SiOx/C Composite Anode for Lithium-Ion Battery with Improved Performance Using Graphene Quantum Dots and Carbon Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of GQD/SiOx/C Composite
2.2. Electrochemical Analysis of GQD/SiOx/C Composite Anode Material
3. Materials and Methods
3.1. Material Preparation
3.2. Characterization of Prepared Composite
3.3. Lithium Secondary Battery Assembly and Electrochemical Characterization
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, W.; Park, C.; Kim, J.; Kim, Y.; Jeong, G.; Sohn, H. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Choi, G.; Kim, J.; Kang, B. Understanding limited reversible capacity of a SiO electrode during the first cycle and its effect on initial coulombic efficiency. Chem. Mater. 2019, 31, 6097–6104. [Google Scholar] [CrossRef]
- Cui, J.; Cui, Y.; Li, S.; Sun, H.; Wen, Z.; Sun, J. Microsized porous SiOx@C composites synthesized through aluminothermic reduction from rice husks and used as anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 30239–30247. [Google Scholar] [CrossRef]
- Fu, J.; Liu, H.; Liao, L.; Fan, P.; Wang, Z.; Wu, Y. Ultrathin Si/CNTs paper-like composite for flexible li-ion battery anode with high volumetric capacity. Front. Chem. 2018, 6, 624–629. [Google Scholar] [CrossRef]
- Guo, C.; Wang, D.; Liu, T.; Zhu, J.; Lang, X. A three-dimensional SiOx/C@RGO nanocomposite as a high energy anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 3521–3527. [Google Scholar] [CrossRef]
- Hang, T.; Mukoyama, D.; Nara, H.; Yokoshima, T.; Momma, T.; Li, M. Electrochemical impedance analysis of electrodeposited Si–O–C composite thick film on Cu microcones-arrayed current collector for lithium ion battery anode. J. Power Sources 2014, 256, 226–232. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, M.; Wang, J.; Hu, S.; Liu, X.; Shao, Z. High yield and low-cost ball milling synthesis of nano-flake Si@SiO2 with small crystalline grains and abundant grain boundaries as a superior anode for Li-ion batteries. J. Alloys Compd. 2015, 639, 27–35. [Google Scholar] [CrossRef]
- Huang, X.; Pu, H.; Chang, J.; Cui, S.; Hallac, P.B.; Jiang, J. Improved cyclic performance of Si anodes for lithium-ion batteries by forming intermetallic interphases between Si nanoparticles and metal microparticles. ACS Appl. Mater. Inter. 2013, 5, 11965–11970. [Google Scholar] [CrossRef] [PubMed]
- Hubaud, A.A.; Yang, Z.; Schroeder, D.J.; Dogan, F.; Trahey, L.; Vaughey, J.T. Interfacial study of the role of SiO2 on Si anodes using electrochemical quartz crystal microbalance. J. Power Sources 2015, 282, 639–644. [Google Scholar] [CrossRef]
- Hwa, Y.; Park, C.; Sohn, H. Modified SiO as a high-performance anode for Li-ion batteries. J. Power Sources 2013, 222, 129–134. [Google Scholar] [CrossRef]
- Jia, H.; Zheng, J.; Song, J.; Luo, L.; Yi, R.; Estevez, L. A novel approach to synthesize micrometer-sized porous silicon as a high-performance anode for lithium-ion batteries. Nano Energy 2018, 50, 589–597. [Google Scholar] [CrossRef]
- Kataoka, R.; Oda, Y.; Inoue, R.; Kitta, M.; Kiyobayashi, T. Highstrength clad current collector for silicon-based negative electrode in lithium ion battery. J. Power Sources 2016, 301, 355–361. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.; Kim, H.; Kim, Y.; Sohn, H. Electrochemical behavior of SiO anode for Li secondary batteries. J. Electrochem. Chem. 2011, 661, 245–249. [Google Scholar] [CrossRef]
- Kim, M.K.; Jang, B.Y.; Lee, J.S.; Kim, J.S.; Nahm, S. Microstructures and electrochemical performances of nano-sized SiOx (1.18 ≤ x ≤ 1.83) as an anode material for a lithium (Li)-ion battery. J. Power Sources 2013, 244, 115–121. [Google Scholar] [CrossRef]
- Krywko-Cendrowska, A.; Strawski, M.; Szklarczyk, M. Low temperature electrodeposition of SiOx films photoactive in water solution. Electrochim. Acta 2013, 108, 112–117. [Google Scholar] [CrossRef]
- Lee, M.; Yoon, D.; Lee, U.J.; Umirov, N.; Mukanova, A.; Bakenov, Z. The electrochemical performances of n-type extended lattice spaced Si negative electrodes for lithium-ion batteries. Front. Chem. 2019, 7, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Li, K.; Zhao, Y.; Zhang, Y.; Gosselink, D. SiO2/Cu/polyacrylonitrile-C composite as anode material in lithium ion batteries. J. Power Sources 2013, 240, 659–666. [Google Scholar] [CrossRef]
- Li, M.; Zeng, Y.; Ren, Y.; Zeng, C.; Gu, J.; Feng, X. Fabrication and lithium storage performance of sugar apple-shaped SiOx@C nanocomposite spheres. J. Power Sources 2015, 288, 53–61. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Lv, P.; Zhao, H.; Gao, C.; Du, Z.; Wang, J.; Liu, X. SiOxC dual-phase glass for lithium ion battery anode with high capacityand stable cycling performance. J. Power Sources 2015, 274, 542–550. [Google Scholar] [CrossRef]
- Lv, P.; Zhao, H.; Wang, J.; Liu, X.; Zhang, T.; Xia, Q. Facile preparation and electrochemical properties of amorphous SiO2/C composite as anode material for lithium ion batteries. J. Power Sources 2013, 237, 291–294. [Google Scholar] [CrossRef]
- Rahaman, O.; Mortazavi, B.; Rabczuk, T. A first-principles study on the effect of oxygen content on the structural and electronic properties of silicon suboxide as anode material for lithium ion batteries. J. Power Sources 2016, 307, 657–664. [Google Scholar] [CrossRef]
- Rahman, M.A.; Song, G.; Bhatt, A.I.; Wong, Y.C.; Wen, C. Nanostructured silicon anodes for high-performance lithium-ion batteries. Adv. Funct. Mater. 2016, 26, 647–678. [Google Scholar] [CrossRef]
- Si, Q.; Hanai, K.; Ichikawa, T.; Phillipps, M.B.; Hirano, A.; Imanishi, N. Improvement of cyclic behavior of a ball-milled SiO and carbon nanofiber composite anode for lithium-ion batteries. J. Power Sources 2011, 196, 9774–9779. [Google Scholar] [CrossRef]
- Song, K.; Yoo, S.; Kang, K.; Heo, H.; Kang, Y.; Jo, M. Hierarchical SiOx nano for Li-ion battery anodes with structural stability and kinetic enhancement. J. Power Sources 2013, 229, 229–233. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W. Siliconbased nanomaterials for lithium-ion batteries: A review. Adv. Energy Mater. 2014, 4, 1300882–1300887. [Google Scholar] [CrossRef]
- Tao, H.; Fan, L.; Qu, X. Facile synthesis of ordered porous Si@C nanorods as anode materials for Li-ion batteries. Electrochim. Acta 2012, 71, 194–200. [Google Scholar] [CrossRef]
- Vengudusamy, B.; Grafl, A.; Preinfalk, K. Influence of silicon on the wear properties of amorphous carbon under dry and lubricated conditions. Tribol. Lett. 2014, 53, 569–583. [Google Scholar] [CrossRef]
- Wang, D.; Gao, M.; Pan, H.; Wang, J.; Liu, Y. High performance amorphous-Si@SiOx/C composite anode materials for Li-ion batteries derived from ball-milling and in situ carbonization. J. Power Sources 2014, 256, 190–199. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Zhu, Y.; Wu, N.; Tian, W. Electrochemical stability of optimized Si/C composites anode for lithium-ion batteries. Ionics 2015, 21, 579–585. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; He, J.; Wang, C.; Wang, J. Nano-sized SiOx/C composite anode for lithium ion batteries. J. Power Sources 2011, 196, 4811–4815. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, J.; Yang, J.; Xie, J.; Ma, L. Silicon@carbon hollowcore–shell heterostructures novel anode materials for lithium ion batteries. Electrochim. Acta 2013, 87, 663–668. [Google Scholar] [CrossRef]
- Xie, J.; Wang, G.; Huo, Y.; Zhang, S.; Cao, G.; Zhao, X. Nanostructured silicon spheres prepared by a controllable magnesiothermicreduction as anode for lithium ion batteries. Electrochim. Acta 2014, 135, 94–100. [Google Scholar] [CrossRef]
- Xia, M.; Li, Y.; Wu, Y.; Zhang, H.; Yang, J.; Zhou, N.; Zhou, Z.; Xiong, X. Improvingthe electrochemical properties of a SiO@C/graphite composite anode for high_energy lithium-ion batteries by adding lithium fluoride. Appl. Surf. Sci. 2019, 480, 410–418. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, D.; Han, S.; Huang, Y.; Li, S.; He, M.; Zhang, F.; Feng, X. Self-assembledFe2O3/graphene aerogel with high lithium storage performance. ACS Appl. Mater. Interfaces 2013, 5, 3764–3769. [Google Scholar] [CrossRef] [PubMed]
- Doh, C.H.; Park, C.W.; Shin, H.M.; Kim, D.H.; Chung, Y.D.; Jin, B.S.; Kim, H.S.; Veluchamy, A. A new SiO/C anode composition for lithium-ion battery. J. Power Sources 2008, 179, 367–370. [Google Scholar] [CrossRef]
- Wu, W.; Shi, J.; Liang, Y.; Liu, F.; Peng, Y.; Yang, H. A low-cost and advanced SiOx–C composite with hierarchical structure as an anode material for lithium-ion batteries. Phys. Chem. Chem. Phys. 2015, 17, 13451–13456. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Manthiram, A. A facile, low-cost synthesis of high-performance silicon-based composite anodes with high tap density for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 2399–2406. [Google Scholar] [CrossRef]
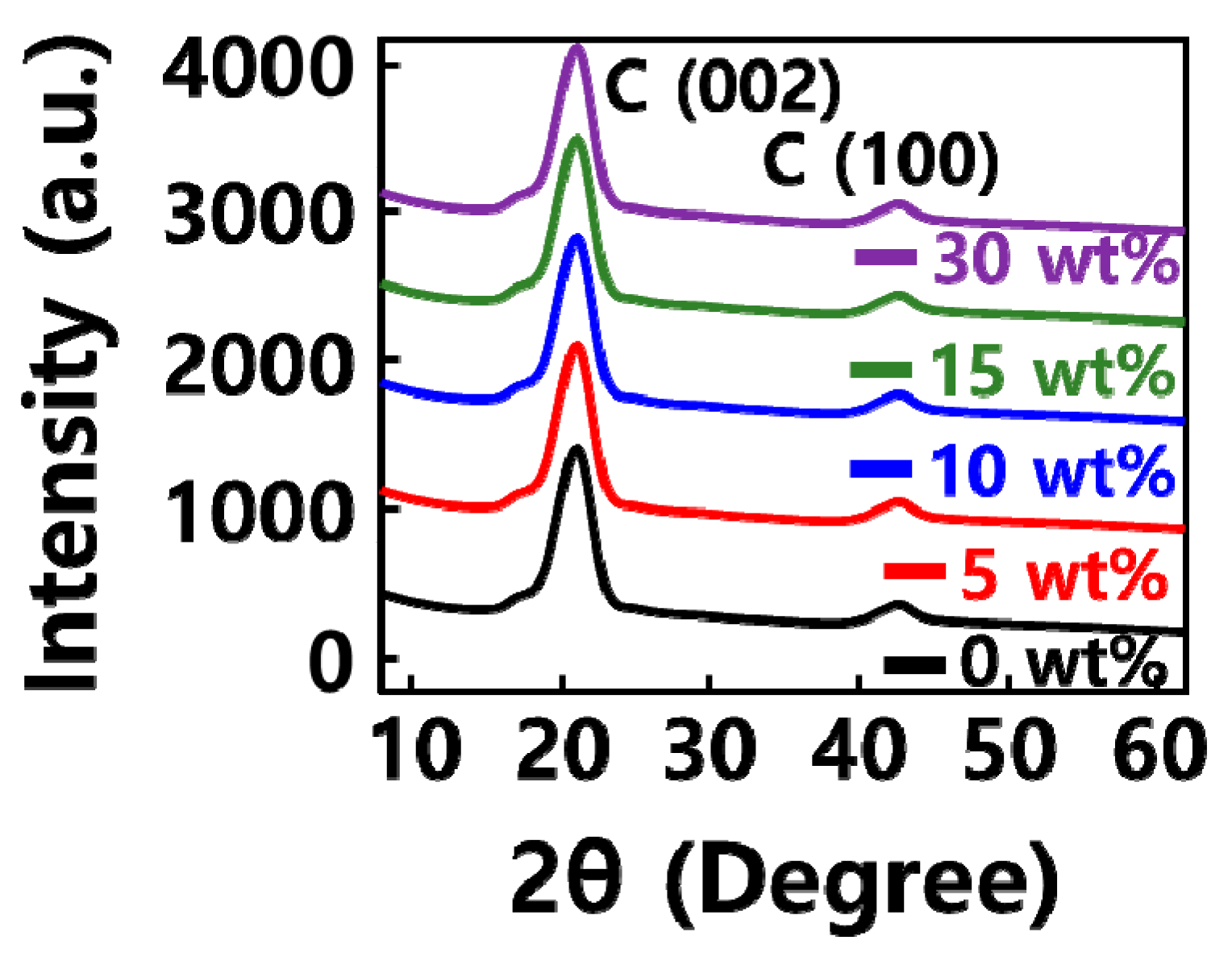

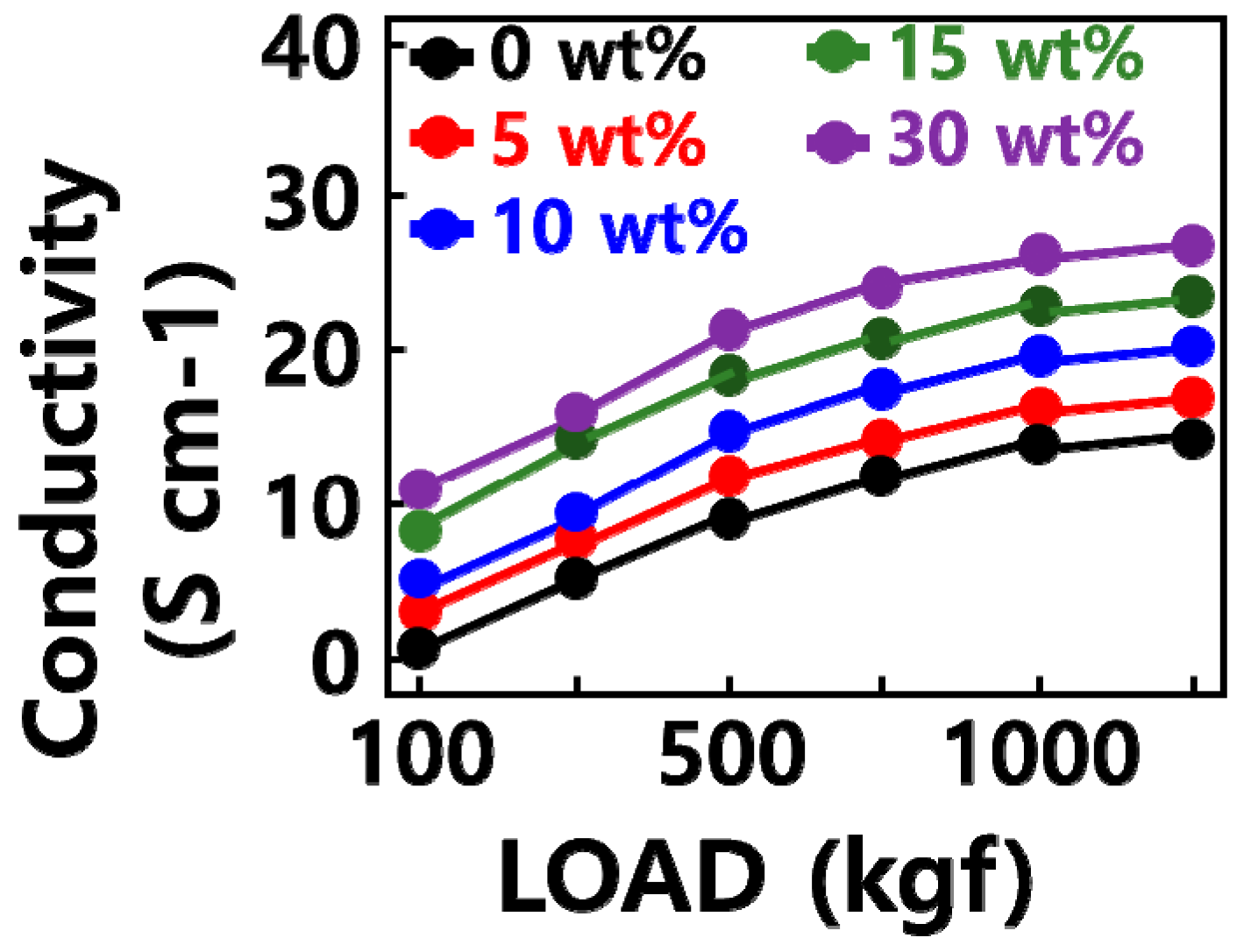

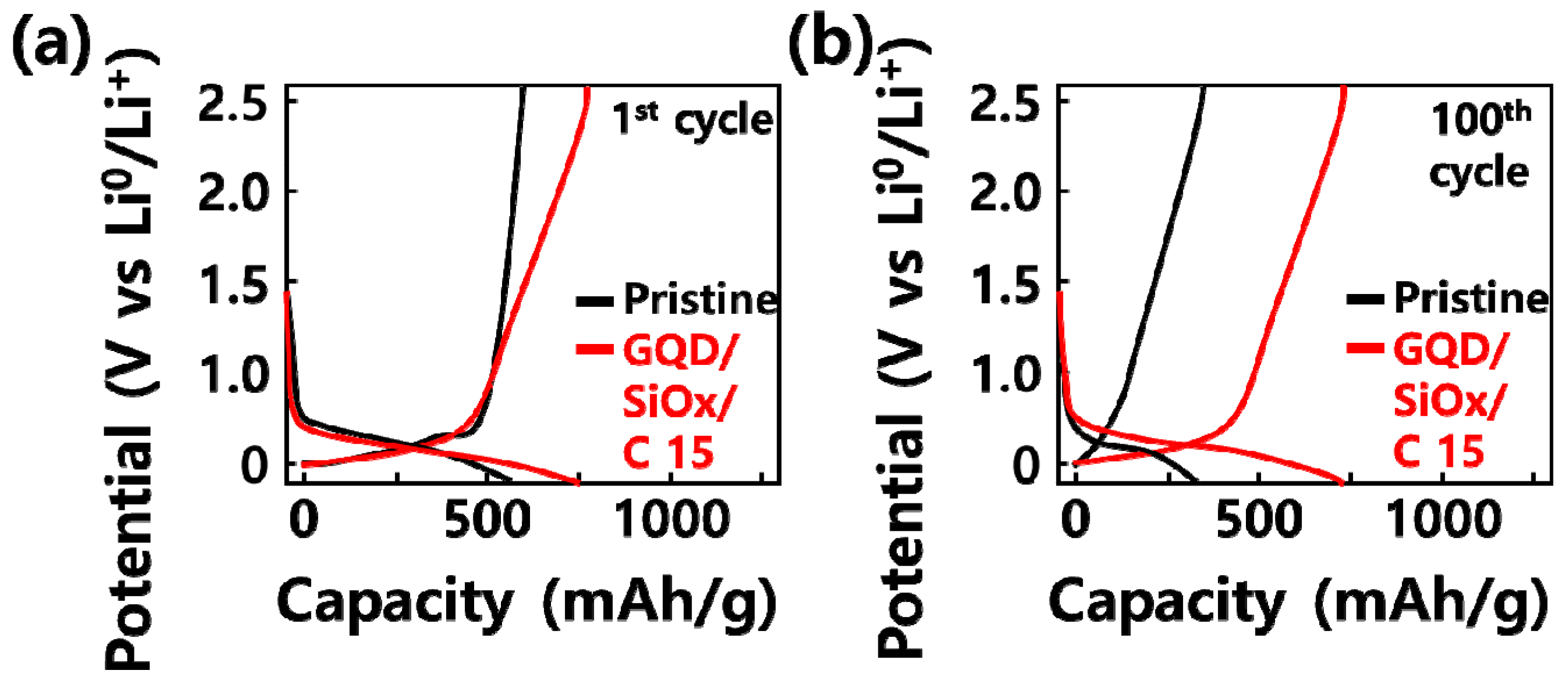
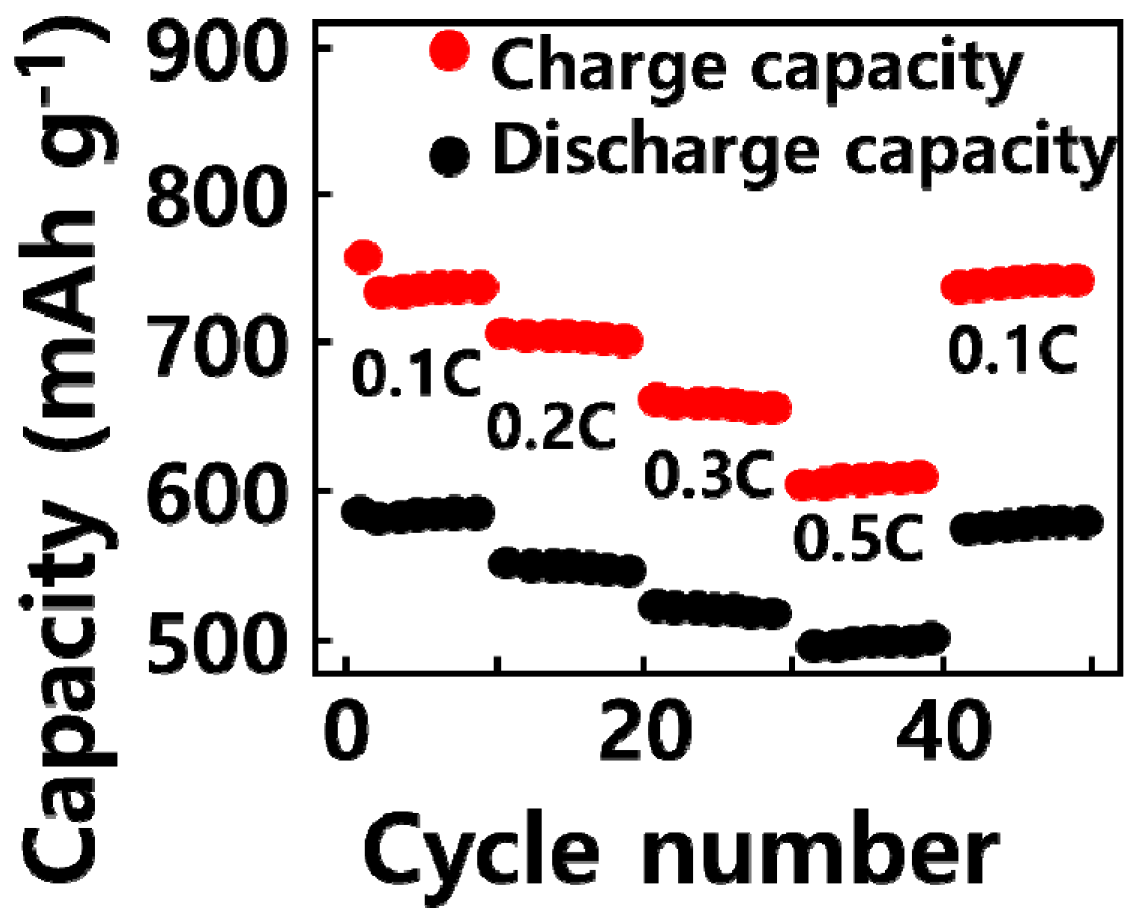

| SiOx (Weight %) | SiOx (g) | Graphene Quantum Dot (g) | Carbon Nanoparticle (g) | |
|---|---|---|---|---|
| GQD/SiOx/C-0 | 0 | 0 | 2.3 | 1.8 |
| GQD/SiOx/C-5 | 5 | 0.54 | 2.3 | 1.8 |
| GQD/SiOx/C-10 | 10 | 1.1 | 2.3 | 1.8 |
| GQD/SiOx/C-15 | 15 | 1.52 | 2.3 | 1.8 |
| GQD/SiOx/C-30 | 30 | 3.04 | 2.3 | 1.8 |
| 1st Charge Capacity (mAh/g) | 1st Discharge Capacity (mAh/g) | Initial Efficient (%) | |
|---|---|---|---|
| GQD/SiOx/C-0 | 594 | 425 | 71.6 |
| GQD/SiOx/C-5 | 615 | 445 | 72.4 |
| GQD/SiOx/C-10 | 634 | 493 | 77.9 |
| GQD/SiOx/C-15 | 768 | 595 | 77.5 |
| GQD/SiOx/C-30 | 867 | 591 | 68.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.W. SiOx/C Composite Anode for Lithium-Ion Battery with Improved Performance Using Graphene Quantum Dots and Carbon Nanoparticles. Molecules 2024, 29, 2578. https://doi.org/10.3390/molecules29112578
Hwang SW. SiOx/C Composite Anode for Lithium-Ion Battery with Improved Performance Using Graphene Quantum Dots and Carbon Nanoparticles. Molecules. 2024; 29(11):2578. https://doi.org/10.3390/molecules29112578
Chicago/Turabian StyleHwang, Sung Won. 2024. "SiOx/C Composite Anode for Lithium-Ion Battery with Improved Performance Using Graphene Quantum Dots and Carbon Nanoparticles" Molecules 29, no. 11: 2578. https://doi.org/10.3390/molecules29112578
APA StyleHwang, S. W. (2024). SiOx/C Composite Anode for Lithium-Ion Battery with Improved Performance Using Graphene Quantum Dots and Carbon Nanoparticles. Molecules, 29(11), 2578. https://doi.org/10.3390/molecules29112578





