In Situ Self-Assembly of Nitrogen-Doped 3D Flower-like Hierarchical Porous Carbon and Its Application for Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
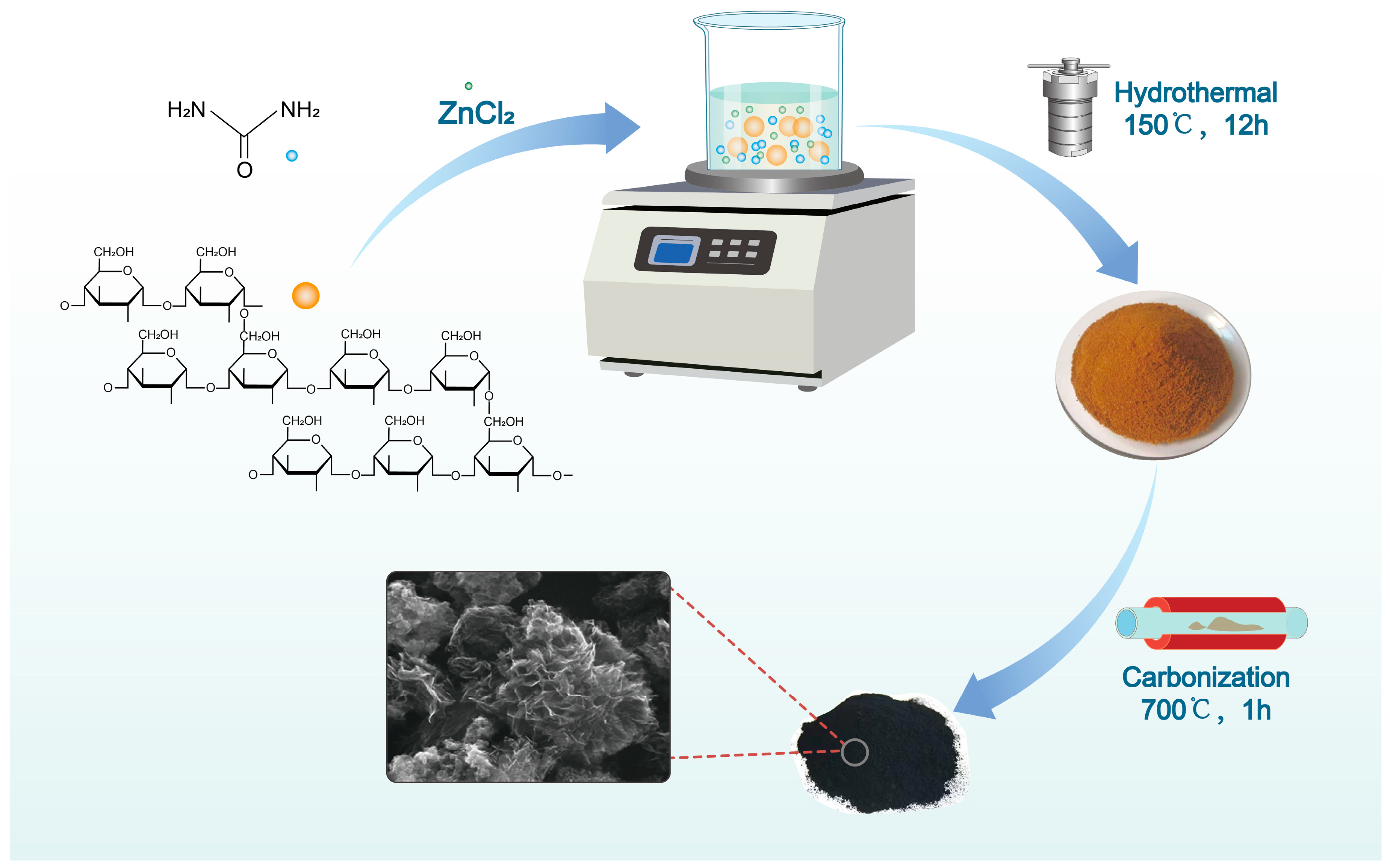
2.1. Formation of NPCs
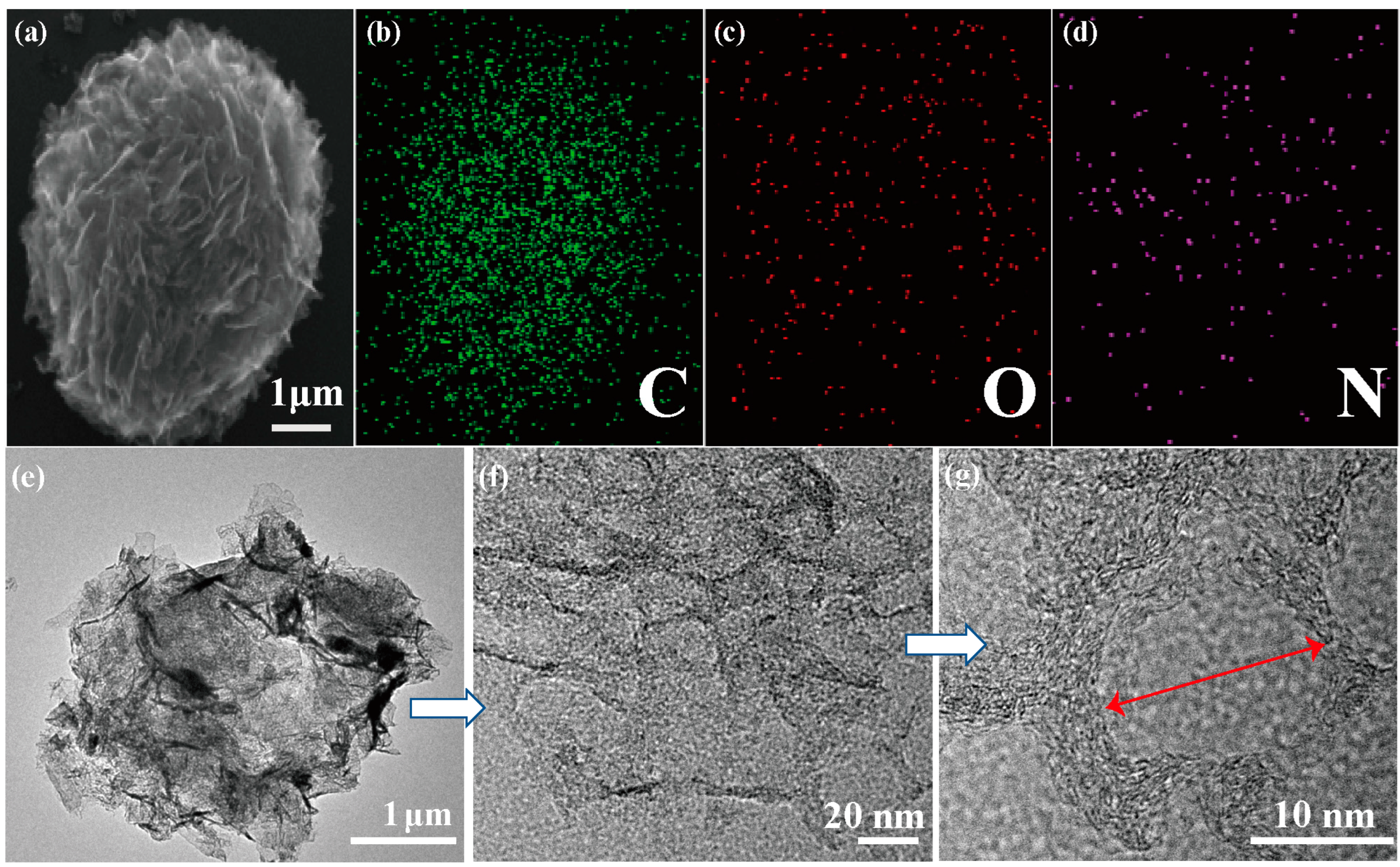
2.2. Morphology of NPCs
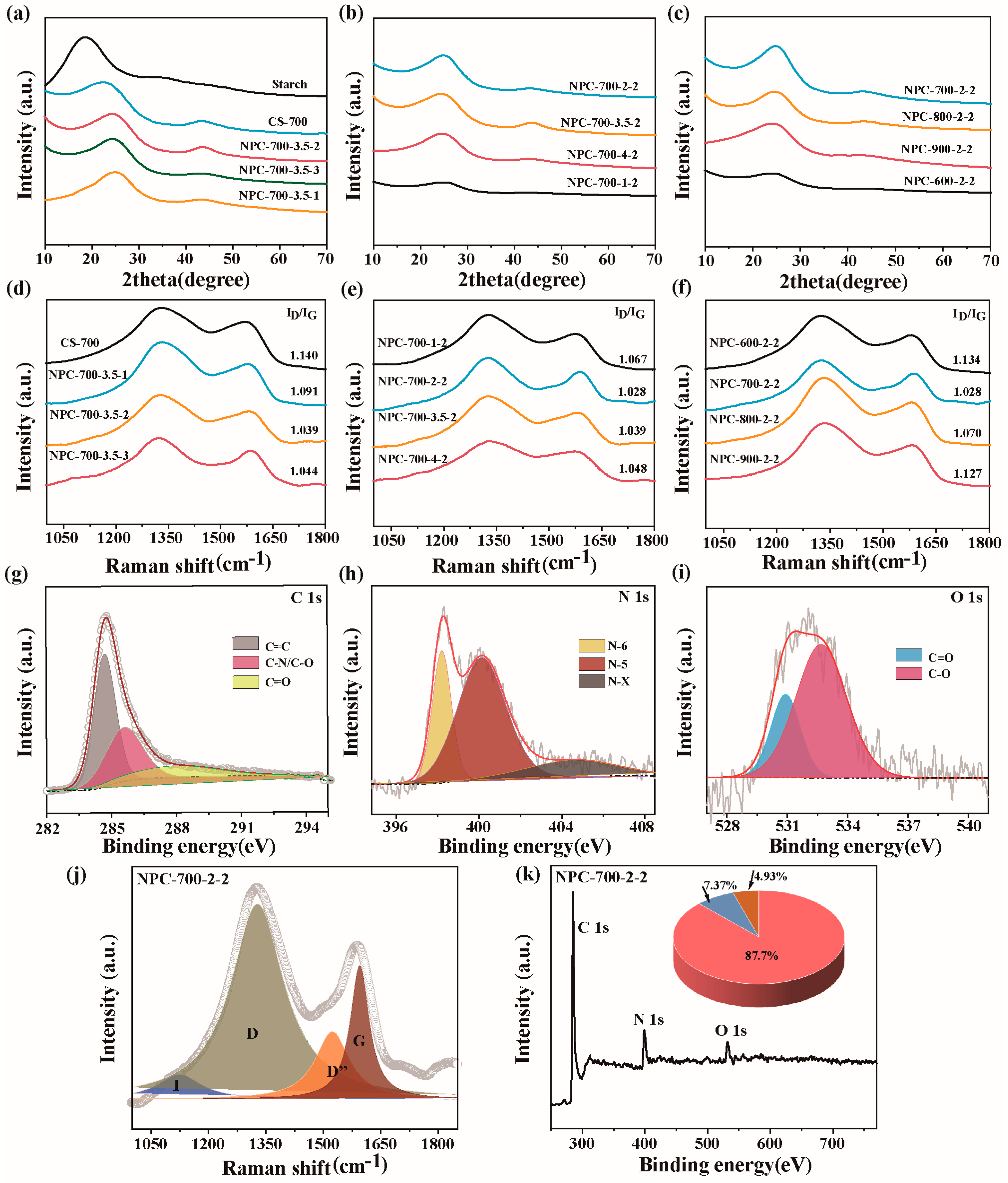
2.3. Phase Structure and Surface Chemistry Characterization
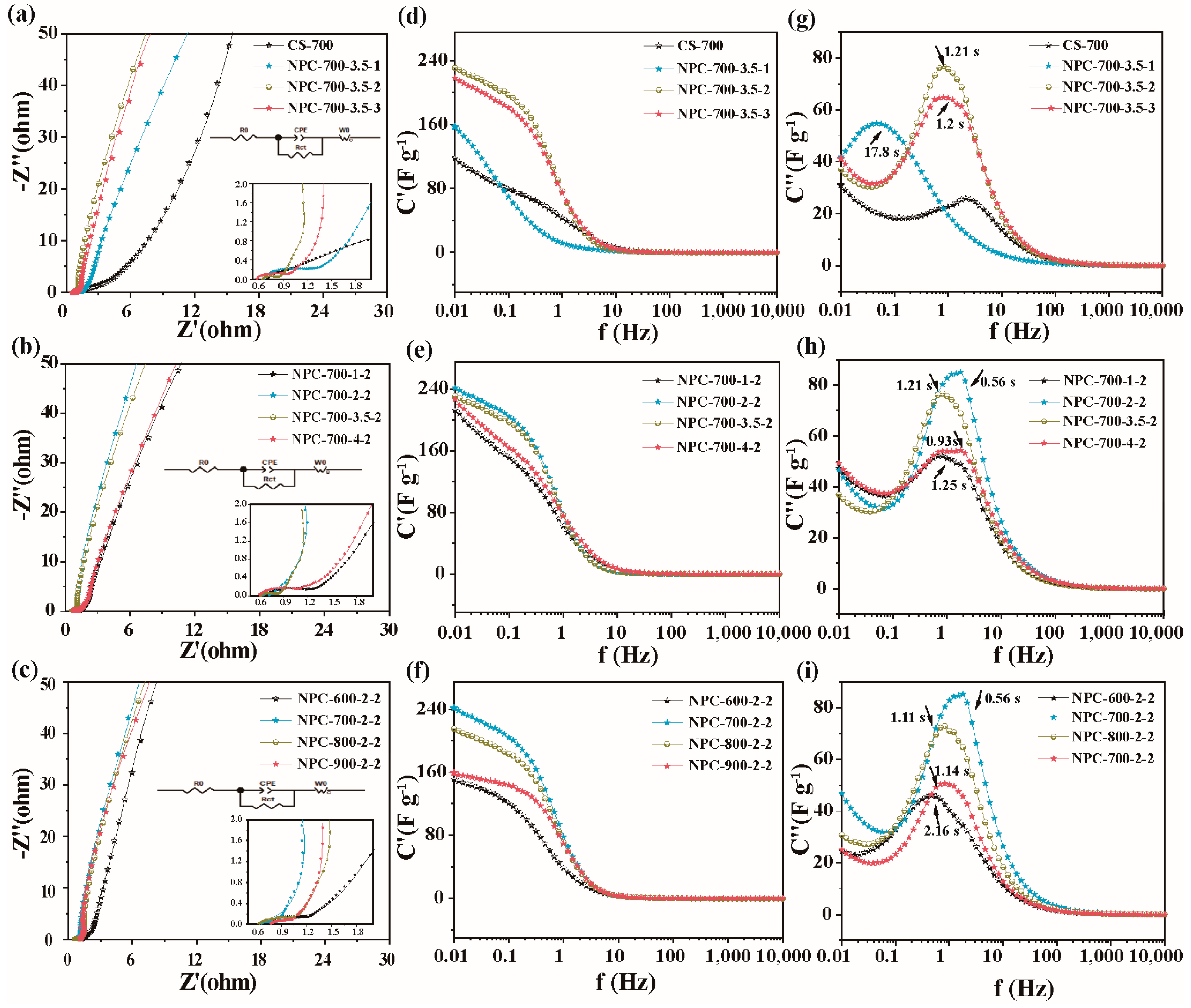
2.4. Textural Structure
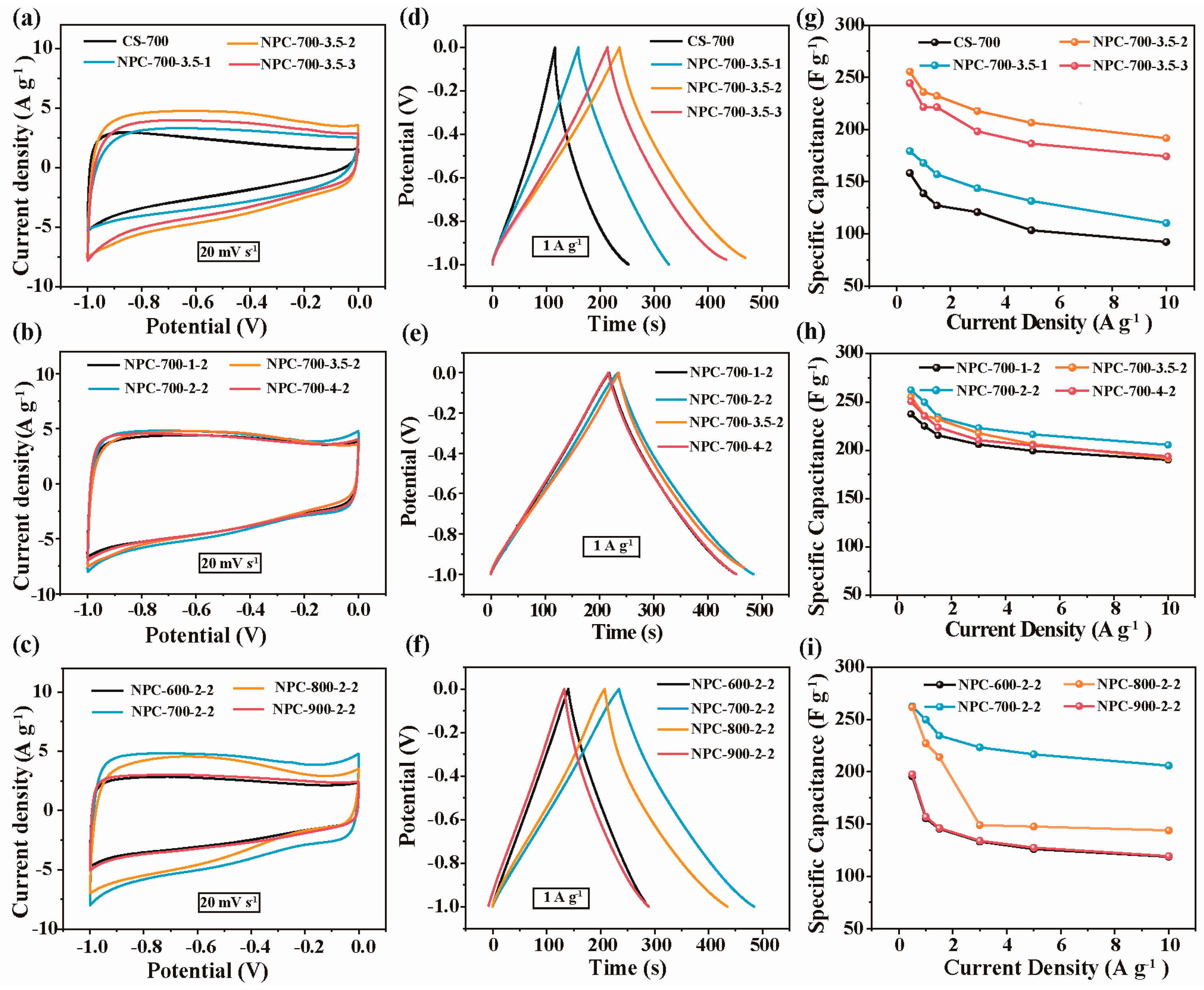
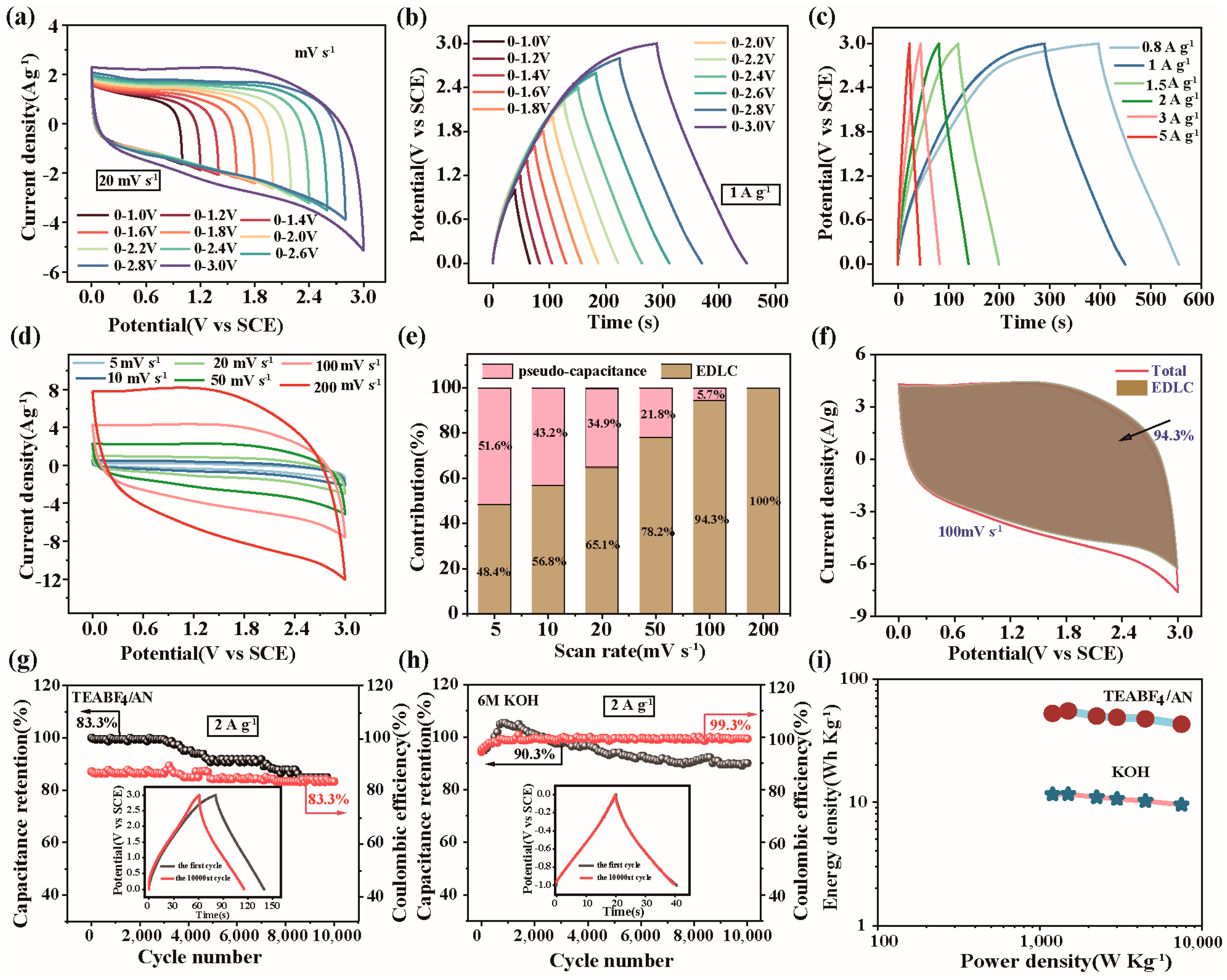
2.5. Electrochemical Performance
2.6. Mechanisms of Energy Storage and the Synergistic Effect


3. Experimental Section
3.1. Synthesis of Starch-Derived Porous Carbon
3.2. Characterization and Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Yin, H.; Wang, Z.; Gao, J.; Jiang, Q.; Xu, Y.; Chen, Z. High-performance Sn-based Anode with Robust Lignin-derived Hard Carbon Support for Sodium-ion Batteries. Asia-Pac. J. Chem. Eng. 2022, 17, e2768. [Google Scholar] [CrossRef]
- Lu, J.; Lin, X.; Wang, S.; Xu, X.; Zhou, Y.; Zhang, Y.; Li, Q.; Liu, H. High Ionic Conductivity and Toughness Hydrogel Electrolyte for High-Performance Flexible Solid-State Zinc-Ion Hybrid Supercapacitors Enabled by Cellulose-Bentonite Coordination Interactions. Green Chem. 2023, 25, 1635–1646. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, Y.; Shu, X.; Xu, T.; Jing, Y. Computational Insights into the Rational Design of Organic Electrode Materials for Metal Ion Batteries. WIREs Comput. Mol. Sci. 2023, 13, e1660. [Google Scholar] [CrossRef]
- Shah, R.A.; Ostertag, T.W.; Tang, S.; Dziubla, T.D.; Hilt, J.Z. Development of Biphenyl Monomers and Associated Crosslinked Polymers with Intramolecular Pi-pi Interactions. J Appl. Polym. Sci. 2021, 138, 50257. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H.E.; Amr, A.E.G.E.; Nossier, E.S.; Elsayed, E.A.; Azmy, E.M. Synthesis, Antimicrobial Activity and Molecular Docking of Novel Thiourea Derivatives Tagged with Thiadiazole, Imidazole and Triazine Moieties as Potential DNA Gyrase and Topoisomerase IV Inhibitors. Molecules 2020, 25, 2766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, W.; Chen, H.; Han, Y.; Liu, J. Electropolymerization of D-A-D Type Monomers Consisting of Thiophene and Quionaxline Moieties for Electrochromic Devices and Supercapacitors. J. Solid State Chem. 2022, 307, 122739. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, S.; Shen, B.; Wang, Y.; Xia, H. Fabrication of Nanosized Layered-MnO2/Activated Carbon Composites Electrodes for High-Performance Supercapacitor. Int. J. Electrochem. Sci. 2020, 15, 7646–7662. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, J.; Zhang, Q.; You, B.; Chen, G. Three Dimensional Ni Foam-Supported Graphene Oxide for Binder-Free Pseudocapacitor. Electrochim. Acta 2015, 152, 216–221. [Google Scholar] [CrossRef]
- Wan, L.; Wei, W.; Xie, M.; Zhang, Y.; Li, X.; Xiao, R.; Chen, J.; Du, C. Nitrogen, Sulfur Co-Doped Hierarchically Porous Carbon from Rape Pollen as High-Performance Supercapacitor Electrode. Electrochim. Acta 2019, 311, 72–82. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent Advances in Shear-thinning and Self-healing Hydrogels for Biomedical Applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, F.; Guo, F.; Han, Y.; Chen, F.; Ma, B.; Yang, J.; Wen, M.; Qing, Y.; Chu, F.; et al. Constructing Monodisperse Blueberry-like Lignin-Based Porous Carbon Nanospheres for High-Performance Supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130237. [Google Scholar] [CrossRef]
- Xu, J.; Mu, Y.; Ruan, C.; Li, P.; Xie, Y. S or N-Monodoping and S,N-Codoping Effect on Electronic Structure and Electrochemical Performance of Tin Dioxide: Simulation Calculation and Experiment Validation. Electrochim. Acta 2020, 340, 135950. [Google Scholar] [CrossRef]
- Xie, Y. Fabrication and Charge Storage Capacitance of PPY/TiO2/PPY Jacket Nanotube Array. J. Polym. Eng. 2021, 41, 137–143. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like Chitosan Porous Carbon Spheres/MXene Composite with High Specific Capacitance and Rate Performance for Supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Ahmad, R.; Iqbal, N.; Noor, T.; Nemani, S.K.; Zhu, L.; Anasori, B. Metal-Organic Framework/Ti3C2Tx MXene-Derived Functional Nanostructures forHigh-Performance Supercapacitors. ACS Appl. Nano Mater. 2024, 7, 253–266. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, M.; Jia, Y.; Lei, H.; Guan, Y.; Mibuy, M.A. Construction of Lanthanide Magnetic Bio-Based Porous Carbon Materials with Beam and Column Structure and Its Synergistic Adsorption Performance. ACS Appl. Mater. Interfaces 2023, 15, 33932–33943. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; O’ Keefe, C.A.; Mostazo López, M.J.; Cazorla Amorós, D.; Herou, S.; Tomani, P.; Grey, C.P.; Titirici, M.M. Hardwood versus Softwood Kraft Lignin-Precursor-Product Relationships in the Manufacture of Porous Carbon Nanofibers for Supercapacitors†. J. Mater. Chem. A 2020, 8, 23543–23554. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.; Ng, K.R.; Chen, W.N. Food Waste Durian Rind-Derived Cellulose Organohydrogels: Toward Anti-Freezing and Antimicrobial Wound Dressing. ACS Sustain. Chem. Eng. 2021, 9, 1304–1312. [Google Scholar] [CrossRef]
- Vazhayal, L.; Wilson, P.; Prabhakaran, K. Waste to Wealth: Lightweight, Mechanically Strong and Conductive Carbon Aerogels from Waste Tissue Paper for Electromagnetic Shielding and CO2 Adsorption. Chem. Eng. J. 2020, 381, 122628. [Google Scholar] [CrossRef]
- Chen, L.; Wang, F.; Tian, Z.; Guo, H.; Cai, C.; Wu, Q.; Du, H.; Liu, K.; Hao, Z.; He, S.; et al. Wood-Derived High-Mass-Loading MnO 2 Composite Carbon Electrode Enabling High Energy Density and High-Rate Supercapacitor. Small 2022, 18, 2201307. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Chi, M.; Guo, C.; Wang, S.; Min, D. Preparation and Performance of Different Carbonized Wood Electrodes. J. For. Eng. 2022, 7, 127–135. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Nie, S.; Li, N.; Cao, H.; Cheng, Z.; Liu, H.; Ni, Y.; Liu, L. Design of B/N Co-Doped Micro/Meso Porous Carbon Electrodes from CNF/BNNS/ZIF-8 Nanocomposites for Advanced Supercapacitors. J. Bioresour. Bioprod. 2023, 8, 292–305. [Google Scholar] [CrossRef]
- Qing, Y.; Liao, J.; Liu, J.; Tian, C.; Xu, H.; Wu, Y. Research progress of wood-derived energy storage materials. J. For. Eng. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.Q.; Wang, Z.F.; Li, B.; Liu, Z.Y.; Huang, J.J.; Fang, Y.T.; Chen, C.M. Biomass-Based Controllable Morphology of Carbon Microspheres with Multi-Layer Hollow Structure for Superior Performance in Supercapacitors. J. Colloid Interface Sci. 2024, 658, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bi, Z.; Xie, L.; Sun, G.; Liu, Z.; Kong, Q.; Wei, X.; Chen, C.M. From Starch to Carbon Materials: Insight into the Cross-Linking Reaction and Its Influence on the Carbonization Process. ACS Sustain. Chem. Eng. 2019, 7, 14796–14804. [Google Scholar] [CrossRef]
- Zhai, Z.; Zheng, Y.; Du, T.; Tian, Z.; Ren, B.; Xu, Y.; Wang, S.; Zhang, L.; Liu, Z. Green and Sustainable Carbon Aerogels from Starch for Supercapacitors and Oil-Water Separation. Ceram. Int. 2021, 47, 22080–22087. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G.; Gao, Q. Starch Nanoparticles-Graphene Aerogels with High Supercapacitor Performance and Efficient Adsorption. ACS Sustain. Chem. Eng. 2019, 7, 14064–14073. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Wang, H.; Hu, H.; Liu, R.; Huang, Z.; Chen, C.; Chen, D.; Feng, Z. Damaged Starch Derived Carbon Foam-Supported Heteropolyacid for Catalytic Conversion of Cellulose: Improved Catalytic Performance and Efficient Reusability. Bioresour. Technol. 2019, 288, 121532. [Google Scholar] [CrossRef]
- Liu, M.C.; Lu, C.; Xu, Y.; Hu, Y.X.; Li, J.; Zhang, H.; Zhang, Y.S.; Zhang, B.M.; Kong, L.B.; Liu, W.W.; et al. Three-Dimensional Interconnected Reticular Porous Carbon From Corn Starch By a Sample Sol-Gel Method Toward High-Performance Supercapacitors With Aqueous and Ionic Liquid Electrolytes. ACS Sustain. Chem. Eng. 2019, 7, 18690–18699. [Google Scholar] [CrossRef]
- Siddiqa, A.; Nagaraju, D.H.; Padaki, M. High-Energy-Density Asymmetric Supercapacitor Based onLayeredDouble-Hydroxide-Derived CoNi2S4 andEco-Friendly BiomassDerived Activated Carbon. Energy Fuels 2022, 36, 13286–13295. [Google Scholar] [CrossRef]
- Lima Oliveira, R.; Ledwa, K.A.; Chernyayeva, O.; Praetz, S.; Schlesiger, C.; Kepinski, L. Cerium Oxide Nanoparticles Confined in Doped Mesoporous Carbons: A Strategy to Produce Catalysts for Imine Synthesis. Inorg. Chem. 2023, 62, 13554–13565. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tanwar, V.; Tiwari, I.; Ingole, P.P.; Nebhani, L. Sustainable Upcycling of Nitrogen-Enriched Polybenzoxazine Thermosets into Nitrogen-Doped Carbon Materials for Contriving High-Performance Supercapacitors. Energy Fuels 2023, 37, 7445–7467. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.; Chi, J.; Lei, E.; Liu, Y.; Yin, Y.; Yang, Z.; Ma, C.; Li, W.; Luo, S.; et al. Soft-Template Hydrothermal Synthesis of N and B Co-Doped Walnut-Shaped Porous Carbon Spheres with Hydrophilic Surfaces for Supercapacitors. Appl. Surf. Sci. 2023, 638, 158016. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Sun, H.; Huang, Y.; An, P.; Yunhua, Y.; Zhao, H. A Manganese Oxide/Biomass Porous Carbon Composite for High-Performance Supercapacitor Electrodes. Electrochim. Acta 2024, 473, 143514. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, R.; Ma, J. Hierarchical Flower-Like Hollow SiO2 @TiO2 Spheres with Enhanced Thermal Insulation and Ultraviolet Resistance Performances for Building Coating. ACS Appl. Mater. Interfaces 2020, 12, 24250–24261. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.Q.; Xu, Z.X.; Duan, P.G.; Fan, H.L.; Hu, X.; Luque, R. Nitrogen- and Sulfur-Doped Carbon Obtained from Direct Hydrothermal Carbonization of Cellulose and Ammonium Sulfate for Supercapacitor Applications. ACS Sustain. Chem. Eng. 2020, 8, 15809–15814. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, X.; Liao, J.; Osman, S.M.; Wu, S.; Luque, R. Effects on the Physicochemical Properties of Hydrochar Originating from Deep Eutectic Solvent (Urea and ZnCl2)-Assisted Hydrothermal Carbonization of Sewage Sludge. ACS Sustain. Chem. Eng. 2022, 10, 4258–4268. [Google Scholar] [CrossRef]
- Yan, B.; Zhao, W.; Zhang, Q.; Kong, Q.; Chen, G.; Zhang, C.; Han, J.; Jiang, S.; He, S. One Stone for Four Birds: A “Chemical Blowing” Strategy to Synthesis Wood-Derived Carbon Monoliths for High-Mass Loading Capacitive Energy Storage in Low Temperature. J. Colloid Interface Sci. 2024, 653, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, Z.; Du, Y.; Wan, J.; Liu, Y.; Ma, F. In-Situ ZnO Template Preparation of Coal Tar Pitch-Based Porous Carbon-Sheet Microsphere for Supercapacitor. J. Colloid Interface Sci. 2021, 602, 721–731. [Google Scholar] [CrossRef]
- Wang, T.; Guo, J.; Guo, Y.; Feng, J.; Wu, D. Nitrogen-Doped Carbon Derived from Deep Eutectic Solvent as a High-Performance Supercapacitor. ACS Appl. Energy Mater. 2021, 4, 2190–2200. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously Mastering Operando Strain and Reconstruction Effects via Phase-Segregation Strategy for Enhanced Oxygen-Evolving Electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Xu, J.; Ruan, C.; Li, P.; Xie, Y. Excessive Nitrogen Doping of Tin Dioxide Nanorod Array Grown on Activated Carbon Fibers Substrate for Wire-Shaped Microsupercapacitor. Chem. Eng. J. 2019, 378, 122064. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, H.; Wang, M.; Zhu, Y.W.; Chen, X.Y.; Zhang, Z.J. Hierarchically N/O-Enriched Nanoporous Carbon for Supercapacitor Application: Simply Adjusting the Composition of Deep Eutectic Solvent as Well as the Ratio with Phenol-Formaldehyde Resin. J. Power Sources 2019, 438, 226982. [Google Scholar] [CrossRef]
- Feng, L.; Wang, M.; Chang, Y.; Song, H.; Hou, W.; Zhang, Y.; Xiao, Y.; Zhu, S.; Han, G. Polymerization-Pyrolysis-Derived Hierarchical Nitrogen-Doped Porous Carbon for Energetic Capacitive Energy Storage. ACS Appl. Energy Mater. 2023, 6, 7147–7155. [Google Scholar] [CrossRef]
- Liao, X.; Denk, J.; Tran, T.; Miyajima, N.; Benker, L.; Rosenfeldt, S.; Schafföner, S.; Retsch, M.; Greiner, A.; Motz, G.; et al. Extremely Low Thermal Conductivity and High Electrical Conductivity of Sustainable Carbon ceramic Electrospun Nonwoven Materials. Sci. Adv. 2023, 9, eade6066. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3−δ Nanocomposite: A New High-Performance Cobalt-Free Triple-Conducting Cathode for Protonic Ceramic Fuel Cells Operating at Reduced Temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, D.; Wang, T.; Jia, D. Nitrogen, Phosphorus Co-Doped Carbon Obtained from Amino Acid Based Resin Xerogel as Efficient Electrode for Supercapacitor. ACS Appl. Energy Mater. 2020, 3, 957–969. [Google Scholar] [CrossRef]
- Chen, L.; Deng, J.; Yuan, Y.; Hong, S.; Yan, B.; He, S.; Lian, H. Hierarchical Porous Graphitized Carbon Xerogel for High Performance Supercapacitor. Diam. Relat. Mater. 2022, 121, 108781. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, C.; Aoki, Y.; Habazaki, H. Starch-Derived Hierarchical Porous Carbon with Controlled Porosity for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 7292–7303. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Barzegar, F.; Mirghni, A.A.; Khaleed, A.A.; Masikhwa, T.M.; Manyala, N. Examination of High-Porosity Activated Carbon Obtained from Dehydration of White Sugar for Electrochemical Capacitor Applications. ACS Sustain. Chem. Eng. 2019, 7, 537–546. [Google Scholar] [CrossRef]
- Wu, L.; Cai, Y.; Wang, S.; Li, Z. Doping of Nitrogen into Biomass-Derived Porous Carbon with Large Surface Area Using N2 Non-Thermal Plasma Technique for High-Performance Supercapacitor. Int. J. Hydrogen Energy 2021, 46, 2432–2444. [Google Scholar] [CrossRef]
- Yin, Q.; Li, X.; Yong, X.; Sha, P.; Zhang, Q.; Dong, H.; Sui, J.; Yu, J.; Yu, L.; Dong, L. An Environmentally Friendly Process to Derive N/O/S-Codoped Porous Carbon from Biomass Waste with High Yield for High Performance Supercapacitor. Diam. Relat. Mater. 2023, 134, 109798. [Google Scholar] [CrossRef]
- Gang, B.; Zhang, F.; Li, X.; Zhai, B.; Wang, X.; Song, Y. A ulva lactuca-derived porous carbon for high-performance electrode materials in supercapacitor: Synergistic effect of porous structure and graphitization degree. J. Energy Storage 2021, 33, 102132. [Google Scholar] [CrossRef]
- Guo, T.; Xiang, K.; Wen, X.; Zhou, W.; Chen, H. Facile Construction on Flower-like CuS Microspheres and Their Applications for the High-Performance Aqueous Ammonium-Ion Batteries. Mater. Res. Bull. 2024, 170, 112595. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, Y.; Lou, X.; Chen, H.; Jiang, S.; Zhou, W. Vanadium Oxide/Carbonized Chestnut Needle Composites as Cathode Materials for Advanced Aqueous Zinc-Ion Batteries. J. Energy Storage 2024, 77, 109859. [Google Scholar] [CrossRef]
- Xu, J.; Xie, Y. Dual-Defects Induced Band Edge Reconstruction of Tin Dioxide via Cobalt and Nitrogen Co-Doping for Wearable Supercapacitor Application. J. Power Sources 2021, 493, 229685. [Google Scholar] [CrossRef]
- Liu, H.; Fan, W.; Lv, H.; Zhang, W.; Shi, J.; Huang, M.; Liu, S.; Wang, H. N,P-Doped Carbon-Based Freestanding Electrodes Enabled by Cellulose Nanofibers for Superior Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 2327–2338. [Google Scholar] [CrossRef]
- Li, B.; Hu, J.; Xiong, H.; Xiao, Y. Application and Properties of Microporous Carbons Activated by ZnCl2: Adsorption Behavior and Activation Mechanism. ACS Omega 2020, 5, 9398–9407. [Google Scholar] [CrossRef]
- Han, G.; Jia, J.; Liu, Q.; Huang, G.; Xing, B.; Zhang, C.; Cao, Y. Template-Activated Bifunctional Soluble Salt ZnCl2 Assisted Synthesis of Coal-Based Hierarchical Porous Carbon for High-Performance Supercapacitors. Carbon 2022, 186, 380–390. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Y.; Ke, X.; Zhou, Q.; Cui, Y.; Wang, C.; Guo, M.; Jiao, J. Resistance of Ytterbium Silicate Environmental Barrier Coatings against Molten Calcium-Magnesium-Aluminosilicate (CMAS): A Comprehensive Study. Surf. Coat. Technol. 2024, 479, 130540. [Google Scholar] [CrossRef]



| Sample | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | |||
|---|---|---|---|---|---|
| SBET | Smicro | Vtotal | Vmicro | Daver (nm) | |
| CS-700 | 332 | 302 | 0.22 | 0.11 | 1.0 |
| NPC-700-3.5-1 | 450 | 432 | 0.25 | 0.14 | 2.0 |
| NPC-700-3.5-2 | 1178 | 839 | 1.69 | 0.38 | 7.8 |
| NPC-700-3.5-3 | 779 | 775 | 0.75 | 0.23 | 6.7 |
| NPC-700-1-2 | 695 | 531 | 0.95 | 0.19 | 7.3 |
| NPC-700-2-2 | 1498 | 1163 | 1.93 | 0.44 | 6.9 |
| NPC-700-4-2 | 1009 | 893 | 0.88 | 0.31 | 5.2 |
| NPC-600-2-2 | 637 | 589 | 0.44 | 0.18 | 4.7 |
| NPC-800-2-2 | 934 | 859 | 0.68 | 0.29 | 6.5 |
| NPC-900-2-2 | 839 | 742 | 0.76 | 0.24 | 4.5 |
| Electrode Material | Activator/Template | Cs (F g−1) | Electrolyte | Ref |
|---|---|---|---|---|
| Carbon microspheres (starch) | CTAB | 242.5 (1 A g−1) | 6 M KOH | [24] |
| Microspheres (cellulose) | Ammonium Sulfate | 227.3 (1 A g−1) | 6 M KOH | [36] |
| Nanoporous carbon (PF resin) | Urea/ZnCl2 | 204 (1 A g−1) | 6 M KOH | [43] |
| NCs (tyrosine) | Urea/ZnCl2 | 235.8 (1 A g−1) | 6 M KOH | [40] |
| Xerogel (tyrosine) | KOH | 302 (0.5 A g−1) | 6 M KOH | [47] |
| HPC (starch) | Mg (NO3)2 | 229 (1 A g−1) | 6 M KOH | [49] |
| Activated Carbon (sugar) | H2SO4/KOH | 242.67 (1 A g−1) | 6 M KOH | [50] |
| Porous carbon (lotus) | KOH | 214.5 (1 A g−1) | 6 M KOH | [51] |
| Porous carbon (biomass waste) | CaCO3 | 232.8 (1 A g−1) | 2 M KOH | [52] |
| Porous carbon (ulva lactuca) | Ni catalyst | 167 (1 A g−1) | 6 M KOH | [53] |
| NPCs (starch) | Urea/ZnCl2 | 249.7 (1 A g−1) | 6 M KOH | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Liu, H.; He, C.; He, S.; Liu, L.; Zhang, Q. In Situ Self-Assembly of Nitrogen-Doped 3D Flower-like Hierarchical Porous Carbon and Its Application for Supercapacitors. Molecules 2024, 29, 2532. https://doi.org/10.3390/molecules29112532
Qiu L, Liu H, He C, He S, Liu L, Zhang Q. In Situ Self-Assembly of Nitrogen-Doped 3D Flower-like Hierarchical Porous Carbon and Its Application for Supercapacitors. Molecules. 2024; 29(11):2532. https://doi.org/10.3390/molecules29112532
Chicago/Turabian StyleQiu, Liqing, Hangzhong Liu, Chenweijia He, Shuijian He, Li Liu, and Qian Zhang. 2024. "In Situ Self-Assembly of Nitrogen-Doped 3D Flower-like Hierarchical Porous Carbon and Its Application for Supercapacitors" Molecules 29, no. 11: 2532. https://doi.org/10.3390/molecules29112532
APA StyleQiu, L., Liu, H., He, C., He, S., Liu, L., & Zhang, Q. (2024). In Situ Self-Assembly of Nitrogen-Doped 3D Flower-like Hierarchical Porous Carbon and Its Application for Supercapacitors. Molecules, 29(11), 2532. https://doi.org/10.3390/molecules29112532







