Impact of Condition Variations on Bioelectrochemical System Performance: An Experimental Investigation of Sulfamethoxazole Degradation
Abstract
1. Introduction
2. Results and Discussion
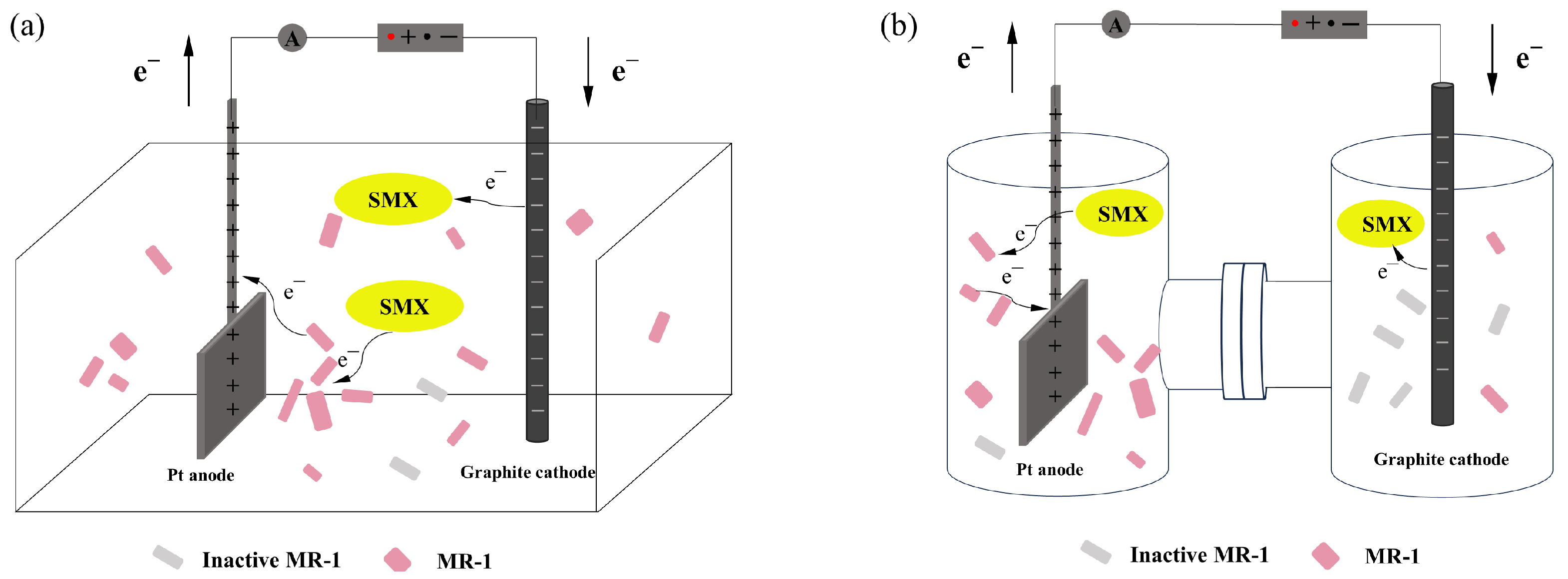
2.1. Reaction Process in Bioelectrochemical Systems
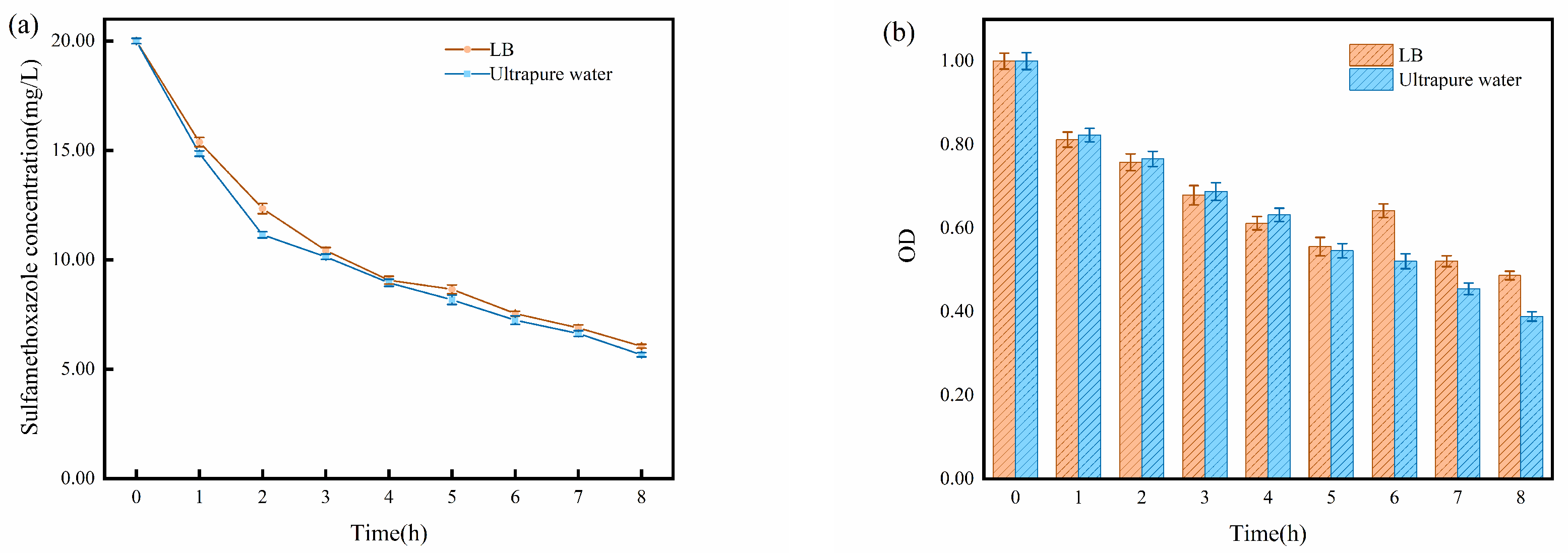
2.2. Impact of Solution Matrix
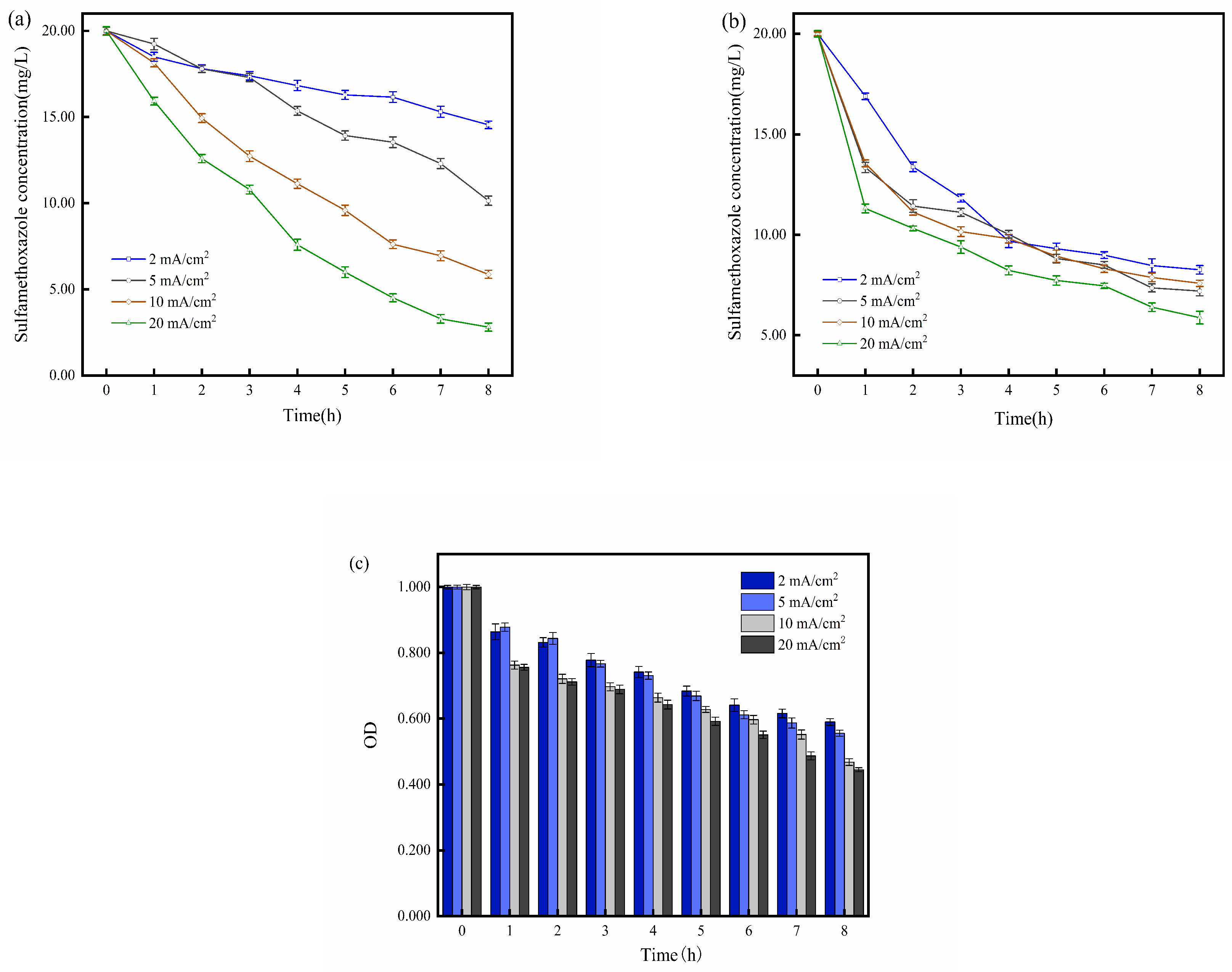
2.3. Impact of Current Density
2.4. Impact of Electrode Distance
3. Materials and Methods
3.1. Materials
3.2. Microbiological Culture
3.3. Reactor Setup and Experimental Procedure
3.4. Sampling and Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Li, L.; Pan, Z.; Zhu, Y.; Shao, Y.; Wang, Y.; Yu, K. Degradation of sulfamethoxazole by UV/persulfate in different water samples: Influential factors, transformation products and toxicity. Chem. Eng. J. 2020, 379, 122354. [Google Scholar] [CrossRef]
- Amina; Si, X.; Wu, K.; Si, Y.; Yousaf, B. Synergistic effects and mechanisms of hydroxyl radical-mediated oxidative degradation of sulfamethoxazole by Fe(II)-EDTA catalyzed calcium peroxide: Implications for remediation of antibiotic-contaminated water. Chem. Eng. J. 2018, 353, 80–91. [Google Scholar] [CrossRef]
- Kolpin, D.; Furlong, E.; Meyer, M.; Thurman, E.; Zaugg, S.; Barber, L.; Buxton, H. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams. 2005, Volume 36. Available online: https://www.researchgate.net/publication/227580982_Pharmaceuticals_Hormones_and_Other_Organic_Wastewater_Contaminants_in_US_Streams (accessed on 20 March 2024).
- Dirany, A.; Sires, I.; Oturan, N.; Oturan, M.A. Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere 2010, 81, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Bing Li, T.Z. Biodegradation and Adsorption of Antibiotics in the Activated. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet. Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, I.R.; Watanabe, N.; Harter, T.; Glaser, B.; Radke, M. Effect of sulfonamide antibiotics on microbial diversity and activity in a Californian Mollic Haploxeralf. J. Soils Sediments 2010, 10, 537–544. [Google Scholar] [CrossRef]
- Dong, H.; Yuan, X.; Wang, W.; Qiang, Z. Occurrence and removal of antibiotics in ecological and conventional wastewater treatment processes: A field study. J. Environ. Manag. 2016, 178, 11–19. [Google Scholar] [CrossRef]
- Song, H.; Yan, L.; Jiang, J.; Ma, J.; Pang, S.; Zhai, X.; Zhang, W.; Li, D. Enhanced degradation of antibiotic sulfamethoxazole by electrochemical activation of PDS using carbon anodes. Chem. Eng. J. 2018, 344, 12–20. [Google Scholar] [CrossRef]
- Pallares-Vega, R.; Blaak, H.; van der Plaats, R.; de Roda Husman, A.M.; Hernandez Leal, L.; van Loosdrecht, M.C.M.; Weissbrodt, D.G.; Schmitt, H. Determinants of presence and removal of antibiotic resistance genes during WWTP treatment: A cross-sectional study. Water Res. 2019, 161, 319–328. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, R.; Li, J.; Cheng, Z.; Luo, C.; Wang, Y.; Yu, K.; Zhang, G. Occurrence and distribution of antibiotics in multiple environmental media of the East River (Dongjiang) catchment, South China. Environ. Sci. Pollut. Res. Int. 2017, 24, 9690–9701. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, G. Hazard of Sulfonamides and Detection Technology Research Progress. IOP Conf. Ser. Earth Environ. Sci. 2017, 100, 012040. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, M.; Zhong, H.; Liu, M.; Sui, Q.; Zheng, L.; Tong, J.; Wei, Y. Deciphering the factors influencing the discrepant fate of antibiotic resistance genes in sludge and water phases during municipal wastewater treatment. Bioresour. Technol. 2018, 265, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, S. Overview of sulfonamide biodegradation and the relevant pathways and microorganisms. Sci. Total Environ. 2018, 640–641, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Mulla, S.I.; Hu, A.; Sun, Q.; Li, J.; Suanon, F.; Ashfaq, M.; Yu, C.-P. Biodegradation of sulfamethoxazole in bacteria from three different origins. J. Environ. Manag. 2018, 206, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, O.N.; Méjean, V.; Iobbi-Nivol, C. The Shewanella genus: Ubiquitous organisms sustaining and preserving aquatic ecosystems. Fems Microbiol. Rev. 2020, 44, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Liu, X.; Wu, K.; Zhou, C.; Si, Y. Biodegradation of sulfonamides by Shewanella oneidensis MR-1 and Shewanella sp. strain MR-4. Biodegradation 2018, 29, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L. Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: An overview. Radiat. Phys. Chem. 2016, 125, 56–64. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Latsos, A.; Gidarakos, E. Performance of electroremediation in real contaminated sediments using a big cell, periodic voltage and innovative surfactants. J. Hazard. Mater. 2016, 320, 376–385. [Google Scholar] [CrossRef]
- Reddy, K.R.; Saichek, R.E. Effect of soil type on electrokinetic removal of phenanthrene using surfactants and cosolvents. J. Environ. Eng. 2003, 129, 336–346. [Google Scholar] [CrossRef]
- Liu, G.; Liu, M.; Shi, H.; Jia, H.; Zou, H.; Tao, N. Efficient electrochemical decomposition of sulfamethoxazole using a novel free-standing TiN anode. Sustain. Horiz. 2023, 7, 100059. [Google Scholar] [CrossRef]
- Gao, G.; Kang, J.; Shen, J.; Chen, Z.; Chu, W. Catalytic ozonation of sulfamethoxazole by composite iron-manganese silicate oxide: Cooperation mechanism between adsorption and catalytic reaction. Environ. Sci. Pollut. Res. 2016, 23, 21360–21368. [Google Scholar] [CrossRef] [PubMed]
- Sopaj, F.; Rodrigo, M.A.; Oturan, N.; Podvorica, F.I.; Pinson, J.; Oturan, M.A. Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin. Chem. Eng. J. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, T.; Wu, X.; Mao, J. Efficient sonoelectrochemical decomposition of sulfamethoxazole adopting common Pt/graphite electrodes: The mechanism and favorable pathways. Ultrason. Sonochem. 2017, 38, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.C.; Hong, Y.T.; Lee, T.W.; Huang, J.F. Facile fabrication of ascorbic acid reduced graphene oxide-modified electrodes toward electroanalytical determination of sulfamethoxazole in aqueous environments. Chem. Eng. J. 2018, 352, 188–197. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Y.; Dong, Z. Enhanced degradation of contaminants of emerging concern by electrochemically activated peroxymonosulfate: Performance, mechanism, and influencing factors. Chem. Eng. J. 2021, 415, 128938. [Google Scholar] [CrossRef]
- Sires, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Yang, K.; Ji, M.; Liang, B.; Zhao, Y.; Zhai, S.; Ma, Z.; Yang, Z. Bioelectrochemical degradation of monoaromatic compounds: Current advances and challenges. J. Hazard. Mater. 2020, 398, 122892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Liu, Y.; Wei, Y.; Lan, F.; Wang, R.; Yang, Y.; Chen, J. Research progress and trend of antibiotics degradation by electroactive biofilm: A review. J. Water Process Eng. 2024, 58, 104846. [Google Scholar] [CrossRef]
- Hassan, M.; Zhu, G.; Lu, Y.-Z.; Al-Falahi, A.H.; Lu, Y.; Huang, S.; Wan, Z. Removal of antibiotics from wastewater and its problematic effects on microbial communities by bioelectrochemical Technology: Current knowledge and future perspectives. Environ. Eng. Res. 2020, 26, 190405. [Google Scholar] [CrossRef]
- Yan, W.; Xiao, Y.; Yan, W.; Ding, R.; Wang, S.; Zhao, F. The effect of bioelectrochemical systems on antibiotics removal and antibiotic resistance genes: A review. Chem. Eng. J. 2019, 358, 1421–1437. [Google Scholar] [CrossRef]
- Wu, D.; Sun, F.; Chua, F.J.D.; Zhou, Y. Enhanced power generation in microbial fuel cell by an agonist of electroactive biofilm—Sulfamethoxazole. Chem. Eng. J. 2020, 384, 123238. [Google Scholar] [CrossRef]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol 2009, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Song, H.; Wei, S.; Liu, Q.; Li, X.; Qian, X. Effect of direct electrical stimulation on decolorization and degradation of azo dye reactive brilliant red X-3B in biofilm-electrode reactors. Biochem. Eng. J. 2015, 93, 294–302. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, L.; Sun, Y. Recovery of flakey cobalt from aqueous Co(II) with simultaneous hydrogen production in microbial electrolysis cells. Int. J. Hydrogen Energy 2014, 39, 654–663. [Google Scholar] [CrossRef]
- Seymour, J.D.; Gupta, R.B. Oxidation of Aqueous Pollutants Using Ultrasound: Salt-Induced Enhancement. Ind. Eng. Chem. Res. 1997, 36, 3453–4357. [Google Scholar] [CrossRef]
- She, P.; Song, B.; Xing, X.H.; Van Loosdrecht, M.; Liu, Z. Electrolytic stimulation of bacteria Enterobacter dissolvens by a direct current. Biochem. Eng. J. 2006, 28, 23–29. [Google Scholar] [CrossRef]
- Jackman, S.A.; Maini, G.; Sharman, A.K.; Knowles, C.J. The effects of direct electric current on the viability and metabolism of acidophilic bacteria. Enzym. Microb. Technol. 1999, 24, 316–324. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron–graphite electrode. Chem. Eng. J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Ding, A.; Yang, Y.; Sun, G.; Wu, D. Impact of applied voltage on methane generation and microbial activities in an anaerobic microbial electrolysis cell (MEC). Chem. Eng. J. 2016, 283, 260–265. [Google Scholar] [CrossRef]
- Giladi, M.; Porat, Y.; Blatt, A.; Wasserman, Y.; Kirson, E.D.; Dekel, E.; Palti, Y. Microbial growth inhibition by alternating electric fields. Antimicrob. Agents Chemother. 2008, 52, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zou, H.; Ji, M.; Li, X.; Li, L.; Tang, T. Enhanced electrokinetic remediation of lead-contaminated soil by complexing agents and approaching anodes. Environ. Sci. Pollut. Res. Int. 2014, 21, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Guo, S.; Li, S.; Zhang, L.; Wang, S. Comparison of approaching and fixed anodes for avoiding the ‘focusing’ effect during electrokinetic remediation of chromium-contaminated soil. Chem. Eng. J. 2012, 203, 231–238. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Q.; Chen, Z.; Xie, W.; Zhang, S.; Jiang, J.; Sun, G. Impact of Condition Variations on Bioelectrochemical System Performance: An Experimental Investigation of Sulfamethoxazole Degradation. Molecules 2024, 29, 2276. https://doi.org/10.3390/molecules29102276
Xue Q, Chen Z, Xie W, Zhang S, Jiang J, Sun G. Impact of Condition Variations on Bioelectrochemical System Performance: An Experimental Investigation of Sulfamethoxazole Degradation. Molecules. 2024; 29(10):2276. https://doi.org/10.3390/molecules29102276
Chicago/Turabian StyleXue, Qun, Zhihui Chen, Wenjing Xie, Shuke Zhang, Jie Jiang, and Guoxin Sun. 2024. "Impact of Condition Variations on Bioelectrochemical System Performance: An Experimental Investigation of Sulfamethoxazole Degradation" Molecules 29, no. 10: 2276. https://doi.org/10.3390/molecules29102276
APA StyleXue, Q., Chen, Z., Xie, W., Zhang, S., Jiang, J., & Sun, G. (2024). Impact of Condition Variations on Bioelectrochemical System Performance: An Experimental Investigation of Sulfamethoxazole Degradation. Molecules, 29(10), 2276. https://doi.org/10.3390/molecules29102276




