Cyanoacetohydrazide as a Novel Derivatization Agent for the Determination of UHPLC-HRMS Steroids in Urine
Abstract
1. Introduction
2. Results
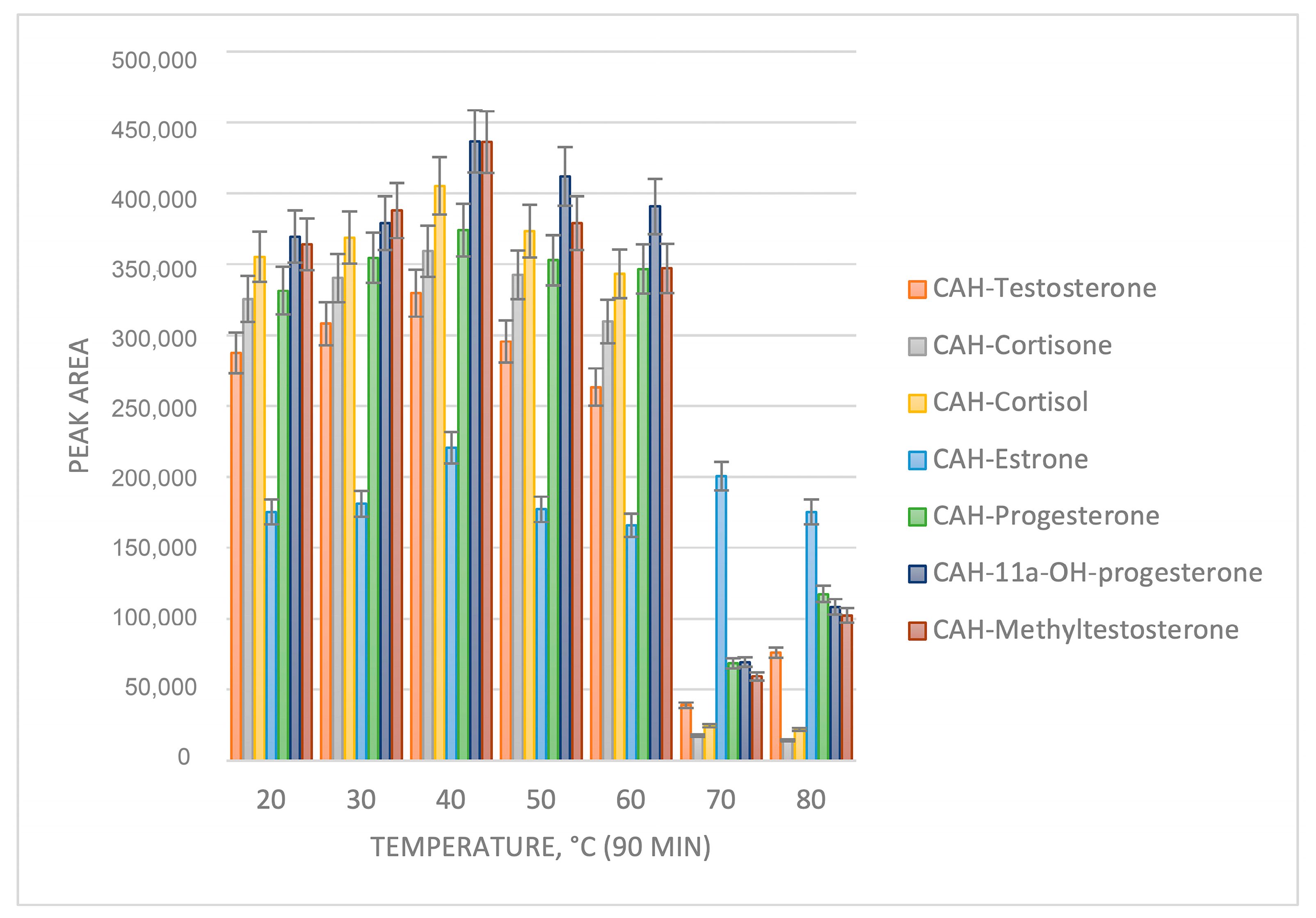
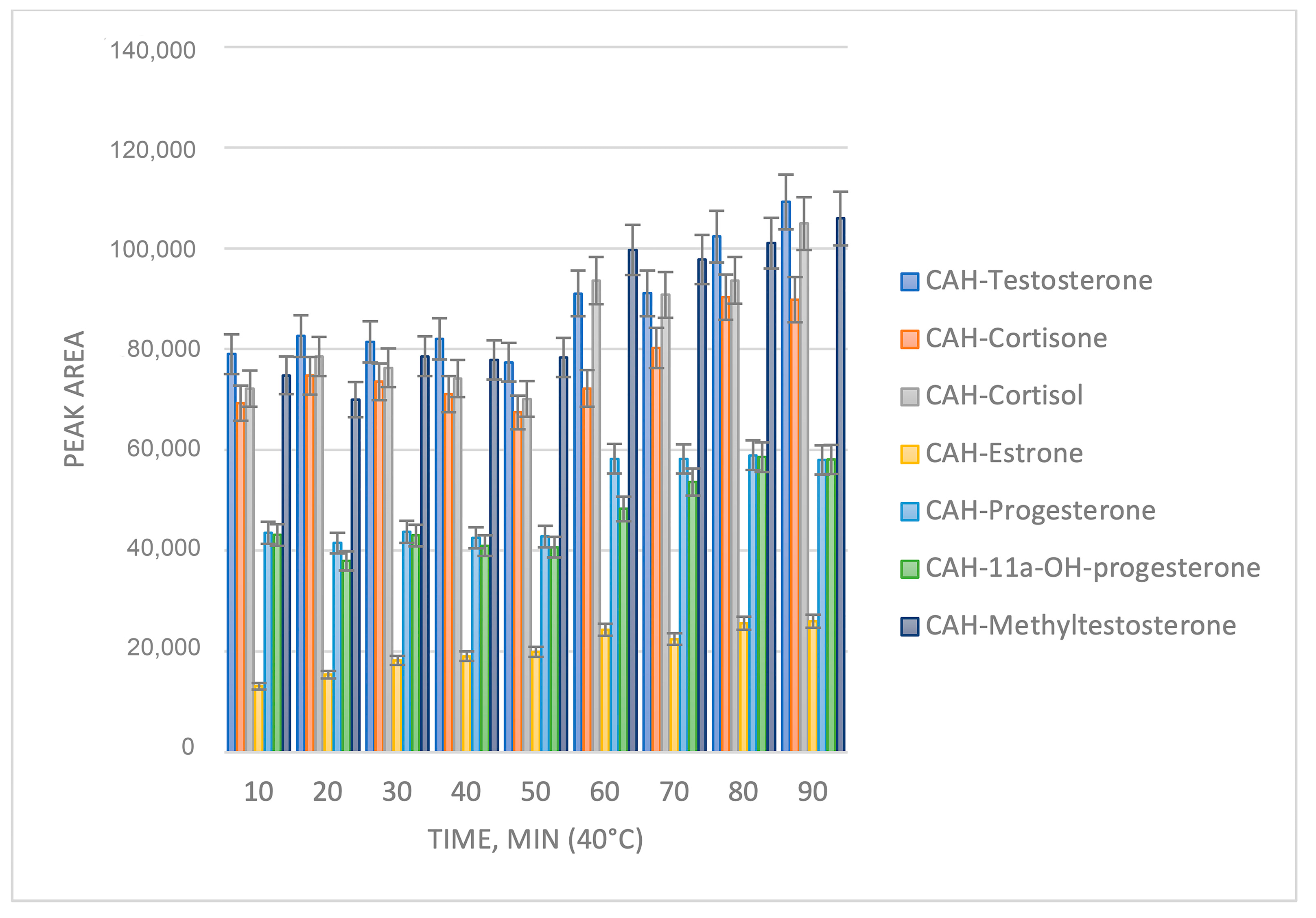
2.1. Sample Preparation Optimization
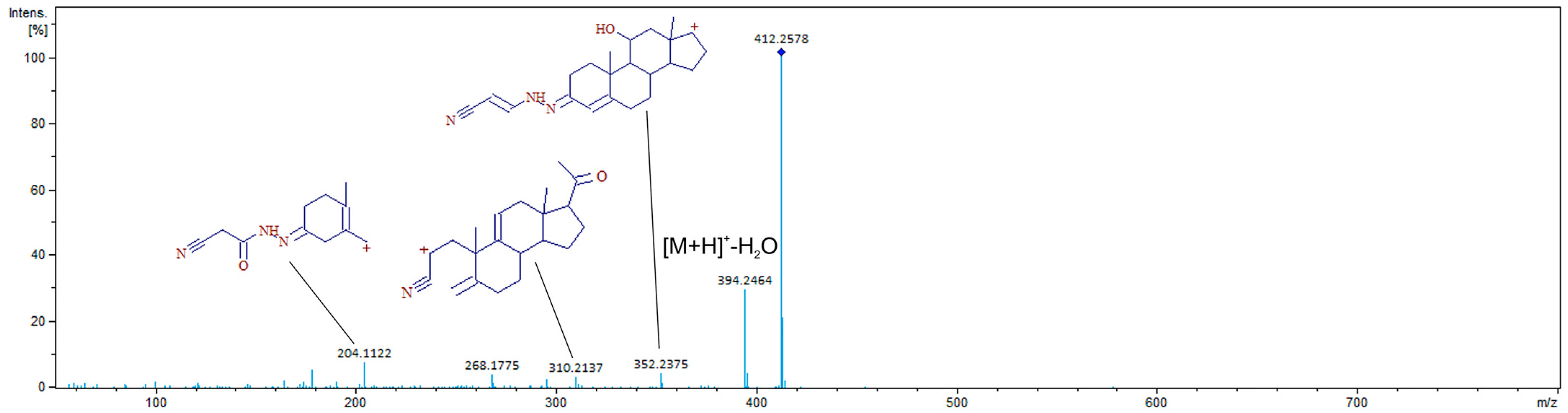
2.2. Chromatographic Separation and MS Detection
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Instrumentation
4.3. Urine Sample Preparation
4.4. Preparation of Standard and Stock Solutions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Son, H.H.; Yun, W.S.; Cho, S.H. Development and validation of an LC-MS/MS method for profiling 39 urinary steroids (estrogens, androgens, corticoids, and progestins). Biomed. Chromatogr. 2020, 34, e4723. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hartmann, M.F.; Wudy, S.A. Targeted LC–MS/MS analysis of steroid glucuronides in human urine. J. Steroid Biochem. Mol. Biol. 2021, 205, 105774. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.G.; Mackenzie, P.I.; Chowdhury, J.R.; Guillemette, C.; Gregory, P.A.; Ishii, Y.; Hansen, A.J.; Kessler, F.K.; Kim, P.M.; Chowdhury, N.R.; et al. Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab. Dispos. 2004, 32, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Kalogera, E.; Pistos, C.; Provatopoulou, X.; Athanaselis, S.; Spiliopoulou, C.; Gounaris, A. Androgen glucuronides analysis by liquid chromatography tandem-mass spectrometry: Could it raise new perspectives in the diagnostic field of hormone-dependent malignancies? J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 940, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.T.; Xu, X.; Keefer, L.; Veenstra, T.D.; Zieger, R.G. A Liquid Chromatography-Mass Spectrometry Method for the Simultaneous Measurement of Fifteen Urinary Estrogens and Estrogen Metabolites: Assay Reproducibility and Inter-individual Variability. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.W.; Gilligan, L.C.; Idkowiak, J.; Arlt, W.; Foster, P.A. The regulation of steroid action by sulfation and desulfation. Endocr. Rev. 2015, 36, 526–563. [Google Scholar] [CrossRef]
- Labrie, F.; Bélanger, A.; Bélanger, P.; Bérubé, R.; Martel, C.; Cusan, L.; Gomez, J.; Candas, B.; Castiel, I.; Chaussade, V.; et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J. Steroid Biochem. Mol. Biol. 2006, 99, 182–188. [Google Scholar] [CrossRef]
- Storbeck, K.H.; Schiffer, L.; Baranowski, E.S.; Chortis, V.; Prete, A.; Barnard, L.; Gilligan, L.C.; Taylor, A.E.; Idkowiak, J.; Arlt, W.; et al. Steroid Metabolome Analysis in Disorders of Adrenal Steroid Biosynthesis and Metabolism. Endocr. Rev. 2019, 40, 1605–1625. [Google Scholar] [CrossRef]
- Olesti, E.; Boccard, J.; Visconti, G.; González-Ruiz, V.; Rudaz, S. From a single steroid to the steroidome: Trends and analytical challenges. J. Steroid Biochem. Mol. Biol. 2021, 206, 105797. [Google Scholar] [CrossRef]
- Emond, J.P.; Lacombe, L.; Caron, P.; Turcotte, V.; Simonyan, D.; Aprikian, A.; Saad, F.; Carmel, M.; Chevalier, S.; Guillemette, C.; et al. Urinary oestrogensteroidome as an indicator of the risk of localised prostate cancer progression. Br. J. Cancer 2021, 125, 78–84. [Google Scholar] [CrossRef]
- Girel, S.; Markin, P.A.; Tobolkina, E.; Boccard, J.; Moskaleva, N.E.; Rudaz, S.; Appolonova, S.A. Comprehensive plasma steroidomics reveals subtle alterations of systemic steroid profile in patients at different stages of prostate cancer disease. Sci. Rep. 2024, 14, 1577. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, C.; Pozo, O.J.; Marcos, J. GC/MS in recent years has defined the normal and clinically disordered steroidome: Will it soon be surpassed by LC/Tandem MS in This Role? J. Endocr. Soc. 2018, 2, 974–996. [Google Scholar] [CrossRef] [PubMed]
- Temerdashev, A.; Nesterenko, P.; Dmitrieva, E.; Zhurkina, K.; Feng, Y.Q. GC-MS/MS Determination of Steroid Hormones in Urine Using Solid-Phase Derivatization as an Alternative to Conventional Methods. Molecules 2022, 27, 5796. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Jacobsen, N.W.; Nielsen, F.K.; Björklund, E.; Styrishave, B.; Halling-Sørensen, B. Determination of steroid hormones in blood by GC-MS/MS. Anal. Bioanal. Chem. 2011, 400, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, K.-M. Bond-Dissociation Energies of Cations—Pushing the Limits to Quantum State Resolution. Mass Spectrom. Rev. 2011, 30, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Temerdashev, A.; Dmitrieva, E.; Podolskiy, I. Analytics for steroid hormone profiling in body fluids. Microchem. J. 2021, 168, 106395. [Google Scholar] [CrossRef]
- Matysik, S.; Liebisch, G. Quantification of steroid hormones in human serum by liquid chromatography-high resolution tandem mass spectrometry. J. Chromatogr. A 2017, 1526, 112–118. [Google Scholar] [CrossRef]
- Liere, P.; Schumacher, M. Mass spectrometric analysis of steroids: All that glitters is not gold. Expert Rev. Endocrinol. Metab. 2015, 10, 463–465. [Google Scholar] [CrossRef]
- Ney, L.J.; Felmingham, K.L.; Bruno, R.; Matthews, A.; Nichols, D.S. Chloroform-based liquid-liquid extraction and LC–MS/MS quantification of endocannabinoids, cortisol and progesterone in human hair. J. Pharm. Biomed. Anal. 2021, 201, 114103. [Google Scholar] [CrossRef] [PubMed]
- Domenech-Coca, C.; Mariné-Casadó, R.; Caimari, A.; Arola, L.; del Bas, J.M.; Bladé, C.; Rodriguez-Naranjo, M.I. Dual liquid-liquid extraction followed by LC-MS/MS method for the simultaneous quantification of melatonin, cortisol, triiodothyronine, thyroxine and testosterone levels in serum: Applications to a photoperiod study in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1108, 11–16. [Google Scholar] [CrossRef]
- Keevil, B.G. Novel liquid chromatography tandem mass spectrometry (LC-MS/MS) methods for measuring steroids. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, E.V.; Temerdashev, A.Z.; Zorina, M.O.; Feng, Y.Q.; Nesterenko, P.N. Solid-phase analytical derivatization as a tool for the quantification of steroid hormones in human urine with HPLC-Q-ToF detection. J. Pharm. Biomed. Anal. 2022, 214, 114736. [Google Scholar] [CrossRef]
- Dévier, M.H.; Labadie, P.; Togola, A.; Budzinski, H. Simple methodology coupling microwave-assisted extraction to SPE/GC/MS for the analysis of natural steroids in biological tissues: Application to the monitoring of endogenous steroids in marine mussels Mytilus sp. Anal. Chim. Acta 2010, 657, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Márta, Z.; Bobály, B.; Fekete, J.; Magda, B.; Imre, T.; Mészáros, K.V.; Bálint, M.; Szabó, P.T. Simultaneous determination of thirteen different steroid hormones using micro UHPLC-MS/MS with on-line SPE system. J. Pharm. Biomed. Anal. 2018, 150, 258–267. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. Deep eutectic solvent as a novel disperser in dispersive liquid-liquid microextraction based on solidification of floating organic droplet (DLLME-SFOD) for preconcentration of steroids in water samples: Assessment of the method deleterious impact on the environment using Analytical Eco-Scale and Green Analytical Procedure Index. Microchem. J. 2019, 149, 103988. [Google Scholar]
- Dmitrieva, E.V.; Temerdashev, A.Z.; Osipova, A.K. Determination of Ketosteroids in Human Urine Using Dispersive Liquid-Liquid Microextraction and Ultra High-Performance Liquid Chromatography-High Resolution Mass Spectrometry. J. Anal. Chem. 2021, 76, 1305–1311. [Google Scholar] [CrossRef]
- Srivastava, A.; Godbole, M.M.; Shrivastava, A. Estimation of Steroid Hormones in Biological Samples Using Micro Extraction and Advanced Chromatography Techniques. Austin J. Anal. Pharm. Chem. 2022, 9, 1150. [Google Scholar] [CrossRef]
- Olesti, E.; Garcia, A.; Rahban, R.; Rossier, M.F.; Boccard, J.; Nef, S.; González-Ruiz, V.; Rudaz, S. Steroid profile analysis by LC-HRMS in human seminal fluid. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1136, 121929. [Google Scholar] [CrossRef]
- Elmongy, H.; Masquelier, M.; Ericsson, M. Development and validation of a UHPLC-HRMS method for the simultaneous determination of the endogenous anabolic androgenic steroids in human serum. J. Chromatogr. A 2020, 1613, 460686. [Google Scholar] [CrossRef]
- Davis, D.E.; Leaptrot, K.L.; Koomen, D.C.; May, J.C.; Cavalcanti, G.D.A.; Padilha, M.C.; Pereira, H.M.G.; McLean, J.A. Multidimensional Separations of Intact Phase II Steroid Metabolites Utilizing LC-Ion Mobility-HRMS. Anal. Chem. 2021, 93, 10990–10998. [Google Scholar] [CrossRef]
- Fabresse, N.; Grassin-Delyle, S.; Etting, I.; Alvarez, J.C. Detection and quantification of 12 anabolic steroids and analogs in human whole blood and 20 in hair using LC-HRMS/MS: Application to real cases. Int. J. Legal Med. 2017, 131, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jia, P.; Cai, J.; Gu, L.; Yan, H.; Zhao, H.; Qin, S. Simultaneous quantitative analysis of seven steroid hormones in human saliva: A novel method based on O-ethylhydroxylamine hydrochloride as derivatization reagent. Rapid Commun. Mass Spectrom. 2022, 36, e9242. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Gao, H.; Xie, W.; Sun, Q.; Liang, K.; Li, Y. Quantitative MALDI-MS assay of steroid hormones in plasma based on hydroxylamine derivatization. Anal. Biochem. 2021, 616, 114089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chi, Q.; Fan, R.T.; Tian, H.D.; Wang, X. Quantitative-Profiling Method of Serum Steroid Hormones by Hydroxylamine-Derivatization HPLC–MS. Nat. Prod. Bioprospect. 2019, 9, 201–208. [Google Scholar] [CrossRef]
- Faqehi, A.M.; Denham, S.G.; Naredo, G.; Cobice, D.F.; Khan, S.; Simpson, J.P.; Sabil, G.; Upreti, R.; Gibb, F.; Homer, N.Z.; et al. Derivatization with 2-hydrazino-1-methylpyridine enhances sensitivity of analysis of 5α-dihydrotestosterone in human plasma by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2021, 1640, 461933. [Google Scholar] [CrossRef]


| Compound | Retention Time, min | [M + H]+ Theoretical, m/z | [M + H]+ Observed, m/z | Mass Error, ppm |
|---|---|---|---|---|
| CAH-testosterone peak 1 | 7.9 | 370.2489 | 370.2486 | 0.81 |
| CAH-testosterone peak 2 | 8.3 | |||
| CAH-cortisone peak 1 | 6.4 | 442.2336 | 442.2339 | −0.68 |
| CAH-cortisone peak 2 | 6.7 | |||
| CAH-cortisol peak 1 | 6.2 | 444.2493 | 444.2492 | 0.23 |
| CAH-cortisol peak 2 | 6.6 | |||
| CAH-estrone | 7.9 | 352.2020 | 352.2016 | 1.14 |
| CAH-progesterone peak 1 | 9.5 | 396.2656 | 396.2657 | −0.25 |
| CAH-progesterone peak 2 | 9.7 | |||
| CAH-11α-hydroxyprogesterone peak 1 | 7.4 | 412.2595 | 412.2599 | −0.97 |
| CAH-11α-hydroxyprogesterone peak 2 | 8.0 | |||
| CAH-methyltestosterone peak 1 | 8.2 | 384.2656 | 384.2651 | 1.30 |
| CAH-methyltestosterone peak 2 | 8.6 | |||
| Testosterone | 8.0 | 289.2162 | 289.2161 | 0.35 |
| Cortisone | 6.3 | 361.2010 | 361.1995 | 4.15 |
| Cortisol | 6.2 | 363.2166 | 363.2165 | 0.27 |
| Progesterone | 9.7 | 315.2319 | 315.2319 | 0.00 |
| Estrone | 7.9 | [M − H]−: 269.1547 | [M − H]−: 269.1540 | 2.60 |
| 11α-hydroxyprogesterone | 7.4 | 331.2268 | 331.2256 | 3.62 |
| Methyltestosterone | 8.4 | 303.2319 | 303.2312 | 2.31 |
| HA-testosterone peak 1 | 8.1 | 304.2271 | 304.2267 | 1.21 |
| HA-testosterone peak 2 | 8.2 | |||
| HA-cortisone peak 1 | 6.3 | 391.2227 | 391.2225 | 0.34 |
| HA-cortisone peak 2 | 6.4 | |||
| HA-cortisol peak 1 | 6.2 | 393.238 | 393.2378 | 0.55 |
| HA-cortisol peak 2 | 6.3 | |||
| HA-estrone | 8.0 | 286.1802 | 268.1797 | 1.75 |
| HA-progesterone peak 1 | 9.7 | 345.2537 | 345.2525 | 3.38 |
| HA-progesterone peak 2 | 9.8 | |||
| HA-11α-hydroxyprogesterone peak 1 | 7.6 | 361.2486 | 361.2476 | 2.79 |
| HA11α-hydroxyprogesterone peak 2 | 7.7 | |||
| HA-methyltestosterone peak 1 | 8.4 | 318.2428 | 318.2418 | 3.02 |
| HA-methyltestosterone peak 2 | 8.5 |
| Compounds Ratio | Peaks Area Ratio |
|---|---|
| CAH-testosterone (peak 2)/CAH-testosterone (peak 1) | 3.2 |
| CAH-cortisone (peak 2)/CAH-cortisone (peak 1) | 3.6 |
| CAH-cortisol (peak 2)/CAH-cortisol (peak 1) | 3.6 |
| CAH-cortisol (peak 2)/CAH-cortisol (peak 1) | 2:1 |
| CAH- progesterone (peak 2)/CAH- progesterone (peak 1) | 2.4 |
| CAH-11α-hydroxyprogesterone (peak 2)/ CAH-11α-hydroxyprogesterone (peak 1) | 2.6 |
| CAH-methyltestosterone (peak 2)/ CAH-methyltestosterone (peak 1) | 2 |
| Compounds Ratio | Peaks Area Ratio |
|---|---|
| CAH-testosterone (peak 2)/Testosterone | 5.3 |
| CAH-cortisone (peak 2)/Cortisone | 4 |
| CAH-cortisol (peak 2)/Cortisol | 3.8 |
| CAH-progesterone (peak 2)/Progesterone | 1.4 |
| CAH-11α-hydroxyprogesterone (peak 2)/11α-hydroxyprogesterone | 2.4 |
| CAH-methyltestosterone (peak 2)/Methyltestosterone | 3 |
| Compound | Concentration, ng/mL | |
|---|---|---|
| Native | CAH-Derivatives | |
| Testosterone | 12 ± 2 | 16 ± 3 |
| Cortisone | 10 ± 2 | 12 ± 2 |
| Cortisol | 4.2 ± 0.8 | 5.5 ± 1.0 |
| Compound | Oximes | CAH-Derivatives | ||||
|---|---|---|---|---|---|---|
| LOD, ng/mL | Linear Range, ng/mL | R2 | LOD, ng/mL | Linear Range, ng/mL | R2 | |
| Testosterone | 0.25 | 1.0–100 | 0.9987 | 2.5 | 5–100 | 0.9991 |
| Cortisone | 0.5 | 2.5–100 | 0.9962 | 2.5 | 5–100 | 0.9973 |
| Cortisol | 0.5 | 2.5–100 | 0.9976 | 2.5 | 5–100 | 0.9974 |
| Progesterone | 0.25 | 1.0–100 | 0.9945 | 2.5 | 5–100 | 0.9967 |
| Estrone | 0.5 | 2.5–100 | 0.9912 | 2.5 | 5–100 | 0.9945 |
| 11α-hydroxyprogesterone | 0.25 | 1.0–100 | 0.9889 | 2.5 | 5–100 | 0.9925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temerdashev, A.; Zorina, M.; Feng, Y.-Q.; Gashimova, E.; Dotsenko, V.V.; Ioutsi, V.; Atapattu, S.N. Cyanoacetohydrazide as a Novel Derivatization Agent for the Determination of UHPLC-HRMS Steroids in Urine. Molecules 2024, 29, 2433. https://doi.org/10.3390/molecules29112433
Temerdashev A, Zorina M, Feng Y-Q, Gashimova E, Dotsenko VV, Ioutsi V, Atapattu SN. Cyanoacetohydrazide as a Novel Derivatization Agent for the Determination of UHPLC-HRMS Steroids in Urine. Molecules. 2024; 29(11):2433. https://doi.org/10.3390/molecules29112433
Chicago/Turabian StyleTemerdashev, Azamat, Maria Zorina, Yu-Qi Feng, Elina Gashimova, Victor V. Dotsenko, Vitalij Ioutsi, and Sanka N. Atapattu. 2024. "Cyanoacetohydrazide as a Novel Derivatization Agent for the Determination of UHPLC-HRMS Steroids in Urine" Molecules 29, no. 11: 2433. https://doi.org/10.3390/molecules29112433
APA StyleTemerdashev, A., Zorina, M., Feng, Y.-Q., Gashimova, E., Dotsenko, V. V., Ioutsi, V., & Atapattu, S. N. (2024). Cyanoacetohydrazide as a Novel Derivatization Agent for the Determination of UHPLC-HRMS Steroids in Urine. Molecules, 29(11), 2433. https://doi.org/10.3390/molecules29112433






