Encapsulation of Probiotics within Double/Multiple Layer Beads/Carriers: A Concise Review
Abstract
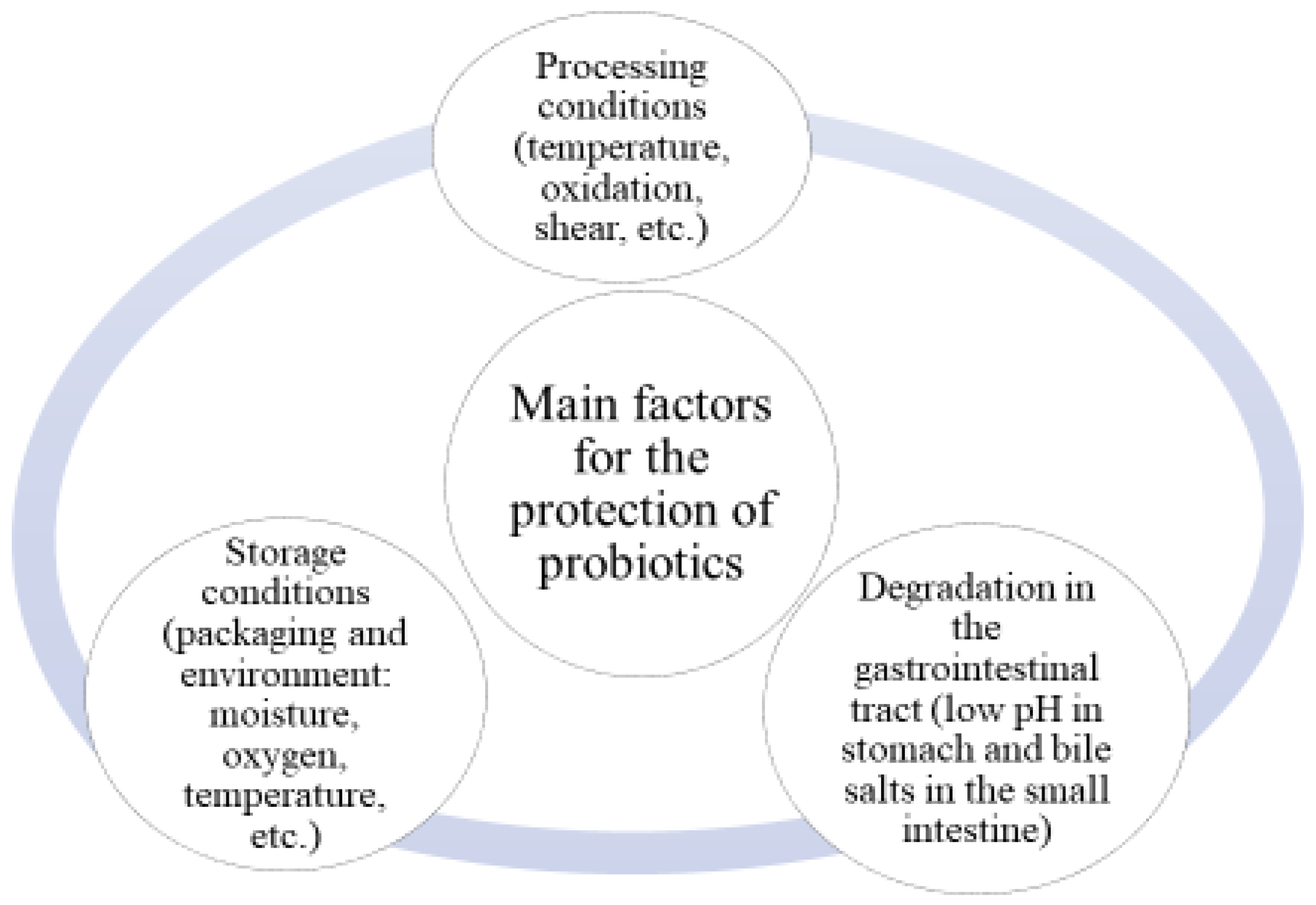
1. Introduction
2. Encapsulation of Probiotics in Monolayer Beads/Carriers—Fundamentals and Mechanisms
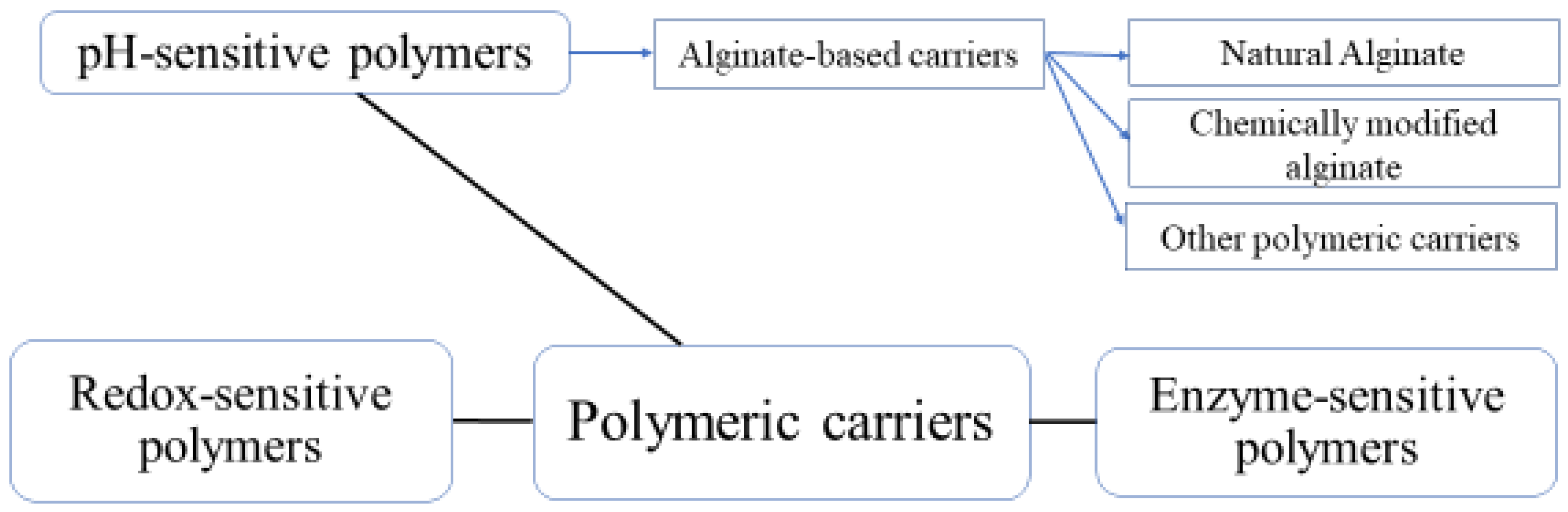
3. Monolayer versus Double/Multilayer Coatings
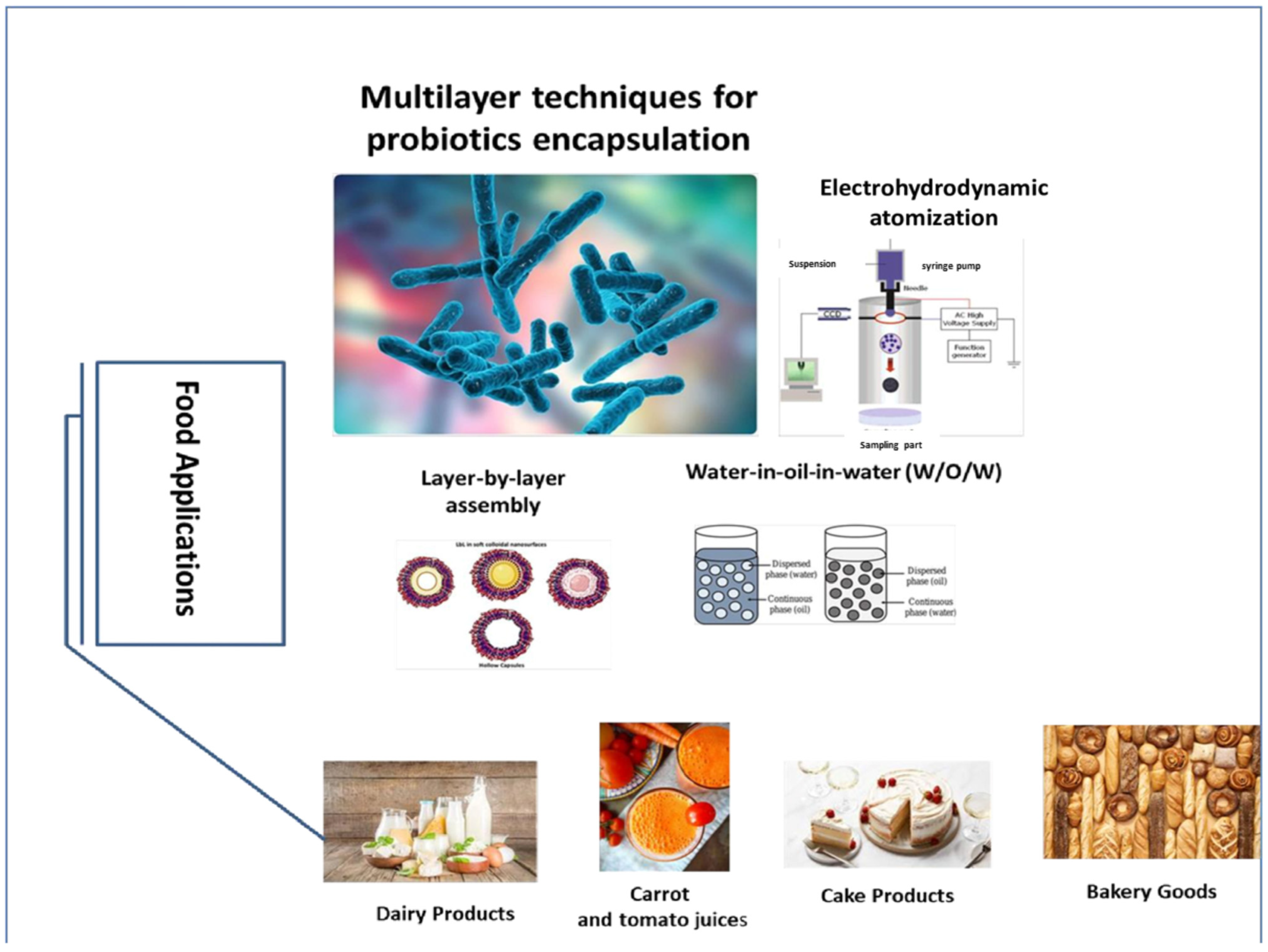
4. Different Multilayer Techniques for Encapsulation of Probiotics
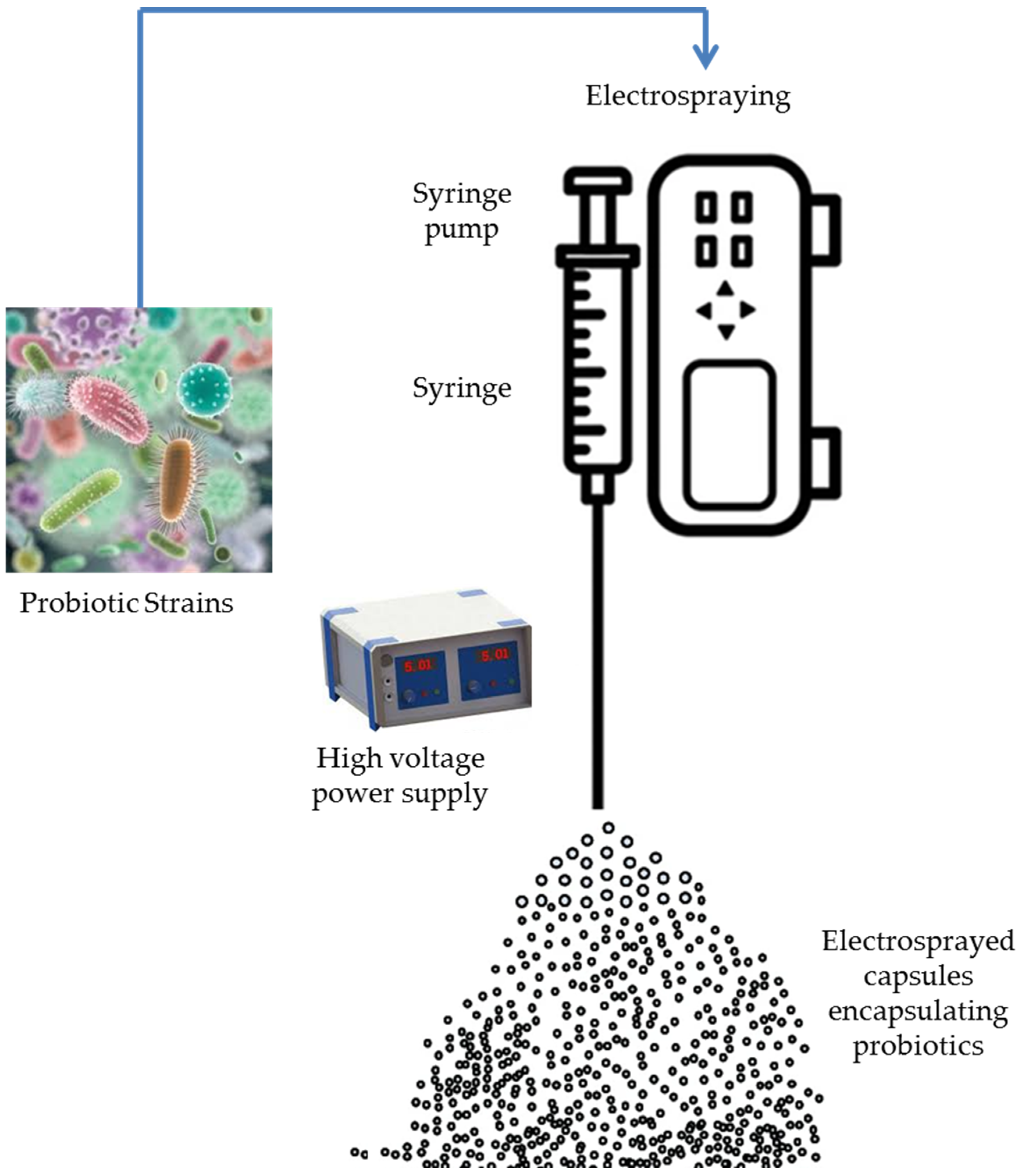
4.1. Electrohydrodynamic Atomization
4.2. Layer-by-Layer Assembly
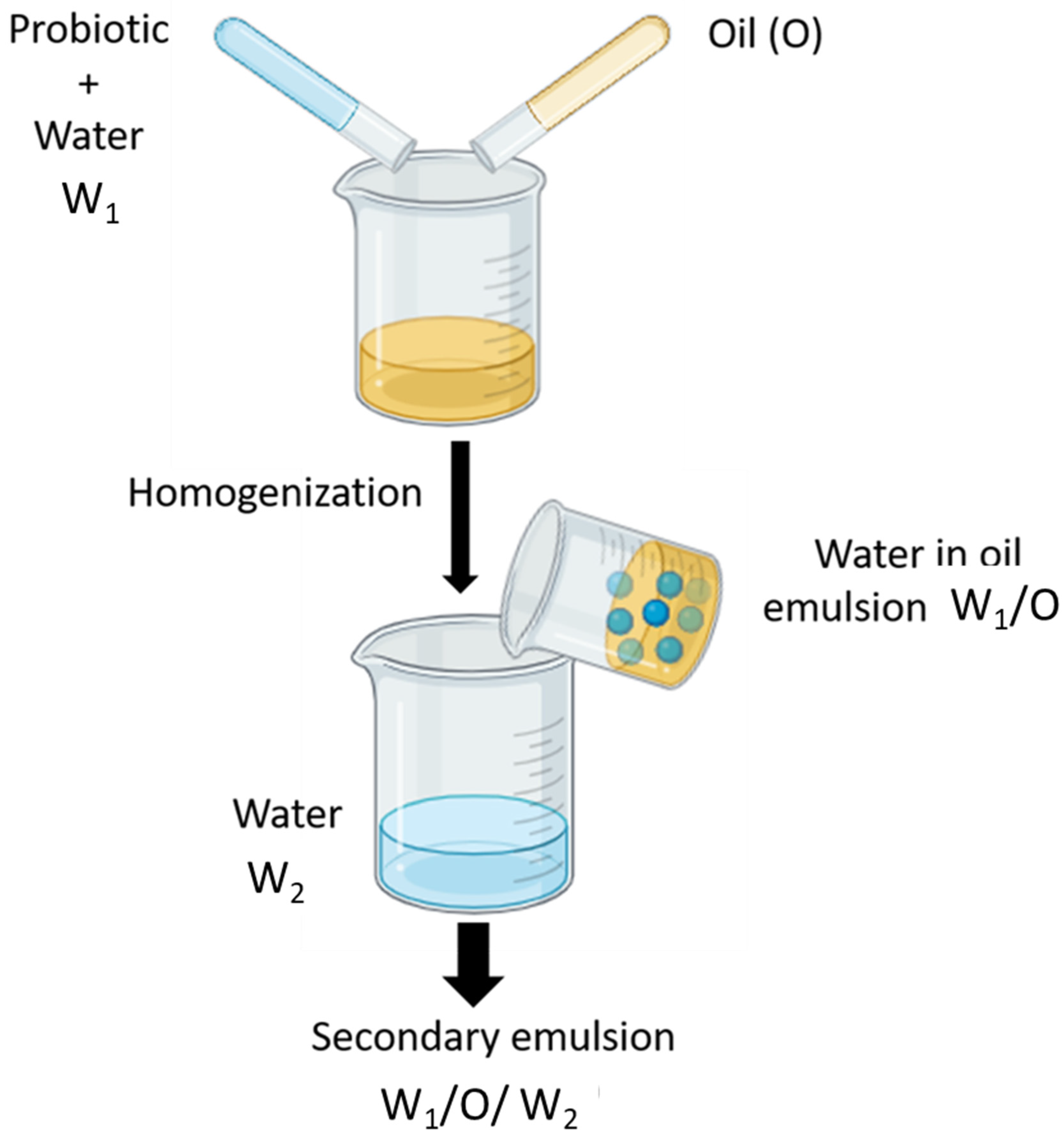
4.3. Water-in-Oil-in-Water (W1/O/W2) Emulsions
4.4. Multiple Pickering Emulsions
5. Applications of Multilayer Encapsulated Probiotics in Food Products
6. The Resistance and Viability of Probiotics Loaded in Multilayer Carriers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fischer, A.R.H.; Reinders, M.J. Chapter 18—Consumer acceptance of novel foods. In Innovation Strategies in the Food Industry, 2nd ed.; Galanakis, C.M., Ed.; Academic Press: New York, NY, USA, 2022; pp. 307–333. [Google Scholar] [CrossRef]
- Lavilla, M.; Gayán, E. Chapter 7—Consumer Acceptance and Marketing of Foods Processed through Emerging Technologies. In Innovative Technologies for Food Preservation; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press: New York, NY, USA, 2018; pp. 233–253. [Google Scholar] [CrossRef]
- Siegrist, M.; Hartmann, C. Consumer acceptance of novel food technologies. Nat. Food 2020, 1, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Chaari, M.; Elhadef, K.; Akermi, S.; Hlima, H.B.; Fourati, M.; Mtibaa, A.C.; D’Amore, T.; Ali, D.S.; Mellouli, L.; Ennouri, M.; et al. Potentials of beetroot (Beta vulgaris L.) peel extract for quality enhancement of refrigerated beef meat. Qual. Assur. Saf. Crops Foods 2023, 15, 99–115. [Google Scholar] [CrossRef]
- Elhadef, K.; Chaari, M.; Akermi, S.; Nilesh Prakash, N.; Khaneghah, A.M.; Abdelkafi, S.; Michaud, P.; Diyar Salahuddin, A.; Mellouli, L.; Smaoui, S. Production of functional raw chicken meat by incorporation of date palm seed extract: An assessment of microbiological, chemical and sensory properties. J. Food Meas. Charact. 2023, 17, 5117–5133. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Nemecek, T.; Chaudhary, A.; Mathys, A. Assessing nutritional, health, and environmental sustainability dimensions of agri-food production. Glob. Food Secur. 2020, 26, 100406. [Google Scholar] [CrossRef]
- Kohut, M.; Lohne, O.; Leker, J.; Bröring, S. Market convergence from a start-up perspective: The case of probiotics. PharmaNutrition 2021, 15, 100243. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: ftp://ftp.fao.org/es/esn/food/wgreport2.pdf (accessed on 24 April 2024).
- Yoha, K.S.; Nida, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics Antimicrob. Proteins 2022, 14, 15–48. [Google Scholar] [CrossRef]
- Kaistha, S.D.; Deshpande, N. Traditional Probiotics, Next-Generation Probiotics and Engineered Live Biotherapeutic Products in Chronic Wound Healing. In Wound Healing Research: Current Trends and Future Directions; Kumar, P., Kothari, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 247–284. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Y.; Kang, X.; Liu, P.; Wang, G. Research progress on the association between mastitis and gastrointestinal microbes in dairy cows and the effect of probiotics. Microb. Pathog. 2022, 173, 105809. [Google Scholar] [CrossRef]
- Naeem, H.; Hassan, H.U.; Shahbaz, M.; Imran, M.; Memon, A.G.; Hasnain, A.; Murtaza, S.; Alsagaby, S.A.; Al Abdulmonem, W.; Hussain, M.; et al. Role of Probiotics against Human Cancers, Inflammatory Diseases, and Other Complex Malignancies. J. Food Biochem. 2024, 2024, 6632209. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z.; et al. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Tungare, K.; De, A.; Jobby, R. Probiotics and Cancer: A Review on the anti-cancer activity of probiotics. J. Environ. Pathol. Toxicol. Oncol. 2024, 43, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhang, Y.; Tang, H.; Zhou, T.; Khan, A. A Review of the Use of Native and Engineered Probiotics for Colorectal Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 3896. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, J.; Thanigaivel, S.; Vickram, S.; Dey, N.; Mihaylova, D.; Desseva, I. Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability. Appl. Sci. 2022, 12, 455. [Google Scholar] [CrossRef]
- Fijan, S.; ter Haar, J.A.; Varga, L. Chapter 1—Probiotic Microorganisms and Their Benefit to Human Health. In Advances in Probiotics; Dhanasekaran, D., Sankaranarayanan, A., Eds.; Academic Press: New York, NY, USA, 2021; pp. 3–22. [Google Scholar] [CrossRef]
- Swe, Z.M.; Chumphon, T.; Panya, M.; Pangjit, K.; Promsai, S. Evaluation of Nano-Wall Material for Production of Novel Lyophilized-Probiotic Product. Foods 2022, 11, 3113. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Sharma, S.; Bajwa, J.; Chugh, R.; Kumar, D. Polymeric carriers in probiotic delivery system. Carbohydr. Polym. Technol. Appl. 2023, 5, 100301. [Google Scholar] [CrossRef]
- Aamir, M.; Afzaal, M.; Saeed, F.; Yasmin, I.; Nouman, M. Chapter 19—The effect of innovative processing technologies on probiotics stability. In Advances in Dairy Microbial Products; Singh, J., Vyas, A., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 287–294. [Google Scholar] [CrossRef]
- Ramazanidoroh, F.; Hosseininezhad, M.; Shahrampour, D.; Wu, X. Edible Packaging as a Functional Carrier of Prebiotics, Probiotics, and Postbiotics to Boost Food Safety, Quality, and Shelf Life. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef] [PubMed]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef]
- Kowalska, E.; Ziarno, M.; Ekielski, A.; Żelaziński, T. Materials Used for the Microencapsulation of Probiotic Bacteria in the Food Industry. Molecules 2022, 27, 3321. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rashidinejad, A.; Jafari, S.M. Application of Spray Dried Encapsulated Probiotics in Functional Food Formulations. Food Bioprocess Technol. 2022, 15, 2135–2154. [Google Scholar] [CrossRef]
- Harel, M.; Tang, Q. Chapter 29—Protection and delivery of probiotics for use in foods. In Microencapsulation in the Food Industry, 2nd ed.; Sobel, R., Ed.; Academic Press: New York, NY, USA, 2023; pp. 463–480. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging Technologies and Coating Materials for Improved Probiotication in Food Products: A Review. Food Bioprocess Technol. 2022, 15, 998–1039. [Google Scholar] [CrossRef]
- Ferrer, J.; Jiang, Q.; Menner, A.; Bismarck, A. An approach for the scalable production of macroporous polymer beads. J. Colloid Interface Sci. 2022, 616, 834–845. [Google Scholar] [CrossRef]
- Smaoui, S.; Ben Hlima, H.; Ben Braïek, O.; Ennouri, K.; Mellouli, L.; Mousavi Khaneghah, A. Recent advancements in encapsulation of bioactive compounds as a promising technique for meat preservation. Meat Sci. 2021, 181, 108585. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Schofield, T.; Valtchev, P.; Schindeler, A.; Kavanagh, J.M.; Adil, Q.; Dehghani, F. Biopolymer-Based Multilayer Microparticles for Probiotic Delivery to Colon. Adv. Healthc. Mater. 2022, 11, 2102487. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wu, F.; Zhou, D.; Tan, B.; Chen, T. Commercial probiotic products in public health: Current status and potential limitations. Crit. Rev. Food Sci. Nutr. 2023, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Ramírez-Corona, N.; López-Malo, A. Advances in probiotic incorporation into cereal-based baked foods: Strategies, viability, and effects—A review. Appl. Food Res. 2023, 3, 100330. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Assadpour, E.; Jafari, S.M. Recent advances in probiotic breads; a market trend in the functional bakery products. Crit. Rev. Food Sci. Nutr. 2023, 1–12. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Kwiecień, I.; Kwiecień, M. Application of Polysaccharide-Based Hydrogels as Probiotic Delivery Systems. Gels 2018, 4, 47. [Google Scholar] [CrossRef]
- Castro-López, C.; Romero-Luna, H.E.; García, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Key Stress Response Mechanisms of Probiotics During Their Journey Through the Digestive System: A Review. Probiotics Antimicrob. Proteins 2023, 15, 1250–1270. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.S.; Kwon, M.; Son, N.; Kim, S.-K.; Son, M.-Y. Multilayer Coating with Red Ginseng Dietary Fiber Improves Intestinal Adhesion and Proliferation of Probiotics in Human Intestinal Epithelial Models. J. Microbiol. Biotechnol. 2023, 33, 1309–1316. [Google Scholar] [CrossRef]
- Centurion, F.; Basit, A.W.; Liu, J.; Gaisford, S.; Rahim, M.A.; Kalantar-Zadeh, K. Nanoencapsulation for Probiotic Delivery. ACS Nano 2021, 15, 18653–18660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sadeghi, A.; Karaca, A.C.; Zhang, J.; Jafari, S.M. Carbohydrate polymer-based carriers for colon targeted delivery of probiotics. Crit. Rev. Food Sci. Nutr. 2023, 1–21. [Google Scholar] [CrossRef]
- Asgari, S.; Pourjavadi, A.; Licht, T.R.; Boisen, A.; Ajalloueian, F. Polymeric carriers for enhanced delivery of probiotics. Adv. Drug Deliv. Rev. 2020, 161–162, 1–21. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Nagarajan, M.; Kumar, P.K.; Singh, S.S.; Manvi, D.; Gowda, N.N. A comprehensive review on microencapsulation of probiotics: Technology, carriers and current trends. Appl. Food Res. 2023, 3, 100248. [Google Scholar] [CrossRef]
- Xu, C.; Gantumur, M.-A.; Sun, J.; Guo, J.; Ma, J.; Jiang, Z.; Wang, W.; Zhang, J.; Ma, Y.; Hou, J.; et al. Design of probiotic delivery systems for targeted release. Food Hydrocoll. 2024, 149, 109588. [Google Scholar] [CrossRef]
- Xie, A.; Zhao, S.; Liu, Z.; Yue, X.; Shao, J.; Li, M.; Li, Z. Polysaccharides, proteins, and their complex as microencapsulation carriers for delivery of probiotics: A review on carrier types and encapsulation techniques. Int. J. Biol. Macromol. 2023, 242, 124784. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C.; Zhang, H.; Hossen, M.A.; Sameen, D.E.; Dai, J.; Qin, W.; Liu, Y.; Li, S. In vitro digestion of sodium alginate/pectin co-encapsulated Lactobacillus bulgaricus and its application in yogurt bilayer beads. Int. J. Biol. Macromol. 2021, 193, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, W.; Xiang, Z.; Yu, X.; Li, P.; Chen, H.; Yao, M.; Fei, Y.; Huang, Y.; Yin, Y.; Xiao, H. The Challenge of Applications of Probiotics in Gastrointestinal Diseases. Adv. Gut Microb. Res. 2023, 2023, e1984200. [Google Scholar] [CrossRef]
- Pech-Canul, A.d.l.C.; Ortega, D.; García-Triana, A.; González-Silva, N.; Solis-Oviedo, R.L. A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings 2020, 10, 197. [Google Scholar] [CrossRef]
- Kari, H.J.; You, S.; Kwon, M.; Shin, M.; Kim, S.-K.; Jung, Y.H. Multilayer coatings containing red ginseng dietary fibre improve the survivability and stability of probiotic bacteria. Int. J. Food Sci. Technol. 2023, 58, 1497–1505. [Google Scholar] [CrossRef]
- Safeer Abbas, M.; Afzaal, M.; Saeed, F.; Asghar, A.; Jianfeng, L.; Ahmad, A.; Ullah, Q.; Elahi, S.; Ateeq, H.; Shah, Y.A.; et al. Probiotic viability as affected by encapsulation materials: Recent updates and perspectives. Int. J. Food Prop. 2023, 26, 1324–1350. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Tarapoulouzi, M.; Varzakas, T.; Jafari, S.M. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms 2023, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Paula, D.d.A.; Martins, E.M.F.; Costa, N.d.A.; de Oliveira, P.M.; de Oliveira, E.B.; Ramos, A.M. Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. Int. J. Biol. Macromol. 2019, 133, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V.; Kourkoutas, Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. LWT Food Sci. Technol. 2019, 116, 108501. [Google Scholar] [CrossRef]
- Kil, B.J.; Yoon, S.J.; Yun, C.-H.; Huh, C.-S. The Effect of Milk Protein on the Biological and Rheological Properties of Probiotic Capsules. J. Microbiol. Biotechnol. 2020, 30, 1870–1875. [Google Scholar] [CrossRef]
- How, Y.-H.; Yeo, S.-K. Oral probiotic and its delivery carriers to improve oral health: A review. Microbiology 2021, 167, 001076. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Xia, Y.; Song, X.; Ai, L. Characteristics of Probiotic Preparations and Their Applications. Foods 2022, 11, 2472. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.d.A.; Silveira, L.R.; Amaral, E.d.P.; Pereira, G.C.; Paula, D.d.A.; Vieira, É.N.R.; Martins, E.M.F.; Stringheta, P.C.; Leite Júnior, B.R.d.C.; Ramos, A.M. Use of maltodextrin, sweet potato flour, pectin and gelatin as wall material for microencapsulating Lactiplantibacillus plantarum by spray drying: Thermal resistance, in vitro release behavior, storage stability and physicochemical properties. Food Res. Int. 2023, 164, 112367. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Sekhavatizadeh, S.S.; Hosseinzadeh, S. Milk dessert containing Lactobacillus reuteri (ATCC 23272) encapsulated with sodium alginate, Ferula assa-foetida and Zedo (Amygdalus scoparia) gum as three layers of wall materials. Food Bioprod. Process. 2021, 127, 244–254. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Ejaz, A.; Afzaal, M.; Saeed, F.; Waliat, S.; Shah, Y.A.; Imran, A.; Akram, N.; Asghar, A.; Ateeq, H.; Alomar, S.Y.; et al. Development and characterization of symbiotic microcapsules to enhance the viability of probiotic under stressed conditions. Int. J. Food Prop. 2023, 26, 2838–2853. [Google Scholar] [CrossRef]
- Qin, X.-S.; Luo, Z.-G.; Li, X.-L. An enhanced pH-sensitive carrier based on alginate-Ca-EDTA in a set-type W1/O/W2 double emulsion model stabilized with WPI-EGCG covalent conjugates for probiotics colon-targeted release. Food Hydrocoll. 2021, 113, 106460. [Google Scholar] [CrossRef]
- Tan, L.L.; Sampathkumar, K.; Wong, J.H.; Loo, S.C.J. Divalent cations are antagonistic to survivability of freeze-dried probiotics encapsulated in cross-linked alginate. Food Bioprod. Process. 2020, 124, 369–377. [Google Scholar] [CrossRef]
- Le, H.D.; Trinh, K.S. Survivability of Lactobacillus acidophilus, Bacillus clausii and Sacharomyces boulardii encapsulated in alginate gel microbeads. Carpathian J. Food Sci. Technol. 2018, 10, 95. [Google Scholar]
- Sekhavatizadeh, S.S.; Afrasiabi, F.; Montaseri, Z. Encapsulation of probiotic Lactobacillus acidophilus ATCC 4356 in alginate–galbanum (Ferula Gummosa Boiss) gum microspheres and evaluation of the survival in simulated gastrointestinal conditions in probiotic Tahini halva. Braz. J. Microbiol. 2023, 54, 1589–1601. [Google Scholar] [CrossRef]
- Han, J.; McClements, D.J.; Liu, X.; Liu, F. Oral delivery of probiotics using single-cell encapsulation. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13322. [Google Scholar] [CrossRef]
- Enayati, M.; Chang, M.-W.; Bragman, F.; Edirisinghe, M.; Stride, E. Electrohydrodynamic preparation of particles, capsules and bubbles for biomedical engineering applications. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 154–164. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Ajalloueian, F.; Guerra, P.R.; Bahl, M.I.; Torp, A.M.; Hwu, E.T.; Licht, T.R.; Boisen, A. Multi-layer PLGA-pullulan-PLGA electrospun nanofibers for probiotic delivery. Food Hydrocoll. 2022, 123, 107112. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Tromp, R.H. Electrospray assisted fabrication of hydrogel microcapsules by single- and double-stage procedures for encapsulation of probiotics. Food Bioprod. Proc. 2017, 102, 250–259. [Google Scholar] [CrossRef]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Librán, C.M.; Castro, S.; Lagaron, J.M. Encapsulation by electrospray coating atomization of probiotic strains. Inn. Food Sci. Emerg. Technol. 2017, 39, 216–222. [Google Scholar] [CrossRef]
- Mojaveri, S.J.; Hosseini, S.F.; Gharsallaoui, A. Viability improvement of Bifidobacterium animalis Bb12 by encapsulation in chitosan/poly(vinyl alcohol) hybrid electrospun fiber mats. Carbohydr. Polym. 2020, 241, 116278. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, C.; Yu, H.; Feng, Z.; Yu, W.; Gu, L.; Liu, Z.; Chen, L.; Jiang, Z.; Hou, J. Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology. Food Hydrocoll. 2021, 111, 106381. [Google Scholar] [CrossRef]
- Yu, H.; Liu, W.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. Targeting Delivery System for Lactobacillus Plantarum Based on Functionalized Electrospun Nanofibers. Polymers 2020, 12, 1565. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, D.; Kang, X. Electrospinning as a novel strategy for the encapsulation of living probiotics in polyvinyl alcohol/silk fibroin. Inn. Food Sci. Emerg. Technol. 2021, 71, 102726. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.D.; Abreu, R.E.F.; Costa, M.M.d.; Melo, N.F.d.; Oliveira, H.P.d. Investigation of Lactobacillus paracasei encapsulation in electrospun fibers of Eudragit® L100. Polímeros 2020, 30, e2020025. [Google Scholar] [CrossRef]
- Castro Coelho, S.; Nogueiro Estevinho, B.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Masiá, R.; López-Nicolás, R.; Periago, M.J.; Ros, G.; Lagaron, J.M.; López-Rubio, A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015, 168, 124–133. [Google Scholar] [CrossRef]
- Wandrey, C.; Bartkowiak, A.; Harding, S.E. Materials for Encapsulation. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 31–100. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Mendes, A.C.; Jacobsen, C.; Chronakis, I.S. Biopolymers for the Nano-microencapsulation of Bioactive Ingredients by Electrohydrodynamic Processing. In Polymers for Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 447–479. [Google Scholar] [CrossRef]
- Niamah, A.K.; Gddoa Al-Sahlany, S.T.; Ibrahim, S.A.; Verma, D.K.; Thakur, M.; Singh, S.; Patel, A.R.; Aguilar, C.N.; Utama, G.L. Electro-hydrodynamic processing for encapsulation of probiotics: A review on recent trends, technological development, challenges and future prospect. Food Biosci. 2021, 44, 101458. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Mendes, A.C.; Sevilla Moreno, J.; Hanif, M.; Douglas, T.E.L.; Chen, M.; Chronakis, I.S. Morphological, Mechanical and Mucoadhesive Properties of Electrospun Chitosan/Phospholipid Hybrid Nanofibers. Int. J. Mol. Sci. 2018, 19, 2266. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Škrlec, K.; Kocbek, P.; Kristl, J.; Berlec, A. Effects of Electrospinning on the Viability of Ten Species of Lactic Acid Bacteria in Poly(Ethylene Oxide) Nanofibers. Pharmaceutics 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Film. 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.-D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Luo, X.; Song, H.; Yang, J.; Han, B.; Feng, Y.; Leng, Y.; Chen, Z. Encapsulation of Escherichia coli strain Nissle 1917 in a chitosan―alginate matrix by combining layer-by-layer assembly with CaCl2 cross-linking for an effective treatment of inflammatory bowel diseases. Colloids Surf. B Biointerfaces 2020, 189, 110818. [Google Scholar] [CrossRef] [PubMed]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Humblet-Hua, K.P.; Scheltens, G.; van der Linden, E.; Sagis, L.M.C. Encapsulation systems based on ovalbumin fibrils and high methoxyl pectin. Food Hydrocoll. 2011, 25, 569–576. [Google Scholar] [CrossRef]
- McClements, D.J. Theoretical Analysis of Factors Affecting the Formation and Stability of Multilayered Colloidal Dispersions. Langmuir 2005, 21, 9777–9785. [Google Scholar] [CrossRef] [PubMed]
- Peyratout, C.S.; Dähne, L. Tailor-Made Polyelectrolyte Microcapsules: From Multilayers to Smart Containers. Angew. Chem. Int. Ed. Engl. 2004, 43, 3762–3783. [Google Scholar] [CrossRef]
- Cui, C.; Li, M.; He, X.; Ji, N.; Qin, Y.; Dai, L.; Xiong, L.; Sun, Q. In vitro digestion and fecal fermentation of encapsulated starch constructed via layer-by-layer coating of calcium alginate. Food Hydrocoll. 2024, 148, 109441. [Google Scholar] [CrossRef]
- Xiang, N.; Lyu, Y.; Narsimhan, G. Characterization of fish oil in water emulsion produced by layer by layer deposition of soy β-conglycinin and high methoxyl pectin. Food Hydrocoll. 2016, 52, 678–689. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bousserrhine, N.; Alphonse, V.; Carbonnier, B. From beta-cyclodextrin polyelectrolyte to layer-by-layer self-assembly microcapsules: From inhibition of bacterial growth to bactericidal effect. Food Hydrocoll. 2019, 95, 219–227. [Google Scholar] [CrossRef]
- Kiprono, S.J.; Ullah, M.W.; Yang, G. Surface Engineering of Microbial Cells: Strategies and Applications. Eng. Sci. 2018, 9, 33–45. [Google Scholar] [CrossRef]
- Yucel Falco, C.; Sotres, J.; Rascón, A.; Risbo, J.; Cárdenas, M. Design of a potentially prebiotic and responsive encapsulation material for probiotic bacteria based on chitosan and sulfated β-glucan. J. Colloid Interface Sci. 2017, 487, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Tzortzis, G.; Khutoryanskiy, V.V.; Charalampopoulos, D. Layer-by-layer coating of alginate matrices with chitosan —Alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J. Mater. Chem. B 2013, 1, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Nualkaekul, S.; Lenton, D.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012, 90, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, A.M.; Bhathena, J.; Prakash, S. Live encapsulated Lactobacillus acidophilus cells in yogurt for therapeutic oral delivery: Preparation and in vitro analysis of alginate–chitosan microcapsules. Can. J. Physiol. Pharmacol. 2007, 85, 884–893. [Google Scholar] [CrossRef]
- Thomas, M.B.; Vaidyanathan, M.; Radhakrishnan, K.; Raichur, A.M. Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan–dextran sulfate polyelectrolytes. J. Food Eng. 2014, 136, 1–8. [Google Scholar] [CrossRef]
- Priya, A.J.; Vijayalakshmi, S.P.; Raichur, A.M. Enhanced Survival of Probiotic Lactobacillus acidophilus by Encapsulation with Nanostructured Polyelectrolyte Layers through Layer-by-Layer Approach. J. Agric. Food Chem. 2011, 59, 11838–11845. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef] [PubMed]
- Chehreara, A.; Tabandeh, F.; Otadi, M.; Alihosseini, A.; Partovinia, A. Enhanced survival of Lacticaseibacillus rhamnosus in simulated gastrointestinal conditions using layer-by-layer encapsulation. Biotechnol. Lett. 2022, 44, 1277–1286. [Google Scholar] [CrossRef]
- Kurapati, R.; Groth, T.W.; Raichur, A.M. Recent Developments in Layer-by-Layer Technique for Drug Delivery Applications. ACS Appl. Bio Mater. 2019, 2, 5512–5527. [Google Scholar] [CrossRef]
- Huang, S.; Xiong, Y.; Zou, Y.; Dong, Q.; Ding, F.; Liu, X.; Li, H. A novel colorimetric indicator based on agar incorporated with Arnebia euchroma root extracts for monitoring fish freshness. Food Hydrocoll. 2019, 90, 198–205. [Google Scholar] [CrossRef]
- Jiang, Z.; Tian, J.; Bai, X.; McClements, D.J.; Ma, C.; Liu, X.; Liu, F. Improving probiotic survival using water-in-oil-in-water (W1/O/W2) emulsions: Role of fish oil in inner phase and sodium alginate in outer phase. Food Chem. 2023, 417, 135889. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Cai, Y.; Tai, K.; Guo, Q.; Zhu, S.; Mao, L.; Gao, Y.; Yuan, F.; der Meeren, P.V. High-internal-phase emulsions (HIPEs) for co-encapsulation of probiotics and curcumin: Enhanced survivability and controlled release. Food Funct. 2021, 12, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Y.; Wang, Y.; Xie, L.; Liang, S.; Li, D.; Wang, Y.; Wang, J.; Zhan, X. Carboxymethyl konjac glucomannan-chitosan complex nanogels stabilized double emulsions incorporated into alginate hydrogel beads for the encapsulation, protection and delivery of probiotics. Carbohydr. Polym. 2022, 289, 119438. [Google Scholar] [CrossRef]
- Eslami, P.; Davarpanah, L.; Vahabzadeh, F. Encapsulating role of β-cyclodextrin in formation of pickering water-in-oil-in-water (W1/O/W2) double emulsions containing Lactobacillus dellbrueckii. Food Hydrocoll. 2017, 64, 133–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Zhong, Q. S/O/W emulsions prepared with sugar beet pectin to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocoll. 2016, 52, 804–810. [Google Scholar] [CrossRef]
- Marefati, A.; Pitsiladis, A.; Oscarsson, E.; Ilestam, N.; Bergenståhl, B. Encapsulation of Lactobacillus reuteri in W1/O/W2 double emulsions: Formulation, storage and in vitro gastro-intestinal digestion stability. LWT Food Sci. Technol. 2021, 146, 111423. [Google Scholar] [CrossRef]
- El Kadri, H.; Lalou, S.; Mantzouridou, F.; Gkatzionis, K. Utilisation of water-in-oil-water (W1/O/W2) double emulsion in a set-type yogurt model for the delivery of probiotic Lactobacillus paracasei. Food Res. Int. 2018, 107, 325–336. [Google Scholar] [CrossRef]
- Pimentel-González, D.J.; Campos-Montiel, R.G.; Lobato-Calleros, C.; Pedroza-Islas, R.; Vernon-Carter, E.J. Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Res. Int. 2009, 42, 292–297. [Google Scholar] [CrossRef]
- Yin, M.; Chen, L.; Chen, M.; Yuan, Y.; Liu, F.; Zhong, F. Encapsulation of Lactobacillus rhamnosus GG in double emulsions: Role of prebiotics in improving probiotics survival during spray drying and storage. Food Hydrocoll. 2024, 151, 109792. [Google Scholar] [CrossRef]
- Eslami, P.; Forootan, K.; Davarpanah, L.; Vahabzadeh, F. Incorporation of Lactobacillus casei into the Inner Phase of the Water-in-Oil-in-Water (W1/O/W2) Emulsion Prepared with β-Cyclodextrin and Bacterial Survival in a Model Gastric Environment. Appl. Food Biotechnol. 2020, 7, 171–182. [Google Scholar] [CrossRef]
- Tavasoli, S.; Liu, Q.; Jafari, S.M. Development of Pickering emulsions stabilized by hybrid biopolymeric particles/nanoparticles for nutraceutical delivery. Food Hydrocoll. 2022, 124, 107280. [Google Scholar] [CrossRef]
- Klojdová, I.; Stathopoulos, C. The Potential Application of Pickering Multiple Emulsions in Food. Foods 2022, 11, 1558. [Google Scholar] [CrossRef] [PubMed]
- Haji, F.; Cheon, J.; Baek, J.; Wang, Q.; Tam, K.C. Application of Pickering emulsions in probiotic encapsulation-A review. Curr. Res. Food Sci. 2022, 5, 1603–1615. [Google Scholar] [CrossRef]
- Rattanaburi, P.; Charoenrat, N.; Pongtharangkul, T.; Suphantharika, M.; Wongkongkatep, J. Hydroxypropyl methylcellulose enhances the stability of o/w Pickering emulsions stabilized with chitosan and the whole cells of Lactococcus lactis IO-1. Food Res. Int. 2019, 116, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Rojas, O.J.; McClements, D.J. Recent Innovations in Emulsion Science and Technology for Food Applications. J. Agric. Food Chem. 2021, 69, 8944–8963. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, M.; Zhao, Z.; Chen, X.; Cai, J.; Cao, Y.; Xiao, J. Lactobacillus acidophilus loaded pickering double emulsion with enhanced viability and colon-adhesion efficiency. LWT Food Sci. Technol. 2020, 121, 108928. [Google Scholar] [CrossRef]
- Shi, K.; Yu, H.; Lee, T.-C.; Huang, Q. Improving Ice Nucleation Activity of Zein Film through Layer-by-Layer Deposition of Extracellular Ice Nucleators. ACS Appl. Mater. Interfaces 2013, 5, 10456–10464. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Nie, W.; He, C.; Zhou, X.; Chen, L.; Qiu, K.; Wang, W.; Yin, Z. Effect of pH-Responsive Alginate/Chitosan Multilayers Coating on Delivery Efficiency, Cellular Uptake and Biodistribution of Mesoporous Silica Nanoparticles Based Nanocarriers. ACS Appl. Mater Interfaces 2014, 6, 8447–8460. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, W.; Qin, X. Ultrasound-assisted multilayer Pickering emulsion fabricated by WPI-EGCG covalent conjugates for encapsulating probiotics in colon-targeted release. Ultrason. Sonochem. 2023, 97, 106450. [Google Scholar] [CrossRef]
- Shi, A.; Feng, X.; Wang, Q.; Adhikari, B. Pickering and high internal phase Pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 109, 106117. [Google Scholar] [CrossRef]
- Xu, B.; Liu, C.; Sun, H.; Wang, X.; Huang, F. Highly Surface-Active Chaperonin Nanobarrels for Oil-in-Water Pickering Emulsions and Delivery of Lipophilic Compounds. J. Agric. Food Chem. 2019, 67, 10155–10164. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, V.; Pellicano, L.; Farina, H.; Pargoletti, E.; Annunziata, L.; Ortenzi, M.A.; Stori, A.; Cappelletti, G. Design of New Polyacrylate Microcapsules to Modify the Water-Soluble Active Substances Release. Polymers 2021, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, H.; Li, J.; Liu, L.; Huang, J.; Cao, Y.; Zhao, T.; McClements, D.J.; Chen, J.; Liu, C.; et al. Improving probiotic (Lactobacillus casei) viability by encapsulation in alginate-based microgels: Impact of polymeric and colloidal fillers. Food Hydrocoll. 2023, 134, 108028. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Liu, C.; Cao, H.; Li, Y.; Li, B.; Zhang, Y.; Liu, S. Water-in-water Pickering emulsion: A fascinating microculture apparatus for embedding and cultivation of Lactobacillus helveticus. Food Hydrocoll. 2024, 147, 109398. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, C.; Zhang, J.; Li, Y.; Li, B.; Liu, S. Crosslinking alginate at water-in-water Pickering emulsions interface to control the interface structure and enhance the stress resistance of the encapsulated probiotics. J. Colloid Interface Sci. 2024, 655, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Xie, Y.; Zhou, C.; Li, Y.; Li, B.; Zhang, Y.; Liu, S. Biomacromolecule based water-in-water Pickering emulsion: A fascinating artificial cell-like compartment for the encapsulation of Lactobacillus plantarum. Food Biosci. 2023, 55, 102916. [Google Scholar] [CrossRef]
- da Silva, S.Â.D.; Batista, L.d.S.P.; Diniz, D.S.; Nascimento, S.S.d.C.; Morais, N.S.; de Assis, C.F.; Passos, T.S.; de Sousa Júnior, F.C. Microencapsulation of Probiotics by Oil-in-Water Emulsification Technique Improves Cell Viability under Different Storage Conditions. Foods 2023, 12, 252. [Google Scholar] [CrossRef]
- Karakaş, C.Y.; Yildirim, R.M.; Karadag, A. Encapsulation of Lactobacillus plantarum ELB90 by electrospraying in a double emulsion (W1/O/W2) loaded alginate beads to improve the gastrointestinal survival and thermal stability. J. Sci. Food Agric. 2023, 103, 3427–3436. [Google Scholar] [CrossRef]
- Mardani Ghahfarokhi, V.; Pescarmona, P.P.; Euverink, G.-J.W.; Poortinga, A.T. Encapsulation of Lactobacillus casei (ATCC 393) by Pickering-Stabilized Antibubbles as a New Method to Protect Bacteria against Low pH. Colloids Interfaces 2020, 4, 40. [Google Scholar] [CrossRef]
- Pandey, P.; Mettu, S.; Mishra, H.N.; Ashokkumar, M.; Martin, G.J.O. Multilayer co-encapsulation of probiotics and γ-amino butyric acid (GABA) using ultrasound for functional food applications. LWT Food Sci. Technol. 2021, 146, 111432. [Google Scholar] [CrossRef]
- Mahmoodi Pour, H.; Marhamatizadeh, M.H.; Fattahi, H. Encapsulation of Different Types of Probiotic Bacteria within Conventional/Multilayer Emulsion and Its Effect on the Properties of Probiotic Yogurt. J. Food Qual. 2022, 2022, e7923899. [Google Scholar] [CrossRef]
- Jasińska, U.T.; Skąpska, S.; Owczarek, L.; Dekowska, A.; Lewińska, D. Immobilization of Bifidobacterium infantis Cells in Selected Hydrogels as a Method of Increasing Their Survival in Fermented Milkless Beverages. J. Food Qual. 2018, 2018, e9267038. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.; Song, Y.; Shu, G.; Chen, H. Effect of xanthan-chitosan-xanthan double layer encapsulation on survival of Bifidobacterium BB01 in simulated gastrointestinal conditions, bile salt solution and yogurt. LWT Food Sci. Technol. 2017, 81, 274–280. [Google Scholar] [CrossRef]
- Yee, W.L.; Yee, C.L.; Lin, N.K.; Phing, P.L. Microencapsulation of Lactobacillus acidophilus NCFM incorporated with mannitol and its storage stability in mulberry tea. Ciênc. Agrotec. 2019, 43, e005819. [Google Scholar] [CrossRef]
- Naga Sivudu, S.; Ramesh, B.; Umamahesh, K.; Vijaya Sarathi Reddy, O. Probiotication of tomato and carrot juices for shelf-life enhancement using micro-encapsulation. J. Food Biosci. Technol. 2016, 6, 13–22. Available online: https://www.sid.ir/paper/680034/fa (accessed on 25 April 2024).
- Arslan-Tontul, S.; Erbas, M.; Gorgulu, A. The Use of Probiotic-Loaded Single- and Double-Layered Microcapsules in Cake Production. Probiotics Antimicrob. Proteins 2019, 11, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Mirzamani, S.S.; Bassiri, A.; Tavakolipour, H.; Azizi, M.H.; Kargozari, M. Fluidized Bed Microencapsulation of Lactobacillus Sporogenes with Some Selected Hydrocolloids for Probiotic Bread Production. J. Food Biosci. Technol. 2021, 11, 23–24. [Google Scholar]
- Wong, C.H.; Mak, I.E.K.; Li, D. Bilayer edible coating with stabilized Lactobacillus plantarum 299v improved the shelf life and safety quality of fresh-cut apple slices. Food Pack. Shelf Life 2021, 30, 100746. [Google Scholar] [CrossRef]
- Jantarathin, S.; Borompichaichartkul, C.; Sanguandeekul, R. Microencapsulation of probiotic and prebiotic in alginate-chitosan capsules and its effect on viability under heat process in shrimp feeding. Mater. Today Proc. 2017, 4, 6166–6172. [Google Scholar] [CrossRef]
- Barajas-Álvarez, P.; González-Ávila, M.; Espinosa-Andrews, H. Recent Advances in Probiotic Encapsulation to Improve Viability under Storage and Gastrointestinal Conditions and Their Impact on Functional Food Formulation. Food Rev. Int. 2023, 39, 992–1013. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.-T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Liao, L.-K.; Wei, X.-Y.; Gong, X.; Li, J.-H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus casei LK-1 by spray drying related to its stability and in vitro digestion. LWT Food Sci. Technol. 2017, 82, 82–89. [Google Scholar] [CrossRef]
- Nunes, G.L.; Etchepare, M.d.A.; Cichoski, A.J.; Zepka, L.Q.; Jacob Lopes, E.; Barin, J.S.; Flores, É.M.d.M.; da Silva, C.d.B.; de Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT Food Sci. Technol. 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Rather, S.A.; Akhter, R.; Masoodi, F.A.; Gani, A.; Wani, S.M. Effect of double alginate microencapsulation on in vitro digestibility and thermal tolerance of Lactobacillus plantarum NCDC201 and L. casei NCDC297. LWT Food Sci. Technol. 2017, 83, 50–58. [Google Scholar] [CrossRef]
- Coman, M.M.; Cecchini, C.; Verdenelli, M.C.; Silvi, S.; Orpianesi, C.; Cresci, A. Functional foods as carriers for SYNBIO®, a probiotic bacteria combination. Int. J. Food Microbiol. 2012, 157, 346–352. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Jannin, V.; Cuppok, Y. Hot-melt coating with lipid excipients. Int. J. Pharm. 2013, 457, 480–487. [Google Scholar] [CrossRef]
- Lopes, D.G.; Salar-Behzadi, S.; Zimmer, A. Designing optimal formulations for hot-melt coating. Int. J. Pharm. 2017, 533, 357–363. [Google Scholar] [CrossRef]
- Jacobsen, N.M.Y.; Nedergaard, H.B.; Kock, A.; Caglayan, I.; Laursen, M.M.; Lange, E.-M.; Marcial-Coba, M.S.; Bar-Shalom, D.; Nielsen, D.S.; Müllertz, A. Development of gastro-resistant coated probiotic granulates and evaluation of viability and release during simulated upper gastrointestinal transit. LWT Food Sci. Technol. 2021, 144, 111174. [Google Scholar] [CrossRef]
- Moussavi, M.; Barouei, J.; Evans, C.; Adams, M.C.; Baines, S. Viability and In Vitro Gastrointestinal Transit Tolerance of Multispecies Probiotic Combinations Incorporated into Orange Juice and Drinking Water. Foods 2023, 12, 2249. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.C.; Messora, M.R. Effectiveness of Multi-strain Versus Single-strain Probiotics: Current Status and Recommendations for the Future. J. Clin. Gastroenterol. 2018, 52, S35. [Google Scholar] [CrossRef] [PubMed]
- Wendel, U. Assessing Viability and Stress Tolerance of Probiotics—A Review. Front. Microbiol. 2022, 12, 818468. [Google Scholar] [CrossRef] [PubMed]


| Probiotic Strain | Polymer | Solvent | Processing Conditions | Average Diameter | Reference |
|---|---|---|---|---|---|
| L. plantarum | Ca-alginate (A)/chitosan (Ch) | A: water Ch: water at pH 3.5 | 9.5 kV 100 mm 5 mL/h | 300–550 μm | [71] |
| L. acidophilus | Core: alginate/glycerol Shell: egg albumen and stearic acid | Water | 8 kV 6 cm 10 mL/h | 450 μm | [72] |
| Bifidobacterium longum | WPC, Fibersol® (F) Maltodextrin (M), Zein (Z); PVP | F and M: water WPC: skimmed milk Z: ethanol PVP: water | Not specified | WPC: 2.47 μm F: 87 μm M: 1.95 μm | [73] |
| B. animalis subsp. lactis Bb12 | Novel nanofiber mats consisting of chitosan (CS)/poly(vinyl alcohol) (PVA), inulin (INU) as a prebiotic | CS inacetic acid (0.5 M) PVA in water | 18 kV Tip-to-collector distance: 15 cm 0.1 mL/h | 117.5 to 217.6 nm | [74] |
| Lactobacillus strains | Gum Arabic (GA)-based nanofibers and pullulan | Deionized water | 16 kV 0.4 mL/h, Tip-to-collector distance: 10 cm | Nanofibers with a smaller diameter | [75] |
| L. plantarums | Polylactic acid and fructooligosaccharide | Dichloromethane and N, N-Dimethylformamide | 16 kV, 0.1–0.25 mL/h | Electrospun fibers | [76] |
| PVA/silk fibroin | n/a | n/a | [77] | ||
| L. paracasei | PVA and sodium-alginate | Water | 15–27 kV 0.4–1.6 mL/h | [78] | |
| Eudragit L100 and Na-alginate | Alcohol | 15 kV 1.0 mL/h | [79] |
| Matrix/Carrier | Food | Probiotic | Reference |
|---|---|---|---|
| Chitosan–alginate | - | Bifidobacterium breve | [101] |
| Chitosan-coated alginate beads | Pomegranate juice | Lactobacillus plantarum | [102] |
| Alginate–chitosan | Yogurt | Lactobacillus acidophilus | [103] |
| Chitosan/dextran sulfate multilayer polyelectrolytes | - | Saccharomyces boulardii | [104] |
| Nanostructured polyelectrolyte layers | - | Lactobacillus acidophilus | [105] |
| Chitosan and alginate | - | Bacillus coagulans | [106] |
| Single bilayer of alginate–chitosan and its double bilayer | - | Lacticaseibacillus rhamnosus | [107] |
| Chitosan and sulfated oat β-glucan | Oat β-glucan | L. acidophilus | [100] |
| Positively charged inner soy β-conglycinin and negatively charged outer high methoxyl pectin | Fish oil in water emulsions | [97] | |
| Na-alginate shells around Ca-alginate/starch beads | Corn starch | Bacteroides, Prevotellaceae | [96] |
| Probiotic | Emulsifier | Reference |
|---|---|---|
| L. salivarius NRRL B- 30514 | Sugar beet pectin | [114] |
| L. reuteri | Carboxymethyl konjac glucomannan–chitosan | [112] |
| L. plantarum | Polyglycerol poliricinoleate (PGPR) | [54] |
| L. reuteri | MCT oil (Miglyol® 812) | [115] |
| L. paracase | PGPR | [116] |
| L. rhamnosus | Sweet whey | [117] |
| L. rhamnosus | Inulin | [118] |
| L. delbrueckii | β-cyclodextrin | [113] |
| L. casei | β-cyclodextrin | [119] |
| Emulsion | Probiotics | Pickering Stabilizer | References |
|---|---|---|---|
| O/W HIPE | L. plantarum | Whey protein isolate (WPI)/(-)-epigallocatechin-3-gallate | [63] |
| L. rhamnosus GG | β-lactoglobulin-propylene glycol alginate composite nanoparticles | [111] | |
| W/O | None tested | Butyl methacrylate derivatives | [131] |
| O/W | L. casei | Calcium alginate | [132] |
| W/W | L. helveticus | Microcrystal celluloses | [133] |
| L. helveticus CICC 22536 | Alginate | [134] | |
| O/W | L. rhamnosus GG (LGG, ATCC 53103) | β-lactoglobulin-propylene glycol alginate | [111] |
| W/W | L. plantarum | Hydroxypropyl methylcellulose and dextran | [135] |
| O/W | L. acidophilus NRRL B-4495, Lactiplantibacillus plantarum NRRL B-4496 | Gelatin | [136] |
| Lactobacillus acidophilus BC | Nanoparticles | [125] | |
| L. plantarum | Alginate beads (emulbeads) | [137] | |
| L. casei ATCC 393 | Silica particles | [138] |
| Material | Encapsulation Technique | Purpose of the Encapsulation | Food Matrix | Encapsulated Probiotic Strain | Reference |
|---|---|---|---|---|---|
| Dextran γ-aminobutyric acid (GABA) and whey protein | Double emulsion (W1/O/W2) microcapsules | Stability, viability | Food formulations | L. plantarum NCDC 414 | [139] |
| Chitosan(Chi), alginate (Alg), and CaCl2(Ca) | Ultrasound-assisted multilayer W/O/W PE | The role of ultrasonic homogenization on the morphology of W1/O/W2 double emulsions, viability | Granular food | L. plantarum | [128] |
| Multilayer emulsions | Cell viability and physicochemical, rheological, structural, and sensorial properties of yogurts | Probiotic yogurts | L. rhamnosus and L. plantarum | [140] | |
| Alginate or low-methoxyl pectin hydrogel particles | Extrusion | Survival rate | Fermented nonmilk beverages | B. infantis ATCC15697 | [141] |
| Sodium alginate, Ferula assa-foetida gum, and Zedo (Amygdalus scoparia) | Microencapsulation | Physicochemical properties, sensory attributes, and probiotic survival | Dairy dessert | L. reuteri ATCC 23272 | [60] |
| Xanthan–chitosan–xanthan | Double-layer encapsulation | Viability | Yogurt | B. bifidum BB01 | [142] |
| Alginate, locust bean gum, and mannitol | Microencapsulation | Viability | Mulberry tea | L. acidophilus NCFM, | [143] |
| Alginate-coated chitosan beads | Microencapsulation | Viability | Carrot and tomato juices | L. plantarum, L. casei, L. fermentum, Sc. Boulardii, and Lysinibacillus sphaericus | [144] |
| Chitosan-coated alginate beads | Microencapsulation | Probiotic survival | Pomegranate juice | L. plantarum | [102] |
| Gum Arabic and β-cyclodextrin | Spray-drying and chilling | Textural and sensorial properties of the cake samples and probiotic survival | Cake | Sc. boulardii, L. acidophilus and B. bifidum | [145] |
| Alginate or xanthan gum as the first layer and gellan or chitosan as the outer layer | Microencapsulation | Viability | Bread | L. Sporogenes | [146] |
| Sodium alginate–galbanum (Ferula Gummosa Boiss) gum | Extrusion | Physicochemical, and textural properties of Tahini halva and probiotic viability | Tahini halva | L. acidophilus | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agriopoulou, S.; Smaoui, S.; Chaari, M.; Varzakas, T.; Can Karaca, A.; Jafari, S.M. Encapsulation of Probiotics within Double/Multiple Layer Beads/Carriers: A Concise Review. Molecules 2024, 29, 2431. https://doi.org/10.3390/molecules29112431
Agriopoulou S, Smaoui S, Chaari M, Varzakas T, Can Karaca A, Jafari SM. Encapsulation of Probiotics within Double/Multiple Layer Beads/Carriers: A Concise Review. Molecules. 2024; 29(11):2431. https://doi.org/10.3390/molecules29112431
Chicago/Turabian StyleAgriopoulou, Sofia, Slim Smaoui, Moufida Chaari, Theodoros Varzakas, Asli Can Karaca, and Seid Mahdi Jafari. 2024. "Encapsulation of Probiotics within Double/Multiple Layer Beads/Carriers: A Concise Review" Molecules 29, no. 11: 2431. https://doi.org/10.3390/molecules29112431
APA StyleAgriopoulou, S., Smaoui, S., Chaari, M., Varzakas, T., Can Karaca, A., & Jafari, S. M. (2024). Encapsulation of Probiotics within Double/Multiple Layer Beads/Carriers: A Concise Review. Molecules, 29(11), 2431. https://doi.org/10.3390/molecules29112431














