A New 2-Aminospiropyrazolylammonium Cation with Possible Uses in the Topical Areas of Ionic Liquids
Abstract
1. Introduction
2. Topical Research Areas Related to Ionic Liquids and the Contribution of Spiroammonium Compounds
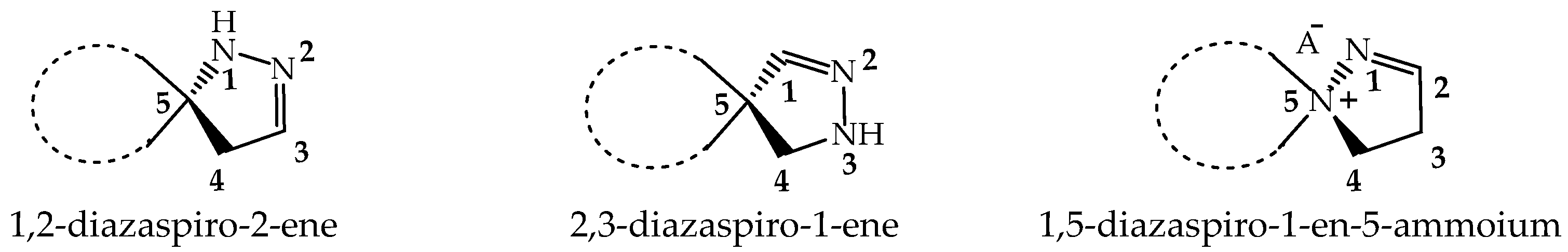
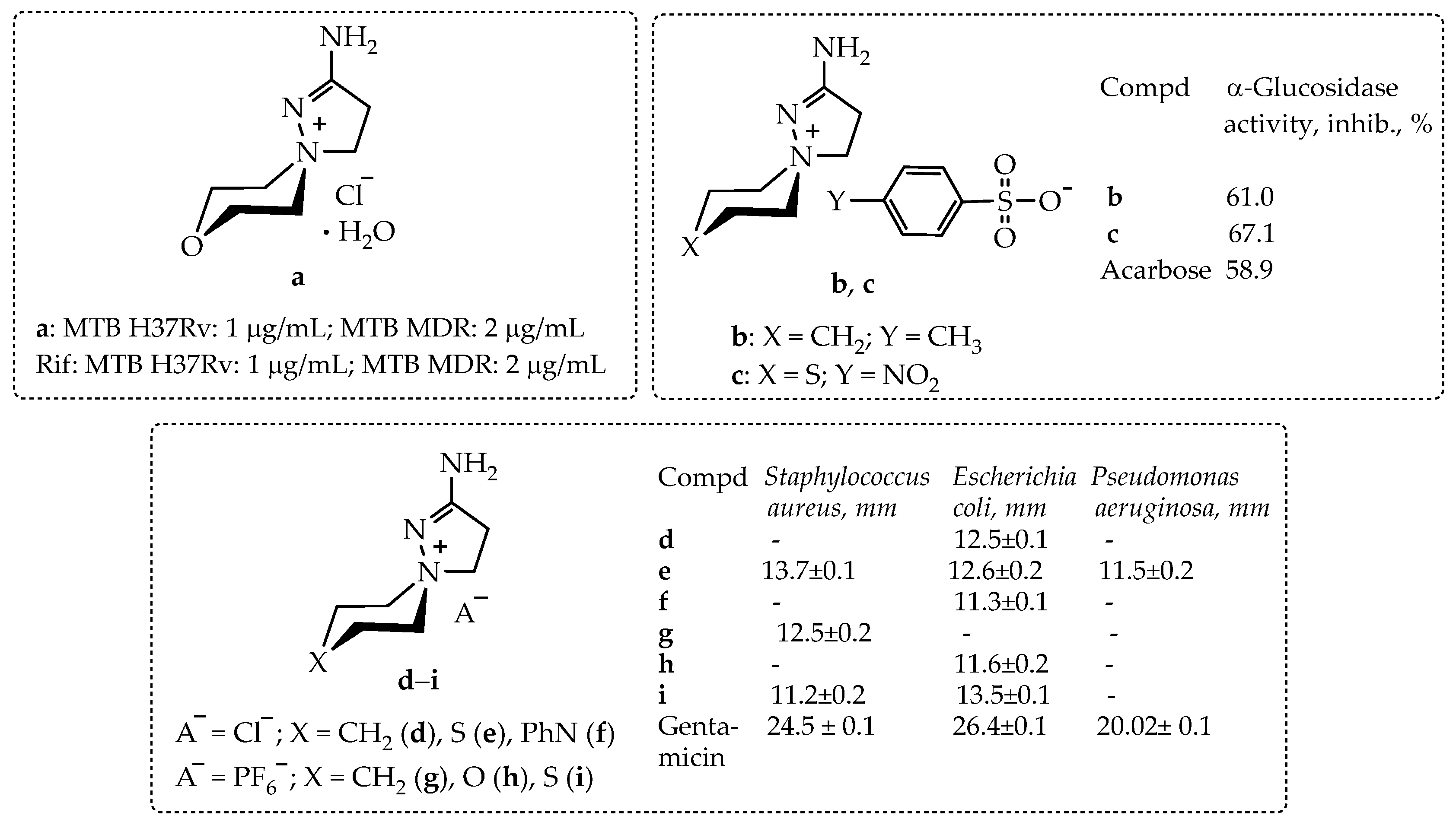
2.1. Synthesis and Structure of the New Biologically Active 2-Aminospiropyrazolylammonium Salts
2.2. Green Chemistry and the IL Concepts
2.2.1. The State of the Art
2.2.2. ILs Used in Industry
2.2.3. The Toxic Properties of ILs; Their Screening with Organisms of Different Trophic Levels
2.2.4. Creation of the Low-Toxic Ionic Liquids and the Variants of Their Use
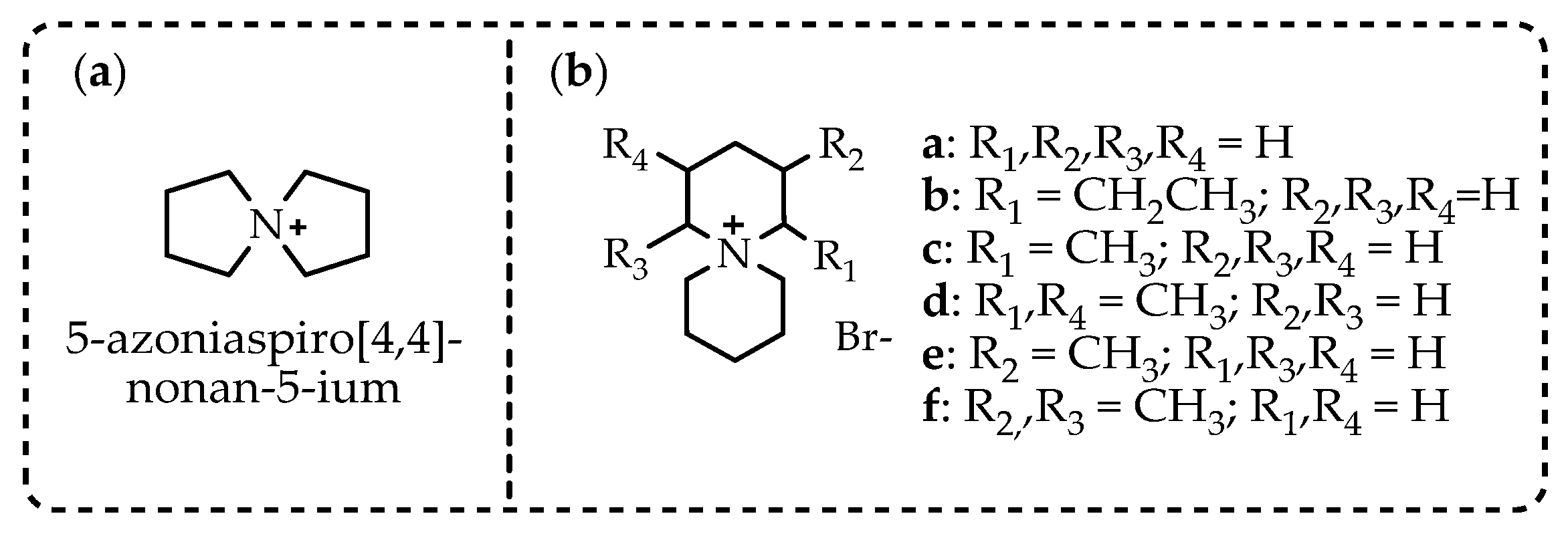
2.3. Azoniaspiroalkanes with IL Properties Used as Electrolytes
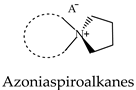
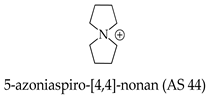
2.4. Green Hydrogen Production and Consumption
2.4.1. The State of the Art
2.4.2. Azoniaspiro Compounds Used as Anion Exchange Membrane Components for Water Electrolysis (AEM-WE) and Fuel Cells (AEM-FC)
2.5. The Use of Metal-Free Small Organic Molecules as Phase-Transfer Catalysts
2.5.1. The State of the Art
2.5.2. Azoniaspiro Compounds as Phase-Transfer Catalysts
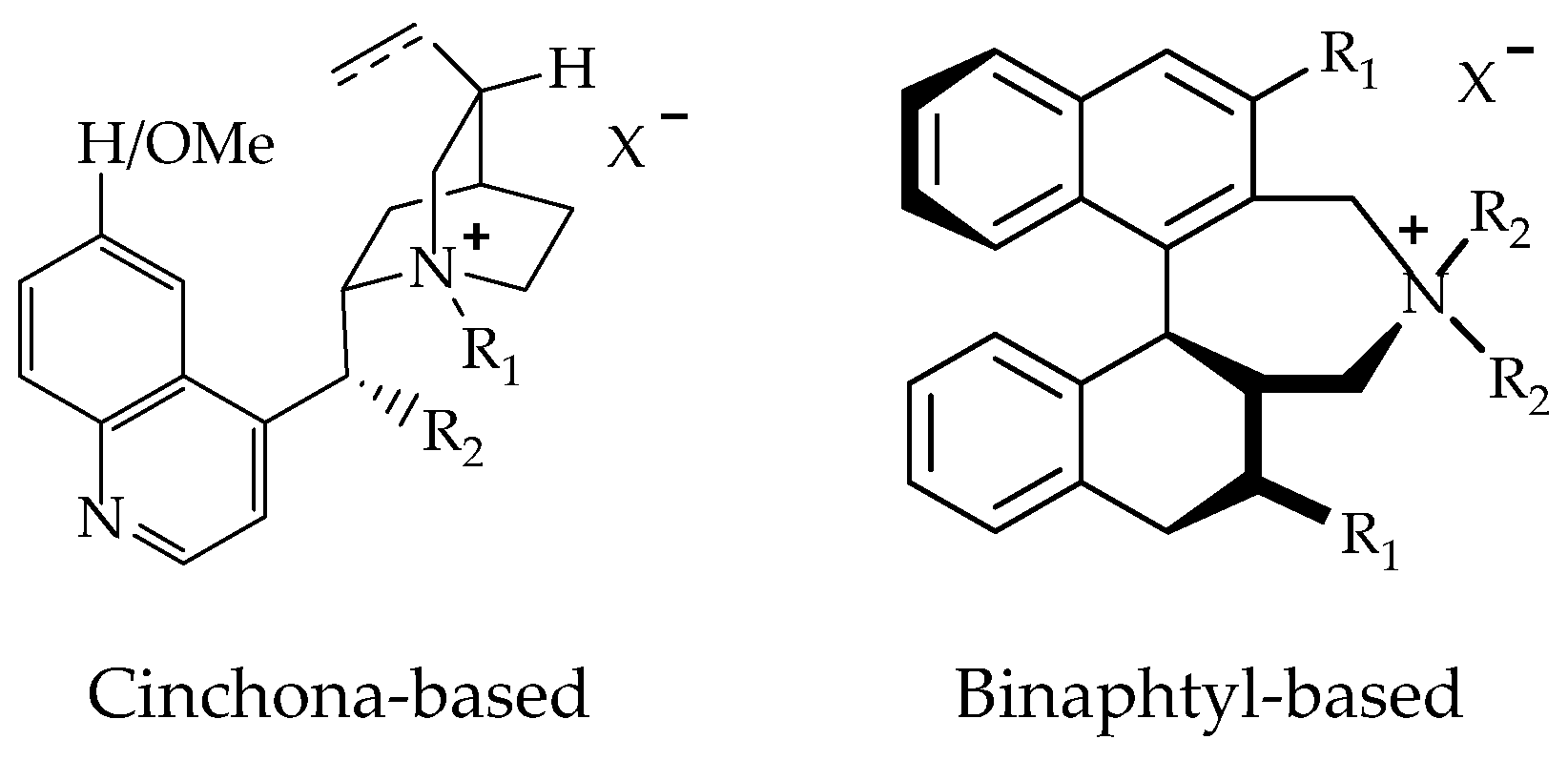
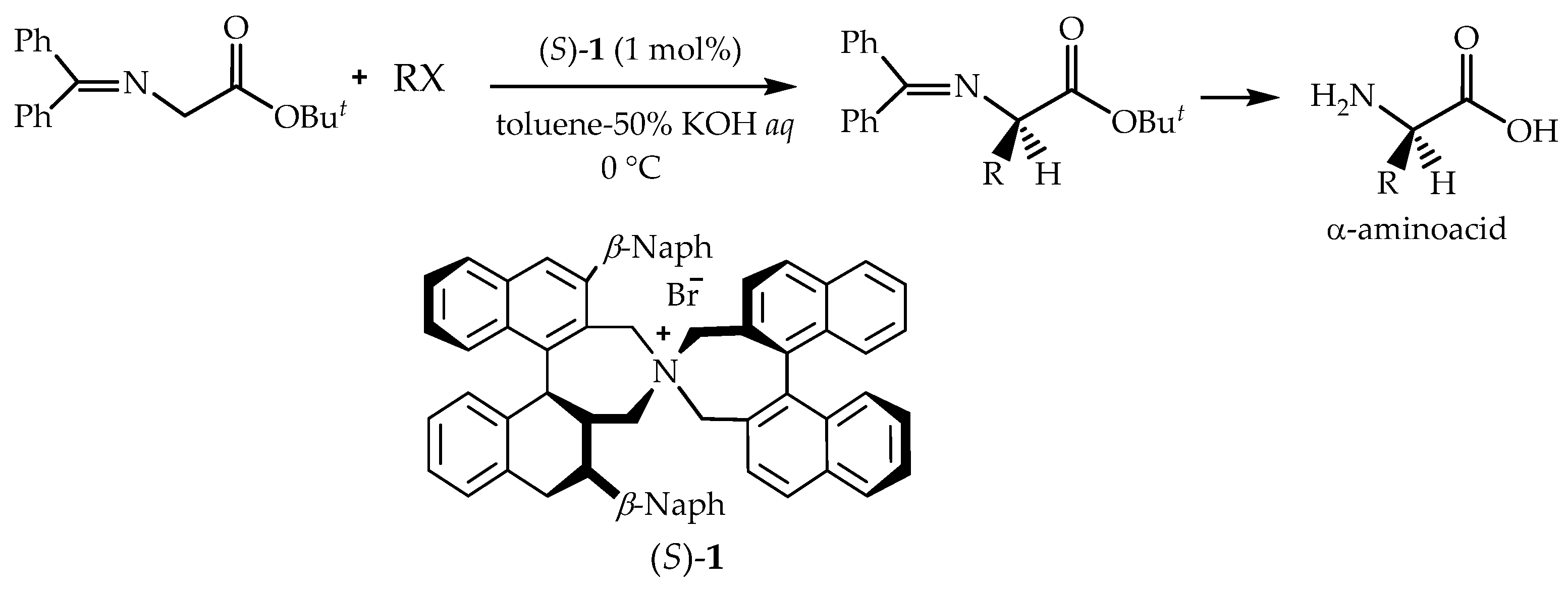
- Catalytic asymmetric alkylation reaction
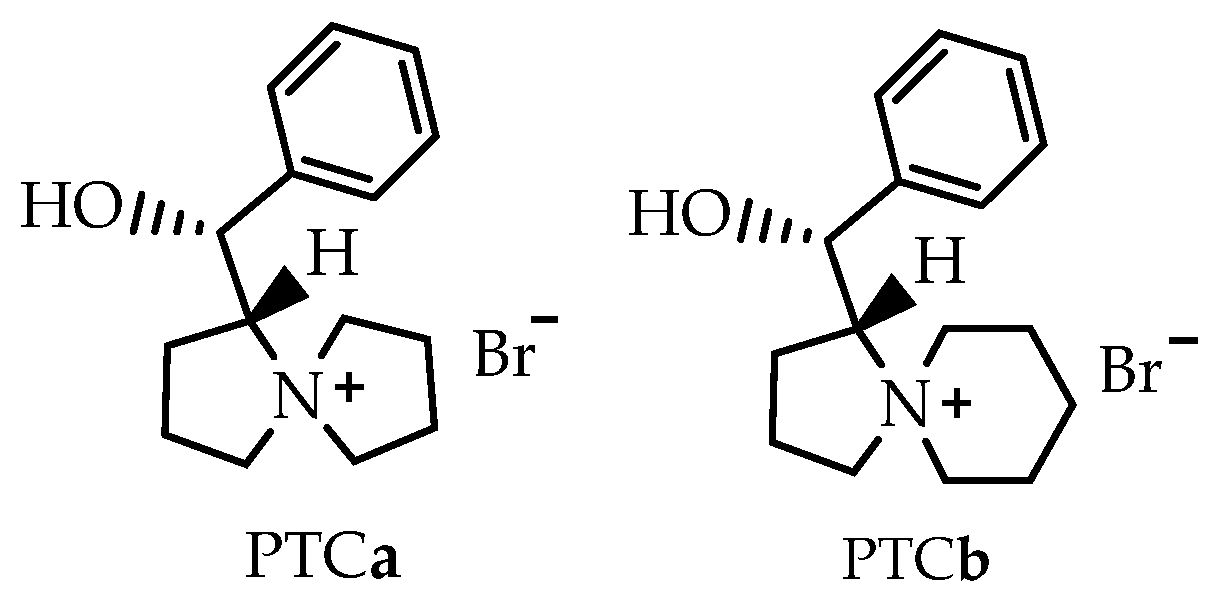
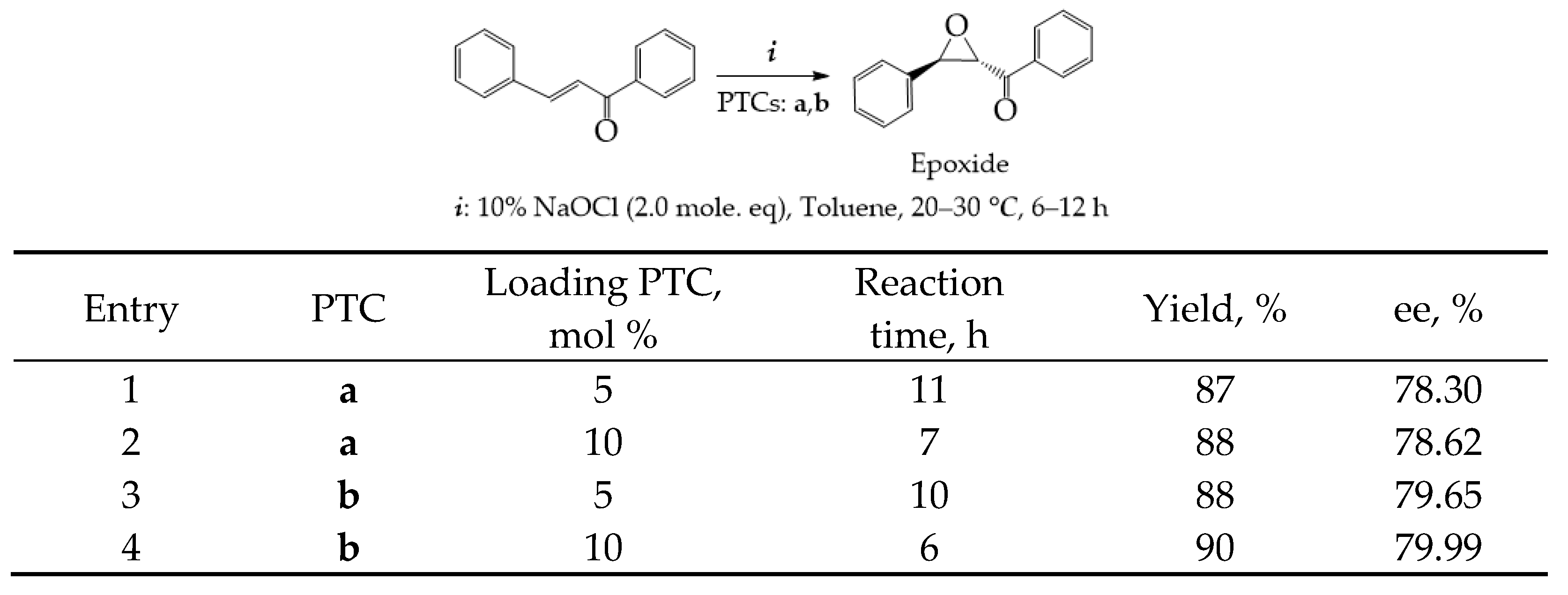
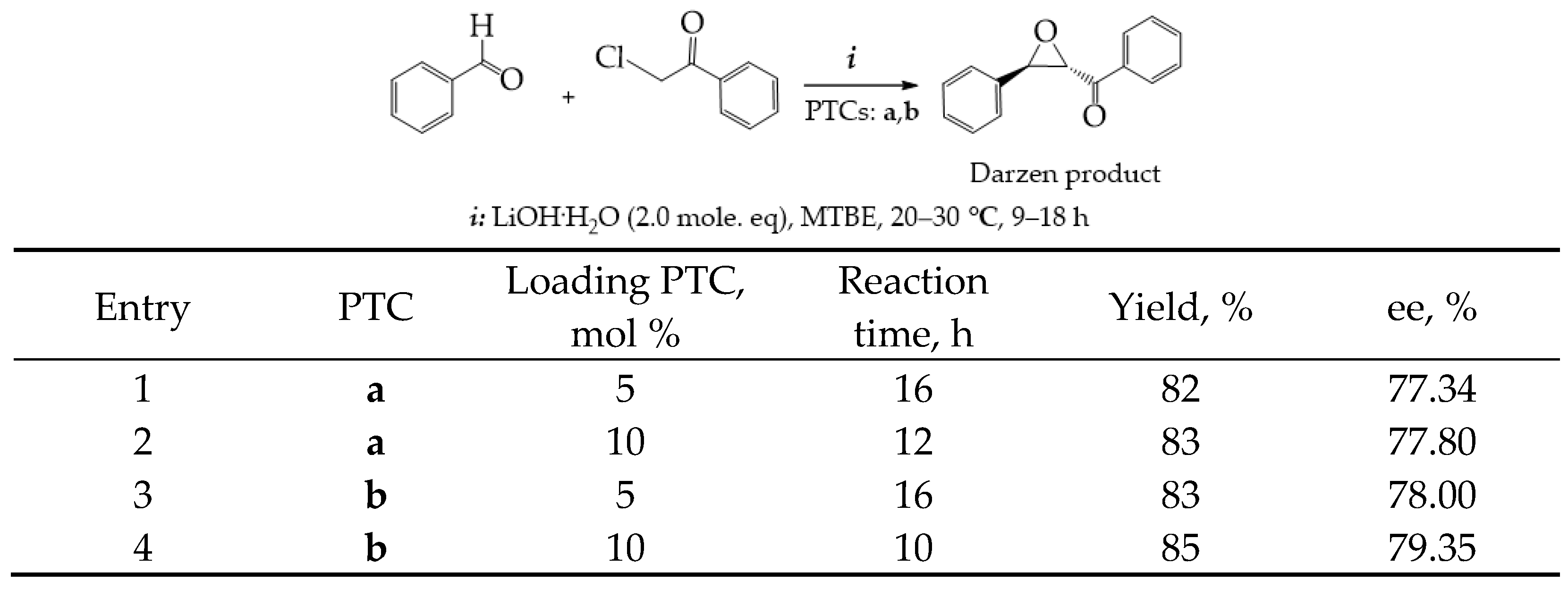
- The Darzen reaction and epoxy condensation
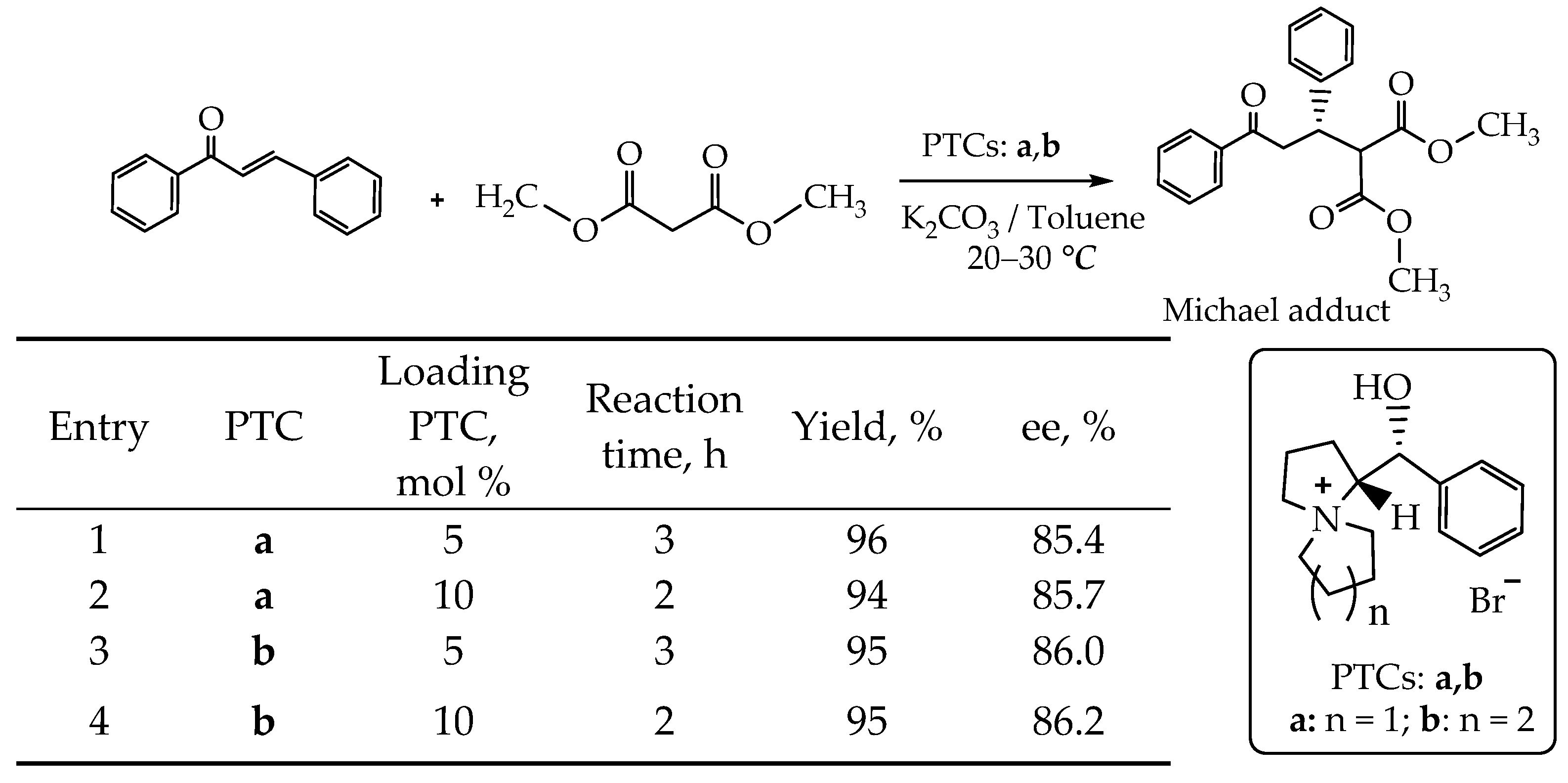
- The Michael addition
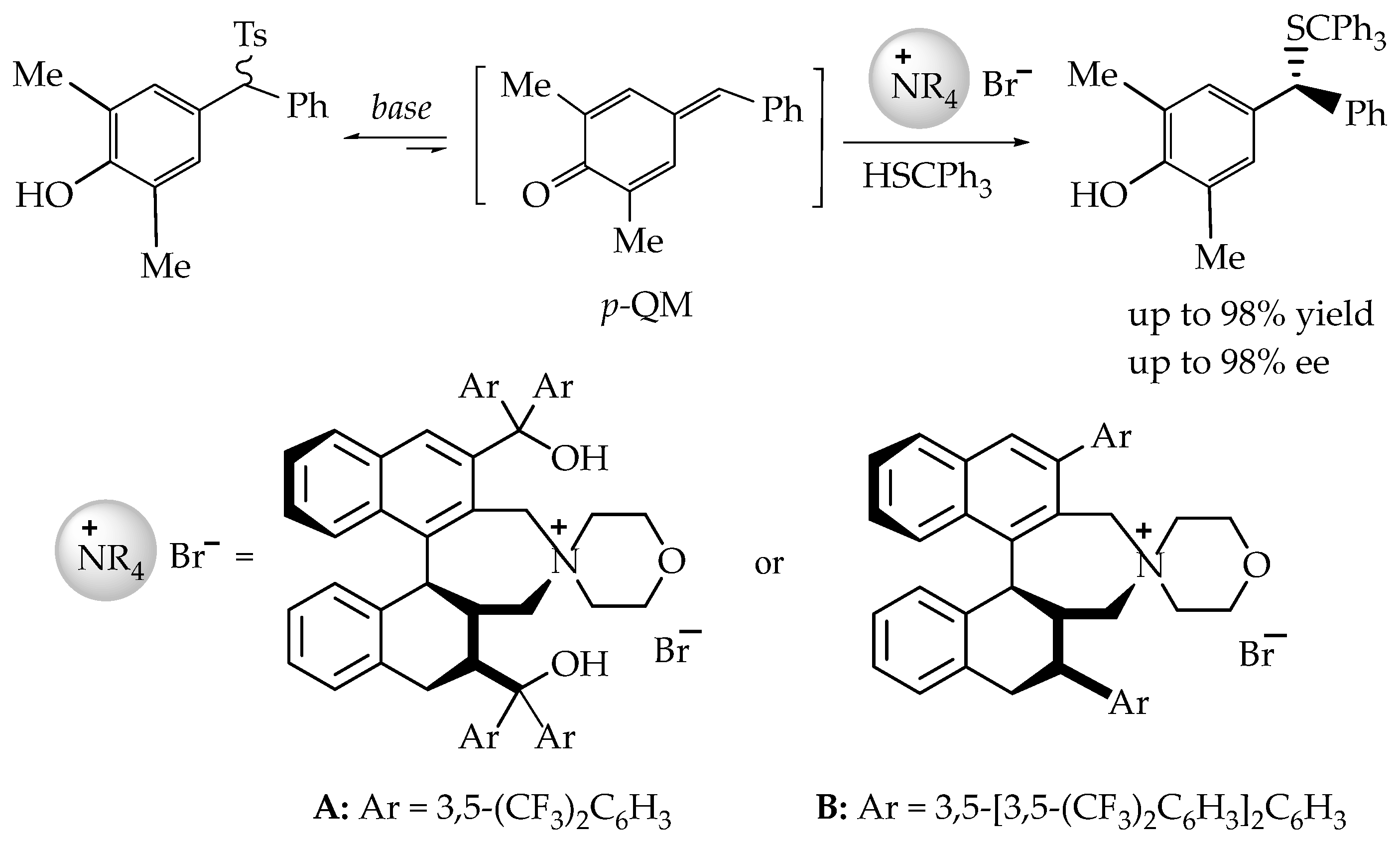
- The catalytic asymmetric 1,6-conjugate addition
2.6. Organic Compounds as Structure-Directing Agents in Zeolite Synthesis
2.6.1. The State of the Art
2.6.2. Azoniaspiro Compounds as Structure-Directing Agents
3. Conclusions
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ILs | ionic liquids |
| DIPEA | diisopropylethylamine |
| MTB H37Rv | drug-sensitive strains of M. tuberculosis |
| MTB MDR | Multidrug resistant strains of M. tuberculosis |
| GC | Green Chemistry |
| TSCA | Toxic Substances Control Act |
| r.t. | room temperature |
| TSCA | Toxic Substances Control Act |
| m.p. | melting point |
| [emim]Cl−AlCl3 | 1-ethyl-3-methylimidazolium chloride aluminum chloride |
| HF | hydrogen fluoride |
| FSI | bis(fluorosulfonyl)imide |
| [PF6] | hexafluorophosphate |
| Emim-Cl | 1-ethyl-3-methylimidazolium chloride |
| Bmim-Cl | 1-butyl-3-methylimidazolium chloride |
| Bmpy-Cl | 1-butyl-1-methylpyrrolidinium chloride |
| NBuPy-Cl | n-butylpyridinium chloride |
| LOELs | lowest observed effect levels |
| [Ch][AA] ILs | cholinium amino acid ionic liquids |
| [Ch][Ac] | cholinium acetate |
| Td | degradation temperature |
| EC50 | the concentration of ionic liquids at which the growth of microorganisms and cells is halved compared to that without the ionic liquid |
| QA | quaternary ammonium |
| LOELs | lowest observed effect levels |
| Tonset | thermal stability of ionic liquids—decomposition temperature, °C |
| FP | flash point |
| TGA/DSC | thermogravimetry and differential scanning calorimetry |
| TGA-FTIR | thermogravimetric analysis coupled with Fourier transformed infrared spectrometry |
| AA ILs | сholinium and amino acid-based ionic liquids |
| [Ch][AcO] | choline acetate |
| [Ch][Cl] | choline chloride |
| [Ch][For] | choline formate |
| [Ch][Lac] | choline lactate |
| [Ch][Pro] | choline propionate |
| [Ch][Ole] | choline oleate |
| BHET | bis-hydroxyethyl terephthalate |
| CHILs | carbohydrate-based ILs |
| MWCNTs | multiwalled carbon nanotubes |
| GTA | N-[2-(d-glucopyranosyl)ethyl]-N,N,N-trimethylammonium) cations |
| [N(SO2CF3)2]− | bis((trifluoromethyl)sulfonyl)methanide |
| QA BF4 | quaternary ammonium tetrafluoro borate |
| UNFCCC | United Nations Framework Convention on Climate Change |
| GHG | greenhouse gas |
| FCHEA | Fuel Cell and Hydrogen Energy Association |
| A-WE | alkaline water electrolysis |
| AEM | anion exchange membrane |
| AEM-WE | anion exchange membrane water electrolysis |
| PEM-WE | proton exchange membrane water electrolysis |
| SO-WE | solid oxide water electrolysis |
| AEM-FCs | anion exchange membrane fuel cells |
| PEM-FCs | proton exchange membrane fuel cells |
| ASD | 5-azonia-spiro-[4.5]-decane |
| ASU | 6-azonia-spiro-[5.5]-undecane |
| PTC | phase-transfer catalysis |
| p-QM | para-quinone methides |
| TBAB | tetrabutylammonium bromide |
| MTBE | methyl tert-butyl ether |
| IZA | International Zeolite Association |
| SDAs | structure-directing agents |
References
- Cuevas-Yañez, E.; Unnamatla, M.V.B.; García-Eleno, M.A.; Chakroborty, S. 12.20–Systems with a Spirocyclic Heteroatom. In Comprehensive Heterocyclic Chemistry IV, 4th ed.; Aldabbagh, F., Ed.; Elsevier: Amsterdam, The Netherlands; Kingston University: London, UK, 2022; Voluem 3, pp. 154–196. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Vologzhanina, A.V.; Ergaliyeva, E.M. Spiropyrazolinium Compounds as a Result of β-Aminopropioamidoximes Intramolecular Rearrangements (Monograph); Daryn: Kazakhstan, Almaty, 2022; 156p. [Google Scholar] [CrossRef]
- Doulou, I.; Kontogiorgis, C.; Koumbis, A.E.; Evgenidou, E.; Hadjipavlou-Litina, D.; Fylaktakidou, K.C. Synthesis of stable aromatic and heteroaromatic sulfonyl-amidoximes and evaluation of their antioxidant and lipid peroxidation activity. Eur. J. Med. Chem. 2014, 80, 145–153. [Google Scholar] [CrossRef]
- Tiemann, F. Ueber die Einwirkung von Benzolsulfonsäurechlorid auf Amidoxime. Eur. J. Inorg. Chem. 1891, 24, 4162–4167. [Google Scholar] [CrossRef]
- Bakunov, S.A.; Rukavishnikov, A.V.; Tkachev, A.V. Modification of the Tiemann rearrangement: One-pot synthesis of N,N-disubstituted cyanamides from amidoximes. Synthesis 2000, 8, 1148–1159. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsieh, T.H.; Liao, P.Y.; Liao, Z.Y.; Chang, C.W.; Shih, Y.C.; Yeh, W.H.; Chien, T.C. Practical synthesis of N-substitutedcyanamides via Tiemann rearrangement of amidoximes. Org. Lett. 2014, 16, 892–895. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Praliyev, K.D.; Myrzabek, A.B.; Kainarbayeva, Z.N. Arylsulfochlorination of β-aminopropioamidoximes giving 2-aminospiropyrazolylammonium arylsulfonates. Rus. Chem. Bul. (Int. Ed.) 2020, 69, 496–503. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Baitursynova, G.P.; Yergaliyeva, E.M.; Zhaksylyk, B.A.; Yelibayeva, N.S.; Kurmangaliyeva, A.B. Arylsulphonates of spiropyrazolines and O-tosilate-β-(benzimidazol-1-yl)propioamidoxime as the products of β-aminopropioamidoximestosylation. Chem. J. Kaz. 2021, 2, 22–32. [Google Scholar] [CrossRef]
- Kayukova, L.; Vologzhanina, A.; Dorovatovskii, P.; Baitursynova, G.; Yergaliyev, E.; Kurmangaliyeva, A.; Shulgau, Z.; Adekenov, S.; Shaimerdenova, Z.; Akata, K. Reaction Products of β-Aminopropioamidoximes Nitrobenzenesulfochlorination: Linear and Rearranged to Spiropyrazolinium Salts with Antidiabetic Activity. Molecules 2022, 27, 2181. [Google Scholar] [CrossRef]
- Kayukova, L.; Vologzhanina, A.; Dorovatovskii, P.; Yergaliyeva, E.; Uzakova, A.; Duissenali, A. N-N(+) Bond-Forming Intramolecular Cyclization of O-Tosyloxy β-Aminopropioamidoximes and Ion Exchange Reaction for the Synthesis of 2-Aminospiropyrazolilammonium Chlorides and Hexafluorophosphates. Int. J. Mol. Sci. 2023, 24, 11315. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Uzakova, A.B.; Vologzhanina, A.V.; Akatan, K.; Shaymardan, E.; Kabdrakhmanova, S.K. Rapid Boulton–Katritzky rearrangement of 5-aryl-3-[2-(piperidin-1-yl)ethyl]-1,2,4-oxadiazoles upon exposure to water and HCl. Chem. Heterocycl. Compd. 2018, 54, 643–649. [Google Scholar] [CrossRef]
- Kayukova, L.; Vologzhanina, A.; Praliyev, K.; Dyusembaeva, G.; Baitursynova, G.; Uzakova, A.; Bismilda, V.; Chingissova, L.; Akatan, K. Boulton-Katritzky Rearrangement of 5-Substituted Phenyl-3-[2-(morpholin-1-yl)ethyl]-1,2,4-oxadiazoles as a Synthetic Path to Spiropyrazoline Benzoates and Chloride with Antitubercular Properties. Molecules 2021, 26, 967. [Google Scholar] [CrossRef]
- Dadiboyena, S. Cycloadditions and condensations as essential tools in spiropyrazoline synthesis. Eur. J. Med. Chem. 2013, 63, 347–377. [Google Scholar] [CrossRef]
- Sadiq, Z.; Naz, S.; Hussain, A.E.; Aslam, U. Spiropyrazolines: A Worthy Insight into the Recent Strategies and Synthetic Applications. Lett. Org. Chem. 2019, 16, 357–391. [Google Scholar] [CrossRef]
- Dadiboyena, S.; Valente, E.J.; Hamme, A.T., II. Synthesis and Tautomerism of Spiro-Pyrazolines. Tetrahedron Lett. 2014, 5514, 2208–2211. [Google Scholar] [CrossRef]
- Sustainable Development Goals. United Nations Programme. 2022. Available online: https://www.undp.org/sustainable-development-goals#responsible-consumption-and-production (accessed on 10 March 2024).
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Buch, D.H. ACS GCI: Past, Present, and Future. Chem. Eng. News 2010, 88, 63. [Google Scholar] [CrossRef]
- Chapter 3-Legislation and Law. In Lees’ Loss Prevention in the Process Industries, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1, pp. 42–50. [CrossRef]
- Green Chemistry Considerations in the Design of Ionic Liquids. Available online: https://www.researchgate.net/publication/267358606 (accessed on 7 September 2023).
- Vedavathi, T.; Srinivas, T. Ionic Liquids—A New Era in Green Chemistry. Int. J. Eng. Comput. Sci. IJECS 2017, 6, 19916–19920. [Google Scholar] [CrossRef]
- Ratti, R. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014, 2014, 729842. [Google Scholar] [CrossRef]
- Freemantle, M. Designer solvents: Ionic liquids may boost clean technology development. Chem. Eng. News 1998, 76, 32–38. [Google Scholar] [CrossRef]
- Boeck, G. Paul Walden (1863–1957): The man behind the Walden inversion, the Walden rule, the Ostwald-Walden-Bredig rule and Ionic liquids. Chem. Texts 2019, 5, 6. [Google Scholar] [CrossRef]
- Walden, P. Ueber die Molekulargroesse und elektrische Leitfähigkeit einiger geschmolzenen Salze. Bull. Acad. Imp. Sci. St-Petersbourg 1914, VII, 405–422. Available online: https://www.mathnet.ru/eng/im/v8/i6/p405 (accessed on 9 May 2024).
- Wu, J.; Xie, P.; Hao, W.; Lu, D.; Qi, Y.; Mi, Y. Ionic liquids as electrolytes in aluminum electrolysis. Front. Chem. 2022, 10, 1014893. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef]
- Ionic Liquids. Introduction. Available online: http://www.sigmaaldrich.com (accessed on 9 May 2024).
- Amrutkar, R.D.; Varpe, V.D.; Sonawane, R.S.; Bhamare, S.V. Ionic liquids: A green solvent for organic synthesis. Curr. Trends Pharm. Pharm. Chem. 2023, 5, 58–62. [Google Scholar] [CrossRef]
- Qureshi, Z.S.; Deshmukh, K.M.; Bhanage, B.M. Applications of ionic liquids in organic synthesis and catalysis. Clean Techn. Environ. Policy 2014, 16, 1487–1513. [Google Scholar] [CrossRef]
- Sheldon_Gabrić, B.; Sander, A.; Cvjetko Bubalo, M.; Macut, D. Extraction of S- and N-Compounds from the Mixture of Hydrocarbons by Ionic Liquids as Selective Solvents. Sci. World J. 2013, 2013, 512953. [Google Scholar] [CrossRef]
- Zhao, Q.; Anderson, J.L. 2.11 Ionic Liquids. Comprehensive Sampling and Sample Preparation. Ion. Liq. 2012, 2, 213–242. [Google Scholar] [CrossRef]
- Bogolitsyn, K.G.; Skrebets, T.E.; Makhova, T.A. Physicochemical properties of 1-butyl-3-methylimidazolium acetate. Russ. J. Gen. Chem. 2009, 79, 125–128. [Google Scholar] [CrossRef]
- Rogstad, D.T.; Einarsrud, M.-A.; Svensson, A.M. High-Temperature Performance of Selected Ionic Liquids as Electrolytes for Silicon Anodes in Li-ion Batteries. J. Electrochem. Soc. 2022, 169, 110531. [Google Scholar] [CrossRef]
- Somers, A.; Howlett, P.; MacFarlane, D.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Bermúdez, M.-D.; Jiménez, A.-E.; Sanes, J.; Carrión, F.-J. Ionic Liquids as Advanced Lubricant Fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. Advanced lubricant fluids. In Biolubricants; Woodhead Publishing: Sawston, UK, 2013; pp. 824–846. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Rynkowska, E. Advancements in proton exchange membranes for high-performance high-temperature proton exchange membrane fuel cells (HT-PEMFC). Rev. Chem. Eng. 2022, 38, 327–346. [Google Scholar] [CrossRef]
- Das, G.; Choi, J.-H.; Nguyen, P.K.T.; Kim, D.-J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef]
- Karimi, N.; Zarrabeitia, M.; Mariani, A.; Gatti, D.; Varzi, A.; Passerini, S. Nonfluorinated Ionic Liquid Electrolytes for Lithium Metal Batteries: Ionic Conduction, Electrochemistry, and Interphase Formation. Adv. Energy Mater. 2021, 11, 2003521. [Google Scholar] [CrossRef]
- Shiflett, M.B. (Ed.) Commercial Applications of Ionic Liquids; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Castro, C.N.; Ribeiro, A.P.C.; Vieira, S.I.C.; Franca, J.M.P.; Lourenco, M.J.V.; Santos, F.V.; Murshed, S.M.S.; Goodrich, P.; Hardacre, C. Synthesis, Properties and Physical Applications of IoNanofluids’. In Ionic Liquids: New Aspects for the Future; Kadokawa, J., Ed.; InTech: London, UK, 2013; pp. 165–193. [Google Scholar] [CrossRef][Green Version]
- Matuszek, K.; Piper, S.L.; Brzeczek-Szafran, A.; Roy, B.; Saher, S.; Pringle, J.M.; MacFarlane, D.R. Unexpected Energy Applications of Ionic Liquids. Adv. Mater. 2024, e2313023. [Google Scholar] [CrossRef]
- Matuszek, K.; Kar, M.; Pringle, J.M.; Macfarlane, D.R. Phase Change Materials for Renewable Energy Storage at Intermediate Temperatures. Chem. Rev. 2023, 123, 491–514. [Google Scholar] [CrossRef]
- Piper, S.L.; Kar, M.; MacFarlane, D.R.; Matuszek, K.; Pringle, J.M. Ionic liquids for renewable thermal energy storage—A perspective. Green Chem. 2022, 24, 102–117. [Google Scholar] [CrossRef]
- Ionic Liquid Market Size, Share, Demand. CAGR of 9.5% Global Ionic Liquid Market. Published Date: January 2024. Report ID: 24071. Number of Pages: 300. One Stop Shop for Market Research Reports. Available online: https://market.us (accessed on 9 May 2024).
- Available online: https://www.mordorintelligence.com/industry-reports/ionic-liquid-market/companies (accessed on 9 May 2024).
- Available online: https://www.chemeurope.com/en/companies/ionic-liquids/order_t/ (accessed on 9 May 2024).
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Samir, I. Ionic Liquids Recycling for Reuse. Ionic Liquids–Classes and Properties; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Liu, Y.; Meyer, A.S.; Nie, Y.; Zhang, S.; Thomsen, K. Low energy recycling of ionic liquids via freeze crystallization during cellulose spinning. Green Chem. 2018, 20, 493–501. [Google Scholar] [CrossRef]
- Elsayed, S.; Hellsten, S.; Guizani, C.; Witos, J.; Rissanen, M.; Rantamäki, A.H.; Varis, P.S.; Wiedmer, K.; Sixta, H. Recycling of Superbase-Based Ionic Liquid Solvents for the Production of Textile-Grade Regenerated Cellulose Fibers in the Lyocell Process. ACS Sustain. Chem. Eng. 2020, 8, 14217–14227. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Abd Hamid, S.B.; Khalid, K.; Chowdhury, Z.Z.; Mohd Zain, S.; Abd Hamid, S.B.; Khalid, K. Catalytic role of ionic liquids for dissolution and degradation of biomacromolecules. BioResources 2014, 9, 1787–1823. [Google Scholar] [CrossRef]
- Tremblay, J.-F. Polyester made from coal? China is betting on it. Petrochemicals 2019, 97, 22–24. Available online: https://cen.acs.org/business/petrochemicals/Polyester-made-coal-China-betting/97/i3 (accessed on 9 May 2024).
- Timken, H.K.; Luo, H.; Chang, B.K.; Carter, E.; Cole, M. ISOALKY™ Technology: Next-Generation Alkylate Gasoline Manufacturing Process Technology Using Ionic Liquid Catalyst. In Commercial Applications of Ionic Liquids. Green Chemistry and Sustainable Technology; Shiflett, M., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Liquid Webshop “Proionic”. Available online: https://www.proionic.com/ (accessed on 22 August 2023).
- Pham, T.P.; Cho, C.W.; Yun, Y.S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Santos, A.; Tojo, J.; Rodriguez, A. Toxicity and biodegradability of imidazolium ionic liquids. J. Hazard. Mater. 2008, 151, 268–273. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity-Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Kuroda, K. A simple overview of toxicity of ionic liquids and designs of biocompatible ionic liquids. New J. Chem. 2022, 46, 20047–20052. [Google Scholar] [CrossRef]
- Thamke, V.R.; Tapase, S.R.; Kodam, K.M. Evaluation of risk assessment of new industrial pollutant, ionic liquids on environmental living systems. Water Res. 2017, 125, 237–248. [Google Scholar] [CrossRef]
- Pawłowska, B.; Telesi´nski, A.; Biczak, R. Phytotoxicity of Ionic Liquids. Chemosphere 2019, 237, 124436. [Google Scholar] [CrossRef]
- Kumari, P.; Pillai, V.V.S.; Benedetto, A. Mechanisms of action of ionic liquids on living cells: The state of the art. Biophys. Rev. 2020, 12, 1187–1215. [Google Scholar] [CrossRef]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; Gonçalves, A.M.M.; Sintra, T.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Designing ionic liquids: The chemical structure role in the toxicity. Ecotoxicology 2013, 22, 1–12. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Slivac, I.; Srček, V.G. Toxicity mechanisms of ionic liquids. Arch. Ind. Hyg. Toxicol. 2017, 68, 171–179. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Z.; Wang, L.; Sun, P.; Wang, Z. Assessment of bromide-based ionic liquid toxicity toward aquatic organisms and QSAR analysis. Ecotoxicol. Environ. Saf. 2015, 115, 112–118. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Sintra, T.E.; Neves, C.M.; Shimizu, K.; Canongia Lopes, J.N.; Gonçalves, F.; Ventura, S.P.; Freire, M.G.; Santos, L.M.; Coutinho, J.A. The effect of the cation alkyl chain branching on mutual solubilities with water and toxicities. Phys. Chem. Chem. Phys. 2014, 16, 19952–19963. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, J.; Firoz, A.; Kamli, M.R.; Patel, R. Improved Antibacterial Activity of Peptide Nisin with Pyrrole-Based Ionic Liquids Having Bis(trifluoromethylsulfonyl)imide as a Counterion: A Synergistic Approach to Combat Bacterial Infections. ACS Omega 2023, 9, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Kebaili, H.; Pérez de los Ríos, A.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Kameche, M.; Hernández-Fernández, J.; Hernández-Fernández, F.J. Evaluating the Toxicity of Ionic Liquids on Shewanella sp. for Designing Sustainable Bioprocesses. Front. Mater. 2020, 7, 578411. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicity studies of select ionic liquids (1-ethyl-3-methylimidazolium chloride, 1-butyl 3-methylimidazolium chloride, 1-butyl-1-methylpyrrolidinium chloride, and n-butylpyridiniumchloride) administered in drinking water to Sprague Dawley (Hsd:Sprague Dawley SD) rats and B6C3F1/N mice. Toxic Rep. Ser. 2022, 103, NTP-TOX-103. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, D.; Zhang, H.; Jiang, J.; Zhang, X.; He, T.; Wang, J.; Liu, S.-H. Thermal hazard evaluation and risk assessment of 1-allyl-3-methylimidazole nitrate ionic liquid. AIP Adv. 2024, 14, 035210. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chen, C.-C.; Zhang, B.; Wu, J.-H. Fire and explosion hazards of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. RSC Adv. 2020, 10, 22468–22479. [Google Scholar] [CrossRef]
- Efimova, A.; Varga, J.; Matuschek, G.; Saraji-Bozorgzad, M.R.; Denner, T.; Zimmermann, R.; Schmidt, P. Thermal Resilience of Imidazolium-Based Ionic Liquids-Studies on Short- and Long-Term Thermal Stability and Decomposition Mechanism of 1-Alkyl-3-methylimidazolium Halides by Thermal Analysis and Single-Photon Ionization Time-of-Flight Mass Spectrometry. J. Phys. Chem. B 2018, 122, 8738–8749. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 1800, 405–422. [Google Scholar]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xing, Y.; Yue, H.; Chen, T.; Diao, Y.; Wei, W.; Zhang, S. Ionic liquids revolutionizing biomedicine: Recent advances and emerging opportunities. Chem. Soc. Rev. 2023, 52, 7262–7293. [Google Scholar] [CrossRef] [PubMed]
- Moshikur, R.M.; Carrier, R.L.; Moniruzzaman, M.; Goto, M. Recent Advances in Biocompatible Ionic Liquids in Drug Formulation and Delivery. Pharmaceutics 2023, 15, 1179. [Google Scholar] [CrossRef]
- Kortstee, G.J.J. The aerobic decomposition of choline by microorganisms. Archiv. Mikrobiol. 1970, 71, 235–244. [Google Scholar] [CrossRef]
- Fukaya, Y.; Iizuka, Y.; Sekikawa, K.; Ohno, H. Bio ionic liquids: Room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007, 9, 1155. [Google Scholar] [CrossRef]
- Le Donne, A.; Bodo, E. Cholinium amino acid-based ionic liquids. Biophys. Rev. 2021, 13, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-D.; Liu, Q.-P.; Smith, T.J.; Li, N.; Zong, M.-H. Evaluation of Toxicity and Biodegradability of Cholinium Amino Acids Ionic Liquids. PLoS ONE 2013, 8, e59145. [Google Scholar] [CrossRef]
- Jesus, A.R.; Soromenho, M.R.C.; Raposo, L.R.; Esperança, J.M.S.S.; Baptista, P.V.; Fernandes, A.R.; Reis, P.M. Enhancement of water solubility of poorly water-soluble drugs by new biocompatible N-acetyl amino acid N-alkyl cholinium-based ionic liquids. Eur. J. Pharm. Biopharm. 2019, 137, 227–232. [Google Scholar] [CrossRef]
- Jesus, A.R.; Raposo, L.R.; Soromenho, M.R.C.; Agostinho, D.A.S.; Esperança, J.M.S.S.; Baptista, P.V.; Fernandes, A.R.; Reis, P.M. New Non-Toxic N-alkyl Cholinium-Based Ionic Liquids as Excipients to Improve the Solubility of Poorly Water-Soluble Drugs. Symmetry 2021, 13, 2053. [Google Scholar] [CrossRef]
- Xuan, X.-Y.; Cheng, Y.-L.; Acosta, E. Lecithin-Linker Microemulsion Gelatin Gels for Extended Drug Delivery. Pharmaceutics 2012, 4, 104–129. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Chowdhury, M.R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic Liquid-In-Oil Microemulsions Prepared with Biocompatible Choline Carboxylic Acids for Improving the Transdermal Delivery of a Sparingly Soluble Drug. Pharmaceutics 2020, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Marullo, S.; Rizzo, C.; Dintcheva, N.T.; D’Anna, F. Amino Acid-Based Cholinium Ionic Liquids as Sustainable Catalysts for PET Depolymerization. ACS Sustain. Chem. Eng. 2021, 9, 15157–15165. [Google Scholar] [CrossRef]
- Jopp, S.; Fleischhammer, T.; Lavrentieva, A.; Kara, S.; Meyer, J. Synthesis, biocompatibility, and antimicrobial properties of glucose-based ionic liquids. RSC Sustain. 2023, 1, 1751–1764. [Google Scholar] [CrossRef]
- Reiß, M.; Brietzke, A.; Eickner, T.; Stein, F.; Villinger, A.; Vogel, C.; Kragl, U.; Jopp, S. Synthesis of novel carbohydrate based pyridinium ionic liquids and cytotoxicity of ionic liquids for mammalian cells. RSC Adv. 2020, 10, 14299–14304. [Google Scholar] [CrossRef]
- Szelwicka, A.; Erfurt, K.; Jurczyk, S.; Boncel, S.; Chrobok, A. Outperformance in Acrylation: Supported D-Glucose-Based Ionic Liquid Phase on MWCNTs for Immobilized Lipase B from Candida antarctica as Catalytic System. Materials 2021, 14, 3090. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P.; Jopp, S. Novel Glucosylimidazolium Ionic-Liquid-Supported Novozym 435 Catalysts—A Proof of Concept for an Acrylation Reaction. Chem. Open 2022, 11, e202200135. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, S.; Fattahi, A. Design of ionic liquids containing glucose and choline as drug carriers, finding the link between QM and MD studies. Sci. Rep. 2022, 12, 21941. [Google Scholar] [CrossRef] [PubMed]
- Suksaeree, J.; Maneewattanapinyo, P. Ionic liquid drug-based polymeric matrices for transdermal delivery of lidocaine and diclofenac. J. Polym. Environ. 2020, 28, 2771–2779. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.; Araújo, M.; Fernandes, A.; Costa, J.; Santos de Almeida, T. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Więcek, P.; Guzik, M.; Chrobok, A. Combining amino acids and carbohydrates into readily biodegradable, task specifc ionic liquids. RSC Adv. 2020, 10, 18355–18359. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Open Source Drug Discovery Consortium; Lynn, A. g_mmpbsa, A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.; Dhariwal, J.; Saha, M.; Trivedi, S.; Banjare, M.K.; Kanaoujiya, R.; Behera, K. Ionic liquids: Environmentally sustainable materials for energy conversion and storage applications. Environ. Sci. Pollut. Res. Int. 2024, 31, 10296–10316. [Google Scholar] [CrossRef]
- Shahzad, S.; Shah, A.; Kowsari, E.; Iftikhar, F.J.; Nawab, A.; Piro, B.; Akhter, M.S.; Rana, U.A.; Zou, Y. Ionic Liquids as Environmentally Benign Electrolytes for High-Performance Supercapacitors. Glob. Chall. 2018, 3, 1800023. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; McIntosh, A.J.; Rowe, R.; Welton, T.; White, A.J. Azoniaspiro salts: Towards bridging the gap between room-temperature ionic liquids and molten salts. Phys. Chem. Chem. Phys. 2016, 18, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Teramoto, K.; Ooyabu, R.; Matsumoto, H. Non-Aqueous Electrolyte Magnesium Secondary Battery. EP3206249, 16 August 2017. [Google Scholar]
- Kubota, K.; Teramoto, K.; Ooyabu, R.; Matsumoto, H. Non-Aqueous Electrolyte Magnesium Secondary Battery. U.S. Patent 10998574B2, 31 August 2021. [Google Scholar]
- Lee, S.; Lee, K.M.; Kim, K. Electrochemical Properties of Trimethylammonium Tetrafluoroborate in Electrochemical Double-Layer Capacitors. J. Electrochem. Sci. Technol. 2022, 13, 254–260. [Google Scholar] [CrossRef]
- Hong, J.; Hwang, B.; Lee, J.; Kim, K. Effects of Cyclic Structure of Ammonium Ions on Capacitance in Electrochemical Double Layer Super capacitors. J. Electrochem. Sci. Technol. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Yu, X.; Ruan, D.; Wu, C.; Wang, J.; Shi, Z. Spiro-(1,1′)-bipyrrolidinium tetrafluoroborate salt as high voltage electrolyte for electric double layer capacitors. J. Power Sources 2014, 265, 309–316. [Google Scholar] [CrossRef]
- Hosseini-Bab-Anari, E. Synthesis of Novel Fluorine-Free Salts for Battery Electrolytes. Ph.D. Thesis, Department of Chemistry and Chemical Engineering Chalmers HALMERS University of Technology, Göteborg, Sweden, 2019. [Google Scholar]
- Jonsson, E.; Armand, M.B.; Johansson, J.P. Anions and Derived Salts with High Dissociation in Non-Protogenic Solvents. U.S. Patent US9269987B2, 23 February 2016. [Google Scholar]
- Jonsson, E.; Armand, M.; Johansson, P. Novel pseudo-delocalized anions for lithium battery electrolytes. Phys. Chem. Chem. Phys. 2012, 14, 6021–6025. [Google Scholar] [CrossRef]
- The United Nations Framework Convention on Climate Change. Available online: http://www.dissertations.se (accessed on 23 May 2016).
- Fay, M.; Hallegatte, S.; Vogt-Schilb, A.; Rozenberg, J.; Narloch, U.; Kerr, T. Decarbonizing Development: Three Steps to a Zero-Carbon Future. Climate Change and Development; World Bank: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Ghosh, B.K. Fossil Fuel Consumption Trend and Global Warming Scenario: Energy Overview. Glob. J. Eng. Sci. 2020, 5, 2039–2641. [Google Scholar] [CrossRef]
- Yusaf, T.; Laimon, M.; Alrefae, W.; Kadirgama, K.; Dhahad, H.A.; Ramasamy, D.; Kamarulzaman, M.K.; Yousif, B. Hydrogen Energy Demand Growth Prediction and Assessment (2021–2050) Using a System Thinking and System Dynamics Approach. Appl. Sci. 2022, 12, 781. [Google Scholar] [CrossRef]
- Christopher, K.; Dimitrios, R. A review on exergy comparison of hydrogen production methods from renewable energy sources. Energy Environ. Sci. 2012, 5, 6640–6651. [Google Scholar] [CrossRef]
- U.S. Fuel Cell Council, National Hydrogen Association Merge. Business Wire, 28 October 2010.
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via pow er-to-gas and power-to-liquids: A review. Renew. Sust. Energ. Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T. Prospects for alkaline anionexchange membranes in low temperature fuel cell. Fuel Cells 2005, 5, 187–200. [Google Scholar] [CrossRef]
- Chand, K.; Paladino, O. Recent developments of membranes and electrocatalysts for the hydrogen production by Anion Exchange Membrane Water Electrolysers: A review. Arab. J. Chem. 2022, 16, 104451. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Hwang, G.J.; Lim, S.G.; Bong, S.Y.; Ryu, C.H.; Choi, H.S. Preparation of anion exchange membrane using polyvinyl chloride (PVC) for alkaline water electrolysis. Korean J. Chem. Eng. 2015, 32, 1896–1901. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 21, 1803–1815. [Google Scholar] [CrossRef]
- Marshall, A.; Børresen, B.; Hagen, G.; Tsypkin, M.; Tunold, R. Hydrogen production by advanced proton exchange membrane (PEM) water electrolysers—Reduced energy consumption by improved electrocatalysis. Energy 2007, 32, 431–436. [Google Scholar] [CrossRef]
- Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability. Available online: www.eesc.europa.eu (accessed on 9 May 2024).
- Cousins, I.T.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Patton, S.; Scheringer, M.; Trier, X.; Vierke, L.; et al. The concept of essential use for determining when uses of PFASs can be phased out. Environ. Sci. Process. Impacts 2019, 21, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114. [Google Scholar] [CrossRef]
- Vinodh, R.; Kalanur, S.S.; Natarajan, S.K.; Pollet, B.G. Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review. Polymers 2023, 15, 2144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, P.; Zheng, X.; Sun, W.; Dou, S.X.; Ma, T.; Pan, H. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 2022, 51, 9620–9693. [Google Scholar] [CrossRef]
- Peterson, D.S. Ion exchange membranes. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Boston, MA, USA, 2014. [Google Scholar]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V.; Pintauro, P.N. Anion exchange membrane fuel cells. Electrochem. Soc. Interface 2010, 19, 31–35. [Google Scholar] [CrossRef]
- Gu, L.; Dong, H.; Sun, Z.; Li, Y.; Yan, F. Spirocyclic quaternary ammonium cations for alkaline anion exchange membrane applications: An experimental and theoretical study. RSC Adv. 2016, 6, 94387–94398. [Google Scholar] [CrossRef]
- Pham, T.H.; Olsson, J.S.; Jannasch, P. N-Spirocyclic Quaternary Ammonium Ionenes for Anion-Exchange Membranes. J. Am. Chem. Soc. 2017, 139, 2888–2891. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, J.; Liu, X.; Huang, T.; Jiang, H.; Yin, Y.; Qin, Y.; Guiver, M.D. Toward alkaline-stable anion exchange membranes in fuel cells: Cycloaliphatic quaternary ammonium-based anion conductors. Electrochem. Energy Rev. 2022, 5, 348–400. [Google Scholar] [CrossRef]
- Gu, S.; Wang, J.H.; Kaspar, R.B.; Fang, Q.; Zhang, B.; Bryan, C.E.; Yan, Y. Permethyl cobaltocenium (Cp*2Co+) as an ultra-stable cation for polymer hydroxide-exchange membranes. Sci. Rep. 2015, 5, 11668. [Google Scholar] [CrossRef] [PubMed]
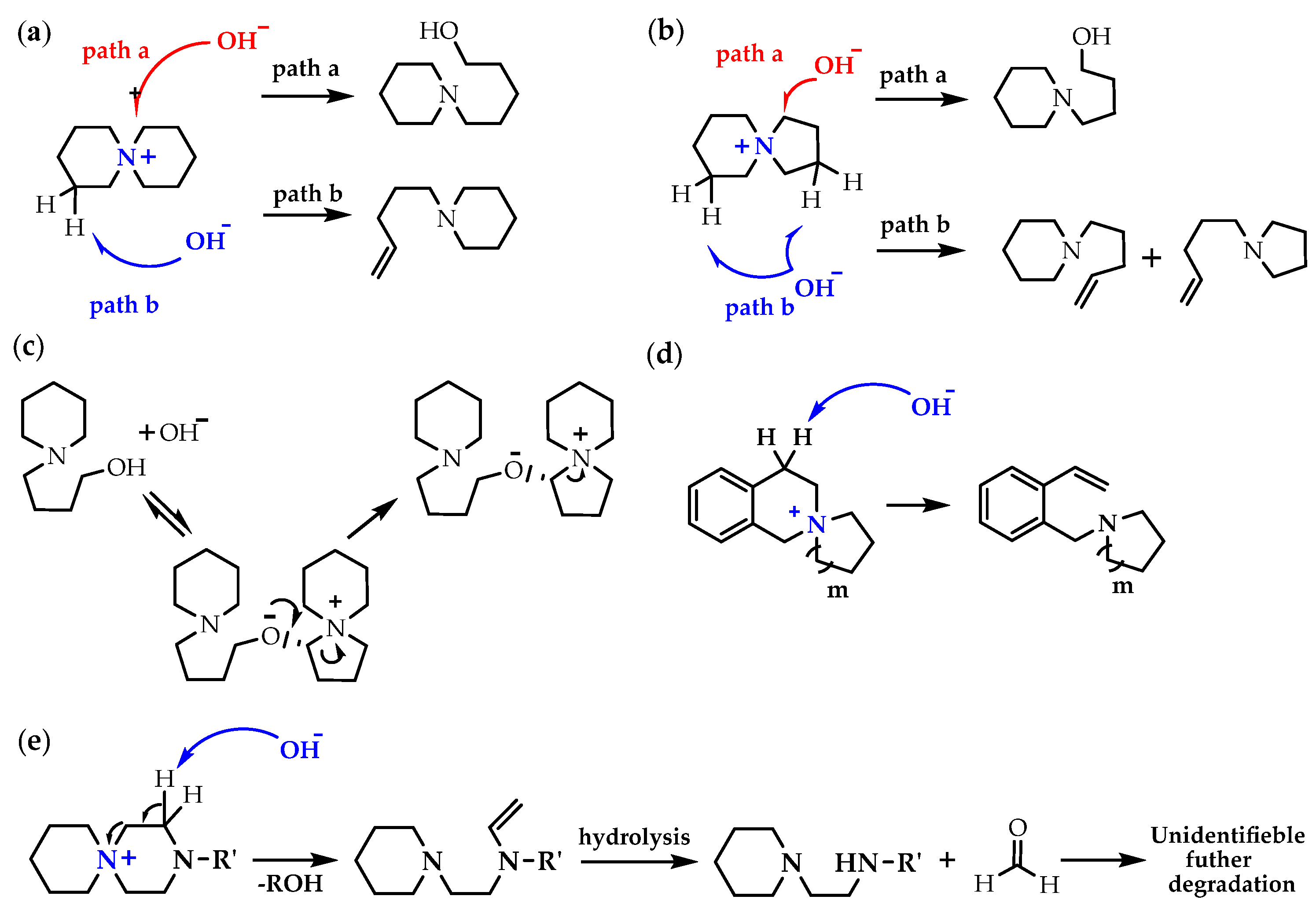
- You, W.; Hugar, K.M.; Selhorst, R.C.; Treichel, M.; Peltier, C.R.; Noonan, K.J.T.; Coates, G.W. Degradation of organic cations under alkaline conditions. J. Org. Chem. 2020, 86, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Comprehensive heterocyclic chemistry II. In 8.40–Systems with a Spirocyclic Heteroatom; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; pp. 1109–1133. [Google Scholar]
- Ahmad, N.M. Comprehensive heterocyclic chemistry III. In 12.20–Systems with a Spirocyclic Heteroatom; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; pp. 1037–1063. [Google Scholar]
- Marino, M.G.; Kreuer, K.D. Alkaline stability of quaternary ammonium cations for alkaline fuel cell membranes and ionic liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, S.A.; Capparelli, C.; Hickner, M.A. N-Alkyl Interstitial Spacers and Terminal Pendants Influence the Alkaline Stability of Tetraalkylammonium Cations for Anion Exchange Membrane Fuel Cells. Chem. Mater. 2016, 28, 2589–2598. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Functionalizing Polystyrene with N-Alicyclic Piperidine-Based Cations via Friedel-Crafts Alkylation for Highly Alkali-Stable Anion-Exchange Membranes. Macromolecules 2020, 53, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.D.; Tignor, S.E.; Krause, J.A.; Choe, Y.K.; Bae, C. Systematic alkaline stability study of polymer backbones for anion exchange membrane applications. Macromolecules 2016, 49, 3361–3372. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Two-dimensional NMR spectroscopy reveals cation triggered backbone degradation in polysulfone-based anion exchange membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490–2495. [Google Scholar] [CrossRef]
- Strasser, D.J.; Graziano, B.J.; Knauss, D.M. Base stable poly(diallylpiperidinium hydroxide) multiblock copolymers for anion exchange membranes. J. Mater. Chem. A 2017, 5, 9627–9640. [Google Scholar] [CrossRef]
- Pham, T.H.; Olsson, J.S.; Jannasch, P. Poly(arylene alkylene)s with pendant N-spirocyclic quaternary ammonium cations for anion exchange membranes. J. Mater. Chem. A 2018, 6, 16537–16547. [Google Scholar] [CrossRef]
- Chen, N.; Long, C.; Li, Y.; Lu, C.; Zhu, H. Ultrastable and high ion-conducting polyelectrolyte based on six-membered N-spirocyclic ammonium for hydroxide exchange membrane fuel cell applications. ACS Appl. Mater. Interfaces 2018, 10, 15720–15732. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Yan, X.; Zhang, F.; Wang, X.; Wu, X.; Pang, B.; Wang, J.; He, G. Ether spaced N-spirocyclic quaternary ammonium functionalized crosslinked polysulfone for high alkaline stable anion exchange membranes. J. Membr. Sci. 2020, 598, 117650. [Google Scholar] [CrossRef]
- Makosza, M. Phase-transfer catalysis. A general green methodology in organic synthesis. Pure Appl. Chem. 2000, 72, 1399–1403. [Google Scholar] [CrossRef]
- Starks, C.M. Phase-Transfer Catalysis, I. Heterogonous Reactions Involving Anion Transfer by Quaternary Ammonium andPhosponium Salts. J. Am. Chem. Soc. 1971, 93, 195–199. [Google Scholar] [CrossRef]
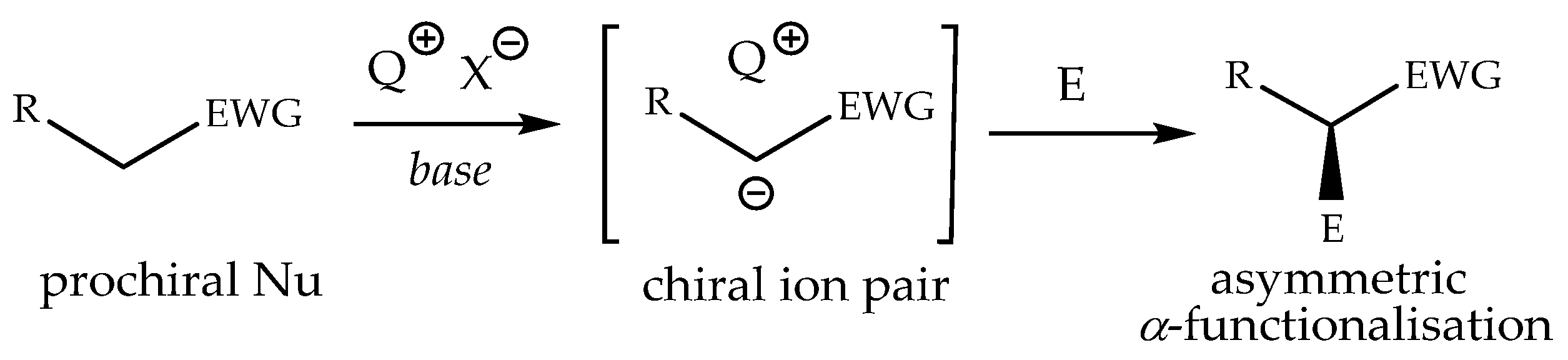
- Kitamura, M.; Shirakawa, S.; Maruoka, K. Powerful chiral phase-transfer catalysts for the asymmetric synthesis of alphaalkyl- and alpha, alpha-dialkyl-alpha-amino acids. Angew. Chem. Int. Ed. 2005, 44, 1549–1551. [Google Scholar] [CrossRef] [PubMed]
- Faisca Phillips, A.M. Thematic Issue: Organocatalysis. Mini Rev. Org. Chem. 2014, 11, 117. [Google Scholar] [CrossRef]
- Ooi, T.; Arimura, Y.; Hiraiwa, Y.; Yuan, L.; Kano, T.; Inoue, T.; Matsumoto, J.; Maruoka, K. Highly Enantioselective Monoalkylation of p-Chlorobenzaldehyde Imine of Glycine tert-Butyl Ester under Mild Phase-Transfer Conditions. Tetrahedron Asymmetry 2006, 17, 603–606. [Google Scholar] [CrossRef]
- Schörgenhumer, J.; Tiffner, M.; Waser, M. Chiral phase-transfer catalysis in the asymmetric α-heterofunctionalization of prochiral nucleophiles. Beilstein, J. Org. Chem. 2017, 13, 1753–1769. [Google Scholar] [CrossRef]
- Maruoka, K. Design of high-performance chiral phase-transfer catalysts with privileged structures. Proc. Jpn. Acad. Ser. B 2019, 95, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, W. A Minireview of Phase-Transfer Catalysis and Recent Trends. Biomed. J. Sci. Tech. Res. 2022, 45, 36691–36702. [Google Scholar] [CrossRef]
- Wang, H. Chiral Phase-Transfer Catalysts with Hydrogen Bond: A Powerful Tool in the Asymmetric Synthesis. Catalysts 2019, 9, 244. [Google Scholar] [CrossRef]
- Melnyk, N.; Garcia, M.R.; Iribarren, I.; Trujillo, C. Evolution of design approaches in asymmetric organocatalysis over the last decade. Tetrahedron Chem. 2023, 5, 100035. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wu, Y.; Zhao, T.; Liu, J.; Zheng, J.; Wang, L.; Lv, J.; Zhang, T. Fast, highly enantioselective, and sustainable fluorination of 4-substituted pyrazolones catalyzed by amide-based phase-transfer catalysts. Org. Chem. Front. 2023, 10, 2226–2233. [Google Scholar] [CrossRef]
- Ammonium Salts—Catalysts. Alfa Chemistry. Available online: https://www.alfachemic.com/catalysts/products/ammonium-salts.html (accessed on 9 May 2024).
- Ötvös, S.B.; Kappe, C.O. Continuous flow asymmetric synthesis of chiral active pharmaceutical ingredients and their advanced intermediates. Green Chem. 2021, 23, 6117–6138. [Google Scholar] [CrossRef]
- Ooi, T.; Kameda, M.; Tannai, H.; Maruoka, K. Facile synthesis of l-Dopa tert-butyl ester by catalytic enantioselective phase-transfer alkylation. Tetrahedron Lett. 2000, 41, 8339–8342. [Google Scholar] [CrossRef]
- Mahajan, D.P.; Godbole, H.M.; Singh, G.P.; Shenoy, G.G. Synthesis of novel phase transfer catalysts derived from proline-mandelic acid/tartaric acid: Their evaluation in enantioselective epoxidation and Darzen condensation. J. Chem. Sci. 2019, 131, 22. [Google Scholar] [CrossRef]
- Mahajan, D.P.; Godbole, H.M.; Singh, G.P.; Shenoy, G.G. Enantioselective Michael addition of malonic esters to benzalacetophenone by using chiral phase transfer catalysts derived from proline-mandelic acid/tartaric acid. J. Chem. Sci. 2019, 131, 67. [Google Scholar] [CrossRef]
- Wang, L.; Shirakawa, S.; Maruoka, K. Asymmetric Neutral Amination of Nitroolefins Catalyzed by Chiral Bifunctional Ammonium Salts in Water-Rich Biphasic Solvent. Angew. Chem. 2011, 123, 5439–5442. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, L.; Li, S. Catalytic asymmetric 1,6-conjugate addition of in situ generated para-quinone methides with tritylthiol. Org. Chem. Front. 2018, 5, 1820–1824. [Google Scholar] [CrossRef]
- Qu, C.; Huang, R.; Li, Y.; Liu, T.; Chen, Y.; Song, G. Selective sulfonylation and isonitrilation of para-quinonemethides employing TosMIC as a source of sulfonyl group or isonitrilegroup. Beilstein J. Org. Chem. 2021, 17, 2822–2831. [Google Scholar] [CrossRef]
- Van Wauwe, J.; Aerts, F.; Cools, M.; Deroose, F.; Freyne, E.; Goossens, J.; Hermans, B.; Lacrampe, J.; Van Genechten, H.; Van Gerven, F.; et al. Identification of R146225 as a novel, orally active inhibitor of interleukin-5 biosynthesis. Pharmacol. Exp. Ther. 2000, 295, 655–661. [Google Scholar]
- Přech, J.; Pizarro, P.; Serrano, D.P.; Čejka, J. From 3D to 2D zeolite catalytic materials. Chem. Soc. Rev. 2018, 47, 8263–8306. [Google Scholar] [CrossRef]
- Zeolites Market Forecast, Trend Analysis & Competition Tracking–Global Market Insights. Available online: http://www.factmr.com (accessed on 20 October 2023).
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 20 May 2020).
- Davis, M.E.; Lobo, R.F. Zeolite and molecular sieve synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Shi, C.; Li, L.; Yang, L.; Li, Y. Molecular simulations of host-guest interactions between zeolite framework STW and its organic structure-directing agents. Chin. Chem. Lett. 2020, 31, 1951–1955. [Google Scholar] [CrossRef]
- Gómez-Hortigüela, L.; Camblor, M.Á. Introduction to the Zeolite Structure-Directing Phenomenon by Organic Species: General Aspects. In Insights into the Chemistry of Organic Structure-Directing Agents in the Synthesis of Zeolitic Materials; Gómez-Hortigüela, L., Ed.; Structure and Bonding; Springer: Cham, Switzerland, 2017; Volume 175. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Sebakhy, K.O. A Review on the Effects of Organic Structure-Directing Agents on the Hydrothermal Synthesis and Physicochemical Properties of Zeolites. Chemistry 2022, 4, 431–446. [Google Scholar] [CrossRef]
- Gómez-Hortigüela, L.; Mayora, Á.; Liu, H.; Sierra, L.; Vaquerizo, L.; Mompeán, C.; Pérez-Pariente, J. Synthesis of large-pore zeolites from chiral structure-directing agents with two L-prolinol units. Dalton Trans. 2020, 49, 9618. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.F.; Zones, S.I.; Davis, M.E. Structure-direction in zeolite synthesis. J. Incl. Phenom. Mol. Recognit. Chem. 1995, 21, 47–78. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Lima, R.B.; Pergher, S.B.C.; Caldeira, V.P.S. Hierarchical Zeolite Synthesis by Alkaline Treatment: Advantages and Applications. Catalysts 2023, 13, 316. [Google Scholar] [CrossRef]
- Structure Directing Agents Market Outlook (2023 to 2033). Market Insights on Structure Directing Agents Covering Sales outlook, Demand Forecast and Up-to-Date Key Trends. Structure Directing Agents Market by Type, End Use Application & Region|Forecast 2023 to 2033. June 2023 Rep-GB-13068 Upcoming Chemical & Materials. Available online: http://www.futuremarketinsights.com (accessed on 1 May 2023).
- Yuan, R.; Claes, N.; Verheyen, E.; Tuel, A.; Bals, S.; Breynaert, E.; Martens, J.A.; Kirschhock, C.E.A. Synthesis of IWW-type germanosilicate zeolite using 5-azoniaspiro [4,4]nonane as structure directing agent. New J. Chem. 2016, 40, 4319–4324. [Google Scholar] [CrossRef]
- Millini, R.; Perego, C.; Frigerio, F.; Carluccio, L.; Bellussi, G.L. Azoniaspiro compounds as structure directing agents: A computation study. Stud. Surf. Sci. Catal. 2004, 154, 275–282. [Google Scholar] [CrossRef]
- Shvets, O.V.; Kasian, N.; Zukal, A.; Pinkas, J.; Cejka, J. The Role of Template Structure and Synergism between Inorganic and Organic Structure Directing Agents in the Synthesis of UTL Zeolite. Chem. Mater. 2010, 22, 3482–3495. [Google Scholar] [CrossRef]



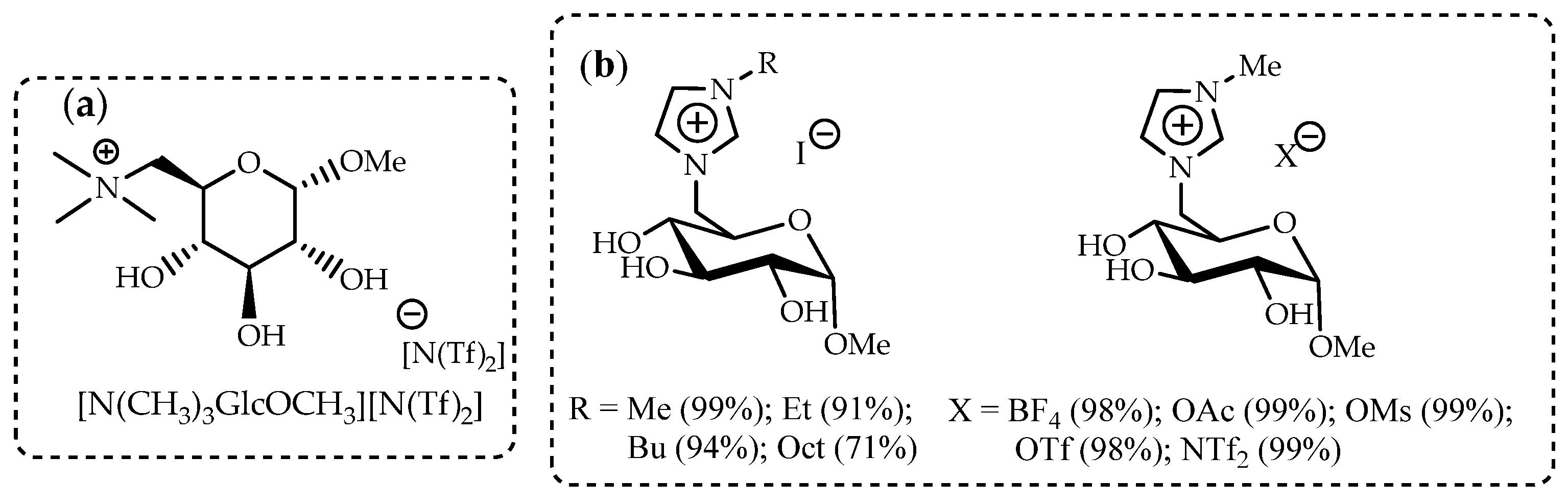
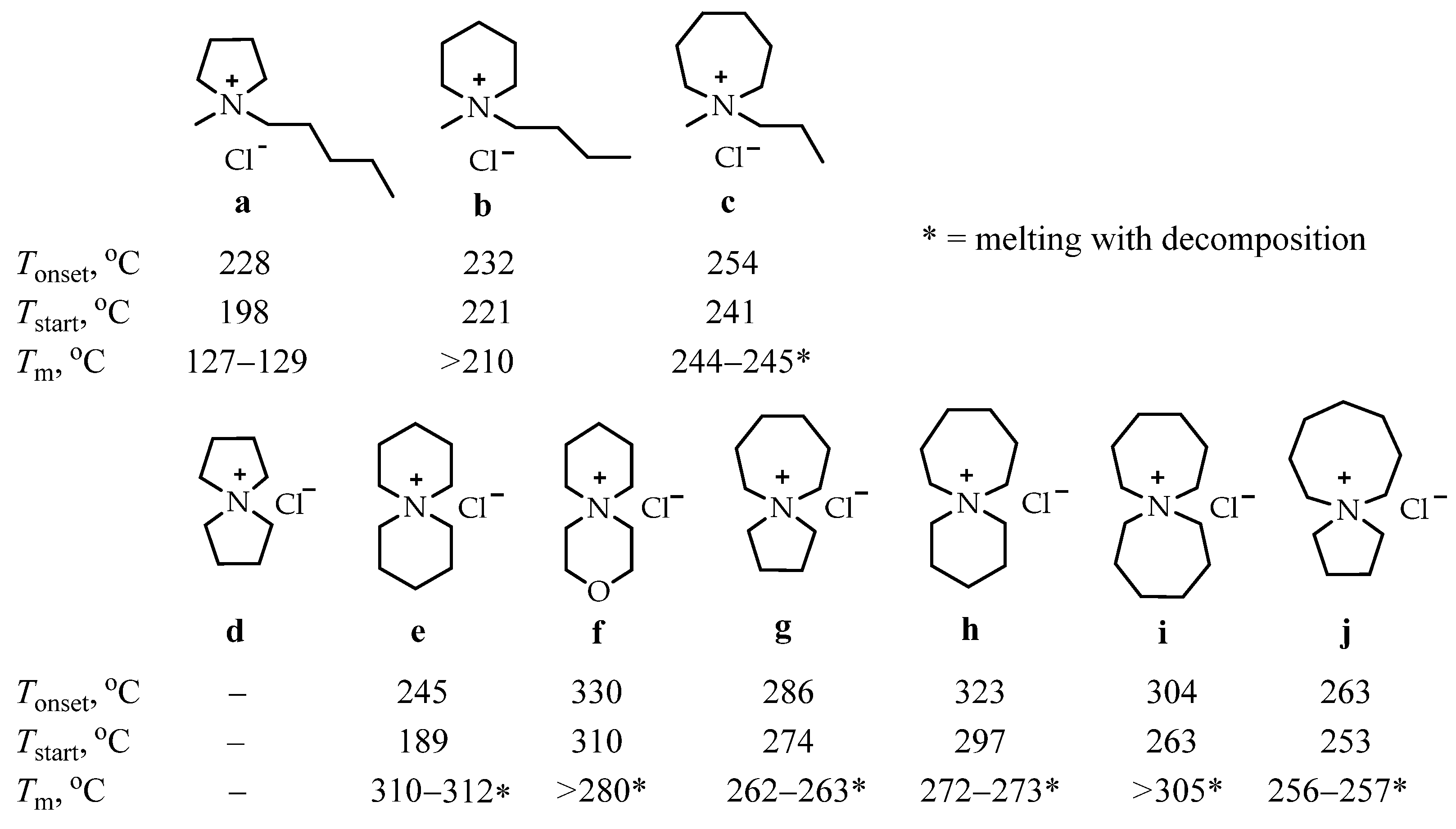


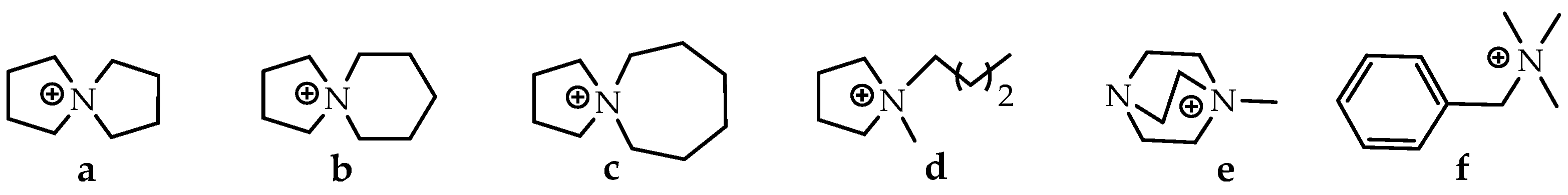

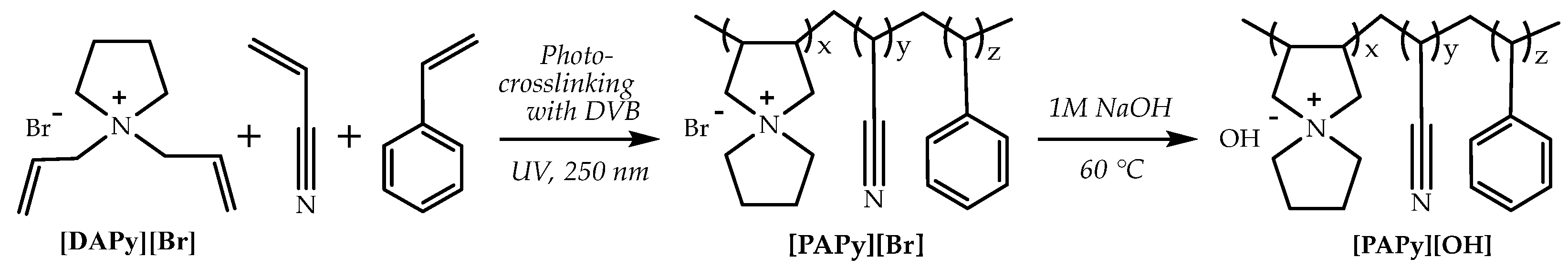

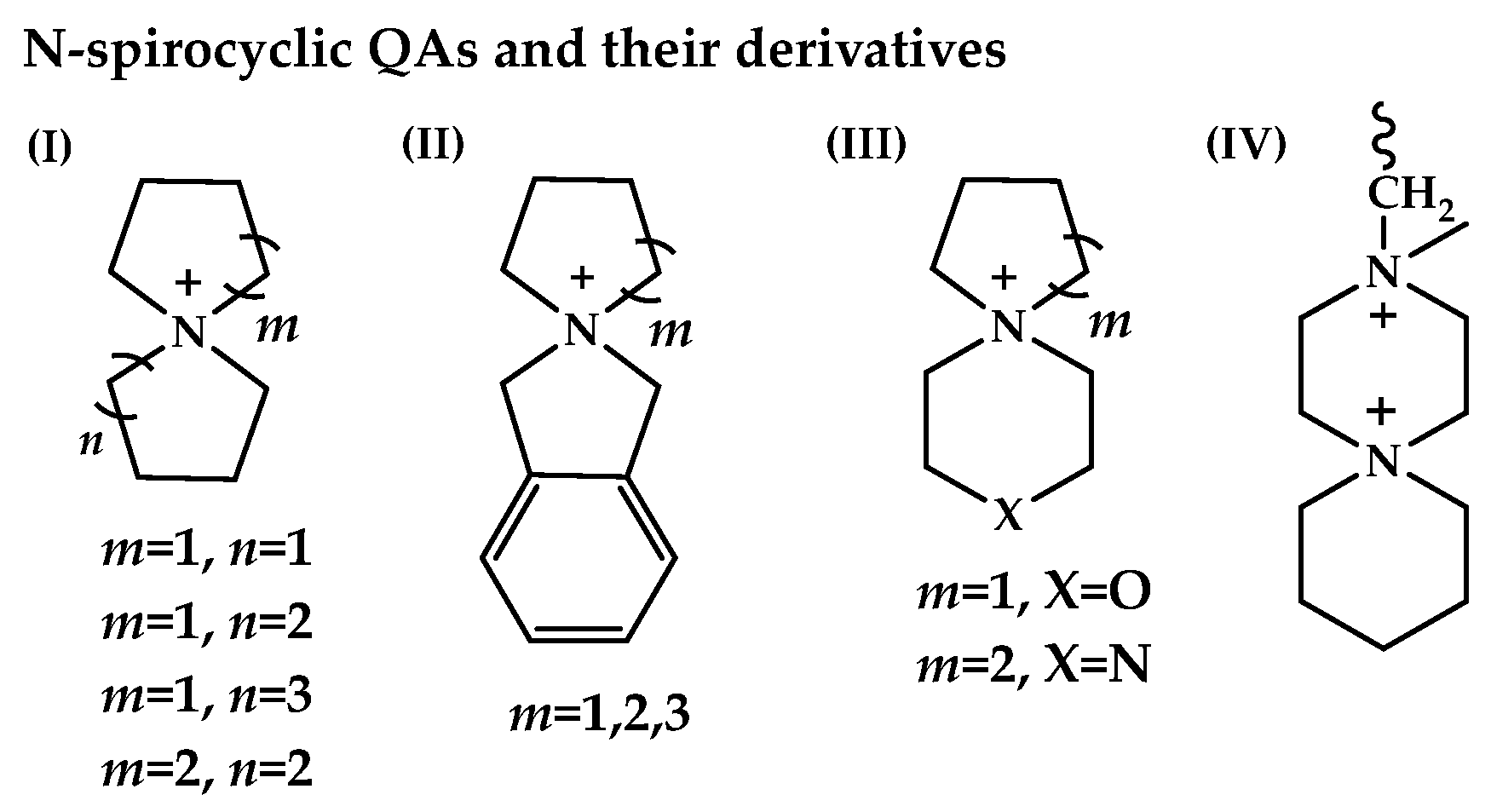
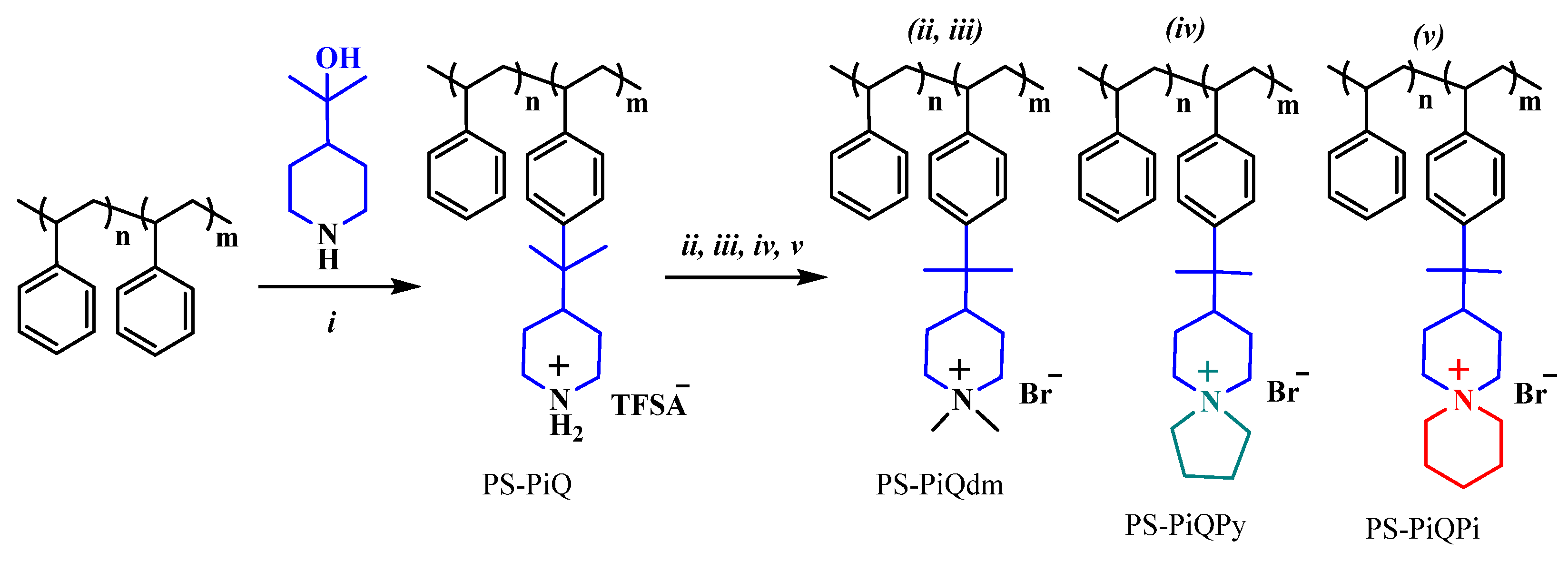

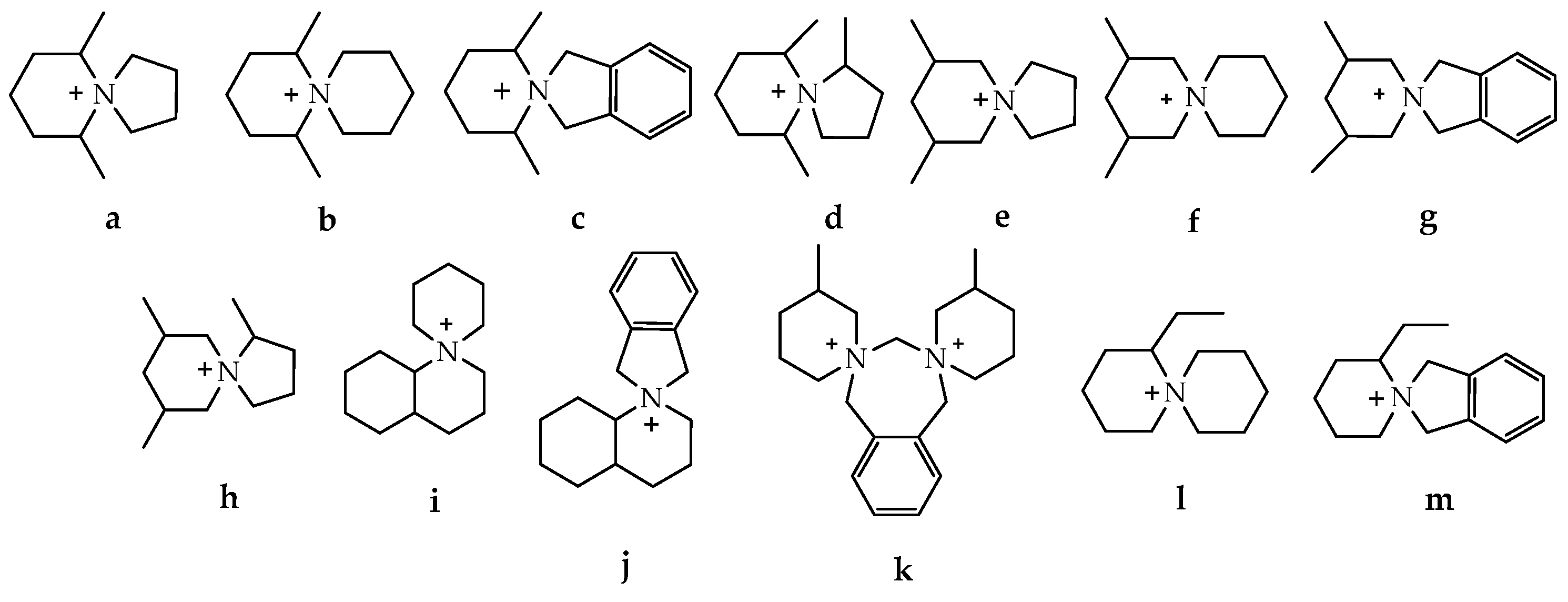
| Parameters | A-WE | PEM-WE | AEM-WE |
|---|---|---|---|
| Separation | Diaphragm | PEM | AEM |
| Cathode | Nickelmolybdenum alloys | Pt group metals | Transition metals |
| Anode | Nickelcobalt alloys | RuOx, IrOx | Transition metals |
| Current collector plate | Nickel | Copper | Copper |
| Bipolar plate | — | Graphite and Ti | Ni or SS |
| Electrolyte | KOH | Pure water | Alkali solution, pure water |
| Current density | <0.5 A cm−2 | 1–2 A cm−2 | 1–2 A cm−2 |
| Operating temperature | 60–90 °C | 50–90 °C | 40–80 °C |
| Gas purity | >99.5% | >99.99% | >99.99% |
| Lifetime | ~100 kh | <10 kh | <2 kh |
| Estimated cost | Low | High | — |
| Technology status | Mature | Commercial for small scale | Research and Development |
| Nitrogen-Containing Groups | Nitrogen-Free Groups |
|---|---|
| Quaternary ammonium/tertiary diamines | Phosphonium |
| (Benz)Imidazolium | Sulphonium |
| Guanidinium | Metal cations (Ruthenium, Nickel, Cobalt) |
| Pyridinium |
| AEMs | Structure/Cation | IEC (mmol·g−1) | WU(%) | SR(%) | HC(mS·cm−1) | Ts (MPa) | Eb (%) | Stabil./ 80 °C |
|---|---|---|---|---|---|---|---|---|
| 80 °C | ||||||||
| Cr-ASD-PSF 2.05 |  | 2.05 | 92.7 | 39.6 | 85.7 | 10.5 | 12.8 | 94.9% (1 M KOH, 720 h) |
| Cr-ASU-PSF 1.92 | 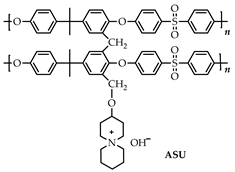 | 1.92 | 91.1 | 37.7 | 78.8 | 8.3 | 17.8 | 95.6% (1 M KOH, 720 h) |
| Entry | Chiral PTC | Solvent | Time, h | Yield, % | ee, % |
|---|---|---|---|---|---|
| 1 | 5 mol%, TBAB | CH2Cl2 | 2.0 | 90 | 0 |
| 2 | 5 mol%, A | CH2Cl2 | 0.5 | 98 | +90 |
| 3 | 5 mol%, B | CH2Cl2 | 1.5 | 90 | 0 |
| 4 | 5 mol%, A | toluene | 0.5 | 90 | +97 |
| 5 | 5 mol%, A | MTBE | 0.5 | 98 | +98 |
| 6 | 1 mol%, A | MTBE | 3.0 | 98 | +96 |
| 7 | 1 mol%, A | MTBE | 5.0 | 95 | +96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayukova, L.; Vologzhanina, A. A New 2-Aminospiropyrazolylammonium Cation with Possible Uses in the Topical Areas of Ionic Liquids. Molecules 2024, 29, 2326. https://doi.org/10.3390/molecules29102326
Kayukova L, Vologzhanina A. A New 2-Aminospiropyrazolylammonium Cation with Possible Uses in the Topical Areas of Ionic Liquids. Molecules. 2024; 29(10):2326. https://doi.org/10.3390/molecules29102326
Chicago/Turabian StyleKayukova, Lyudmila, and Anna Vologzhanina. 2024. "A New 2-Aminospiropyrazolylammonium Cation with Possible Uses in the Topical Areas of Ionic Liquids" Molecules 29, no. 10: 2326. https://doi.org/10.3390/molecules29102326
APA StyleKayukova, L., & Vologzhanina, A. (2024). A New 2-Aminospiropyrazolylammonium Cation with Possible Uses in the Topical Areas of Ionic Liquids. Molecules, 29(10), 2326. https://doi.org/10.3390/molecules29102326







