric. Food Chem. 1999, 47, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Bas, D.; Boyaci, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Cusano, E.; Consonni, R.; Petrakis, E.A.; Astraka, K.; Cagliani, L.R.; Polissiou, M.G. Integrated analytical methodology to investigate bioactive compounds in Crocus sativus L. flowers. Phytochem. Anal. 2018, 29, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Díaz, J.; Ana, M.; Sánchez, A.M.; Maggi, L.; Martínez-Tomé, M.; García-Diz, L.; Murcia, M.A.; Alonso, G.L. Increasing the applications of Crocus sativus flowers as natural antioxidants. J. Food Sci. 2012, 77, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Nile, S.H.; Zhang, Y.; Qin, L.; El-Seedi, H.R.; Daglia, M.; Kai, G. Novel insight into utilization of flavonoid glycosides and biological properties of saffron (Crocus sativus L.) flower byproducts. J. Agric. Food Chem. 2020, 68, 10685–10696. [Google Scholar] [CrossRef] [PubMed]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Thermal degradation of acylated and nonacylated anthocyanins. J. Food Sci. 2006, 71, 504–512. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D.A. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Villa, C.; Caviglia, D.; Robustelli della Cuna, F.S.; Zuccari, G.; Russo, E. NaDES application in cosmetic and pharmaceutical fields: An overview. Gels 2024, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Morgana, N.M.; Magdalena, E.; Fernandez, M.d.L.A.; Fernanda, S.M. NADES for food industry innovation: Novel bioadditives based on olive oil byproducts. Food Bioprod. Process. 2022, 134, 193–201. [Google Scholar] [CrossRef]
- De Luca, M.; Tuberoso, C.I.G.; Pons, R.; García, M.T.; Morán, M.d.C.; Ferino, G.; Vassallo, A.; Martelli, G.; Caddeo, C. Phenolic fingerprint, bioactivity and nanoformulation of Prunus spinosa L. fruit extract for skin delivery. Pharmaceutics 2023, 15, 1063:1–1063:18. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
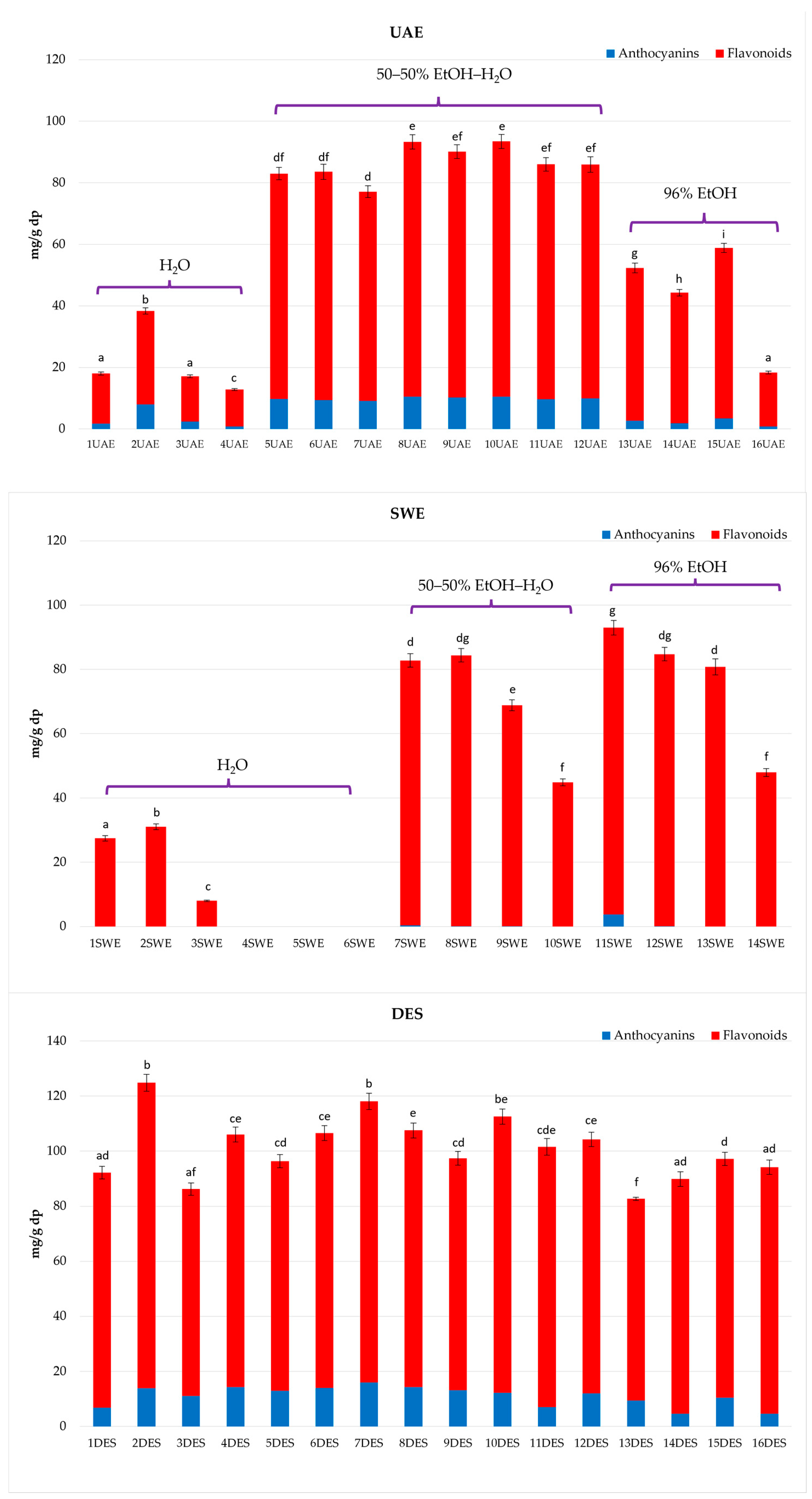
| Sample Code * | Extraction Parameters | ||
|---|---|---|---|
| Amplitude | Impulse | Solvent | |
| 1UAE | 100 | 60 | 100% H2O |
| 2UAE | 60 | 20 | |
| 3UAE | 60 | 100 | |
| 4UAE | 20 | 60 | |
| 5UAE | 100 | 100 | EtOH:H2O (50:50, v/v) |
| 6UAE | 100 | 20 | |
| 7UAE | 60 | 60 | |
| 8UAE | 60 | 60 | |
| 9UAE | 60 | 60 | |
| 10UAE | 60 | 60 | |
| 11UAE | 20 | 100 | |
| 12UAE | 20 | 20 | |
| 13UAE | 100 | 60 | 96% EtOH |
| 14UAE | 60 | 20 | |
| 15UAE | 60 | 100 | |
| 16UAE | 20 | 60 | |
| Temperature (°C) | Solvent | ||
| 1SWE | 125 | 100% H2O | |
| 2SWE | 150 | ||
| 3SWE | 175 | ||
| 4SWE | 200 | ||
| 5SWE | 225 | ||
| 6SWE | 250 | ||
| 7SWE | 125 | EtOH:H2O (50:50, v/v) | |
| 8SWE | 150 | ||
| 9SWE | 175 | ||
| 10SWE | 200 | ||
| 11SWE | 125 | 96% EtOH | |
| 12SWE | 150 | ||
| 13SWE | 175 | ||
| 14SWE | 200 | ||
| Extraction solvent | |||
| 1DES | Choline chloride:urea 1:2–H2O content (80–20, v,v) | ||
| 2DES | Choline chloride:N-methyl urea 1:3–H2O (80–20, v,v) | ||
| 3DES | Choline chloride:thiourea 1:2–H2O (80–20, v,v) | ||
| 4DES | Choline chloride:xylitol 1:1–H2O (80–20, v,v) | ||
| 5DES | Choline chloride:sorbitol 1:1–H2O (80–20, v,v) | ||
| 6DES | Choline chloride:acetamide 1:2–H2O (80–20, v,v) | ||
| 7DES | Choline chloride:butane-1,4-diol 1:2–H2O (80–20, v,v) | ||
| 8DES | Choline chloride:ethane-1,2-diol 1:2–H2O (80–20, v,v) | ||
| 9DES | Choline chloride:glycerol 1:2–H2O (80–20, v,v) | ||
| 10DES | Choline chloride:oxalic acid 1:1–H2O (80–20, v,v) | ||
| 11DES | Choline chloride:1,3-dimethylurea 1:2–H2O (80–20, v,v) | ||
| 12DES | Choline chloride:maleic acid 1:1–H2O (80–20, v,v) | ||
| 13DES | Choline chloride:malic acid 1:1–H2O (80–20, v,v) | ||
| 14DES | Choline chloride:malonic acid 1:1–H2O (80–20, v,v) | ||
| 15DES | Choline chloride:lactic acid 1:2–H2O (80–20, v,v) | ||
| 16DES | Choline chloride:levulinic 1:2–H2O (80–20, v,v) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masala, V.; Jokić, S.; Aladić, K.; Molnar, M.; Tuberoso, C.I.G. Exploring Phenolic Compounds Extraction from Saffron (C. sativus) Floral By-Products Using Ultrasound-Assisted Extraction, Deep Eutectic Solvent Extraction, and Subcritical Water Extraction. Molecules 2024, 29, 2600. https://doi.org/10.3390/molecules29112600
Masala V, Jokić S, Aladić K, Molnar M, Tuberoso CIG. Exploring Phenolic Compounds Extraction from Saffron (C. sativus) Floral By-Products Using Ultrasound-Assisted Extraction, Deep Eutectic Solvent Extraction, and Subcritical Water Extraction. Molecules. 2024; 29(11):2600. https://doi.org/10.3390/molecules29112600
Chicago/Turabian StyleMasala, Valentina, Stela Jokić, Krunoslav Aladić, Maja Molnar, and Carlo Ignazio Giovanni Tuberoso. 2024. "Exploring Phenolic Compounds Extraction from Saffron (C. sativus) Floral By-Products Using Ultrasound-Assisted Extraction, Deep Eutectic Solvent Extraction, and Subcritical Water Extraction" Molecules 29, no. 11: 2600. https://doi.org/10.3390/molecules29112600
APA StyleMasala, V., Jokić, S., Aladić, K., Molnar, M., & Tuberoso, C. I. G. (2024). Exploring Phenolic Compounds Extraction from Saffron (C. sativus) Floral By-Products Using Ultrasound-Assisted Extraction, Deep Eutectic Solvent Extraction, and Subcritical Water Extraction. Molecules, 29(11), 2600. https://doi.org/10.3390/molecules29112600











