Efficacy of Glycyrrhetinic Acid in the Treatment of Acne Vulgaris Based on Network Pharmacology and Experimental Validation
Abstract
1. Introduction
2. Results
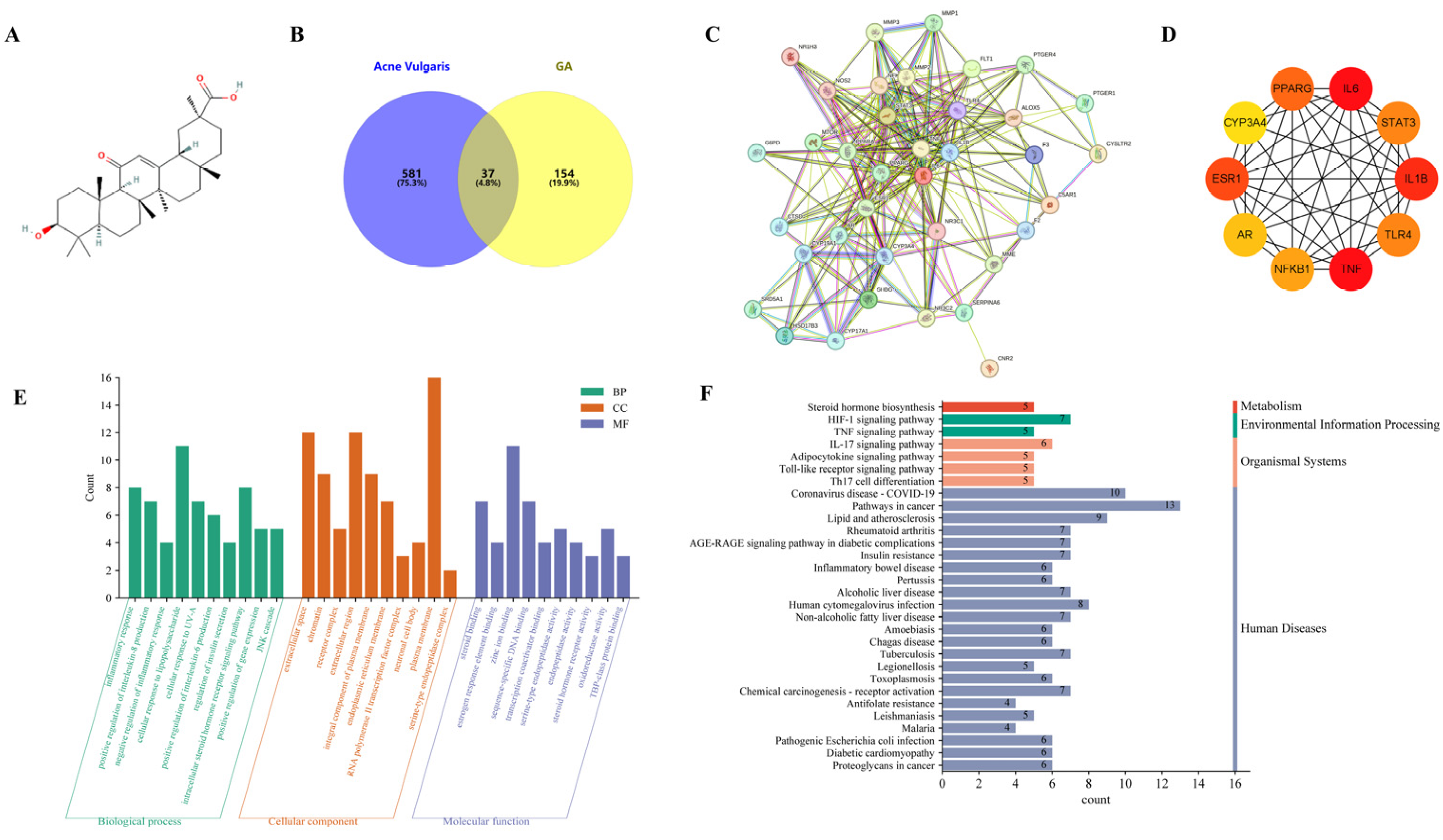
2.1. Network Pharmacology Analysis of GA against Acne Vulgaris
2.2. Molecular Docking
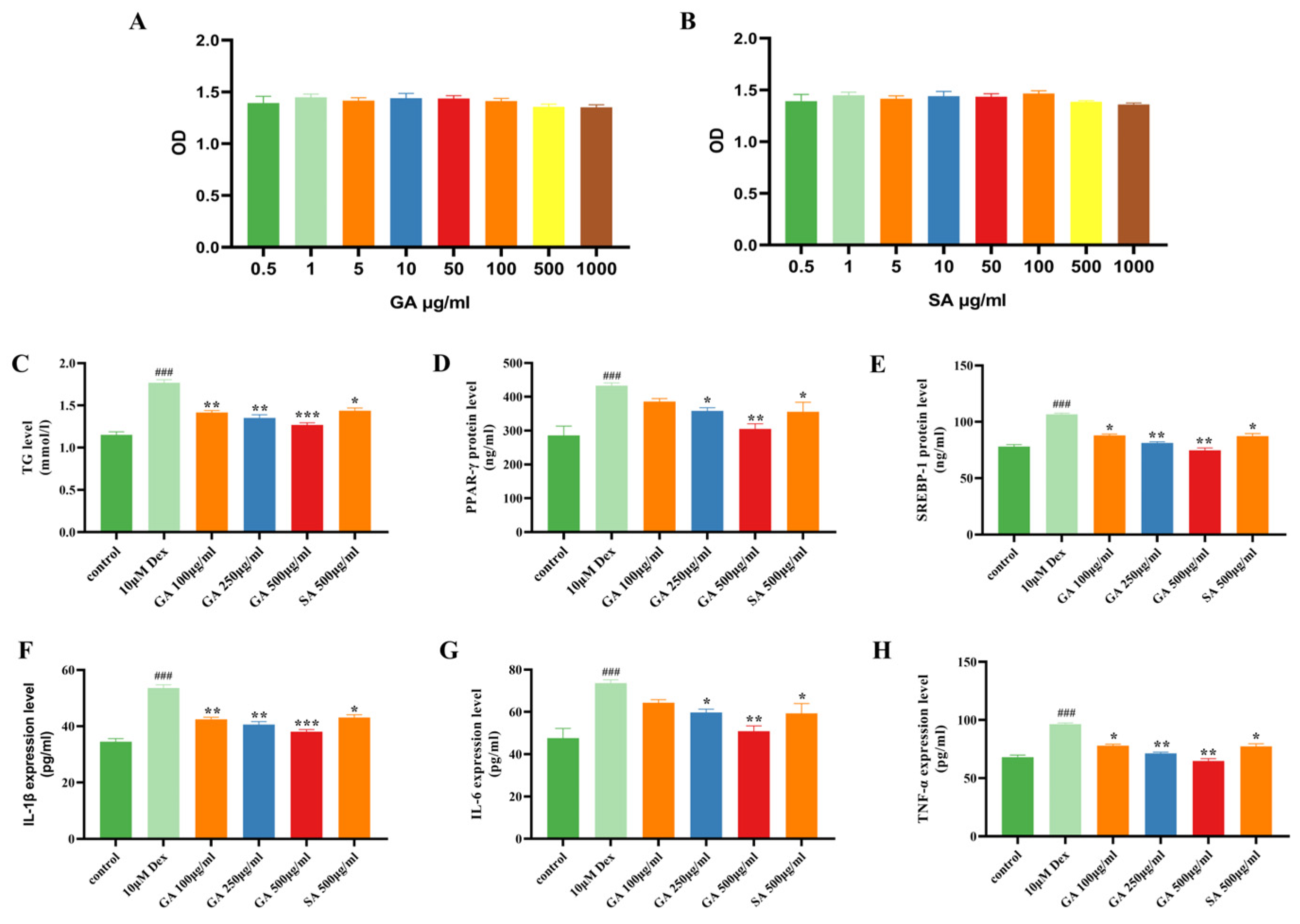
2.3. GA Inhibits Dex-Induced Lipogenesis and Inflammation in Human SZ95 Sebocytes
2.4. Effect of GA on a Mouse Model of Acne Vulgaris
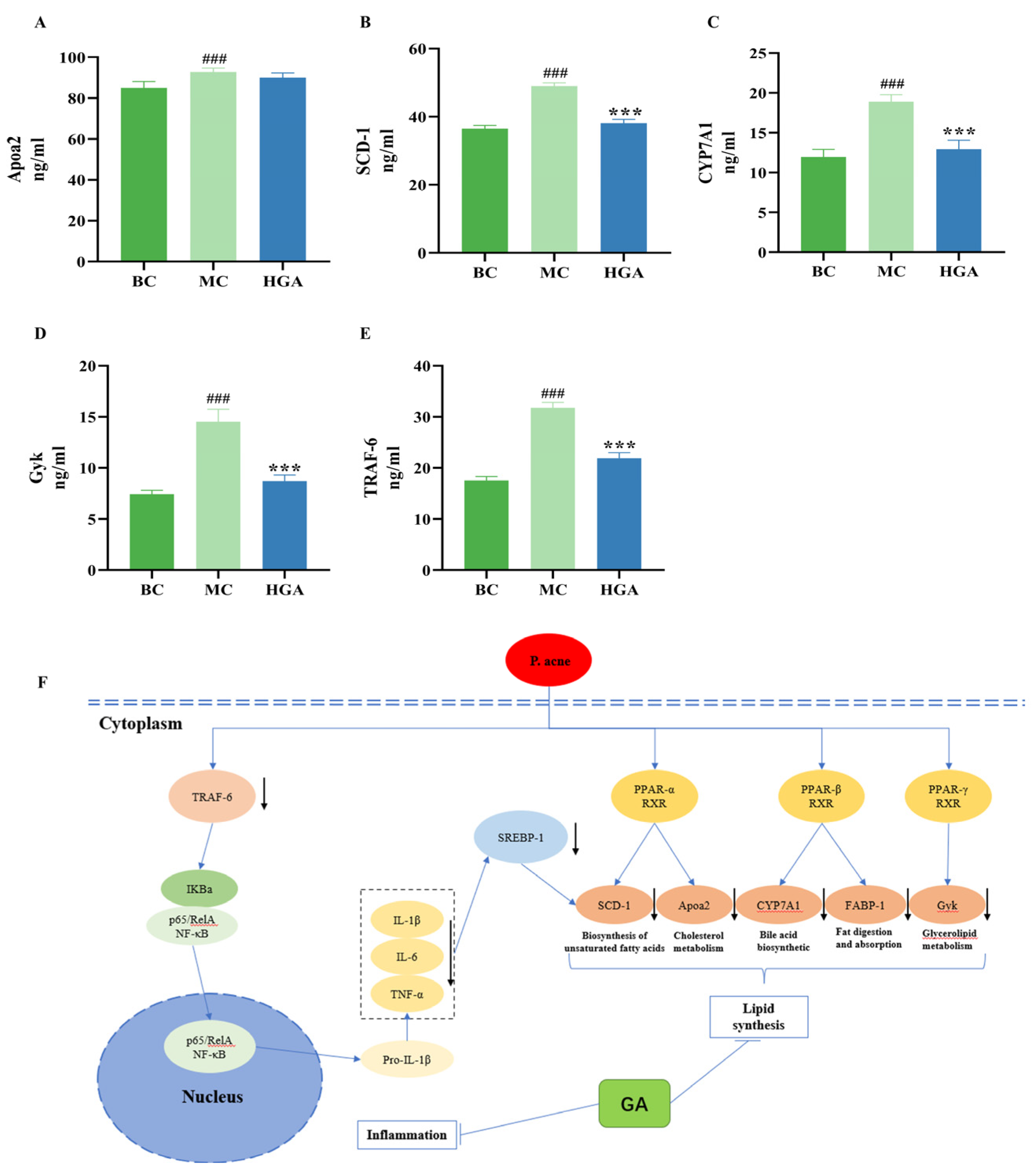
2.5. GA Suppresses Sebogenesis and Pro-Inflammatory Cytokines in a Mouse Model of Acne Vulgaris
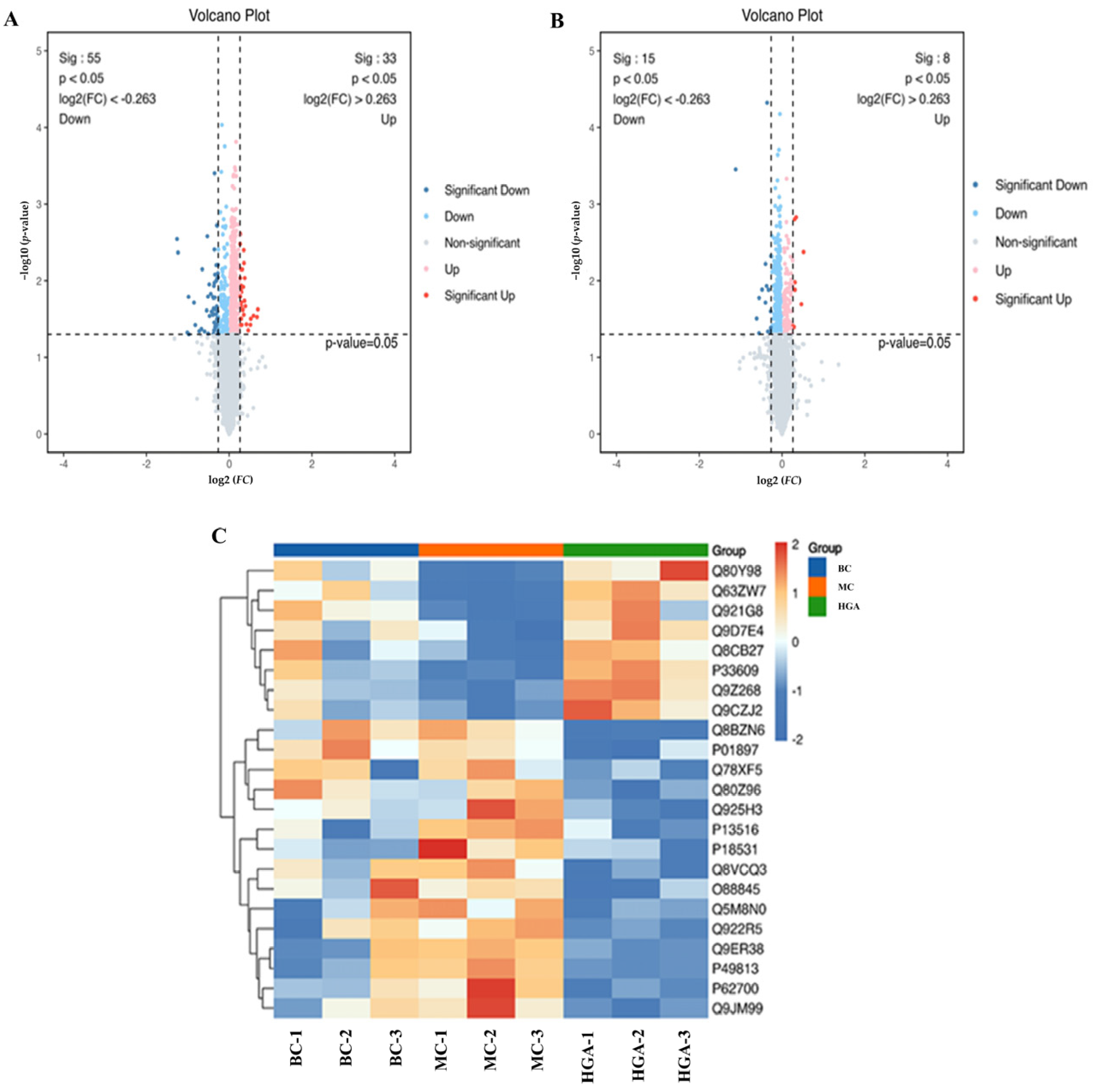
2.6. Differentially Expressed Proteins (DEPs) Analysis
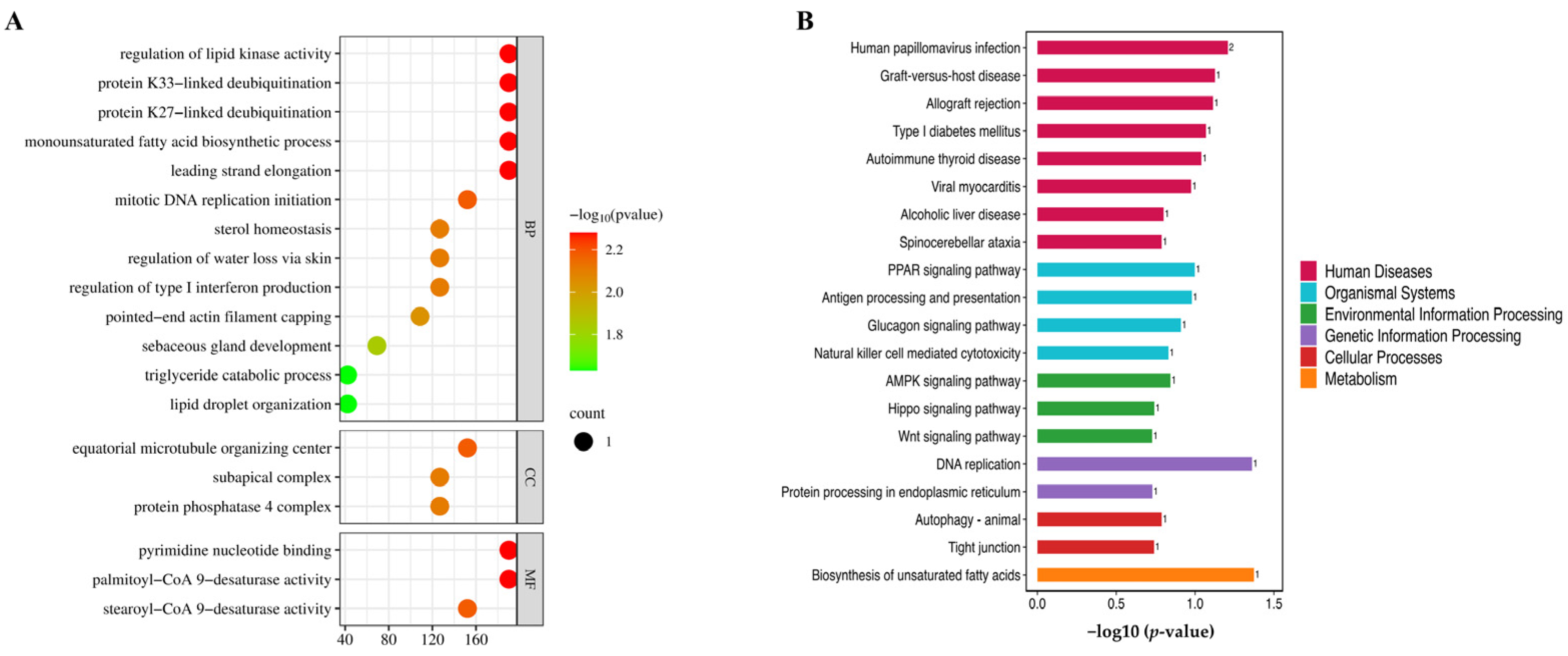
2.7. GO and KEGG Pathway Analysis
2.8. Validation of Proteins Identified by Proteomics Analyses
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Collecting Potential Targets of GA
4.3. PPI Analysis
4.4. Molecular Docking
4.5. Cell Culture
4.6. Cell Viability Essay
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Mice Treatments
4.9. Histopathological Examination
4.10. Immunohistochemistry
4.11. Establishment of a Target Database for Acne Vulgaris
4.12. Protein Digestion and TMT Labeling
4.13. LC-MS/MS Analysis
4.14. Proteomic Bioinformatic Analysis
4.15. Statistical Method
4.16. Ethics Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiang, C.; Ward, M.; Gooderham, M. Dermatology: How to manage acne in skin of colour. Drugs Context 2022, 11, 2021-10-9. [Google Scholar] [CrossRef]
- Collier, C.N.; Harper, J.C.; Cafardi, J.A.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The prevalence of acne in adults 20 years and older. J. Am. Acad. Dermatol. 2008, 58, 56–59. [Google Scholar] [CrossRef]
- Picardo, M.; Eichenfield, L.F.; Tan, J. Acne and Rosacea. Dermatol. Ther. 2017, 7 (Suppl. S1), 43–52. [Google Scholar] [CrossRef]
- Ju, Q.; Tao, T.; Hu, T.; Karadağ, A.S.; Al-Khuzaei, S.; Chen, W. Sex hormones and acne. Clin. Dermatol. 2017, 35, 130–137. [Google Scholar] [CrossRef]
- Nagy, I.; Pivarcsi, A.; Kis, K.; Koreck, A.; Bodai, L.; McDowell, A.; Seltmann, H.; Patrick, S.; Zouboulis, C.C.; Kemény, L. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect 2006, 8, 2195–2205. [Google Scholar] [CrossRef]
- Geueke, A.; Niemann, C. Stem and progenitor cells in sebaceous gland development, homeostasis and pathologies. Exp. Dermatol. 2021, 30, 588–597. [Google Scholar] [CrossRef]
- Schneider, M.R.; Paus, R. Sebocytes, multifaceted epithelial cells: Lipid production and holocrine secretion. Int. J. Biochem. Cell Biol. 2010, 42, 181–185. [Google Scholar] [CrossRef]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Propionibacterium acnes and sebaceous lipogenesis: A love-hate relationship? J. Investig. Dermatol. 2009, 129, 2093–2096. [Google Scholar] [CrossRef]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef]
- Hussain, H.; Ali, I.; Wang, D.; Hakkim, F.L.; Westermann, B.; Ahmed, I.; Ashour, A.M.; Khan, A.; Hussain, A.; Green, I.R.; et al. Glycyrrhetinic acid: A promising scaffold for the discovery of anticancer agents. Expert Opin. Drug Discov. 2021, 16, 1497–1516. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.F.; Hu, Y.; Wu, W.F.; Du, Q.Q.; Wang, Z.X.; Chen, T.T.; Shen, Q.; Liu, L.; Jiang, C.P.; Li, H.; et al. Explore the Anti-Acne Mechanism of Licorice Flavonoids Based on Metabonomics and Microbiome. Front Pharmacol. 2022, 13, 832088. [Google Scholar] [CrossRef]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, W.J.; Dou, J.H.; Gong, L.K. Research progress on the protective effects of licorice-derived 18β-glycyrrhetinic acid against liver injury. Acta Pharmacol. Sin. 2021, 42, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Y.; Wang, B.; Liu, L.; Chen, Y.; Chen, C.; Feng, L.; Chen, J.; Dong, N. Glycyrrhizin protects against particulate matter-induced lung injury via regulation of endoplasmic reticulum stress and NLRP3 inflammasome-mediated pyroptosis through Nrf2/HO-1/NQO1 signaling pathway. Int. Immunopharmacol. 2023, 120, 110371. [Google Scholar] [CrossRef]
- Shinu, P.; Gupta, G.L.; Sharma, M.; Khan, S.; Goyal, M.; Nair, A.B.; Kumar, M.; Soliman, W.E.; Rahman, A.; Attimarad, M.; et al. Pharmacological Features of 18β-Glycyrrhetinic Acid: A Pentacyclic Triterpenoid of Therapeutic Potential. Plants 2023, 12, 1086. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Peng, G.; Han, X. Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1. Int. Immunopharmacol. 2018, 60, 9–17. [Google Scholar] [CrossRef]
- Chen, F.N.; Wang, X.L.; Xu, R.R.; Wang, X.J.; Ruan, J.H. Preparation of tanshinone Ⅱ_A-glycyrrhetinic acid solid lipid nanoparticles and its inhibitory effect on acne. Zhongguo Zhong Yao Za Zhi 2022, 47, 2449–2456. [Google Scholar]
- Hao, D.C.; Xiao, P.G. Network pharmacology: A Rosetta Stone for traditional Chinese medicine. Drug Dev. Res. 2014, 75, 299–312. [Google Scholar] [CrossRef]
- Kaminsky, A. Less common methods to treat acne. Dermatology 2003, 206, 68–73. [Google Scholar] [CrossRef]
- Shalita, A.R. Comparison of a salicylic acid cleanser and a benzoyl peroxide wash in the treatment of acne vulgaris. Clin. Ther. 1989, 11, 264–267. [Google Scholar]
- Lee, S.E.; Kim, J.M.; Jeong, M.K.; Zouboulis, C.C.; Lee, S.H. 11β-hydroxysteroid dehydrogenase type 1 is expressed in human sebaceous glands and regulates glucocorticoid-induced lipid synthesis and toll-like receptor 2 expression in SZ95 sebocytes. Br. J. Dermatol. 2013, 168, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The protective effects of melittin on Propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Su, C.P.; Wang, Q.; Wu, F.J.; Bai, R.; Zhang, H.M.; Liu, J.Y.; Lu, W.J.; Wang, W.; Lan, F.; et al. Chlorogenic acid: A potent molecule that protects cardiomyocytes from TNF-α-induced injury via inhibiting NF-κB and JNK signals. J. Cell Mol. Med. 2019, 23, 4666–4678. [Google Scholar] [CrossRef]
- Tanghetti, E.A. The role of inflammation in the pathology of acne. J. Clin. Aesthet. Dermatol. 2013, 6, 27–35. [Google Scholar] [PubMed]
- Li, Z.J.; Choi, D.K.; Sohn, K.C.; Seo, M.S.; Lee, H.E.; Lee, Y.; Seo, Y.J.; Lee, Y.H.; Shi, G.; Zouboulis, C.C.; et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J. Investig. Dermatol. 2014, 134, 2747–2756. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Seltmann, H.; Alestas, T. Zileuton prevents the activation of the leukotriene pathway and reduces sebaceous lipogenesis. Exp. Dermatol. 2010, 19, 148–150. [Google Scholar] [CrossRef]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef]
- Ragab, M.; Hassan, E.M.; Elneily, D.; Fathallah, N. Association of interleukin-6 gene promoter polymorphism with acne vulgaris and its severity. Clin. Exp. Dermatol. 2019, 44, 637–642. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology-divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Zeng, H.; Qin, H.; Liao, M.; Zheng, E.; Luo, X.; Xiao, A.; Li, Y.; Chen, L.; Wei, L.; Zhao, L.; et al. CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing. Mol. Metab. 2022, 57, 101428. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Xia, L.; Akamatsu, H.; Seltmann, H.; Fritsch, M.; Hornemann, S.; Rühl, R.; Chen, W.; Nau, H.; Orfanos, C.E. The human sebocyte culture model provides new insights into development and management of seborrhoea and acne. Dermatology 1998, 196, 21–31. [Google Scholar] [CrossRef]
- Melnik, B.C. Western diet-induced imbalances of FoxO1 and mTORC1 signalling promote the sebofollicular inflammasomopathy acne vulgaris. Exp. Dermatol. 2016, 25, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.W.; Betzel, B.; Stoopen, G.; Berends, F.J.; Janssen, I.M.; Peijnenburg, A.A.; Kersten, S. The impact of PPARα activation on whole genome gene expression in human precision cut liver slices. BMC Genom. 2015, 16, 760. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Lee, Y.J.; Kim, H.J.; Choi, H.; Choi, Y.; Seok, J.W.; Kim, J.W. Monoacylglycerol O-acyltransferase 1 is regulated by peroxisome proliferator-activated receptor γ in human hepatocytes and increases lipid accumulation. Biochem. Biophys. Res. Commun. 2015, 460, 715–720. [Google Scholar] [CrossRef]
- Lin, D.; Alberton, P.; Caceres, M.D.; Volkmer, E.; Schieker, M.; Docheva, D. Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing. Cell Death Dis. 2017, 8, e3116. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xi, Q.Y.; Wei, S.; Wu, D.; Ye, R.S.; Chen, T.; Qi, Q.E.; Jiang, Q.Y.; Wang, S.B.; Wang, L.N.; et al. Critical role of miR-125b in lipogenesis by targeting stearoyl-CoA desaturase-1 (SCD-1). J. Anim. Sci. 2016, 94, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y.; Yang, T.; Gong, N.; Chen, X.; Liu, G.; Xiao, J. Integration of network pharmacology and molecular docking to explore the molecular mechanism of Cordycepin in the treatment of Alzheimer’s disease. Front Aging Neurosci. 2022, 14, 1058780. [Google Scholar] [CrossRef]
- Assenov, Y.; Ramírez, F.; Schelhorn, S.E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2008, 24, 282–284. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-H.; Shu, P.; Li, Y.-L.; Li, M.; Ye, Z.-H.; Chu, S.; Du, Z.-Y.; Dong, C.-Z.; Meunier, B.; Chen, H.-X. GDU-952, a novel AhR agonist ameliorates skin barrier abnormalities and immune dysfunction in DNFB-induced atopic dermatitis in mice. Biochem. Pharmacol. 2023, 217, 115835. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cheung, M.-K.; Yue, G.G.-L.; Leung, P.-C.; Wong, C.-K.; Lau, C.B.-S. Integrated Network Pharmacology Analysis and In Vitro Validation Revealed the Potential Active Components and Underlying Mechanistic Pathways of Herba Patriniae in Colorectal Cancer. Molecules 2021, 26, 6032. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | PDB ID/ Uniprot ID | Interactions | Cohesive Energy kcal/mol |
|---|---|---|---|
| PPAR-γ | 6C5Q | Pi-Alkyl, Pi-Sigma | −11.0 |
| TNF | 2AZ5 | Conventional hydrogen bond | −9.2 |
| IL-1β | 4DEP | Conventional hydrogen bond | −7.4 |
| IL-6 | 1P9M | Conventional hydrogen bond | −8.0 |
| TLR4 | 2Z62 | Conventional hydrogen bond, Pi-Alkyl | −8.0 |
| NFκB1 | 1VKV | Conventional hydrogen bond | −7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Ma, C.; Li, X.; Chen, H.; Han, P.; Lin, L.; Huang, W.; Xu, M.; Lu, H.; Du, Z. Efficacy of Glycyrrhetinic Acid in the Treatment of Acne Vulgaris Based on Network Pharmacology and Experimental Validation. Molecules 2024, 29, 2345. https://doi.org/10.3390/molecules29102345
Xie L, Ma C, Li X, Chen H, Han P, Lin L, Huang W, Xu M, Lu H, Du Z. Efficacy of Glycyrrhetinic Acid in the Treatment of Acne Vulgaris Based on Network Pharmacology and Experimental Validation. Molecules. 2024; 29(10):2345. https://doi.org/10.3390/molecules29102345
Chicago/Turabian StyleXie, Lingna, Congwei Ma, Xinyu Li, Huixiong Chen, Ping Han, Li Lin, Weiqiang Huang, Menglu Xu, Hailiang Lu, and Zhiyun Du. 2024. "Efficacy of Glycyrrhetinic Acid in the Treatment of Acne Vulgaris Based on Network Pharmacology and Experimental Validation" Molecules 29, no. 10: 2345. https://doi.org/10.3390/molecules29102345
APA StyleXie, L., Ma, C., Li, X., Chen, H., Han, P., Lin, L., Huang, W., Xu, M., Lu, H., & Du, Z. (2024). Efficacy of Glycyrrhetinic Acid in the Treatment of Acne Vulgaris Based on Network Pharmacology and Experimental Validation. Molecules, 29(10), 2345. https://doi.org/10.3390/molecules29102345






