The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks
Abstract
1. Introduction
2. Pathogenesis of Neurodegenerative Diseases
2.1. Neuroinflammation
2.2. Oxidative Stress
2.3. Mitochondrial Dysfunction
2.4. Abnormally Folded Proteins
2.5. Axonal Transport Defects
2.6. Gene Mutations
2.7. Disorders in Gut Microbiota
3. Potential Active Compounds from Ganoderma lucidum against Neurodegenerative Diseases
3.1. Polysaccharides
3.2. Triterpenoids
| No. | Compound Name | Structure | Reference |
|---|---|---|---|
| 1 | Ganoderic acid C1 | 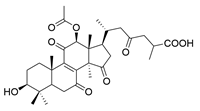 | [75] |
| 2 | Ganoderiol F | 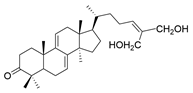 | [76] |
| 3 | Ganodermanondiol | 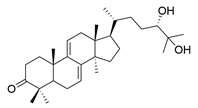 | [76] |
| 4 | Lucidumol B | 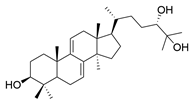 | [76] |
| 5 | Methyl ganodenate J | 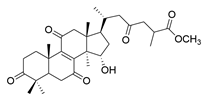 | [76] |
| 6 | Methyl lucidenate A | 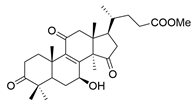 | [76] |
| 7 | Methyl lucidenate N | 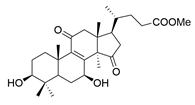 | [76] |
| 8 | Butyl lucidenate D2 | 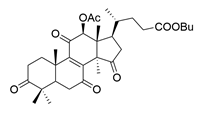 | [76] |
| 9 | Butyl lucidenate E2 | 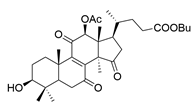 | [76] |
| 10 | Butyl lucidenate H | 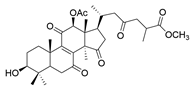 | [76] |
| 11 | Butyl lucidenate N | 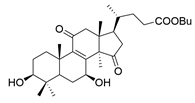 | [76] |
| 12 | Butyl lucidenate P | 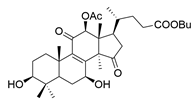 | [76] |
| 13 | Butyl lucidenate Q | 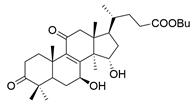 | [76] |
| 14 | Ganoderic acid A | 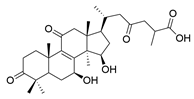 | [77] |
| 15 | Methyl ganoderate A |  | [77] |
| 16 | Methyl ganoderate H | 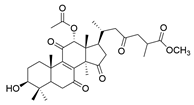 | [77] |
| 17 | Ganosidone A | 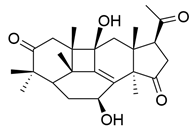 | [77] |
| 18 | Ganodermanon triol | 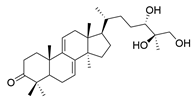 | [77] |
| 19 | Ganolucidic acid A | 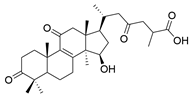 | [76] |
| 20 | Ganolucidic acid E |  | [77] |
| 21 | Lucidumol A | 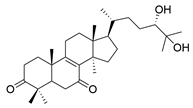 | [77] |
| 22 | Lucidumol C |  | [77] |
| 23 | Ganoderic acid D2 | 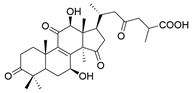 | [80] |
| 24 | Ganoderic acid H | 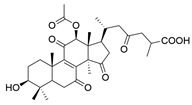 | [80] |
| 25 | Ganoderiol B |  | [80] |
| 26 | Lucidumol B | 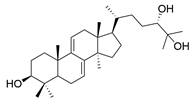 | [80] |
| 27 | Lingzhine E |  | [80] |
| 28 | Lingzhine F | 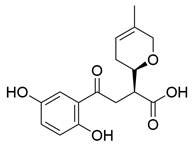 | [80] |
3.3. Proteins and Peptides
3.4. Other Components
4. The Effects of Ganoderma lucidum on the Pathological Hallmarks of Neurodegenerative Diseases
4.1. Target on Pathogenic Proteins
4.2. Regulation of Inflammatory Factors
4.3. Antioxidative Effects
4.4. Anti-Aging Effects
4.5. Rejuvenating Neurogenesis
4.6. Other Roles
| Mechanism | Ganoderma lucidum Ingredients | Model | Factors Related to NDDs | Potential Mechanism (↓: Upregulate, ↑: Downregulate) | Year/ References |
|---|---|---|---|---|---|
| Antipathogenic protein | GAA | BV2 microglial cells/Rats (Aβ42) | AD | Aβ42 ↓ | 2021 [96] |
| Antipathogenic protein | GAA/ganoderenic acid B | Molecular docking and dynamics simulation | AD | high binding affinity and stable interactions with MARK4 | 2022 [95] |
| Antipathogenic protein | GAA/GAB | PC12 cells (okadaic acid) | AD | tau hyperphosphorylation at S199 and T231 ↓ | 2023 [92] |
| Antipathogenic protein | ergosterol and ganoderic acid DM | SH-SY5Y cells (Aβ42 fibrils) C57BL/6 mice (Aβ42 fibrils) | AD | cell viability ↑ LDH level ↓ Cognitive dysfunction ↓ hippocampus neuron loss ↓ Nrf2-Keap1 signaling pathway ↑ | 2024 [98] |
| Antipathogenic protein | MGE | C. elegans | AD/PD | α-Syn, Aβ ↓ | 2024 [124] |
| Anti-neuroinflammation | GLE | microglia (LPS/MPP+) | PD | pro-inflammatory cytokines: NO, TNF-α, IL-1β ↓ | 2011 [99] |
| Anti-neuroinflammation | GLE | BV2(LPS) | Neuroinflammation | pro-inflammatory cytokines: G-CSF, Il-1α, MCP-5, MIP3α ↓ (NFB signaling) | 2020 [100] |
| Anti-neuroinflammation | GLP | BV2 and primary mouse microglia (LPS/(Aβ42) | AD | Anti-inflammatory cytokines: TGF-β ↑ pro-inflammatory cytokines: IL-1β, IL-6, iNOS, MCP-1 ↓ | 2017 [101] |
| Anti-neuroinflammation | GLT | Rats (D-galactose) | Cognitive impairment | Anti-inflammatory cytokines: IL-2 ↑ pro-inflammatory cytokines: NO, iNOS, TNF-α, IL-6 ↓ (PI3K/AKT/mTOR pathway) | 2020 [102] |
| Anti-neuroinflammation | DeGA F | BV2 Microglia/zebrafish embryos/mice model (LPS) | Neuroinflammation | Anti-inflammatory cytokines: IL-10 ↑ pro-Inflammatory Cytokines: NO, iNOS, TNF-α, IL-6, IL-1β ↓ (NF-κB Pathway) | 2019 [105] |
| Anti-neuroinflammation | GAA | BV2 Microglia (LPS) | Neuroinflammation | M1 and M2 cell surface markers: iNOS ↓, Arg-1 ↑ Pro-inflammatory Cytokines: IL-1β, IL-6 and TNF-α ↓ | 2021 [103] |
| Anti-neuroinflammation | GAA | BALB/c Mice (D-galactose) | AD | pro-inflammatory Cytokines: IL-17A, IL-17F, IL-21, and IL-22 ↓ Anti-inflammatory cytokines: TGF-β1, IL-10, and IL-35 ↑ (inhibition of the JAK/STAT signaling pathway by Regulating the Imbalance of the Th17/Tregs Axis) | 2021 [104] |
| Anti-neuroinflammation | Ganoderterpene A, | BV-2 Cells (LSP) | Neuroinflammation | pro-inflammatory Cytokines: NO ↓ (MAPK and TLR-4/NF-κB Pathways) | 2021 [106] |
| Anti-oxidative effects | Fermented GLE | PC12 cells (H2O2) | Oxidative stress | Oxidative stress-related factor: LDH ↓ apoptosis-related protein: caspase-3 ↓ | 2015 [109] |
| Anti-oxidative effects | GLP | primary dopaminergic cell (MPP+/Rot) | PD | Free radical scavenging ability ↑ mitochondrial complex I, ΔΨm ↑ Oxidative stress-related factors: ROS ↓ | 2016 [110] |
| Anti-oxidative effects | GLP | SH-SY5Y Cells (H2O2) | Mitochondrial dysfunction | ΔΨm, SOD ↑ MDA ↓ Bax, Caspase-3 ↓ Bcl-2 ↑ fission proteins (Fis1 and p-Drp1) ↓ fusion proteins (OPA1, Mfn1, and Mfn2) ↑ | 2024 [111] |
| Anti-oxidative effects | GLT | SH-SY5Y cells (H2O2/Aβ25–35) | AD | Free radical scavenging ability ↑ | 2019 [79] |
| Anti-oxidative effects | GLT | APP/PS1 Transgenic Mice. (Aβ25–35) | AD | SOD, Nrf2, NQO1, and HO1 ↑ MDA, LDH ↓ Bax, caspase 3/cleaved caspase 3 ↓ Bcl-2 ↑ (ROCK Signal Pathway) | 2020 [91] |
| Anti-oxidative effects | GLT | Rats (D-galactose) | Cognitive impairment | Oxidative stress-related factor: MDA, AGEs ↓ Antioxidative factor: T-AOC, GSH-Px, T-SOD, CAT ↑ | 2020 [102] |
| Anti-oxidative effects | GLS | rat hippocampus (STZ) | AD | Oxidative stress-related factor: MDA ↓ Antioxidative factor: GR, GSH, ATP and CytOx ↑ | 2012 [112] |
| Anti-oxidative effects | GLFE | PC-12 cells (H2O2) | oxidative stress | SOD, CAT, and GSH-Px ↑ PI3K and Akt ↑ Caspase-3 ↓ | 2024 [113] |
| Regulation of autophagy | GLE | Mice/Mice and neuroblastoma neuro-2a cells (MPTP/MPP+) | PD | protect dopaminergic neurons: TH, PINK1, Parkin ↑ autophagy related factors: BNIP3L ↑ cytochrome C, LC3-II/LC3-I ratio, AMPK, mTOR, ULK1 ↓ (AMPK/mTOR and PINK1/Parkin signaling pathway.) | 2018 [11] |
| Regulation of autophagy | GAA | BV2 microglial cells/Rats (Aβ42) | AD | autophagy related factors: LC3B-II, Axl and Pak1 phosphorylation ↑ (Axl/Pak1 signaling pathway) | 2021 [119] |
| Regulation of autophagy | GAA | HT22 cells (Aβ25–35) | AD | senescence and autophagy-related factors: P16, P21, Hmgal, LC3B I/II ↓ ATG5, Beclin 1, PADI4 ↑ (Akt/mTOR pathway) | 2021 [97] |
| Stimulation of Neurotrophic factor synthesis and neurite outgrowth | GLE | PC12 cells | AD | Phosphorylation of ERK1/2 and CREB ↑ | 2000 [129] |
| Stimulation of Neurotrophic factor synthesis and neurite outgrowth | GLE | PC12 cells | AD | neurite outgrowth ↑ (MEK/ERK1/2 and P13K/Akt signaling pathways) ↑ | 2013 [130] |
| Stimulation of Neurotrophic factor synthesis and neurite outgrowth | GLP | Primary dopaminergic cell cultures prepared (MPP+/Rot) | PD | neurites of dopaminergic neurons ↑ | 2016 [110] |
| Promote neural stem cell proliferation | GLP | APP/PS1 transgenic AD mice | AD | NPC ↑ (FGFR and ERK/AKT pathways) | 2017 [132] |
| Stimulate β adrenergic receptors | GAA | SH-SY5Y/ PC12 cell (SNP) | NO stress injury | adrenaline ↑ NO ↓ | 2020 [134] |
| Inhibition of AChE | GLT | Assay of AChE activity | _ | AChE ↓ IC50 value = 9.40 µM~31.03 µM | 2011 [136] |
| Inhibition of AChE | 11β-hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid | Assay of AChE activity | _ | AChE ↓ IC50 value = 10.8 μM | 2017 [137] |
| Inhibition of AChE | dayaolingzhiols D and E | Assay of AChE activity | _ | AChE ↓ IC50 values: Dayaolingzhiols D = 8.52 ± 1.90 μM Dayaolingzhiols E = 7.37 ± 0.52 μM | 2019 [138] |
| Inhibition of AChE | GLBR | Assay of AChE activity | _ | AChE ↓ IC50 value = 1.01 mg/mL | 2013 [138] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Kaushiki, B. Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation. J. Neuroimmunol. 2023, 383, 578180. [Google Scholar]
- Dar, N.J.; John, U.; Bano, N.; Khan, S.; Bhat, S.A. Oxytosis/Ferroptosis in Neurodegeneration: The Underlying Role of Master Regulator Glutathione Peroxidase 4 (GPX4). Mol. Neurobiol. 2023, 61, 1507–1526. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Barrientos, F.A.; Luque-Campos, N.; Araya, M.M.; Lara-Barba, E.; de Solminihac, J.; Pradenas, C.; Molina, L.; Herrera-Luna, Y.; Utreras-Mendoza, Y.; Elizondo-Vega, R.; et al. Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J. Transl. Med. 2023, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- Pravin, H.; Kratika, M.; Gitanjali, S.; Sharad, G.; Dhiraj, B. Endocytic pathways of pathogenic protein aggregates in neurodegenerative diseases. Traffic 2023, 24, 434–452. [Google Scholar]
- Adriano, A.; Martin, K. Neurodegeneration enters the era of functional genomics. Science 2023, 381, eadk5693. [Google Scholar]
- Xiaoman, Y.; Zhuoran, M.; Piaopiao, L.; Yan, X.; Xuebing, C. Common mechanisms underlying axonal transport deficits in neurodegenerative diseases: A mini review. Front. Mol. Neurosci. 2023, 16, 1172197. [Google Scholar]
- Phan, C.-W.; David, P.; Naidu, M.; Wong, K.-H.; Sabaratnam, V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: Diversity, metabolite, and mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Veena, R.K.; Ramya, H.; Janardhanan, K.K.; George, V. Gano oil: A novel antinociceptive agent extracted from Ganoderma lucidum inhibits paw oedema and relieves pain by hypnotic and analgesic actions of fatty acid amides. J. Ethnopharmacol. 2020, 263, 113144. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, E.; Ziala, A.; Gamian, A. Health-Promoting of Polysaccharides Extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, C.; Xing, Z.; Ahmad, Z.; Li, J.-S.; Chang, M.-W. Pharmacological effects of natural Ganoderma and its extracts on neurological diseases: A comprehensive review. Int. J. Biol. Macromol. 2019, 121, 1160–1178. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A Mechanistic Review on Medicinal Mushrooms-Derived Bioactive Compounds: Potential Mycotherapy Candidates for Alleviating Neurological Disorders. Planta Medica 2020, 86, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, C.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Liu, Y.-Y.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Socala, K.; Nieoczym, D.; Grzywnowicz, K.; Stefaniuk, D.; Wlaz, P. Evaluation of Anticonvulsant, Antidepressant-, and Anxiolytic-like Effects of an Aqueous Extract from Cultured Mycelia of the Lingzhi or Reishi Medicinal Mushroom Ganoderma lucidum (Higher Basidiomycetes) in Mice. Int. J. Med. Mushrooms 2015, 17, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.W.; David, P.; Sabaratnam, V. Edible and Medicinal Mushrooms: Emerging Brain Food for the Mitigation of Neurodegenerative Diseases. J. Med. Food 2017, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed]
- Christidi, F.; Migliaccio, R.; Santamaria-Garcia, H.; Santangelo, G.; Trojsi, F. Social Cognition Dysfunctions in Neurodegenerative Diseases: Neuroanatomical Correlates and Clinical Implications. Behav. Neurol. 2018, 2018, 1849794. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Ng, C.C.; Kanagasabapathy, G.; Yow, Y.Y.; Sabaratnam, V. An Overview of Culinary and Medicinal Mushrooms in Neurodegeneration and Neurotrauma Research. Int. J. Med. Mushrooms 2017, 19, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Peferoen, L.A.N.; Vogel, D.Y.S.; Breur, M.; van der Valk, P.; Baker, D.; van Noort, J.M. Inflammation in neurodegenerative diseases-an update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Bernhardi, E.R.; Bernhardi, E.L.; EEugenin, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.-L.; Lee, K.-H.; Vidyadaran, S.; Lajis, N.H.; Akhtar, M.N.; Israf, D.A.; Syahida, A. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-κB signalling pathway. Int. Immunopharmacol. 2012, 12, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.F.; Ramalho, T.C. Future Therapeutic Perspectives into the Alzheimer’s Disease Targeting the Oxidative Stress Hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef] [PubMed]
- Pasteuning-Vuhman, S.; de Jongh, R.; Timmers, A.; Pasterkamp, R.J. Towards Advanced iPSC-based Drug Development for Neurodegenerative Disease. Trends Mol. Med. 2021, 27, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F.; Hyman, B.T.; Koroshetz, W. Do defects in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci. 1993, 16, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schlehe, J.S.; LaVoie, M.J.; Schwarz, T.L. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J. Cell Biol. 2014, 206, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Wei, D.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration 2021, 1, 20210115. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Daniele, S.; Martini, C. Potential biomarkers and novel pharmacological targets in protein aggregation-related neurodegenerative diseases. Biochem. Pharmacol. 2017, 131, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bignante, E.A.; Heredia, F.; Morfini, G.; Lorenzo, A. Amyloid β precursor protein as a molecular target for amyloid β--induced neuronal degeneration in Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2525–2537. [Google Scholar] [CrossRef] [PubMed]
- Chun, W.; Johnson, G.V. The role of tau phosphorylation and cleavage in neuronal cell death. Front. Biosci. 2007, 12, 733–756. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.M.; Ardiles, A.O.; Barraza, N.; Baéz-Matus, X.; Caviedes, P. Role of tau protein in neuronal damage in Alzheimer’s disease and Down syndrome. Arch. Med. Res. 2012, 43, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Burré, J. The Synaptic Function of α-Synuclein. J. Park. Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Peña, S. Role of oxidative DNA damage in mitochondrial dysfunction and Huntington’s disease pathogenesis. Free Radic. Biol. Med. 2013, 62, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Cario, A.; Berger, C.L. Tau, microtubule dynamics, and axonal transport: New paradigms for neurodegenerative disease. Bioessays 2023, 45, e2200138. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Park, J.H.; Lu, H.C. Axonal energy metabolism, and the effects in aging and neurodegenerative diseases. Mol. Neurodegener. 2023, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Leitão, A.D.G.; Fang, S.; Gu, Y.; Barber, S.; Gilliard-Telefoni, R.; Castro, A.; Sung, K.; Shen, R.; Florio, J.B.; et al. Overexpression of alpha synuclein disrupts APP and Endolysosomal axonal trafficking in a mouse model of synucleinopathy. Neurobiol. Dis. 2023, 178, 106010. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Altman, T.; Perlson, E. Looking for answers far away from the soma-the (un)known axonal functions of TDP-43, and their contribution to early NMJ disruption in ALS. Mol. Neurodegener. 2023, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.M.; van Duijn, C.M. In search of genes involved in neurodegenerative disorders. Mutat. Res. 2005, 592, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.T.; Wells, S.A.; Hsu, C.L.; Cheng, Y.Y.; Römer, R.A. The interplay of mutations and electronic properties in disease-related genes. Sci. Rep. 2012, 2, 272. [Google Scholar] [CrossRef] [PubMed]
- Bordner, A.J.; Zorman, B. Predicting non-neutral missense mutations and their biochemical consequences using genome-scale homology modeling of human protein complexes. arXiv 2013, arXiv:1308.4433. [Google Scholar]
- Bayraktar, A.; Onal-Suzek, T.; Suzek, B.E.; Baysal, O. Meta-analysis of Gene Expression in Neurodegenerative Diseases Reveals Patterns in GABA Synthesis and Heat Stress Pathways. arXiv 2019, arXiv:1909.07469. [Google Scholar]
- Ruffini, N.; Klingenberg, S.; Schweiger, S.; Gerber, S. Common Factors in Neurodegeneration: A Meta-Study Revealing Shared Patterns on a Multi-Omics Scale. Cells 2020, 9, 2642. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Zhou, Q.G.; Xu, C.; Taleb, A.; Meng, F.; Ahmed, B.; Zhang, Y.; Fukunaga, K.; Han, F. Gut-brain axis: A matter of concern in neuropsychiatric disorders...! Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110051. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, N.U.; Islam, M.M.; Hong, S.T.; Chung, H.J. Dysbiosis of the gut microbiota and its effect on α-synuclein and prion protein misfolding: Consequences for neurodegeneration. Front. Cell Infect. Microbiol. 2024, 14, 1348279. [Google Scholar] [CrossRef] [PubMed]
- Flossmann, V.; Cross, A.; Issa, F. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Transplantation 2020, 104, 1112. [Google Scholar] [CrossRef]
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. A Timeline for Parkinson’s Disease. Chem. Senses 2009, 34, A22. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. Gut microbiome, short-chain fatty acids, alpha-synuclein, neuroinflammation, and ROS/RNS: Relevance to Parkinson’s disease and therapeutic implications. Redox Biol. 2024, 71, 103092. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.S.; Lee, K.J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef] [PubMed]
- Cör Andrejč, D.; Knez, Ž.; Knez Marevci, M. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front. Pharmacol. 2022, 13, 934982. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Liang, Y.C.; Lee, S.S.; Chiang, B.L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Kou, F.; Ge, Y.; Wang, W.; Mei, Y.; Cao, L.; Wei, X.; Xiao, H.; Wu, X. A review of Ganoderma lucidum polysaccharides: Health benefit, structure-activity relationship, modification, and nanoparticle encapsulation. Int. J. Biol. Macromol. 2023, 243, 125199. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F.; Ahmad, F.A.; Khan, M.I.; Alsayegh, A.A.; Wahab, S.; Alam, M.I.; Ahmed, F. Ganoderma lucidum: A potential source to surmount viral infections through β-glucans immunomodulatory and triterpenoids antiviral properties. Int. J. Biol. Macromol. 2021, 187, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, W.; Liu, S. Optimization of ultrasonic-assisted extraction of polysaccharides and triterpenoids from the medicinal mushroom Ganoderma lucidum and evaluation of their in vitro antioxidant capacities. PLoS ONE 2020, 15, e0244749. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Yan, X.H.; Zhang, J.L.; Wang, L.Y.; Xue, H.; Jiang, G.C.; Ma, X.T.; Liu, X.J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, J.; Xu, Z.; Li, H.; Hu, J.; Ning, H.; Qin, Z.; Pei, H.; Sun, T.; Zhang, X. Effect of heat stress on production and in-vitro antioxidant activity of polysaccharides in Ganoderma lucidum. Bioprocess Biosyst. Eng. 2018, 41, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Ma, B.; Xue, F.; Xing, Y.; Wu, P.; Li, T.; Shi, F.; Xu, C. Structure Characterization and Anti-Inflammatory Activity of Polysaccharides from Lingzhi or Reishi Medicinal Mushroom Ganoderma lucidum (Agaricomycetes) by Microwave-Assisted Freeze-Thaw Extraction. Int. J. Med. Mushrooms 2022, 24, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, Q.; Hou, K.; Ding, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Regulatory effects of Ganoderma atrum polysaccharides on LPS-induced inflammatory macrophages model and intestinal-like Caco-2/macrophages co-culture inflammation model. Food Chem. Toxicol. 2020, 140, 111321. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Feng, J.; Tang, C.; Yan, M.; Zhou, S.; Chen, W.; Wang, W.; Liu, Y. Multiple Fingerprint-Activity Relationship Assessment of Immunomodulatory Polysaccharides from Ganoderma lucidum Based on Chemometric Methods. Molecules 2023, 28, 2913. [Google Scholar] [CrossRef] [PubMed]
- Gokce, E.C.; Kahveci, R.; Atanur, O.M.; Gürer, B.; Aksoy, N.; Gokce, A.; Sargon, M.F.; Cemil, B.; Erdogan, B.; Kahveci, O. Neuroprotective effects of Ganoderma lucidum polysaccharides against traumatic spinal cord injury in rats. Injury 2015, 46, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.R.; Yue, Q.X.; Wu, Z.Y.; Song, X.Y.; Tao, S.J.; Wu, X.H.; Xu, P.P.; Liu, X.; Guan, S.H.; Guo, D.A. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry 2010, 71, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F. Ganoderic Acid A targeting leucine-rich repeat kinase 2 involved in Parkinson’s disease—A computational study. Aging Med. 2023, 6, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dunkin, D.; Lai, J.; Song, Y.; Ceballos, C.; Benkov, K.; Li, X.M. Anti-inflammatory Effects of Ganoderma lucidum Triterpenoid in Human Crohn’s Disease Associated with Downregulation of NF-κB Signaling. Inflamm. Bowel Dis. 2015, 21, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Nguyen, V.T.; Tae, N.; Lee, S.; Ryoo, S.; Min, B.S.; Lee, J.H. Anti-inflammatory and heme oxygenase-1 inducing activities of lanostane triterpenes isolated from mushroom Ganoderma lucidum in RAW264.7 cells. Toxicol. Appl. Pharmacol. 2014, 280, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.H.; Chae, H.J.; Lee, J.H.; Suh, S.S.; Youn, U.J. Antiinflammatory lanostane triterpenoids from Ganoderma lucidum. Nat. Prod. Res. 2021, 35, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Ćilerdžić, J.L.; Sofrenić, I.V.; Tešević, V.V.; Brčeski, I.D.; Duletić-Laušević, S.N.; Vukojević, J.B.; Stajić, M.M. Neuroprotective Potential and Chemical Profile of Alternatively Cultivated Ganoderma lucidum Basidiocarps. Chem. Biodivers 2018, 15, e1800036. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, L.; Zhang, L.; Hu, B.; Wang, Q.; Liang, L. Antioxidant Properties of Triterpenoids Isolated from Bagasse-Cultivated Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), at Different Developmental Stages. Int. J. Med. Mushrooms 2022, 24, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Aursuwanna, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Puthong, S.; Reamtong, O.; Karnchanatat, A. Investigating the cellular antioxidant and anti-inflammatory effects of the novel peptides in lingzhi mushrooms. Heliyon 2022, 8, e11067. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, C.; Zhang, J.; Sun, M.; Xu, J.; Xu, C.; Kuang, H.; Xu, L. Chiral nanoparticle-remodeled gut microbiota alleviates neurodegeneration via the gut-brain axis. Nat. Aging 2023, 3, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, F.; Li, J.; Peng, P.; Liu, B.; Zhao, L. Ganoderma lucidum protease hydrolyzate on lipid metabolism and gut microbiota in high-fat diet fed rats. Food Biosci. 2022, 47, 101460. [Google Scholar] [CrossRef]
- Tulsawani, R.; Sharma, P.; Manimaran, M.; Koganti, P.; Singh, M.; Meena, D.K.; Negi, P.S.; Misra, K. Effects of Extraction Temperature on Efficacy of Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), Aqueous Extract against Oxidative Stress. Int. J. Med. Mushrooms 2020, 22, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, Y.; Ji, H.; Yang, Y.; Xu, C.; Li, Q.; Ran, L.; Wu, C.; Zhou, Q.; Wu, S. Adenosine-rich extract of Ganoderma lucidum: A safe and effective lipid-lowering substance. iScience 2022, 25, 105214. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lan, P. Total Syntheses and Biological Evaluation of the Ganoderma lucidum Alkaloids Lucidimines B and C. ACS Omega 2018, 3, 3471–3481. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Z.; Cheng, P.G.; Abdulrahman, A.Y.; Teoh, T.C. The identification of active compounds in Ganoderma lucidum var. antler extract inhibiting dengue virus serine protease and its computational studies. J. Biomol. Struct. Dyn. 2020, 38, 4273–4288. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, X.; Yin, X.; Zhang, Q.; Wang, Y.; Guo, C.; Chen, Y. Ganoderma lucidum aqueous extract inducing PHGPx to inhibite membrane lipid hydroperoxides and regulate oxidative stress based on single-cell animal transcriptome. Sci. Rep. 2022, 12, 3139. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Deng, Z.; Zhang, L.L.; Pan, Y.; Fu, J.; Zou, L.; Bai, Z.F.; Xiao, X.H.; Sheng, F.Y. Therapeutic potential of the medicinal mushroom Ganoderma lucidum against Alzheimer’s disease. Biomed. Pharmacother. 2024, 172, 116222. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.; Yu, M.S.; Yuen, W.H.; So, K.F.; Zee, S.Y.; Chang, R.C. Antagonizing β-amyloid peptide neurotoxicity of the anti-aging fungus Ganoderma lucidum. Brain Res. 2008, 1190, 215–224. [Google Scholar] [CrossRef]
- Yu, N.; Huang, Y.; Jiang, Y.; Zou, L.; Liu, X.; Liu, S.; Chen, F.; Luo, J.; Zhu, Y. Ganoderma lucidum Triterpenoids (GLTs) Reduce Neuronal Apoptosis via Inhibition of ROCK Signal Pathway in APP/PS1 Transgenic Alzheimer’s Disease Mice. Oxid Med. Cell Longev. 2020, 2020, 9894037. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Meng, Y.H.; Wang, Z.W.; Wang, J.; Shi, D.F.; Liu, D. Ganoderic Acids A and B Reduce Okadaic Acid-Induced Neurotoxicity in PC12 Cells by Inhibiting Tau Hyperphosphorylation. Biomed. Environ. Sci. 2023, 36, 103–108. [Google Scholar] [PubMed]
- Zhao, H.L.; Cui, S.Y.; Qin, Y.; Liu, Y.T.; Cui, X.Y.; Hu, X.; Kurban, N.; Li, M.Y.; Li, Z.H.; Xu, J.; et al. Prophylactic effects of sporoderm-removed Ganoderma lucidum spores in a rat model of streptozotocin-induced sporadic Alzheimer’s disease. J. Ethnopharmacol. 2021, 269, 113725. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Abid, M.; Hasan, G.M.; Islam, A.; Hassan, M.I. Therapeutic targeting of microtubule affinity-regulating kinase 4 in cancer and neurodegenerative diseases. J. Cell Biochem. 2023, 124, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Singh, G.; Soni, H.; Tandon, S. Identification of potential neuroprotective compound from Ganoderma lucidum extract targeting microtubule affinity regulation kinase 4 involved in Alzheimer’s disease through molecular dynamics simulation and MMGBSA. Aging Med. 2023, 6, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Liu, S.; Liu, Y.; Li, P.; Xu, X. Ganoderic Acid A Promotes Amyloid-β Clearance (In Vitro) and Ameliorates Cognitive Deficiency in Alzheimer’s Disease (Mouse Model) through Autophagy Induced by Activating Axl. Int. J. Mol. Sci. 2021, 22, 5559. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, X.; Lv, H.; Shi, Y.; Xiao, L. PADI4 mediates autophagy and participates in the role of ganoderic acid A monomers in delaying the senescence of Alzheimer’s cells through the Akt/mTOR pathway. Biosci. Biotechnol. Biochem. 2021, 85, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Han, H.H.; Zang, D.D.; Zha, X.Q.; Zhou, A.; Zhang, F.Y.; Luo, J.P. Rapid Discovery of Aβ42 Fibril Disintegrators from Ganoderma lucidum via Ligand Fishing and Their Neuroprotective Effects on Alzheimer’s Disease. J. Agric. Food Chem. 2024, 72, 4127–4141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, S.; Cai, Y.; Zhou, M.; Zuo, X.; Chan, P. Ganoderma lucidum Protects Dopaminergic Neuron Degeneration through Inhibition of Microglial Activation. Evid Based Complement. Altern. Med. 2011, 2011, 156810. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, A.; Mendonca, P.; Soliman, K.F.A. Involvement of NFƙB and MAPK signaling pathways in the preventive effects of Ganoderma lucidum on the inflammation of BV-2 microglial cells induced by LPS. J. Neuroimmunol. 2020, 345, 577269. [Google Scholar] [CrossRef]
- Cai, Q.; Li, Y.; Pei, G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflamm. 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xiao, C.; Zheng, J.; Ye, C.; Dai, Z.; Wu, Q.; Liu, J.; Strappe, P.; Zhou, Z. Terpenoids of Ganoderma lucidum reverse cognitive impairment through attenuating neurodegeneration via suppression of PI3K/AKT/mTOR expression in vivo model. J. Funct. Foods 2020, 73, 104142. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, D.; Yin, H.; Li, H.; Du, J.; Bao, H. Ganoderic Acid A Attenuates LPS-Induced Neuroinflammation in BV2 Microglia by Activating Farnesoid X Receptor. Neurochem. Res. 2021, 46, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Yang, X.; Yang, X.; Xue, J.; Yang, Y. Ganoderic Acid A To Alleviate Neuroinflammation of Alzheimer’s Disease in Mice by Regulating the Imbalance of the Th17/Tregs Axis. J. Agric. Food Chem. 2021, 69, 14204–14214. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Zhang, L.; Wang, S.; Yang, L.; Li, P. Deacetyl Ganoderic Acid F Inhibits LPS-Induced Neural Inflammation via NF-κB Pathway Both In Vitro and In Vivo. Nutrients 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.W.; Gao, Y.Q.; Xia, B.; Wang, J.Y.; Liu, X.N.; Tang, J.J.; Yin, X.; Gao, J.M. Ganoderterpene A, a New Triterpenoid from Ganoderma lucidum, Attenuates LPS-Induced Inflammation and Apoptosis via Suppressing MAPK and TLR-4/NF-κB Pathways in BV-2 Cells. J. Agric. Food Chem. 2021, 69, 12730–12740. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chang, C.Y.; Li, J.R.; Wang, J.D.; Wu, C.C.; Kuan, Y.H.; Liao, S.L.; Wang, W.Y.; Chen, C.J. Anti-inflammatory and Neuroprotective Effects of Fungal Immunomodulatory Protein Involving Microglial Inhibition. Int. J. Mol. Sci. 2018, 19, 3678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Q.; Deng, W.; Li, Y.; Xing, G.; Shi, X.; Du, Y. Neuroprotective effect of pretreatment with Ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus. Neural Regen Res. 2014, 9, 1446–1452. [Google Scholar] [PubMed]
- Yang, H.S.; Choi, Y.J.; Jo, J.H.; Lee, S.C.; Kim, K.J.; Jin, S.W.; Park, T.Y.; Huh, C.K. Neuroprotective activities of fermented Ganoderma lucidum extracts by lactic acid bacteria against H2O2-stimulated oxidative stress in PC12 cells. Food Sci. Biotechnol. 2015, 24, 1413–1420. [Google Scholar] [CrossRef]
- Guo, S.S.; Cui, X.L.; Rausch, W.D. Ganoderma lucidum polysaccharides protect against MPP+ and rotenone-induced apoptosis in primary dopaminergic cell cultures through inhibiting oxidative stress. Am. J. Neurodegener. Dis. 2016, 5, 131–144. [Google Scholar] [PubMed]
- Li, Y.B.; Wang, J.W.; Liu, X.Q.; Guo, M.F.; Niu, X.J.; Meng, T.; Su, Q.; Wang, H.B.; Yang, L.Z.; Ma, C.G.; et al. Regulatory effect of Ganoderma lucidum polysaccharides on H2O2-induced apoptosis and mitochondrial dysfunction in SH-SY5Y cells. Chin. J. Tissue Eng. Res. 2024, 28, 4041–4047. [Google Scholar]
- Zhou, Y.; Qu, Z.Q.; Zeng, Y.S.; Lin, Y.K.; Li, Y.; Chung, P.; Wong, R.; Hägg, U. Neuroprotective effect of preadministration with Ganoderma lucidum spore on rat hippocampus. Exp. Toxicol. Pathol. 2012, 64, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, X.; Wu, S.; Wang, F.; Lu, M.; Wang, F.; Hu, R. Optimization of flavonoid compound extraction from Ganoderma lucidum and its antioxidant activity on PC12 cells exposed to H2O2. J. Food Meas. Charact. 2024, 18, 105–116. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Lou, P.; Dai, T.; Chen, Y.; Zhuge, A.; Yuan, Y.; Li, L. The Relationship between the Gut Microbiome and Neurodegenerative Diseases. Neurosci Bull. 2021, 37, 1510–1522. [Google Scholar] [CrossRef] [PubMed]
- Shadfar, S.; Parakh, S.; Jamali, M.S.; Atkin, J.D. Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Pizza, V.; Agresta, A.; D’Acunto, C.W.; Festa, M.; Capasso, A. Neuroinflamm-aging and neurodegenerative diseases: An overview. CNS Neurol Disord Drug Targets 2011, 10, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, Y.; Yuan, Z.; Zeng, Y.; Wang, S.; Fan, X.; Yang, D.; Yang, M. Iron Metabolism in Aging and Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 3612. [Google Scholar] [CrossRef]
- Kitchener, E.J.A.; Dundee, J.M.; Brown, G.C. Activated microglia release β-galactosidase that promotes inflammatory neurodegeneration. Front. Aging Neurosci. 2024, 15, 1327756. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, S.; Müller, L.; Wenger, E.; Düzel, S.; Pawelec, G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 2017, 75, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.F.; Zhao, Y.; Ruan, L.H.; Zhu, L.N.; Jin, K.L.; Zhuge, Q.C.; Su, D.M.; Zhao, Y. Impact of aging immune system on neurodegeneration and potential immunotherapies. Prog. Neurobiol. 2017, 157, 2–28. [Google Scholar] [CrossRef] [PubMed]
- Yanar, K.; Aydın, S.; Cakatay, U.; Mengi, M.; Buyukpınarbaşılı, N.; Atukeren, P.; Sitar, M.E.; Sönmez, A.; Uslu, E. Protein and DNA oxidation in different anatomic regions of rat brain in a mimetic ageing model. Basic Clin. Pharmacol. Toxicol. 2011, 109, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Qi, L.; Guo, Y.; Zhu, X.; Tang, X.; Yong, T.; Xie, Y.; Wu, Q.; Zhang, M.; Chen, D. Long-Term Administration of Triterpenoids From Ganoderma lucidum Mitigates Age-Associated Brain Physiological Decline via Regulating Sphingolipid Metabolism and Enhancing Autophagy in Mice. Front. Aging Neurosci. 2021, 13, 628860. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Xiang, L.; Matsuura, A.; Zhang, Y.; Huang, Q.; Qi, J. Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg. Med. Chem. 2010, 18, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Okoro, N.O.; Odiba, A.S.; Han, J.; Osadebe, P.O.; Omeje, E.O.; Liao, G.; Liu, Y.; Jin, C.; Fang, W.; Liu, H.; et al. Ganoderma lucidum methyl ganoderate E extends lifespan and modulates aging-related indicators in Caenorhabditis elegans. Food Funct. 2024, 15, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Akman, K.; Calimport, S.R.; Wuttke, D.; Stolzing, A.; de Magalhães, J.P. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res. 2012, 15, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Guo, Y.; Chen, D.; Tang, X.; Shuai, O.; Yong, T.; Wang, D.; Xiao, C.; Zhou, G.; Xie, Y.; et al. Alcohol Extracts From Ganoderma lucidum Delay the Progress of Alzheimer’s Disease by Regulating DNA Methylation in Rodents. Front. Pharmacol. 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Sulistio, Y.A.; Heese, K. Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy. Mol. Neurobiol. 2017, 54, 7401–7459. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Neurotrophin receptors in the pathogenesis, diagnosis and therapy of neurodegenerative diseases. Pharmacol. Res. 2017, 121, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.M.; Hui, W.S.; Chu, P.W.; Chiu, S.W.; Ip, N.Y. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000, 486, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ling-Sing Seow, S.; Naidu, M.; David, P.; Wong, K.H.; Sabaratnam, V. Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells. BMC Complement. Altern Med. 2013, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.A.; Cheung, M. Neural crest stem cells and their potential therapeutic applications. Dev. Biol. 2016, 419, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Mao, J.; Ding, K.; Zhou, Y.; Zeng, X.; Yang, W.; Wang, P.; Zhao, C.; Yao, J.; Xia, P.; et al. Polysaccharides from Ganoderma lucidum Promote Cognitive Function and Neural Progenitor Proliferation in Mouse Model of Alzheimer’s Disease. Stem. Cell Rep. 2017, 8, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Knott, A.B.; Bossy-Wetzel, E. Nitric oxide in health and disease of the nervous system. Antioxid. Redox Signal. 2009, 11, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.R.; Jia, W.H.; Liu, C.; Wang, H.Q.; Yang, H.G.; He, G.R.; Chen, R.Y.; Du, G.H. Ganoderic acid A protects neural cells against NO stress injury in vitro via stimulating β adrenergic receptors. Acta Pharmacol. Sin. 2020, 41, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Akıncıoğlu, H.; Gülçin, İ. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini. Rev. Med. Chem. 2020, 20, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ahn, B.; Choi, J.; Hattori, M.; Min, B.; Bae, K. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.; Wang, A.H.; Wei, Y.L.; Huo, X.K.; Tian, X.G.; Feng, L.; Ma, X.C.; Wang, C.; Huang, S.S.; Jia, J.M. Chemical characteristics of the fungus Ganoderma lucidum and their inhibitory effects on acetylcholinesterase. J. Asian Nat. Prod. Res. 2018, 20, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, Z.-L.; Cheng, Y.-X.; Dayaolingzhiols, A.-E. AchE inhibitory meroterpenoids from Ganoderma lucidum. Tetrahedron 2019, 75, 2910–2915. [Google Scholar] [CrossRef]
- Hasnat, A.; Pervin, M.; Lim, B.O. Acetylcholinesterase inhibition and in vitro and in vivo antioxidant activities of Ganoderma lucidum grown on germinated brown rice. Molecules 2013, 18, 6663–6678. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef]
- Kuypers, K.P.C. Self-Medication with Ganoderma lucidum (“Reishi”) to Combat Parkinson’s Disease Symptoms: A Single Case Study. J. Med. Food. 2021, 24, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Gao, Y.; Chen, G.; Gao, H.; Dai, X.; Ye, J.; Chan, E.; Huang, M.; Zhou, S. A randomized, double-blind and placebo-controlled study of a Ganoderma lucidum polysaccharide extract in neurasthenia. J. Med. Food. 2005, 8, 53–58. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, W.; Yang, X.; Duan, Q.; Li, J.; Zhao, Y.; Yu, C.; He, T.; Sun, T.; Zhao, Y.; Wang, W. The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks. Molecules 2024, 29, 2516. https://doi.org/10.3390/molecules29112516
Lian W, Yang X, Duan Q, Li J, Zhao Y, Yu C, He T, Sun T, Zhao Y, Wang W. The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks. Molecules. 2024; 29(11):2516. https://doi.org/10.3390/molecules29112516
Chicago/Turabian StyleLian, Wenhui, Xu Yang, Qidong Duan, Jie Li, Yuting Zhao, Chunhui Yu, Tianzhu He, Tianxia Sun, Yu Zhao, and Weinan Wang. 2024. "The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks" Molecules 29, no. 11: 2516. https://doi.org/10.3390/molecules29112516
APA StyleLian, W., Yang, X., Duan, Q., Li, J., Zhao, Y., Yu, C., He, T., Sun, T., Zhao, Y., & Wang, W. (2024). The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks. Molecules, 29(11), 2516. https://doi.org/10.3390/molecules29112516






