Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets
Abstract
1. Introduction
2. Methods
3. Therapeutic Efficacies of Plant Extracts
3.1. Effects of Plant Extracts on Dermal Papilla Cells In Vitro
3.2. Effects of Plant Extracts on Hair Follicles Ex Vivo
3.3. Effects of Plant Extracts on Hair Growth in Animal Models In Vivo
3.4. Clinical Studies on the Hair Growth Promotion or Suppression Efficacy of Plant Extracts
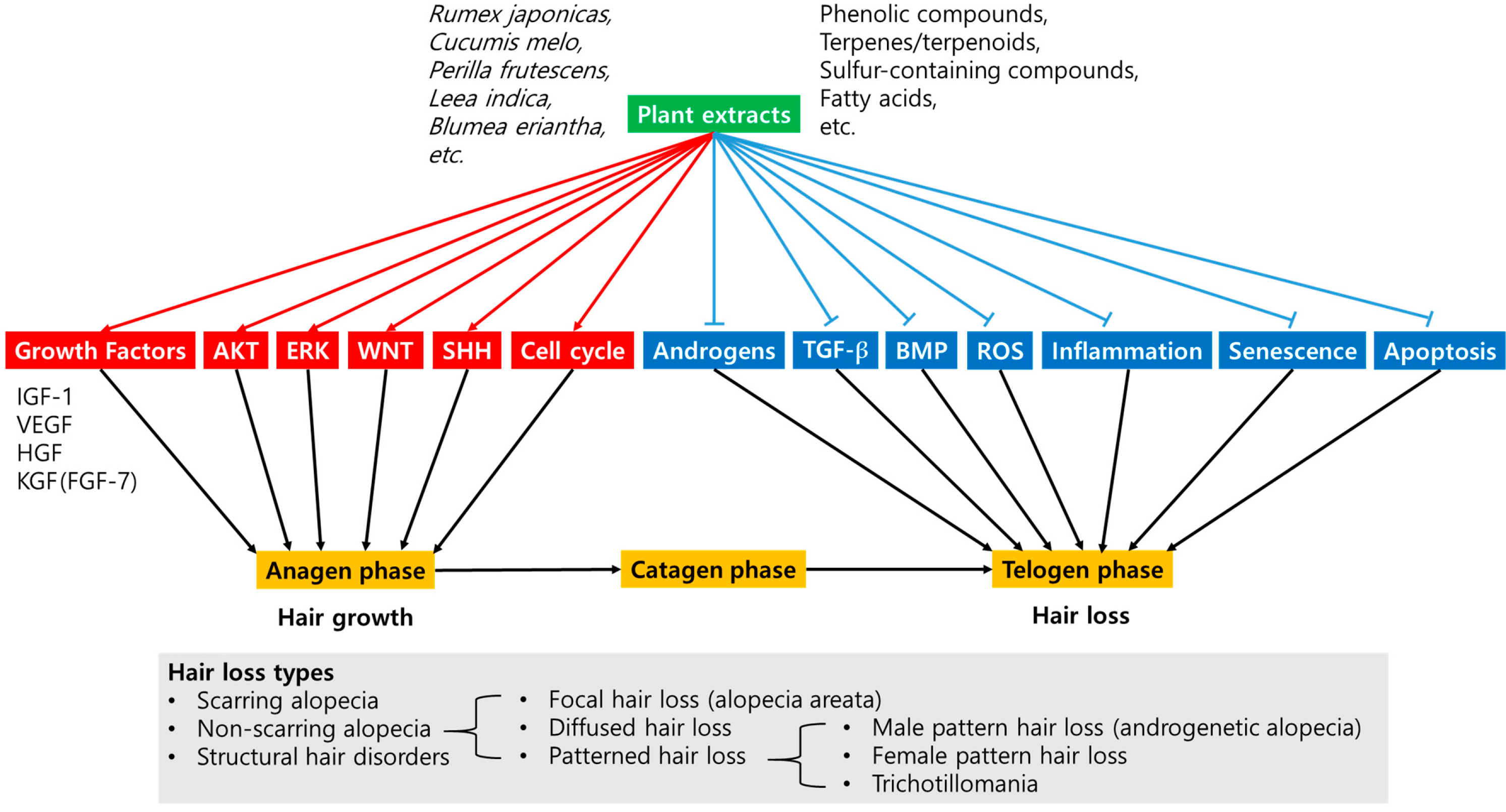
4. Phytochemical Components and Active Compounds in Plant Extracts
4.1. Phenolic Compounds
4.2. Terpenes and Terpenoids
4.3. Sulfur-Containing Compounds, Fatty Acids, and Other Compounds
5. Modulatory Targets of Plant Extracts
5.1. Antioxidant, Anti-Inflammatory, and Anti-Senescence Effects of Plant Extracts
5.2. Effects of Plant Extracts on the Apoptotic Cell Death Pathway
5.3. Effects of Plant Extracts on Male Hormones
5.4. Effects of Plant Extracts on Cell Cycle
5.5. Effects of Plant Extracts on the Expression Levels of Growth Factors
5.6. Effects of Plant Extracts on the AKT and Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways
5.7. Effects of Plant Extracts on the Wingless and Int-1 (WNT) Signaling Pathways
5.8. Effects of Plant Extracts on the Sonic Hedgehog (SHH) Signaling Pathways
5.9. Effects of Plant Extracts on the Transforming Growth Factor (TGF)-β and Bone Morphogenetic Protein (BMP) Signaling Pathways
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.C.; Zhang, Y.; Rheinstädter, M.C. The structure of people’s hair. PeerJ 2014, 2, e619. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The Hair Follicle as a Dynamic Miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, X.M.; Liu, Z.N.; Wang, Y.; Han, X.; Lian, A.B.; Mu, Y.; Jin, M.H.; Liu, J.Y. Human hair follicle-derived mesenchymal Stem. cells: Isolation, expansion, and differentiation. World J. Stem. Cells. 2020, 12, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Buffoli, B.; Rinaldi, F.; Labanca, M.; Sorbellini, E.; Trink, A.; Guanziroli, E.; Rezzani, R.; Rodella, L.F. The human hair: From anatomy to physiology. Int. J. Dermatol. 2014, 53, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Piyaman, P.; Patchanee, K.; Oonjitti, T.; Ratanalekha, R.; Yodrabum, N. Surgical anatomy of vascularized submental lymph node flap: Sharing arterial supply of lymph nodes with the skin and topographic relationship with anterior belly of digastric muscle. J. Surg. Oncol. 2020, 121, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.A. The Dermal Papilla: An Instructive Niche for Epithelial Stem. and Progenitor Cells in Development and Regeneration of the Hair Follicle. CSH Perspect Med. 2014, 4, a015180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, T. Local and systemic mechanisms that control the hair follicle Stem. cell niche. Nat. Rev. Mol. Cell Biol. 2024, 25, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.F.; Zhu, Z.Y.; Sun, X.Y.; Fu, X.B. Functional hair follicle regeneration: An updated review. Signal Transduct. Target Ther. 2021, 6, 66. [Google Scholar] [CrossRef]
- Alzoabi, N.M.; Alsharif, H.S.r.; Alawami, A.M.; Habarah, H.H.; Alhawaj, H.A.; Bin Rubaian, N.; Alqahtani, J.M. Assessing the Impact of Alopecia on Quality of Life, Depression, and Self-Esteem in Saudi Arabia. Cureus J. Med. Sci. 2023, 15, e49864. [Google Scholar] [CrossRef]
- Gokce, N.; Basgoz, N.; Kenanoglu, S.; Akalin, H.; Ozkul, Y.; Ergoren, M.C.; Beccari, T.; Bertelli, M.; Dundar, M. An overview of the genetic aspects of hair loss and its connection with nutrition. J. Prev. Med. Hyg. 2022, 63, E228–E238. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Juma, H.; Eid, F.A.; Alaswad, H.A.; Ali, W.M.; Aladraj, F.J. Effects of Hormones and Endocrine Disorders on Hair Growth. Cureus 2022, 14, e32726. [Google Scholar] [CrossRef] [PubMed]
- Zeberkiewicz, M.; Rudnicka, L.; Malejczyk, J. Immunology of alopecia areata. Cent. Eur. J. Immunol. 2020, 45, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Cash, T.F. The psychology of hair loss and its implications for patient care. Clin. Dermatol. 2001, 19, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Borrelli, M.R.; Tapking, C.; Popp, D.; Puladi, B.; Ooms, M.; Chelliah, M.P.; Rein, S.; Pförringer, D.; Thor, D.; et al. Molecular Mechanisms of Hair Growth and Regeneration: Current Understanding and Novel Paradigms. Dermatology 2020, 236, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Mulinari-Brenner, F.; Bergfeld, W.F. Hair loss: Diagnosis and management. Cleve Clin. J. Med. 2003, 70, 705–706, 709–710, 712. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mysore, V. Classifications of Patterned Hair Loss: A Review. J. Cutan Aesthet Surg. 2016, 9, 3–12. [Google Scholar] [CrossRef]
- Cardoso, C.O.; Tolentino, S.; Gratieri, T.; Cunha-Filho, M.; Lopez, R.F.V.; Gelfuso, G.M. Topical Treatment for Scarring and Non-Scarring Alopecia: An Overview of the Current Evidence. Clin. Cosmet. Investig. Dermatol. 2021, 14, 485–499. [Google Scholar] [CrossRef]
- Olsen, E.A.; Whiting, D.; Bergfeld, W.; Miller, J.; Hordinsky, M.; Wanser, R.; Zhang, P.; Kohut, B. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2007, 57, 767–774. [Google Scholar] [CrossRef]
- Modha, J.D.; Pathania, Y.S. Comprehensive review of oral minoxidil in alopecia. J. Cosmet. Dermatol. 2022, 21, 5527–5531. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Talukder, M.; Venkataraman, M.; Bamimore, M.A. Minoxidil: A comprehensive review. J. Dermatol. Treat. 2022, 33, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Chislett, B.; Chen, D.; Perera, M.L.; Chung, E.; Bolton, D.; Qu, L.G. 5-alpha reductase inhibitors use in prostatic disease and beyond. Transl. Androl. Urol. 2023, 12, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Song, S.; Gao, Z.; Wu, J.; Ma, J.; Cui, Y. The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: A systematic review and meta-analysis. Clin. Interv. Aging. 2019, 14, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Cruz, M.; Magana, M.; Escandon-Perez, S.; Bello-Chavolla, O.Y. Use of 5-Alpha Reductase Inhibitors in Dermatology: A Narrative Review. Dermatol Ther. 2023, 13, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Ntege, E.H.; Sunami, H.; Inoue, Y. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen. Ther. 2022, 21, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Sivamaruthi, B.S.; Thangaleela, S.; Bharathi, M.; Chaiyasut, C. Role and Mechanisms of Phytochemicals in Hair Growth and Health. Pharmaceuticals 2023, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Kowalczyk, T.; Wieezfinska, J.; Merecz-Sadowska, A.; Górski, K.; Sliwinski, T.; Skala, E. Plant Extracts as a Natural Source of Bioactive Compounds and Potential Remedy for the Treatment of Certain Skin Diseases. Curr. Pharm. Design. 2020, 26, 2859–2875. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 1235. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Ei, Z.Z.; Visuttijai, K.; Chanvorachote, P. Potential Natural Products Regulation of Molecular Signaling Pathway in Dermal Papilla Stem. Cells. Molecules 2023, 28, 5517. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G.; Akram, S.; Westgate, G.E.; Tamburic, S. Can plant-derived phytochemicals provide symptom relief for hair loss? A critical review. Int. J. Cosmetic Sci. 2019, 41, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.S.; Park, S.J.; Hwang, S.L.; Lee, M.H.; Kim, C.D.; Lee, I.H.; Chang, S.Y.; Rang, M.J. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J. Dermatol. Sci. 2005, 38, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Han, J.; Kchouk, M.E.; Isoda, H. Hair growth regulation by the extract of aromatic plant Erica multiflora. J. Nat. Med. 2009, 63, 335–339. [Google Scholar] [CrossRef]
- Park, P.J.; Moon, B.S.; Lee, S.H.; Kim, S.N.; Kim, A.R.; Kim, H.J.; Park, W.S.; Choi, K.Y.; Cho, E.G.; Lee, T.R. Hair growth-promoting effect of Aconiti Ciliare Tuber extract mediated by the activation of Wnt/beta-catenin signaling. Life Sci. 2012, 91, 935–943. [Google Scholar] [CrossRef]
- Junlatat, J.; Sripanidkulchai, B. Hair growth-promoting effect of Carthamus tinctorius floret extract. Phytother. Res. 2014, 28, 1030–1036. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, J.Y.; Park, B.C.; Lee, B.H. Platycarya strobilacea S. et Z. Extract Has a High Antioxidant Capacity and Exhibits Hair Growth-promoting Effects in Male C57BL/6 Mice. Prev. Nutr. Food Sci. 2014, 19, 136–144. [Google Scholar] [CrossRef]
- Park, G.H.; Park, K.Y.; Cho, H.I.; Lee, S.M.; Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C.; et al. Red ginseng extract promotes the hair growth in cultured human hair follicles. J. Med. Food. 2015, 18, 354–362. [Google Scholar] [CrossRef]
- Lee, H.; Kim, N.H.; Yang, H.; Bae, S.K.; Heo, Y.; Choudhary, I.; Kwon, Y.C.; Byun, J.K.; Yim, H.J.; Noh, B.S.; et al. The Hair Growth-Promoting Effect of Rumex japonicus Houtt. Extract. Evid. Based Complement. Alternat. Med. 2016, 2016, 1873746. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Gupta, B.; Park, H.G.; Son, M.; Jun, J.H.; Yong, C.S.; Kim, J.A.; Kim, J.O. Preclinical and Clinical Studies Demonstrate That the Proprietary Herbal Extract DA-5512 Effectively Stimulates Hair Growth and Promotes Hair Health. Evid. Based Complement. Alternat. Med. 2017, 2017, 4395638. [Google Scholar] [CrossRef]
- Boisvert, W.A.; Yu, M.; Choi, Y.; Jeong, G.H.; Zhang, Y.L.; Cho, S.; Choi, C.; Lee, S.; Lee, B.H. Hair growth-promoting effect of Geranium sibiricum extract in human dermal papilla cells and C57BL/6 mice. BMC Complement. Altern Med. 2017, 17, 109. [Google Scholar] [CrossRef]
- Somsukskul, I.; De-Eknamkul, W.; Tengamnuay, P. Effect of Orthosiphon stamineus plant extract on in vitro dermal papilla cell proliferation and ex vivo hair growth. Chulalongkorn Med. J. 2017, 61, 41–50. [Google Scholar] [CrossRef]
- Wen, T.C.; Li, Y.S.; Rajamani, K.; Harn, H.J.; Lin, S.Z.; Chiou, T.W. Effect of Cinnamomum osmophloeum Kanehira Leaf Aqueous Extract on Dermal Papilla Cell Proliferation and Hair Growth. Cell Transplant. 2018, 27, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.Y.; Choi, Y.H.; Jang, M.; Nam, Y.J.; Lee, S.Y.; Jeon, J.; Jin, M.H.; Lee, S. Hair Growth Promoting Effect of Hottuynia cordata Extract in Cultured Human Hair Follicle Dermal Papilla Cells. Biol. Pharm. Bull. 2019, 42, 1665–1673. [Google Scholar] [CrossRef]
- Kang, M.G.; Park, D.; Han, H.Y.; Shim, H.; Hong, Y.; Moon, J.; Yoon, S.; Kwon, B. RE-ORGA, a Korean Herb Extract, Can Prevent Hair Loss Induced by Dihydrotestosterone in Human Dermal Papilla Cells. Ann. Dermatol. 2019, 31, 530–537. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, Y.H.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.R.; Zhang, Y.L.; Yap, J.; Boisvert, W.A.; Lee, B.H. Hair growth potential of Salvia plebeia extract and its associated mechanisms. Pharm. Biol. 2020, 58, 400–409. [Google Scholar] [CrossRef]
- Yamada, N.; Miki, K.; Yamaguchi, Y.; Takauji, Y.; Yamakami, Y.; Hossain, M.N.; Ayusawa, D.; Fujii, M. Extract of Plumbago zeylanica enhances the growth of hair follicle dermal papilla cells with down-regulation of 5α-reductase type II. J. Cosmet. Dermatol. 2020, 19, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Serruya, R.; Maor, Y. Hair growth-promotion effects at the cellular level and antioxidant activity of the plant-based extract Phyllotex™. Heliyon 2021, 7, e07888. [Google Scholar] [CrossRef]
- Park, H.J.; Jin, G.R.; Jung, J.H.; Hwang, S.B.; Lee, S.H.; Lee, B.H. Hair Growth Promotion Effect of Nelumbinis Semen Extract with High Antioxidant Activity. Evid. Based Complement. Alternat. Med. 2021, 2021, 6661373. [Google Scholar] [CrossRef]
- Ramadhani, F.J.; Bak, D.H.; Kang, S.H.; Park, C.H.; Park, S.H.; Chung, B.Y.; Bai, H.W. The effects of centipedegrass extract on hair growth via promotion of anagen inductive activity. PLoS ONE 2022, 17, e0265532. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E.; et al. Phytochemical Constitution, Anti-Inflammation, Anti-Androgen, and Hair Growth-Promoting Potential of Shallot (Allium ascalonicum L.) Extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Chen, T.; Ma, L. Hair growth-promoting effects of Camellia seed cake extract in human dermal papilla cells and C57BL/6 mice. J. Cosmet. Dermatol. 2022, 21, 5018–5025. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Kim, J.H.; Park, S.D.; Shim, J.J.; Lee, J.L. Lactobacillus paracasei HY7015 and Lycopus lucidus Turcz. Extract Promotes Human Dermal Papilla Cell Cytoprotective Effect and Hair Regrowth Rate in C57BL/6 Mice. Molecules 2022, 27, 8235. [Google Scholar] [CrossRef]
- Tan, Y.F.; Koay, Y.S.; Zulkifli, R.M.; Hamid, M.A. In Vitro hair growth and hair tanning activities of mangosteen pericarp extract on hair dermal papilla cells. J. Herb. Med. 2022, 36, 100594. [Google Scholar] [CrossRef]
- You, J.; Woo, J.; Roh, K.B.; Ryu, D.; Jang, Y.; Cho, E.; Park, D.; Jung, E. Assessment of the anti-hair loss potential of Camellia japonica fruit shell extract in vitro. Int. J. Cosmet. Sci. 2023, 45, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Lin, Y.H.; Lin, Y.K.; Chiang, C.F. Hair growth-promotion effects and antioxidant activity of the banana flower extract HappyAngel?: Double-blind, placebo-controlled trial. Food Sci. Hum. Well. 2023, 12, 1917–1923. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Ogura, K.; Hagiwara, K.; Ueno, S.; Kitamura, H.; Yamanishi, H.; Tsunekawa, Y.; Kiso, A. Ginsenosides in Panax ginseng Extract Promote Anagen Transition by Suppressing BMP4 Expression and Promote Human Hair Growth by Stimulating Follicle-Cell Proliferation. Biol. Pharm. Bull. 2024, 47, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, X.; Ji, T.; Hu, H.; Chang, L. Gynostemma pentaphyllum Makino extract induces hair growth and exhibits an anti-graying effect via multiple mechanisms. J. Cosmet. Dermatol. 2024, 23, 648–657. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Woo, J.; Roh, K.B.; Jeon, K.; Jang, Y.; Choi, S.A.; Ryu, D.; Cho, E.; Park, D.; Lee, J.; et al. Evaluation of efficacy of Silybum marianum flower extract on the mitigating hair loss in vitro and in vivo. J. Cosmet. Dermatol. 2024, 23, 529–542. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, X. Brassica oleracea extract, glucosinlates, and sulforaphane promote hair growth in vitro and ex vivo. J. Cosmet. Dermatol. 2022, 21, 1178–1184. [Google Scholar] [CrossRef]
- Ma, L.; Shen, H.; Fang, C.; Chen, T.; Wang, J. Camellia Seed Cake Extract Supports Hair Growth by Abrogating the Effect of Dihydrotestosterone in Cultured Human Dermal Papilla Cells. Molecules 2022, 27, 6443. [Google Scholar] [CrossRef]
- Woo, M.J.; Kang, H.Y.; Paik, S.J.; Choi, H.J.; Uddin, S.; Lee, S.; Kim, S.Y.; Choi, S.; Jung, S.K. The In Vivo and In Vitro Effects of Terminalia bellirica (Gaertn.) Roxb. Fruit Extract on Testosterone-Induced Hair Loss. J. Microbiol. Biotechnol. 2023, 33, 1467–1474. [Google Scholar] [CrossRef]
- Bullwinkel, J.; Baron-Lühr, B.; Lüdemann, A.; Wohlenberg, C.; Gerdes, J.; Scholzen, T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J. Cell Physiol. 2006, 206, 624–635. [Google Scholar] [CrossRef]
- Pi, L.Q.; Lee, W.S.; Min, S.H. Hot water extract of oriental melon leaf promotes hair growth and prolongs anagen hair cycle: In vivo and in vitro evaluation. Food Sci. Biotechnol. 2016, 25, 575–580. [Google Scholar] [CrossRef]
- Li, J.J.; Li, Z.; Gu, L.J.; Choi, K.J.; Kim, D.S.; Kim, H.K.; Sung, C.K. The promotion of hair regrowth by topical application of a Perilla frutescens extract through increased cell viability and antagonism of testosterone and dihydrotestosterone. J. Nat. Med. 2018, 72, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kawai, Y.; Masutani, T.; Tanaka, K.; Ito, K.; Iddamalgoda, A. Effects of watercress extract fraction on R-spondin 1-mediated growth of human hair. Int. J. Cosmetic. Sci. 2022, 44, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Fard, S.G.; Shamsabadi, F.T.; Emadi, M.; Meng, G.Y.; Muhammad, K.; Mohamed, S. Ethanolic Extract of Eucheuma cottonii Promotes in vivo Hair Growth and Wound Healing. J. Anim. Vet. Adv. 2011, 10, 601–605. [Google Scholar] [CrossRef]
- Rajan, P.; Natraj, P.; Kim, N.H.; Kim, J.H.; Choi, H.J.; Han, C.H. Effects of Cudrania tricuspidata and Sargassum fusiforme extracts on hair growth in C57BL/6 mice. Lab. Anim. Res. 2023, 39, 4. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kondo, R.; Sakai, K.; Shoyama, Y.; Sato, H.; Ueno, T. Steroid 5alpha-reductase inhibitory activity and hair regrowth effects of an extract from Boehmeria nipononivea. Biosci. Biotechnol. Biochem. 2000, 64, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.S.; Kim, C.D.; Lee, M.H.; Hwang, S.L.; Rang, M.J.; Yoon, Y.K. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J. Dermatol. Sci. 2002, 30, 43–49. [Google Scholar] [CrossRef]
- Datta, K.; Singh, A.T.; Mukherjee, A.; Bhat, B.; Ramesh, B.; Burman, A.C. Eclipta alba extract with potential for hair growth promoting activity. J. Ethnopharmacol. 2009, 124, 450–456. [Google Scholar] [CrossRef]
- Murkute, A.V.; Sahu, M.S.; Mali, P.Y.; Rangari, V.D. Development and evaluation of formulations of microbial biotransforMed. extract of tobacco leaves for hair growth potential. Pharmacogn. Res. 2010, 2, 300–303. [Google Scholar] [CrossRef]
- Park, H.J.; Zhang, N.; Park, D.K. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J. Ethnopharmacol. 2011, 135, 369–375. [Google Scholar] [CrossRef]
- Upadhyay, S.; Ghosh, A.K.; Singh, V. Hair Growth Promotant Activity of Petroleum Ether Root Extract of Glycyrrhiza Glabra L (Fabaceae) in Female Rats. Trop. J. Pharm. Res. 2012, 11, 753–758. [Google Scholar] [CrossRef][Green Version]
- Sandhya, S.; Chandrasekhar, J.; Vinod, K.; Banji, D. Potentiality of aqueous leaf extract of Trichosanthes cucumerina Linn. on hair growth promotion in Wistar albino rats. Indian J. Nat. Prod. Resour. 2012, 3, 14–19. [Google Scholar]
- Hou, I.C.; Oi, Y.; Fujita, H.; Yano, Y.; Fukami, H.; Yoshikawa, M. A hair growth-promoting effect of Chinese black tea extract in mice. Biosci. Biotechnol. Biochem. 2013, 77, 1606–1607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, N.N.; Park, D.K.; Park, H.J. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement. Altern Med. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Noguchi, K.; Kondo, M.; Onishi, M.; Watanabe, N.; Okamura, K.; Matsuda, H. Promotion of hair growth by Rosmarinus officinalis leaf extract. Phytother Res. 2013, 27, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jung, S.K.; Jeon, M.H.; Moon, J.N.; Moon, W.S.; Ji, Y.H.; Choi, I.S.; Wook Son, S. Effects of Lycopersicon esculentum extract on hair growth and alopecia prevention. J. Cosmet. Sci. 2013, 64, 429–443. [Google Scholar] [PubMed]
- Choi, J.S.; Jeon, M.H.; Moon, W.S.; Moon, J.N.; Cheon, E.J.; Kim, J.W.; Jung, S.K.; Ji, Y.H.; Son, S.W.; Kim, M.R. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol. Pharm. Bull. 2014, 37, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Gu, L.; Begum, S.; Wang, Y.; Sun, B.; Lee, M.; Sung, C. Chrysanthemum zawadskii extract induces hair growth by stimulating the proliferation and differentiation of hair matrix. Int. J. Mol. Med. 2014, 34, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Punasiya, R.; Verma, R.; Pillai, S. In vitro hair growth promoting activity of various leaves extract of Hibiscus syriacus L. on albino rats. Int. J. Pharm. Life Sci. 2014, 5, 3565–3579. [Google Scholar]
- Rozianoor, M.W.; Nadia, M.F.; Dzulsuhaimi, D. In vivo evaluation of hair growth potential of Stachytarpheta jamaicensis ethanolic leaves extract on Sprague Dawley rats. Nat. Prod. Ann. Indian J. 2014, 10, 17–21. [Google Scholar]
- Begum, S.; Gu, L.J.; Lee, M.R.; Li, Z.; Li, J.J.; Hossain, M.J.; Wang, Y.B.; Sung, C.K. In vivo hair growth-stimulating effect of medicinal plant extract on BALB/c nude mice. Pharm. Biol. 2015, 53, 1098–1103. [Google Scholar] [CrossRef]
- Park, S.O.; Park, B.S.; Noh, G.Y. Action mechanism of natural plant extracts for hair loss prevention and hair growth promotion in C57BL/6 mice. Int. J. Pharmacol. 2015, 11, 588–595. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, D.; Ganapaty, S.; Sushrutha, M. Preliminary phytochemical analysis and evaluation of hair growth stimulating potential of ethanol extract from L. (Asteraceae) leaves in Wistar albino Eclipta alba rats. Asian J. Pharm. Pharmacol. 2016, 2, 121–127. [Google Scholar]
- Imtiaz, F.; Islam, M.; Saeed, H.; Saleem, B.; Asghar, M.; Saleem, Z. Impact of Trigonella foenum-graecum Leaves Extract on Mice Hair Growth Leaves Extract on Mice Hair Growth. Pak. J. Zool. 2017, 49, 1405–1412. [Google Scholar] [CrossRef]
- Zhu, H.L.; Gao, Y.H.; Yang, J.Q.; Li, J.B.; Gao, J. Serenoa repens extracts promote hair regeneration and repair of hair loss mouse models by activating TGF-β and mitochondrial signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4000–4008. [Google Scholar] [PubMed]
- Nanashima, N.; Horie, K. Blackcurrant Extract with Phytoestrogen Activity Alleviates Hair Loss in Ovariectomized Rats. Molecules 2019, 24, 1272. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.X.; Pang, S.L.; Zhou, J.; Cai, J.; Shang, J. Alcohol extract from Vernonia anthelmintica willd (L.) seed counteracts stress-induced murine hair follicle growth inhibition. BMC Complement. Altern. Med. 2019, 19, 372. [Google Scholar] [CrossRef] [PubMed]
- Amin, J.; Djajadisastra, J.; Syafhan, N.F.; Simamora, E.L.P.; Wulandari, K. Green tea [Camellia sinensis (L.) kuntze] leaves extract and hibiscus (Hibiscus tilliaceus L.) leaves extract as topical hair growth promoter in microemulsion. Agr. Nat. Resour. 2019, 53, 139–147. [Google Scholar] [CrossRef]
- Lee, T.K.; Kim, B.; Kim, D.W.; Ahn, J.H.; Sim, H.; Lee, J.C.; Yang, G.E.; Her, Y.; Park, J.H.; Kim, H.S.; et al. Effects of Decursin and Angelica gigas Nakai Root Extract on Hair Growth in Mouse Dorsal Skin via Regulating Inflammatory Cytokines. Molecules 2020, 25, 3697. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Park, Y.E.; Kim, B.; Park, C.W.; Sim, T.H.; Lee, T.K.; Lee, J.C.; Park, J.H.; Kim, J.D.; Lee, H.S.; et al. Hair Growth is Promoted in Mouse Dorsal Skin by a Mixture of Platycladus orientalis (L.) Franco Leaf Extract and Alpha-Terpineol by Increasing Growth Factors and wnt3/β-Catenin. Nat. Prod Commun. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Rose, L.C.; Rusdi, N.N.S.; Asari, A.; Abd Wahid, M.E.; Suhaimi, H. Potential hair growth of crude extract from Hibiscus rosa-sinensis Linn. Arch. Pharm. Pract. 2020, 11, 13–19. [Google Scholar]
- Putra, I.B.; Jusuf, N.K.; Sumantri, I.B. The Potency of Hibiscus rosa-sinensis Linn. Leaves Ethanol Extract as Hair Growth. Open Access Maced J. Med. Sci. 2020, 8, 89–92. [Google Scholar] [CrossRef]
- Chavan, R.R.; Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S. In vivo and in vitro hair growth-promoting effect of silver and iron nanoparticles synthesized via Blumea eriantha DC plant extract. J. Cosmet. Dermatol. 2021, 20, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Sakib, S.A.; Tareq, A.M.; Islam, A.; Rakib, A.; Islam, M.N.; Uddin, M.A.; Rahman, M.M.; Seidel, V.; Emran, T.B. Anti-Inflammatory, Thrombolytic and Hair-Growth Promoting Activity of the n-Hexane Fraction of the Methanol Extract of Leea indica Leaves. Plants 2021, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Savali, A.S.; Chittapurkar, H.R. Screening of hair growth promoting activity of Punica granatum L. (pomegranate) leaves extracts and its potential to exhibit antidandruff and anti-lice effect. Heliyon 2021, 7, e06903. [Google Scholar] [CrossRef] [PubMed]
- Madhunithya, E.; Venkatesh, G.; Shyamala, G.; Manjari, V.; Ramesh, S.; Karuppaiah, A.; Sankar, V. Development of ethosome comprising combined herbal extracts and its effect on hair growth. Adv. Tradit. Med. 2021, 21, 131–141. [Google Scholar] [CrossRef]
- Nursiyah, N.; Saputri, R.K.; Al-Bari, A. Hair Growth Activity Test of Hair Tonic That Contain Green Tea Leaf Extract, Celery Leaf Extract and Combination of Green Tea Leaf and Celery Leaf Extract. Ad-Dawaa: J. Pharm. Sci. 2021, 4, 87–98. [Google Scholar] [CrossRef]
- Rahmi, I.A.; Mun’im, A.; Jufri, M. Formulation and evaluation of phytosome lotion from Nothopanax scutellarium leaf extract for hair growth. Int. J. Appl Pharm. 2021, 13, 178–185. [Google Scholar] [CrossRef]
- Her, Y.; Lee, T.K.; Sim, H.; Lee, J.C.; Kim, D.W.; Choi, S.Y.; Hong, J.K.; Lee, J.W.; Kim, J.D.; Won, M.H.; et al. Pinus thunbergii bark extract rich in flavonoids promotes hair growth in dorsal skin by regulating inflammatory cytokines and increasing growth factors in mice. Mol. Med. Rep. 2022, 25, 100. [Google Scholar] [CrossRef]
- Bhinge, S.D.; Jadhav, N.R.; Randive, D.S.; Bhutkar, M.A.; Chavan, R.; Kumbhar, B.V. Isolation and identification of hair growth potential fraction from active plant extract of Blumea eriantha DC grown in Western Ghat of India: In silico study. J. Ayurveda Integr. Med. 2022, 13, 100542. [Google Scholar] [CrossRef]
- Jung, H.; Jung, D.M.; Lee, S.S.; Kim, E.M.; Yoon, K.; Kim, K.K. Mangifera Indica leaf extracts promote hair growth via activation of Wnt signaling pathway in human dermal papilla cells. Anim. Cells Syst. 2022, 26, 129–136. [Google Scholar] [CrossRef]
- Gunawan, E.; Mochta Mano, D.F.; Dewi, K.; Pratiwi, R.D. Hair Growth Test in Male Rabbits (Oryctolagus cuniculus) With Variations in The Concentration of Ethanol Extract Terentang (Campnosperma brevipetiolatum Volkens) Stem. Barks. J. Adv. Pharm. Pract. 2022, 4, 30–35. [Google Scholar] [CrossRef]
- Leny, L.; Fitri, K.; Lase, Y.K.; Hafiz, I.; Iskandar, B. Formulation of Hair Tonic from Ethanol Extract of Sea Hibiscus (Hibiscus tileaceus L.) Leaves in Promoting Hair Growth on Guinea Pig (Cavia porcellus). J. Drug Deliv. Ther. 2022, 12, 1–5. [Google Scholar] [CrossRef]
- Shibato, J.; Takenoya, F.; Kimura, A.; Min, C.W.; Yamashita, M.; Gupta, R.; Kim, S.T.; Rakwal, R.; Shioda, S. Examining the Effect of Notocactus ottonis Cold Vacuum Isolated Plant Cell Extract on Hair Growth in C57BL/6 Mice Using a Combination of Physiological and OMICS Analyses. Molecules 2023, 28, 1565. [Google Scholar] [CrossRef] [PubMed]
- Dangi, I.; Sahu, M.; Verma, L.; Banweer, J. Hair growth stimulating effect and phytochemical evaluation hydoalcoholic extract of Carica papaya leaves. World J. Pharm. Res. 2023, 12, 169–776. [Google Scholar] [CrossRef]
- Tendri Adjeng, A.N.; Puspita Sarry, E.; Muhammad Ali, N.F.; Suryani, S. Hair Growth-Promoting Activity of Hair Tonic containing Delipidated Ethanol Extract of Capsicum frutescens L. Leaves on Male Rabbit (Oryctolagus cuniculus). Res. J. Pharm. Technol. 2023, 16, 3305–3310. [Google Scholar] [CrossRef]
- Lailiyah, M. Hair Growth Cream Formulation from Shoe Flower Leaf Ethanol Extract (Hibiscus rosa-sinensis L.) As a Hair Grower in Rabbit (Oryctolagus cuniculus). J. Eduhealth 2023, 14, 720–728. [Google Scholar]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, S.Y.; Yoo, M.; Park, W.S.; Lee, S.J.; Boo, Y.C.; Koh, J.S. Effects of a new mild shampoo for preventing hair loss in Asian by a simple hand-held phototrichogram technique. Int. J. Cosmet. Sci. 2011, 33, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, M.; McCoy, J.; Goren, A.; Situm, M.; Stanimirovic, A.; Liu, W.; Tan, Y.; Vano-Galvan, S.; Shapiro, J.; Sinclair, R. Novel shampoo reduces hair shedding by contracting the arrector pili muscle via the trace amine-associated receptor. J. Cosmet. Dermatol. 2019, 18, 2037–2039. [Google Scholar] [CrossRef]
- Vicente, R.A.; Leite e Silva, V.R.; Baby, A.R.; Velasco, M.V.; Bedin, V. Double-blind, randomized, placebo-controlled trial of a cream containing the Stryphnodendron adstringens (Martius) Coville bark extract for suppressing terminal hair growth. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 410–414. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, J.B.; Moon, W.S.; Moon, J.N.; Son, S.W.; Kim, M.R. Safety and Efficacy of Rice Bran Supercritical CO2 Extract for Hair Growth in Androgenic Alopecia: A 16-Week Double-Blind Randomized Controlled Trial. Biol. Pharm. Bull. 2015, 38, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Srivilai, J.; Nontakhot, K.; Nutuan, T.; Waranuch, N.; Khorana, N.; Wisuthiprot, W.; Scholfield, C.N.; Champachaisri, K.; Ingkaninan, K. Sesquiterpene-Enriched Extract of Curcuma aeruginosa Roxb. Retards Axillary Hair Growth: A Randomised, Placebo-Controlled, Double-Blind Study. Skin Pharmacol. Physiol. 2018, 31, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, E.; Dundar, C.; Turkoglu, M. A proprietary herbal extract against hair loss in androgenetic alopecia and telogen effluvium: A placebo-controlled, single-blind, clinical-instrumental study. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.C.; Nam, G.W.; Jeong, N.H.; Choi, B.Y. Hair Growth Promotion by Extracts of Inula Helenium and Caesalpinia Sappan Bark in Patients with Androgenetic Alopecia: A Pre-clinical Study Using Phototrichogram Analysis. Cosmetics 2019, 6, 66. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, W.Y.; Trinh, T.A.; Pyo, J.S.; Lee, S.; Kim, C.E.; Lee, D.H.; Park, E.S.; Kang, K.S. Hair Growth Effect of Emulsion Extracted Brevilin A, a JAK3 Inhibitor, from Centipeda minima. Processes 2020, 8, 767. [Google Scholar] [CrossRef]
- Ham, S.; Lee, Y.I.; Kim, I.A.; Suk, J.; Jung, I.; Jeong, J.M.; Lee, J.H. Efficacy and safety of persimmon leaf formulated with green tea and sophora fruit extracts (BLH308) on hair growth: A randomized, double-blind, placebo-controlled clinical trial. Skin Res. Technol. 2023, 29, e13448. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Oxidative stress and its impact on skin, scalp and hair. Int. J. Cosmet. Sci. 2021, 43 (Suppl. 1), S9–S13. [Google Scholar] [CrossRef]
- Trüeb, R.M. The impact of oxidative stress on hair. Int. J. Cosmetic Sci. 2015, 37, 25–30. [Google Scholar] [CrossRef]
- Trüeb, R.M.; Henry, J.P.; Davis, M.G.; Schwartz, J.R. Scalp Condition Impacts Hair Growth and Retention via Oxidative Stress. Int. J. Trichol. 2018, 10, 262–270. [Google Scholar] [CrossRef]
- Zhai, X.; Gong, M.; Peng, Y.; Yang, D. Effects of UV Induced-Photoaging on the Hair Follicle Cycle of C57BL6/J Mice. Clin. Cosmet. Investig. Dermatol. 2021, 14, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.S.; Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Particulate Matters Induce Apoptosis in Human Hair Follicular Keratinocytes. Ann. Dermatol. 2020, 32, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Choi, J.Y.; Lee, J.B.; Yun, S.J.; Moon, B.K.; Ahn, Y.G.; Lee, S.Y.; Lee, S.C. Protective Activity against Oxidative Stress in Dermal Papillae with Extracted Herbal Essential Oils. Appl. Sci. 2023, 13, 3985. [Google Scholar] [CrossRef]
- Fernández, E.; Martínez-Teipel, B.; Armengol, R.; Barba, C.; Coderch, L. Efficacy of antioxidants in human hair. J. Photoch. Photobiol. B. 2012, 117, 146–156. [Google Scholar] [CrossRef]
- Muangsanguan, A.; Linsaenkart, P.; Chaitep, T.; Sangta, J.; Sommano, S.R.; Sringarm, K.; Arjin, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; et al. Hair Growth Promotion and Anti-Hair Loss Effects of By-Products Arabica Coffee Pulp Extracts Using Supercritical Fluid Extraction. Foods 2023, 12, 4116. [Google Scholar] [CrossRef] [PubMed]
- Erekat, N.S. ProgramMed. Cell Death in Diabetic Nephropathy: A Review of Apoptosis, Autophagy, and Necroptosis. Med. Sci. Monitor. 2022, 28, e937766. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.W.; Seo, J.; Jeong, M.; Lee, S.; Song, J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012, 45, 496–508. [Google Scholar] [CrossRef]
- Han, Y.H.; Wang, Y.; Lee, S.J.; Jin, M.H.; Sun, H.N.; Kwon, T. Regulation of anoikis by extrinsic death receptor pathways. Cell Commun. Signal. 2023, 21, 227. [Google Scholar] [CrossRef]
- Heilmann-Heimbach, S.; Hochfeld, L.M.; Henne, S.K.; Nothen, M.M. Hormonal regulation in male androgenetic alopecia-Sex hormones and beyond: Evidence from recent genetic studies. Exp. Dermatol. 2020, 29, 814–827. [Google Scholar] [CrossRef]
- Olsen, E.A.; Hordinsky, M.; Whiting, D.; Stough, D.; Hobbs, S.; Ellis, M.L.; Wilson, T.; Rittmaster, R.S.; Dutasteride Alopecia Research, T. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. J. Am. Acad. Dermatol. 2006, 55, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, Z.; Liu, P.; Zhang, Z.; Tong, T. Wild-type p16INK4a suppresses cell growth, telomerase activity and DNA repair in human breast cancer MCF-7 cells. Int. J. Oncol. 2004, 24, 1597–1605. [Google Scholar] [PubMed]
- Trüeb, R.M. Further Clinical Evidence for the Effect of IGF-1 on Hair Growth and Alopecia. Skin Appendage Disor. 2018, 4, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Man, X.Y.; Li, C.M.; Chen, J.Q.; Zhou, J.; Cai, S.Q.; Lu, Z.F.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, M.; Xu, L.; Chang, Z.; Shu, X.; Zhou, L. Therapeutic role of human hepatocyte growth factor (HGF) in treating hair loss. PeerJ 2016, 4, e2624. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.D.; Bazzi, H.; Fantauzzo, K.A.; Waters, J.M.; Crawford, H.; Hynd, P.; Christiano, A.M.; Jahoda, C.A. KGF and EGF signalling block hair follicle induction and promote interfollicular epidermal fate in developing mouse skin. Development 2009, 136, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Gu, L.; Maeda, K. Evaluation of the effect of plant mixture ethanol extracts containing Biota orientalis L. extract on suppression of sebum in cultured sebocytes and on stimulation of growth of keratinocytes co-cultured with hair papilla cells. Cosmetics 2017, 4, 29. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, M.J.; Lee, W.Y.; Pyo, J.; Shin, M.S.; Hwang, G.S.; Shin, D.; Kim, C.E.; Park, E.S.; Kang, K.S. Hair Growth Stimulation Effect of Centipeda minima Extract: Identification of Active Compounds and Anagen-Activating Signaling Pathways. Biomolecules 2021, 11, 976. [Google Scholar] [CrossRef]
- Yudushkin, I. Control of Akt activity and substrate phosphorylation in cells. IUBMB Life 2020, 72, 1115–1125. [Google Scholar] [CrossRef]
- Shimura, T.; Kakuda, S.; Ochiai, Y.; Nakagawa, H.; Kuwahara, Y.; Takai, Y.; Kobayashi, J.; Komatsu, K.; Fukumoto, M. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3β-mediated cyClin. D1 overexpression. Oncogene 2010, 29, 4826–4837. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, H.Y.; Chen, J.Z.; Wang, J.; Liu, J.Y.; Jiang, Y.F. Targeting Akt in cancer for precision therapy. J. Hematol. Oncol. 2021, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, R.; Nikolic-Paterson, D.J. Mitogen-Activated Protein Kinases: Functions in Signal Transduction and Human Diseases. Int. J. Mol. Sci. 2019, 20, 4844. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Xiao, Q.; Xiao, J.N.; Niu, C.X.; Li, Y.Y.; Zhang, X.J.; Zhou, Z.W.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef]
- Lyros, O.; Rafiee, P.; Nie, L.H.; Medda, R.; Jovanovic, N.; Schmidt, J.; Mackinnon, A.; Venu, N.; Shaker, R. Dickkopf-1, the Wnt antagonist, is induced by acidic pH and mediates epithelial cellular senescence in human reflux esophagitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 306, G557–G574. [Google Scholar] [CrossRef][Green Version]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Papukashvili, D.; Rcheulishvili, N.; Liu, C.; Xie, F.F.; Tyagi, D.; He, Y.J.; Wang, P.G. Perspectives on miRNAs Targeting DKK1 for Developing Hair Regeneration Therapy. Cells 2021, 10, 2957. [Google Scholar] [CrossRef]
- Jing, J.J.; Wu, Z.X.; Wang, J.H.; Luo, G.W.; Lin, H.Y.; Fan, Y.; Zhou, C.C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct. Target Ther. 2023, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.S.; Filip, S.; Mokry, J. Signaling Involved in Hair Follicle Morphogenesis and Development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; Spohr, T.C.L.D.E. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Sabol, M.; Trnski, D.; Musani, V.; Ozretic, P.; Levanat, S. Role of GLI Transcription Factors in Pathogenesis and Their Potential as New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 2562. [Google Scholar] [CrossRef] [PubMed]
- Aashaq, S.; Batool, A.; Mir, S.A.; Beigh, M.A.; Andrabi, K.I.; Shah, Z.A. TGF-beta signaling: A recap of SMAD-independent and SMAD-dependent pathways. J. Cell Physiol. 2022, 237, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-beta signal transduction: Biology, function and therapy for diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.R.; Wu, S.L.; Chen, W.; Li, Y.P. The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Yoo, H.G.; Inui, S.; Itami, S.; Kim, I.G.; Cho, A.R.; Lee, D.H.; Park, W.S.; Kwon, O.; Cho, K.H.; et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013, 46, 460–464. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Y.; Xing, Y.; Xu, W.; Guo, H.; Deng, F.; Ma, X.; Li, Y. The balance of Bmp6 and Wnt10b regulates the telogen-anagen transition of hair follicles. Cell Commun. Signal. 2019, 17, 16. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, H.K.; Lee, D.H.; Le, T.N.; Park, H.J.; Kim, M.I. Poly(gamma-Glutamic Acid)/Chitosan Hydrogel Nanoparticles For Effective Preservation And Delivery Of Fermented Herbal Extract For Enlarging Hair Bulb And Enhancing Hair Growth. Int. J. Nanomed. 2019, 14, 8409–8419. [Google Scholar] [CrossRef] [PubMed]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef] [PubMed]
- Horesh, E.J.; Cheret, J.; Paus, R. Growth Hormone and the Human Hair Follicle. Int. J. Mol. Sci. 2021, 22, 13205. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.D.B.; Peters, E.M.J.; Amer, Y.; Atuluru, P.; Cheret, J.; Rosenberg, A.M.; Picard, M.; Paus, R. The impact of perceived stress on the hair follicle: Towards solving a psychoneuroendocrine and neuroimmunological puzzle. Front. Neuroendocrinol. 2022, 66, 101008. [Google Scholar] [CrossRef] [PubMed]
| Plant Extracts | Cell Types | Assays | Effective Concentrations * | Literature |
|---|---|---|---|---|
| Ethanol (EtOH) extract of roots of Asiasarum heterotropoides (or Asiasarum sieboldi) | Human follicle dermal papilla cells (HFDPCs) | [3 H]-thymidine incorporation | 0.1 μg mL−1 | Rho et al., 2005 [38] |
| 70% EtOH extract of Erica multiflora | HFDPCs | 3-(4,5-Dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) reduction | 500 and 5000 μg mL−1 | Kawano et al., 2009 [39] |
| Water extract of tubers of Aconiti Ciliare | Human immortalized dermal papilla cells (iDPCs) | 2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) reduction | 5, 10, and 20 μg mL−1 | Park et al., 2012 [40] |
| 50% EtOH extract of florets of Carthamus tinctorius | HFDPCs | MTT reduction | 5–1250 μg mL−1 | Junlatat and Sripanidkulchai, 2014 [41] |
| 50% methanol (MeOH) extract of Platycarya strobilacea | HFDPCs | CCK-8 assay using 2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8) reduction | 9.8, 19.5, 39.1, and 156.3 μg mL−1 | Kim et al., 2014 [42] |
| Extract of red ginseng (Panax ginseng) | HFDPCs | CCK-8 assay | 300 μg mL−1 | Park et al., 2015 [43] |
| 95% EtOH extract of roots of Rumex japonicus | HFDPCs | MTT reduction | 5, 10, 50, and 100 μg mL−1 | Lee et al., 2016 [44] |
| DA-5512 formula (EtOH extract of herbal mixture: Thea sinensis, Emblica officinalis, Pinus densiflora, Pueraria thunbergiana, Tribulus terrestris, and Zingiber officinale) | HFDPCs | Ki-67 staining | 100 μg mL−1 | Yu et al., 2017 [45] |
| MeOH extract of Geranium sibiricum | HFDPCs | CCK-8 (WST-8) reduction | 19.5 μg mL−1 | Boisvert et al., 2017 [46] |
| Extract of Orthosiphon stamineus | HFDPCs | PrestoBlue assay using resazurin reduction | 25, 50, 125, and 250 μg mL−1 | Somsukskul et al., 2017 [47] |
| Water extract of Cinnamomum osmophloeum | HFDPCs | MTT reduction | 5000 μg mL−1 | Wen et al., 2018 [48] |
| 50% EtOH extract Houttuynia cordata | HFDPCs | Bromodeoxyuridine (BrdU) incorporation | 20 and 50 μg mL−1 | Kim et al., 2019 [49] |
| RE-ORGA (hot water extract of herbal mixture: Panax ginseng, Glycine max, Houttuynia cordata, Lycium chinense, Glycyrrhiza uralensis, Citrus unshiu, Zizyphus jujuba, Perilla frutescens, Camellia sinensis, and Cynanchum wilfordii) | HFDPCs | CCK-8 assay | 10,000, 50,000, and 100,000 μg mL−1 | Kang et al., 2019 [50] |
| 50% EtOH extract of Polygonum multiflorum | HFDPCs | CCK-8 assay | 10 and 100 μg mL−1 | Shin et al., 2020 [51] |
| MeOH extract of Salvia plebeia | HFDPCs | CCK-8 assay | 15.6, 31.3, and 62.5 μg mL−1 | Jin et al., 2020 [52] |
| 50% EtOH extract of Plumbago zeylanica | HFDPCs | Cell counting | 0.2 μg mL−1 | Yamada et al., 2020 [53] |
| Phyllotex™ (a herbal formula: Euterpe oleracea, Olea europea, Tabebuia impetiginosa, and Coffea Arabica) | HFDPCs | MTT reduction | 60–2000 μg mL−1 | Serruya and Maor, 2021 [54] |
| 50% MeOH extract of lotus (Nelumbo nucifera) seeds | HFDPCs | CCK-8 assay | 31.25, 62.5, 125, and 250 μg mL−1 | Park et al., 2021 [55] |
| 80% MeOH extract of centipedegrass (Eremochloa ophiuroides) | HFDPCs | MTT reduction | 6.2, 12.5, 25, and 50 μg mL−1 | Ramadhani et al., 2022 [56] |
| MeOH extract of shallot (Allium ascalonicum) | HFDPCs | Sulforhodamine B (SRB) assay | 100 μg mL−1 | Ruksiriwanich et al., 2022 [57] |
| 60% EtOH extract of Camellia japonica seed cakes | HFDPCs | MTT reduction | 20 μg mL−1 | Wang et al., 2022 [58] |
| Hot water extract of Lycopus lucidus | HFDPCs | CCK-8 assay | 50 μg mL−1 | Lee et al., 2022 [59] |
| Hot water extract of mangosteen (Garcinia mangostana) pericarps | HFDPCs | WST-1 reduction | 62.5, 125, 250, and 500 μg mL−1 | Tan et al., 2022 [60] |
| 70% EtOH extract of fruit shells of Camellia japonica | HFDPCs | Ki-67 staining | 10 and 50 μg mL−1 | You et al., 2023 [61] |
| Water extract of banana (Musa paradisiaca) flowers | HFDPCs | MTT reduction | 62.5 and 125 μg mL−1 | Liang et al., 2023 [62] |
| 20% EtOH extract of Panax ginseng | iDPCs and immortalized human outer root sheath cells (ORSCs) | AlamarBlue assay using resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide) reduction | 50 and 100 μg mL−1 | Iwabuchi et al., 2024 [63] |
| Extract of leaves of Gynostemma pentaphyllum | HFDPCs | CCK-8 assay | 50, 100, 200, and 400 μg mL−1 | Liu et al., 2024 [64] |
| 70% EtOH extract of flowers of Silybum marianum | HFDPCs | MTT reduction | 50 and 100 μg mL−1 | You et al., 2024 [65] |
| Plant Extracts | Cell Types | Androgens | Assays | Effective Concentrations * | Literature |
|---|---|---|---|---|---|
| Extract of Brassica oleracea | HFDPCs | 50 μg mL−1 testosterone | MTT reduction | 30 and 100 μg mL−1 | Luo and Zhang, 2022 [66] |
| 60% EtOH extract of seed cakes of Camellia japonica | HFDPCs | 10 μg mL−1 dihydrotestosterone (DHT) | MTT reduction | 10 and 20 μg mL−1 | Ma et al., 2022 [67] |
| 50% EtOH extract of fruits of Terminalia bellirica | HFDPCs | 100 μM testosterone | 3-(4,5-Dimethyl thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2- (4-sulpho phenyl)-2H-tetrazolium inner salt (MTS) reduction | 6.25, 12.5, and 25 μg mL−1 | Woo et al., 2023 [68] |
| Plant Extracts | Hair Follicles | Hair Growth | Hair Cycle | Cell Proliferation | Literature |
|---|---|---|---|---|---|
| Extract of red ginseng (Panax ginseng) | Human hair follicles | The extract (100 mg mL−1) recovered the number of Ki-67-positive hair matrix keratinocytes reduced by DHT. | Park et al., 2015 [43] | ||
| Water extract from oriental melon (Cucumis melo) leaves | Human hair follicles | The extract (100 μg mL−1) enhanced the elongation of hair (entire hair length). | The extract (100 μg mL−1) extended the anagen-phase duration. | The extract (100 μg mL−1) increased Ki-67-positive hair bulb keratinocytes. | Pi et al., 2016 [70] |
| Extract of Orthosiphon stamineus | Human hair follicles | The extract (500 μg mL−1) enhanced the elongation of hair. | The extract (500 μg mL−1) extended the anagen-phase duration. | Somsukskul et al., 2017 [47] | |
| n-Butanol (BuOH) fraction of Perilla frutescens extract | C57BL/6 mice vibrissa hair follicles | The BuOH fraction (2.5 μg mL−1) enhanced hair shaft growth. | Li et al., 2018 [71] | ||
| 50% aqueous EtOH extract of Houttuynia cordata | Human hair follicles | The extract (20 μg mL−1) extended the anagen-phase duration. | Kim et al., 2019 [49] | ||
| Extract of Polygonum multiflorum | Human hair follicles | The extract (20 or 50 μg mL−1) extended the anagen-phase duration. | Shin et al., 2020 [51] | ||
| Extract of Brassica oleracea | Male C57BL/6 mice hair follicles (whisker pads) | The extract (10 μg mL−1) recovered the elongation of the hair shaft suppressed by testosterone. | Luo and Zhang, 2022 [66] | ||
| Extract of watercress (Nasturtium officinale) | Human hair follicles | The extract (10 mg mL−1) enhanced the elongation of hair. | Hashimoto et al., 2022 [72] | ||
| Extract of Panax ginseng | Human hair follicles | The extract (100 μg mL−1) enhanced the elongation of the hair shaft. | Iwabuchi et al., 2024 [63] |
| Plant Extracts | Animal Models | Vehicle or Formula | Treatments | Hair Growth | Hair Cycle | Literature |
|---|---|---|---|---|---|---|
| Acetone extract of Boehmeria nipononivea | 5-week-old male C57BL/6 mice; dorsal hair shaving and applying a depilatory agent | EtOH | Topical application; 20 days | Vehicle control < 2% extract | Shimizu et al., 2000 [75] | |
| MeOH extract of dried roots of Sophora flavescens | 7-week-old female C57BL/6 mice; dorsal hair shaving | 50% EtOH | Topical application; 30 days | Vehicle control < 1% extract | ↑(telogen to anagen) | Roh et al., 2002 [76] |
| EtOH extract of roots of Asiasarum heterotropoides (or Asiasarum sieboldi) | 7-week-old female C57BL/6 mice; dorsal hair shaving | 40% EtOH | Topical application; 30 days | Vehicle control < 1% extract | Rho et al., 2005 [38] | |
| 7-week-old female C3H mice; dorsal hair shaving | 40% EtOH | Topical application; 45 days | Vehicle control < 1% extract | |||
| 70% EtOH extract of Erica multiflora | 7-week-old male C3H/He mice; dorsal hair shaving | Phosphate-buffered saline (PBS) | Subcutaneous injection; 3 weeks | Vehicle control ≤ 0.05% extract | ↑(telogen to anagen) | Kawano et al., 2009 [39] |
| MeOH extract of Eclipta alba | 62-day-old C57BL/6 mice; dorsal hair shaving | 50% propylene glycol (PG), 30% EtOH, and 20% water | Topical application; 10 days | Number of hair follicles; vehicle control < 1.6 mg extract < 3.2 mg extract | ↑(telogen to anagen) | Datta et al., 2009 [77] |
| Extract of tobacco (Nicotiana tabacum) leaves microbially biotransformed in cow urine | Male albino Wister rats; dorsal hair shaving and applying a hair remover | Lotion | Topical application; 30 days | Vehicle control ≤ 10% extract ≤ 20% extract ≤ 30% extract ≅ 2% minoxidil | Murkute et al., 2010 [78] | |
| Hot water extract of Polygonum multiflorum fermented with Lactobacillus sp. | 6-week-old C57BL6/N mice; dorsal hair shaving | Water containing Lactobacillus sp. | Topical application; 4 weeks | Vehicle control < 4.7 mg extract | ↑(telogen to anagen) | Park et al., 2011 [79] |
| EtOH and aqueous extracts of Eucheuma cottonii | 10–12-week-old male Sprague–Dawley rats; dorsal hair shaving | Water | Oral administration; 15 days | Vehicle control < aqueous extract < honey < EtOH extract (100 mg kg−1) | Fard et al., 2011 [73] | |
| Extract of Aconiti Ciliare tubers | 7-week-old male C57BL/6 mice; dorsal hair shaving | 50% EtOH, 30% water, and 20% PG | Topical application; 35 days | Vehicle control < 2% minoxidil < 1% extract | ↑(telogen to anagen) | Park et al., 2012 [40] |
| Extract of Glycyrrhiza Glabra | Female Wistar albino rats; dorsal hair shaving and applying a depilatory cream | Paraffin oil | Topical application; 30 days | Vehicle control < 2% minoxidil < 2% extract | ↑(telogen to anagen) | Upadhyay et al., 2012 [80] |
| Water extract of Trichosanthes cucumerina leaves | Wistar albino rats; dorsal hair shaving and applying a depilatory cream | Water | Topical application; 30 days | Vehicle control < 0.03% extract ≤ 2% minoxidil | Sandhya et al., 2012 [81] | |
| Extract of Chinese black tea (Camellia sinensis or Camellia taliensis) fermented with Aspergillus sp. | 6-week-old male C3H/He mice; dorsal hair shaving | 50% PG, 30% EtOH, and 20% water | Topical application; 2 weeks | Vehicle control ≅ 0.05% capsaicin < 3.5% extract < 0.05% capsaicin plus 3.5% extract | Hou et al., 2013 [82] | |
| Hot water extract of Thuja orientalis | 6-week-old male C57BL/6N mice; dorsal hair shaving | 48.25% PG, 1.75% dimethyl sulfoxide (DMSO), and 50% water | Topical application; 21 days | Vehicle control < 1% minoxidil ≤ 30% extract | ↑(telogen to anagen) | Zhang et al., 2013 [83] |
| Extract from leaves of Rosmarinus officinalis | 7-week-old male C57BL/6NCrSlc mice; dorsal hair shaving and topical application of testosterone | 80% EtOH | Topical application; 30 days | Testosterone model < model with 2% extract ≤ control without testosterone | Murata et al., 2013 [84] | |
| 7-week-old male C3H/He mice; dorsal hair shaving | 80% EtOH | Topical application; 30 days | Vehicle control < 2% extract ≤ 1% minoxidil | |||
| Extract of tomato (Lycopersicon esculentum) | 6-week-old C57BL/6 mice; dorsal hair shaving | 10% EtOH | Topical application; 4 weeks | Vehicle control ≅ 3% ethyl acetate extract) < 3% supercritical CO2 extract < 3% lycopene-enriched extract ≤ 3% minoxidil | ↑(telogen to anagen) | Choi et al., 2013 [85] |
| Supercritical CO2 extract from rice (Oryza sativa) brans | 6-week-old female C57BL/6 mice; dorsal hair shaving | 10% EtOH | Topical application; 4 weeks | Vehicle control < 3% minoxidil ≅ 3% extract | ↑(telogen to anagen) | Choi et al., 2014 [86] |
| EtOH extract from florets of Carthamus tinctorius | 6-week-old female C57BL/6 mice; dorsal hair shaving | 50% PG, 20% EtOH, and 30% water | Topical application; 15 days | Non-treated ≅ vehicle control ≤ 0.05 mg mL−1 extract ≤ 0.1 mg mL−1 minoxidil ≅ 0.1 mg mL−1 extract < 0.5 mg mL−1 extract | ↑(telogen to anagen) | Junlatat and Sripanidkulchai, 2014 [41] |
| 70% EtOH extract of Chrysanthemum zawadskii | 8-week-old female C57BL/6 mice; dorsal hair shaving and applying a depilatory cream | 70% EtOH | Topical application; 30 days | Vehicle control < 1.6 g kg−1 BuOH fraction < 0.6 g kg −1 water fraction | ↑(telogen to anagen) | Li et al., 2014 [87] |
| Extract of Platycarya strobilacea | 6-week-old male C57BL/6 mice; dorsal hair shaving | DMSO | Topical application; 3 weeks | Vehicle control ≅ 0.1 % extract ≅ 5% minoxidil | ↑(telogen to anagen) | Kim et al., 2014 [42] |
| Extract of Hibiscus syriacus leaves | 21-day-old albino rats; dorsal hair shaving and applying a depilatory cream | Liquid paraffin | Topical application; 30 days | Vehicle control < 10% extract | ↑(telogen to anagen) | Punasiya et al., 2014 [88] |
| EtOH extract of Stachytarpheta jamaicensis leaves | Male Sprague–Dawley rats; dorsal hair shaving | Solution | Topical application; 30 days | Vehicle control < 2% extract ≤ 2% minoxidil | Rozianoor et al., 2014 [89] | |
| Extract of red ginseng (Panax ginseng) | 7-week-old C57BL/6 mice; dorsal hair shaving | Normal saline | Subcutaneous injection; 7 weeks | Vehicle control < 3% extract ≅ 0.5% minoxidil (topical) | ↑(telogen to anagen) | Park et al., 2015 [43] |
| MeOH extracts of Chrysanthemum zawadskii (CZ) and Polygonum multiflorum (PM) | 7-week-old male athymic BALB/c nude mice | 67% PG, 30% EtOH, and 3% DMSO | Topical application; 40 days | Vehicle control ≅ 10 mg PM extract per mouse < 10 mg CZ extract per mouse ≅ 2% minoxidil | ↑(telogen to anagen) | Begum et al., 2015 [90] |
| Hot water extract of herbal mixture: Acorus calamus, Morus alba, Glycyrrhiza uralensis, Pinus densiflora, Sophora angustifolia, Ligusticum chuanxiong, and Angelica gigas | 7-week-old male C57BL/6 mice; dorsal hair shaving and applying a depilatory cream | Ointment base | Topical application; 18 days | Vehicle control < 5% minoxidil ≤ extract-containing ointment | ↑(telogen to anagen) | Park et al., 2015 [91] |
| Extract of Rumex japonicus roots | 7-week-old C57BL/6 mice; dorsal hair shaving | 60% MeOH and 40% PBS | Topical application; 25 days | Vehicle control ≤ 0.4% extract ≤ 0.8% extract < 5% minoxidil | ↑(telogen to anagen) | Lee et al., 2016 [44] |
| Water extract of oriental melon (Cucumis melo) leaves | 7-week-old female C57BL/6 mice; dorsal hair shaving | Dulbecco’s phosphate-buffered saline | Topical application; 28 days | Vehicle control < 0.3% extract ≅ 5% minoxidil | Pi et al., 2016 [70] | |
| 90% EtOH extract of Eclipta alba leaves | Wistar albino rats; dorsal hair shaving and applying a depilatory cream | Water | Topical application; 30 days | Vehicle control < 10% extract ≤ 2% minoxidil | Mondal et al., 2016 [92] | |
| DA-5512 formula (EtOH extract of herbal mixture: Thea sinensis, Emblica officinalis, Pinus densiflora, Pueraria thunbergiana, Tribulus terrestris, and Zingiber officinale) | 8-week-old male C57BL/6 mice; dorsal hair shaving and applying a depilatory cream | 30% EtOH | Topical application; 14 days | Vehicle control (30% EtOH) < 1% DA-5512 < 3% minoxidil ≅ 5% DA-5512 | ↑(telogen to anagen) | Yu et al., 2017 [45] |
| MeOH extract of Geranium sibiricum | 6-week-old male C57BL/6 mice; dorsal hair shaving | 1% DMSO | Topical application; 3 weeks | Vehicle control ≅ 0.1% extract ≅ 5% minoxidil | ↑(telogen to anagen) | Boisvert et al., 2017 [46] |
| Extract of Trigonella foenum-graecum leaves | Male albino mice; dorsal hair shaving and applying a depilatory cream | 65% water, 25% EtOH, and 10% butylene glycol | Topical application; 21 days | Vehicle control < 5% minoxidil ≤ 10% extract | Imtiaz et al., 2017 [93] | |
| Water extract of Cinnamomum osmophloeum | 8-week-old male C57BL/6 mice; dorsal hair shaving and applying a calcium thioglycolate solution | Water | Topical spraying; 30 days | Vehicle control < 20% extract ≤ 1% extract ≤ 0.5 mM minoxidil | ↑(telogen to anagen) | Wen et al., 2018 [48] |
| BuOH fraction of Perilla frutescens extract | 8-week-old C57BL/6 mice; dorsal hair removal by applying a depilatory cream | 67% PG, 30% EtOH, and 3% DMSO | Topical application; 25 days | Vehicle control < 2.5% BuOH fraction ≅ 2.5% minoxidil | ↑(telogen to anagen) | Li et al., 2018 [71] |
| 7-week-old male C57BL/6NCrSlc mice; dorsal hair removal and topical application of testosterone or DHT | 70% EtOH | Topical application; 15 days | DHT model < testosterone model < DHT with 2 mg BuOH fraction ≤ testosterone with 2 mg BuOH fraction < control without hormones | ↑(telogen to anagen) | ||
| Extract of Serenoa repens | 6–8-week-old male C57BL/6 mice; dorsal hair shaving and applying a depilatory cream | DMSO | Oral administration; 5 weeks | DHT model < model with 50% extract < model with 0.01% finasteride | Zhu et al., 2018 [94] | |
| Extract of blackcurrant (Ribes nigrum) | 12-week-old ovariectomized female Sprague–Dawley rats | AIN-93M diet | Feeding a diet containing 3% extract; 3 months | Number of hair shafts per follicular unit; ovariectomy control < ovariectomy plus 3% extract ≅ sham control without ovariectomy | Nanashima and Horie, 2019 [95] | |
| 60% EtOH extract of Vernonia anthelmintica seeds | 5–6-week-old male C57BL/6 mice; dorsal hair shaving | 0.5% sodium carboxymethylcellulose | Oral administration; 23 days | Chronic restraint stress model < model with 5% minoxidil ≅ model with extract (80 mg kg−1) | Wang et al., 2019 [96] | |
| 70% EtOH extract of Camellia sinensis (CS) leaves and Hibiscus tilliaceus (HT) leaves | 7–8-week-old male Sprague–Dawley rats; dorsal hair shaving and applying a depilatory cream | Microemulsion | Topical application; 21 days | Vehicle control < 2.5% minoxidil ≤ 7.5% CS extract < 7.5% HT extract | Amin et al., 2019 [97] | |
| EtOH extract of Angelica gigas | 6–7-week-old male C57/BL6 mice; dorsal hair shaving | Water | Topical application; 17 days | Vehicle control < 0.15% decursin ≅ 2% extract | Lee et al., 2020 [98] | |
| MeOH extract of Salvia plebeian | 6-week-old male C57BL/6 mice; dorsal hair shaving | DMSO | Topical application; 21 days | Vehicle control < 0.1% extract ≅ 3% minoxidil | ↑(telogen to anagen) | Jin et al., 2020 [52] |
| 70% EtOH extract of Platycladus orientalis leaves | 6-week-old male C57BL/6 mice; dorsal hair shaving | Water | Topical application; 17 days | Vehicle control < 3% extract plus 1% α-terpineol | ↑(telogen to anagen) | Ahn et al., 2020 [99] |
| The extract of Hibiscus rosa-sinensis | Sprague–Dawley rats; dorsal hair shaving and applying a depilatory cream | Liquid paraffin | Topical application; 42 days | Vehicle control < 1% extract | Rose et al., 2020 [100] | |
| 96% EtOH extract of Hibiscus rosa-sinensis leaves | Wistar albino rats; dorsal hair shaving | Liquid paraffin | Topical application; 25 days | Vehicle control < 2.5% extract < 5% extract < 10% extract | Putra et al., 2020 [101] | |
| EtOH extract of Blumea eriantha | Male and female Swiss albino mice; dorsal hair shaving | Ag or Fe nanoparticles in 95% EtOH | Topical application; 30 days | Vehicle control < 2% or 5% Fe nanoparticles ≤ 2% or 5% Ag nanoparticles ≤ 2% minoxidil | ↑(telogen to anagen) | Chavan et al., 2021 [102] |
| n-Hexane fraction of the MeOH extract of Leea indica leaves | Male and female Swiss albino mice; dorsal hair shaving and applying a surgical hair removal cream | 1% Tween 80 in water | Topical application; 21 days | Vehicle control ≤ 5% minoxidil (100 μL) ≤ 1% extract (10 μL) | Sakib et al., 2021 [103] | |
| EtOH and water extracts of Punica granatum | Male and female Swiss Albino mice; dorsal hair shaving | 95% EtOH | Topical application; 30 days | Vehicle control < 2% minoxidil ≤ 3% extract | ↑(telogen to anagen) | Bhinge et al., 2021 [104] |
| Extract of Phyllanthus niruri leaves, Zingiber officinale rhizomes, and Croton tiglium seeds | 6–8-month-old male Wistar rats; dorsal hair shaving | 80% EtOH, 10% PG, and 10% water | Topical application; 21 days | Vehicle control < 2% finasteride < 2% extract | ↑(telogen to anagen) | Madhunithya et al., 2021 [105] |
| 50% MeOH extract of lotus (Nelumbo nucifera) seeds | 4-week-old male C57BL/6 mice; dorsal hair shaving | DMSO | Oral administration; 3 weeks | Vehicle control < 3% minoxidil < 0.1% extract | ↑(telogen to anagen) | Park et al., 2021 [55] |
| 96% EtOH extract of green tea (Camellia sinensis) leaves and celery (Apium gravelens) leaves | Guinea pigs; dorsal hair shaving | Tonic | Topical application; 28 days | Vehicle control < hair tonic containing 2.5% green tea extract and 7.5% celery extract | Nursiyah et al., 2021 [106] | |
| Extract of mangkokan (Nothopanax scutellarium) leaves | 4–5-month-old male New Zealand rabbits; dorsal hair shaving | Lotion | Topical application; 4 weeks | Vehicle control < 2% minoxidil < 10% extract | Rahmi et al., 2021 [107] | |
| Extract of Pinus thunbergii barks | 7-week-old male C57BL/6 mice; dorsal hair shaving | Water | Topical application; 17 days | Vehicle control < 1% minoxidil (100 μL) < 2% extract ≅ 4% extract | Her et al., 2022 [108] | |
| Extract of centipedegrass (Eremochloa ophiuroides) | 6-week-old female C57BL/6 mice; dorsal hair shaving | 50% glycerol, 25% EtOH, and 25% water | Topical application; 14 days | Vehicle control < 1% extract < 5% minoxidil | ↑(telogen to anagen) | Ramadhani et al., 2022 [56] |
| EtOH extract of Blumea eriantha | Male and female albino mice; dorsal hair shaving | 95% EtOH | Topical application; 30 days | Control (normal saline) < 1% extract ≤ 1% minoxidil ≤ 3% extract | ↑(telogen to anagen) | Bhinge et al., 2022 [109] |
| 60% EtOH extract of camellia (Camellia japonica) seed cakes | 7-week-old male C57BL/6J mice; dorsal shaving and applying 6% Na2S solution | Water | Topical application; 21 days | Vehicle control < 10% extract < 5% minoxidil | Wang et al., 2022 [58] | |
| Hot water extract of Lycopus lucidus | Female 7-week-old male C57BL/6 mice; dorsal hair shaving | Diet | Oral feeding; 5 weeks | Control diet < diet supplemented with 0.01% extract | Lee et al., 2022 [59] | |
| Hot water extract of mango (Mangifera Indica) leaves | 8-week-old male C57BL/6J mice; dorsal hair shaving and applying a depilatory cream | 82.5% water, 12.5% EtOH, and 0.05% jojoba oil | Topical application; 11 days | Vehicle control < 1% extract ≤ 0.3% minoxidil | Jung et al., 2022 [110] | |
| 96% EtOH extract of terentang (Campnosperma brevipetiolatum) stem barks | Male rabbits; dorsal hair shaving | Water | Topical application; 21 days | Vehicle control < 0.5% extract < 1% extract < 5% extract ≤ 5% minoxidil | Gunawan et al., 2022 [111] | |
| EtOH extract of sea hibiscus (Hibiscus tileaceus) leaves | Male guinea pigs; dorsal hair shaving | Tonic | Topical application; 3 weeks | Vehicle control < 30% extract ≤ 2% minoxidil | Leny et al., 2022 [112] | |
| Cold vacuum extract of Notocactus ottonis | 8-week-old male C57BL/6 mice; dorsal hair shaving | 50% PG, 30% EtOH, and 20% water | Topical application; 27 days | Vehicle control < 10% extract ≤ 5% minoxidil | Shibato et al., 2023 [113] | |
| EtOH extract of Terminalia bellirica fruits | 7-week-old male C57BL/6 mice; dorsal hair shaving, applying a depilatory cream, and subcutaneous injection of testosterone | Water | Oral administration; 14 days | Testosterone model ≅ model with 2 mg kg−1 finasteride < model with 20 mg kg−1 extract ≅ model with 100 mg kg−1 extract < control without testosterone | Woo et al., 2023 [68] | |
| 50% EtOH extract of Cudrania tricuspidata and Sargassum fusiforme | 7-week-old female C57BL/6 mice; dorsal hair shaving and applying a depilatory cream | Water | Oral administration; 21 days | Vehicle control < 50 mg kg−1 extract < 60 μg kg−1 minoxidil | Rajan et al., 2023 [74] | |
| Topical application; 21 days | Vehicle control < 250 mg kg−1 minoxidil < 50 mg kg−1 extract | |||||
| 75% EtOH extract of Carica papaya leaves | Sprague–Dawley rats | Ointment base | Topical application; 30 days | Vehicle control < 2% minoxidil < 5% extract | Dangi et al., 2023 [114] | |
| 96% EtOH extract of Capsicum frutescens leaves | Male rabbits; dorsal hair shaving and applying a depilatory cream | Tonic | Topical application; 21 days | Vehicle control < 20% extract ≤ 2% minoxidil | Tendri Adjeng et al., 2023 [115] | |
| 70% EtOH extract of Hibiscus rosa-sinensis leaves | White rabbits; dorsal hair shaving | Cream | Topical application; 21 days | Vehicle control < 20% extract ≤ a minoxidil product | Lailiyah, 2023 [116] | |
| Extract of Gynostemma pentaphyllum leaves | 4-week-old male C57BL/6 mice; dorsal hair shaving | Water | Topical application; 28 days | Vehicle control ≅ 0.5% extract ≤ 2% minoxidil < 1% extract ≅ 2% extract | ↑(telogen to anagen) | Liu et al., 2024 [64] |
| Study Format and Subjects | Plant Extracts | Formulas | Treatments | Outcomes | Literature |
|---|---|---|---|---|---|
| Double-blind, randomized, placebo-controlled trial on 44 subjects with male or female pattern alopecia (aged 18 to 60 years) | Extract from Thuja occidentalis seeds | A shampoo containing 0.17% extract | Topical application; twice daily for 16 weeks | The shampoo increased total hair count compared to the placebo group. | Baek et al., 2011 [118] |
| Double-blind, randomized, placebo-controlled trial on 50 women subjects (aged 18 years or over) | Extract from barks of Stryphnodendron adstringens | A cream containing 6.0% extract | Topical application; twice daily for 6 months | The cream reduced terminal hair growth. | Vicente et al., 2009 [120] |
| Double-blind, randomized, controlled single-center trial on 50 alopecia patients including 22 women and 28 men (aged 18 years or over, 42.0 ± 11.37 years) | Supercritical CO2 extract of brans of Oryza sativa | A tonic containing 0.5% extract | Topical application; twice a day for 16 weeks | The tonic increased hair diameter and the density of hairs per skin area in male subjects. | Choi et al., 2015 [121] |
| Double-blind, placebo-controlled, randomized clinical trial on 23 subjects with mild alopecia (aged 20 to 60 years) | DA-5512 formula (EtOH extract of herbal mixture: Thea sinensis, Emblica officinalis, Pinus densiflora, Pueraria thunbergiana, Tribulus terrestris, and Zingiber officinale) | A solution | Topical application on the shaved head skin twice daily for 16 weeks | Hair density, hair shaft diameter, and hair growth rate; placebo (n = 8) < 5% DA-5512 (n = 8) ≅ 3% minoxidil (n = 7). | Yu et al., 2017 [45] |
| Double-blind, randomized, placebo-controlled study on 30 women (aged 20 to 52 years) | n-Hexane extract of Curcuma aeruginosa | A lotion containing 5% extract | Topical application; twice daily for 12 weeks | The lotion reduced the growth rates of axillary hairs. | Srivilai et al., 2018 [122] |
| Randomized, placebo-controlled, single-blind, clinical study on 120 subjects with androgenetic alopecia and telogen effluvium (aged 20 to 55 years, 36.9 ± 9.8 years) | A mixture of herbal extracts: Urtica urens, Urtica dioica, Matricaria chamomilla, Achillea millefolium, Ceratonia siliqua, and Equisetum arvense. | A shampoo and a solution | Topical application of active shampoo (3 to 4 min), 3 times a week, and/or active solution (4 to 6 h) daily for 6 months | Effectiveness in preventing and reducing hair loss; placebo shampoo plus placebo solution (n = 30) < active shampoo (n = 30) ≤ active solution (n = 30) ≤ active shampoo plus active solution (n = 30). | Pekmezci et al., 2018 [123] |
| Double-blind, randomized controlled study on 47 subjects including male and female patients with androgenic alopecia (aged 18 to 54 years) | Extracts of Inula helenium (IH) roots and Caesalpinia sappan (CS) barks | A shampoo containing 0.3% IH root extract and 0.1% CS bark extract | Topical application twice daily for 24 weeks | The treatment group (n = 23) showed a higher hair density and total hair count than the placebo group (n = 24). | Choi et al., 2019 [124] |
| Randomized, double-blind, placebo-controlled study on 72 patients with mild to moderate vertex balding (aged 37 to 54 years, 46.6 ± 8.5 years) | Extract of Centipeda minima | A tonic | Topical application daily for 24 weeks | The treatment group (n = 34) showed a higher hair count than the placebo group (n= 32). | Kim et al., 2020 [125] |
| Double-blind, randomized controlled study on 46 male subjects (aged 20 to 55 years) | Extract of watercress (Nasturtium officinale) | A lotion containing 2% extract | Topical application twice daily for 6 months | The treatment group (n = 23) showed a higher hair thickness and hair density than the placebo group (n = 23). | Hashimoto et al., 2022 [72] |
| Randomized, double-blind, placebo-controlled clinical study on 50 subjects including 7 males and 43 females (aged 20 years or over) | Water extract of banana (Musa paradisiaca) flowers | A sachet containing 16% extract | Oral administration daily for 12 weeks | The sachet uptake increased the hair root diameter and reduced hair loss and scalp redness compared to the placebo group. | Liang et al., 2023 [62] |
| Randomized, double-blind, placebo-controlled clinical study on 88 subjects including 34 males and 54 females (aged 19 to 60 years, 38.52 ± 7.98 years) | Extract of persimmon (Diospyros kaki) leaves, green tea (Camellia sinensis) leaves, and sophora (Sophora Japonica) fruits | A tablet containing 30% extract | Oral administration twice daily for 24 weeks | The treatment group (n = 44) showed a higher hair density and hair diameter compared with the placebo group (n = 44). | Ham et al., 2023 [126] |
| Randomized, double-blind, placebo-controlled clinical study on 42 subjects including male and female patients with androgenetic alopecia (aged 18 to 54 years, 46.096 ± 6.60 years) | EtOH extract from flowers of Silybum marianum | A shampoo containing 0.05% extract | Topical application; once a day for 24 weeks | The shampoo increased the hair density and total hair count compared with those in the placebo group. | You et al., 2024 [65] |
| Plant Extracts | Main Phytochemical Components and Active Compounds | Literature |
|---|---|---|
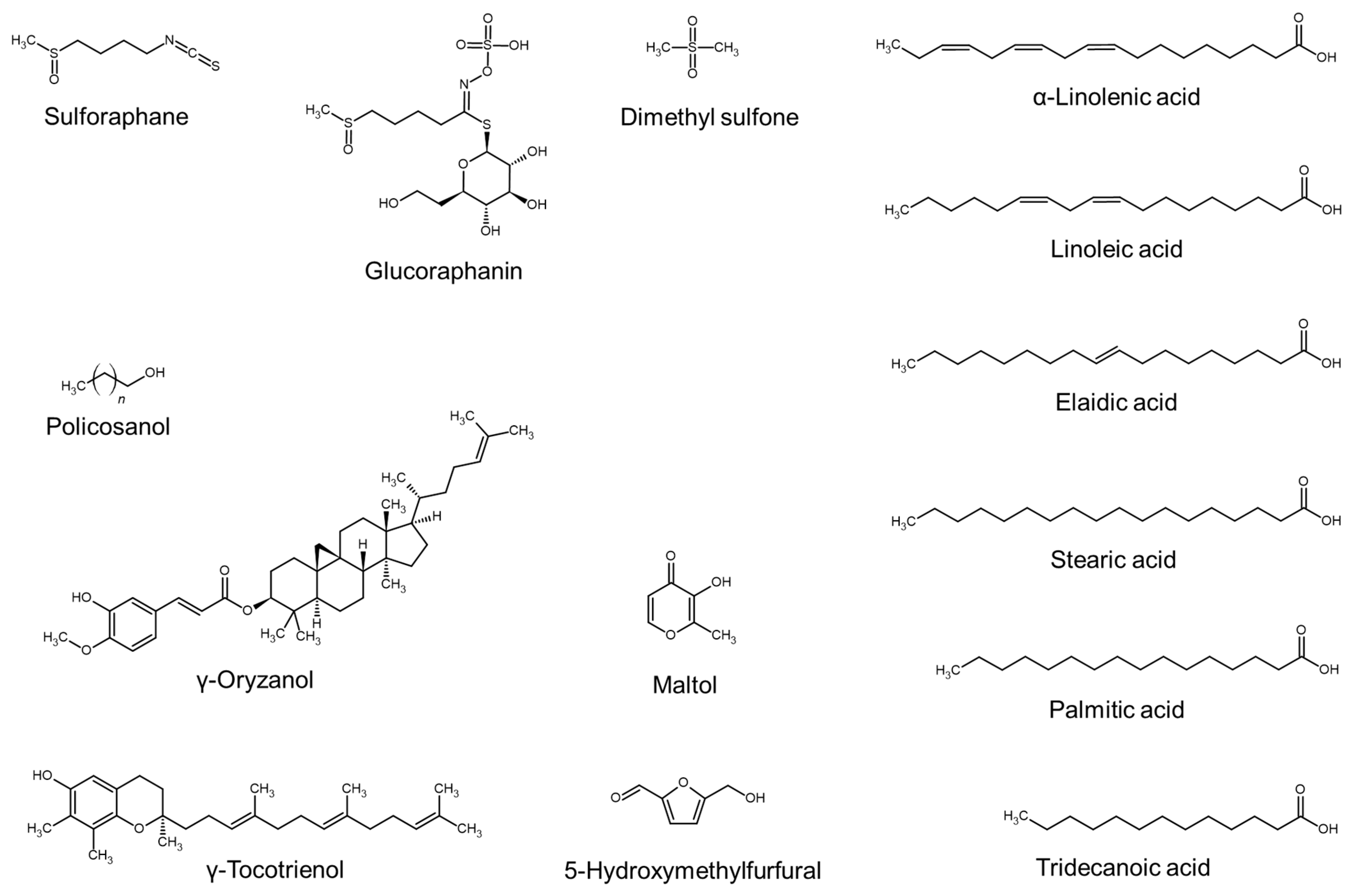
| Acetone extract of Boehmeria nipononivea | α-Linolenic acid, linoleic acid, palmitic acid, elaidic acid, oleic acid, and stearic acid | Shimizu et al., 2000 [75] |
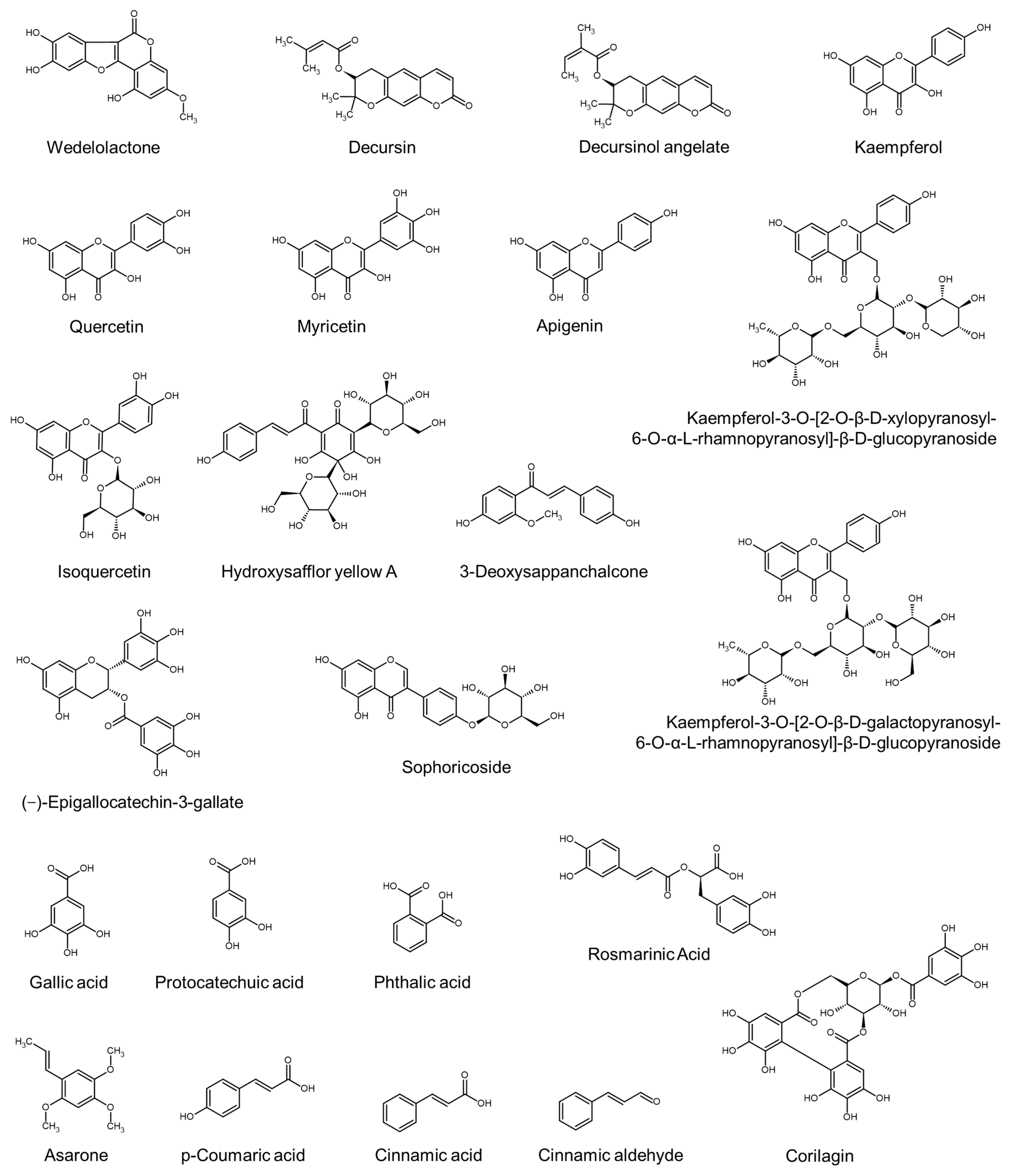
| MeOH extract of Eclipta alba | Coumestans (e.g., Wedelolactone), flavonoids, triterpenoid glycosides, triterpenoid saponins, and thiophene derivatives | Datta et al., 2009 [77] |
| Hot water extract of Thuja orientalis | Kaempferol and isoquercetin | Zhang et al., 2013 [83] |
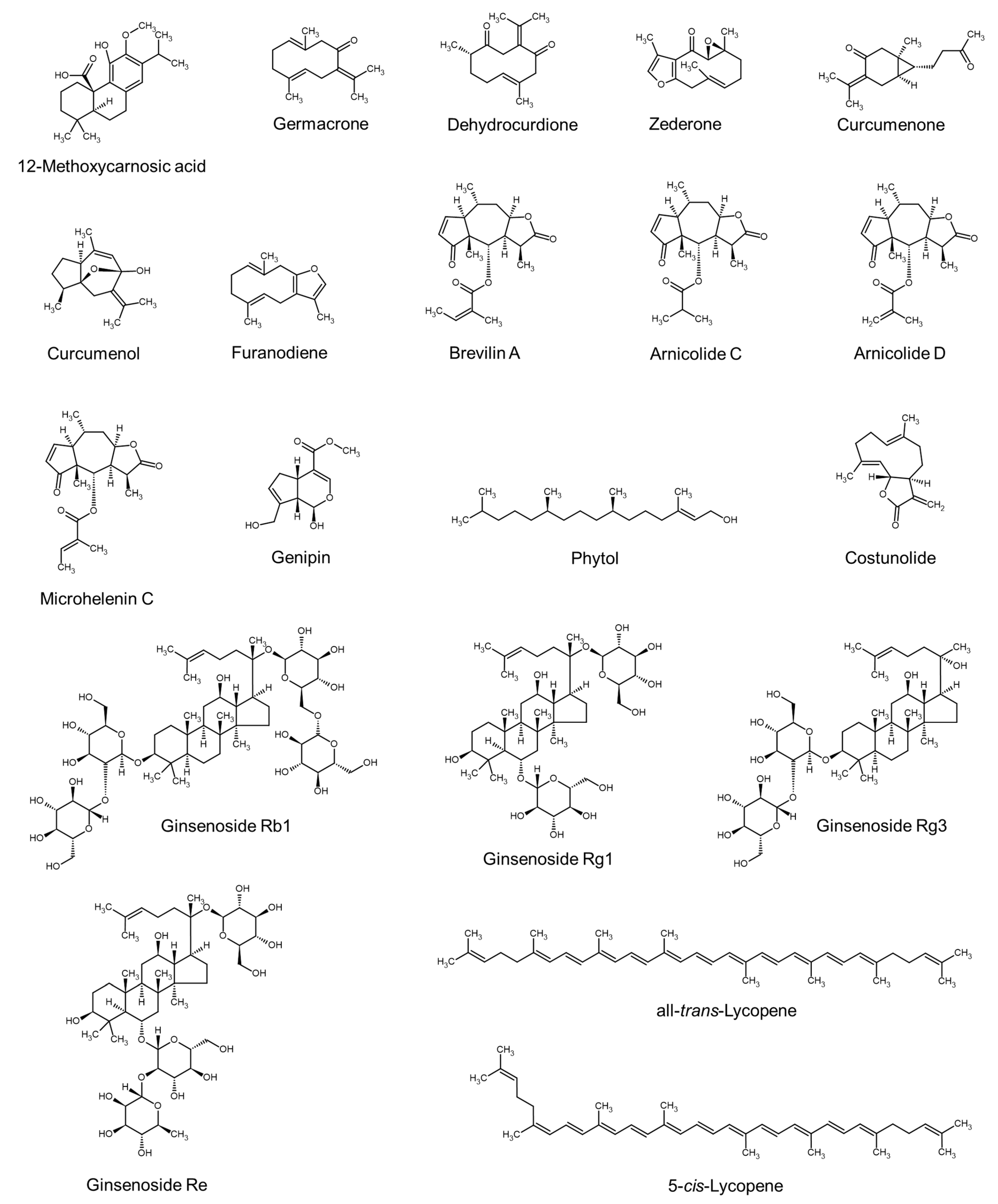
| Extract of Rosmarinus officinalis leaves | 12-Methoxycarnosic acid (a diterpenoid) | Murata et al., 2013 [84] |
| Extract of tomato (Lycopersicon esculentum) | all-trans-Lycopene and 5-cis-lycopene | Choi et al., 2013 [85] |
| Supercritical CO2 extract of rice (Oryza sativa) brans | Linoleic acid, policosanol, γ-oryzanol, and γ-tocotrienol | Choi et al., 2014 [86] |
| 50% EtOH extract of Carthamus tinctorius florets | Hydroxysafflor yellow A (a chalcone glycoside) | Junlatat and Sripanidkulchai, 2014 [41] |
| EtOH extract of Stachytarpheta jamaicensis leaves | Genipin, phytol, α-linolenic acid, palmitic acid, and tridecanoic acid | Rozianoor et al., 2014 [89] |
| Extract of red ginseng (Panax ginseng) | Ginsenoside Rb1 and ginsenoside Rg3 | Park et al., 2015 [43] |
| Hot water extract of an herbal mixture: Acorus calamus, Morus alba, Glycyrrhiza uralensis, Pinus densiflora, Sophora angustifolia, Ligusticum chuanxiong, and Angelica giga | Asarone and p-coumaric acid | Park et al., 2015 [91] |
| MeOH extract of Geranium sibiricum | Corilagin and gallic acid | Boisvert et al., 2017 [46] |
| Water extract of Cinnamomum osmophloeum | Cinnamic aldehyde and cinnamic acid | Wen et al., 2018 [48] |
| BuOH fraction of Perilla frutescens extract | Rosmarinic acid | Li et al., 2018 [71] |
| n-Hexane extract of Curcuma aeruginosa | Germacrone and other sesquiterpenoids (e.g., dehydrocurdione, zederone, cucumenone, curcumenol, and furanodiene) | Srivilai et al., 2018 [122] |
| A mixture of herbal extracts: Urtica urens, Urtica dioica, Matricaria chamomilla, Achillea millefolium, Ceratonia siliqua, and Equisetum arvense | Kaempferol, quercetin, and myricetin | Pekmezci et al., 2018 [123] |
| Extracts of Inula helenium (IH) roots and Caesalpinia sappan (CS) barks | Costunolide (from IH) and 3-deoxysappanchalcone (from CS) | Choi et al., 2019 [124] |
| Extract of Centipeda minima | Brevilin A and other sesquiterpene lactones (e.g., arnicolide C, arnicolide D, and microhelenin C) | Kim et al., 2020 [125] |
| EtOH extract of Angelica gigas | Decursin and decursinol angelate | Lee et al., 2020 [98] |
| n-Hexane fraction of the MeOH extract of Leea indica leaves | Phthalic acid, palmitic acid, n-octadecane, n-eicosane, n-heptadecane, and farnesol | Sakib et al., 2021 [103] |
| EtOH and water extract of Punica granatum | Volatile compounds (e.g., maltol and 5-hydroxymethylfurfural) | Bhinge et al., 2021 [104] |
| MeOH extract of shallot (Allium ascalonicum) | Rosmarinic acid, p-coumaric acid, and quercetin | Ruksiriwanich et al., 2022 [57] |
| Extract of Brassica oleracea | Sulforaphane and glucoraphanin (a glucosinolate of sulforaphane) | Luo and Zhang, 2022 [66] |
| EtOH extract of Blumea eriantha | Dimethyl sulfone | Bhinge et al., 2022 [109] |
| 60% EtOH extract of seed cakes of Camellia japonica | Kaempferol-3-O-[2-O-β-D-galactopyranosyl-6-O-α-L-rhamnopyranosyl]-β-D-glucopyranoside and kaempferol-3-O-[2-O-β-D-xylopyranosyl-6-O-α-L-rhamnopyranosyl]-β-D-glucopyranoside | Ma et al., 2022 [67] |
| Hot water extract of Lycopus lucidus | Rosmarinic acid | Lee et al., 2022 [59] |
| 70% EtOH extract of fruit shells of Camellia japonica | Protocatechuic acid gallic acid | You et al., 2023 [61] |
| Extract of persimmon (Diospyros kaki) leaves, green tea (Camellia sinensis) leaves, and sophora (Sophora Japonica) fruits | Tannic acids (from persimmon), (−)-epigallocatechin-3-gallate (from green tea), and sophoricoside (from sophora) | Ham et al., 2023 [126] |
| Extract of Panax ginseng | Ginsenoside Rb1, ginsenoside Rg1, and ginsenoside Re | Iwabuchi et al., 2024 [63] |
| 70% EtOH extract of flowers of Silybum marianum | Apigenin | You et al., 2024 [65] |
| Plant Extract Sources | Model | Antioxidant, Anti-Inflammatory, and Anti-Senescence Effects | Literature |
|---|---|---|---|
| Platycarya strobilacea | In vitro | The extract had 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging capacity. | Kim et al., 2014 [42] |
| Geranium sibiricum | In vitro | The extract had a DPPH radical-scavenging capacity. | Boisvert et al., 2017 [46] |
| Panax ginseng, Glycine max, Houttuynia cordata, Lycium chinense, Glycyrrhiza uralensis, Citrus unshiu, Zizyphus jujuba, Perilla frutescens, Camellia sinensis, and Cynanchum wilfordii | Mast cell-1 | The extract suppressed the production of tumor necrosis factor-alpha (TNF-α) in cells stimulated with phorbol-12-myristate 13-acetate (PMA) plus calcium ionophore A23187. | Kang et al., 2019 [50] |
| Angelica gigas | Male C57/BL6 mice | The extract reduced pro-inflammatory cytokines, such as TNF-α and interleukin (IL)-1β, while increasing anti-inflammatory cytokines, such as IL-4 and IL-13, in the dorsal skin. | Lee et al., 2020 [98] |
| Leea indica | In silico | Molecular docking analysis identified some phytochemicals, such as including phthalic acid, that showed high ligand efficiencies towards prostaglandin D2 synthase. | Sakib et al., 2021 [103] |
| Euterpe oleracea, Olea europea, Tabebuia impetiginosa, and Coffea Arabica | HFDPCs | The extract enhanced the viability of cells exposed to 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH) radical. | Serruya and Maor, 2021 [54] |
| Nelumbo nucifera | In vitro | The extract had a DPPH radical-scavenging capacity. | Park et al., 2021 [55] |
| Pinus thunbergii | Male C57/BL6 mice | The extract reduced pro-inflammatory cytokines, such as TNF-α and IL-1β, while increasing anti-inflammatory cytokines, such as IL-4 and IL-13, in the dorsal skin. | Her et al., 2022 [108] |
| Camellia japonica | HFDPCs | The extract suppressed the production of IL-6 and IL-1α in cells stimulated with DHT. It also reduced the expression of senescence-associated β-galactosidase (SA-β-gal) in DHT-treated cells. | Ma et al., 2022 [67] |
| Lycopus lucidus | HFDPCs | The extract reduced IL-1β levels in cells exposed to hydrogen peroxide (H2O2). | Lee et al., 2022 [59] |
| Camellia japonica | In vitro | The extract had DPPH radical-scavenging capacity. | You et al., 2023 [61] |
| HFDPCs | The extract reduced intracellular reactive oxygen species (ROS) levels and enhanced the viability of cells exposed to H2O2. It reduced SA-β-gal expression in cells exposed to H2O2. | ||
| Musa paradisiaca | HFDPCs | The extract reduced intracellular ROS levels exposed to H2O2. | Liang et al., 2023 [62] |
| Coffea arabica | In vitro | The extract had 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical and DPPH radical-scavenging capacities. | Muangsanguan et al., 2023 [135] |
| Silybum marianum | HFDPCs | The extract reduced intracellular ROS levels and enhanced the viability of cells exposed to H2O2. It reduced the expression of SA-β-gal and IL-6 in senescent cells and young cells exposed to H2O2. | You et al., 2024 [65] |
| Plant Extract Sources | Models | BCL-2 | BAX | BAD | Literature |
|---|---|---|---|---|---|
| Rumex japonicus | HFDPCs | ↑(protein) | ↓(protein) | Lee et al., 2016 [44] | |
| Serenoa repens | C57BL/6 mice | ↑(protein) | ↓(protein) | Zhu et al., 2018 [94] | |
| Houttuynia cordata | HFDPCs | ↑(mRNA) ↑(protein) | ↓(mRNA) | =(mRNA) | Kim et al., 2019 [49] |
| Panax ginseng, Glycine max, Houttuynia cordata, Lycium chinense, Glycyrrhiza uralensis, Citrus unshiu, Zizyphus jujuba, Perilla frutescens, Camellia sinensis, and Cynanchum wilfordii | HFDPCs | ↓(mRNA) ↓(protein) | Kang et al., 2019 [50] | ||
| Polygonum multiflorum | HFDPCs | ↑(mRNA) | ↓(mRNA) | Shin et al., 2020 [51] | |
| Salvia plebeia | HFDPCs | ↑(protein) | =(protein) | Jin et al., 2020 [52] | |
| Brassica oleracea | HFDPCs | =(mRNA) | ↓(mRNA) | Luo and Zhang, 2022 [66] | |
| Camellia japonica | HFDPCs | ↑(mRNA) ↑(protein) | ↓(mRNA) | Ma et al., 2022 [67] |
| Plant Extract Sources | Models | Testosterone | Androgen Receptor | Steroid 5α-Reductase Type II | Literature |
|---|---|---|---|---|---|
| Sophora flavescens | In vitro | ↓(activity) | Roh et al., 2002 [76] | ||
| Nicotiana tabacum | Male albino Wister rats | ↓(protein) | Murkute et al., 2010 [78] | ||
| Rosmarinus officinalis | In vitro | ↓(activity) | Murata et al., 2013 [84] | ||
| Panax ginseng | HFDPCs | ↓(mRNA) | Park et al., 2015 [43] | ||
| Panax ginseng, Glycine max, Houttuynia cordata, Lycium chinense, Glycyrrhiza uralensis, Citrus unshiu, Zizyphus jujuba, Perilla frutescens, Camellia sinensis, and Cynanchum wilfordii | HFDPCs | ↓(protein) | Kang et al., 2019 [50] | ||
| Polygonum multiflorum | HFDPCs | ↓(protein) | Shin et al., 2020 [51] | ||
| Plumbago zeylanica | HFDPCs | ↓(protein) | Yamada et al., 2020 [53] | ||
| Allium ascalonicum | Prostate cancer cell line Du-145 | ↓(mRNA) | Ruksiriwanich et al., 2022 [57] | ||
| Mangifera indica | HFDPCs | ↓(mRNA) | Jung et al., 2022 [110] | ||
| Camellia japonica | HFDPCs | ↓(mRNA) | ↓(mRNA) | Ma et al., 2022 [67] | |
| In vitro | ↓(activity) | You et al., 2023 [61] | |||
| Musa paradisiaca | HFDPCs | ↓(mRNA) | ↓(mRNA) | Liang et al., 2023 [62] | |
| Coffea arabica | HFDPCs | ↓(mRNA) | Muangsanguan et al., 2023 [135] |
| Plant Extract Sources | Models | CDKs | p16 INK4 | p53 | Cell Cycle Phase | Literature |
|---|---|---|---|---|---|---|
| Erica multiflora | HFDPCs | ↓(G0/G1), =(S), ↑(G2/M) | Kawano et al., 2009 [39] | |||
| Houttuynia cordata | HFDPCs | =(mRNA, CDK1 and CDK2), ↑(mRNA, CDK4) | ↓(protein) | =(mRNA) | Kim et al., 2019 [49] | |
| Camellia japonica | HFDPCs | ↓(mRNA) | Ma et al., 2022 [67] | |||
| HFDPCs | ↓(G0/G1), ↑(S), ↑(G2/M) | Wang et al., 2022 [58] |
| Plant Extract Sources | Models | IGF-1 | VEGF | HGF | KGF (FGF-7) | Literature |
|---|---|---|---|---|---|---|
| Sophora flavescens | HFDPCs | ↑(mRNA) | =(mRNA) | =(mRNA) | ↑(mRNA) | Roh et al., 2002 [76] |
| Asiasarum heterotropoides | HFDPCs | =(mRNA) | ↑(mRNA) | =(mRNA) | =(mRNA) | Rho et al., 2005 [38] |
| Eclipta alba | C57BL/6 mice | ↑(protein) | Datta et al., 2009 [77] | |||
| Lycopersicon esculentum | C57BL/6 mice | ↑(mRNA) | ↑(mRNA) | ↑(mRNA) | Choi et al., 2013 [85] | |
| Oryza sativa | C57BL/6 mice | ↑(mRNA) | ↑(mRNA) | ↑(mRNA) | Choi et al., 2014 [86] | |
| Carthamus tinctorius | HFDPCs | ↑(mRNA) | ↑(mRNA) | Junlatat and Sripanidkulchai, 2014 [41] | ||
| Platycarya strobilacea | HFDPCs | ↓(mRNA) | =(mRNA) | Kim et al., 2014 [42] | ||
| Panax ginseng | HFDPCs | =(mRNA) | =(mRNA) | =(mRNA) | Park et al., 2015 [43] | |
| Acorus calamus, Morus alba, Glycyrrhiza uralensis, Pinus densiflora, Sophora angustifolia, Ligusticum chuanxiong, and Angelica gigas | C57BL/6 mice | ↑(mRNA) | ↑(mRNA) | Park et al., 2015 [91] | ||
| Geranium sibiricum | HFDPCs | ↑(mRNA) | ↑(mRNA) | Boisvert et al., 2017 [46] | ||
| C57BL/6 mice | ↓(mRNA) | ↓(mRNA) | ||||
| Biota orientalis, Eclipta thermalis, Sophora angustifolia, Cnidium monnieri, Ligusticum chuanxiong, and Panax notoginseng | HFDPCs | ↑(mRNA) | ↑(mRNA) | Zeng et al., 2017 [148] | ||
| Cinnamomum osmophloeum | HFDPCs | =(mRNA) | ↑(mRNA) | =(mRNA) | ↑(mRNA) | Wen et al., 2018 [48] |
| Houttuynia cordata | HFDPCs | =(protein) | ↑(protein) | =(protein) | Kim et al., 2019 [49] | |
| Polygonum multiflorum | HFDPCs | ↑(protein) | Shin et al., 2020 [51] | |||
| Salvia plebeia | HFDPCs | ↑(mRNA) | Jin et al., 2020 [52] | |||
| Platycladus orientalis | C57BL/6 mice | ↑(protein) | ↑(protein) | Ahn et al., 2020 [99] | ||
| Nelumbo nucifera | C57BL/6 mice | ↑(mRNA) | ↑(mRNA) | Park et al., 2021 [55] | ||
| Centipeda minima | HFDPCs | ↑(protein) | ↑(mRNA) ↑(protein) | Kim et al., 2021 [149] | ||
| Brassica oleracea | HFDPCs | =(mRNA) | ↑(mRNA) | ↓(mRNA) | Luo and Zhang, 2022 [66] | |
| Pinus thunbergii | C57BL/6 mice | ↑(protein) | ↑(protein) | Her et al., 2022 [108] | ||
| Eremochloa ophiuroides | HFDPCs | ↑(mRNA) | ↑(mRNA) | Ramadhani et al., 2022 [56] | ||
| Allium ascalonicum | HFDPCs | ↑(mRNA) | Ruksiriwanich et al., 2022 [57] | |||
| Camellia japonica | HFDPCs | ↑(mRNA) | ↑(mRNA) | ↑(mRNA) | Wang et al., 2022 [58] | |
| Lycopus lucidus | HFDPCs | ↑(protein) =(mRNA) | Lee et al., 2022 [59] | |||
| C57BL/6 mice | ↑(protein) | ↑(protein) | ||||
| Camellia japonica | HFDPCs | ↑(mRNA) ↓(protein) | You et al., 2023 [61] | |||
| Coffea arabica | HFDPCs | ↑(mRNA) | Muangsanguan et al., 2023 [135] | |||
| Cudrania tricuspidata and Sargassum fusiforme | C57BL/6 mice | ↑(mRNA) | Rajan et al., 2023 [74] | |||
| Silybum marianum | HFDPCs | ↑(mRNA) | ↑(protein) | ↑(mRNA) | You et al., 2024 [65] |
| Plant Extract Sources | Models | AKT | ERK | JNK | p38 MAPK | Literature |
|---|---|---|---|---|---|---|
| Panax ginseng | HFDPCs | ↑(phospho) | ↑(phospho) | Park et al., 2015 [43] | ||
| Rumex japonicus | HFDPCs | ↑(phospho) | ↑(phospho) | =(phospho) | =(phospho) | Lee et al., 2016 [44] |
| Houttuynia cordata | HFDPCs | ↑(phospho) | ↑(phospho) | Kim et al., 2019 [49] | ||
| Salvia plebeia | HFDPCs | ↑(phospho) | ↑(phospho) | Jin et al., 2020 [52] | ||
| Euterpe oleracea, Olea europea, Tabebuia impetiginosa, and Coffea Arabica | HFDPCs | ↑(phospho) | Serruya and Maor, 2021 [54] | |||
| Eremochloa ophiuroides | HFDPCs | ↑(phospho) | Ramadhani et al., 2022 [56] | |||
| Camellia japonica | HFDPCs | ↑(phospho) | ↑(phospho) | Wang et al., 2022 [58] | ||
| Centipeda minima | HFDPCs | ↑(phospho) | ↑(phospho) | ↓(phospho) | Kim et al., 2021 [149] |
| Plant Extract Sources | Models | WNTs | DKK1 | GSK3β | β-Catenin | LEF1 | c-Myc | Cyclin D1 | Literature |
|---|---|---|---|---|---|---|---|---|---|
| Polygonum multiflorum | C57BL6/N | ↑(protein) | Park et al., 2011 [79] | ||||||
| Aconiti Ciliare | iDPCs | ↑(protein) | Park et al., 2012 [40] | ||||||
| Thuja orientalis | C57BL/6N mice | ↑(protein) | Zhang et al., 2013 [83] | ||||||
| Rumex japonicus | HFDPCs | ↑(phospho) | ↑(protein) | Lee et al., 2016 [44] | |||||
| Polygonum multiflorum | HFDPCs | ↓(protein) | Shin et al., 2020 [51] | ||||||
| Salvia plebeia | HFDPCs | ↑(phospho) | ↓(phospho) ↑(protein) | Jin et al., 2020 [52] | |||||
| Platycladus orientalis | C57BL/6 mice | WNT3 ↑(protein) | ↑(protein) | Ahn et al., 2020 [99] | |||||
| Centipeda minima | HFDPCs | WNT5a ↑(mRNA) | ↑(phospho) | ↑(protein) | Kim et al., 2021 [149] | ||||
| Brassica oleracea | HFDPCs | =(mRNA) | Luo and Zhang, 2022 [66] | ||||||
| Eremochloa ophiuroides | HFDPCs | ↑(phospho) | ↑(protein) | Ramadhani et al., 2022 [56] | |||||
| Allium ascalonicum | HFDPCs | ↑(mRNA) | Ruksiriwanich et al., 2022 [57] | ||||||
| Mangifera indica | HFDPCs | ↓(mRNA) | ↑(mRNA) | Jung et al., 2022 [110] | |||||
| Nasturtium officinale | Human hair follicles | ↓(protein) | Hashimoto et al., 2022 [72] | ||||||
| Camellia japonica | HFDPCs | WNT1 ↑(mRNA) | ↓(protein) | ↑(mRNA) | ↑(mRNA) | You et al., 2023 [61] | |||
| Terminalia bellirica | C57BL/6 mice | ↑(protein) | ↑(protein) | Woo et al., 2023 [68] | |||||
| Cudrania tricuspidata and Sargassum fusiforme | C57BL/6 mice | WNT5a, WNT7b ↑(mRNA) | Rajan et al., 2023 [74] | ||||||
| Gynostemma pentaphyllum | HFDPCs | WNT5a ↑(mRNA) ↑(protein) | ↓(mRNA) | ↑(mRNA) ↑(protein) | ↑(mRNA) | Liu et al., 2024 [64] |
| Plant Extract Sources | Models | SHH | SMO | GLI1 | Literature |
|---|---|---|---|---|---|
| Eclipta alba | C57BL/6 mice | ↑(protein) | Datta et al., 2009 [77] | ||
| Polygonum multiflorum | C57BL6/N | ↑(protein) | Park et al., 2011 [79] | ||
| Thuja orientalis | C57BL/6N mice | ↑(protein) | Zhang et al., 2013 [83] | ||
| Eremochloa ophiuroides | C57BL/6 mice | ↑(protein) | Ramadhani et al., 2022 [56] | ||
| Allium ascalonicum | HFDPCs | ↑(mRNA) | ↑(mRNA) | ↑(mRNA) | Ruksiriwanich et al., 2022 [57] |
| Coffea arabica | HFDPCs | ↑(mRNA) | ↑(mRNA) | ↑(mRNA) | Muangsanguan et al., 2023 [135] |
| Plant Extract Sources | Models | TGF-β1 | TGF-β2 | BMP4 | SMAD2 | SMAD3 | Literature |
|---|---|---|---|---|---|---|---|
| Asiasarum heterotropoides | HFDPCs | =(mRNA) | Rho et al., 2005 [38] | ||||
| Eclipta alba | C57BL/6 mice | ↓(protein) | Datta et al., 2009 [77] | ||||
| Lycopersicon esculentum | C57BL/6 mice | =(mRNA) | Choi et al., 2013 [85] | ||||
| Oryza sativa | C57BL/6 mice | ↓(mRNA) | Choi et al., 2014 [86] | ||||
| Carthamus tinctorius | HFDPCs | ↓(mRNA) | Junlatat and Sripanidkulchai, 2014 [41] | ||||
| Platycarya strobilacea | HFDPCs | =(mRNA) | Kim et al., 2014 [42] | ||||
| Geranium sibiricum | HFDPCs | =(mRNA) | Boisvert et al., 2017 [46] | ||||
| C57BL/6 mice | ↓(mRNA) | ||||||
| Cinnamomum osmophloeum | HFDPCs | ↑(mRNA) | Wen et al., 2018 [48] | ||||
| Serenoa repens | C57BL/6 mice | ↓(protein) | Zhu et al., 2018 [94] | ||||
| Panax ginseng, Glycine max, Houttuynia cordata, Lycium chinense, Glycyrrhiza uralensis, Citrus unshiu, Zizyphus jujuba, Perilla frutescens, Camellia sinensis, and Cynanchum wilfordii | HFDPCs | ↓(mRNA) | Kang et al., 2019 [50] | ||||
| Salvia plebeia | HFDPCs | ↓(mRNA) | ↓(protein) | ↓(protein) | Jin et al., 2020 [52] | ||
| Euterpe oleracea, Olea europea, Tabebuia impetiginosa, and Coffea Arabica | HFDPCs | ↓(protein) | Serruya and Maor, 2021 [54] | ||||
| Nelumbo nucifera | C57BL/6 mice | ↓(mRNA) | Park et al., 2021 [55] | ||||
| Brassica oleracea | HFDPCs | =(mRNA) | Luo and Zhang, 2022 [66] | ||||
| Camellia japonica | HFDPCs | ↓(mRNA) | Wang et al., 2022 [58] | ||||
| Acorus calamus, Morus alba, Glycyrrhiza uralensis, Pinus densiflora, Sophora angustifolia, Ligusticum chuanxiong, and Angelica gigas | C57BL/6 mice | ↓(mRNA) | Muangsanguan et al., 2023 [135] | ||||
| Panax ginseng | ↓(mRNA) | Iwabuchi et al., 2024 [63] | |||||
| Gynostemma pentaphyllum | HFDPCs | ↓(mRNA) ↓(protein) | Liu et al., 2024 [64] | ||||
| Silybum marianum | HFDPCs | ↓(mRNA) | You et al., 2024 [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Boo, M.Y.; Boo, Y.C. Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Molecules 2024, 29, 2288. https://doi.org/10.3390/molecules29102288
Choi JY, Boo MY, Boo YC. Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Molecules. 2024; 29(10):2288. https://doi.org/10.3390/molecules29102288
Chicago/Turabian StyleChoi, Joon Yong, Min Young Boo, and Yong Chool Boo. 2024. "Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets" Molecules 29, no. 10: 2288. https://doi.org/10.3390/molecules29102288
APA StyleChoi, J. Y., Boo, M. Y., & Boo, Y. C. (2024). Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Molecules, 29(10), 2288. https://doi.org/10.3390/molecules29102288









