Insights into the Origin of Activity Enhancement via Tuning Electronic Structure of Cu2O towards Electrocatalytic Ammonia Synthesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
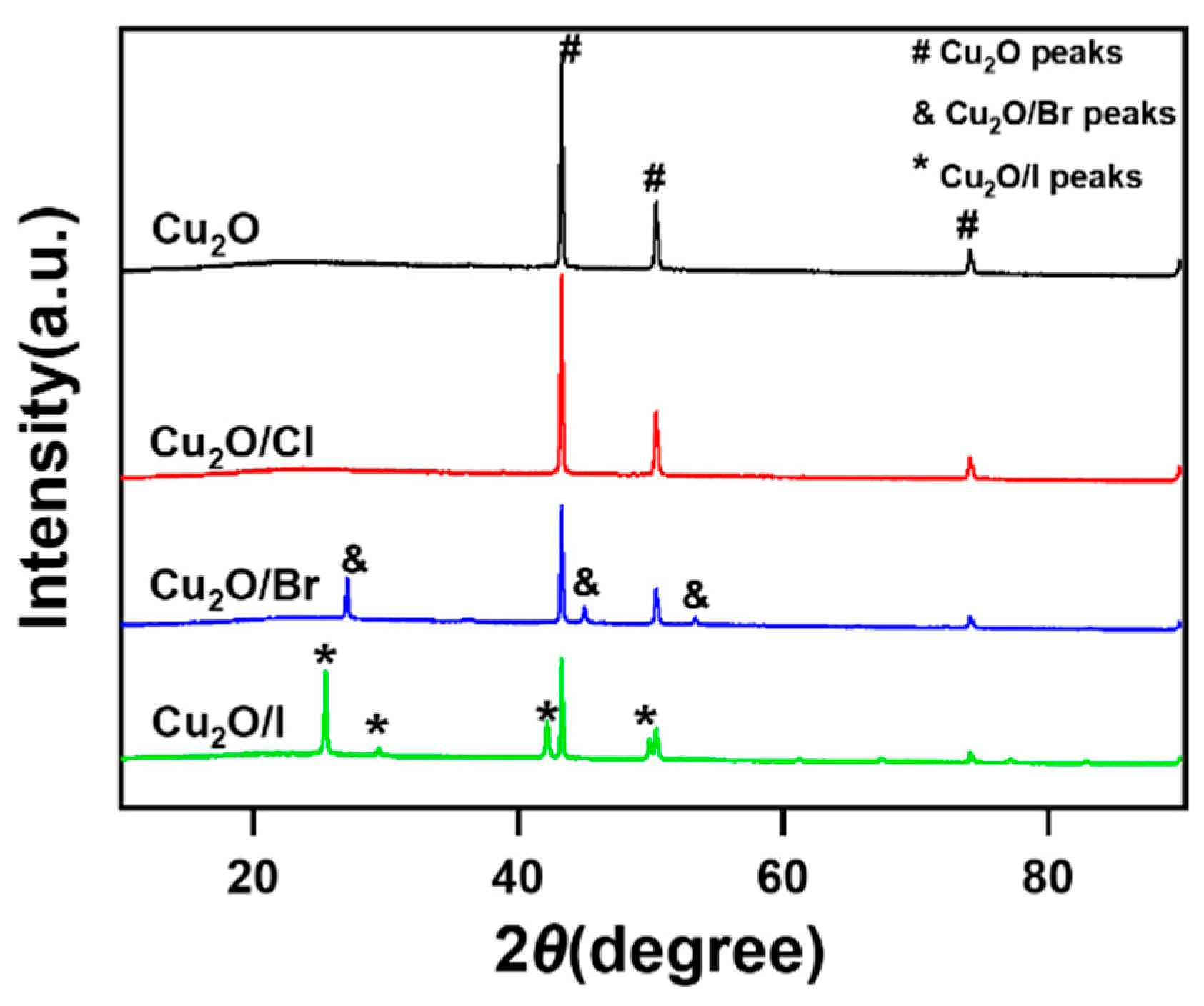
2.1.1. XRD Analysis
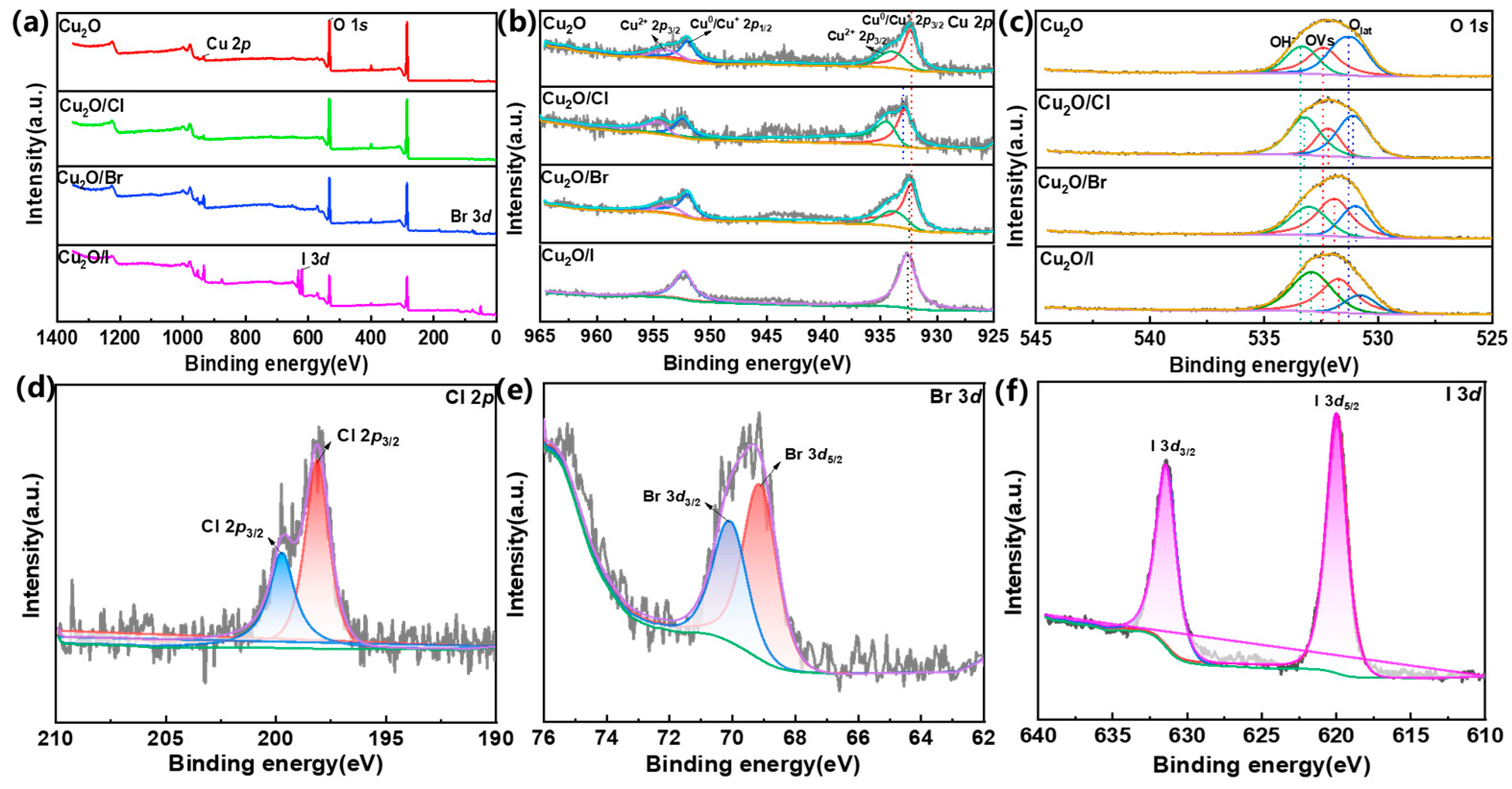
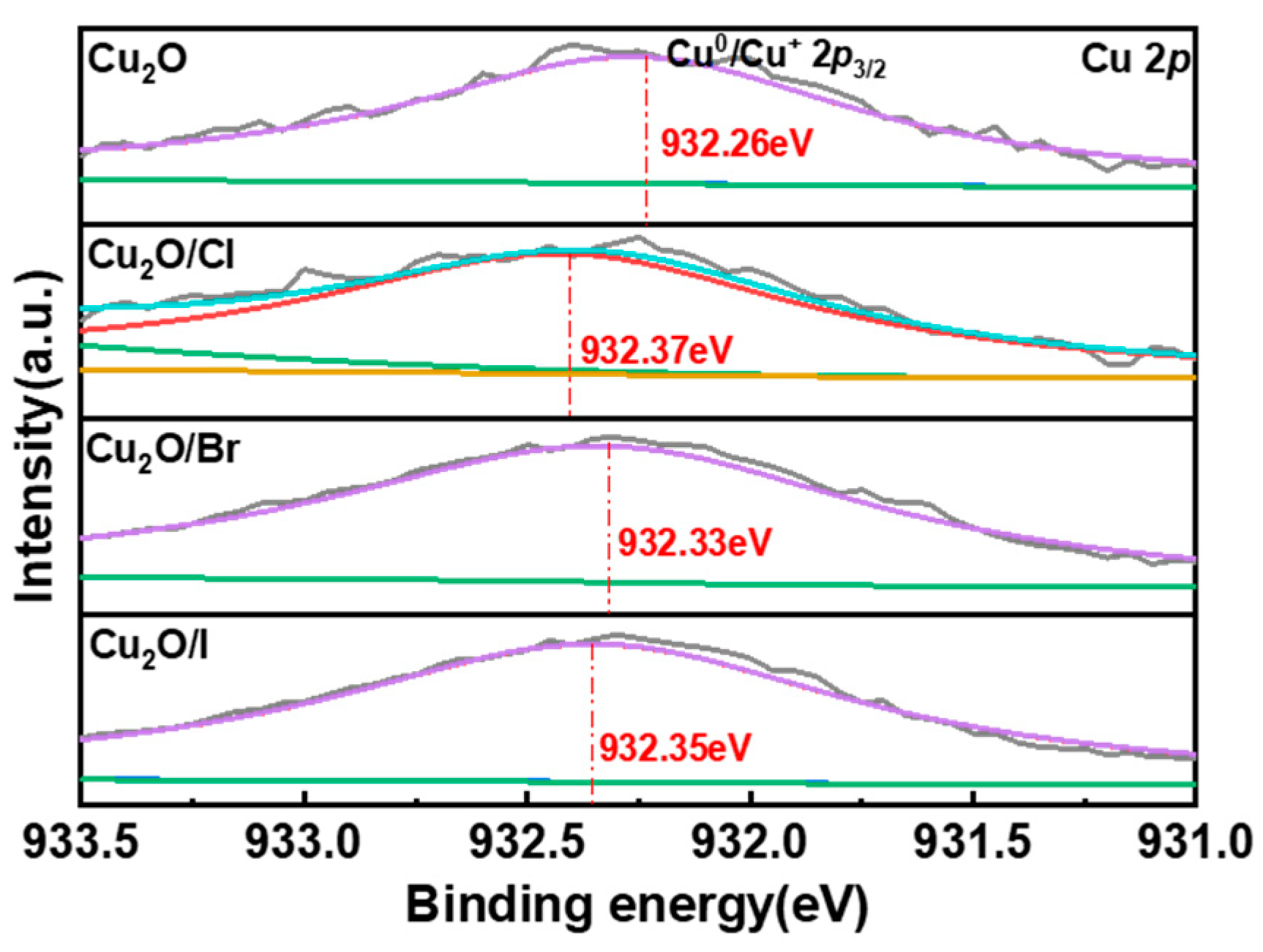
2.1.2. XPS Analysis
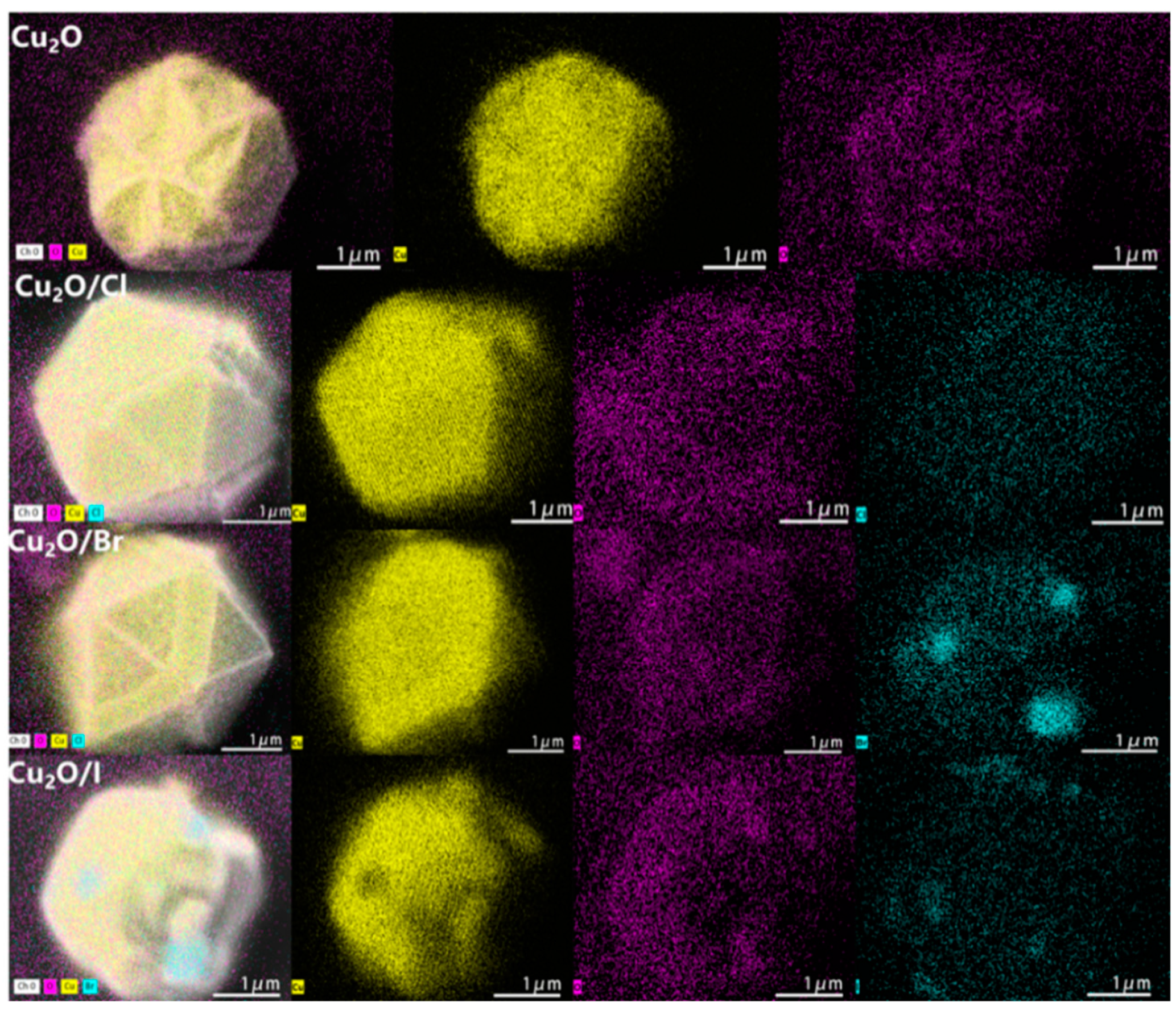
2.1.3. SEM Mapping Analysis
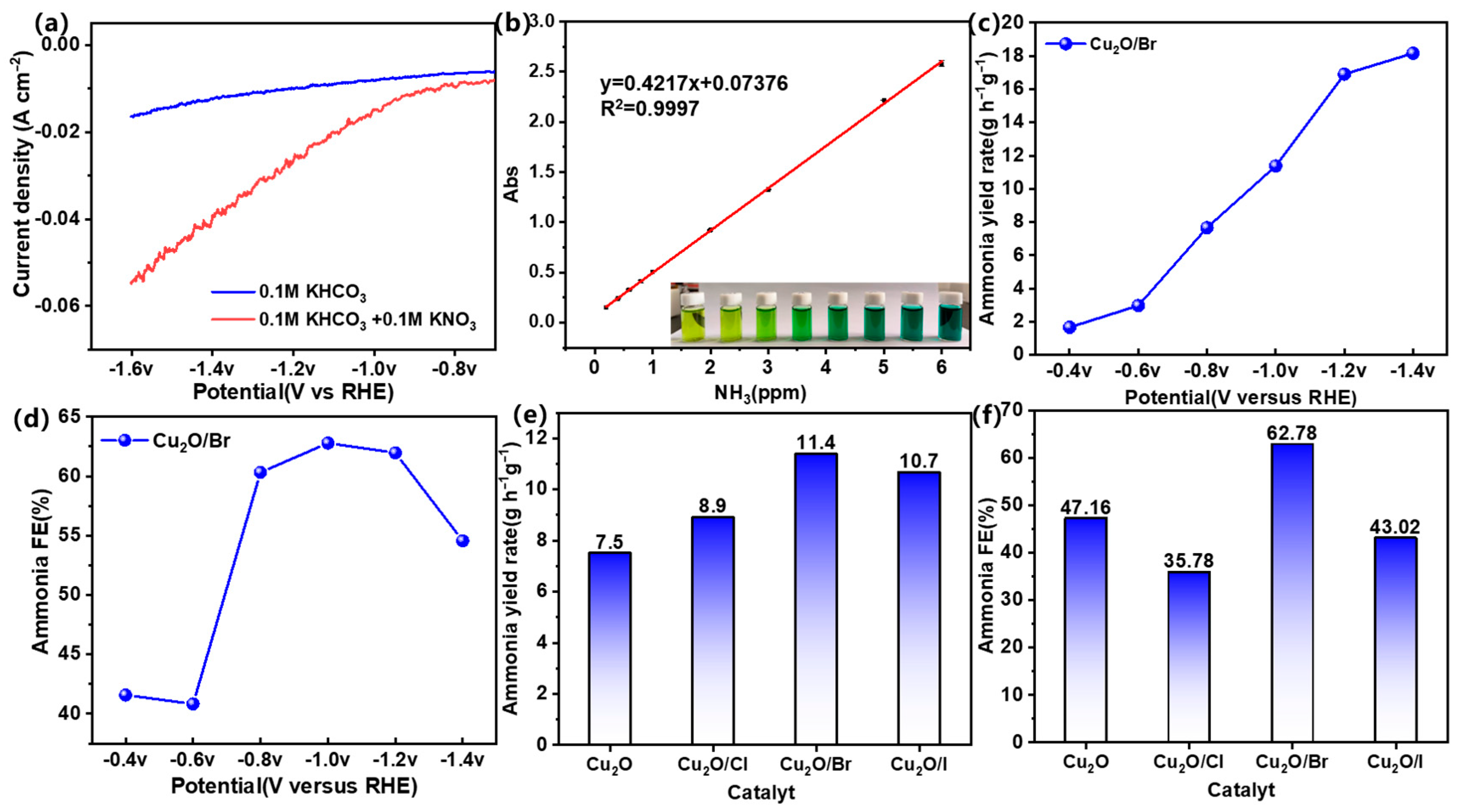
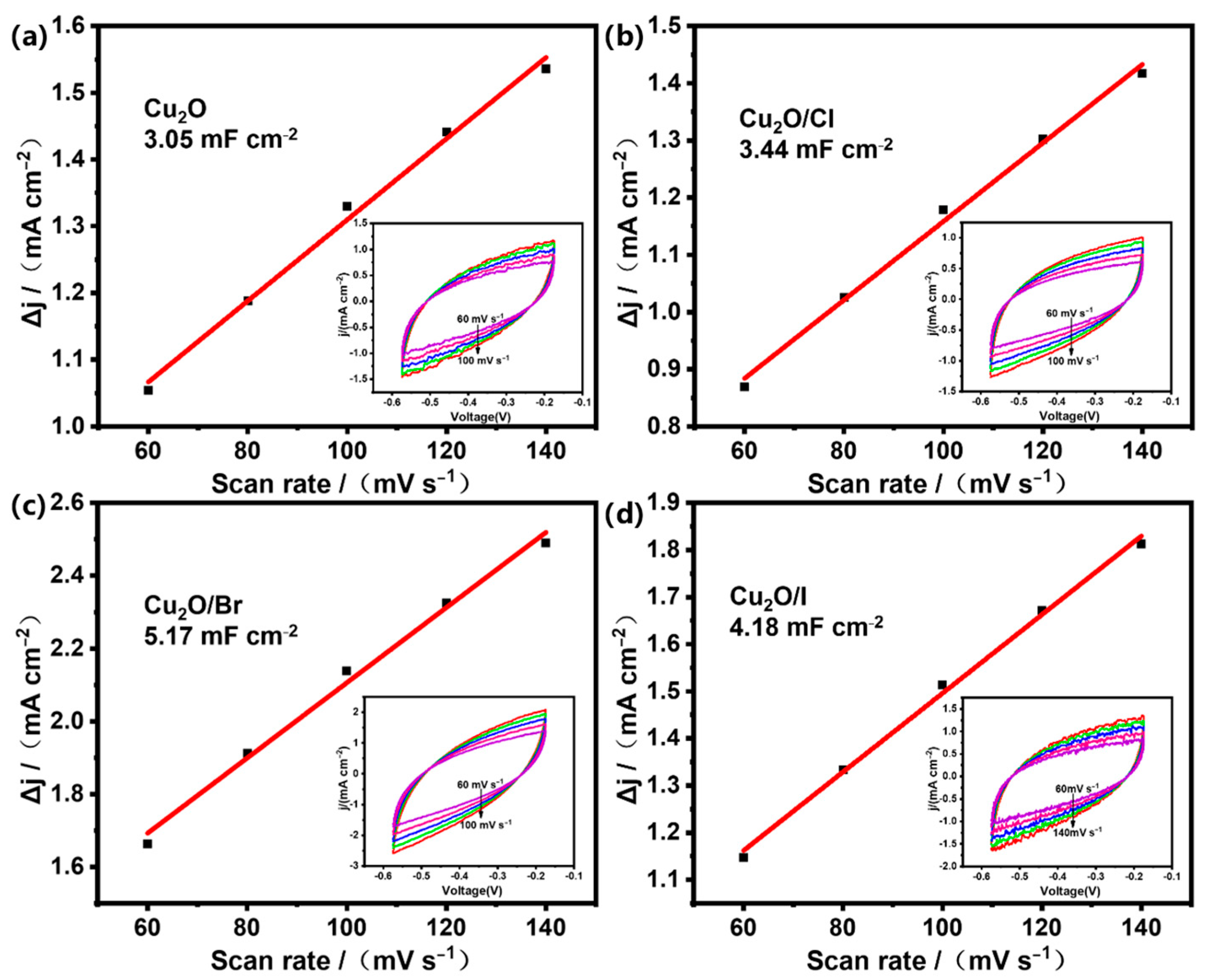
2.2. Electrocatalytic Performance
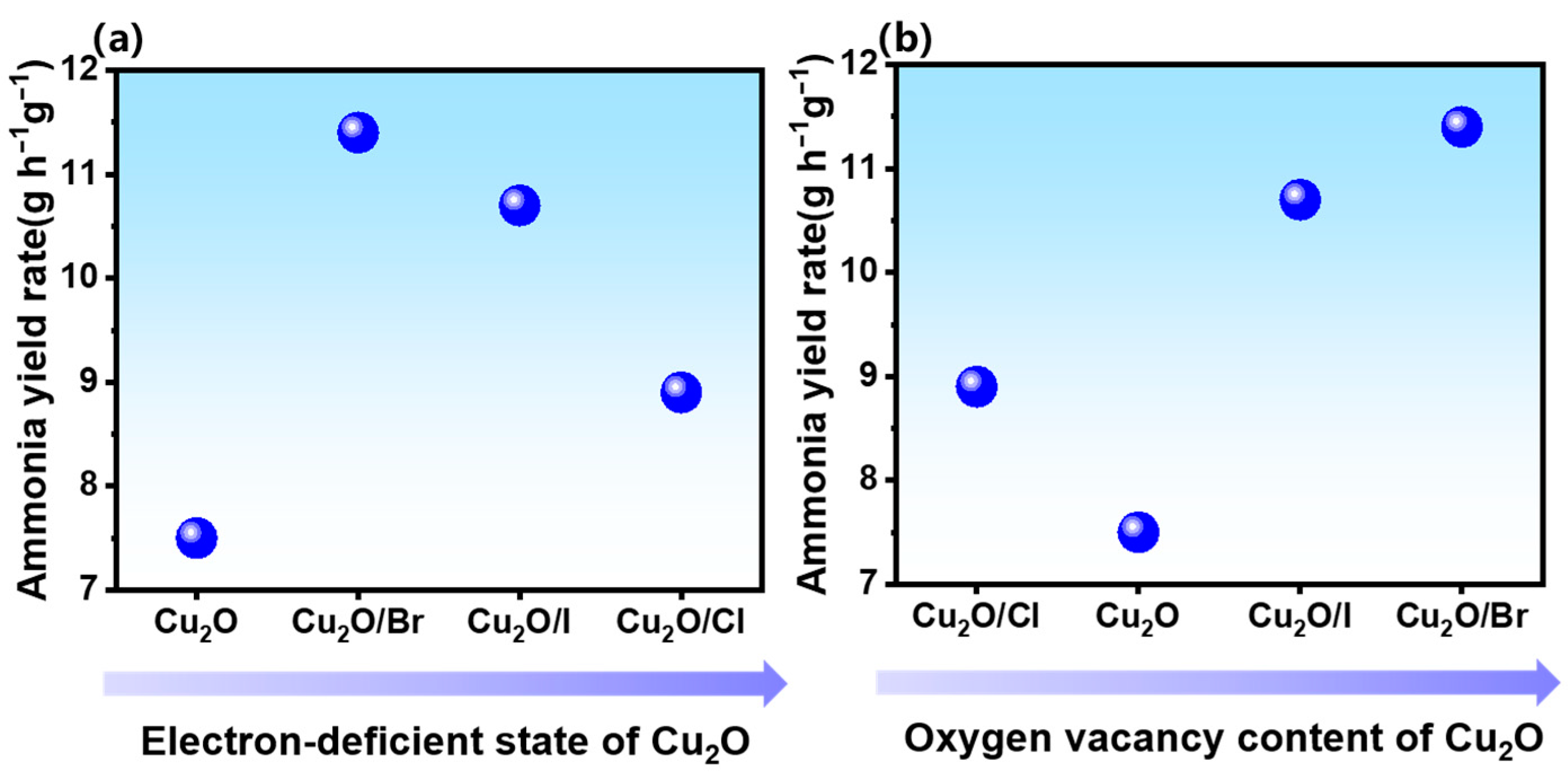
2.3. Relationship between Electronic Structure and Activity of Catalysts
3. Materials and Method
3.1. Reagents
3.2. Synthesis of Cuprous Oxide (Cu2O) and Cu2O/X (X = Cl, Br, I)
3.3. Materials Characterization
3.4. Electrochemical Measurements
3.5. Product Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Tu, X.; Wei, X.; Wang, D.; Zhang, X.; Chen, W.; Chen, C.; Wang, S. C-Bound or O-Bound Surface: Which One Boosts Electrocatalytic Urea Synthesis? Angew. Chem. Int. Ed. 2023, 62, e202300387. [Google Scholar] [CrossRef] [PubMed]
- Légaré, M.-A.; Bélanger-Chabot, G.; Dewhurst, R.D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen fixation and reduction at boron. Science 2018, 359, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xia, M.; Wang, H.; Huang, K.; Qian, C.; Maravelias, C.T.; Ozin, G.A. Greening ammonia toward the solar ammonia refinery. Joule 2018, 2, 1055–1074. [Google Scholar] [CrossRef]
- Guo, C.; Ran, J.; Vasileff, A.; Qiao, S.-Z. Rational design of electrocatalysts and photo (electro) catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energ. Environ. Sci. 2018, 11, 45–56. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.-Y. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Park, J.; Chen, Y.; Qiu, Y.; Cheng, Y.; Srivastava, K.; Gu, S.; Shanks, B.H.; Roling, L.T.; Li, W. Electrocatalytic nitrate reduction on oxide-derived silver with tunable selectivity to nitrite and ammonia. ACS Catal. 2021, 11, 8431–8442. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Thonemann, N.; Pizzol, M. Consequential life cycle assessment of carbon capture and utilization technologies within the chemical industry. Energy Environ. Sci. 2019, 12, 2253–2263. [Google Scholar] [CrossRef]
- Li, W.; Li, K.; Ye, Y.; Zhang, S.; Liu, Y.; Wang, G.; Liang, C.; Zhang, H.; Zhao, H. Efficient electrocatalytic nitrogen reduction to ammonia with aqueous silver nanodots. Commun. Chem. 2021, 4, 10. [Google Scholar] [CrossRef]
- Shipman, M.A.; Symes, M.D. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef]
- Deng, J.; Iñiguez, J.A.; Liu, C. Electrocatalytic nitrogen reduction at low temperature. Joule 2018, 2, 846–856. [Google Scholar] [CrossRef]
- Suryanto, B.H.; Du, H.-L.; Wang, D.; Chen, J.; Simonov, A.N.; MacFarlane, D.R. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2019, 2, 290–296. [Google Scholar] [CrossRef]
- Ouyang, L.; Liang, J.; Luo, Y.; Zheng, D.; Sun, S.; Liu, Q.; Hamdy, M.S.; Sun, X.; Ying, B. Recent advances in electrocatalytic ammonia synthesis. Chin. J. Catal. 2023, 50, 6–44. [Google Scholar] [CrossRef]
- Shao, J.; Jing, H.; Wei, P.; Fu, X.; Pang, L.; Song, Y.; Ye, K.; Li, M.; Jiang, L.; Ma, J. Electrochemical synthesis of ammonia from nitric oxide using a copper-tin alloy catalyst. Nat. Energy 2023, 8, 1273–1283. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, W.; Wang, P.; Li, R.; Liu, K.; Omer, K.M.; Jin, Z.; Li, P. Pyridine-N-rich Cu single-atom catalyst boosts nitrate electroreduction to ammonia. Appl. Catal. B-Environ. Energy 2024, 340, 123228. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Zheng, Q.-Z.; Lou, Y.-Y.; Zhao, K.-M.; Hu, S.-N.; Li, G.; Akdim, O.; Huang, X.-Y.; Sun, S.-G. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 2022, 13, 7899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, L.; Yang, Y.; Liu, D.; Peng, X.; Liang, S.; Jiang, L. Insights into electrocatalytic nitrate reduction to ammonia via Cu-based bimetallic catalysts. ACS Sustain. Chem. Eng. 2023, 11, 2468–2475. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Y.; Zhao, F.; Han, W.; Yuan, Q.; Kou, M.; Zhao, J.; Chen, C.; Wang, S. Identifying the facet-dependent active sites of Cu2O for selective C-N coupling toward electrocatalytic urea synthesis. Appl. Catal. B-Environ. Energy 2024, 340, 123265. [Google Scholar] [CrossRef]
- Li, S.; Sha, X.; Gao, X.; Peng, J. Al-Doped Octahedral Cu2O Nanocrystal for Electrocatalytic CO2 Reduction to Produce Ethylene. Int. J. Mol. Sci. 2023, 2, 12680. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.; Tian, B.; Gao, X.; Han, L.; Zhong, Y.; Song, S.; Wang, Z.; Li, Y.; Gui, J. Synergistic Cu+/Cu0 on Cu2O-Cu interfaces for efficient and selective C2+ production in electrocatalytic CO2 conversion. Sci. China Mater. 2023, 66, 1801–1809. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Xie, B.; Ni, Z.; Xia, S. Br doping promotes the transform of Cu2O (100) to Cu2O (111) and facilitates efficient photocatalytic degradation of tetracycline. Mol. Catal. 2023, 548, 113431. [Google Scholar] [CrossRef]
- Zhou, Y.; Ganganahalli, R.; Verma, S.; Tan, H.R.; Yeo, B.S. Production of C3–C6 acetate esters via CO electroreduction in a membrane electrode assembly cell. Angew. Chem. Int. Ed. 2022, 61, e202202859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, L.; Bai, G.; Lan, X. Engineering single Cu sites into covalent organic framework for selective photocatalytic CO2 reduction. Small 2023, 19, 2300035. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Zhang, H.; Gao, X.; Wang, X.; Wang, S.; Tang, Z.; Li, S.; Nie, K.; Xie, J. Deciphering the Stability Mechanism of Cu Active Sites in CO2 Electroreduction via Suppression of Antibonding Orbital Occupancy in the O 2p-Cu 3d Hybridization. ACS Catal. 2024, 14, 1351–1362. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, M.; Dong, Q.; Gao, Q.; Cai, X.; Zhang, S.; Yuan, T.; Peng, F.; Fang, Y.; Yang, S. Photoinduced Cu+/Cu2+ interconversion for enhancing energy conversion and storage performances of CuO based Li-ion battery. J. Energy Chem. 2023, 79, 83–91. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Xu, M.; Liu, X.; Qi, H.; Zhang, L.; Yang, X.; Niu, S.; Zhou, D.; Liu, Y. Dynamic behavior of single-atom catalysts in electrocatalysis: Identification of Cu-N3 as an active site for the oxygen reduction reaction. J. Am. Chem. Soc. 2021, 143, 14530–14539. [Google Scholar] [CrossRef]
- Teng, Z.; Yi, X.; Zhang, C.; He, C.; Yang, Y.; Hao, Q.; Dou, B.; Bin, F. CO self-sustained catalytic combustion over morphological inverse model CeO2/Cu2O catalysts exposing (100),(111) and (110) planes. Appl. Catal. B-Environ. Energy 2023, 339, 123119. [Google Scholar] [CrossRef]
- Sun, D.; Li, Z.; Huang, S.; Chi, J.; Zhao, S. Efficient mercury removal in chlorine-free flue gas by doping Cl into Cu2O nanocrystals. J. Hazard. Mater. 2021, 419, 126423. [Google Scholar] [CrossRef]
- Nie, J.; Yu, X.; Liu, Z.; Zhang, J.; Ma, Y.; Chen, Y.; Ji, Q.; Zhao, N.; Chang, Z. Energy band reconstruction mechanism of Cl-doped Cu2O and photocatalytic degradation pathway for levofloxacin. J. Clean. Prod. 2022, 363, 132593. [Google Scholar] [CrossRef]
- Benaissa, M.; Abdelkader, H.S.; Merad, G. Electronic and optical properties of halogen (H= F, Cl, Br)-doped Cu2O by hybrid density functional simulations. Optik 2020, 207, 164440. [Google Scholar] [CrossRef]
- Bouaziz, L.; Dubus, M.; Si-Ahmed, K.; Kerdjoudj, H.; Özacar, M.; Bessekhouad, Y. Effectiveness of I-doped ZnO as polyvalent material for both anti-bacterial and photocatalytic treatments. J. Mol. Struct. 2022, 1255, 132391. [Google Scholar] [CrossRef]
- Okoye, P.; Azi, S.; Qahtan, T.; Owolabi, T.; Saleh, T. Synthesis, properties, and applications of doped and undoped CuO and Cu2O nanomaterials. Mater. Today Chem. 2023, 30, 101513. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, G.F.; Savateev, O.; Xue, J.; Ding, L.X.; Liang, Z.; Antonietti, M.; Wang, H. Enabled efficient ammonia synthesis and energy supply in a zinc–nitrate battery system by separating nitrate reduction process into two stages. Angew. Chem. Int. Ed. 2023, 62, e202218717. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.D.; Mahajani, S.M.; Suresh, A.; Sarkar, A. Electrochemical reduction of CO2 on activated copper: Influence of surface area. Mater. Res. Bull. 2020, 123, 110702. [Google Scholar] [CrossRef]
- Martínez-Hincapié, R.; Wegner, J.; Anwar, M.U.; Raza-Khan, A.; Franzka, S.; Kleszczynski, S.; Čolić, V. The determination of the electrochemically active surface area and its effects on the electrocatalytic properties of structured nickel electrodes produced by additive manufacturing. Electrochim. Acta 2024, 476, 143663. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, Y.; Huang, J.; Chen, S. A combinatorial descriptor for volcano relationships of electrochemical nitrogen reduction reaction. Chin. J. Catal. 2022, 43, 2881–2888. [Google Scholar] [CrossRef]
- Chen, J.; Ji, Y. Locating the cocktail and scaling-relation breaking effects of high-entropy alloy catalysts on the electrocatalytic volcano plot. Chin. J. Catal. 2022, 43, 2889–2897. [Google Scholar] [CrossRef]
- Liu, X.; Kumar, P.V.; Chen, Q.; Zhao, L.; Ye, F.; Ma, X.; Liu, D.; Chen, X.; Dai, L.; Hu, C. Carbon nanotubes with fluorine-rich surface as metal-free electrocatalyst for effective synthesis of urea from nitrate and CO2. Appl. Catal. B-Environ. Energy 2022, 316, 121618. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S.; Liu, H.; Liu, S.; Yuan, Y.; Meng, Y.; Wang, M.; Shen, C.; Peng, Q.; Chen, J. Breaking local charge symmetry of iron single atoms for efficient electrocatalytic nitrate reduction to ammonia. Angew. Chem. Int. Edit. 2023, 62, e202308044. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, C.; Bai, X.; Wang, Z.; Yu, X.; Tong, X.; Wang, Z.; Zhang, H.; Pang, H.; Zhou, L. Facile synthesis of carbon nanobelts decorated with Cu and Pd for nitrate electroreduction to ammonia. ACS Appl. Mater. Interfaces 2022, 14, 30969–30978. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, K.; Chen, L.; Wang, X.; Zhao, Q.; Liu, B.; Ye, Z. Achieving high selectivity for nitrate electrochemical reduction to ammonia over MOF-supported RuxOy clusters. J. Mater. Chem. 2022, 10, 3963–3969. [Google Scholar] [CrossRef]
- Lei, F.; Xu, W.; Yu, J.; Li, K.; Xie, J.; Hao, P.; Cui, G.; Tang, B. Electrochemical synthesis of ammonia by nitrate reduction on indium incorporated in sulfur-doped graphene. Chem. Eng. J. 2021, 426, 131317. [Google Scholar] [CrossRef]
- Jiang, M.; Su, J.; Song, X.; Zhang, P.; Zhu, M.; Qin, L.; Tie, Z.; Zuo, J.-L.; Jin, Z. Interfacial reduction nucleation of noble metal nanodots on redox-active metal-organic frameworks for high-efficiency electrocatalytic conversion of nitrate to ammonia. Nano Lett. 2022, 22, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Ren, K.; Wang, M.; Liu, M.; Wang, Z.; Wang, H.; Li, X.; Wang, L.; Xu, Y. Concave-convex surface oxide layers over copper nanowires boost electrochemical nitrate-to-ammonia conversion. Chem. Eng. J. 2021, 426, 130759. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Ren, K.; Ren, T.; Liu, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Atomic defects in pothole-rich two-dimensional copper nanoplates triggering enhanced electrocatalytic selective nitrate-to-ammonia transformation. J. Mater. Chem. 2021, 9, 16411–16417. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Zheng, M.; Jin, X.; Shen, Z.; Li, Z.; Wang, Y.; Wang, Q.; Wang, X.; Wei, H. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 14, 3634. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhong, W.; He, Z.; Liu, Q.; Chen, H.; Zhou, D.; Zhang, N.; Kang, X.; Chen, Y. Regulating surface oxygen species on copper (I) oxides via plasma treatment for effective reduction of nitrate to ammonia. Appl. Catal. B-Environ. Energy 2022, 305, 121021. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, H.; Zhang, H.; Zhang, Y.; Shi, P.; Qu, K.; Cheng, S.-B.; Wang, A.-L.; Lu, Q. Filling mesopores of conductive metal-organic frameworks with Cu clusters for selective nitrate reduction to ammonia. ACS Appl. Mater. Interfaces 2022, 14, 32176–32182. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, X.; Hu, X.; He, K.; Yu, Y.; Li, T.; Tu, Q.; Qian, X.; Yue, Q.; Wasielewski, M.R. Alternative route for electrochemical ammonia synthesis by reduction of nitrate on copper nanosheets. Appl. Mater. Today 2020, 19, 100620. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Zhang, B.; Yu, Y. Self-template synthesis of hierarchically structured Co3O4@ NiO bifunctional electrodes for selective nitrate reduction and tetrahydroisoquinolines semi-dehydrogenation. Sci. China Mater. 2020, 63, 2530–2538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, M.; Yuan, Y.; Zhao, R.; Wang, Y.; Zhao, J.; Yuan, Q.; Zhao, J. Insights into the Origin of Activity Enhancement via Tuning Electronic Structure of Cu2O towards Electrocatalytic Ammonia Synthesis. Molecules 2024, 29, 2261. https://doi.org/10.3390/molecules29102261
Kou M, Yuan Y, Zhao R, Wang Y, Zhao J, Yuan Q, Zhao J. Insights into the Origin of Activity Enhancement via Tuning Electronic Structure of Cu2O towards Electrocatalytic Ammonia Synthesis. Molecules. 2024; 29(10):2261. https://doi.org/10.3390/molecules29102261
Chicago/Turabian StyleKou, Meimei, Ying Yuan, Ruili Zhao, Youkui Wang, Jiamin Zhao, Qing Yuan, and Jinsheng Zhao. 2024. "Insights into the Origin of Activity Enhancement via Tuning Electronic Structure of Cu2O towards Electrocatalytic Ammonia Synthesis" Molecules 29, no. 10: 2261. https://doi.org/10.3390/molecules29102261
APA StyleKou, M., Yuan, Y., Zhao, R., Wang, Y., Zhao, J., Yuan, Q., & Zhao, J. (2024). Insights into the Origin of Activity Enhancement via Tuning Electronic Structure of Cu2O towards Electrocatalytic Ammonia Synthesis. Molecules, 29(10), 2261. https://doi.org/10.3390/molecules29102261





