A Review of the Effect of Defect Modulation on the Photocatalytic Reduction Performance of Carbon Dioxide
Abstract
1. Introduction
2. Basic Principles of the Photocatalytic Reduction of CO2
3. Defect Types and the Properties of Photocatalysts
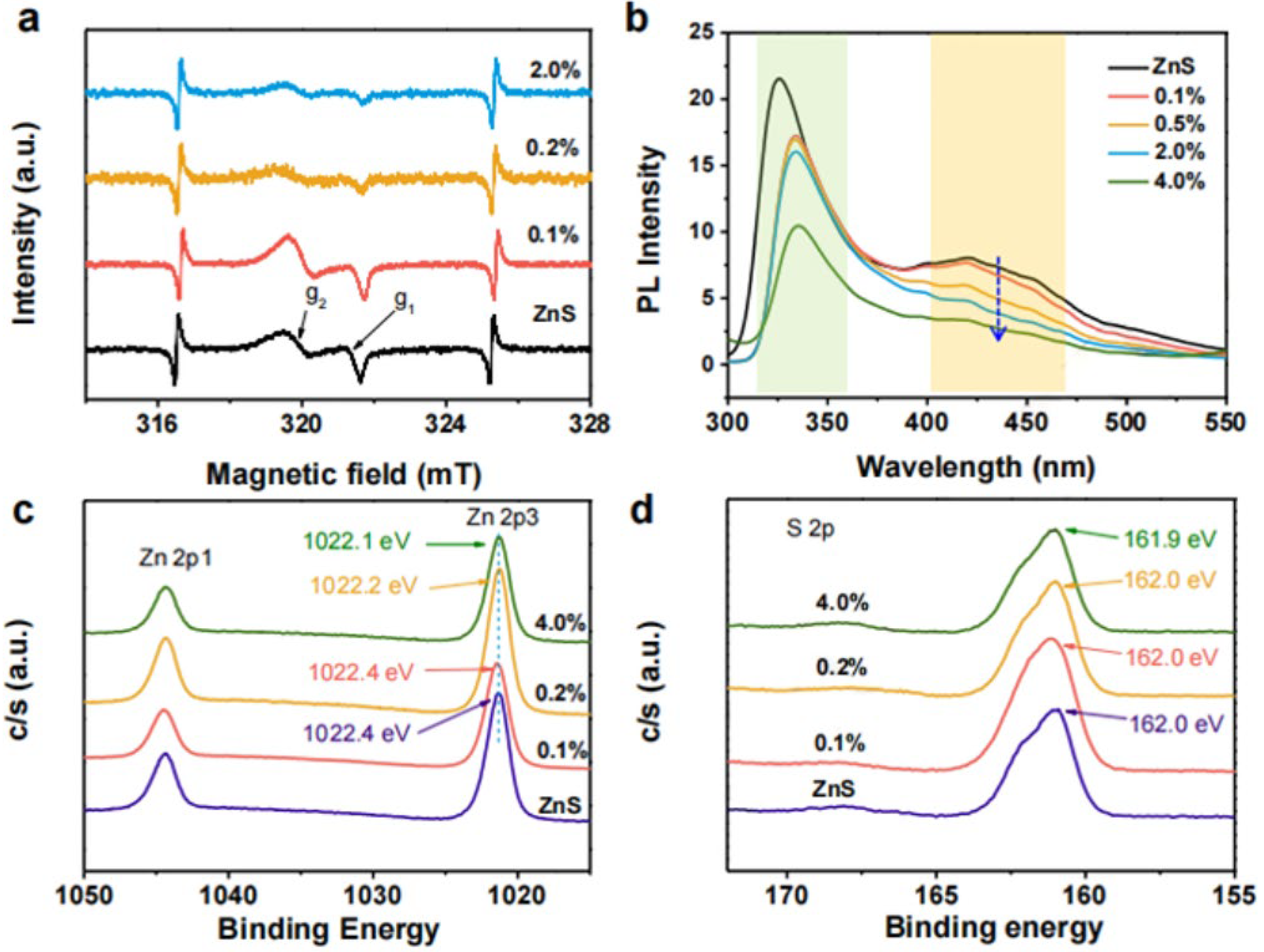
3.1. Metallic and Metal-Free Vacancy Defects
3.2. Metal and Metal-Free Doping Defects
4. The Effect of Defect Modulation
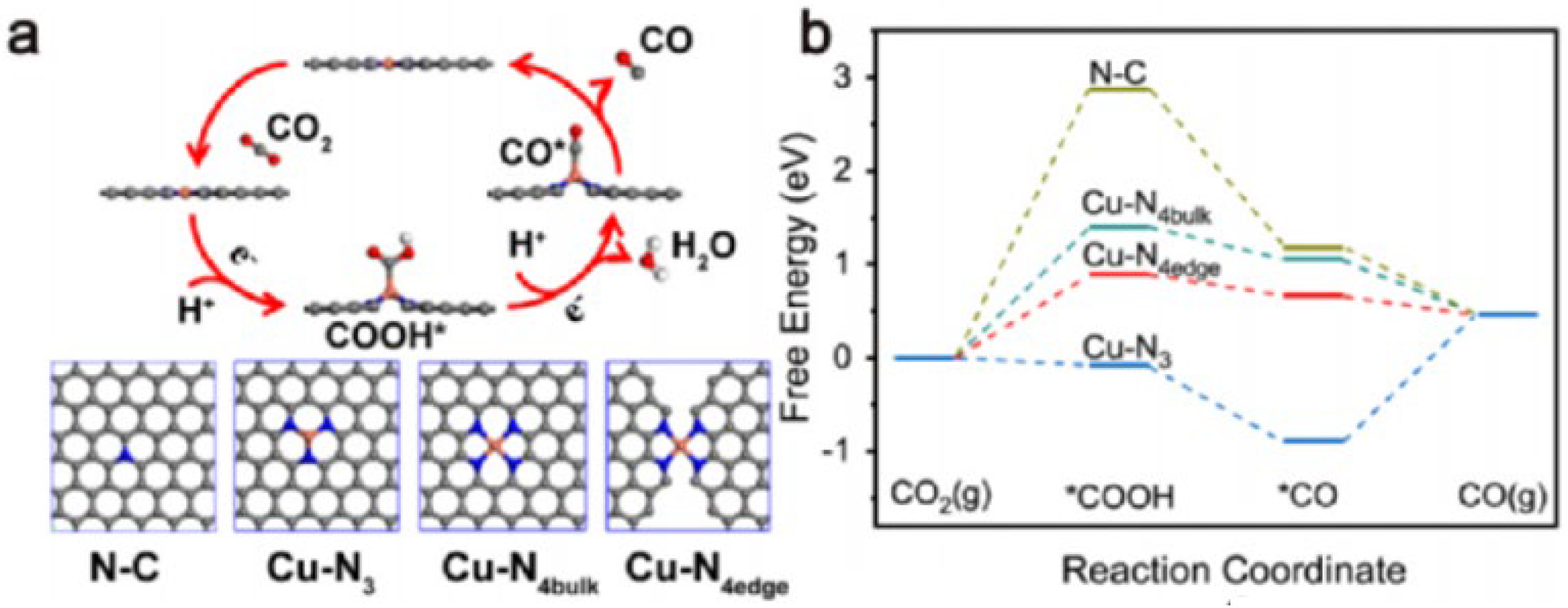
4.1. Effect of Defect Types on CO2 Reduction Efficiency
4.2. Effect of Defect Concentration on the Selectivity of CO2 Reduction
4.3. Effect of Defect Distribution on the Stability of CO2 Reduction
4.4. Relationship between Defect Modulation and Photocatalytic Activity
5. Optimization Methods for Defect Modulation Strategies
5.1. Optimization Strategies for Defect Distribution
5.2. Synergistic Effects of Compound Defects
5.3. Precise Control of Defect Concentration
6. Conclusions
- Employing advanced analytical techniques such as electron paramagnetic resonance (EPR), X-ray absorption near-edge structures (XANES), and extended X-ray absorption fine structures (EXAFS), the electronic structure and the intricate local coordination environment surrounding the defects can be meticulously delineated. This fine-level analysis facilitates careful study of the reaction process at the microscopic scale, revealing the complex ways in which defects affect the performance of photocatalysts. The subtle nuances of how defects can either enhance or impede photocatalytic activity can be uncovered by dissecting the reaction mechanisms at such a fundamental level. This profound understanding is crucial for the strategic manipulation of defects to optimize the efficiency of photocatalytic systems. It can achieve the customization of defect characteristics to achieve the desired photocatalytic performance, thereby driving innovation in the development of advanced photocatalytic materials.
- With the aid of advanced computational methods and simulation software, the existence and distribution of defects in different photocatalysts can be simulated, and their impact on photocatalytic reaction activity can be predicted. This can not only reveal the influence of defects on the electronic structure and band structure of photocatalysts but also predict the impact of defects on the performance of photocatalysts, such as in the light absorption, separation, and transport of photogenerated carriers. Theoretical simulations allow for the identification of potential photocatalyst candidates, which can then be experimentally verified. By comparing the simulation results with experimental data, a more accurate understanding of how defects affect photocatalytic performance is obtained, thereby guiding the design and optimization of novel efficient photocatalysts. Simultaneously, based on the simulation and experimental findings, improvements in composition, structure, and preparation methods can be made to enhance the overall photocatalytic performance.
- The application of the photocatalytic reduction of CO2 into high-value fuels is very promising, but unfavorable factors such as low spectral utilization and the short lifetime of photogenerated charges have been restricting the industrial application process. Therefore, in order to meet production requirements, it is necessary to develop a low-cost and simple synthesis method with a simple preparation process to prepare highly efficient and stable photocatalysts, which will make it possible to achieve practical applications and energy sustainability in the field of photocatalysis. The distribution of defects is important in the performance of photocatalysts. Uniformly distributed defects contribute to the separation efficiency of photogenerated carriers and the stability of the photocatalysts. For example, uniformly distributed defects could be formed on the surface of photocatalysts by specific synthesis methods, such as via solution or template methods. These methods help to improve the activity and selectivity of the photocatalyst while maintaining long-term stability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omer, A.M. Energy, environment and sustainable development. Renew. Sust. Energ. Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Zhu, X.L.; Zong, H.B.; Yuan, Z.M.; Wang, S.H.; Zeng, G.X.; Xu, H.; Jiang, Z.Y.; Ozin, G.A. Supercharged CO2 photothermal catalytic methanation: High conversion, rate, and selectivity. Angew. Chem. Int. Edit. 2023, 62, e202218694. [Google Scholar] [CrossRef] [PubMed]
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.S.; Yan, X.H.; Wang, L.H. Synthesis, structural characterization, Hirschfeld surface analysis, density functional theory, and photocatalytic CO2 reduction activity of a new Ca(II) complex with a Bis-Schiff base ligand. Molecules 2024, 29, 1047. [Google Scholar] [CrossRef] [PubMed]
- Albero, J.; Peng, Y.; Garcia, H. Photocatalytic CO2 reduction to C2+ products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Shinde, G.Y.; Mote, A.S.; Gawande, M.B. Recent Advances of Photocatalytic Hydrogenation of CO2 to Methanol. Catalysts. 2022, 12, 94. [Google Scholar] [CrossRef]
- Wang, L.H.; Tai, X.S. Synthesis, structural characterization, hirschfeld surface analysis and photocatalytic CO2 reduction activity of a new dinuclear Gd (III) complex with 6-phenylpyridine-2-carboxylic acid and 1, 10-phenanthroline ligands. Molecules 2023, 28, 7595. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, Y.; Wang, J.; Fang, H.; Xi, S.; Zhao, X.; Xu, D.; Xu, H.; Yu, W.; Hai, X.; et al. Ordered clustering of single atomic Te vacancies in atomically thin PtTe2 promotes hydrogen evolution catalysis. Nat. Commun. 2021, 12, 2351. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Sun, W.; Miao, W.K. Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst. Adv. Sci. 2019, 6, 1900289. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ahamad, T.; Veses, R.C.; Stadler, F.J. Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: The triggering effect of Ag and RGO. Chem. Eng. J. 2019, 370, 148–165. [Google Scholar] [CrossRef]
- Duic, N.; Guzovic, Z.; Vyatcheslav, K.; Klemes, J.J.; Mathiessen, B.V.; Yan, J.Y. Sustainable development of energy, water and environment systems. Appl. Energy 2013, 101, 3–5. [Google Scholar] [CrossRef]
- Yang, X.G.; Wang, D.W. Photocatalysis: From fundamental principles to materials and applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Baur, E.; Perret, A. The action of light on dissolved silver salts in the presence of zinc oxide. Hel. Chim. Acta 1924, 7, 910–915. [Google Scholar] [CrossRef]
- Goodeve, C.F.; Kitchener, J.A. The mechanism of photosensitisation by solids. Trans. Faraday Soc. 1938, 34, 902–908. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Halmann, M. Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature 1978, 275, 115–116. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, J.; Li, Y.; Zhao, W.; Zeng, Y.; Wang, C. Photocatalytic CO2 reduction over SrTiO3:Correlation between surface structure and activity. Appl. Surf. Sci. 2018, 447, 627–635. [Google Scholar] [CrossRef]
- Hao, X.Q.; Zhou, J.; Cui, Z.W.; Wang, Y.C.; Wang, Y.; Zou, Z. Zn-vacancy mediated electron-hole separation in ZnS/g-C3N4 heterojunction for efficient visible-light photocatalytic hydrogen production. Appl. Catal. B-Environ. 2018, 229, 41–51. [Google Scholar]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (040) binary heterojunction photocatalysts with enhanced photocatalytic activity for ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. Engineering approach in stimulating photocatalytic H2 production in a slurry and monolithic photoreactor systems using Ag-bridged Z-scheme pCN/TiO2 nanocomposite. Chem. Eng. J. 2019, 374, 1076–1095. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. 2D-montmorillonite-dispersed g-C3N4/TiO2 2D/0D nanocomposite for enhanced photo-induced H2 evolution from glycerol-water mixture. Appl. Surf. Sci. 2019, 471, 1053–1064. [Google Scholar] [CrossRef]
- Chen, C.; Jin, J.; Chen, S.; Wang, T.; Xiao, J.; Peng, T. In-situ growth of ultrafine ZnO on g-C3N4 layer for highly active and selective CO2 photoreduction to CH4 under visible light. Mater. Res. Bull. 2021, 137, 111177. [Google Scholar] [CrossRef]
- Han, P.; Mih, A.A.; Ferre-borrull, J. Interplay between morphology, optical properties, and electronic structure of solution-processed Bi2S3 colloidal nanocrystals. J. Phys. Chem. C 2015, 119, 10693–10699. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Rungtaweevoranit, B.; BParlinska-Wojtan, M.; Pei, X.; Yaghi, O.M.; Behm, R.J. Highly active and stable single-atom Cu catalysts supported by a metal—Organic framework. J. Am. Chem. Soc. 2019, 141, 5201–5210. [Google Scholar] [CrossRef]
- Niu, P.; Yang, Y.; Jimmy, C.Y.; Liu, G.; Cheng, H.-M. Switching the selectivity of the photoreduction reaction of carbon dioxide by controlling the band structure of a g-C3N4 photocatalyst. Chem. Commun. 2014, 50, 10837–10840. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M. A noble metal-free reduced graphene oxide-CdS nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2014, 2, 3407. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Ji, X.Y.; Li, Z.S.; Lin, Z.D.; Pan, W.G. Recent advances and perspectives of MoS2-based materials for photocatalytic dyes degradation: A review. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125836. [Google Scholar] [CrossRef]
- An, X.; Li, K.; Tang, J. Inside Cover Picture: Cu2O/Reduced Graphene Oxide Composites for the Photocatalytic Conversion of CO2. ChemSusChem 2014, 7, 1086. [Google Scholar] [CrossRef] [PubMed]
- Shown, I.; Hsu, H.C.; Chang, Y.C.; Lin, C.H.; Roy, P.K.; Ganguly, A.; Wang, C.H.; Chang, J.K.; Wu, C.I.; Chen, L.C.; et al. Highly Efficient Visible Light Photocatalytic Reduction of CO2 to Hydrocarbon Fuels by Cu-Nanoparticle Decorated Graphene Oxide. Nano Lett. 2014, 14, 6097–6103. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, D.; Rungtaweevoranit, B.; Trickett, C.A.; Barmanbek, J.T.; Alshammari, A.S.; Yang, P.; Yaghi, O.M. Plasmon-enhanced photocatalytic CO2 conversion within metal-organic frameworks under visible light. J. Am. Chem. Soc. 2017, 139, 356–362. [Google Scholar] [CrossRef]
- Gondal, M.A.; Dastageer, M.A.; Oloore, L.E.; Baig, U. Laser induced selective photo-catalytic reduction of CO2 into methanol using In2O3-WO3 nano-composite. J. Photochem. Photobiol. A 2017, 343, 40–50. [Google Scholar] [CrossRef]
- Mao, J.; Li, K.; Peng, T. Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal. Sci. Technol. 2013, 3, 2481–2498. [Google Scholar] [CrossRef]
- Humphrey, V.; Zscheischler, J.; Ciais, P.; Gudmundsson, L.; Sitch, S.; Seneviratne, S.I. Sensitivity of atmospheric CO2 growth rate to observed changes in terrestrial water storage. Nature 2018, 560, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Soden, B.J.; Collins, W.D.; Feldman, D.R. Reducing uncertainties in climate models. Science 2018, 361, 326–327. [Google Scholar] [CrossRef]
- Xu, Q.L.; Xia, Z.H.; Zhang, J.M.; Wei, Z.Y.; Guo, Q.; Jin, H.; Tang, H.; Li, S.; Pan, X.; Su, Z.; et al. Recent advances in solar-driven CO2 reduction over g-C3N4-based photocatalysts. Carbon Energy 2023, 5, e205. [Google Scholar] [CrossRef]
- Zhao, W.G.; Wang, F.; Zhao, K.Y.; Liu, K.Y.; Zhu, X.T.; Yan, L.; Yin, Y.; Xu, Q.; Yin, D.L. Recent advances in the catalytic production of bio-based diol 2,5-bis (hydroxymethyl) furan. Carbon Resour. Convers. 2023, 6, 116–131. [Google Scholar] [CrossRef]
- Lo, A.Y.; Chung, Y.C.; Xie, P.J.; Delbari, H.; Yang, H.; Taghipour, H. Effect of Ag-doping strategies on the Lewis acid/base behavior of mesoporous TiO2 photocatalyst and its performance in CO2 photoreduction. Appl. Mater. Today 2023, 32, 101811. [Google Scholar] [CrossRef]
- Li, X.; Yu, G.J.; Jaroniec, M.; Chen, X. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.X.; Shi, J.L. Defect Engineering of Photocatalysts towards Elevated CO2 Reduction Performance. ChemSusChem 2021, 14, 2635–2654. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Meng, X.; Li, P.; Chang, K.; Zhou, W.; Wang, X.; Zhang, X.; Jevasuwan, W.; Fukata, N.; Wang, D.; et al. Cation vacancy-initiated CO2 photoreduction over ZnS for efficient formate production. ACS Energy Lett. 2019, 4, 1387–1393. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-mediated electron-hole separation in one-unit-cell ZnIn2S4 layers for boosted solar-driven CO2 reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef]
- Wang, Q.L.; Dong, P.M.; Huang, Z.F.; Zhang, X.W. Synthesis of Ag or Pt nanoparticle-deposited TiO2 nanorods for the highly efficient photoreduction of CO2 to CH4. Chem. Phys. Lett. 2015, 639, 11–16. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, Y.Z.; Zhao, J.T.; Xiong, Z.; Zhao, Y.C.; Zhang, J.Y. PtCu alloy cocatalysts for efficient photocatalytic CO2 reduction into CH4 with 100% selectivity. Catal. Sci. Technol. 2022, 12, 3454–3463. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, L.; Jiang, K.; Liu, K.; Wang, Z.; Qiu, X.; Li, H.; Hu, J.; Pan, H.; Lu, Y.-R.; et al. Activation of CO2 on graphitic carbon nitride supported single-atom cobalt sites. Chem. Eng. J. 2021, 415, 128982. [Google Scholar] [CrossRef]
- Li, Y.; Gong, F.; Zhou, Q.; Feng, X.H.; Fan, J.J.; Xiang, Q.J. Crystalline isotype heptazine-/triazine-based carbon nitride heterojunctions for an improved hydrogen evolution. Appl. Catal. B Environ. 2020, 268, 118381. [Google Scholar] [CrossRef]
- Zhou, G.; Shan, Y.; Hu, Y.Y.; Xu, X.Y.; Long, L.Y.; Zhang, J.L.; Dai, J.; Guo, J.H.; Shen, J.C.; Li, S.; et al. Half-metallic carbon nitride nanosheets with microgrid mode resonance structure for efficient photocatalytic hydrogen evolution. Nat. Commun. 2018, 9, 3366. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.F.; Borjigin, T.; Zhang, Y.H.; Liu, H.; Liu, B.H.; Guo, H. Z-scheme Au@Void@ g-C3N4/SnS yolk-shell heterostructures for superior photocatalytic CO2 reduction under visible light. ACS Appl. Mater. Interfaces 2018, 10, 34123–34131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.X.; Ai, L.H.; Jiang, J. MXene-derived TiO2@C/g-C3N4 heterojunctions for highly efficient nitrogen photofixation. J. Mater. Chem. A 2018, 6, 4102–4110. [Google Scholar] [CrossRef]
- Shen, Y.; Han, Q.T.; Hu, J.Q.; Gao, W.; Wang, L.; Yang, L.Q.; Gao, C.; Shen, Q.; Wu, C.P.; Wang, X.Y.; et al. Artificial trees for artificial photosynthesis: Construction of dendrite-structured α-Fe2O3/g-C3N4 Z-Scheme system for efficient CO2 reduction into solar fuels. ACS Appl. Energy Mater. 2018, 3, 6561–6572. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2- reduction performance. Appl. Catal. B-Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Ruan, D.; Kim, S.; Fujitsuka, M.; Majima, T. Defects rich g-C3N4 with mesoporous structure for efficient photocatalytic H2 production under visible light irradiation. Appl. Catal. B Environ. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Tay, Q.; Kanhere, P.; Ng, C.F.; Chen, S.; Chakraborty, S.; Huan, A.C.H.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect engineered g-C3N4 for efficient visible light photocatalytic hydrogen production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Sun, S.Q.; Wu, Y.C.; Zhu, J.F.; Lu, C.J.; Sun, Y.; Wang, Z.; Chen, J. Stabilizing plasmainduced highly nitrogen-deficient g-C3N4 by heteroatom-refilling for excellent lithium-ion battery anodes. Chem. Eng. J. 2022, 427, 131032. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Wang, M.; Tian, J.; Jin, X.; Guo, L.; Wang, L.; Shi, J. Carbon-vacancy modified graphitic carbon nitride: Enhanced CO2 photocatalytic reduction performance and mechanism probing. J. Mater. Chem. A 2019, 7, 1556–1563. [Google Scholar] [CrossRef]
- Zhang, T.; Low, J.X.; Huang, X.X.; Al-Sharab, J.F.; Yu, J.; Asefa, T. Copper-Decorated Microsized Nanoporous Titanium Dioxide Photocatalysts for Carbon Dioxide Reduction by Water. Chemcatchem 2017, 9, 3054–3062. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, S.S.; Zhao, Y.; Zheng, M.; Hou, D.D.; Xiao, J. Enhancing visible light photocatalytic performance with N-doped TiO2 nanotube arrays assisted by H2O2. Int. J. Mod. Phys. B 2019, 33, 1940025. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhang, Y.H.; Zhang, H.F.; Bai, L.Q.; Hao, L.; Ma, T.Y.; Huang, H.W. Defect engineering in metal sulfides for energy conversion and storage. Coordin. Chem. Rev. 2021, 448, 214147. [Google Scholar] [CrossRef]
- Abdellah, M.; El-Zohry, A.M.; Antila, L.J.; Windle, C.D.; Reisner, E.; Hammarström, L. Time-Resolved IR Spectroscopy Reveals a. Mechanism with TiO2 as a Reversible Electron Acceptor in a TiO2-Re Catalyst System for CO2 Photoreduction. J. Am. Chem. Soc. 2017, 139, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, L.C.; Gong, Y.; Niu, P.; Wang, J.Q.; Gu, L.; Chen, X.; Liu, G.; Wang, L.; Cheng, H.M. An Unusual Strong Visible-Light Absorption Band in Red Anatase TiO2 Photocatalyst Induced by Atomic Hydrogen-Occupied Oxygen Vacancies. Adv. Mater. 2018, 30, 1704479. [Google Scholar] [CrossRef]
- Li, F.; Yue, X.Y.; Zhang, D.N.; Fan, J.J.; Xiang, Q.J. Targeted regulation of exciton dissociation in graphitic carbon nitride by vacancy modification for efficient photocatalytic CO2 reduction. Appl. Catal. B-Environ. 2021, 292, 120179. [Google Scholar]
- Ikreedeegh, R.R. Recent developments of Fe-based metal-organic frameworks and their composites in photocatalytic applications: Fundamentals, synthesis and challenges. Russ. Chem. Rev. 2022, 91, RCR5064. [Google Scholar] [CrossRef]
- Li, Y.; Ho, W.; Lv, K.; Zhu, B.; Lee, S.C. Carbon vacancy-induced enhancement of the visible light-driven photocatalytic oxidation of NO over g-C3N4 nanosheets. Appl. Surf. Sci. 2018, 430, 380–389. [Google Scholar] [CrossRef]
- Pang, H.; Meng, X.; Song, H.; Zhou, W.; Yang, G.; Zhang, H.; Izumi, Y.; Takei, T.; Jewasuwan, W.; Fukata, N.; et al. Probing the role of nickel dopant in aqueous colloidal ZnS nanocrystals for efficient solar-driven CO2 reduction. Appl. Catal. B 2019, 244, 1013–1020. [Google Scholar] [CrossRef]
- Shi, H.N.; Long, S.R.; Hou, J.G.; Ye, L.; Sun, Y.; Ni, W.; Song, C.; Li, K.; Gurzadyan, G.G.; Guo, X. Defects Promote ultrafast charge separation in graphitic carbon nitride for enhanced visible-light-driven CO2 reduction activity. Chem.-Eur. J. 2018, 25, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.R.; Liu, J.; Zhang, J.T. Defect Engineering in 2D Photocatalytic Materials for CO2 Reduction. ChemNanoMat 2021, 7, 737–747. [Google Scholar] [CrossRef]
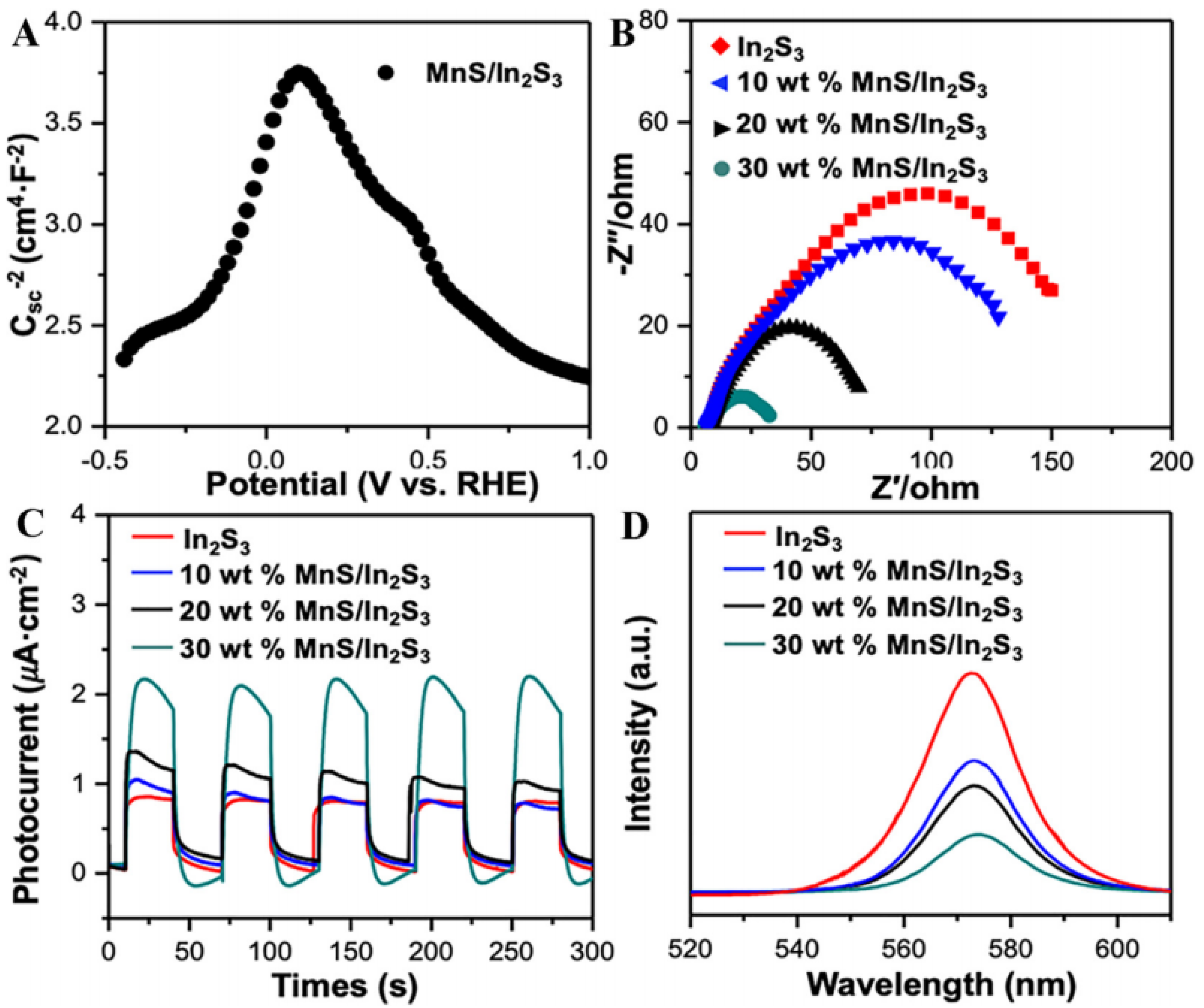
- Tan, J.; Yu, M.; Cai, Z.; Lou, X.; Wang, J.; Li, Z. MOF-derived synthesis of MnS/In2S3 p-n heterojunctions with hierarchical structures for efficient photocatalytic CO2 reduction. J. Colloid Interface Sci. 2021, 588, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.W.; Xuan, Y.M.; Zhang, K.; Liu, X.L. Highly Selective Production of Ethanol over Hierarchical Bi@Bi2MoO6 composite via Bicarbonate-Assisted Photocatalytic CO2 Reduction. ChemSusChem 2021, 14, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qileng, A.; Wubulikasimu, N.; Liu, Z.X.; Liu, W.P.; Liu, Y.J. Self-sacrificial templated tynthesis of Fe/N co-doping TiO2 for enhanced CO2 photocatalytic reduction. ChemNanoMat 2023, 9, e202300343. [Google Scholar] [CrossRef]
- Guo, Q.; Fu, L.M.; Yan, T.; Tian, W.; Ma, D.F.; Li, J.M.; Jiang, Y.N.; Wang, X.T. Improved photocatalytic activity of porous ZnO nanosheets by thermal deposition graphene-like g-C3N4 for CO2 reduction with H2O vapor. Appl. Surf. Sci. 2020, 509, 144773. [Google Scholar] [CrossRef]
- Ding, C.; Lu, X.X.; Tao, B.; Yang, L.Q. Interlayer spacing regulation by single-atom indium Inδ+__N4 on carbon nitride for boosting CO2/CO photo-conversion. Adv. Funct. Mater. 2023, 33, 2302824. [Google Scholar] [CrossRef]
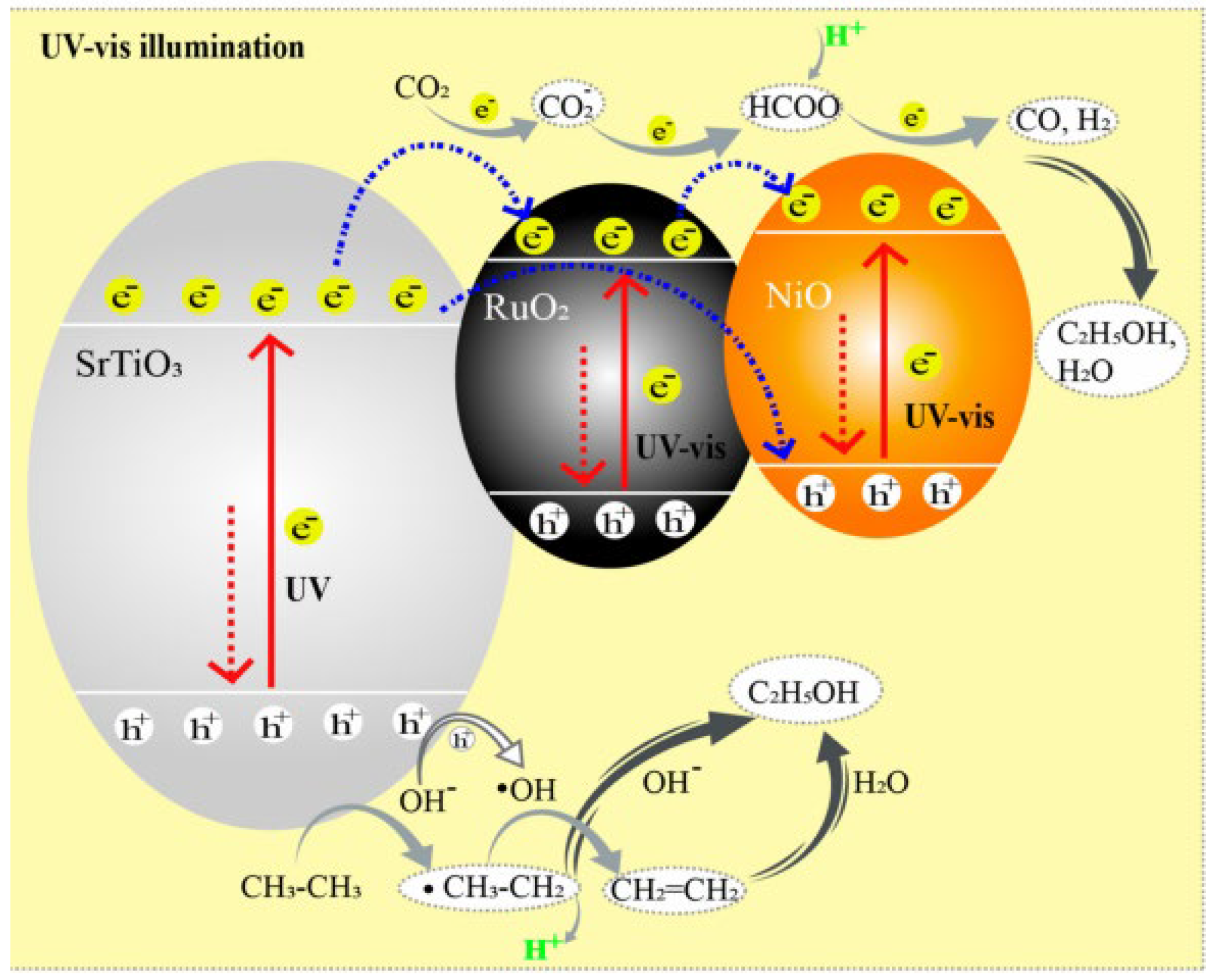
- Paulista, L.O.; Albero, J.; Martins, R.J.E.; Boaventura, R.A.R.; Vilar, V.J.P.; Silva, T.F.C.V.; García, H. Turning carbon dioxide and ethane into ethanol by solar-driven heterogeneous photocatalysis over RuO2- and NiO-co-Doped SrTiO3. Catalysts 2021, 11, 461. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, X.; Li, X.; Nie, X.; Fan, S.; Feng, M.; Fan, Z.; Tan, M.; Chen, Y.; He, G. Construction of atomically dispersed Cu-N4 sites via engineered coordination environment for high-efficient CO2 electroreduction. Chem. Eng. J. 2021, 407, 126842. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. A critical review in recent developments of metal-organic-frameworks (MOFs) with band engineering alteration for photocatalytic CO2 reduction to solar fuels. J. CO2 Utili. 2021, 43, 101381. [Google Scholar] [CrossRef]
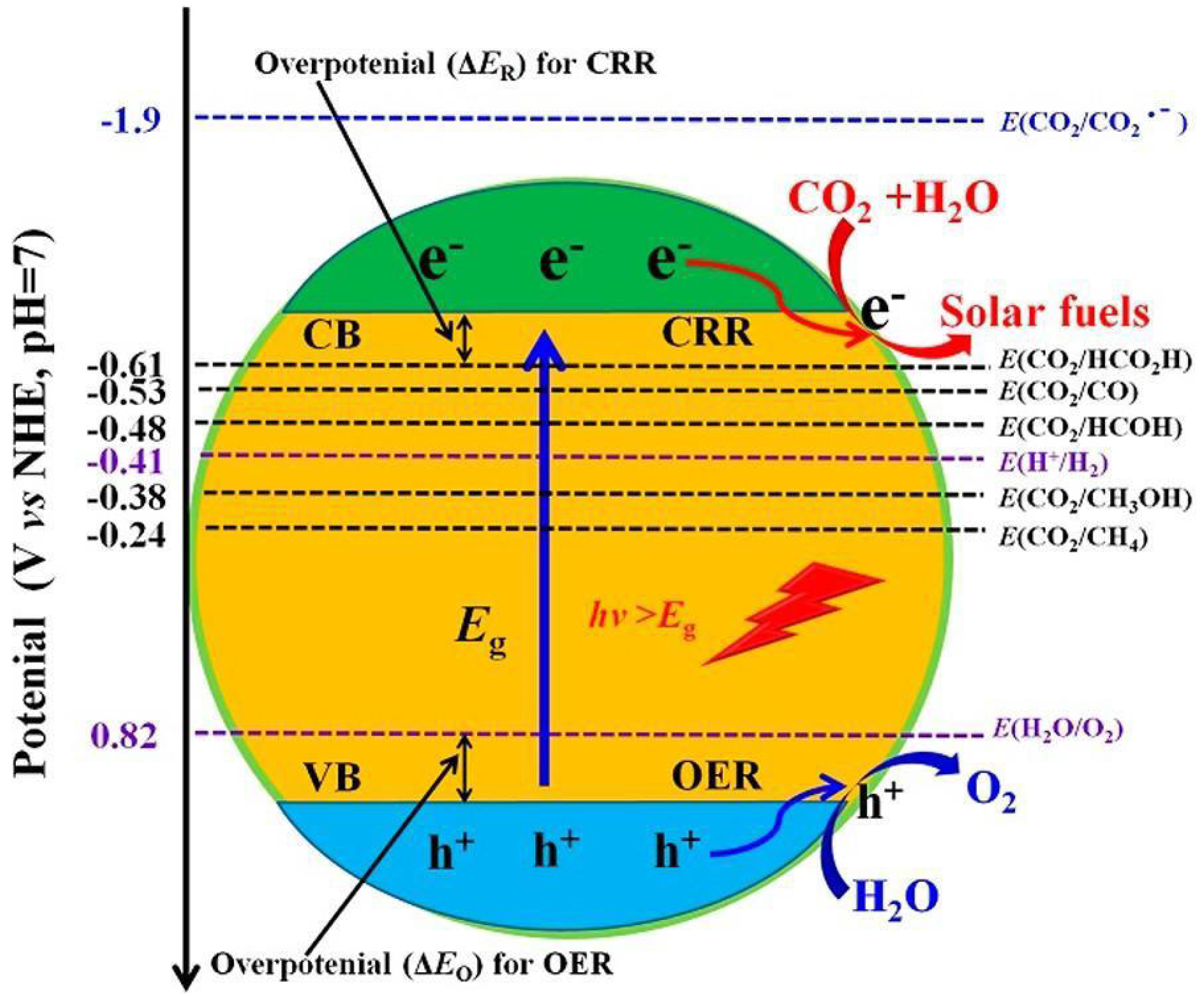

| Specific Reaction | Redox Potentials E° (V) versus SHE at pH = 7 |
|---|---|
| CO + e− → CO− | −1.9 |
| CO2 + 2H+ + 2e− → HCOOH | −0.61 |
| CO2 + 2H+ + 2e− → CO + H2O | −0.53 |
| CO2 + 4H+ + 4e− → HCHO + H2O | -0.48 |
| CO2 + 6H+ + 6e− → CH3OH + H2O | −0.38 |
| CO2 + 8H+ + 8e− → CH4 + 2H2O | −0.24 |
| 2CO2 + 12H+ + 12e− → C2H4 + 4H2O | −0.34 |
| 2CO2 + 12H+ + 12e− → C2H5OH + 3H2O | −0.33 |
| 2CO2 + 14H+ + 14e− → C2H6 + 4H2O | −0.27 |
| 3CO2 + 18H+ + 18e−→ C3H7OH + 5H2O | −0.32 |
| 3CO2 + 20H+ + 20e− → C3H8 + 6H2O | −0.33 |
| 2H+ + 2e− → H2 | −0.41 |
| 2H2O + 4h+ → 4H+ + O2 | 0.82 |
| Type of Defect | Defective Photocatalyst | Product | Yield and Selectivity | Roles of Defects on Photocatalysis | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Cation vacancy | ZnS | HCOOH | Selectivity up to 86.6% | Lowering the barrier of CO2RR and suppressing proton adsorption | Changing the electronic states of density | [41] |
| Cation vacancy | ZnIn2S4 | CO | 33.2 μmol g−1 h−1 | Increasing the light absorption, improving the CO2 adsorption capacity, and enhancing surface hydrophilicity | Increased charge density | [42] |
| Metal vacancy | Ag-TiO2 | CH4 | 16.0 ppm/g h | Promoting the separation of photo-induced electron-hole pairs | Forming a Schottky barrier and surface plasmon resonance | [43] |
| Metal vacancy | PtCu/TiO2 | CH4 | Selectivity up to 100% | Enhancing the adsorption/activation of CO2/CO and the further hydrogenation of CO | Reducing the activation energy barriers of *CO2 and *CHO and inhibiting the desorption of *CO | [44] |
| Metal vacancy | Co-CN | CO | 94.9 umol/g/h | Reducing the energy barrier of CO2 adsorption/activation and promoting the | Strong interaction between electrons | [45] |
| Carbon vacancy | GCN | CO | 4.18 mmol g−1 h−1 | Enhancing CO2 adsorption/activation, upshifting the conduction band and elevating the charge carrier concentration and lifetime | Attenuating the exciton effect and facilitating charge carrier generation | [55] |
| Doping | Cu-TiO2 | CH4 | 8.04 μmol g−1 h−1 | Increasing visible-light absorption in the materials, and suppressing photogenerated electron-hole recombination | Serving as electron traps | [56] |
| Doping | O-doped g-C3N4 | CH3OH | 0.88 µmol g-1 h-1 | Improving light utilization efficiency and CO2 affinity, and separation efficiency of photogenerated charge carriers | Optimizing the band structure | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, C.; Tang, X.; Wang, H.; Su, Q. A Review of the Effect of Defect Modulation on the Photocatalytic Reduction Performance of Carbon Dioxide. Molecules 2024, 29, 2308. https://doi.org/10.3390/molecules29102308
Zuo C, Tang X, Wang H, Su Q. A Review of the Effect of Defect Modulation on the Photocatalytic Reduction Performance of Carbon Dioxide. Molecules. 2024; 29(10):2308. https://doi.org/10.3390/molecules29102308
Chicago/Turabian StyleZuo, Cheng, Xiao Tang, Haiquan Wang, and Qian Su. 2024. "A Review of the Effect of Defect Modulation on the Photocatalytic Reduction Performance of Carbon Dioxide" Molecules 29, no. 10: 2308. https://doi.org/10.3390/molecules29102308
APA StyleZuo, C., Tang, X., Wang, H., & Su, Q. (2024). A Review of the Effect of Defect Modulation on the Photocatalytic Reduction Performance of Carbon Dioxide. Molecules, 29(10), 2308. https://doi.org/10.3390/molecules29102308






