Development of a Convenient and Quantitative Method for Evaluating Photosensitizing Activity Using Thiazolyl Blue Formazan Dye
Abstract
1. Introduction
2. Results and Discussion
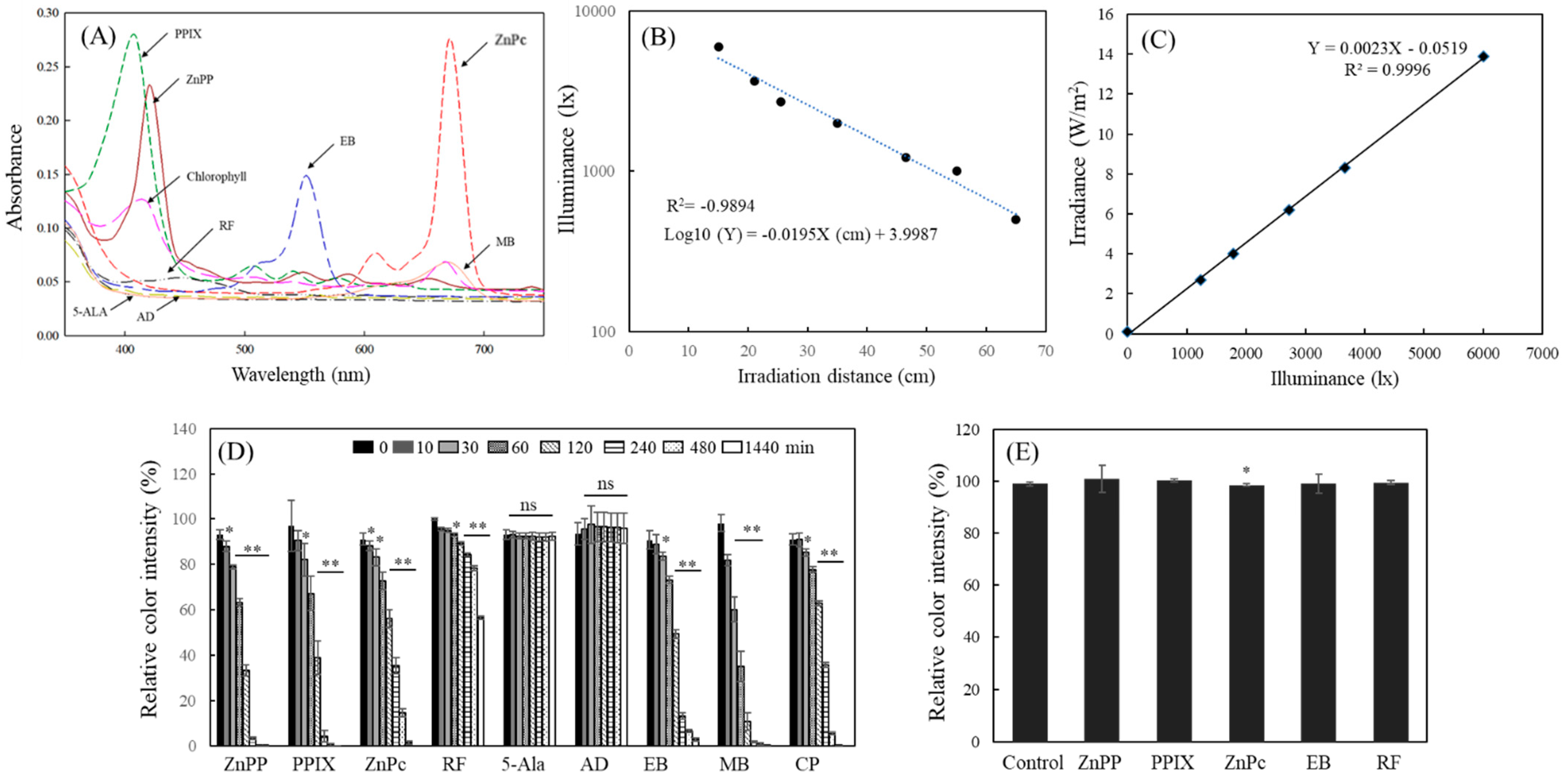
2.1. Decolorization of MTT-F by Photosensitizing Agents under Light
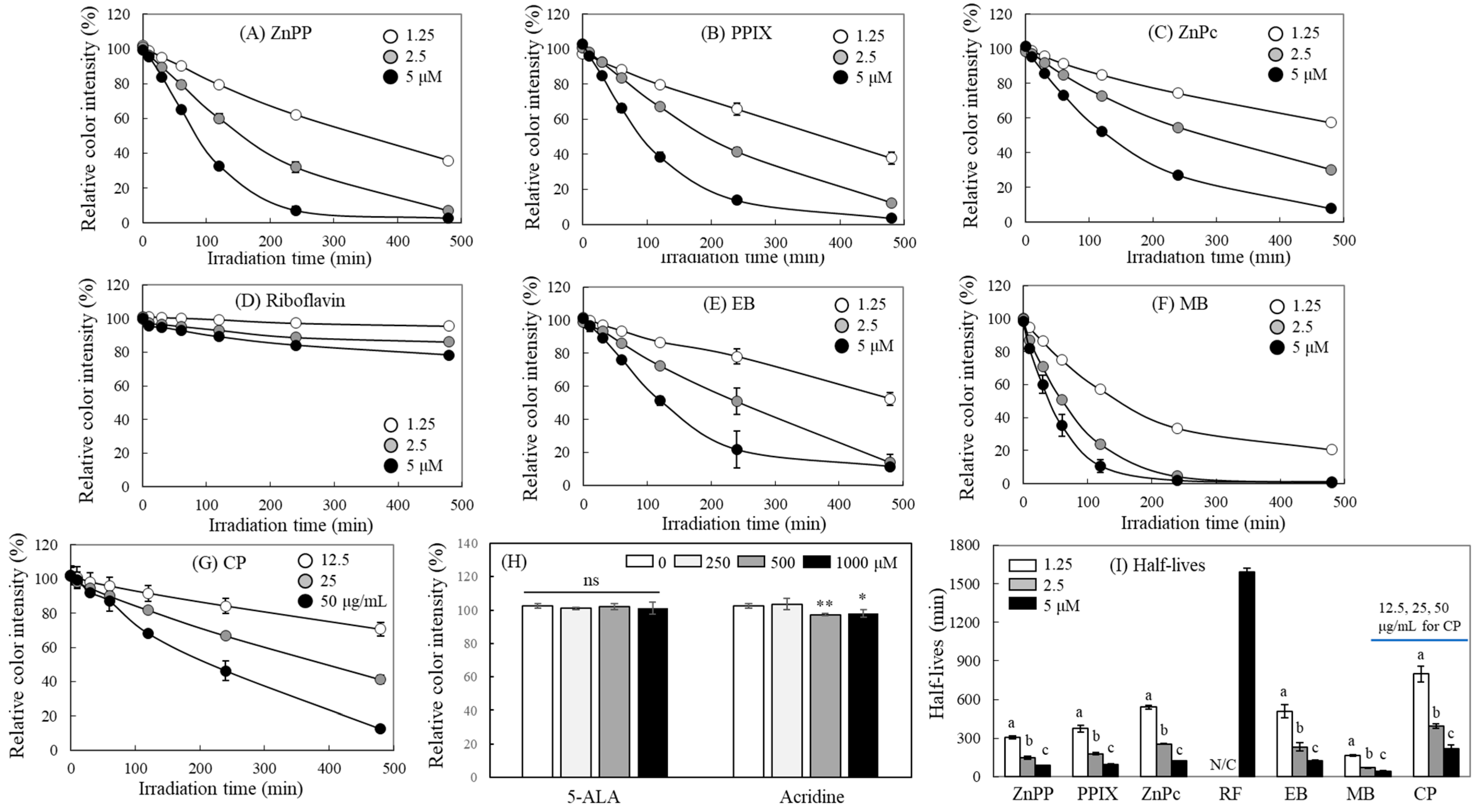
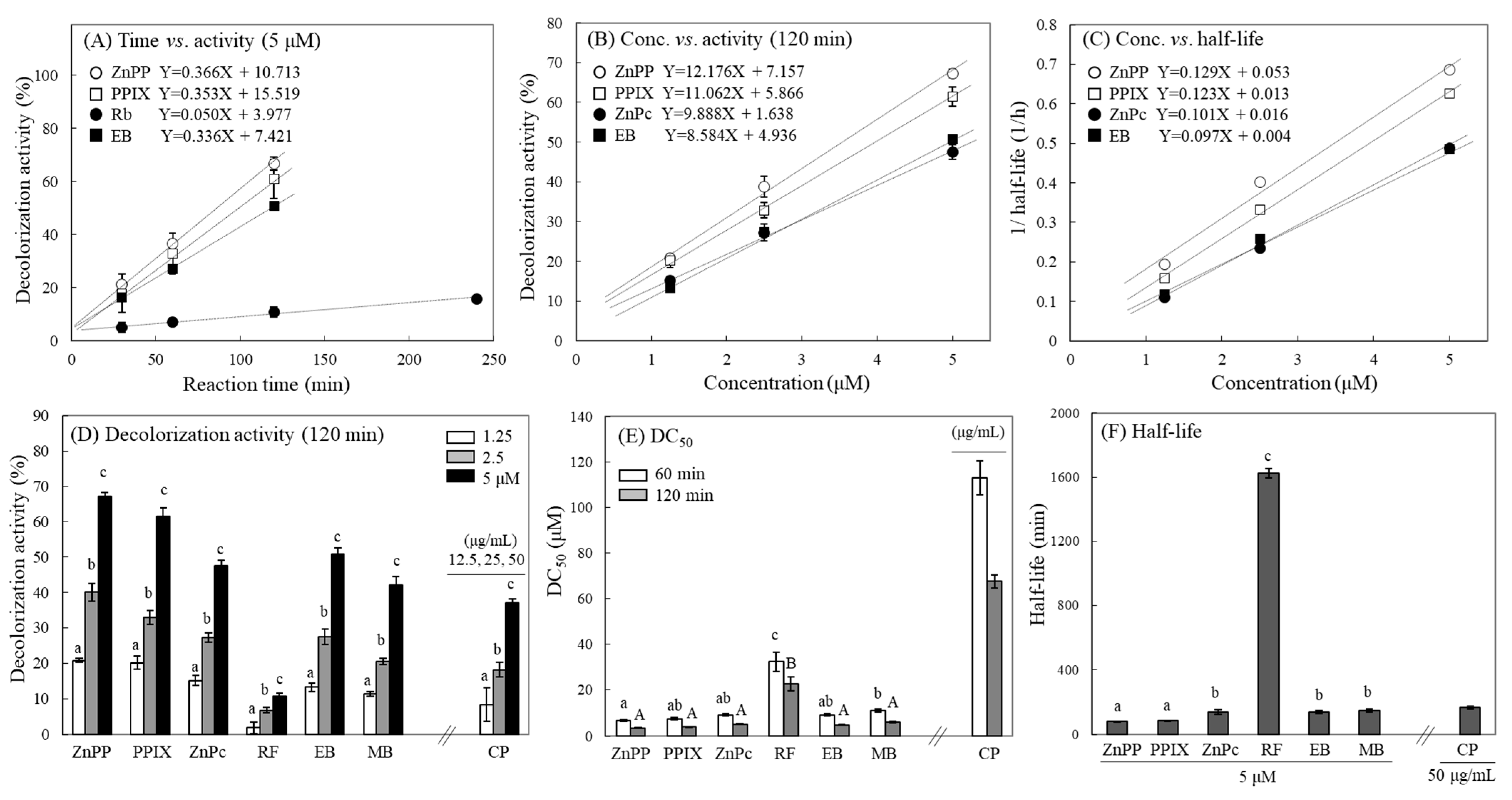
2.2. Concentration-Dependent Effects of Photosensitizers on MTT-F Decolorization
2.3. Illuminance-Dependent Effect on MTT-F Decolorization by Photosensitizing Agents
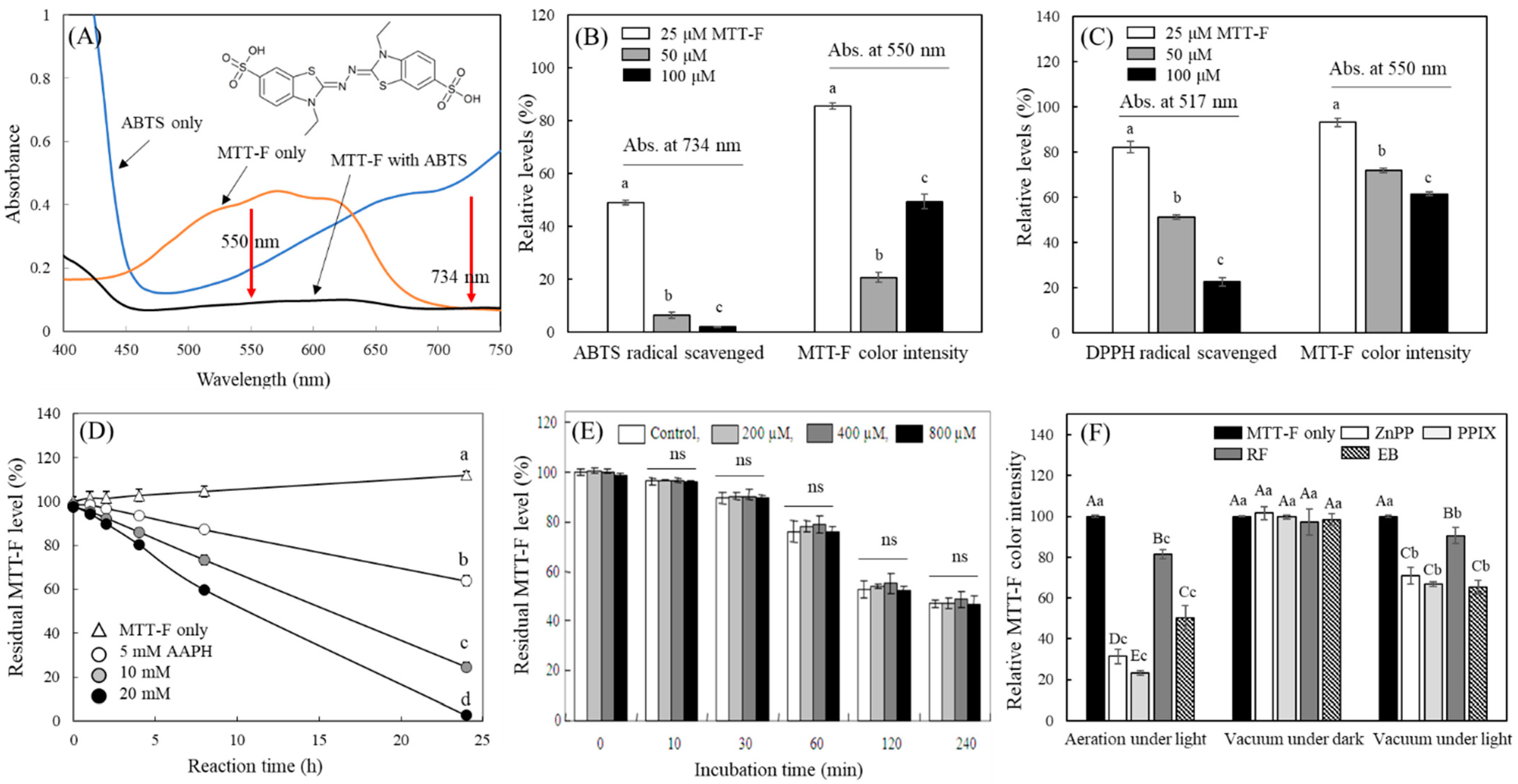
2.4. Decolorization Pattern of MTT-F under Various Conditions
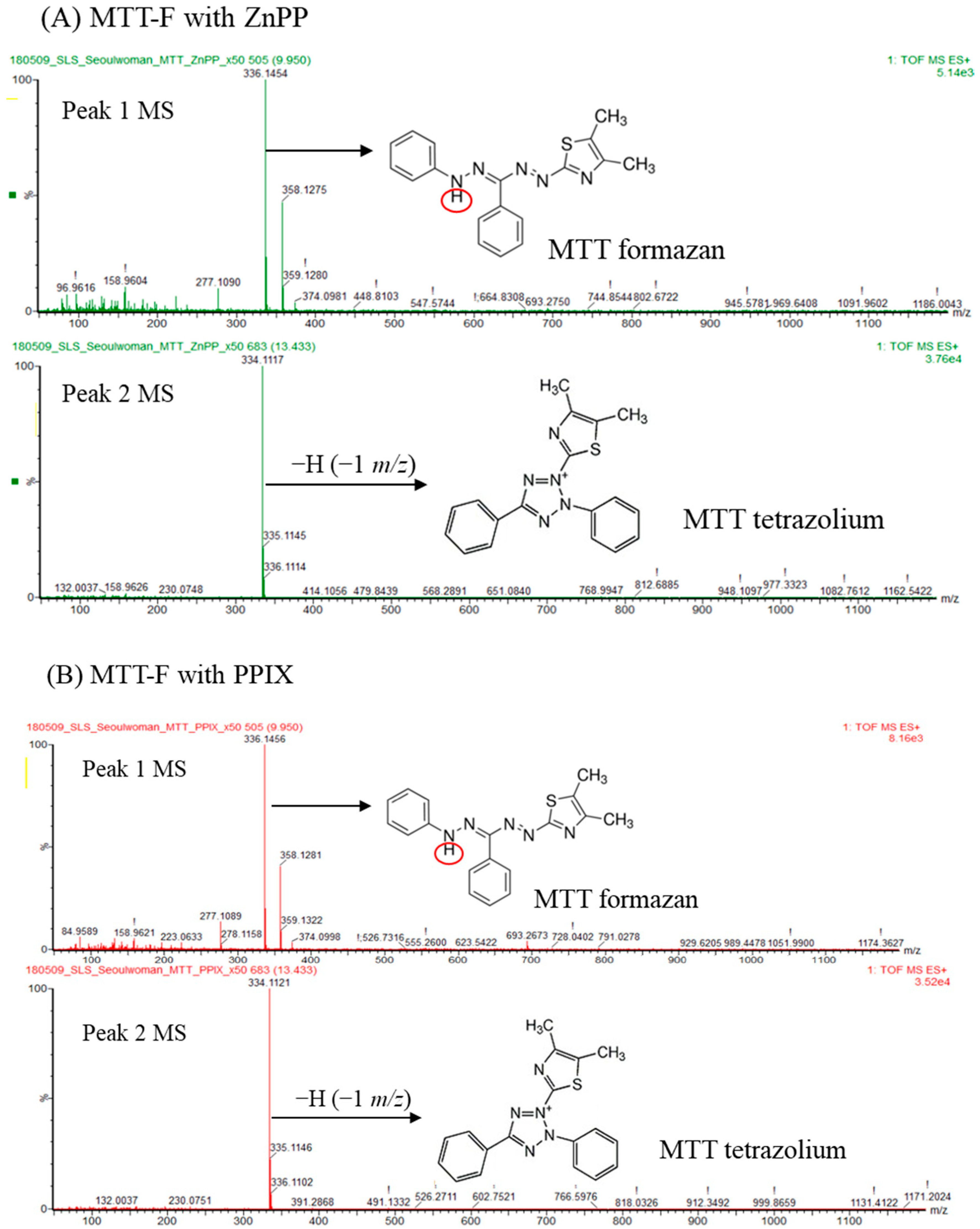
2.5. Determination of MTT-F Product Decolorized by Photosensitizers
2.6. Quantification of Photosensitizing Activity Using MTT-F
3. Materials and Methods
3.1. Experimental Materials
3.2. Fluorescent Lamp Lighting System
3.3. Analysis of MTT-F Decolorization by Photosensitizers under Light Irradiation
3.4. Characterization of MTT-F Decolorization by Different Radicals and in a Vacuum Condition
3.5. Evaluation of MTT-F Decolorization by Photosensitizers in a Vacuum Condition
3.6. Analysis of MTT-F Decolorization Product Using LC-MS/MS and Its Reduction by Cells
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nineham, A.W. The chemistry of formazans and tetrazolium salts. Chem. Rev. 1955, 55, 355–483. [Google Scholar] [CrossRef]
- Rajeena, C.K.; Sumayya, P.C.; Muraleedharan, K. Structure and non-covalent interactions of (E, Z) 3-benzoyl-1, 5-bis (4-ethoxyphenyl) formazan: A crystallographic and DFT/TD-DFT study. J. Mol. Struct. 2022, 1266, 133501. [Google Scholar] [CrossRef]
- Morgan, D.M.L. Tetrazolium (MTT) assay for cellular viability and activity. In Polyamine Protocols, Methods in Molecular Biology™; Humana Press: London, UK, 1998; Volume 79, pp. 179–184. [Google Scholar] [CrossRef]
- Twentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, P.; Kumar, S.; Alex, S.A.; Kumar, R.; Mehta, S.K.; Mukherjee, A.; Umar, A. A comparative multi-assay approach to study the toxicity behaviour of Eu2O3 nanoparticles. J. Mol. Liq. 2018, 269, 783–795. [Google Scholar] [CrossRef]
- Oh, Y.J.; Hong, J. Application of the MTT-based colorimetric method for evaluating bacterial growth using different solvent systems. Lwt 2022, 153, 112565. [Google Scholar] [CrossRef]
- Petty, R.D.; Sutherland, L.A.; Hunter, E.M.; Cree, I.A. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J. Biolumin. Chemilumin. 1995, 10, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Benov, L. Improved formazan dissolution for bacterial MTT assay. Microbiol. Spectr. 2021, 9, e01637-21. [Google Scholar] [CrossRef] [PubMed]
- Sladowski, D.; Steer, S.J.; Clothier, R.H.; Balls, M. An improved MIT assay. J. Immunol. Methods 1993, 157, 203–207. [Google Scholar] [CrossRef]
- Choi, B.H.; Kim, M.R.; Jung, Y.N.; Kang, S.; Hong, J. Interfering with Color Response by Porphyrin-Related Compounds in the MTT Tetrazolium-Based Colorimetric Assay. Int. J. Mol. Sci. 2023, 24, 562. [Google Scholar] [CrossRef]
- Holder, A.L.; Goth-Goldstein, R.; Lucas, D.; Koshland, C.P. Particle-induced artifacts in the MTT and LDH viability assays. Chem. Res. Toxicol. 2012, 25, 1885–1892. [Google Scholar] [CrossRef]
- Luis, C.; Castaño-Guerrero, Y.; Soares, R.; Sales, G.; Fernandes, R. Avoiding the interference of doxorubicin with MTT measurements on the MCF-7 breast cancer cell line. Methods Protoc. 2019, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Park, K.A.; Choi, H.A.; Kim, M.R.; Choi, Y.M.; Kim, H.J.; Hong, J.I. Changes in color response of MTT formazan by zinc protoporphyrin. Korean J. Food Sci. Technol. 2011, 43, 754–759. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [PubMed]
- Ochsner, M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B Biol. 1997, 39, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sharman, W.M.; Allen, C.M.; Van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Namiki, M. Antioxidants/antimutagens in food. Crit. Rev. Food Sci. Nutr. 1990, 29, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Alloy, M.M.; Finch, B.E.; Ward, C.P.; Redman, A.D.; Bejarano, A.C.; Barron, M.G. Recommendations for Advancing Test Protocols Examining the Photo-induced Toxicity of Petroleum and Polycyclic Aromatic Compounds. Aquat. Toxicol. 2023, 256, 106390. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Shiraishi, Y.; Ogawa, K.; Komasawa, I. Effect of photosensitizer and hydrogen peroxide on desulfurization of light oil by photochemical reaction and liquid− liquid extraction. Ind. Eng. Chem. Res. 1997, 36, 530–533. [Google Scholar] [CrossRef]
- Hall, N.K.; Chapman, T.M.; Kim, H.J.; Min, D.B. Antioxidant mechanisms of Trolox and ascorbic acid on the oxidation of riboflavin in milk under light. Food Chem. 2010, 118, 534–539. [Google Scholar] [CrossRef]
- Scotter, M.J.; Castle, L. Chemical interactions between additives in foodstuffs: A review. Food Addit. Contam. 2004, 21, 93–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, H.; Shang, M.; Sun, D.; Liu, C.; Che, G. Recent analytical methodologies and analytical trends for riboflavin (vitamin B2) analysis in food, biological and pharmaceutical samples. Trends. Anal. Chem. 2021, 143, 116412. [Google Scholar] [CrossRef]
- Passaretti, M.G.; Ninago, M.D.; Villar, M.A.; López, O.V. Protective packaging for light-sensitive foods. In Food Packaging; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–31. [Google Scholar]
- Verduin, J.; Den Uijl, M.J.; Peters, R.J.B.; Van Bommel, M.R. Photodegradation products and their analysis in food. J. Food Sci. Nutr. 2020, 6, 24966. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, S.; Partoazar, A.; Chiniforush, N.; Goudarzi, R. The potential application of natural photosensitizers used in antimicrobial photodynamic therapy against oral infections. Pharmaceuticals 2022, 15, 767. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Abou Saoud, W.; Kane, A.; Le Cann, P.; Gérard, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Innovative photocatalytic reactor for the degradation of VOCs and microorganism under simulated indoor air conditions: Cu-Ag/TiO2-based optical fibers at a pilot scale. Chem. Eng. J. 2021, 411, 128622. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.; Ye, Y.S.; Kim, T.I. Materials and device design for advanced phototherapy systems. Adv. Drug Deliv. Rev. 2022, 186, 114339. [Google Scholar] [CrossRef] [PubMed]
- Pushpan, S.K.; Venkatraman, S.; Anand, V.G.; Sankar, J.; Parmeswaran, D.; Ganesan, S.; Chandrashekar, T.K. Porphyrins in photodynamic therapy-a search for ideal photosensitizers. Curr. Med. Chem. Anticancer Agents 2002, 2, 187–207. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Zhang, B.; Kang, H.; Du, L.; Li, M. Nanostructures of an amphiphilic zinc phthalocyanine polymer conjugate for photodynamic therapy of psoriasis. Colloids Surf. B Biointerfaces 2015, 128, 405–409. [Google Scholar] [CrossRef]
- Minnock, A.; Vernon, D.I.; Schofield, J.; Griffiths, J.; Parish, J.H.; Brown, S.B. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J. Photochem. Photobiol. B 1996, 32, 159–164. [Google Scholar] [CrossRef]
- Konings, E.J. Committee on food nutrition: Water-soluble vitamins. J. AOAC Int. 2006, 89, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Labbé, R.F.; Vreman, H.J.; Stevenson, D.K. Zinc protoporphyrin: A metabolite with a mission. Clin. Chem. 1999, 45, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Buchczyk, D.P.; Klotz, L.O.; Lang, K.; Fritsch, C.; Sies, H. High efficiency of 5-aminolevulinate-photodynamic treatment using UVA irradiation. Carcinogenesis 2001, 22, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2′, 7′-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Papastergiadis, A.; Mubiru, E.; Van Langenhove, H.; De Meulenaer, B. Malondialdehyde measurement in oxidized foods: Evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J. Agric. Food Chem. 2012, 60, 9589–9594. [Google Scholar] [CrossRef]
- Winterbourn, C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013, 528, 3–25. [Google Scholar] [CrossRef]


| ZnPP | PPIX | ZnPc | RF | EB | MB | CP | ||
|---|---|---|---|---|---|---|---|---|
| Range | 2000 lx | 0–5 μM 0–240 min | 0–5 μM 0–240 min | 0–5 μM 0–240 min | 0–10 μM 0–480 min | 0–5 μM 0–240 min | 0–5 μM 0–240 min | 0–50 μg/mL 0–240 min |
| I. Irradiation time vs. Activity | ||||||||
| R2 | 0.9818 | 0.9906 | 0.9768 | 0.9601 | 0.9952 | 0.9994 | 0.9921 | |
| II. Concentration vs. Activity (120 min) | ||||||||
| R2 | 0.9939 | 0.9963 | 0.9955 | 0.9846 | 0.9989 | 0.9996 | 0.9997 | |
| III. Concentration vs. Half-life | ||||||||
| Linear scale | R2 | −0.9046 | −0.9161 | −0.9224 | −0.8973 | −0.9063 | −0.9468 | −0.9151 |
| Log scale | R2 | −0.9689 | −0.9754 | −0.9788 | −0.9646 | −0.9699 | −0.9905 | −0.9046 |
| Reciprocal | R2 | 0.9948 | 0.9992 | 1.0000 | 0.9756 | 0.9985 | 1.0000 | 0.9980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Oh, Y.J.; Kim, M.-R.; Jung, Y.N.; Song, E.; Lee, H.; Hong, J. Development of a Convenient and Quantitative Method for Evaluating Photosensitizing Activity Using Thiazolyl Blue Formazan Dye. Molecules 2024, 29, 2471. https://doi.org/10.3390/molecules29112471
Kang S, Oh YJ, Kim M-R, Jung YN, Song E, Lee H, Hong J. Development of a Convenient and Quantitative Method for Evaluating Photosensitizing Activity Using Thiazolyl Blue Formazan Dye. Molecules. 2024; 29(11):2471. https://doi.org/10.3390/molecules29112471
Chicago/Turabian StyleKang, Smee, Yeong Ji Oh, Mi-Ri Kim, Yu Na Jung, Eiseul Song, Hyowon Lee, and Jungil Hong. 2024. "Development of a Convenient and Quantitative Method for Evaluating Photosensitizing Activity Using Thiazolyl Blue Formazan Dye" Molecules 29, no. 11: 2471. https://doi.org/10.3390/molecules29112471
APA StyleKang, S., Oh, Y. J., Kim, M.-R., Jung, Y. N., Song, E., Lee, H., & Hong, J. (2024). Development of a Convenient and Quantitative Method for Evaluating Photosensitizing Activity Using Thiazolyl Blue Formazan Dye. Molecules, 29(11), 2471. https://doi.org/10.3390/molecules29112471







