Characterization of Key Odor-Active Compounds in Draft Beers for the Chinese Market Using Molecular Sensory Science Approaches
Abstract
1. Introduction
2. Results and Discussion
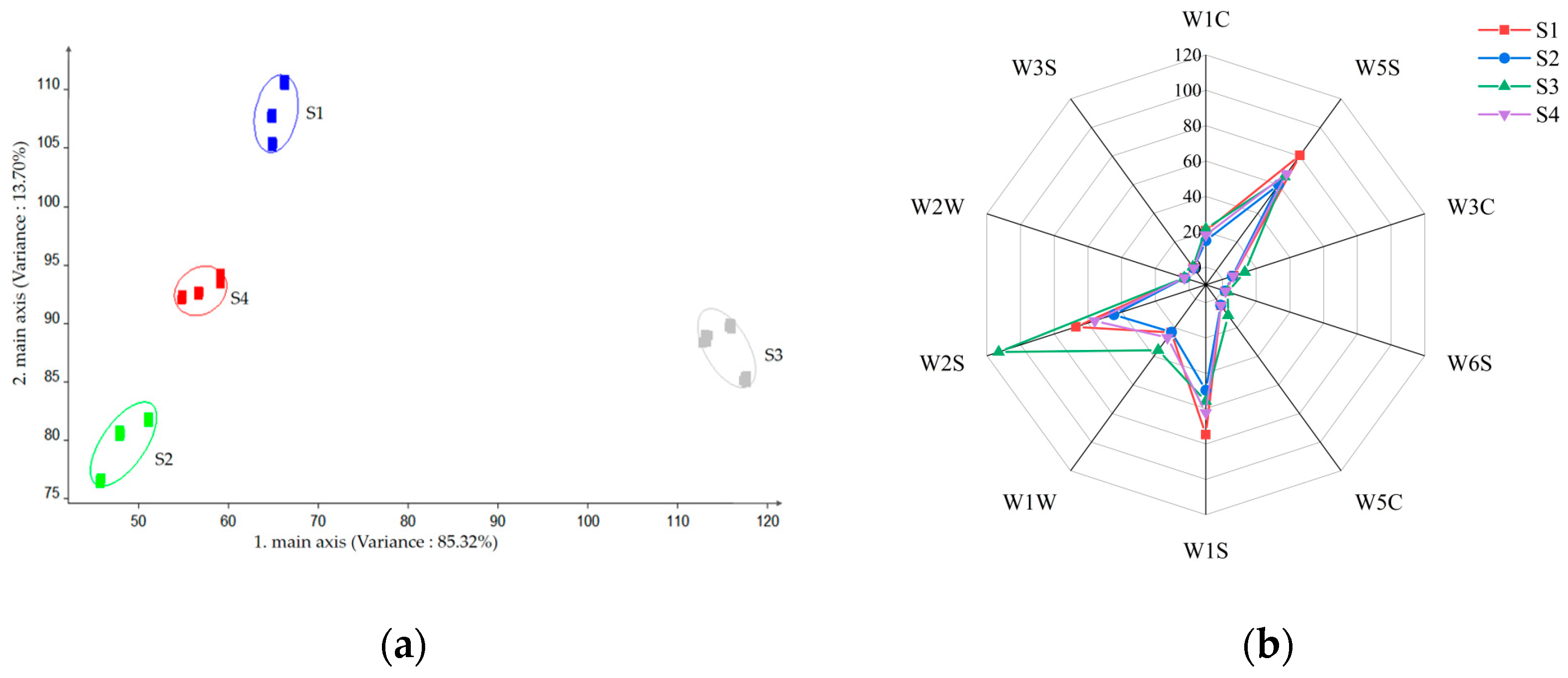
2.1. Analysis of E-Nose
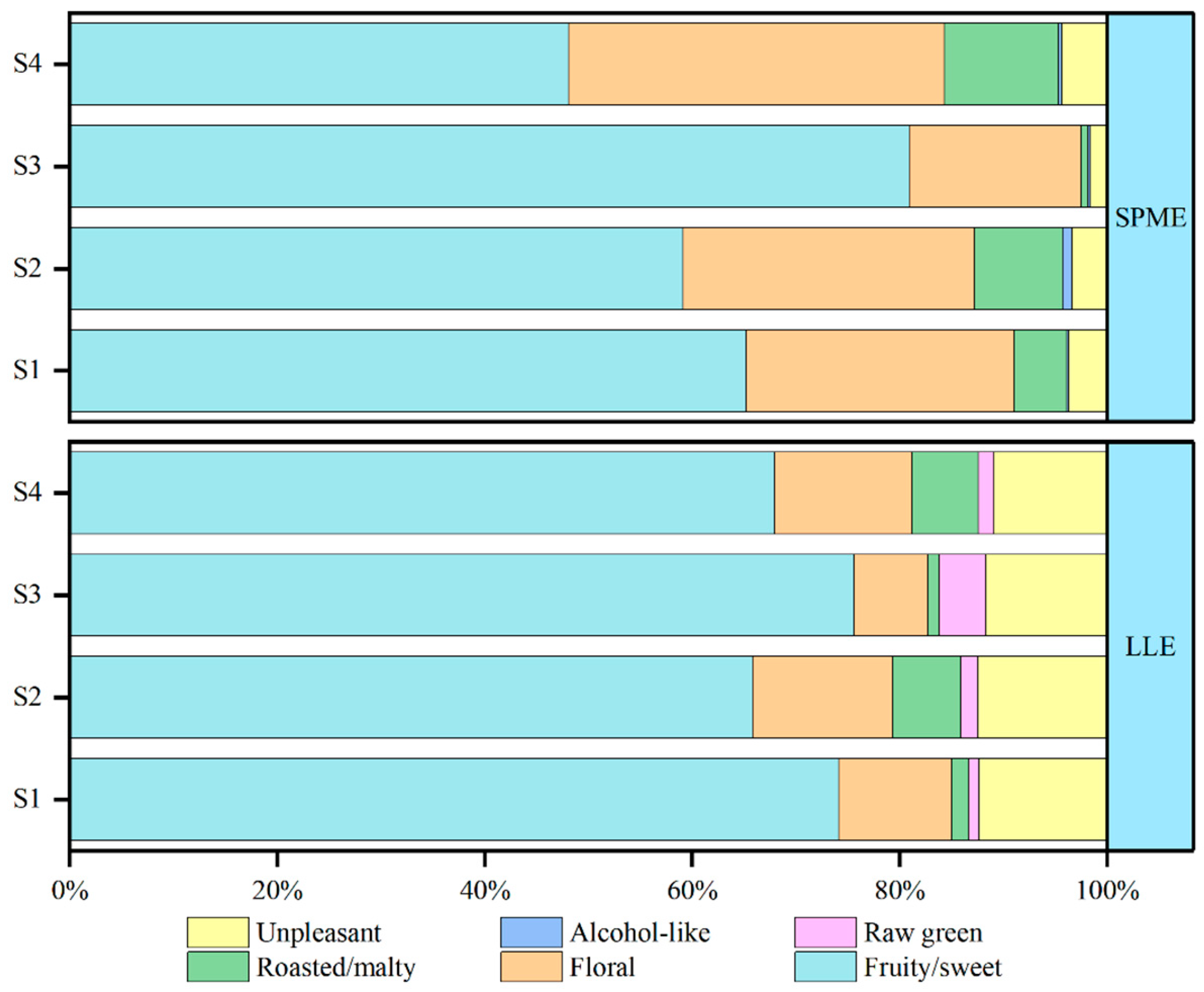
2.2. GC×GC–O–MS Analysis
2.3. Identification of the Important Odor Compounds in Draft Beer
| No. | Compound | Selected Ion 2 (m/z) | Standard Curve | R2 | Odor Threshold (μg/L) | Concentration 3 ± SD 4 (μg/L) (OAVs 5) | |||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||||
| 1 | Ethyl propionate | 102, 57, 75 | y = 0.0556x | 0.9832 | 140 6 | <1 a (<1) | <1 d (<1) | <1 b (<1) | <1 c (<1) |
| 2 | Ethyl butyrate | 101, 43, 71, 88 | y = 0.0649x | 0.9757 | 367 7 | <1 b (<1) | <1 c (<1) | <1 a (<1) | <1 c (<1) |
| 3 | Ethyl isovalerate | 115, 57, 85, 88 | y = 0.0014x | 0.9792 | 1.6 6 | 134.3 ± 1.4 a (84) | 133.6 ± 1.9 a (83) | 37.6 ± 1.6 d (24) | 127.7 ± 0.5 c (80) |
| 4 | 2-Methyl-1-propanol | 74, 31, 41, 43 | y = 0.0008x | 0.9886 | 56.5 6 | 163.3 ± 4.5 a (3) | 66.8 ± 0.6 c (1) | 133.3 ± 2.7 b (2) | 139.6 ± 6.4 b (2) |
| 5 | Isoamyl acetate | 87, 43, 55, 70 | y = 0.023x | 0.9819 | 0.15 6 | 72.7 ± 3.1 a (485) | 34.4 ± 1.5 c (229) | 27.7 ± 1.3 d (184) | 51.8 ± 3.7 b (345) |
| 6 | 1-Butanol | 56, 31, 41, 43 | y = 0.0001x | 0.9947 | 459.2 6 | 1752.2 ± 35.7 a (4) | 757.0 ± 75.2 d (2) | 1622.9 ± 69.7 b (4) | 1346.1 ± 50.1 c (3) |
| 7 | Isoamyl propionate | 101, 43, 57, 70 | y = 0.0035x | 0.9823 | 0.3 6 | <1 c (<1) | <1 b (2) | 1.2 ± 0.1 a (4) | <1 d (<1) |
| 8 | 3-Methyl-1-butanol | 70, 41, 42, 55 | y = 0.0029x | 0.9746 | 176 6 | 6519.9 ± 297.2 b (37) | 6648.7 ± 393.0 b (38) | 6057.4 ± 131.8 b (34) | 14,832.1 ± 454.8 a (84) |
| 9 | Ethyl caproate | 115, 43, 88, 99 | y = 0.0434x | 0.9785 | 1.2 6 | 5.4 ± 0.5 b (5) | 2.8 ± 0.1 d (2) | 7.1 ± 0.1 a (6) | 3.7 ± 0.1 c (3) |
| 10 | 1-Hexanol | 69, 41, 43, 56 | y = 0.0002x | 0.9822 | 5.6 6 | 17.4 ± 0.5 bc (3) | 23.2 ± 0.7 b (4) | 151.7 ± 6.1 a (27) | 13.9 ± 0.6 c (2) |
| 11 | 3-Hydroxy-2-butanone | 88, 43, 45 | y = 0.0005x | 0.9758 | 14 6 | 77.0 ± 1.7 c (6) | 1184.8 ± 75.4 a (85) | 47.0 ± 0.6 c (3) | 159.4 ± 7.4 b (11) |
| 12 | Ethyl lactate | 75, 29, 45 | y = 0.0223x | 0.9802 | 250,000 7 | <1 a (<1) | <1 c (<1) | <1 c (<1) | <1 b (<1) |
| 13 | 1-heptyl acetate | 116, 43, 56, 70, 98 | y = 0.0036x | 0.9706 | 830 6 | 18.3 ± 0.9 a (<1) | 7.1 ± 0.7 c (<1) | 17.0 ± 0.6 a (<1) | 12.7 ± 1.3 b (<1) |
| 14 | Acetic acid | 60, 43, 45 | y = 0.0024x | 0.9786 | 200,000 7 | 402.5 ± 12.7 b (<1) | 251.5 ± 14.6 c (<1) | 74.9 ± 0.5 d (<1) | 477.5 ± 17.2 a (<1) |
| 15 | Decanal | 112, 43, 57, 70, 82 | y = 0.0097x | 0.9925 | 1.5 6 | <1 d (<1) | <1 c (<1) | <1 a (<1) | <1 b (<1) |
| 16 | Butyric acid | 73, 41, 60 | y = 0.0073x | 0.9789 | 1899 7 | 54.8 ± 3.5 a (<1) | 41.1 ± 1.3 b (<1) | 50.9 ± 3.2 a (<1) | 35.9 ± 2.7 b (<1) |
| 17 | Furfuryl alcohol | 98, 97, 81, 53, 41 | y = 0.0031x | 0.9766 | 282 6 | 387.0 ± 12.1 a (1) | 282.4 ± 13.4 b (1) | 359.2 ± 23.2 a (1) | 233.3 ± 11.8 c (<1) |
| 18 | Ethyl caprate | 155, 43, 88, 101 | y = 0.1819x | 0.9703 | 1500 8 | 1.0 ± 0.1 b (<1) | <1 d (<1) | 1.2 ± 0.1 a (<1) | <1 c (<1) |
| 19 | Isovaleric acid | 87, 43, 60, 69 | y = 0.001x | 0.9795 | 1230 7 | 314.5 ± 7.4 c (<1) | 192.4 ± 4.2 d (<1) | 563.1 ± 39.1 a (1) | 396.2 ± 8.7 b (<1) |
| 20 | 2-Phenylethyl acetate | 104, 43, 91 | y = 0.0925x | 0.9696 | 19 6 | 30.8 ± 0.7 a (2) | 5.3 ± 0.1 d (<1) | 11.4 ± 0.7 c (<1) | 21.1 ± 0.4 b (1) |
| 21 | Hexanoic acid | 87, 41, 60, 73 | y = 0.0024x | 0.9718 | 8000 7 | 479.8 ± 7.4 a (<1) | 283.2 ± 9.3 c (<1) | 412.0 ± 9.8 b (<1) | 413.1 ± 20.7 b (<1) |
| 22 | Phenethyl alcohol | 122, 65, 91 | y = 0.0021x | 0.9726 | 140 6 | 2135.8 ± 99.8 b (15) | 2139.1 ± 124.6 b (15) | 1830.4 ± 58.4 c (13) | 9118.6 ± 164.5 a (65) |
2.4. Quantification of Important Odor-Active Compounds and Odor Activity Value (OAV)
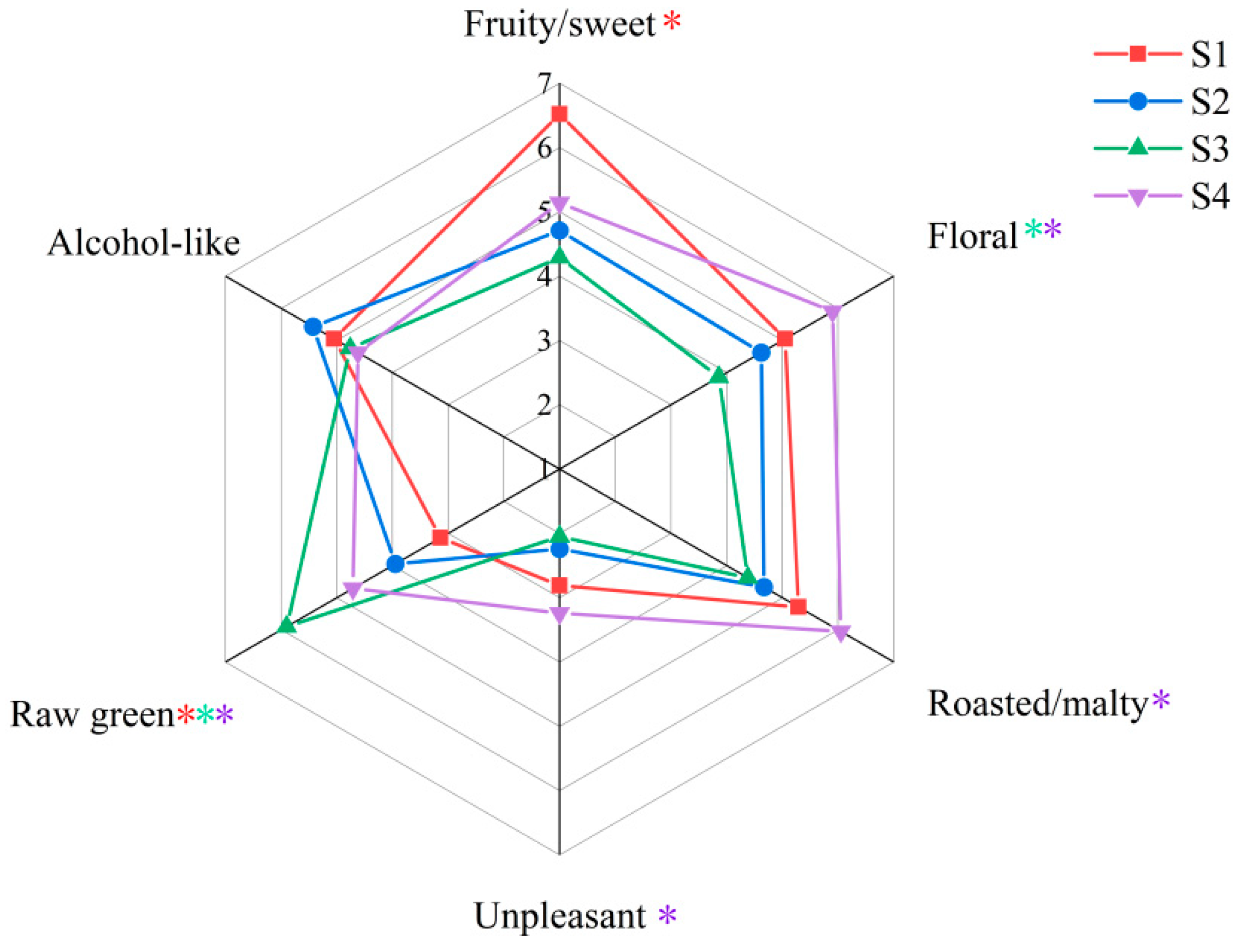
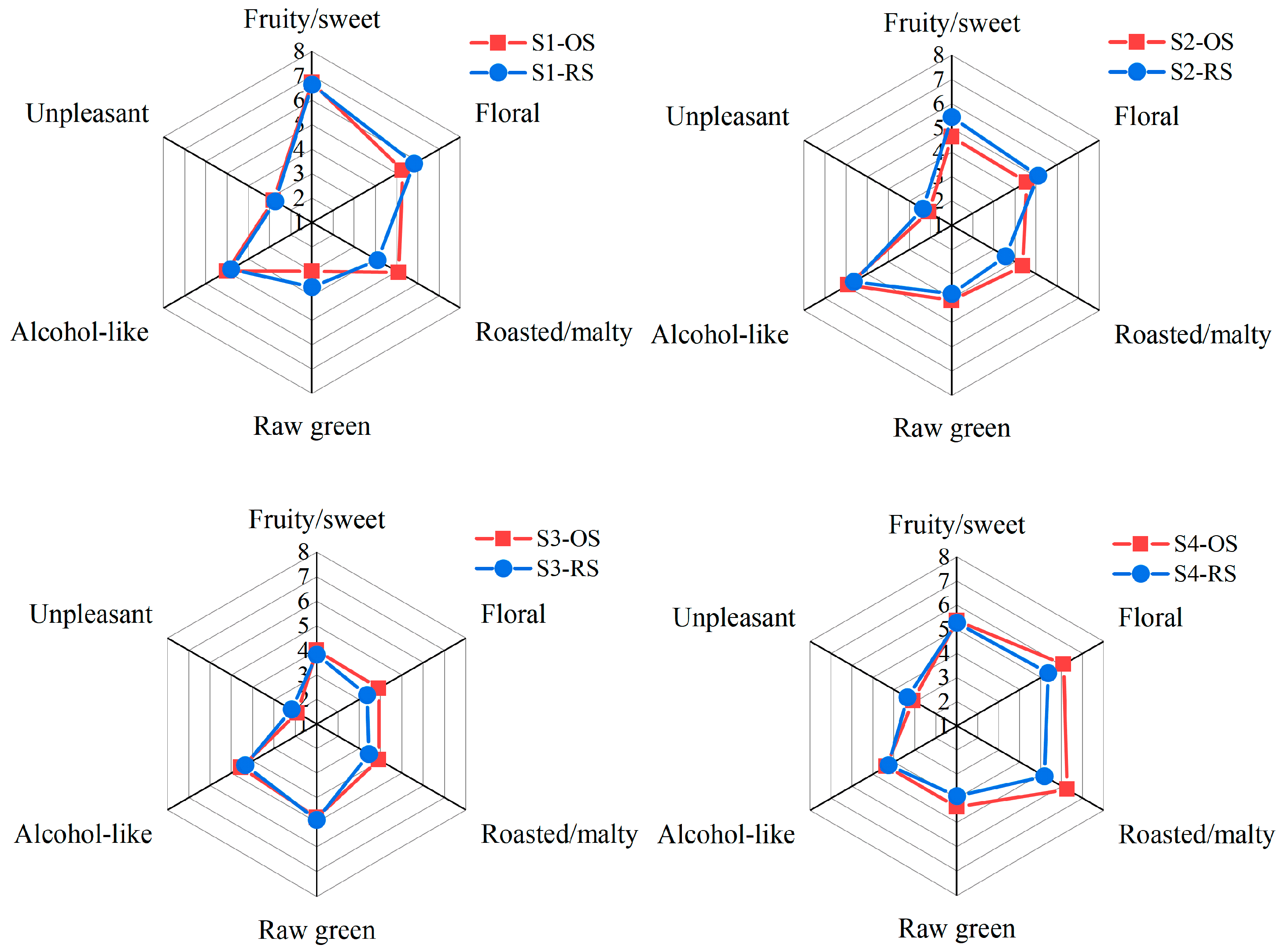
2.5. Sensory Evaluation
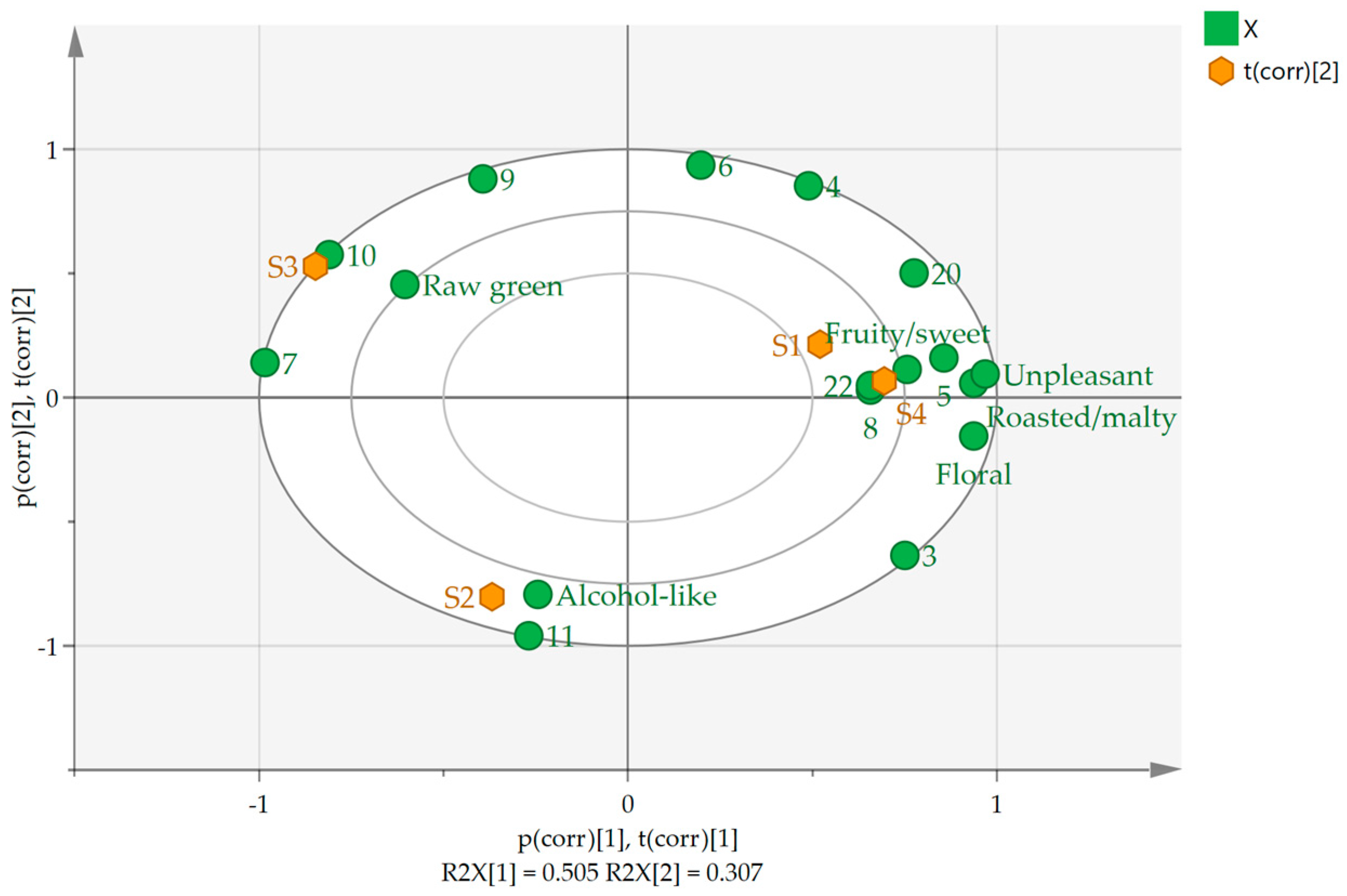
2.6. Correlation between Key Odor-Active Compounds in the Draft Beers and Sensory Evaluation
2.7. Aroma Recombination
3. Materials and Methods
3.1. Samples of Beer
3.2. Chemicals
3.3. Analysis of E-Nose
3.4. Collection of Aroma Compounds from Samples
3.5. Identification of the Odor-Active Compounds in Draft Beer through GC×GC–O–MS
3.6. AEDA
3.7. Quantitative Analysis
3.8. Sensory Evaluation
3.9. Aroma Recombination
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertuzzi, T.; Mulazzi, A.; Rastelli, S.; Donadini, G.; Rossi, F.; Spigno, G. Targeted healthy compounds in small and large-scale brewed beers. Food Chem. 2020, 310, 125935. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. I. Brewing. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- GB 4927-2008; National Bureau of Quality and Technical Supervision. Chinese National Food Safety Standard of Beer: Beijing, China, 2008.
- Bai, Y.L.; Liu, Q.; Xiao, L.; Jia, J.H.; Li, Q.L.; Yang, J.J.; Qiu, R. Application of multivariate statistical analysis techniques in the consistency study of commercially available draft beer. J. Food Saf. Qual. 2024, 15, 225–236. [Google Scholar] [CrossRef]
- Chen, H.L.; Huang, K.X.; Zheng, M.; Yang, Z.X. Analysis of flavor differences among three brands of lager beer based on untargeted flavoromics. J. Food Sci. 2021, 42, 223–228. [Google Scholar] [CrossRef]
- Meyerding, S.G.H.; Bauchrowitz, A.; Lehberger, M. Consumer preferences for beer attributes in Germany: A conjoint and latent class approach. J. Retail. 2019, 47, 229–240. [Google Scholar] [CrossRef]
- Zhang, P.H.; Maurizio, P.; Pietro, F.; Fulvio, M.; Urska, V.; Silvia, C. Application of Comprehensive 2D Gas Chromatography Coupled with Mass Spectrometry in Beer and Wine VOC Analysis. Analytica 2023, 4, 347–373. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Gosetti, F.; Rocio-Bautista, P.; Termopoli, V. Aroma determination in alcoholic beverages: Green MS-based sample preparation approaches. Mass Spectrom. Rev. 2022, e21802. [Google Scholar] [CrossRef]
- Lehnhardt, F.; Becker, T.; Gastl, M. Flavor stability assessment of lager beer: What we can learn by comparing established methods. Eur. Food Res. Technol. 2020, 246, 1105–1118. [Google Scholar] [CrossRef]
- Zhang, P.H.; Carlin, S.; Lotti, C.; Mattivi, F.; Vrhovsek, U. On sample preparation methods for fermented beverage VOCs profiling by GCxGC-TOFMS. Metabolomics 2020, 16, 102. [Google Scholar] [CrossRef]
- Carnol, L.; Schummer, C.; Moris, G. Quantification of six Phthalates and one Odipate in Luxembourgish beer using HS-SPME-GC/MS. Food Anal. Methods 2017, 10, 298–309. [Google Scholar] [CrossRef]
- Zhou, Z.; Ni, W.; Ji, Z.; Liu, S.; Han, X.; Li, X.; Mao, J. Development of a rapid method for determination of main higher alcohols in fermented alcoholic beverages based on dispersive Liquid-Liquid microextraction and Gas Chromatography-Mass Spectrometry. Food Anal. Methods 2020, 13, 591–600. [Google Scholar] [CrossRef]
- De Lima, A.C.; Acena, L.; Mestres, M.; Boque, R. Monitoring the evolution of the aroma profile of lager beer in aluminium cans and glass bottles during the natural ageing process by means of HS-SPME/GC-MS and multivariate analysis. Molecules 2023, 28, 2807. [Google Scholar] [CrossRef] [PubMed]
- Riu-Aumatell, M.; Miro, P.; Serra-Cayuela, A.; Buxaderas, S.; Lopez-Tamames, E. Assessment of the aroma profiles of low-alcohol beers using HS-SPME-GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, X.; Xia, Y.; Chen, M.; Zhang, Y.; Li, Q.; Zhen, D.; Fang, S. Research on the application of liquid-liquid extraction-gas chromatography-mass spectrometry (LLE-GC-MS) and headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) in distinguishing the Baiyunbian aged liquors. Int. J. Food Eng. 2021, 17, 83–96. [Google Scholar] [CrossRef]
- Thompson-Witrick, K.A.; Rouseff, R.L.; Cadawallader, K.R.; Duncan, S.E.; Eigel, W.N.; Tanko, J.M.; O’Keefe, S.F. Comparison of two extraction techniques, solid-phase microextraction versus continuous Liquid-Liquid Extraction/Solvent-Assisted flavor evaporation, for the analysis of flavor compounds in Gueuze Lambic beer. J. Food Sci. 2015, 80, C571–C576. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Tang, X.X.; Zhang, Q.J.; Chen, D.N.; Hui, G.H. An intellectual electronic nose system for distinguishing beer brands. J. Food Sci. 2011, 32, 184–187. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma-a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biot. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonca, M.d.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef] [PubMed]
- Vera, L.; Acena, L.; Guasch, J.; Boque, R.; Mestres, M.; Busto, O. Characterization and classification of the aroma of beer samples by means of an MS e-nose and chemometric tools. Anal. Bioanal. Chem. 2011, 399, 2073–2081. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquie-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. Fems. Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef]
- Viejo, C.G.; Fuentes, S.; Torrico, D.D.; Godbole, A.; Dunshea, F.R. Chemical characterization of aromas in beer and their effect on consumers liking. Food Chem. 2019, 293, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hou, Y.; Li, F.; Piao, Y.; Zhang, X.; Zhang, X.; Li, C.; Zhao, C. Characterization of volatile aroma compounds in different brewing barley cultivars. J. Sci. Food Agric. 2015, 95, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Schieberle, P.; Komarek, D. Changes in key aroma compounds during natural beer aging. Freshness Shelf Life Foods 2003, 836, 70–79. [Google Scholar]
- Rodrigues, F.; Caldeira, M.; Camara, J.S. Development of a dynamic headspace solid-phase microextraction procedure coupled to GC-qMSD for evaluation the chemical profile in alcoholic beverages. Anal. Chim. Acta 2008, 609, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Cramer, A.C.J.; Mattinson, D.S.; Fellman, J.K.; Baik, B.K. Analysis of volatile compounds from various types of barley cultivars. J. Agric. Food Chem. 2005, 53, 7526–7531. [Google Scholar] [CrossRef]
- Moreira, N.; Meireles, S.; Brandao, T.; de Pinho, P.G. Optimization of the HS-SPME-GC-IT/MS method using a central composite design for volatile carbonyl compounds determination in beers. Talanta 2013, 117, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, M.; Mlinaric, N.K.; Kosir, I.J. Comparison of sensory and chemical evaluation of lager beer aroma by gas chroma-tography and gas chromatography/mass spectrometry. J. Sci. Food Agric. 2018, 98, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Niu, C.; Han, Y.; Wang, J.; Zheng, F.; Liu, C.; Li, Y.; Li, Q. Comparative analysis of the effect of protein Z 4 from barley malt and recombinant Pichia pastoris on beer foam stability: Role of N-glycosylation and glycation. Int. J. Biol. Macromol. 2018, 106, 241–247. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Odour Thresholds-Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Kishimoto, T.; Noba, S.; Yako, N.; Kobayashi, M.; Watanabe, T. Simulation of Pilsner-type beer aroma using 76 odor-active compounds. J. Biosci. Bioeng. 2018, 126, 330–338. [Google Scholar] [CrossRef]
- Liu, M.M.; Zeng, Z.R.; Xiong, B. Preparation of novel solid-phase microextraction fibers by sol-gel technology for headspace solid-phase microextraction-gas chromatographic analysis of aroma compounds in beer. J. Chromatogr. A 2005, 1065, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Schieberle, P. Primary odorants of pale lager beer. Z. Lebensm. Unters. Forch. 1991, 193, 558–565. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Song, H. Comparison of SPME versus SAFE processes for the analysis of flavor compounds in watermelon juice. Food Anal. Methods 2018, 11, 1677–1689. [Google Scholar] [CrossRef]
- Shi, Y.; Gong, F.; Wang, M.; Liv, J.; Wu, Y.; Men, H. A deep feature mining method of electronic nose sensor data for iden-tifying beer olfactory information. J. Food Eng. 2019, 263, 437–445. [Google Scholar] [CrossRef]
- Zhai, Y.; Guo, M.; Meng, Q.; Song, H. Characterization of key odor-active compounds in high quality high-salt liquid-state soy sauce. J. Food Compos. Anal. 2023, 117, 105148. [Google Scholar] [CrossRef]
- Jiao, J.H.; Ding, N.Y.; Shi, T.Q.; Chai, X.L.; Cong, P.S.; Zhu, Z.L. Study of chromatographic fingerprint of the flavor in beer by HS-SPME-GC. Anal. Lett. 2011, 44, 648–655. [Google Scholar] [CrossRef]
- Vandendool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Fechir, M.; Reglitz, K.; Mall, V.; Voigt, J.; Steinhaus, M. Molecular insights into the contribution of specialty barley malts to the aroma of bottom-fermented lager Beers. J. Agric. Food Chem. 2021, 69, 8190–8199. [Google Scholar] [CrossRef]
- Zhang, H.W.; Jiang, F.H.; Yang, O.; Dai, Y.; Li, M.Q.; Cheng, J.H. Flavor wheel development and sensory quantitative descriptive analysis of Chinese brewed soy sauce. J. Food Sci. 2023, 44, 258–265. [Google Scholar] [CrossRef]
| No. | RI 2 (DB-WAX) | Compounds 3 | Aroma | FD Factor 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||||||
| LLE | SPME | LLE | SPME | LLE | SPME | LLE | SPME | ||||
| Fruity/sweet odor | |||||||||||
| 1 | 890 | Ethyl acetate | fruity, pineapple-like | - 5 | 1 | - | 2 | - | 1 | - | 1 |
| 2 | 954 | Ethyl propionate | fruity | - | 32 | - | 8 | - | 32 | - | 16 |
| 3 | 969 | Propyl acetate | fruity | - | 2 | - | - | - | - | - | 1 |
| 4 | 1001 | Ethyl butyrate | fruity, pineapple-like | 32 | 16 | 8 | 8 | 64 | 32 | 16 | 8 |
| 5 | 1005 | Isobutyl acetate | fruity | - | 1 | - | 1 | - | 1 | - | 1 |
| 6 | 1021 | Butyl acetate | fruity, banana-like | 2 | - | - | - | - | - | 4 | 2 |
| 7 | 1026 | Ethyl isovalerate | fruity | 4 | 16 | - | 8 | - | 8 | 1 | 16 |
| 8 | 1032 | 1-Propanol | fruity | - | 1 | - | 1 | - | - | - | 4 |
| 9 | 1035 | 2-Methyl-1-propanol | fruity | 512 | 2 | 128 | 2 | 256 | 2 | 512 | 1 |
| 10 | 1068 | Isoamyl acetate | fruity, banana-like | 256 | 128 | 128 | 32 | 64 | 32 | 128 | 64 |
| 11 | 1083 | 1-Butanol | fruity, apple-like | 16 | 8 | 2 | 2 | 16 | 2 | 8 | 4 |
| 12 | 1184 | Isoamyl propionate | fruity | - | 8 | - | 16 | - | 32 | - | 8 |
| 13 | 1185 | Ethyl caproate | fruity, pineapple-like | 32 | 16 | 16 | 8 | 64 | 32 | 16 | 8 |
| 14 | 1188 | 3-Methylbutyl 2-methylpropanoa | fruity | - | - | - | 1 | - | - | - | - |
| 15 | 1202 | Ethyl pyruvate | fruity | 4 | - | 1 | - | - | - | 1 | - |
| 16 | 1217 | Amyl acetate | fruity | - | - | - | - | - | - | - | 4 |
| 17 | 1273 | Ethyl lactate | fruity | 16 | 8 | 2 | 4 | 4 | 4 | 8 | 8 |
| 18 | 1321 | Ethyl heptanoate | fruity | - | 1 | 2 | 1 | 1 | - | 2 | 1 |
| 19 | 1325 | 1-Hexanol | fruity, hawthorn-like | 32 | 4 | 32 | 16 | 64 | 32 | 8 | - |
| 20 | 1401 | Ethyl caprylate | fruity | 2 | 1 | 2 | 4 | 2 | 2 | 2 | 1 |
| 21 | 1462 | Ethyl 3-hydroxybutyrate | fruity | 1 | - | - | - | - | - | - | - |
| 22 | 1471 | Propionic acid | fruity | 1 | - | 2 | - | - | - | - | - |
| 23 | 1487 | 2,3-Butanediol | fruity | 2 | - | 1 | - | 8 | - | 1 | - |
| 24 | 1505 | Ethyl nonanoate | fruity, grape-like | 8 | - | 1 | - | - | - | 8 | - |
| 25 | 1607 | Ethyl caprate | fruity | 4 | 16 | 1 | 8 | 4 | 32 | 4 | 16 |
| 26 | 1626 | Diethyl succinate | fruity | - | - | - | - | - | - | 1 | - |
| 27 | 879 | 2-Methylfuran | sweet | - | 2 | - | 4 | - | - | - | 2 |
| 28 | 969 | 2,3-Butanedione | sweet | - | - | - | 1 | - | - | - | 1 |
| 29 | 1225 | 1-Hydroxyacetone | sweet | 1 | - | 1 | - | - | - | 1 | - |
| 30 | 1228 | Hexyl acetate | sweet | 2 | - | 2 | - | 2 | - | - | - |
| 31 | 1449 | 2-Ethylhexanol | sweet | - | - | 2 | - | 4 | - | - | - |
| 32 | 1596 | Furfuryl alcohol | sweet | 64 | 16 | 32 | 4 | 32 | 8 | 8 | 4 |
| 33 | 1214 | 3-Hydroxy-2-butanone | creamy | 2 | 4 | 16 | 8 | 1 | 2 | 16 | 4 |
| 34 | 1445 | 2-Acetylfuran | caramel-like | 1 | - | 1 | - | 4 | - | 1 | - |
| 35 | 1573 | gamma-Butyrolactone | caramel-like | 2 | - | 1 | - | 4 | - | 2 | - |
| Floral odor | |||||||||||
| 36 | 1164 | 1-Pentanol | flowery | 2 | - | 2 | - | 4 | 4 | 2 | - |
| 37 | 1354 | 1-Heptyl acetate | flowery | 64 | 16 | 8 | 2 | 32 | 8 | 8 | 4 |
| 38 | 1837 | 2-Phenylethyl acetate | flowery, rose-like | 16 | 64 | 4 | 32 | 4 | 32 | 8 | 64 |
| 39 | 1958 | Phenethyl alcohol | flowery, rose-like | 64 | 32 | 64 | 32 | 16 | 8 | 128 | 64 |
| Roasted/malty odor | |||||||||||
| 40 | 917 | 3-Methylbutyraldehyde | malty | - | 4 | - | 2 | - | - | - | 4 |
| 41 | 1170 | 3-Methyl-1-butanol | roasted, malty | 8 | 16 | 32 | 16 | 8 | 2 | 64 | 32 |
| 42 | 1397 | Furfural | nutty-like | 1 | 2 | 1 | 2 | 1 | - | 2 | 4 |
| 43 | 1004 | 2,3-Pentanedione | nutty-like | 4 | - | 1 | - | - | - | - | - |
| 44 | 1469 | Benzaldehyde | nutty-like | 8 | - | 4 | - | - | - | 4 | - |
| Raw green odor | |||||||||||
| 45 | 1053 | Hexanal | fresh aroma | 1 | - | 1 | - | 1 | - | 1 | - |
| 46 | 1447 | Octyl acetate | fresh aroma | 4 | - | - | - | 2 | - | - | - |
| 47 | 1471 | Decanal | cucumber-like | 8 | - | 8 | - | 32 | - | 16 | - |
| Alcohol-like odor | |||||||||||
| 48 | 931 | Ethanol | alcohol-like | - | 1 | - | 1 | - | 1 | - | 1 |
| 49 | 1205 | 2-Methyl-1-butanol | alcohol-like | - | - | - | 1 | - | - | - | - |
| Unpleasant odor | |||||||||||
| 50 | 1372 | Acetic acid | sour | 64 | - | 32 | - | 8 | - | 64 | - |
| 51 | 1563 | Butyric acid | sour | 16 | - | 8 | - | 32 | - | 2 | - |
| 52 | 1861 | Hexanoic acid | sour | 16 | 16 | 4 | 8 | 8 | 4 | 16 | 16 |
| 53 | 1608 | Isovaleric acid | unpleasant | 64 | - | 16 | - | 32 | - | 32 | - |
| 54 | 1664 | 3-Methylthiopropanol | unpleasant | 2 | - | 4 | - | 4 | - | 4 | - |
| 55 | 2092 | Octanoic acid | unpleasant | 4 | - | 8 | - | 8 | 1 | 2 | - |
| Samples | Raw Materials | Wort Concentration (°P) | Alcohol Content (%vol) | Acidity (μmol/L) | pH | Age |
|---|---|---|---|---|---|---|
| S1 | water, malt, rice, hop products | 8.0 | 2.5 | 45.7 | 4.3 | 5 May 2021 |
| S2 | water, malt, rice, hop products | 8.0 | 3.1 | 39.8 | 4.4 | 25 June 2021 |
| S3 | water, malt, rice, hop products, yeast | 8.0 | 3.1 | 52.5 | 4.3 | 4 January 2023 |
| S4 | water, malt, rice, hop products | 8.0 | 2.5 | 53.7 | 4.3 | 8 March 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, S.; Meng, Q.; Song, H.; Wang, X. Characterization of Key Odor-Active Compounds in Draft Beers for the Chinese Market Using Molecular Sensory Science Approaches. Molecules 2024, 29, 2537. https://doi.org/10.3390/molecules29112537
Zhang Y, Li S, Meng Q, Song H, Wang X. Characterization of Key Odor-Active Compounds in Draft Beers for the Chinese Market Using Molecular Sensory Science Approaches. Molecules. 2024; 29(11):2537. https://doi.org/10.3390/molecules29112537
Chicago/Turabian StyleZhang, Yu, Sinuo Li, Qi Meng, Huanlu Song, and Xiaojun Wang. 2024. "Characterization of Key Odor-Active Compounds in Draft Beers for the Chinese Market Using Molecular Sensory Science Approaches" Molecules 29, no. 11: 2537. https://doi.org/10.3390/molecules29112537
APA StyleZhang, Y., Li, S., Meng, Q., Song, H., & Wang, X. (2024). Characterization of Key Odor-Active Compounds in Draft Beers for the Chinese Market Using Molecular Sensory Science Approaches. Molecules, 29(11), 2537. https://doi.org/10.3390/molecules29112537







