Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine
Abstract
1. Introduction
2. Drug Delivery Methods
2.1. Traditional Drug Delivery System (TDDS)
2.2. Advanced Drug Delivery System (ADDS)
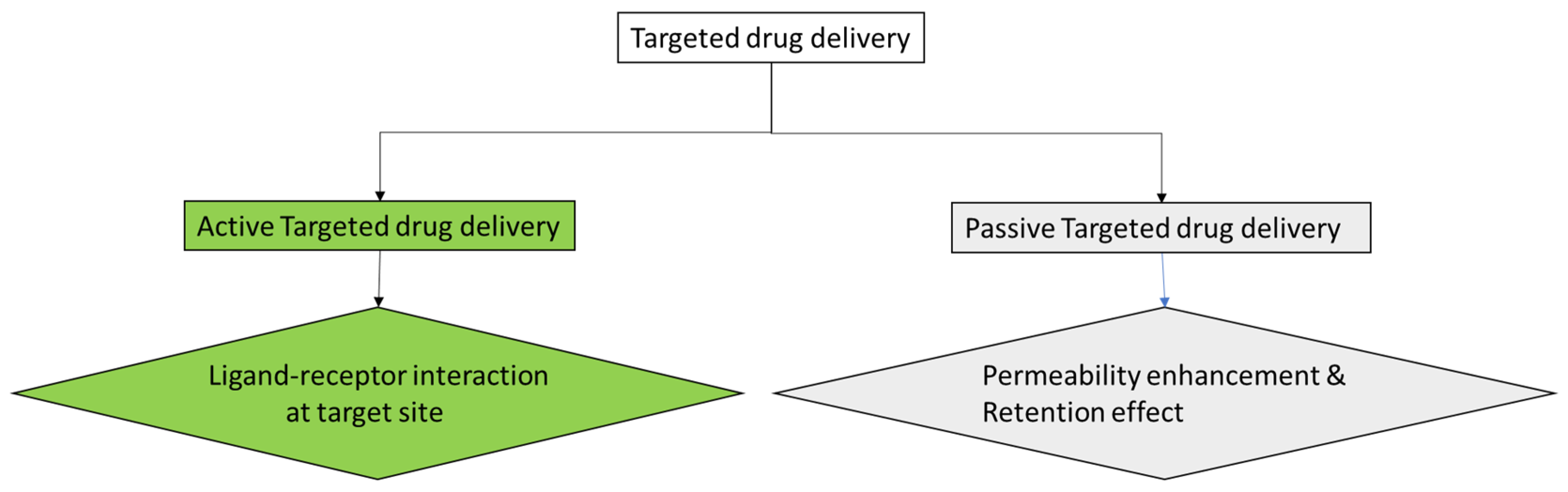
2.2.1. Targeted Drug Delivery
2.2.2. Controlled Drug Release
2.2.3. Improvement in Drug Stability
2.2.4. Improvement of Pharmacokinetic Properties of Drugs
3. Nanoparticles Used in Drug Delivery
3.1. Liposomes
| Drug | Trade Name | Administration Route | Approved Indication | Reference(s) |
|---|---|---|---|---|
| Liposomes | ||||
| Amphotericin B | AmBisome (Astellas) | Intravenous | Fungal infections | [31] |
| Amphotericin B | Fungizone | Intravenous | Fungal infections | [31] |
| Daunorubicin | DaunoXome (Galen) | Intravenous | Leukemia | [32] |
| Doxorubicin | Doxil (Baxter Hlthcare Corp) | Intravenous | Combination therapy with cyclophosphamide in metastatic breast cancer | [33] |
| Verteporfin | Visudyne (Valeant Luxembourg) | Intravenous | Age-related molecular degeneration, pathological myopia, ocular histoplasmosis | [34] |
| Cytarabine | DepoCyt© (Pacira Pharms Inc.) | Spinal | Neoplastic meningitis and lymphomatous meningitis | [35] |
| Morphine sulphate | DepoDur | Epidural | Pain management | [36] |
| Moderna vaccine | Moderna | Intravenous | COVID-19 | [37] |
| Pfizer-BioNTech | Pfizer-BioNTech | Intravenous | COVID-19 | [37] |
| Mifamurtide | Mepact (Takeda) | intravenous | Osteosarcoma, a form of bone cancer | [38] |
| Recombinant varicella-zoster virus glycoprotein E | Shingrix | Intramuscular | Against shingles and post-herpetic neuralgia | [39] |
| Micelles | ||||
| Docetaxel | Taxotere (Sanofi Aventis) | Intravenous | Antineoplastic | [40] |
| Estradiol | Estrasorb™ (Novavax) | Dermal | Menopausal therapy | [41] |
| Dendritic macromolecules | ||||
| VivaGel | VivaGel R© BV (Starpharma) | Dermal | Treatment and symptomatic relief of bacterial vaginosis | [42] |
| Quantum dots | ||||
| Aprepitant | Emend (Merck) | Oral | Vomiting agent | [43,44] |
| Megestrol acetate | MegaceES (Endo Pharms Inc.) | Oral | Anorexia | [45] |
| Dexamethylphenidate HCl | Focalin XR/(Novartis) | Oral | Mental stimulant | [46] |
| Tizanidine HCl | Zanaflex (Covis) | Oral | Muscle relaxant | [47] |
| Nabilone | Cesamet (Bausch) | Oral | Antiemetic | [48] |
| Naproxen sodium | Naprelan (Almatica) | Oral | Anti-inflammation | [49] |
| Griseofulvin | Gris-PEG (Novartis) | Oral | Fungal infection | [48] |
| Active Ingredient | Drug Candidate ID | Phase of Development | Indication | Reference(s) |
|---|---|---|---|---|
| Liposome | ||||
| Cisplatin | SPI-77 | III | Different forms of Cancer | [50] |
| Cisplatin | Lipoplatin | III | Different forms of Cancer | [50] |
| Amphotericin | Ambisome | III | Fungal Infection | [50] |
| Micelles | ||||
| Cisplatin | Nanoplatin | III | Different forms of Cancer | [50] |
| Paclitaxel | NK-105 | III | Breast cancer | [51] |
| Doxorubicin | SP1049-C | III | Cancer | [52] |
3.2. Micelles
3.3. Dendritic Macromolecules
3.4. Quantum Dots
3.5. Carbon Nanotubes
3.6. Metal-Based Nanoparticles
4. Applications of Nanotechnology in Drug Delivery
4.1. Treatment of Resistant Infectious Diseases
4.1.1. Addressing Antimalarial Drug Resistance
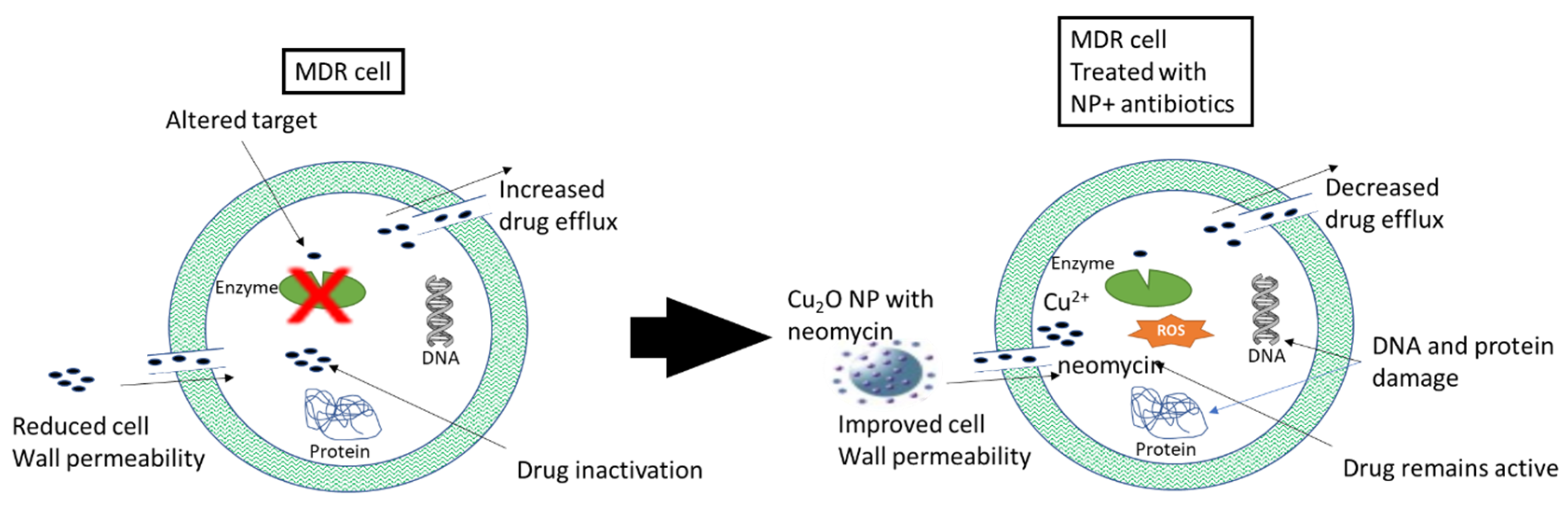
4.1.2. Treatment of Bacterial Multi-Drug Resistance (MDR)
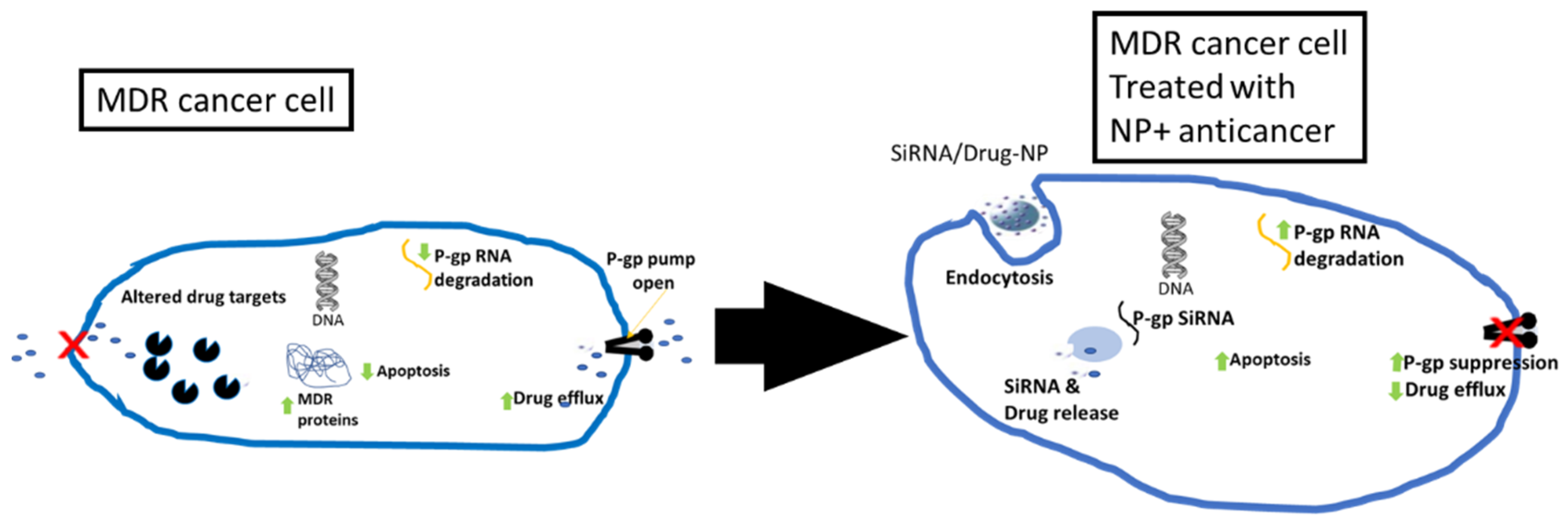
4.2. Cancer Therapy
4.3. Treatment of Cardiovascular Diseases
4.4. Administration of Nutraceuticals
4.5. Gene Therapy
5. Challenges of Nanotechnology
5.1. Toxicity and Biocompatibility
5.2. Cost of Production
5.3. Regulatory Challenges
5.4. Composition of the Drug and Nanoparticles
5.5. Poor Biodistribution
6. Nanomedicine Research Boom and Clinical Bust: The Uphill Task
7. Prospects of Nanotechnology in Medicine
7.1. Tissue Regeneration/Tissue Engineering Construct
7.2. Potentiation of Immunotherapy
7.3. Medical Implants
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Satalkar, P.; Elger, B.S.; Shaw, D.M. Defining Nano, Nanotechnology and Nanomedicine: Why Should It Matter? Sci. Eng. Eth. 2016, 22, 1255–1276. [Google Scholar] [CrossRef]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, J.J. Nanotechnology: An Introduction, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780323393119. [Google Scholar]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-Based Drug Delivery Systems: Conventional Drug Delivery Routes, Recent Developments and Future Prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Gupta, J.; Fatima, M.T.; Islam, Z.; Khan, R.H.; Uversky, V.N.; Salahuddin, P. Nanoparticle Formulations in the Diagnosis and Therapy of Alzheimer’s Disease. Int. J. Biol. Macromol. 2019, 130, 515–526. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Rodriques, P.; Prajapati, B.; Bhattacharya, S.; Rodriques, P.; Prajapati, B. Introductory Chapter: Advanced Drug Delivery Systems. In Advanced Drug Delivery Systems; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for Controlled Delivery of Growth Factors and Cells for Bone Regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Di Stefano, A. Nanotechnology in Targeted Drug Delivery. Int. J. Mol. Sci. 2023, 24, 8194. [Google Scholar] [CrossRef]
- Salahpour Anarjan, F. Active Targeting Drug Delivery Nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active Targeting Schemes for Nanoparticle Systems in Cancer Therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An Overview of Active and Passive Targeting Strategies to Improve the Nanocarriers Efficiency to Tumour Sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siegel, R.A.; Rathbone, M.J. Fundamentals and Applications of Controlled Release Drug Delivery. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–594. [Google Scholar] [CrossRef]
- de Jesus, R.A.; Oliveira, Í.M.; Nascimento, V.R.S.; Ferreira, L.F.R.; Figueiredo, R.T. Porous Nanostructured Metal Oxides as Potential Scaffolds for Drug Delivery. In Novel Platforms for Drug Delivery Applications; Woodhead Publishing: Cambridge, UK, 2023; pp. 437–457. [Google Scholar] [CrossRef]
- Little, T.A. Toxicological Assessment of Degradation Products: Is It Relevant as a Complementary Approach during Stability Testing of Pharmaceuticals? J. Dev. Drugs 2014, 12, 18–24. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. SPJ 2018, 26, 64. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Lu, Y.; Hovgaard, L.; Guan, P.; Tan, Y.; Lian, R.; Qi, J.; Wu, W. Hypoglycemic Activity and Oral Bioavailability of Insulin-Loaded Liposomes Containing Bile Salts in Rats: The Effect of Cholate Type, Particle Size and Administered Dose. Eur. J. Pharm. Biopharm. 2012, 81, 265–272. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Li, T.; Deng, Y. Modulation of the Physicochemical State of Interior Agents to Prepare Controlled Release Liposomes. Coll. Surf. B Biointerfaces 2009, 69, 232–238. [Google Scholar] [CrossRef]
- Santos Giuberti, C.D.; De Oliveira Reis, E.C.; Ribeiro Rocha, T.G.; Leite, E.A.; Lacerda, R.G.; Ramaldes, G.A.; De Oliveira, M.C. Study of the Pilot Production Process of Long-Circulating and PH-Sensitive Liposomes Containing Cisplatin. J. Liposome Res. 2011, 21, 60–69. [Google Scholar] [CrossRef]
- Afergan, E.; Epstein, H.; Dahan, R.; Koroukhov, N.; Rohekar, K.; Danenberg, H.D.; Golomb, G. Delivery of Serotonin to the Brain by Monocytes Following Phagocytosis of Liposomes. J. Control. Release 2008, 132, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Ahmad, Z.; Sharma, S.; Khuller, G.K. Nano-Encapsulation of Azole Antifungals: Potential Applications to Improve Oral Drug Delivery. Int. J. Pharm. 2005, 301, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Paavola, A.; Kilpeläinen, I.; Yliruusi, J.; Rosenberg, P. Controlled Release Injectable Liposomal Gel of Ibuprofen for Epidural Analgesia. Int. J. Pharm. 2000, 199, 85–93. [Google Scholar] [CrossRef]
- Van Den Hoven, J.M.; Van Tomme, S.R.; Metselaar, J.M.; Nuijen, B.; Beijnen, J.H.; Storm, G. Liposomal Drug Formulations in the Treatment of Rheumatoid Arthritis. Mol. Pharm. 2011, 8, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Moles, E.; Urbán, P.; Prohens, R.; Busquets, M.A.; Sevrin, C.; Grandfils, C.; Fernàndez-Busquets, X. Application of Heparin as a Dual Agent with Antimalarial and Liposome Targeting Activities toward Plasmodium-Infected Red Blood Cells. Nanomedicine 2014, 10, 1719–1728. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef]
- Lister, J. Amphotericin B Lipid Complex (Abelcet) in the Treatment of Invasive Mycoses: The North American Experience. Eur. J. Haematol. Suppl. 1996, 57, 18–23. [Google Scholar] [CrossRef]
- Blair, H.A. Daunorubicin/Cytarabine Liposome: A Review in Acute Myeloid Leukaemia. Drugs 2018, 78, 1903. [Google Scholar] [CrossRef]
- Smith, J.A.; Mathew, L.; Burney, M.; Nyshadham, P.; Coleman, R.L. Equivalency Challenge: Evaluation of Lipodox® as the Generic Equivalent for Doxil® in a Human Ovarian Cancer Orthotropic Mouse Model. Gynecol. Oncol. 2016, 141, 357–363. [Google Scholar] [CrossRef]
- Keam, S.J.; Scott, L.J.; Curran, M.P. Verteporfin: A Review of Its Use in the Management of Subfoveal Choroidal Neovascularisation. Drugs 2003, 63, 2521–2554. [Google Scholar] [CrossRef]
- Jaeckle, K.A.; Phuphanich, S.; Van den Bent, M.J.; Aiken, R.; Batchelor, T.; Campbell, T.; Fulton, D.; Gilbert, M.; Heros, D.; Rogers, L.; et al. Intrathecal Treatment of Neoplastic Meningitis Due to Breast Cancer with a Slow-Release Formulation of Cytarabine. Br. J. Cancer 2001, 84, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hartrick, C.T.; Hartrick, K.A. Extended-Release Epidural Morphine (DepoDur): Review and Safety Analysis. Expert Rev. Neurother. 2008, 8, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Liposomes and MRNA: Two Technologies Together Create a COVID-19 Vaccine. Med. Drug Discov. 2021, 12, 100104. [Google Scholar] [CrossRef]
- Kager, L.; Pötschger, U.; Bielack, S. Review of Mifamurtide in the Treatment of Patients with Osteosarcoma. Ther. Clin. Risk Manag. 2010, 6, 279. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Song, S.; Choi, E.; Lee, P.B.; Nahm, F.S. Recombinant Zoster Vaccine (Shingrix®): A New Option for the Prevention of Herpes Zoster and Postherpetic Neuralgia. Korean J. Pain 2020, 33, 201. [Google Scholar] [CrossRef] [PubMed]
- Sanofi Aventis TAXOTERE® (Docetaxel) Injection, for Intravenous Use. Available online: https://products.sanofi.us/taxotere/taxotere.html (accessed on 7 January 2024).
- Novavax. Estradiol-Topical--Novavax: Estrasorb. Drugs R D. 2003, 4, 49–51. [Google Scholar] [CrossRef]
- Pellett Madan, R.; Dezzutti, C.S.; Rabe, L.; Hillier, S.L.; Marrazzo, J.; Mcgowan, I.; Richardson, B.A.; Herold, B.C. Soluble Immune Mediators and Vaginal Bacteria Impact Innate Genital Mucosal Antimicrobial Activity in Young Women. Am. J. Reprod. Immunol. 2015, 74, 323. [Google Scholar] [CrossRef]
- Ju, J.; Rh, M. Nanocrystal Technology, Drug Delivery and Clinical Applications. Int. J. Nanomed. 2008, 3, 295. [Google Scholar] [CrossRef]
- Caster, J.M.; Patel, A.N.; Zhang, T.; Wang, A. Investigational Nanomedicines in 2016: A Review of Nanotherapeutics Currently Undergoing Clinical Trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1416. [Google Scholar] [CrossRef]
- Deschamps, B.; Musaji, N.; Gillespie, J.A. Food Effect on the Bioavailability of Two Distinct Formulations of Megestrol Acetate Oral Suspension. Int. J. Nanomed. 2009, 4, 185–192. [Google Scholar] [CrossRef][Green Version]
- Chavez, B.; Sopko, M.A.; Ehret, M.J.; Paulino, R.E.; Goldberg, K.R.; Angstadt, K.; Bogart, G.T. An Update on Central Nervous System Stimulant Formulations in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Ann. Pharmacother. 2009, 43, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Kaddar, N.; Vigneault, P.; Pilote, S.; Patoine, D.; Simard, C.; Drolet, B. Tizanidine (Zanaflex): A Muscle Relaxant That May Prolong the QT Interval by Blocking I Kr. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.H.; Alsowinea, A.F. Approved and Marketed Nanoparticles for Disease Targeting and Applications in COVID-19. Nanotechnol. Rev. 2021, 10, 1941–1977. [Google Scholar] [CrossRef]
- Ardestani, M.S.; Zaheri, Z.; Mohammadzadeh, P.; Bitarafan-Rajabi, A.; Ghoreishi, S.M. Novel Manganese Carbon Quantum Dots as a Nano-Probe: Facile Synthesis, Characterization and Their Application in Naproxen Delivery (Mn/CQD/SiO2@naproxen). Bioorg. Chem. 2021, 115, 105211. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Q. Development of Liposomal Formulations: From Concept to Clinical Investigations. Asian J. Pharm. Sci. 2013, 8, 81–87. [Google Scholar] [CrossRef]
- Fasol, U.; Frost, A.; Büchert, M.; Arends, J.; Fiedler, U.; Scharr, D.; Scheuenpflug, J.; Mross, K. Vascular and Pharmacokinetic Effects of EndoTAG-1 in Patients with Advanced Cancer and Liver Metastasis. Ann. Oncol. 2012, 23, 1030–1036. [Google Scholar] [CrossRef]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Kim, J.O. Nanomedicine-Based Commercial Formulations: Current Developments and Future Prospects. J. Pharm. Investig. 2022, 53, 19–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric Micelles: Nanocarriers for Cancer-Targeted Drug Delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric Micelles as Drug Delivery Vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to Improve Micelle Stability for Drug Delivery. Nano Res. 2018, 11, 4985. [Google Scholar] [CrossRef] [PubMed]
- Buhleier, E.; Wehner, E.; Vögtle, F. Cascade-Chain like and Nonskid-Chain-like Synthesis of Molecular Cavity Topologies. Synthesis 1978, 2, 155–158. [Google Scholar] [CrossRef]
- Noriega-Luna, B.; Godínez, L.A.; Rodríguez, F.J.; Rodríguez, A.; Zaldívar-Lelo De Larrea, G.; Sosa-Ferreyra, C.F.; Mercado-Curiel, R.F.; Manríquez, J.; Bustos, E. Applications of Dendrimers in Drug Delivery Agents, Diagnosis, Therapy, and Detection. J. Nanomater. 2014, 2014, 507273. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. BioMed Res. Int. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.B.; Pooja, D.; Kulhari, H. Dendrimers in Gene Delivery. In Pharmaceutical Applications of Dendrimers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 211–231. [Google Scholar] [CrossRef]
- Dufès, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in Gene Delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed]
- Farias, E.D.; Bouchet, L.M.; Brunetti, V.; Strumia, M.C. Dendrimers and Dendronized Materials as Nanocarriers. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 429–456. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface Modification of PAMAM Dendrimer Improves Its Biocompatibility. Nanomedicine 2012, 8, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Aurelia Chis, A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomed. 2017, 12, 5421. [Google Scholar] [CrossRef]
- Bruno, J.G. Advantages and Disadvantages of Using Quantum Dots in Lateral Flow and Other Biological Assay Formats. In Application of Quantum Dots in Biology and Medicine: Recent Advances; Springer Nature Singapore Pte Ltd.: Singapore, 2022; pp. 91–102. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Cai, X.; Jia, H.; Liu, Z.; Hou, B.; Luo, C.; Feng, Z.; Li, W.; Liu, J. Polyhydroxylated Fullerene Derivative C60(OH)24 Prevents Mitochondrial Dysfunction and Oxidative Damage in an MPP+-Induced Cellular Model of Parkinson’s Disease. J. Neurosci. Res. 2008, 86, 3622–3634. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Singh, A.; Sharma, P.K.; Fuloria, N.K. Smart Carbon Nanotubes for Drug Delivery System: A Comprehensive Study. J. Drug Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- Nayfeh, M. Toxicity and Safety Issues of Metal-Based Nanoparticles. In Fundamentals and Applications of Nano Silicon in Plasmonics and Fullerines: Current and Future Trends; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–602. ISBN 9780323480574. [Google Scholar]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic Nanoparticles for Antimicrobial Applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron Oxide Nanoparticles: Diagnostic, Therapeutic and Theranostic Applications. Adv. Drug Deliv. Rev. 2019, 138, 302. [Google Scholar] [CrossRef]
- Bajpai, A.; Shinde, S.; Basu, S. Nanobiomaterials for Drug Delivery and Theranostics. In Nanotechnology in Medicine and Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–56. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular Mechanisms of Drug Resistance in Plasmodium Falciparum Malaria. Annu. Rev. Microbiol. 2020, 74, 431. [Google Scholar] [CrossRef]
- WHO. World Malaria Report. 2021. Available online: https://www.who.int/publications/i/item/9789240040496 (accessed on 3 January 2022).
- Chaves, J.B.; Portugal Tavares de Moraes, B.; Regina Ferrarini, S.; Noé da Fonseca, F.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Potential of Nanoformulations in Malaria Treatment. Front. Pharmacol. 2022, 13, 999300. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Lymperaki, E.; Egwu, C.O.; Pouroutzidou, G.K.; Kazeli, K.; Reybier, K.; Bourgeade-Delmas, S.; Valentin, A.; Kontonasaki, E. Effect of Silica Based Nanoparticles against Plasmodium Falciparum and Leishmania Infantum Parasites. J. Xenobiotics 2021, 11, 155–162. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 1370. [Google Scholar] [CrossRef]
- Borah Slater, K.; Kim, D.; Chand, P.; Xu, Y.; Shaikh, H.; Undale, V. A Current Perspective on the Potential of Nanomedicine for Anti-Tuberculosis Therapy. Trop. Med. Infect. Dis. 2023, 8, 100. [Google Scholar] [CrossRef]
- Singh, R.; Smitha, M.S.; Singh, S.P. The Role of Nanotechnology in Combating Multi-Drug Resistant Bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Aabed, K.; Mohammed, A.E. Synergistic and Antagonistic Effects of Biogenic Silver Nanoparticles in Combination with Antibiotics Against Some Pathogenic Microbes. Front. Bioeng. Biotechnol. 2021, 9, 652362. [Google Scholar] [CrossRef]
- Adeniji, O.O.; Ojemaye, M.O.; Okoh, A.I. Antibacterial Activity of Metallic Nanoparticles against Multidrug-Resistant Pathogens Isolated from Environmental Samples: Nanoparticles/Antibiotic Combination Therapy and Cytotoxicity Study. ACS Appl. Bio Mater. 2022, 5, 4814–4826. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Y.; Chen, W.; Fan, J.; Lv, H.; Wu, Q. Integrated Nanotechnology of Synergism-Sterilization and Removing-Residues for Neomycin through Nano-Cu2O. Coll. Surfaces B Biointerfaces 2019, 183, 110371. [Google Scholar] [CrossRef] [PubMed]
- WHO Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 10 February 2023).
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, Y.; Wang, W.; Wang, G.; Zhang, S.; Cheng, H. Emerging Nano-Based Strategies Against Drug Resistance in Tumor Chemotherapy. Front. Bioeng. Biotechnol. 2021, 9, 798882. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- He, J.; Gong, C.; Qin, J.; Li, M.; Huang, S. Cancer Cell Membrane Decorated Silica Nanoparticle Loaded with MiR495 and Doxorubicin to Overcome Drug Resistance for Effective Lung Cancer Therapy. Nanoscale Res. Lett. 2019, 14, 339. [Google Scholar] [CrossRef]
- Babu, A.; Munshi, A.; Ramesh, R. Combinatorial Therapeutic Approaches with RNAi and Anticancer Drugs Using Nanodrug Delivery Systems. Drug Dev. Ind. Pharm. 2017, 43, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Wang, C.; Lu, Q.; Zhao, M.; Ban, F.Q.; Yu, D.H.; Guan, Y.Y.; Luan, X.; Liu, Y.R.; Chen, H.Z.; et al. Nanoparticle-Mediated Drug Delivery to Tumor Neovasculature to Combat P-Gp Expressing Multidrug Resistant Cancer. Biomaterials 2013, 34, 6163–6174. [Google Scholar] [CrossRef] [PubMed]
- Venishetty, V.K.; Chede, R.; Komuravelli, R.; Adepu, L.; Sistla, R.; Diwan, P.V. Design and Evaluation of Polymer Coated Carvedilol Loaded Solid Lipid Nanoparticles to Improve the Oral Bioavailability: A Novel Strategy to Avoid Intraduodenal Administration. Coll. Surf. B Biointerfaces 2012, 95, 1–9. [Google Scholar] [CrossRef]
- Kumar, V.V.; Chandrasekar, D.; Ramakrishna, S.; Kishan, V.; Rao, Y.M.; Diwan, P.V. Development and Evaluation of Nitrendipine Loaded Solid Lipid Nanoparticles: Influence of Wax and Glyceride Lipids on Plasma Pharmacokinetics. Int. J. Pharm. 2007, 335, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Manocha, S.; Dhiman, S.; Grewal, S.A.; Guarve, K. Nanotechnology: An Approach to Overcome Bioavailability Challenges of Nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent Developments in Nanoformulations of Lipophilic Functional Foods. Trends Food Sci. Technol. 2015, 46, 144–157. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of Nutraceuticals: Role of the Food Matrix, Processing Conditions, the Gastrointestinal Tract, and Nanodelivery Systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Herranz, F.; Almarza, E.; Rodríguez, I.; Salinas, B.; Rosell, Y.; Desco, M.; Bulte, J.W.; Ruiz-Cabello, J. The Application of Nanoparticles in Gene Therapy and Magnetic Resonance Imaging. Microsc. Res. Tech. 2011, 74, 577. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Z.; Karim, S. Nanoparticles-Based Drug Delivery and Gene Therapy for Breast Cancer: Recent Advancements and Future Challenges. Semin. Cancer Biol. 2021, 69, 226–237. [Google Scholar] [CrossRef]
- Piperno, A.; Sciortino, M.T.; Giusto, E.; Montesi, M.; Panseri, S.; Scala, A. Recent Advances and Challenges in Gene Delivery Mediated by Polyester-Based Nanoparticles. Int. J. Nanomed. 2021, 16, 5981. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, D.; Cheng, Y.; Yang, M.; Wu, L.P. Polyester Based Nanovehicles for SiRNA Delivery. Mater. Sci. Eng. C 2018, 92, 1006–1015. [Google Scholar] [CrossRef]
- Prazeres, P.H.D.M.; Ferreira, H.; Costa, P.A.C.; da Silva, W.; Alves, M.T.; Padilla, M.; Thatte, A.; Santos, A.K.; Lobo, A.O.; Sabino, A.; et al. Delivery of Plasmid DNA by Ionizable Lipid Nanoparticles to Induce CAR Expression in T Cells. Int. J. Nanomed. 2023, 18, 5891–5904. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282. [Google Scholar] [CrossRef]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J. Nanomater. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Pandey, G.; Jain, P. Assessing the Nanotechnology on the Grounds of Costs, Benefits, and Risks. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 63. [Google Scholar] [CrossRef]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory Landscape of Nanotechnology and Nanoplastics from a Global Perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef] [PubMed]
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU-Regulatory Overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef]
- Fay, F.; Hansen, L.; Hectors, S.J.C.G.; Sanchez-Gaytan, B.L.; Zhao, Y.; Tang, J.; Munitz, J.; Alaarg, A.; Braza, M.S.; Gianella, A.; et al. Investigating the Cellular Specificity in Tumors of a Surface-Converting Nanoparticle by Multimodal Imaging. Bioconjug. Chem. 2017, 28, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Dey, N.S.; Maji, R.; Bhowmik, P.; Das, P.J.; Paul, P. Current Status and Future Scope for Nanomaterials in Drug Delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Avraham Dilmanian, F.; Vadas, T.M.; Smilowitz, H.M. Gold Nanoparticle Hyperthermia Reduces Radiotherapy Dose. Nanomedicine Nanotechnol. Biol. Med. 2014, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.C.; Löwa, N.; Wiekhorst, F.; Taupitz, M.; Wagner, S.; Möller, K.; Baumann, G.; Stangl, V.; Trahms, L.; Ludwig, A. Magnetic Particle Spectroscopy Reveals Dynamic Changes in the Magnetic Behavior of Very Small Superparamagnetic Iron Oxide Nanoparticles During Cellular Uptake and Enables Determination of Cell-Labeling Efficacy. J. Biomed. Nanotechnol. 2016, 12, 337–346. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle Size-Dependent Organ Distribution of Gold Nanoparticles after Intravenous Administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Waite, C.L.; Roth, C.M. Nanoscale Drug Delivery Systems for Enhanced Drug Penetration into Solid Tumors: Current Progress and Opportunities. Crit. Rev. Biomed. Eng. 2012, 40, 21. [Google Scholar] [CrossRef] [PubMed]
- Drexler, K.E.; Peterson, C.; Pergamit, G. Unbounding the Future: The Nanotechnology Revolution, 1st ed.; William Morrow and Company, Inc.: New York, NY, USA, 1991; ISBN 0688125735. [Google Scholar]
- Arachchige, M.C.M.; Reshetnyak, Y.K.; Andreev, O.A. Advanced Targeted Nanomedicine. J. Biotechnol. 2015, 202, 88. [Google Scholar] [CrossRef]
- Ibrahim-Hashim, A.; Estrella, V. Acidosis and Cancer: From Mechanism to Neutralization. Cancer Metastasis Rev. 2019, 38, 149. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Rajivgandhi, G.N.; Quero, F.; Li, W.J. Anti-Quorum Sensing and Anti-Biofilm Activity of Nickel Oxide Nanoparticles against Pseudomonas aeruginosa. J. Environ. Chem. Eng. 2020, 8, 104533. [Google Scholar] [CrossRef]
- Ozdal, M.; Gurkok, S. Recent Advances in Nanoparticles as Antibacterial Agent. ADMET DMPK 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.N.; Mare, A.; Tanase, C.; Bud, E.; Rusu, A. Silver-Deposited Nanoparticles on the Titanium Nanotubes Surface as a Promising Antibacterial Material into Implants. Metals 2021, 11, 92. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Ahmad, I.; Husain, F.M.; Khan, R.A.; Hassan, I. Green Synthesis of Silver Nanoparticles Using Carum Copticum: Assessment of Its Quorum Sensing and Biofilm Inhibitory Potential against Gram Negative Bacterial Pathogens. Microb. Pathog. 2020, 144, 104172. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Chen, M.; Chen, M.; He, J. Cancer Cell Membrane Cloaking Nanoparticles for Targeted Co-Delivery of Doxorubicin and PD-L1 SiRNA. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1635–1641. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and Its Use in Imaging and Drug Delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Kanaoujiya, R.; Saroj, S.K.; Rajput, V.D.; Alimuddin; Srivastava, S.; Minkina, T.; Igwegbe, C.A.; Singh, M.; Kumar, A. Emerging Application of Nanotechnology for Mankind. Emergent Mater. 2023, 6, 439–452. [Google Scholar] [CrossRef]
- Whitney, G.A.; Jayaraman, K.; Dennis, J.E.; Mansour, J.M. Scaffold-Free Cartilage Subjected to Frictional Shear Stress Demonstrates Damage by Cracking and Surface Peeling. J. Tissue Eng. Regen. Med. 2017, 11, 412. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.S. Nanoparticles in Tissue Engineering: Applications, Challenges and Prospects. Int. J. Nanomed. 2018, 13, 5637. [Google Scholar] [CrossRef]
- Santos, A.R., Jr.; Nascimento, V.A.; Genari, S.C.; Lombello, C.B.; Nascimento, V.A.; Genari, S.C.; Lombello, C.B. Mechanisms of Cell Regeneration—From Differentiation to Maintenance of Cell Phenotype. Cells Biomater. Regen. Med. 2014, 37–69. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Yang, Y.; Liu, Y. A Dual Targeting Cyclodextrin/Gold Nanoparticle Conjugate as a Scaffold for Solubilization and Delivery of Paclitaxel. RSC Adv. 2015, 5, 8938–8941. [Google Scholar] [CrossRef]
- Qadri, H.; Shah, A.H.; Alkhanani, M.; Almilaibary, A.; Mir, M.A. Immunotherapies against Human Bacterial and Fungal Infectious Diseases: A Review. Front. Med. 2023, 10, 1135541. [Google Scholar] [CrossRef]
- Kang, S.M.; Compans, R.W. Host Responses from Innate to Adaptive Immunity after Vaccination: Molecular and Cellular Events. Mol. Cells 2009, 27, 5. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492. [Google Scholar] [CrossRef] [PubMed]
- Alfarouk, K.O.; Stock, C.M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to Cancer Chemotherapy: Failure in Drug Response from ADME to P-Gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef]
- Debele, T.A.; Yeh, C.F.; Su, W.P. Cancer Immunotherapy and Application of Nanoparticles in Cancers Immunotherapy as the Delivery of Immunotherapeutic Agents and as the Immunomodulators. Cancers 2020, 12, 3773. [Google Scholar] [CrossRef]
- Pokkalath, A.; Nadar, D.; Ravikumar, P.; Sawarkar, S.P. Nanomaterials for Orthopaedic Implants and Applications. In Handbook on Nanobiomaterials for Therapeutics and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 229–270. [Google Scholar] [CrossRef]
- Tomsia, A.P.; Launey, M.E.; Lee, J.S.; Mankani, M.H.; Wegst, U.G.K.; Saiz, E. Nanotechnology Approaches for Better Dental Implants. Int. J. Oral Maxillofac. Implants 2011, 26, 25. [Google Scholar]


| Type of Nanomaterial | Exploited Property |
|---|---|
| Gold nanoparticles | Surface conjugation and conduction |
| Silver, Gold and other metallic nanoparticles and metallic oxides | Antimicrobial ability |
| Quantum dots | Fluorescence ability |
| Carbon nanotubes | Electromagnetic ability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egwu, C.O.; Aloke, C.; Onwe, K.T.; Umoke, C.I.; Nwafor, J.; Eyo, R.A.; Chukwu, J.A.; Ufebe, G.O.; Ladokun, J.; Audu, D.T.; et al. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules 2024, 29, 2584. https://doi.org/10.3390/molecules29112584
Egwu CO, Aloke C, Onwe KT, Umoke CI, Nwafor J, Eyo RA, Chukwu JA, Ufebe GO, Ladokun J, Audu DT, et al. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules. 2024; 29(11):2584. https://doi.org/10.3390/molecules29112584
Chicago/Turabian StyleEgwu, Chinedu O., Chinyere Aloke, Kenneth T. Onwe, Chukwunalu Igbudu Umoke, Joseph Nwafor, Robert A. Eyo, Jennifer Adaeze Chukwu, Godswill O. Ufebe, Jennifer Ladokun, David Tersoo Audu, and et al. 2024. "Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine" Molecules 29, no. 11: 2584. https://doi.org/10.3390/molecules29112584
APA StyleEgwu, C. O., Aloke, C., Onwe, K. T., Umoke, C. I., Nwafor, J., Eyo, R. A., Chukwu, J. A., Ufebe, G. O., Ladokun, J., Audu, D. T., Agwu, A. O., Obasi, D. C., & Okoro, C. O. (2024). Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules, 29(11), 2584. https://doi.org/10.3390/molecules29112584