Strong Acceptors Based on Derivatives of Benzothiadiazoloimidazole
Abstract
1. Introduction
2. Results and Discussion
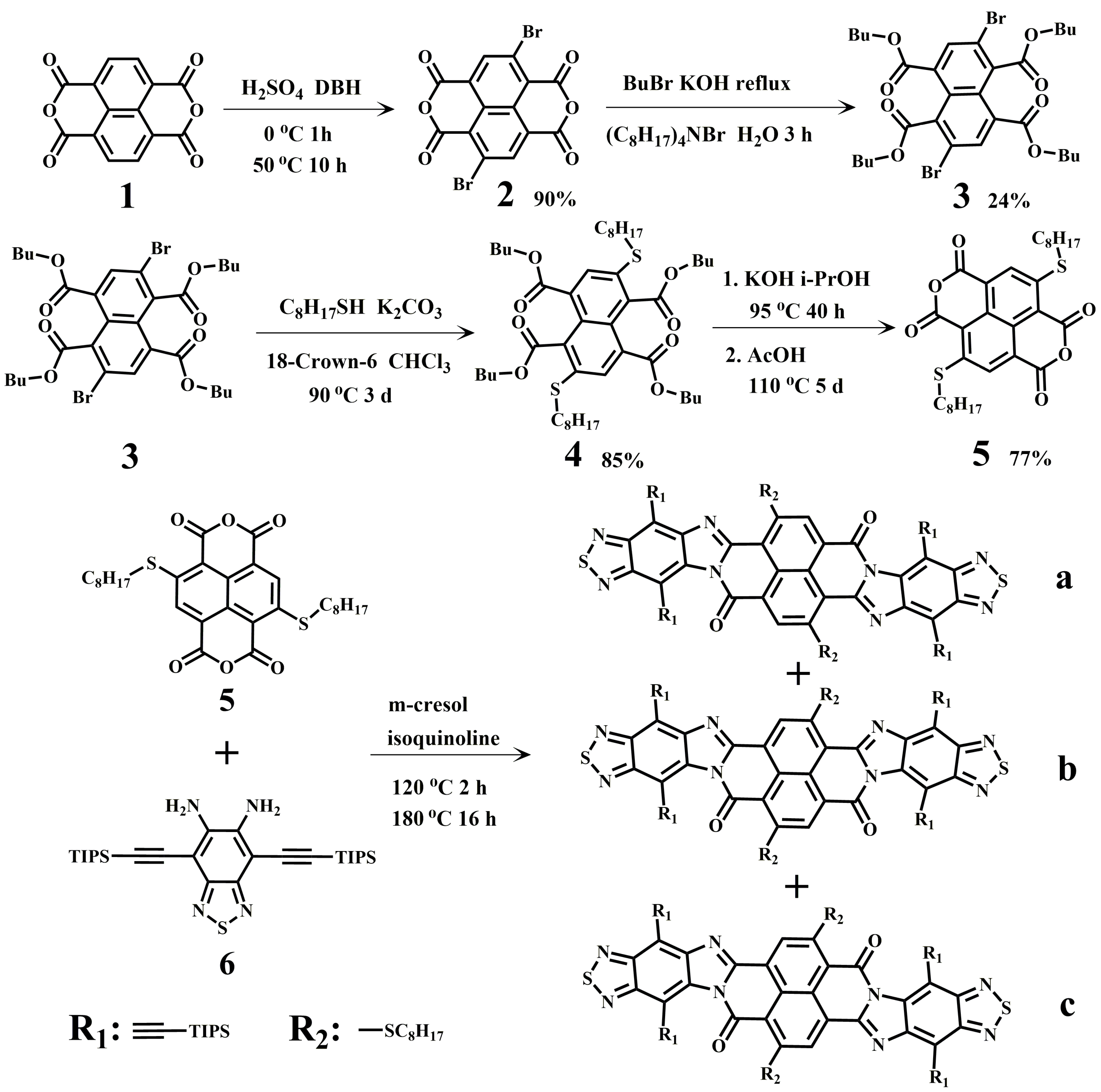
2.1. The Synthesis of BTI-NDI-BTI
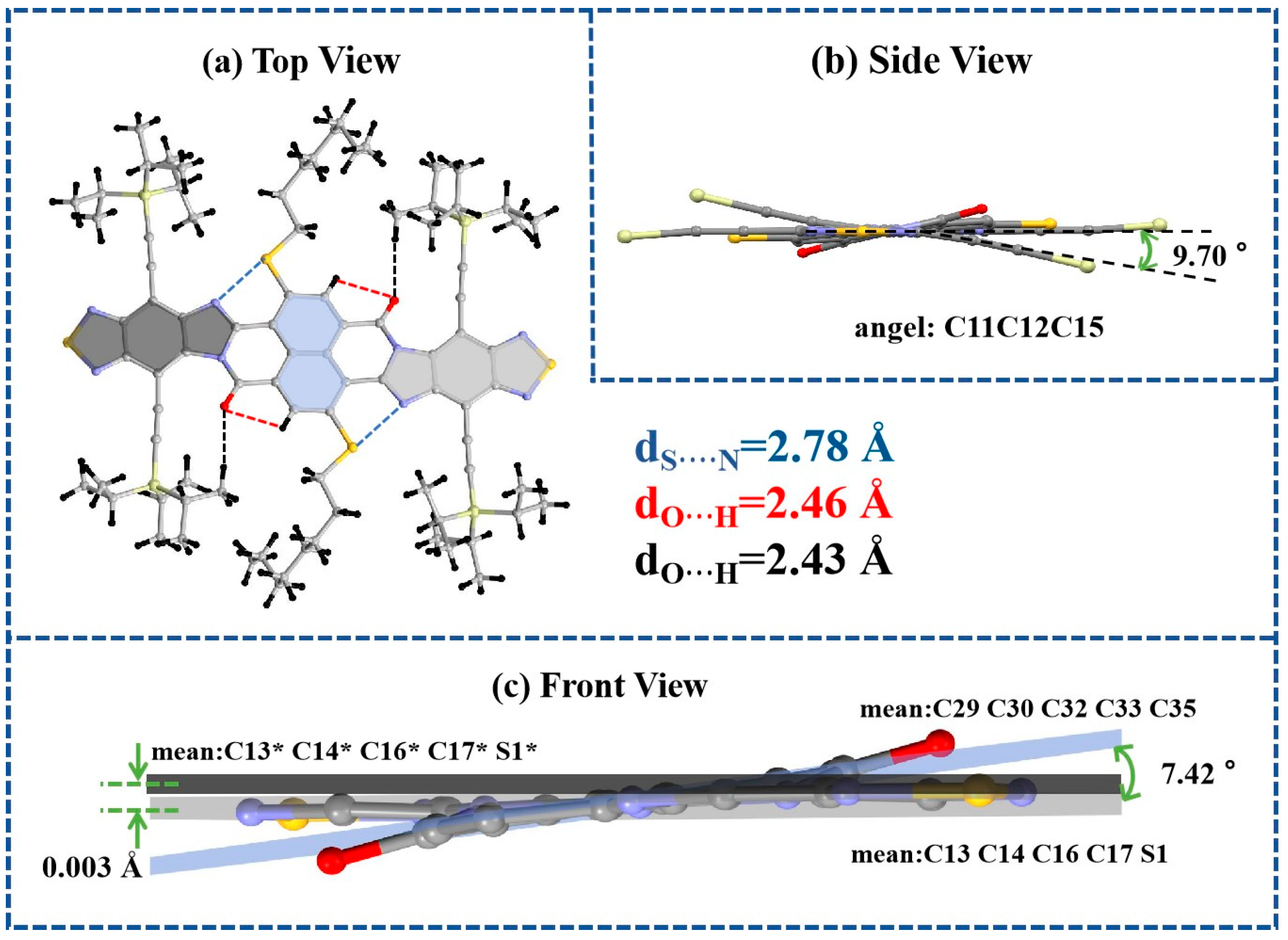
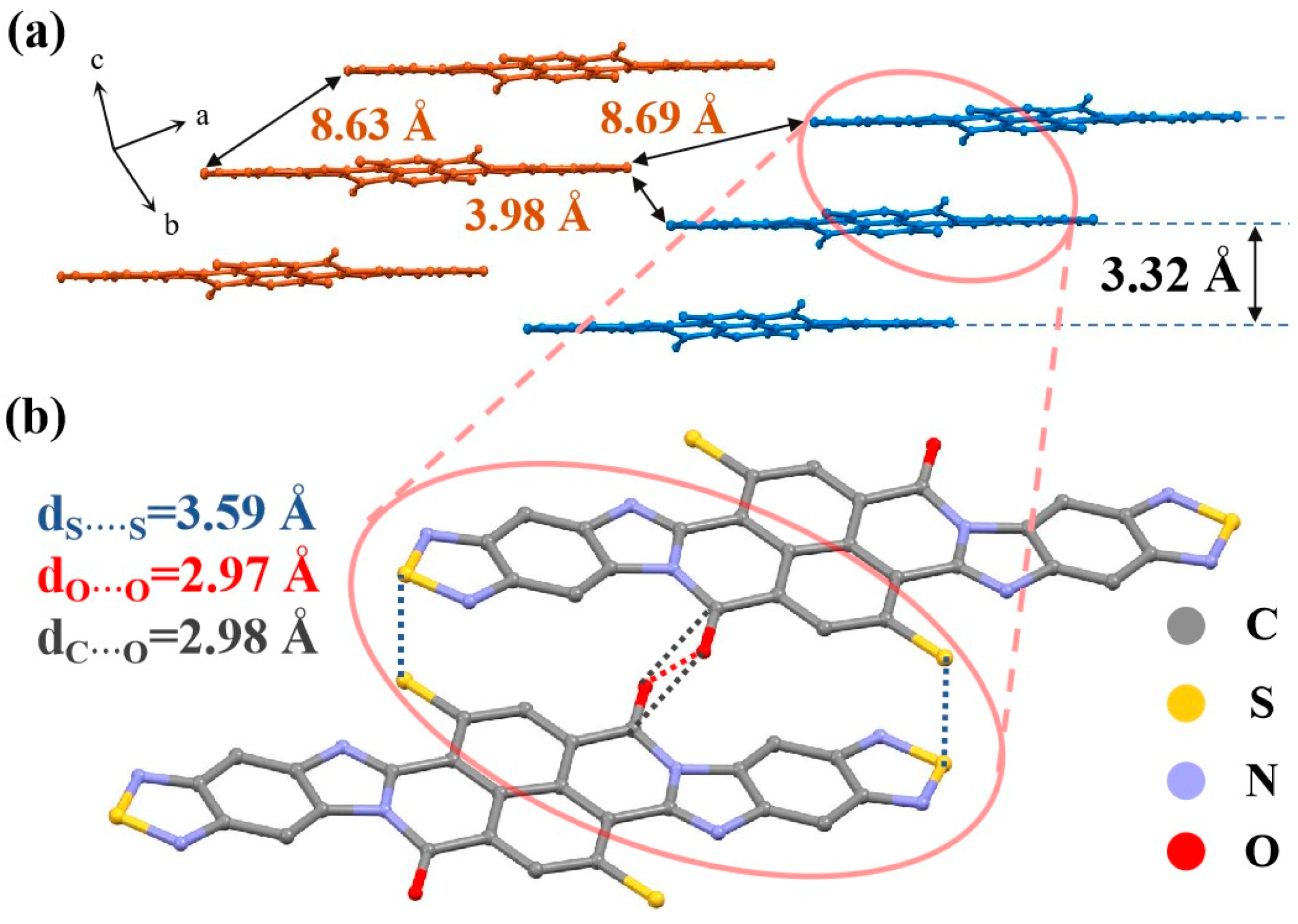
2.2. The Results of Crystals
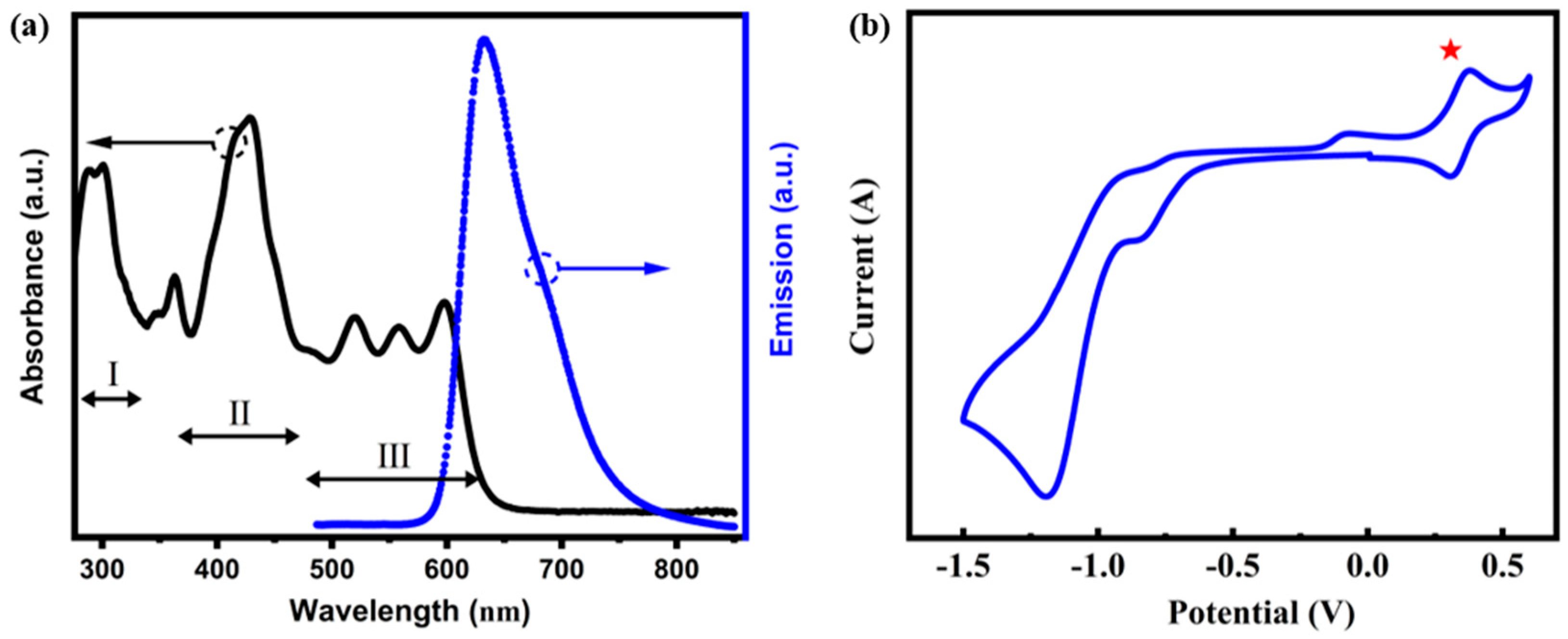
2.3. The Results of UV/Vis Absorption Spectrum and Cyclic Voltammetry
3. Materials and Methods
3.1. Materials
- Synthesis of 2,6-bis(octylthio)naphthalene-1,4,5,8-tetracarboxylic tetrabutyl-ester (4)
- Synthesis of BTI-NDI-BTI-a
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, W.; Guo, Y.L.; Liu, Y.Q. When Flexible Organic Field-Effect Transistors Meet Biomimetics: A Prospective View of the Internet of Things. Adv. Mater. 2020, 32, e1901493. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraja, S.; Nawaz, A.; Liu, Q.; Dubal, D.; Surya, S.G.; Salama, K.N.; Sonar, P. Organic field-effect transistor-based flexible sensors. Chem. Soc. Rev. 2020, 49, 3423–3460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Li, M.; Chen, C.F. Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes. Chem. Soc. Rev. 2020, 49, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.A.; Yang, T.C.J.; Wilson, G.J.; Evans, R.A.; Gupta, A.; Langford, S.J. Naphthalene diimide-based electron transport materials for perovskite solar cells. J. Mater. Chem. A 2021, 9, 27170–27192. [Google Scholar] [CrossRef]
- Gu, C.T.; Su, X.Z.; Li, Y.H.; Liu, B.; Tian, Y.; Tan, W.Q.; Ma, J.P.; Bao, X.C. n-Type polymer electron acceptors for organic solar cells. Mol. Syst. Des. Eng. 2022, 7, 1364–1384. [Google Scholar] [CrossRef]
- Tang, H.R.; Liang, Y.Y.; Liu, C.C.; Hu, Z.C.; Deng, Y.F.; Guo, H.; Yu, Z.D.; Song, A.; Zhao, H.Y.; Zhao, D.K.; et al. A solution-processed n-type conducting polymer with ultrahigh conductivity. Nature 2022, 611, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.F.; Abtahi, A.; Hwang, J.; Chen, K.; Chaudhary, J.; Song, I.; Perera, K.; You, L.Y.; Baustert, K.N.; Graham, K.R.; et al. Highly Conductive and Solution-Processable n-Doped Transparent Organic Conductor. J. Am. Chem. Soc. 2023, 145, 3706–3715. [Google Scholar] [CrossRef] [PubMed]
- Griggs, S.; Marks, A.; Bristow, H.; McCulloch, I. n-Type organic semiconducting polymers: Stability limitations, design considerations and applications. J. Mater. Chem. C 2021, 9, 8099–8128. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Chu, M.; Fan, J.X.; Yang, S.J.; Liu, D.; Ng, C.F.; Dong, H.L.; Ren, A.M.; Miao, Q. Halogenated Tetraazapentacenes with Electron Mobility as High as 27.8 cm V−1 s−1 in Solution-Processed n-Channel Organic Thin-Film Transistors. Adv. Mater. 2018, 30, e1803467. [Google Scholar] [CrossRef]
- Kobaisi, M.A.; Bhosale, S.V.; Latham, K.; Raynor, A.M.; Bhosale, S.V. Functional Naphthalene Diimides: Synthesis, Properties, and Applications. Chem. Rev. 2016, 116, 11685–11796. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhuang, X.M.; Huang, W.; Su, M.Y.; Feng, L.W.; Swick, S.M.; Wang, G.; Chen, Y.; Yu, J.S.; Guo, X.G.; et al. π-Extended Naphthalene Diimide Derivatives for n-Type Semiconducting Polymers. Chem. Mater. 2020, 32, 5317–5326. [Google Scholar] [CrossRef]
- West, S.M.; Tran, D.K.; Guo, J.J.; Chen, S.E.; Ginger, D.S.; Jenekhe, S.A. Phenazine-Substituted Poly(benzimidazobenzophenanthrolinedione): Electronic Structure, Thin Film Morphology, Electron Transport, and Mechanical Properties of an n-Type Semiconducting Ladder Polymer. Macromolecules 2023, 56, 2081–2091. [Google Scholar] [CrossRef]
- Hu, B.L.; Zhang, K.; An, C.; Pisula, W.; Baumgarten, M. Thiadiazoloquinoxaline-Fused Naphthalenediimides for n-Type Organic Field-Effect Transistors (OFETs). Org. Lett. 2017, 19, 6300–6303. [Google Scholar] [CrossRef]
- Hu, B.L.; Li, M.M.; Chen, W.Q.; Wan, X.J.; Chen, Y.S.; Zhang, Q.C. Novel donor-acceptor polymers based on 7-perfluorophenyl-6-[1, 2, 5]thiadiazole [3, 4-]-benzoimidazole for bulk heterojunction solar cells. RSC Adv. 2015, 5, 50137–50145. [Google Scholar] [CrossRef]
- Watanabe, N.; He, W.; Nozaki, N.; Matsumoto, H.; Michinobu, T. Benzothiadiazole versus Thiazolobenzotriazole: A Structural Study of Electron Acceptors in Solution-Processable Organic Semiconductors. Chem. Asian J. 2022, 17, e2200768. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.J.; Duan, X.W.; Zheng, R.; Xie, F.L.; Chen, L.J.; Zhao, Z.; Han, R.J.; Lei, Z.; Hu, J.Y. Two Isomeric Azulene-Decorated Naphthodithiophene Diimide-based Triads: Molecular Orbital Distribution Controls Polarity Change of OFETs Through Connection Position. ACS Appl. Mater. Interfaces 2020, 12, 23225–23235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Zhang, W.F.; Huang, J.Y.; Gao, D.; Wei, C.Y.; Lin, Z.Z.; Wang, L.P.; Yu, G. Fluorinated Dithienylethene-Naphthalenediimide Copolymers for High-Mobility n-Channel Field-Effect Transistors. Macromolecules 2017, 50, 6098–6107. [Google Scholar] [CrossRef]
- Hahn, R.; Bohle, F.; Fang, W.W.; Walther, A.; Grimme, S.; Esser, B. Raising the Bar in Aromatic Donor-Acceptor Interactions with Cyclic Trinuclear Gold(I) Complexes as Strong π-Donors. J. Am. Chem. Soc. 2018, 140, 17932–17944. [Google Scholar] [CrossRef]
- An, C.; Zhou, S.; Baumgarten, M. Condensed Derivatives of Thiadiazoloquinoxaline as Strong Acceptors. Cryst. Growth Des. 2015, 15, 1934–1938. [Google Scholar] [CrossRef]
- Pauling, L. The nature of the chemical bond. II. The one-electron bond and the three-electron bond. J. Am. Chem. Soc. 1931, 53, 3225–3237. [Google Scholar] [CrossRef]
- Pauling, L. The nature of the chemical bond IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 1932, 54, 3570–3582. [Google Scholar] [CrossRef]
- Clar, E. The simple principle of decomposition of aromatic hydrogen carbons and their absorption spectra (Aromatic hydrogen carbons, 20. Announcement). Berichte Dtsch. Chem. Ges. 1936, 69, 607–614. [Google Scholar] [CrossRef]
- Qian, G.; Zhong, Z.; Luo, M.; Yu, D.; Zhang, Z.; Ma, D.; Wang, Z.Y. Synthesis and Application of Thiadiazoloquinoxaline-Containing Chromophores as Dopants for Efficient Near-Infrared Organic Light-Emitting Diodes. J. Phys. Chem. C 2009, 113, 1589–1595. [Google Scholar] [CrossRef]
- Sarkar, A.; Dhiman, S.; Chalishazar, A.; George, S.J. Visualization of Stereoselective Supramolecular Polymers by Chirality-Controlled Energy Transfer. Angew. Chem. Int. Ed. 2017, 56, 13767–13771. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Li, J.; Xiao, Y.; Li, Z.; Qian, X.H. Core-Perfluoroalkylated Perylene Diimides and Naphthalene Diimides: Versatile Synthesis, Solubility, Electrochemistry, and Optical Properties. J. Org. Chem. 2010, 75, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Wallingford, CT, USA, 2016; Available online: https://gaussian.com/gaussian16/ (accessed on 8 May 2024).
- Becke, A.D. A New Mixing of Hartree-Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Romele, P.; Gkoupidenis, P.; Koutsouras, D.A.; Lieberth, K.; Kovács-Vajna, Z.M.; Blom, P.W.M.; Torricelli, F. Multiscale real time and high sensitivity ion detection with complementary organic electrochemical transistors amplifier. Nat. Commun. 2020, 11, 3743–3754. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Lu, T.; Chen, Q.X. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model 1996, 14, 33–38. [Google Scholar] [CrossRef]

| a | (eV) b | EA (eV) c | ELUMO (eV) d | EHOMO (eV) d | |
|---|---|---|---|---|---|
| BTI-NDI-BTI-a | −0.63 | 1.95 | 3.94 | −3.57 | −5.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Chen, B.; Zhang, F. Strong Acceptors Based on Derivatives of Benzothiadiazoloimidazole. Molecules 2024, 29, 2262. https://doi.org/10.3390/molecules29102262
Du H, Chen B, Zhang F. Strong Acceptors Based on Derivatives of Benzothiadiazoloimidazole. Molecules. 2024; 29(10):2262. https://doi.org/10.3390/molecules29102262
Chicago/Turabian StyleDu, Hanyun, Bin Chen, and Fengyuan Zhang. 2024. "Strong Acceptors Based on Derivatives of Benzothiadiazoloimidazole" Molecules 29, no. 10: 2262. https://doi.org/10.3390/molecules29102262
APA StyleDu, H., Chen, B., & Zhang, F. (2024). Strong Acceptors Based on Derivatives of Benzothiadiazoloimidazole. Molecules, 29(10), 2262. https://doi.org/10.3390/molecules29102262





