Influence of Different Types of Surfactants on the Flotation of Natural Quartz by Dodecylamine
Abstract
1. Introduction
2. Results and Discussion
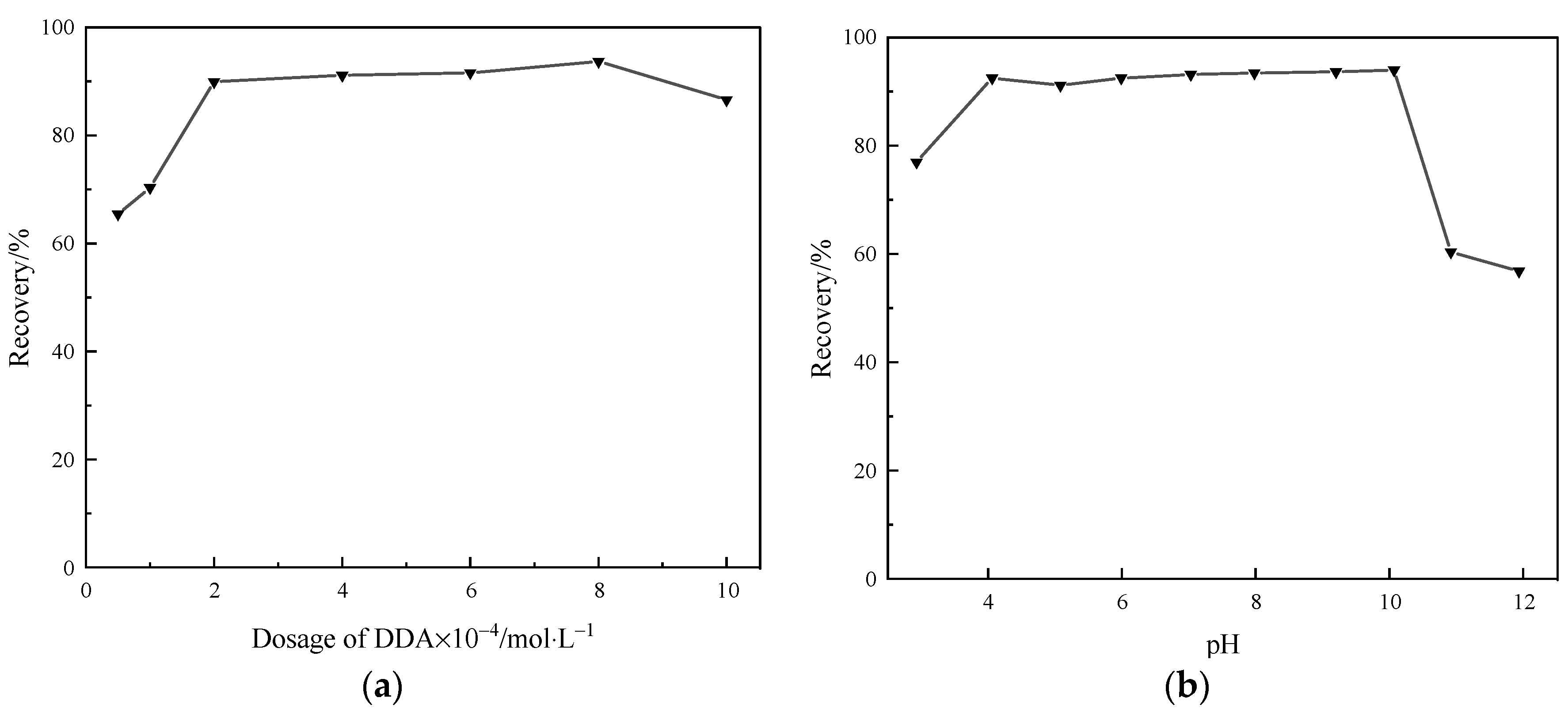
2.1. DDA Flotation Performance
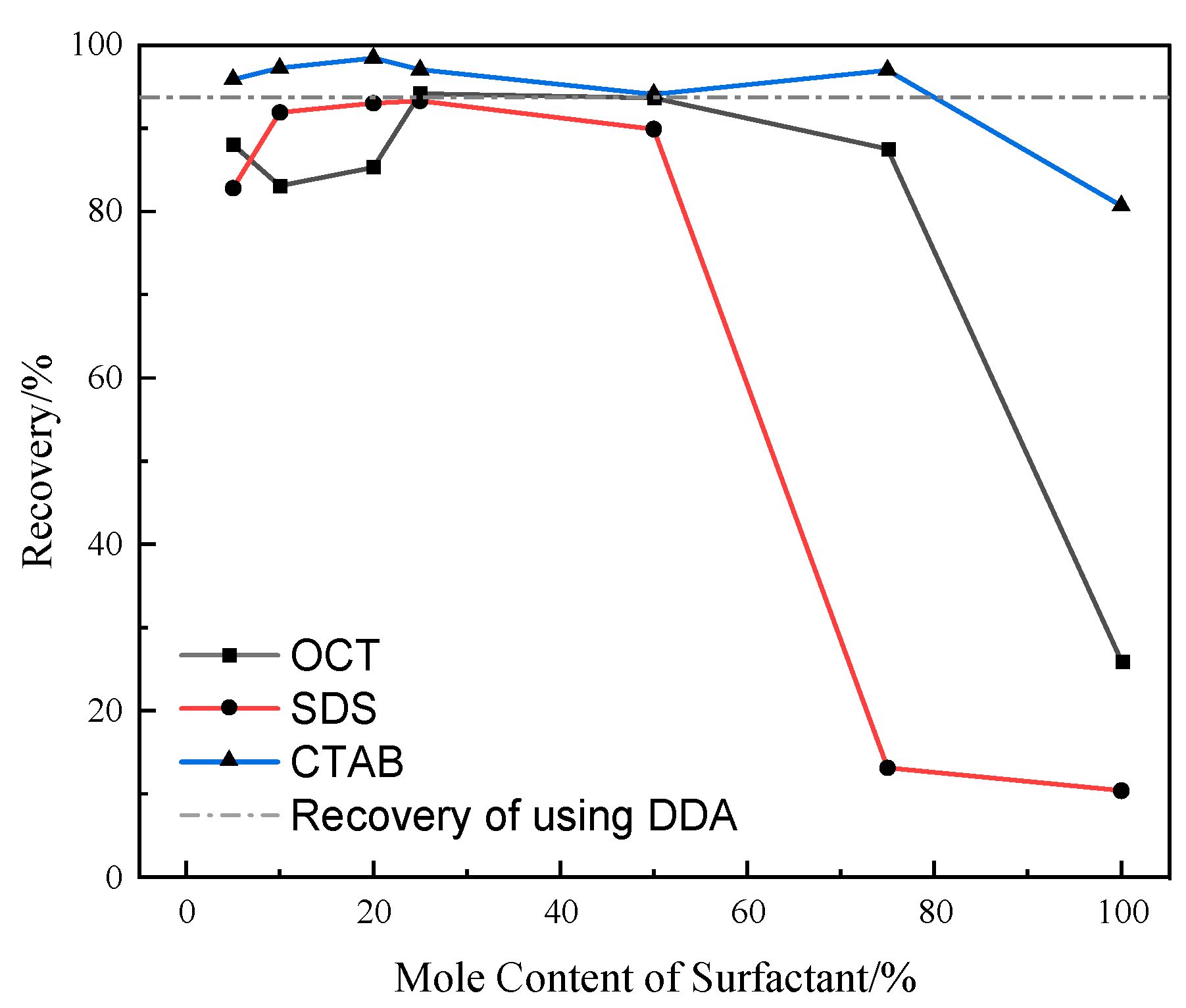
2.2. Combined Collector Flotation Performance
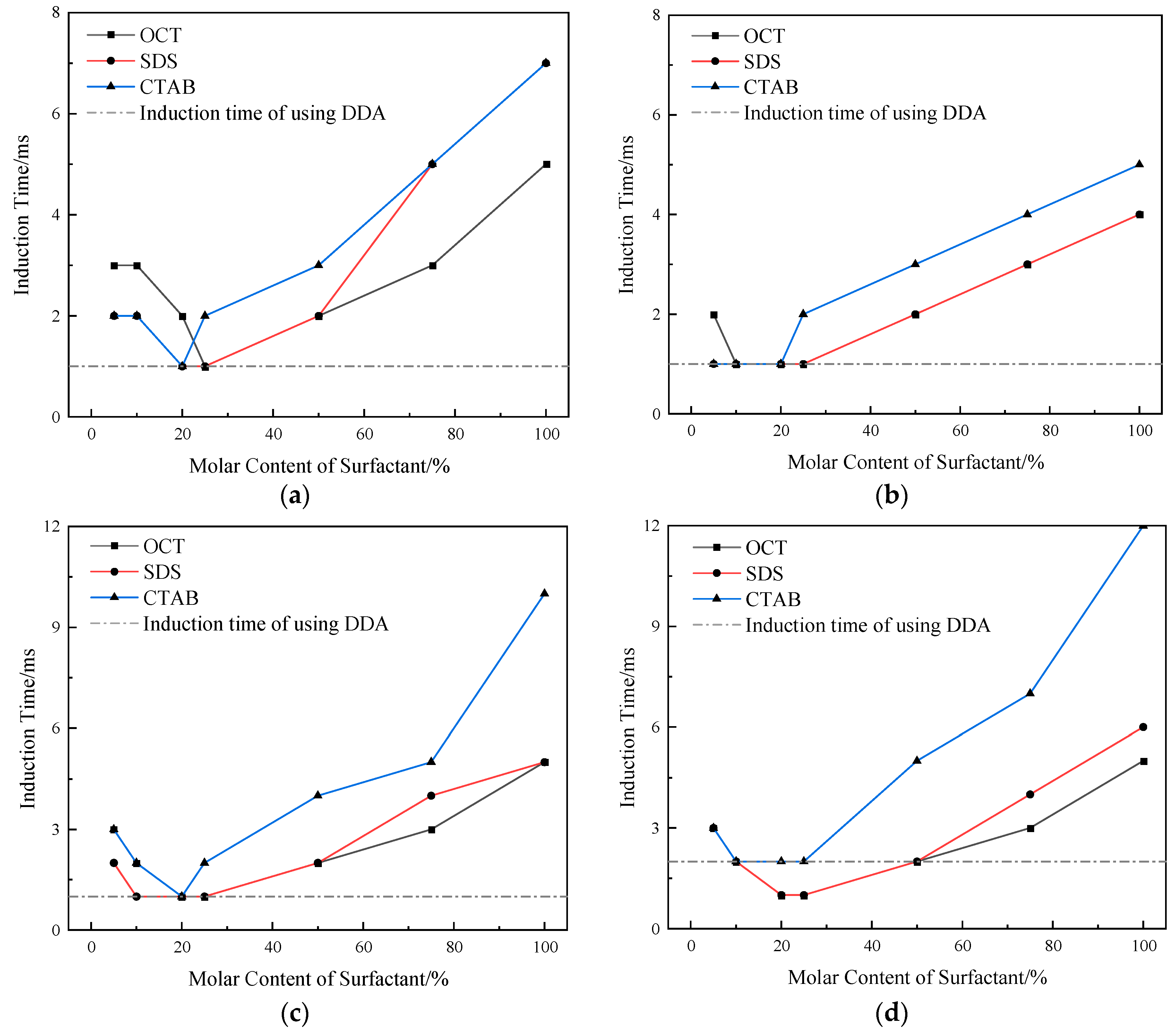
2.3. Induction Time of Bubble–Particle Adhesion
2.4. System Surface Tension
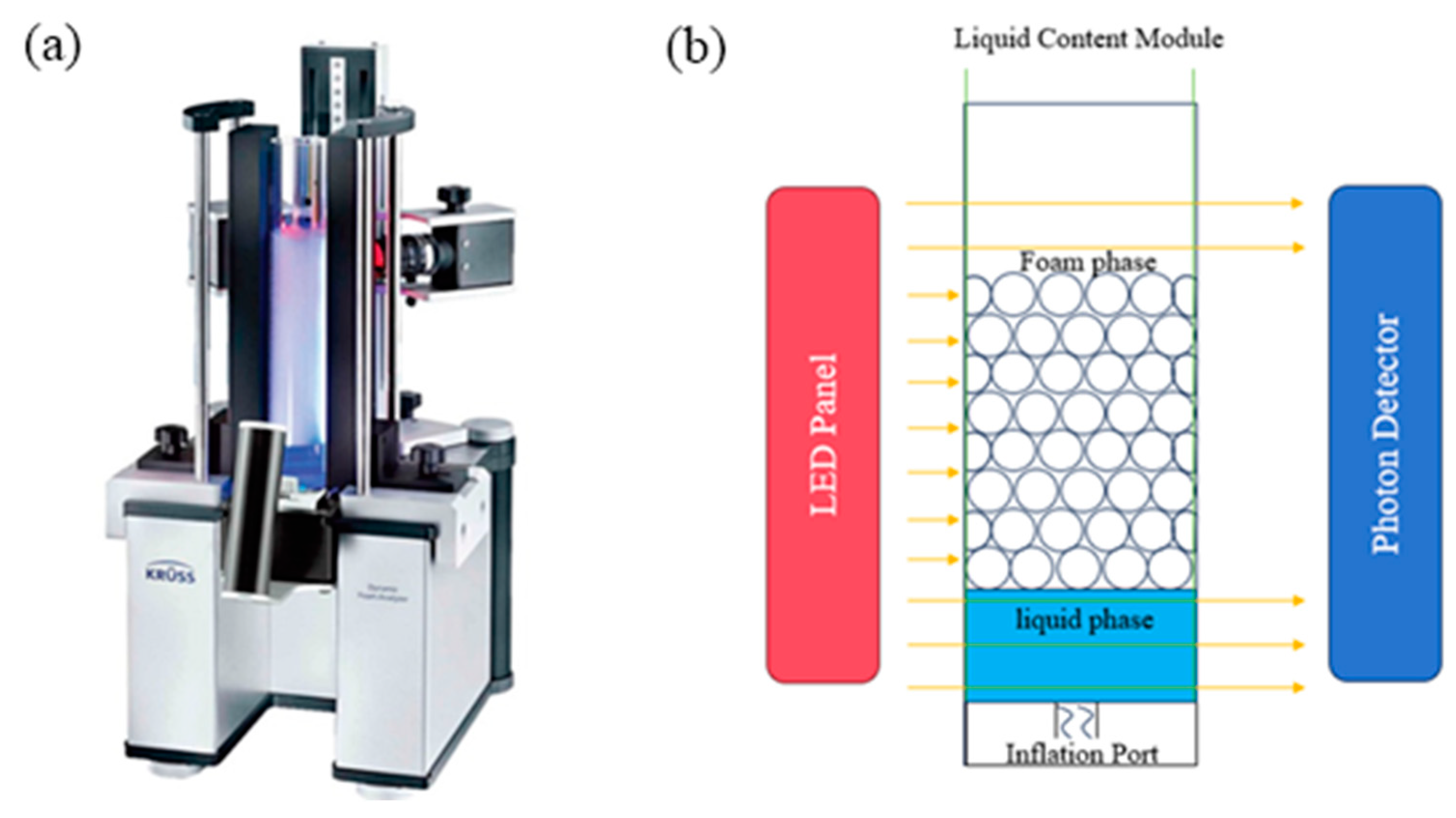
2.5. Properties of Two-Phase Foam
2.5.1. Foaming Properties
2.5.2. Foam Stability
2.5.3. Structure of Generated Foam
3. Materials and Methods
3.1. Materials
3.2. Reagents
3.3. Methods
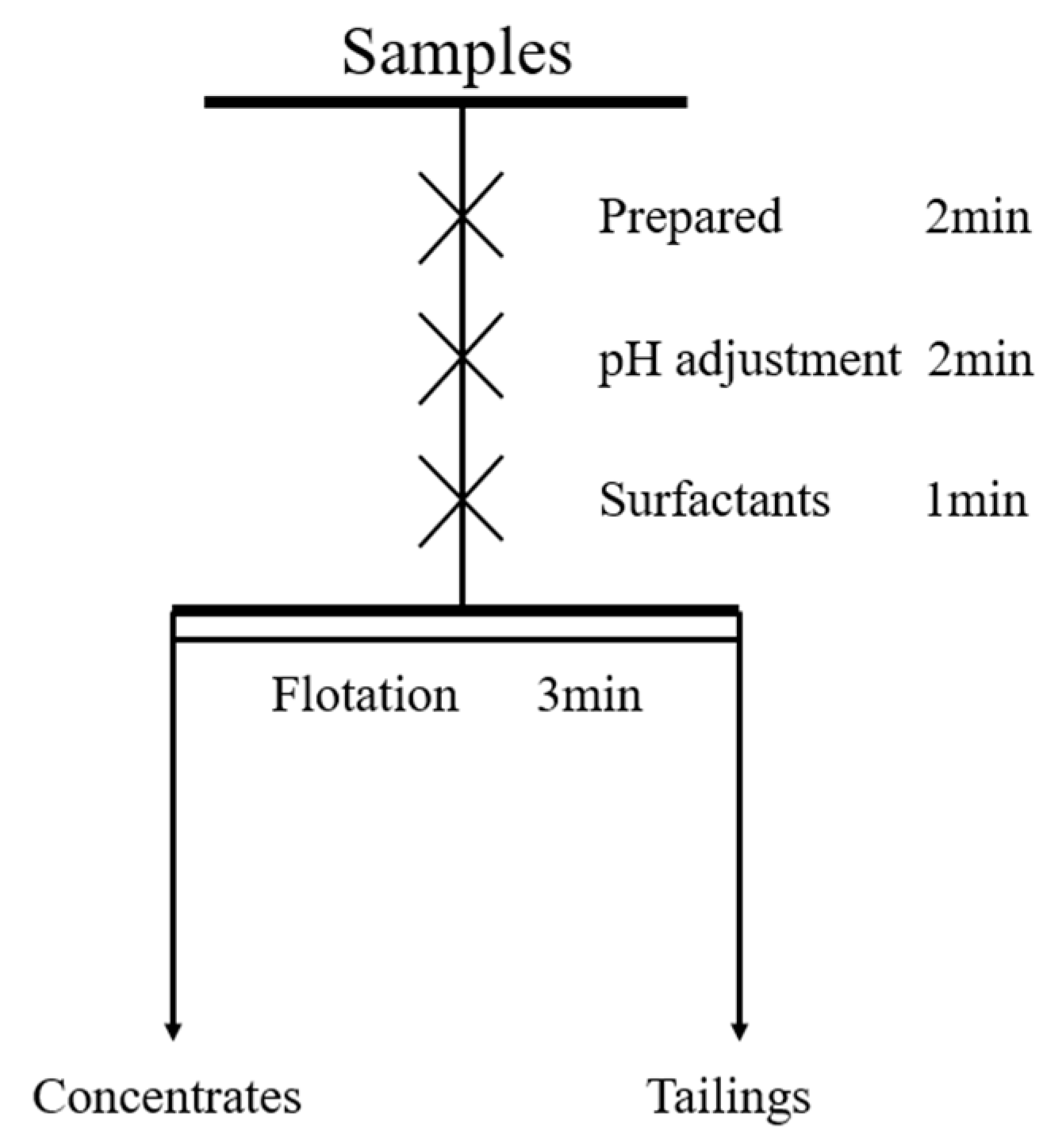
3.3.1. Flotation Experiments
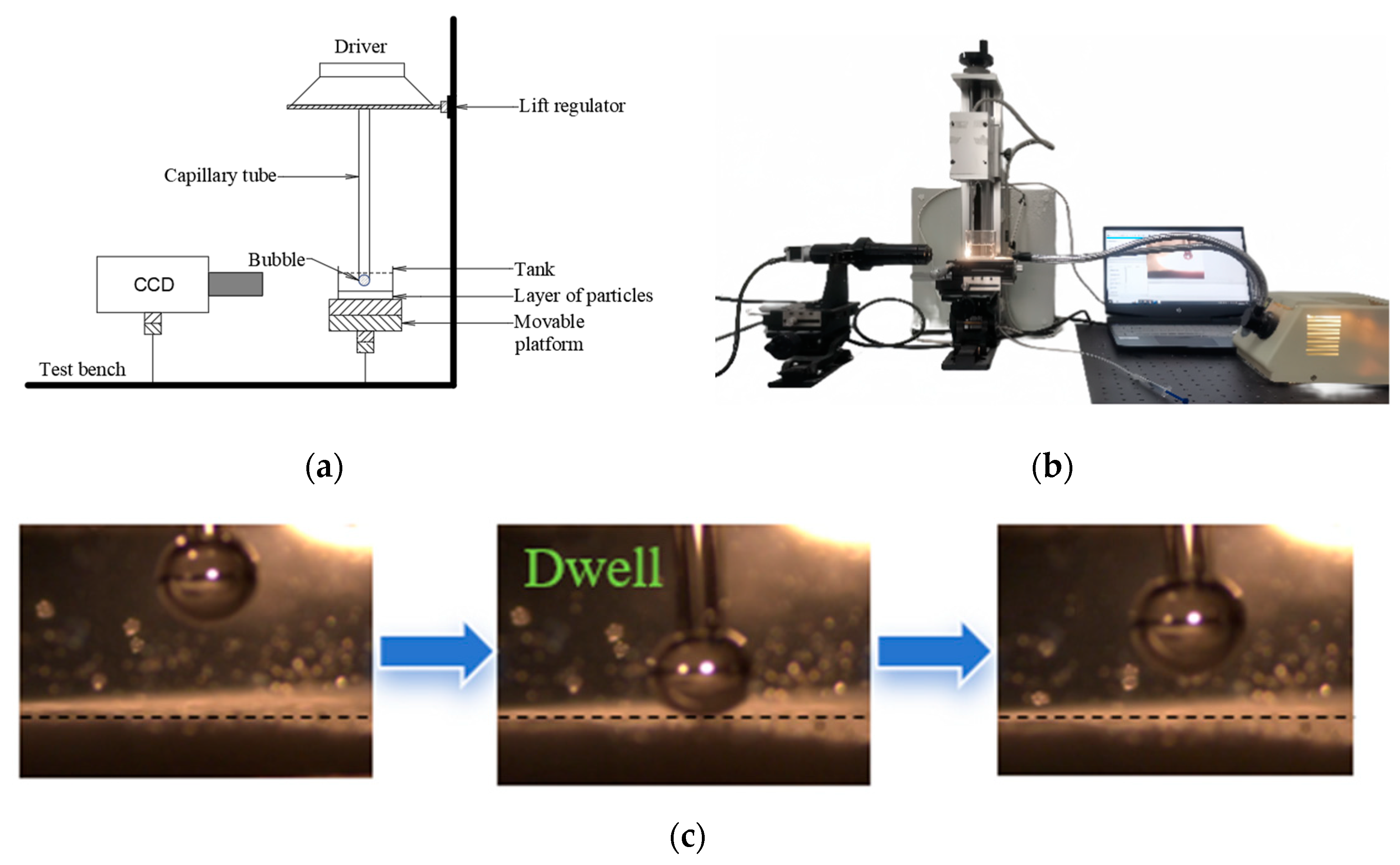
3.3.2. Induction Time Measurements
3.3.3. Foam Performance Test
3.3.4. Surface Tension Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.A.; Liu, J.; Luo, X.P. Study on technological mineralogy and the experimental of hematite in Fengyang Anhui. In Proceedings of the International Conference on Energy, Environment and Sustainable Development (ICEESD 2011), Shanghai Univ Elect Power, Shanghai, China, 21–23 October 2011; pp. 283–287. [Google Scholar]
- Wulandari, W.; Purwasasmita, M.; Sanwani, E.; Malatsih, W.; Fadilla, F. A study of bauxite tailing quality improvement by reverse flotation. In Proceedings of the Mineral Processing and Technology International Conference (MINEPROCET), Jakarta, Indonesia, 23–24 October 2017. [Google Scholar]
- Sun, H.R.; Yin, W.Z.; Yang, B.; Han, F. Simultaneous separation of quartz and dolomite from magnesite using monosodium phosphate as a regulator via reverse flotation. Miner. Eng. 2021, 172, 6. [Google Scholar] [CrossRef]
- Yin, W.Z.; Tang, Y. Interactive effect of minerals on complex ore flotation: A brief review. Int. J. Miner. Metall. Mater. 2020, 27, 571–583. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.Y.; Lu, H.Y.; Ni, T.J.; Li, Y. Waste energy recovery and energy efficiency improvement in China’s iron and steel industry. Appl. Energy 2017, 191, 502–520. [Google Scholar] [CrossRef]
- Ravishankar, S.; Sankar, T.; Khosla, N. Beneficiation studies on alumina-rich Indian iron ore slimes using selective dispersants, flocculants and flotation collectors. In Proceedings of the Proceedings XVIII International Mineral Processing Congress, Sydney, Australia, 23–28 May 1993; pp. 1289–1294. [Google Scholar]
- Fan, G.X.; Wang, L.G.; Cao, Y.J.; Li, C. Collecting Agent-Mineral Interactions in the Reverse Flotation of Iron Ore: A Brief Review. Minerals 2020, 10, 22. [Google Scholar] [CrossRef]
- Zhang, X.L.; Gu, X.T.; Han, Y.X.; Parra-Alvarez, N.; Claremboux, V.; Kawatra, S.K. Flotation of Iron Ores: A Review. Miner. Process Extr. Metall. Rev. 2021, 42, 184–212. [Google Scholar] [CrossRef]
- Araujo, A.C.; Viana, P.R.M.; Peres, A.E.C. Reagents in iron ores flotation. Miner. Eng. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Nakhaei, F.; Irannajad, M. Reagents types in flotation of iron oxide minerals: A review. Miner. Process Extr. Metall. Rev. 2018, 39, 89–124. [Google Scholar] [CrossRef]
- Tang, C.M.; Guo, Z.Q.; Pan, J.; Zhu, D.Q.; Li, S.W.; Yang, C.C.; Tian, H.Y. Current situation of carbon emissions and countermeasures in China’s ironmaking industry. Int. J. Miner. Metall. Mater. 2023, 30, 1633–1650. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ren, J.W. The flotation of quartz from iron minerals with a combined quaternary ammonium salt. Int. J. Miner. Process. 2005, 77, 116–122. [Google Scholar] [CrossRef]
- Wang, J.J.; Gao, Z.Y.; Gao, Y.S.; Hu, Y.H.; Sun, W. Flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Miner. Eng. 2016, 98, 261–263. [Google Scholar] [CrossRef]
- Luo, L.P.; Wu, H.Q.; Xu, L.H.; Meng, J.P.; Lu, J.H.; Zhou, H.; Huo, X.M.; Huang, L.Y. An in situ ATR-FTIR study of mixed collectors BHA/DDA adsorption in ilmenite-titanaugite flotation system. Int. J. Min. Sci. Technol. 2021, 31, 689–697. [Google Scholar] [CrossRef]
- Xia, W.C.; Ma, G.X.; Bu, X.N.; Peng, Y.L. Effect of particle shape on bubble-particle attachment angle and flotation behavior of glass beads and fragments. Powder Technol. 2018, 338, 168–172. [Google Scholar] [CrossRef]
- Albijanic, B.; Ozdemir, O.; Nguyen, A.V.; Bradshaw, D. A review of induction and attachment times of wetting thin films between air bubbles and particles and its relevance in the separation of particles by flotation. Adv. Colloid Interface Sci. 2010, 159, 1–21. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhou, Z.; Nandakumar, K.; Masliyah, J.H.; Xu, Z.G. An induction time model for the attachment of an air bubble to a hydrophobic sphere in aqueous solutions. Int. J. Miner. Process. 2005, 75, 69–82. [Google Scholar] [CrossRef]
- Laskowski, J.; Miller, J. New reagents in coal flotation. In Reagents in the Mineral Industry; Jones, M.J., Oblatt, R., Eds.; The Institute of Mining and Metallurgy: London, UK, 1984; pp. 145–154. [Google Scholar]
- Fuerstenau, D.; Yang, G.; Laskowski, J. Oxidation phenomena in coal flotation part I. Correlation between oxygen functional group concentration, immersion wettability and salt flotation response. Coal Perparation 1987, 4, 161–182. [Google Scholar] [CrossRef]
- Wang, S.; Fan, H.; He, H.; Tang, L.; Tao, X. Effect of particle shape and roughness on the hydrophobicity of low-rank coal surface. Int. J. Coal Prep. Util. 2020, 40, 876–891. [Google Scholar] [CrossRef]
- Wang, S.W.; Tao, X.X. Comparison of the adhesion kinetics between air or oily bubble and long flame coal surface in flotation. Fuel 2021, 291, 120139. [Google Scholar] [CrossRef]
- Liu, W.B.; Peng, X.Y.; Liu, W.G.; Wang, X.Y.; Zhao, Q.; Wang, B.Y. Effect mechanism of the iso-propanol substituent on amine collectors in the flotation of quartz and magnesite. Powder Technol. 2020, 360, 1117–1125. [Google Scholar] [CrossRef]
- Qiao, X.X. Flotation Performance and Mechanism of Reagents Based on Dodecylamine and 2-Octanol. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China, 2020. [Google Scholar]
- Tan, R.; Han, C.; Ao, Y.X.; Chai, L.; Kong, L.H.; Shen, Y.B.; Song, T. Influence of Three Auxiliary Collectors with different Electrical Properties of Polar Groups on Quartz Floatability in Dodecylamine System. Met. Mine 2022, 556, 79–87. [Google Scholar] [CrossRef]
- Gupta, A.K.; Banerjee, P.K.; Mishra, A.; Satish, P.; Pradip. Effect of alcohol and polyglycol bubble size and ether frothers on foam stability, coal flotation. Int. J. Miner. Process. 2007, 82, 126–137. [Google Scholar] [CrossRef]
- Laskowski, J. Particle-Bubble Attachment in Flotation. Miner. Sci. Eng. 1974, 6, 223–235. [Google Scholar]
- Nguyen, A.V.; Schulze, H.J.; Ralston, J. Elementary steps in particle-bubble attachment. Int. J. Miner. Process. 1997, 51, 183–195. [Google Scholar] [CrossRef]
- Li, M.; Xing, Y.W.; Zhu, C.Y.; Liu, Q.S.; Yang, Z.L.; Zhang, R.; Zhang, Y.F.; Xia, Y.C.; Gui, X.H. Effect of roughness on wettability and floatability: Based on wetting film drainage between bubbles and solid surfaces. Int. J. Min. Sci. Technol. 2022, 32, 1389–1396. [Google Scholar] [CrossRef]
- Yoon, R.-H.; Yordan, J.L. Induction time measurements for the quartz—Amine flotation system. J. Colloid Interface Sci. 1991, 141, 374–383. [Google Scholar] [CrossRef]
- Burdukova, E.; Laskowski, J.S. Effect of Insoluble Amine on Bubble Surfaces on Particle-Bubble Attachment in Potash Flotation. Can. J. Chem. Eng. 2009, 87, 441–447. [Google Scholar] [CrossRef]
- Wang, S.W.; Tao, X.X. Effect of prewetting time on different size fraction flotation performances of low rank coal. Int. J. Oil Gas Coal Technol. 2018, 18, 187–199. [Google Scholar] [CrossRef]
- Wang, R.T.; Li, Y.L. Micellar and Interfacial Behavior in Mixtures of Dodecyldiethoxylamine Oxide with Ionic Surfactants. J. Chem. Eng. Data 2013, 58, 2240–2247. [Google Scholar] [CrossRef]
- Xu, M.D.; Guo, F.Y.; Bao, X.C.; Gui, X.H.; Xing, Y.W.; Cao, Y.J. Study on the Strengthening Mechanism of a MIBC-PEG Mixed Surfactant on Foam Stability. ACS Omega 2023, 8, 27429–27438. [Google Scholar] [CrossRef]
- Jiang, H.; Ji, W.Y.; Yang, Q.H.; Xu, L.H.; Zhao, C.; Hu, Y.H. Synergistic Adsorption and Flotation of New Mixed Cationic/Nonionic Collectors on Muscovite. Minerals 2017, 7, 11. [Google Scholar] [CrossRef]
- Xu, M.D. Drainage Process and Dynamics of Two-Phase Foam for Flotation. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2020. [Google Scholar]
- Xie, Q. Study on Foam Stability and Defoaming of Dodecylamine Reverse Flotation of Colloidal Phosphate Rock. Ph.D. Thesis, Guizhou Minzu University, Guiyang, China, 2022. [Google Scholar]
- Han, J.K.; Li, Y.F.; Chen, P. Research Progress on Phenomenon and Mechanism in Flotation Froth Phase. Nonferrous Met. (Miner. Process. Sect.) 2023, 4, 17–28. [Google Scholar] [CrossRef]
- Bhakta, A.; Ruckenstein, E. Decay of standing foams: Drainage, coalescence and collapse. Adv. Colloid Interface Sci. 1997, 70, 1–124. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhuang, L.; Wang, L.; Gao, H.; Zhao, L. The relationship among contact angle, induction time and flotation recovery of coal. Int. J. Coal Prep. Util. 2021, 41, 398–406. [Google Scholar] [CrossRef]
- Xing, Y.W. Interaction Force between Bubble and Particle and the Thinning Dynamics of the Thin Liquid Film. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2018. [Google Scholar]
- Snyder, B.A.; Aston, D.E.; Berg, J.C. Particle-drop interactions examined with an atomic force microscope. Langmuir 1997, 13, 590–593. [Google Scholar] [CrossRef]
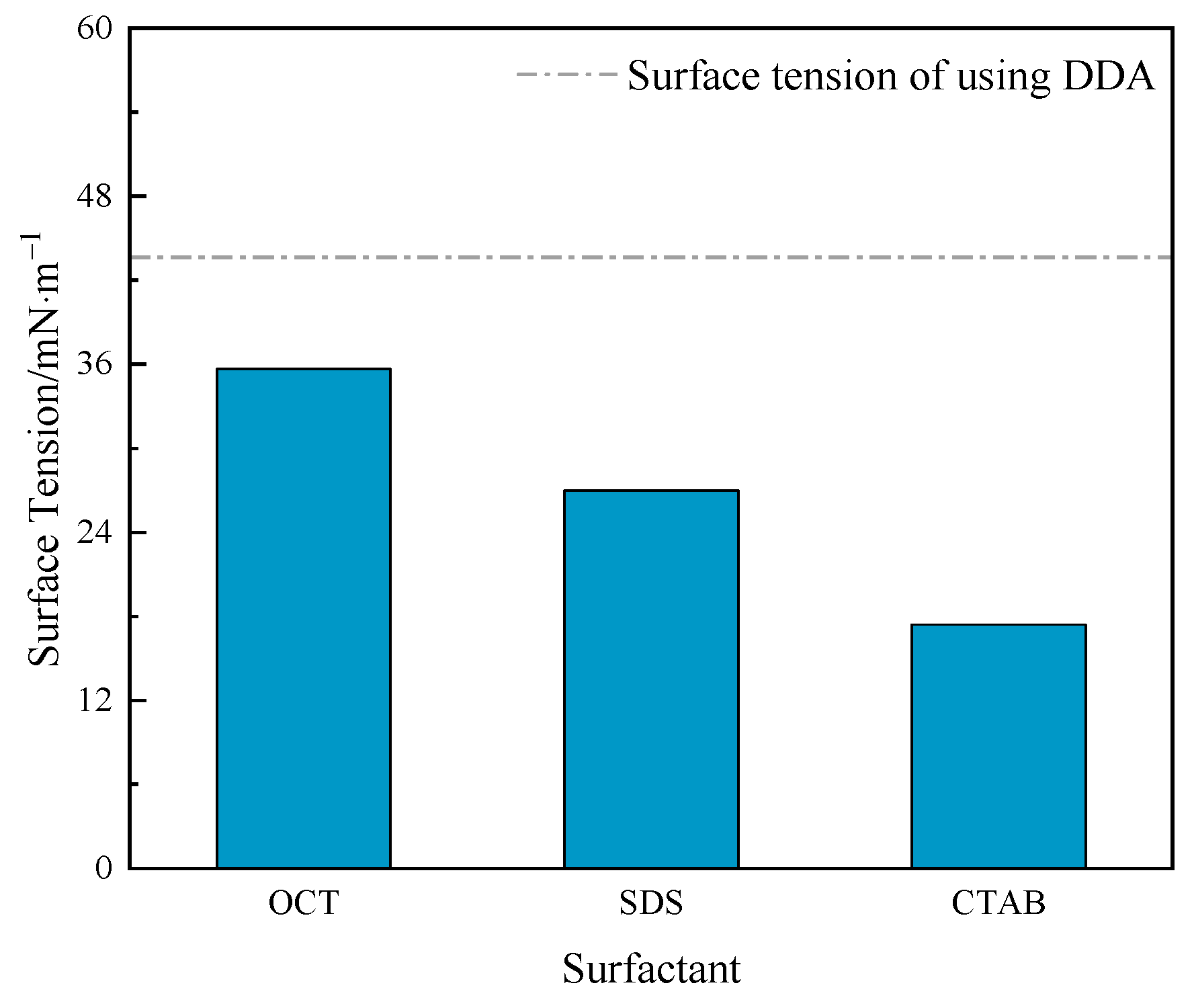
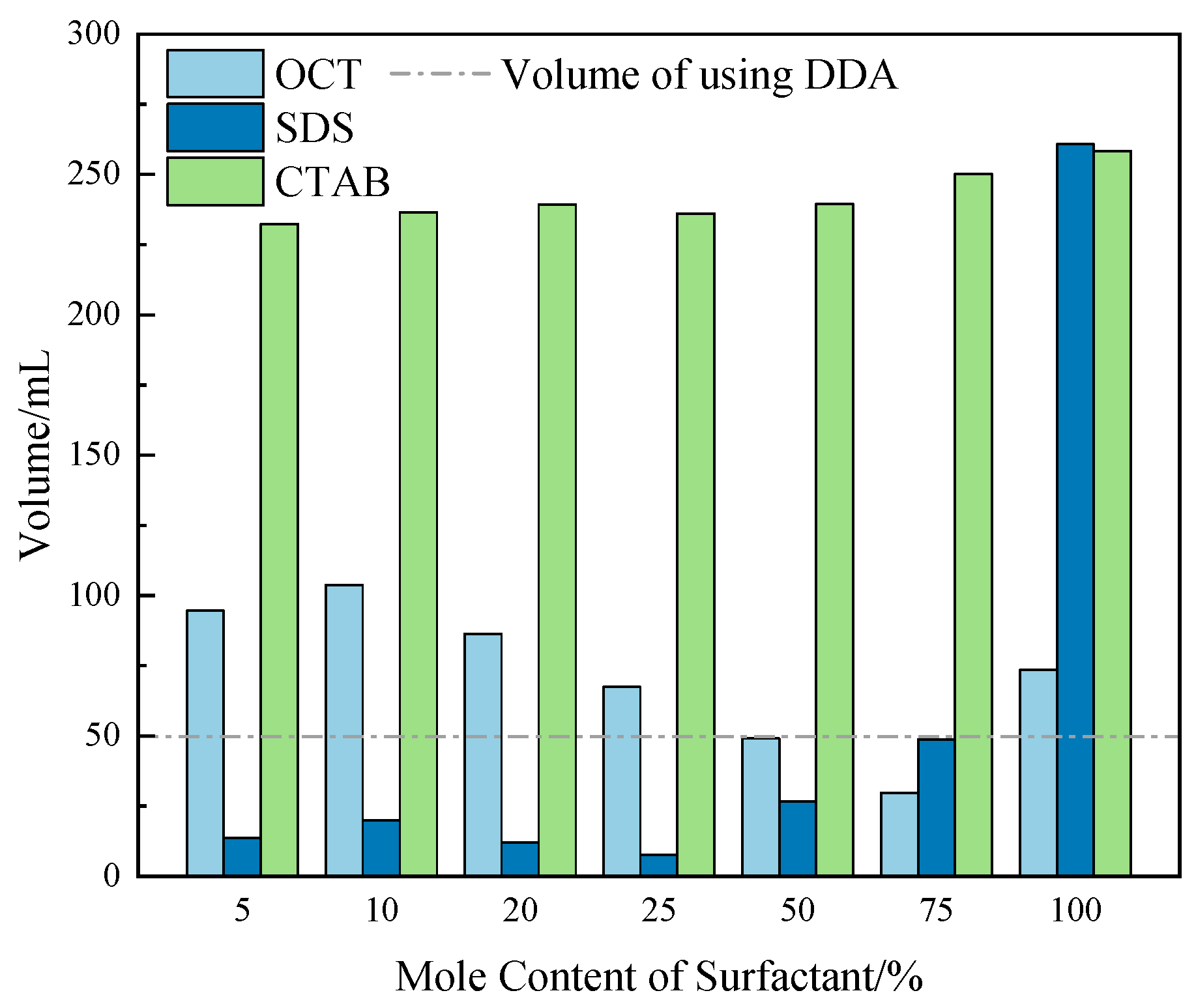
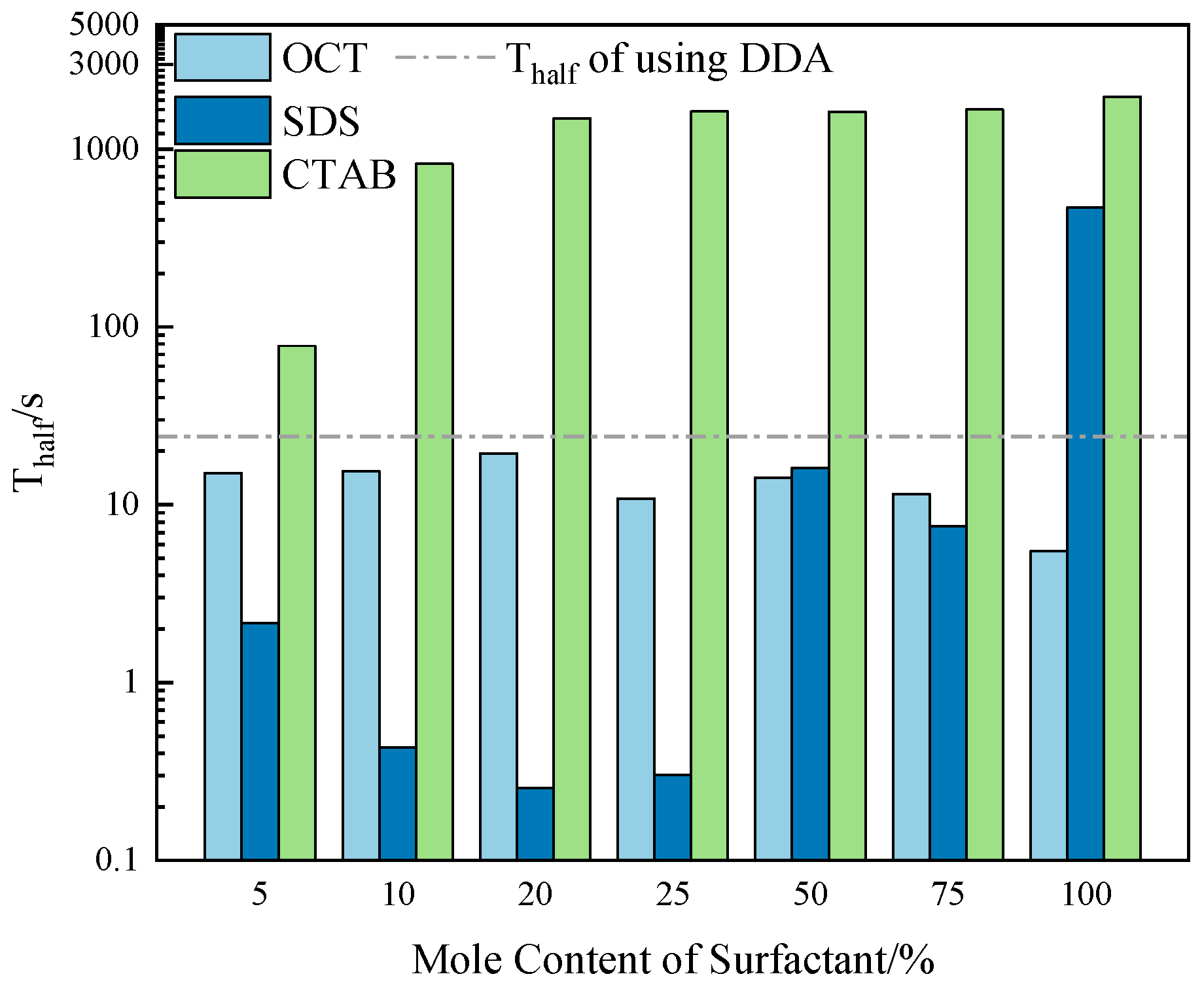
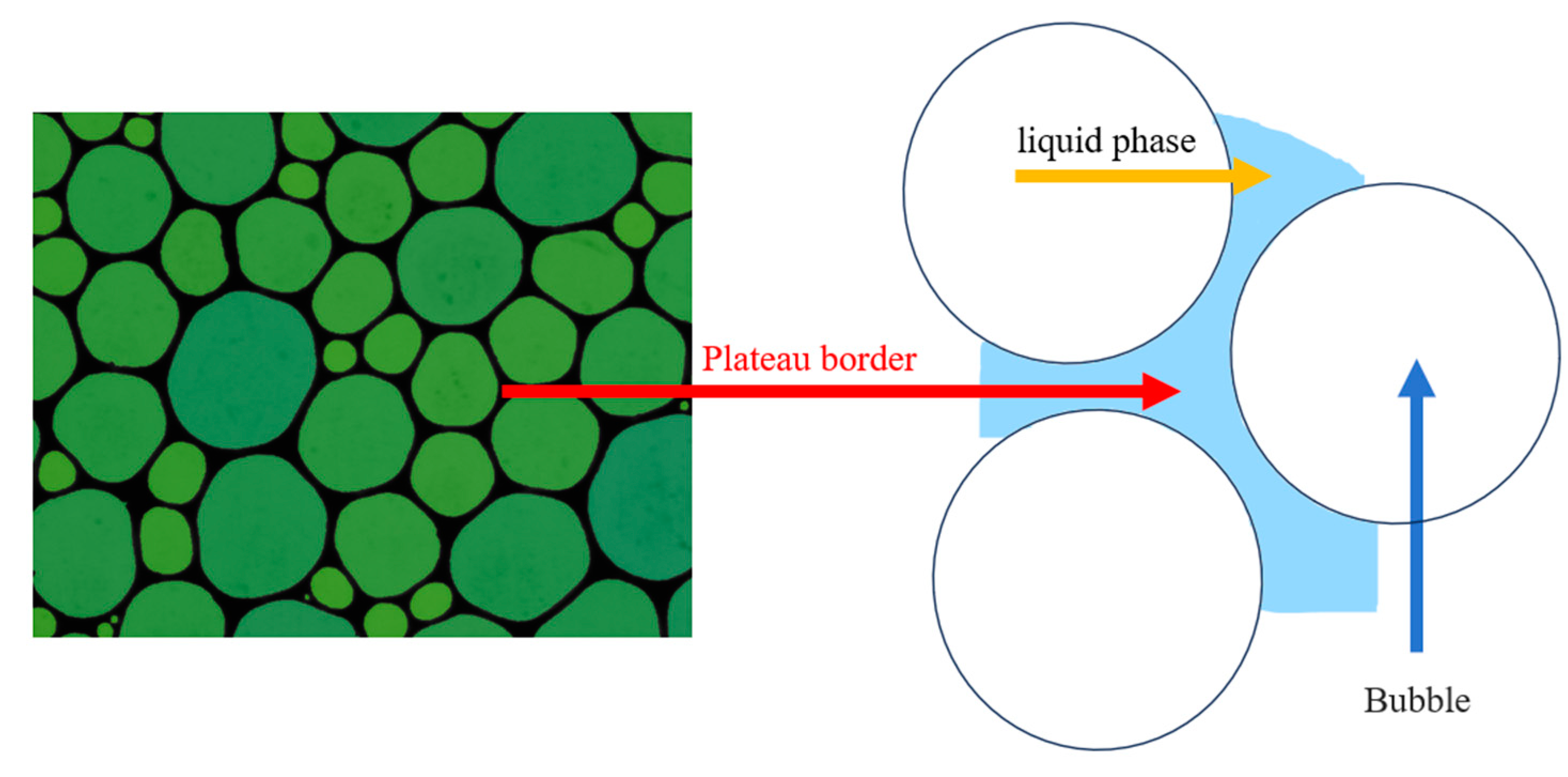
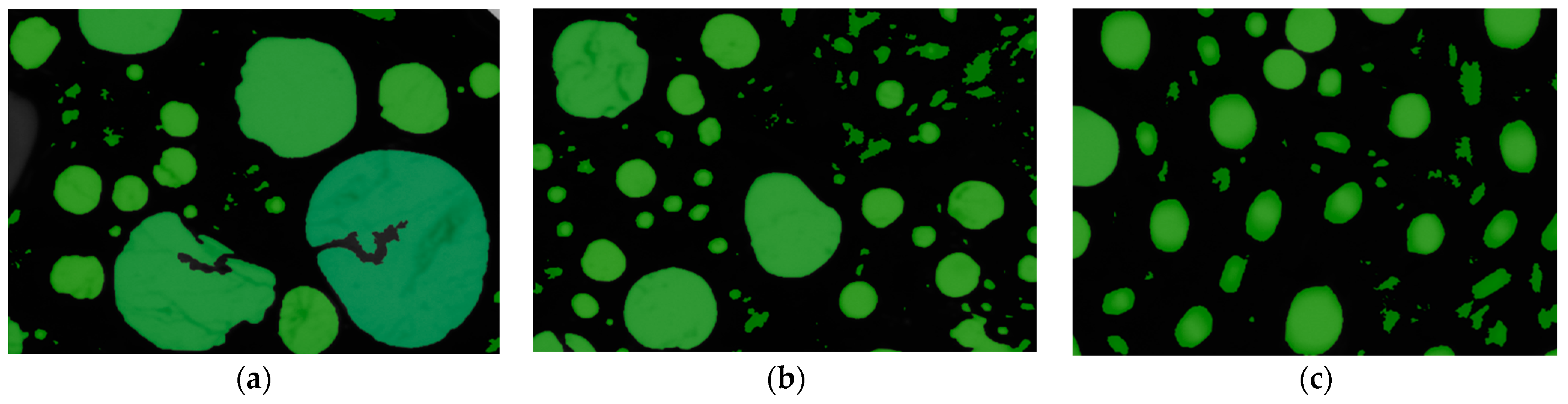
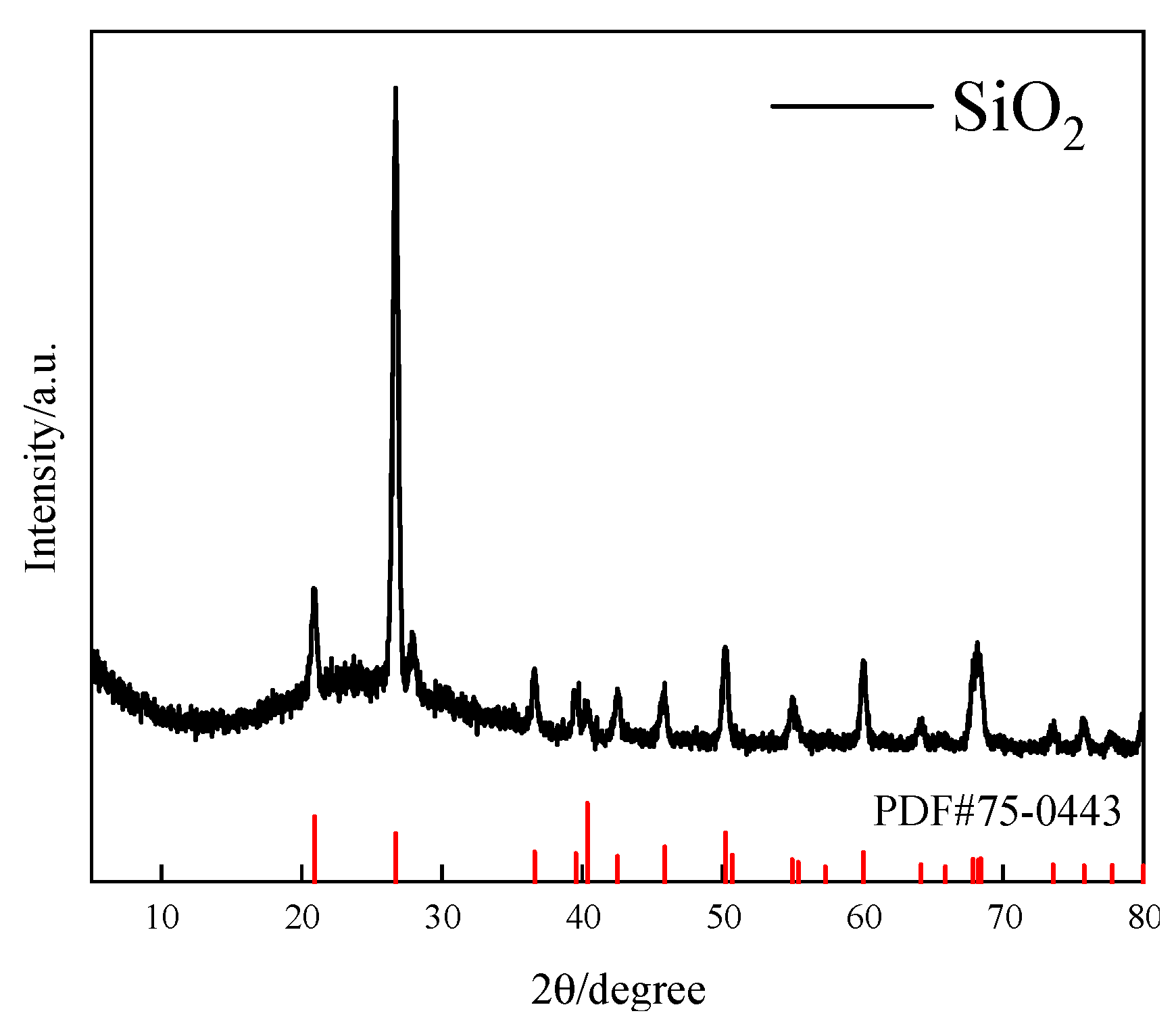
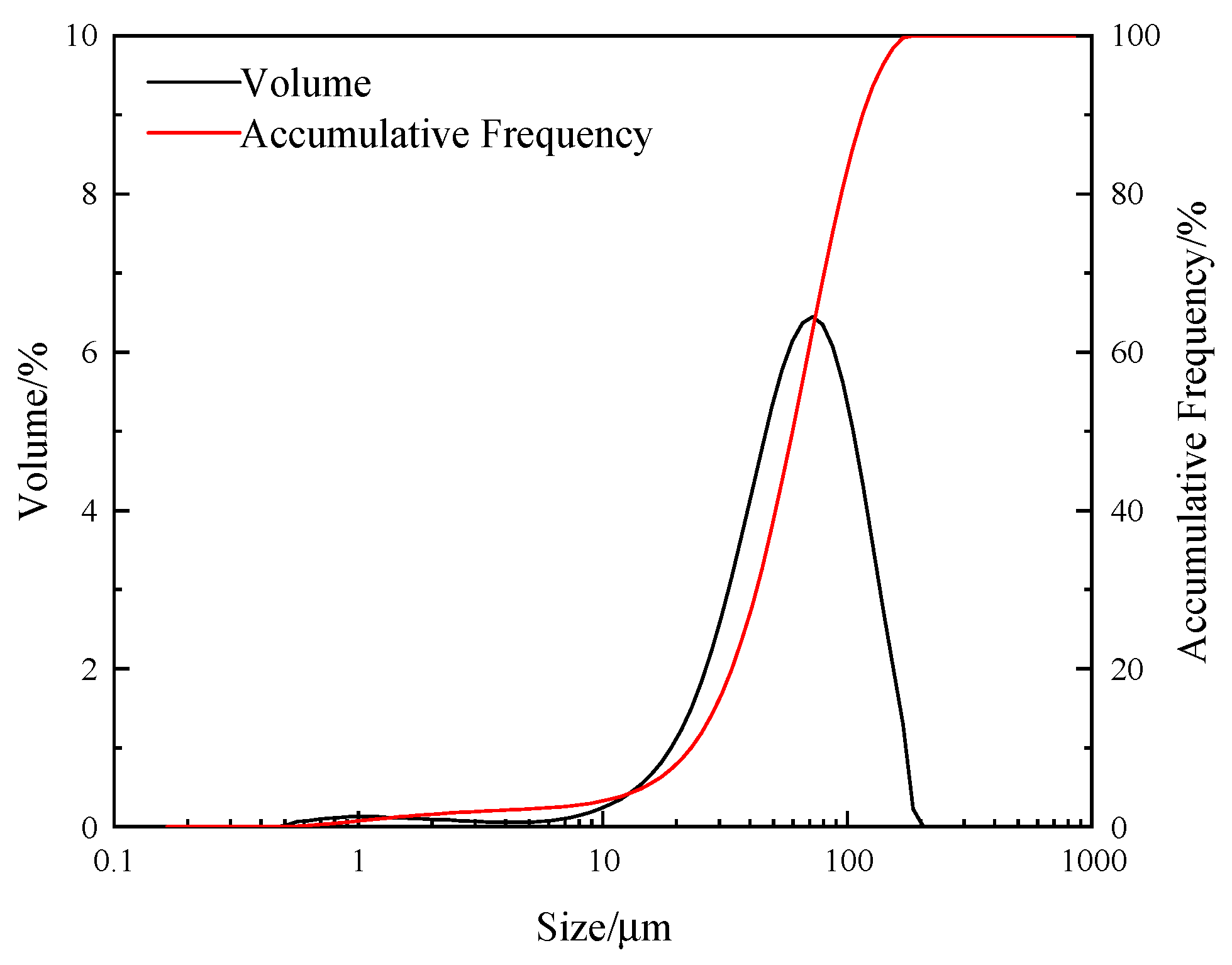
| Collector System | Number of Bubbles per Square Millimeter | /μm2 | /μm2 | /μm2 |
|---|---|---|---|---|
| DDA | 18.643 | 53,639 | 316 | 12,830,746 |
| OCT/DDA | 35.566 | 28,116 | 316 | 1,225,583 |
| CTAB/DDA | 44.685 | 22,379 | 316 | 695,032 |
| Component | SiO2 | Al2O3 | K2O | CaO | TFe |
|---|---|---|---|---|---|
| Content | 99.57% | 0.3019% | 0.0475% | 0.0382% | <0.05% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ao, Y.; Han, C.; Kong, L.; Shen, Y.; Zhao, S.; Liu, W.; Zhou, S. Influence of Different Types of Surfactants on the Flotation of Natural Quartz by Dodecylamine. Molecules 2024, 29, 2256. https://doi.org/10.3390/molecules29102256
Ao Y, Han C, Kong L, Shen Y, Zhao S, Liu W, Zhou S. Influence of Different Types of Surfactants on the Flotation of Natural Quartz by Dodecylamine. Molecules. 2024; 29(10):2256. https://doi.org/10.3390/molecules29102256
Chicago/Turabian StyleAo, Yuxin, Cong Han, Linghao Kong, Yanbai Shen, Sikai Zhao, Wengang Liu, and Shijie Zhou. 2024. "Influence of Different Types of Surfactants on the Flotation of Natural Quartz by Dodecylamine" Molecules 29, no. 10: 2256. https://doi.org/10.3390/molecules29102256
APA StyleAo, Y., Han, C., Kong, L., Shen, Y., Zhao, S., Liu, W., & Zhou, S. (2024). Influence of Different Types of Surfactants on the Flotation of Natural Quartz by Dodecylamine. Molecules, 29(10), 2256. https://doi.org/10.3390/molecules29102256










