Thermochemical Activation of Wood with NaOH, KOH and H3PO4 for the Synthesis of Nitrogen-Doped Nanoporous Carbon for Oxygen Reduction Reaction
Abstract
1. Introduction
2. Results
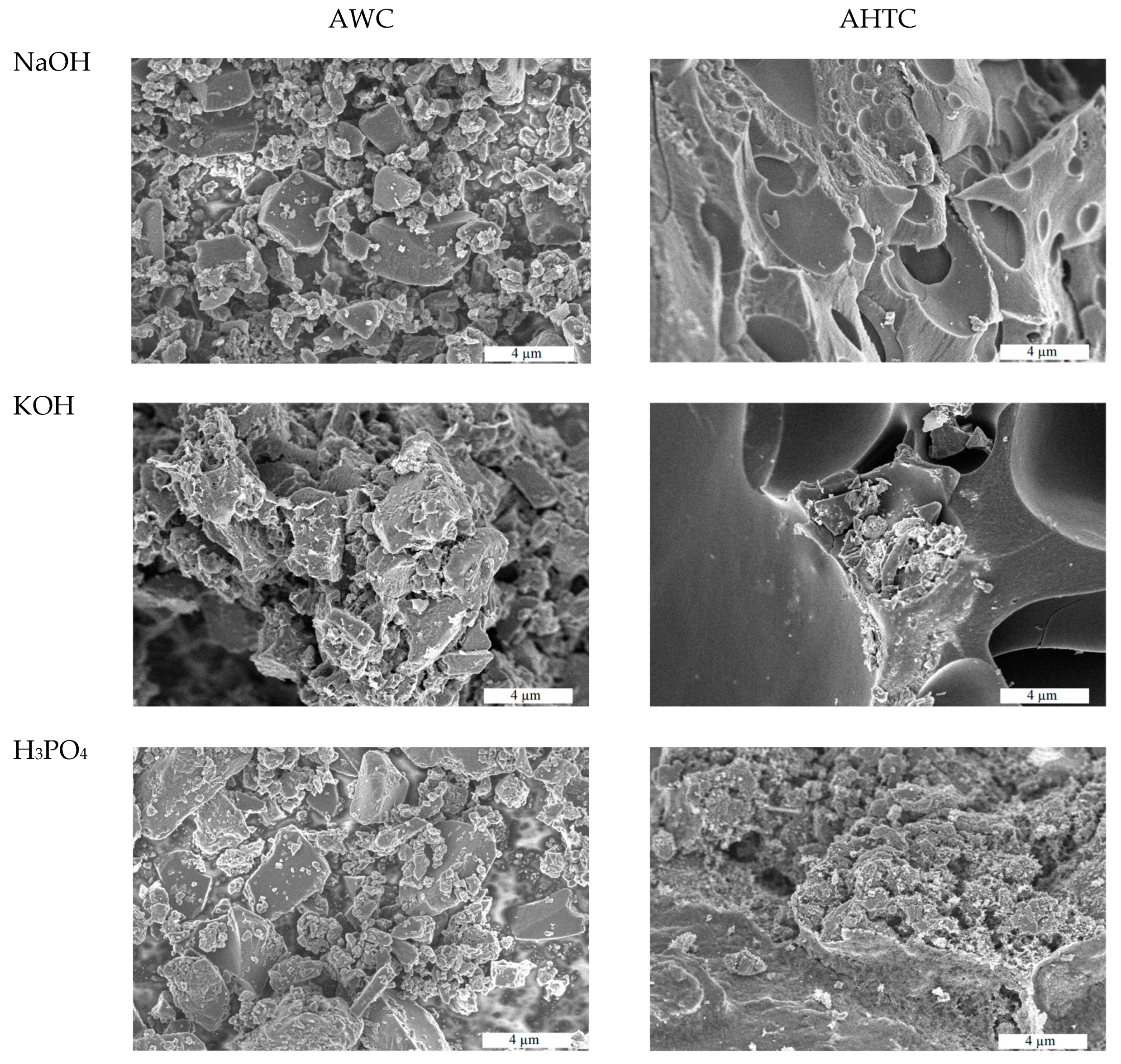
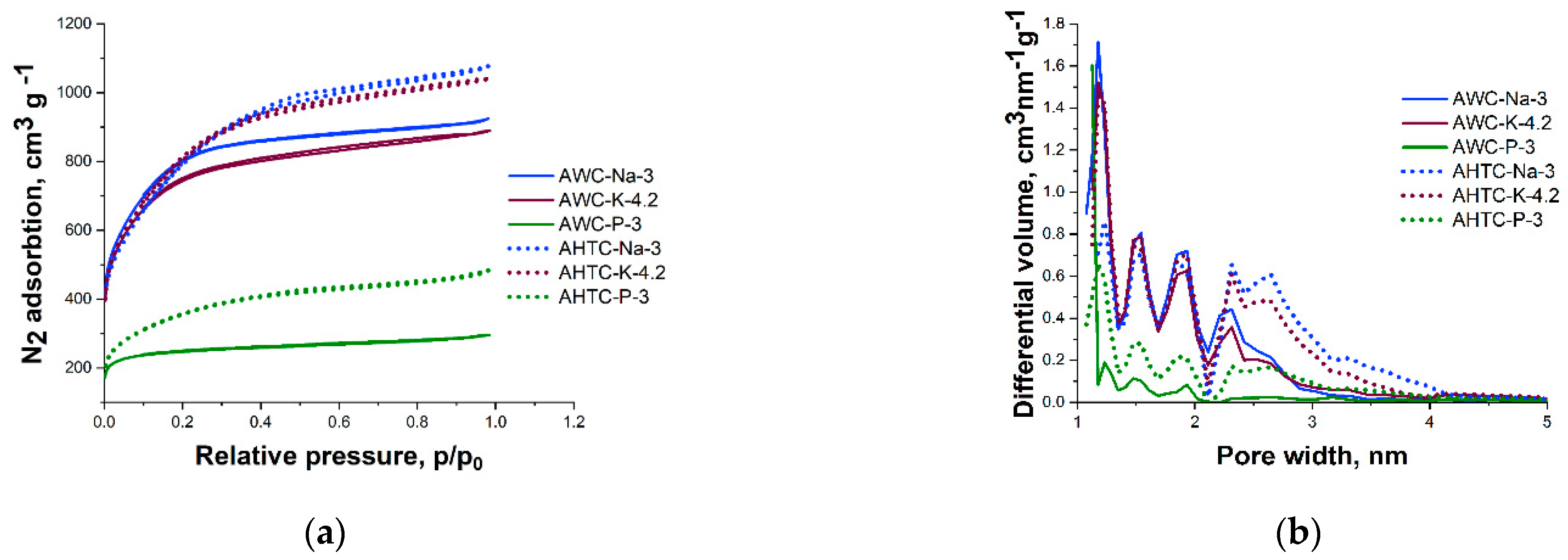
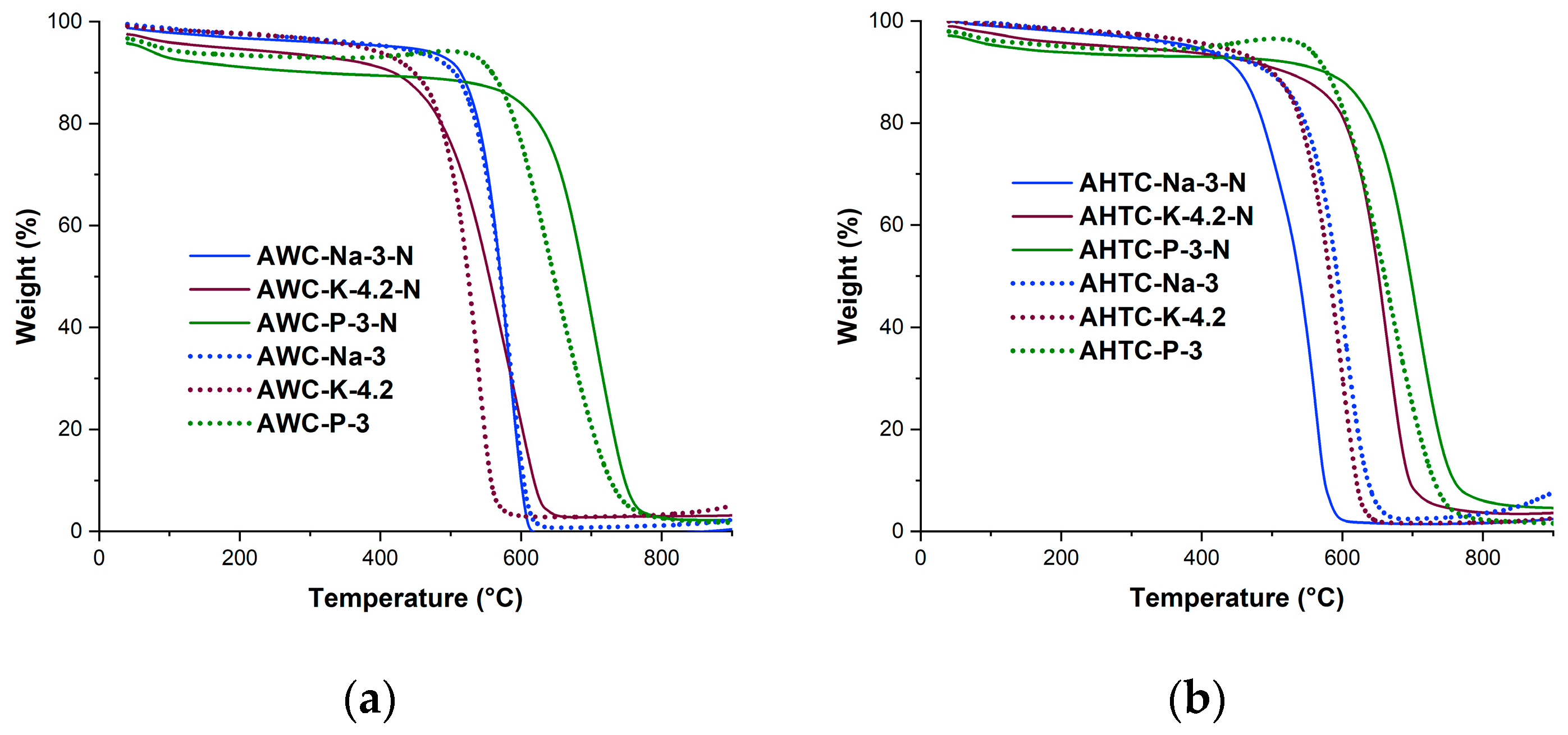
2.1. Characteristics of Chemical Composition and Structure of Carbon Materials
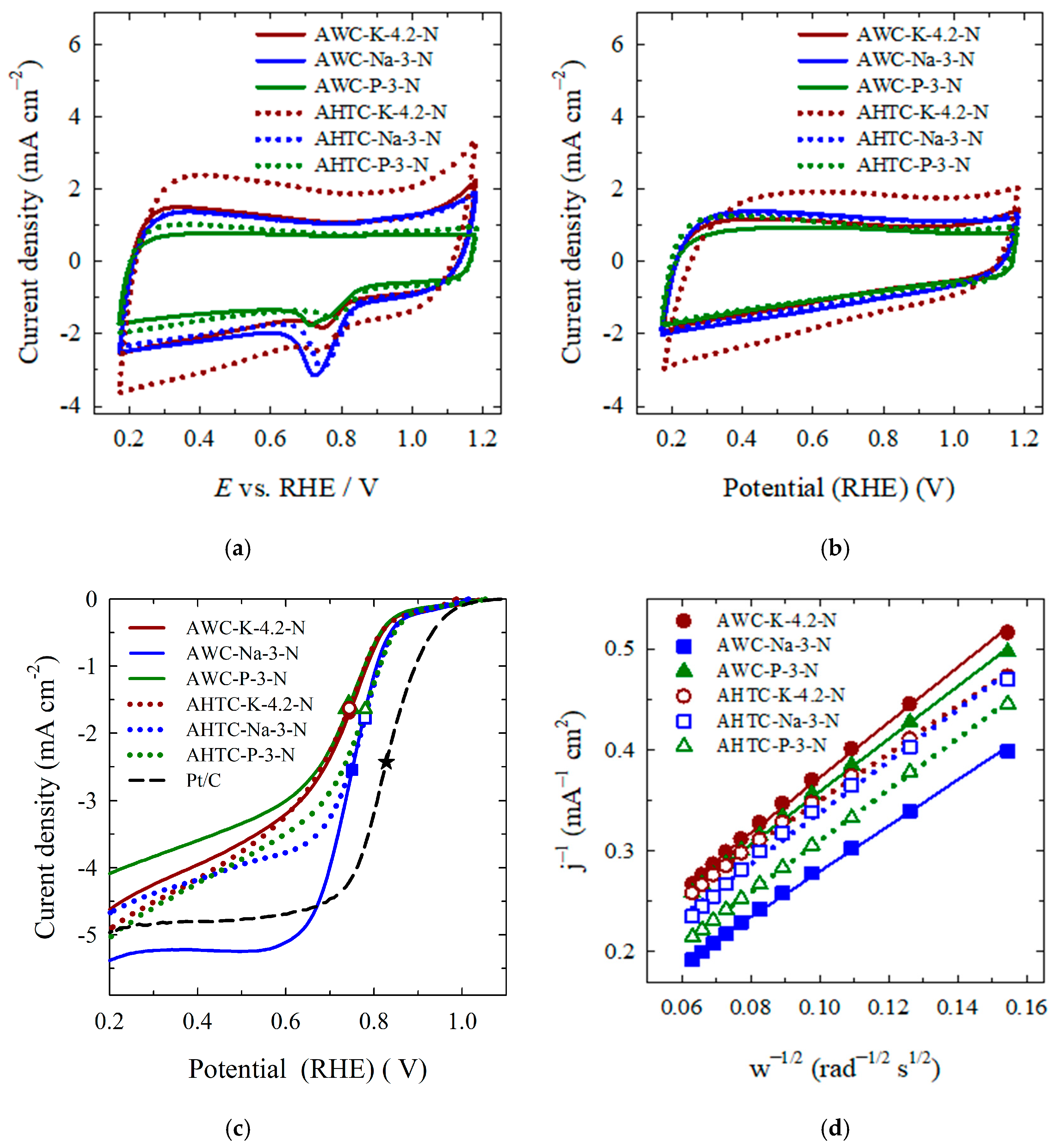
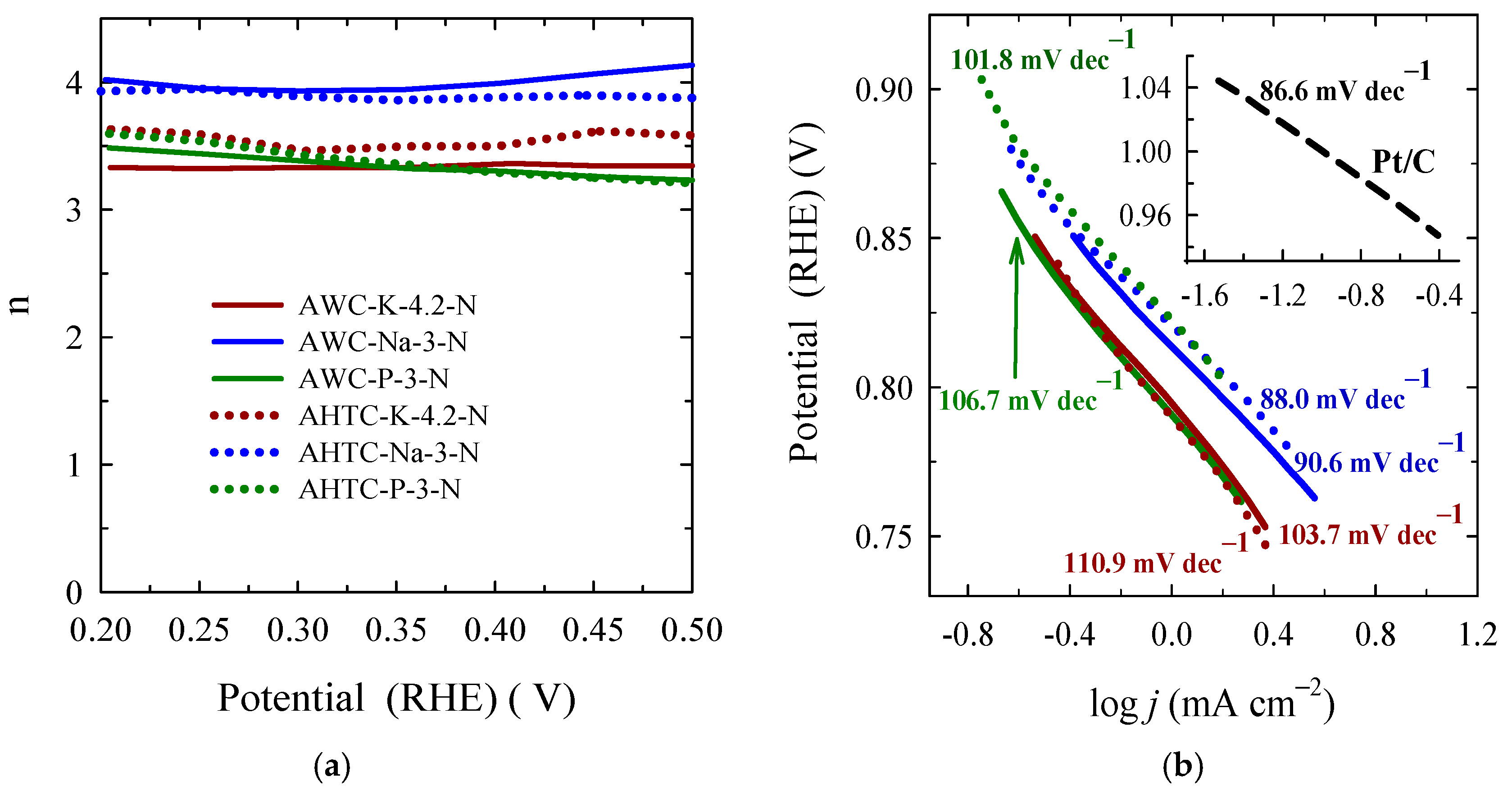
2.2. Catalytic Properties of Nitrogen-Doped Carbon Materials
3. Materials and Methods
3.1. Synthesis of Materials
- H3PO4 (Sigma Adrich, >99%), to carbon 3:1
- KOH (Honeywell Fluka, Puriss. p.a., Charlotte, NC, USA, >98%), to carbon 4.2:1
- NaOH (Honeywell Fluka, Puriss. p.a., >98%), to carbon 3:1
3.2. Characterization of Synthesized Materials
3.3. Electrochemical Measurements of Synthesized Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yagmur, E.; Ozmak, M.; Aktas, Z. A novel method for production of activated carbon from waste tea by chemical activation with microwave energy. Fuel 2008, 87, 3278–3285. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I.; Kaare, K.; Locs, J.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Biomass based activated carbons for fuel cells. Renew. Energy 2019, 141, 40–45. [Google Scholar] [CrossRef]
- Dobele, G.; Vervikishko, D.; Volperts, A.; Bogdanovich, N.; Shkolnikov, E. Characterization of the pore structure of nanoporous activated carbons produced from wood waste. Holzforschung 2013, 67, 587–594. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amorós, D.; Linares-Solano, A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J. Anal. Appl. Pyrolysis 2007, 80, 166–174. [Google Scholar] [CrossRef]
- Yorgun, S.; Yildiz, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Mahmood, T.; Ali, R.; Naeem, A.; Hamayun, M.; Aslam, M. Potential of used Camellia sinensis leaves as precursor for activated carbon preparation by chemical activation with H3PO4; optimization using response surface methodology. Process Saf. Environ. Prot. 2017, 109, 548–563. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys. 2016, 6, 651–658. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Kalderis, D.; Diamadopoulos, E. Numerical analysis of the influence of the impregnation ratio on the microporous structure formation of activated carbons, prepared by chemical activation of waste biomass with phosphoric(V) acid. J. Phys. Chem. Solids 2017, 105, 81–85. [Google Scholar] [CrossRef]
- Dobele, G.; Dizhbite, T.; Gil, M.V.V.; Volperts, A.; Centeno, T.A.A. Production of nanoporous carbons from wood processing wastes and their use in supercapacitors and CO2 capture. Biomass Bioenergy 2012, 46, 145–154. [Google Scholar] [CrossRef]
- Sun, J.; Niu, J.; Liu, M.; Ji, J.; Dou, M.; Wang, F. Biomass-derived nitrogen-doped porous carbons with tailored hierarchical porosity and high specific surface area for high energy and power density supercapacitors. Appl. Surf. Sci. 2018, 427, 807–813. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Liu, B.; Wang, S.; Cao, M. Porous carbon nanofiber webs derived from bacterial cellulose as an anode for high performance lithium ion batteries. Carbon N. Y. 2015, 91, 56–65. [Google Scholar] [CrossRef]
- Zhu, B.; Qiu, K.; Shang, C.; Guo, Z. Naturally derived porous carbon with selective metal- and/or nitrogen-doping for efficient CO2 capture and oxygen reduction. J. Mater. Chem. A 2015, 3, 5212–5222. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Adsorption of Cr(VI) from aqueous phase by high surface area activated carbon prepared by chemical activation with ZnCl2. Process Saf. Environ. Prot. 2017, 109, 63–71. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Rash, T.; Firlej, L.; Kuchta, B.; Yu, P.; Suppes, G.; Wexler, C.; Pfeifer, P. Nanospace engineering of KOH activated carbon. Nanotechnology 2011, 23, 015401. [Google Scholar] [CrossRef]
- Kuzmin, O.; Tamarkina, J.; Shendrik, T.; Zubkova, V.; Koval, O.; Roman, T. Production of active coal from pyrolyzed wood wastes by alkaline activation of KOH. Ukr. Food J. 2017, 6, 443–458. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activation Processes (Thermal or Physical). In Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Otowa, T.; Tanibata, R.; Itoh, M. Production and adsorption characteristics of MAXSORB: High-surface-area active carbon. Gas Sep. Purif. 1993, 7, 241–245. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon N. Y. 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Dobele, G.; Rossinskaja, G.; Telysheva, G.; Meier, D.; Faix, O. Cellulose dehydration and depolymerization reactions during pyrolysis in the presence of phosphoric acid. J. Anal. Appl. Pyrolysis 1999, 49, 307–317. [Google Scholar] [CrossRef]
- Ahmed Hared, I.; Dirion, J.L.; Salvador, S.; Lacroix, M.; Rio, S. Pyrolysis of wood impregnated with phosphoric acid for the production of activated carbon: Kinetics and porosity development studies. J. Anal. Appl. Pyrolysis 2007, 79, 101–105. [Google Scholar] [CrossRef]
- Zuo, S.; Yang, J.; Liu, J. Effects of the heating history of impregnated lignocellulosic material on pore development during phosphoric acid activation. Carbon N. Y. 2010, 48, 3293–3295. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Tascón, J.M.D. Phosphorus-containing carbons: Preparation, properties and utilization. Carbon N. Y. 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Solum, M.S.; Pugmire, R.J.; Jagtoyen, M.; Derbyshire, F. Evolution of carbon structure in chemically activated wood. Carbon N. Y. 1995, 33, 1247–1254. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Suárez-García, F.; Tascón, J.M.D. Synthetic carbons activated with phosphoric acid: I. Surface chemistry and ion binding properties. Carbon N. Y. 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation of Hemp-Derived Activated Carbon Monoliths. Adsorpt. Water Vapor. Ind. Eng. Chem. Res. 2008, 47, 1288–1296. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. HEMP-derived activated carbon fibers by chemical activation with phosphoric acid. Fuel 2009, 88, 19–26. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.G.; Michelin, M.; Romaní, A.; Domingues, L.; Teixeira, J.A. Co-production of biofuels and value-added compounds from industrial Eucalyptus globulus bark residues using hydrothermal treatment. Fuel 2021, 285, 119265. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Zeng, L.; Cui, X.; Shi, J. Engineering crystalline CoOOH anchored on an N-doped carbon support as a durable electrocatalyst for the oxygen reduction reaction. Dalt. Trans. 2018, 47, 6069–6074. [Google Scholar] [CrossRef]
- Teppor, P.; Jäger, R.; Paalo, M.; Palm, R.; Volobujeva, O.; Härk, E.; Kochovski, Z.; Romann, T.; Härmas, R.; Aruväli, J.; et al. Peat-derived carbon-based non-platinum group metal type catalyst for oxygen reduction and evolution reactions. Electrochem. Commun. 2020, 113, 106700. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Gao, Y.; Talreja, N.; Guo, F.; Texter, J.; Yan, C.; Sun, Z. Two-dimensional nanosheets for electrocatalysis in energy generation and conversion. J. Mater. Chem. A 2017, 5, 7257–7284. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Thorum, M.S. Electroreduction of dioxygen for fuel-cell applications: Materials and challenges. Inorg. Chem. 2010, 49, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, H.; Zhang, L.C.; Chen, H.; Zhu, H.; Wu, Y.; Xu, M.; Bao, S.J. Highly efficient Fe-N-C oxygen reduction electrocatalyst engineered by sintering atmosphere. J. Power Sources 2020, 449, 227497. [Google Scholar] [CrossRef]
- Sui, Z.Y.; Li, X.; Sun, Z.Y.; Tao, H.C.; Zhang, P.Y.; Zhao, L.; Han, B.H. Nitrogen-doped and nanostructured carbons with high surface area for enhanced oxygen reduction reaction. Carbon N. Y. 2018, 126, 111–118. [Google Scholar] [CrossRef]
- Wong, W.Y.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Recent progress in nitrogen-doped carbon and its composites as electrocatalysts for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 9370–9386. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Marsh, H. Activated Carbon (Activation Processes); Elsevier: Amsterdam, The Netherlands, 1992; ISBN 0080444636. [Google Scholar]
- Plavniece, A.; Dobele, G.; Volperts, A.; Zhurinsh, A. Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials. Sustainability 2022, 14, 15982. [Google Scholar] [CrossRef]
- Stadler, F.J.; Cheng, G.; Han, C.; Dobele, G.; Plavniece, A.; Volperts, A.; Zhurinsh, A.; Upskuviene, D.; Balciunaite, A.; Jasulaitiene, V.; et al. Effect of Pretreatment on the Nitrogen Doped Activated Carbon Materials Activity towards Oxygen Reduction Reaction. Materials 2023, 16, 6005. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Soares, J.; Oliveros, M.E.; Garin, C.; David, M.V.; Martins, L.G.P.; Almeida, C.A.; Martins-Ferreira, E.H.; Takai, K.; Enoki, T.; Magalhães-Paniago, R.; et al. Structural analysis of polycrystalline graphene systems by Raman spectroscopy. Carbon N. Y. 2015, 95, 646–652. [Google Scholar] [CrossRef]
- Ribeiro-Soares, J.; Cançado, L.G.; Falcão, N.P.S.; Martins Ferreira, E.H.; Achete, C.A.; Jorio, A. The use of Raman spectroscopy to characterize the carbon materials found in Amazonian anthrosoils. J. Raman Spectrosc. 2013, 44, 283–289. [Google Scholar] [CrossRef]
- Jorio, A.; Cançado, L.G. Perspectives on Raman spectroscopy of graphene-based systems: From the perfect two-dimensional surface to charcoal. Phys. Chem. Chem. Phys. 2012, 14, 15246–15256. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A.; Souza Filho, A.G. Raman Studies of Carbon Nanostructures. Annu. Rev. Mater. Res. 2016, 46, 357–382. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The importance of interbands on the interpretation of the raman spectrum of graphene oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Gnawali, C.L.; Shrestha, L.K.; Hill, J.P.; Ma, R.; Ariga, K.; Adhikari, M.P.; Rajbhandari, R.; Pokharel, B.P. Nanoporous Activated Carbon Material from Terminalia chebula Seed for Supercapacitor Application. C J. Carbon Res. 2023, 9, 109. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Khaidarov, A.A.; Pavlikov, A.V.; Minnebaev, K.F. The Field-Effect Transistor Based on a Polyyne–Polyene Structure Obtained via PVDC Dehydrochlorination. J. Compos. Sci. 2023, 7, 264. [Google Scholar] [CrossRef]
- Khosla, K.; Rathour, R.; Maurya, R.; Maheshwari, N.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Biodiesel production from lipid of carbon dioxide sequestrating bacterium and lipase of psychrotolerant Pseudomonas sp. ISTPL3 immobilized on biochar. Bioresour. Technol. 2017, 245, 743–750. [Google Scholar] [CrossRef]
- Trusovas, R.; Ratautas, K.; Račiukaitis, G.; Niaura, G. Graphene layer formation in pinewood by nanosecond and picosecond laser irradiation. Appl. Surf. Sci. 2019, 471, 154–161. [Google Scholar] [CrossRef]
- Upskuviene, D.; Balciunaite, A.; Drabavicius, A.; Jasulaitiene, V.; Niaura, G.; Talaikis, M.; Plavniece, A.; Dobele, G.; Volperts, A.; Zhurinsh, A.; et al. Synthesis of nitrogen-doped carbon catalyst from hydrothermally carbonized wood chips for oxygen reduction. Catal. Commun. 2023, 184, 106797. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Liu, J.; Zhang, T.; Liu, F.X.; Zheng, M.; Shi, K.; Liu, J.P.; Zhang, Y.; Wang, H. Synthesis of novel hollow carbon nanotubes @ Co-Fe alloy/iron phthalocyanine electrocatalyst by self-assembly method for OER and ORR study. Colloids Surf. Physicochem. Eng. Asp. 2024, 684, 133093. [Google Scholar] [CrossRef]
- Sun, S.; Xue, Y.; Wang, Q.; Huang, H.; Miao, H.; Liu, Z. Cerium ion intercalated MnO2 nanospheres with high catalytic activity toward oxygen reduction reaction for aluminum-air batteries. Electrochim. Acta 2018, 263, 544–554. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Gao, W.; Li, P.; Yan, W.; Wu, S.; Cui, Q.; Song, W.; Ding, K. Trivalent cerium-preponderant CeO2/graphene sandwich-structured nanocomposite with greatly enhanced catalytic activity for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 6656–6663. [Google Scholar] [CrossRef]
- Yan, J.; Zheng, X.; Wei, C.; Sun, Z.; Zeng, K.; Shen, L.; Sun, J.; Rümmeli, M.H.; Yang, R. Nitrogen-doped hollow carbon polyhedron derived from salt-encapsulated ZIF-8 for efficient oxygen reduction reaction. Carbon N. Y. 2021, 171, 320–328. [Google Scholar] [CrossRef]
- Wei, X.; Song, S.; Cai, W.; Luo, X.; Jiao, L.; Fang, Q.; Wang, X.; Wu, N.; Luo, Z.; Wang, H.; et al. Tuning the spin state of Fe single atoms by Pd nanoclusters enables robust oxygen reduction with dissociative pathway. Chem 2023, 9, 181–197. [Google Scholar] [CrossRef]
- Li, W.; Jiang, J.; Huang, Z.; Wang, Z.; Zhou, W.; Zhang, M.; Tang, Y.; Yu, Z.; Xie, J. Strontium doped Fe-based porous carbon for highly efficient electrocatalytic ORR and MOR reactions. J. Colloid Interface Sci. 2024, 659, 799–810. [Google Scholar] [CrossRef]
- Liu, J.; Maimaitiyiming, X.; Kuerban, Z. Fe/N-doped porous carbon materials derived from corn stalks as ORR electrocatalysts for DMFC. Mater. Lett. 2024, 354, 135336. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Luyen Doan, T.L.; Prabhakaran, S.; Tran, D.T.; Kim, D.H.; Lee, J.H.; Kim, N.H. Hierarchical Co and Nb dual-doped MoS2 nanosheets shelled micro-TiO2 hollow spheres as effective multifunctional electrocatalysts for HER, OER, and ORR. Nano Energy 2021, 82, 105750. [Google Scholar] [CrossRef]
- Meng, H.; Chen, X.; Gong, T.; Liu, H.; Liu, Y.; Li, H.; Zhang, Y. N, P, S/Fe-codoped Carbon Derived from Feculae Bombycis as an Efficient Electrocatalyst for Oxygen Reduction Reaction. ChemCatChem 2019, 11, 6015–6021. [Google Scholar] [CrossRef]
- Li, D.; Qu, Y.; Liu, X.; Zhai, C.; Liu, Y. Preparation of three-dimensional Fe–N co-doped open-porous carbon networks as an efficient ORR electrocatalyst in both alkaline and acidic media. Int. J. Hydrogen Energy 2021, 46, 18364–18375. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, Q.; He, X. Fabrication of Fe3C caged in N doped carbon nanotube as a desirable ORR electrocatalyst by a facile method. J. Electroanal. Chem. 2020, 871, 114316. [Google Scholar] [CrossRef]
- Yang, L.; Ding, H.; Xu, G.; Zhang, L.; Wei, B. Efficient ORR activity of N-doped porous carbon encapsulated cobalt electrocatalyst derived from a novel bimetal-organic framework. Mater. Res. Bull. 2021, 138, 111237. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Ma, J.; Wang, Z.; Liu, J.; Gong, X. ORR and OER of Co–N codoped carbon-based electrocatalysts enhanced by boundary layer oxygen molecules transfer. Carbon N. Y. 2021, 172, 556–568. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, H.; Yang, L.; Yang, Y.; Wang, J.; Liang, H. Core@shell MOFs derived Co2P/CoP@NPGC as a highly-active bifunctional electrocatalyst for ORR/OER. J. Ind. Eng. Chem. 2022, 106, 492–502. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Basic Potential Step Methods. In Electrochemical Methods: Fundamentals and Applications; John Wiley& Sons: New York, NY, USA, 2001; pp. 156–225. [Google Scholar]
- Kaare, K.; Yu, E.; Käämbre, T.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Niaura, G.; TamasauskaiteTamasiunaite, L.; Norkus, E.; Kruusenberg, I. Biomass-derived Graphene-like Catalyst Material for Oxygen Reduction Reaction. ChemNanoMat 2021, 7, 307–313. [Google Scholar] [CrossRef]
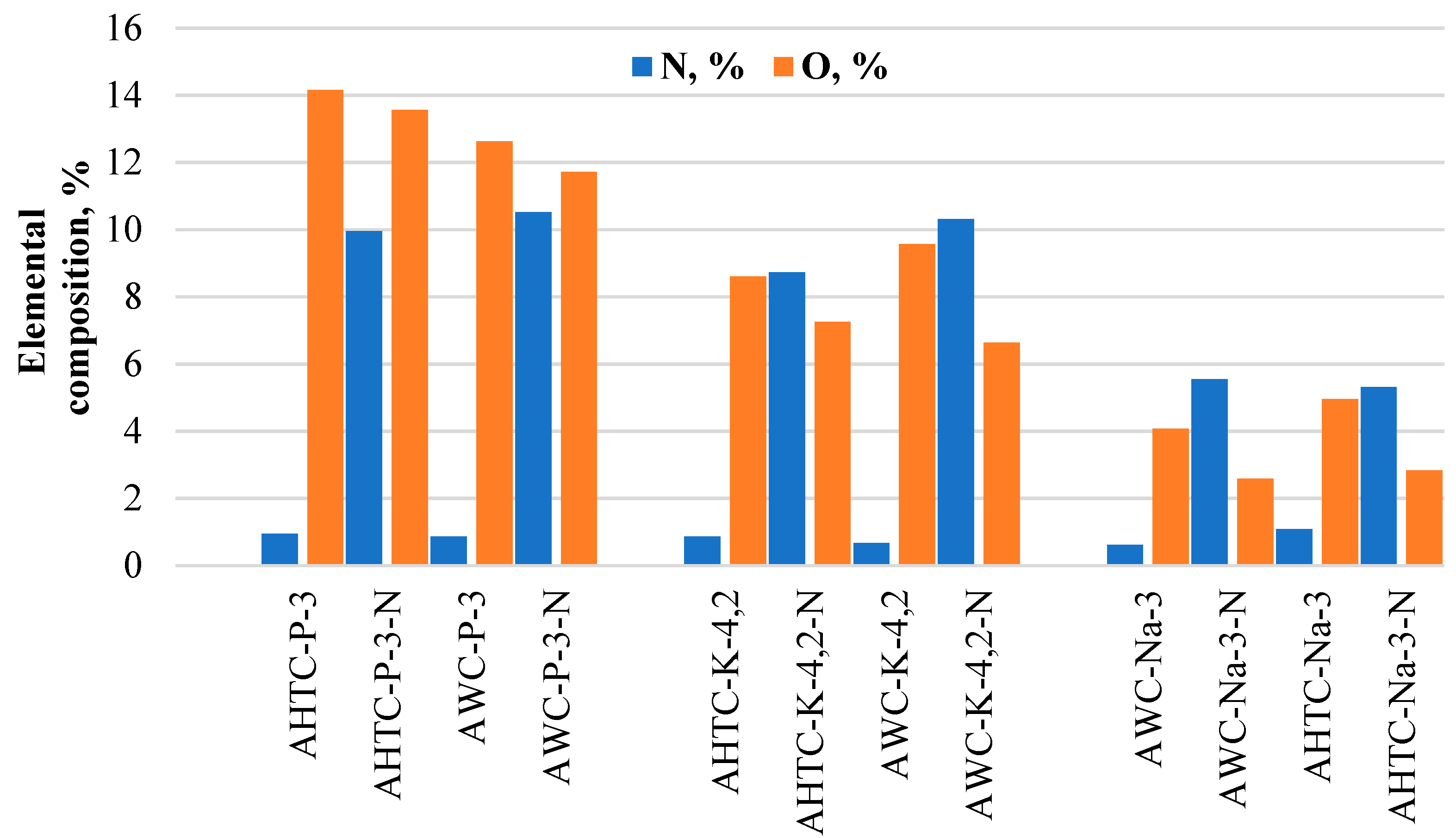
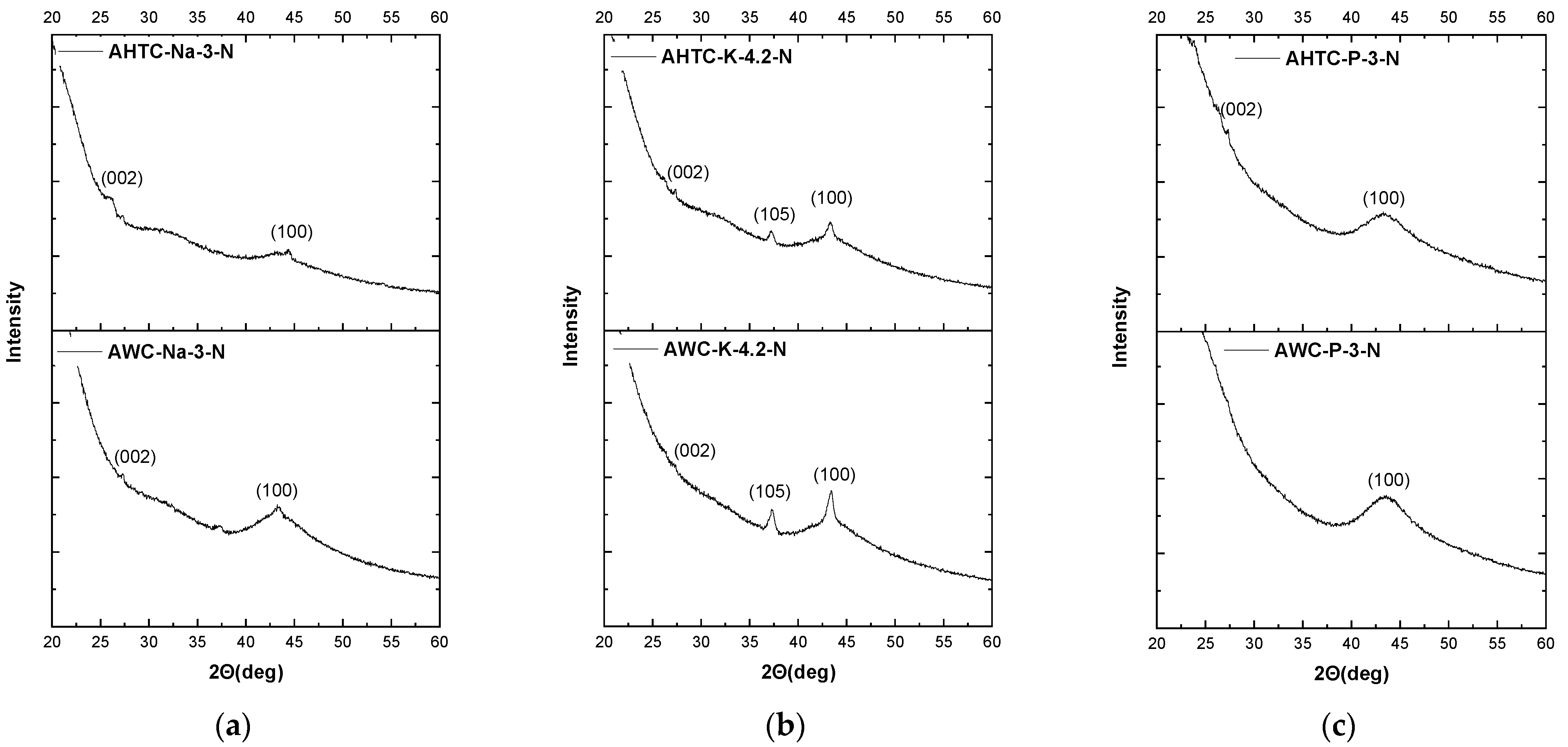
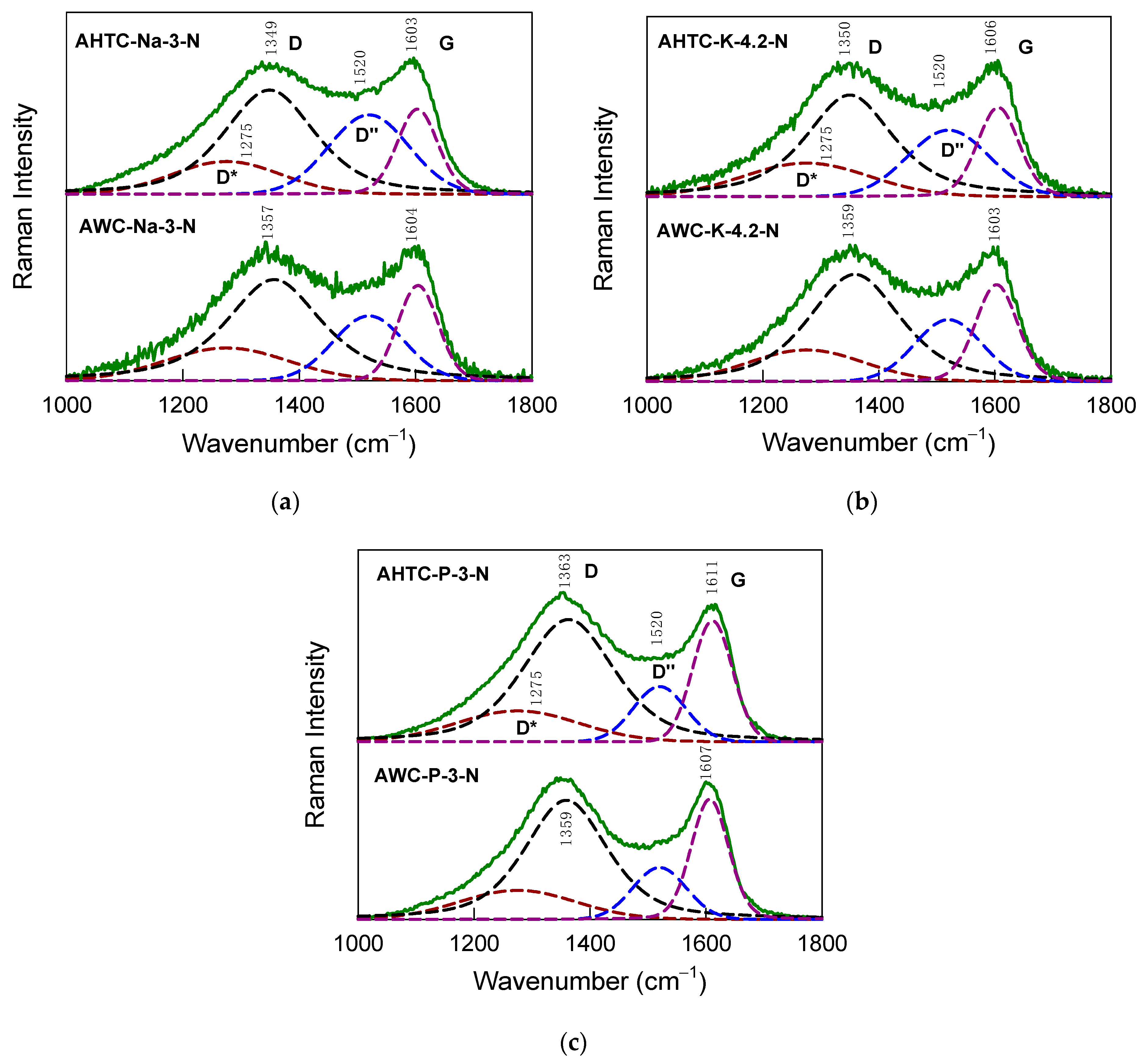
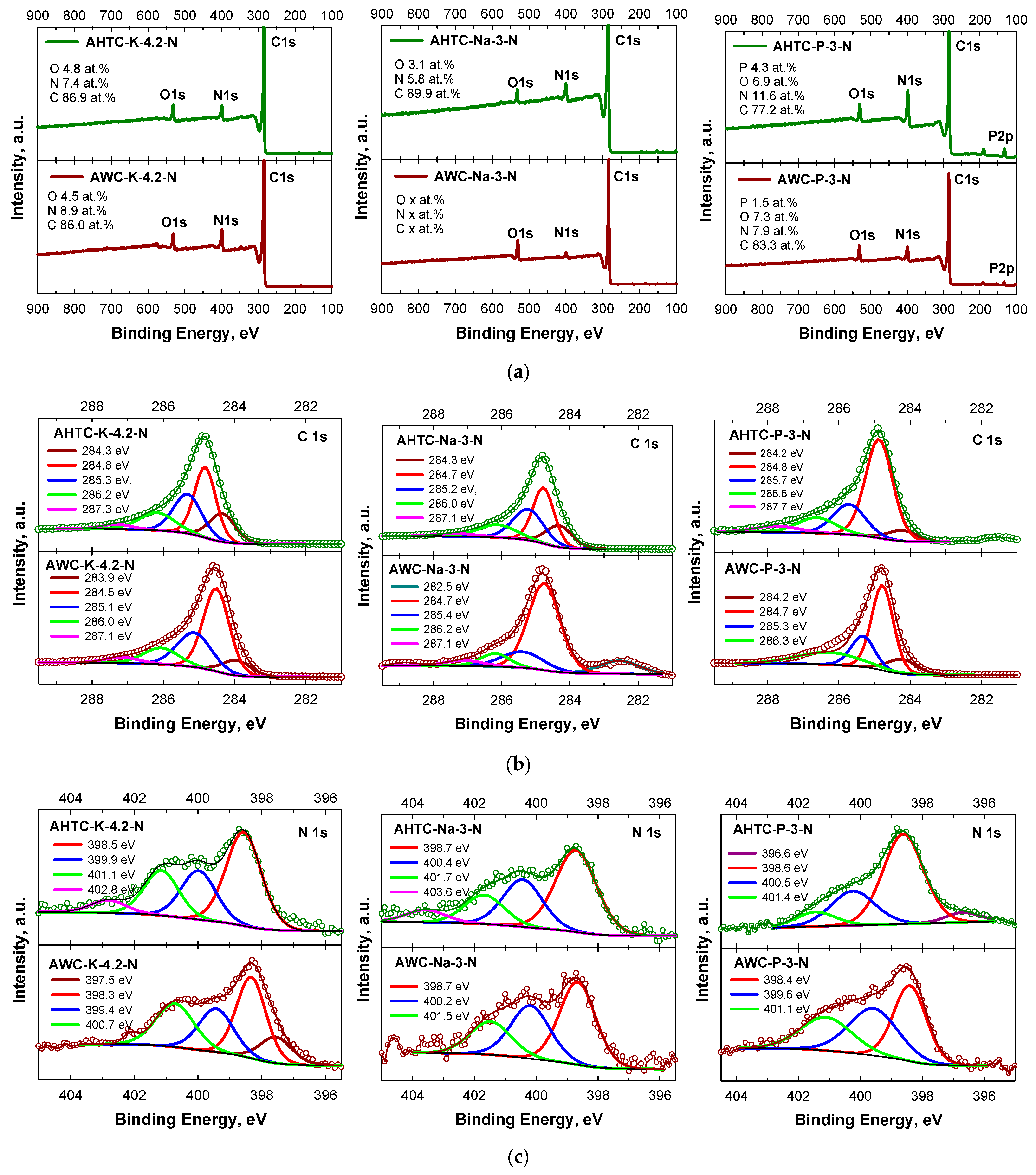
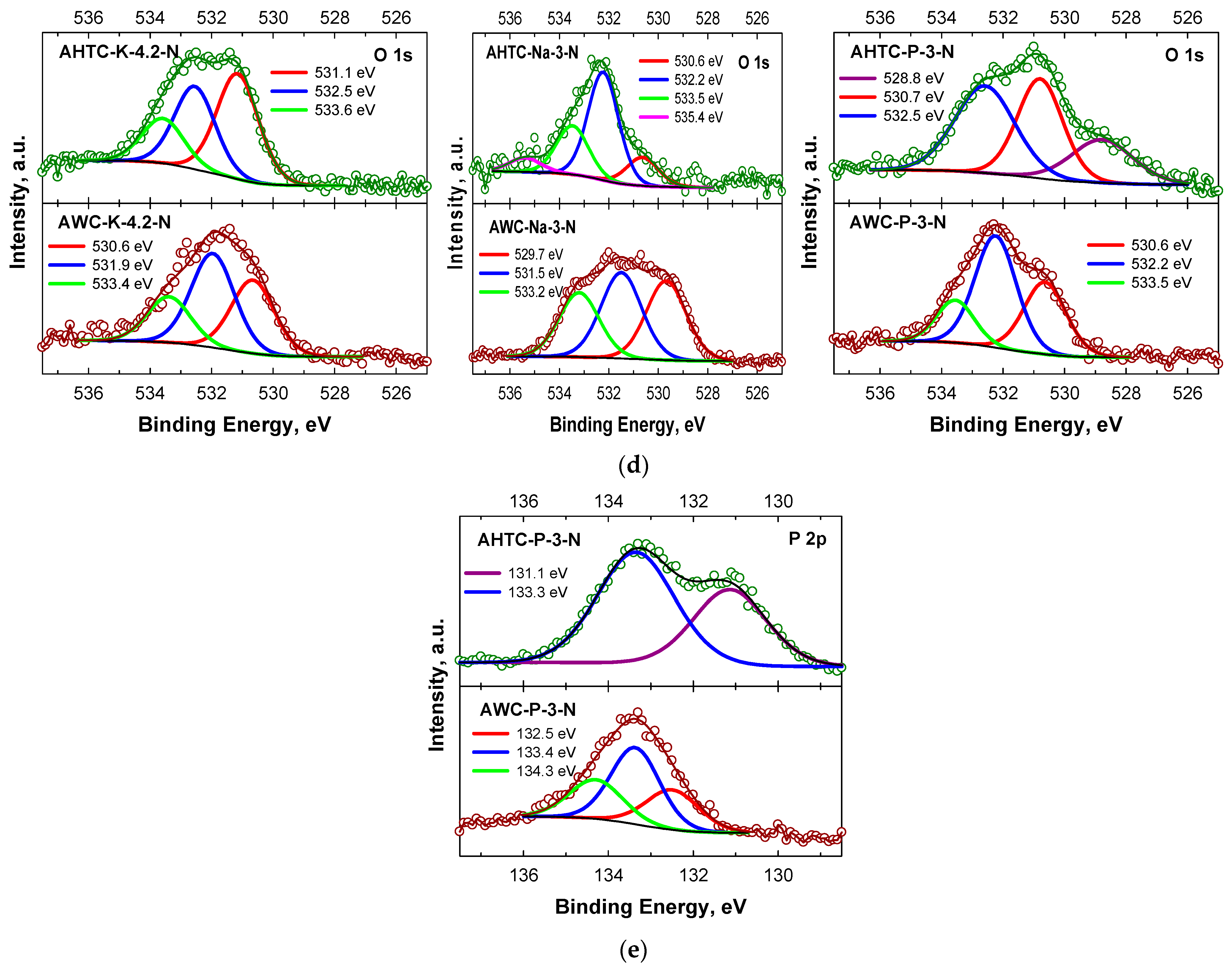
| Sample | Elemental Composition | Ash, % | |||
|---|---|---|---|---|---|
| N, % | C, % | H, % | * O, % | ||
| AHTC-P-3 | 0.95 | 82.01 | 2.89 | 14.15 | 1.9 |
| AHTC-P-3-N | 9.95 | 73.51 | 2.98 | 13.56 | 3.7 |
| AWC-P-3 | 0.86 | 83.61 | 2.91 | 12.62 | 2.3 |
| AWC-P-3-N | 10.52 | 74.73 | 3.04 | 11.71 | 2.8 |
| AHTC-K-4.2 | 0.86 | 89.49 | 1.05 | 8.60 | 1.7 |
| AHTC-K-4.2-N | 8.73 | 81.32 | 2.69 | 7.26 | 2.7 |
| AWC-K-4.2 | 0.67 | 88.88 | 0.88 | 9.57 | 2.0 |
| AWC-K-4.2-N | 10.31 | 80.24 | 2.82 | 6.63 | 1.8 |
| AWC-Na-3 | 0.62 | 94.85 | 0.46 | 4.07 | 1.7 |
| AWC-Na-3-N | 5.55 | 90.97 | 0.89 | 2.59 | 1.2 |
| AHTC-Na-3 | 1.09 | 93.3 | 0.65 | 4.96 | 1.5 |
| AHTC-Na-3-N | 5.31 | 90.05 | 1.81 | 2.83 | 1.4 |
| Samples | Specific Surface Area (BET), m2 g−1 | Pore Volume, cm3 g−1 | Mesopores from Vt, % | ||
|---|---|---|---|---|---|
| Total (Vt) | micro * | meso ** | |||
| AHTC-P-3 | 1739 | 0.73 | 0.41 | 0.32 | 43 |
| AHTC-P-3-N | 921 | 0.55 | 0.32 | 0.23 | 42 |
| AWC-P-3 | 769 | 0.41 | 0.34 | 0.07 | 17 |
| AWC-P-3-N | 520.9 | 0.28 | 0.22 | 0.06 | 21 |
| AHTC-K-4.2 | 2926 | 1.61 | 0.96 | 0.65 | 40 |
| AHTC-K-4.2-N | 2029 | 1.039 | 0.66 | 0.38 | 36 |
| AWC-K-4.2 | 2662 | 1.38 | 0.93 | 0.45 | 32 |
| AWC-K-4.2-N | 1679 | 0.82 | 0.583 | 0.24 | 29 |
| AWC-Na-3 | 2909 | 1.67 | 0.92 | 0.74 | 45 |
| AWC-Na-3-N | 2497 | 1.34 | 0.86 | 0.48 | 36 |
| AHTC-Na-3 | 2892 | 1.43 | 0.97 | 0.46 | 32 |
| AHTC-Na-3-N | 2521 | 1.289 | 0.831 | 0.46 | 36 |
| Sample | FWHM(G) (cm–1) | La (nm) | I(D″)/I(G) |
|---|---|---|---|
| AHTC-Na-3-N | 82.2 | 5.5 | 0.93 |
| AWC-Na-3-N | 81.5 | 5.7 | 0.68 |
| AHTC-K-4.2-N | 84.6 | 5.0 | 0.74 |
| AWC-K-4.2-N | 85.7 | 4.7 | 0.64 |
| AHTC-P-3-N | 80.7 | 5.9 | 0.46 |
| AWC-P-3-N | 76.1 | 7.1 | 0.43 |
| Element | AWC-K-4.2-N | AWC-Na-3-N | AWC-P-3-N | AHTC-K-4.2-N | AHTC-Na-3-N | AHTC-P-3-N |
|---|---|---|---|---|---|---|
| at. % | at. % | at. % | at. % | at. % | at. % | |
| N | 8.9 ± 0.1 | 3.6 ± 0.1 | 7.9 ± 0.1 | 7.4 ± 0.1 | 5.8 ± 0.1 | 11.6 ± 0.1 |
| O | 4.5 ± 0.1 | 10.6 ± 0.1 | 7.3 ± 0.1 | 4.8 ± 0.1 | 3.1 ± 0.1 | 6.9 ± 0.1 |
| C | 86.0 ± 0.1 | 85.8 ± 0.1 | 83.3 ± 0.1 | 86.9 ± 0.1 | 89.9 ± 0.1 | 77.2 ± 0.1 |
| P | - | - | 1.5 ± 0.1 | - | - | 4.3 ± 0.1 |
| Sample | Eonset, V | E1/2, V | Average Number of Electrons Transferred, n | Tafel Slope, mV dec–1 | Ref. |
|---|---|---|---|---|---|
| AWC-K-4.2-N | 0.97 | 0.74 | 3.3 | −103.7 | This work |
| AWC-Na-3-N | 0.97 | 0.75 | 4.0 | −90.6 | This work |
| AWC-P-3-N | 0.94 | 0.74 | 3.3 | −106.7 | This work |
| AHTC-K-4.2-N | 0.95 | 0.74 | 3.6 | −110.9 | This work |
| AHTC-Na-3-N | 0.95 | 0.78 | 3.9 | −88.0 | This work |
| AHTC-P-3-N | 0.90 | 0.78 | 3.3 | −101.8 | This work |
| Pt/C | 0.99 | 0.83 | 4.2 | −86.6 | This work |
| CNT@Co2-Fe1/FePc | 0.953 | 0.844 | 3.81 | - | [57] |
| 4.8% Ce-MnO2/C | 0.872 | 0.783 | 3.95–3.97 | −90 | [58] |
| CeGS | 0.92 | 0.81 | 3.6–4 | −111 | [59] |
| NHCP-1000 | 0.98 | 0.56 | ~4 | −72 | [60] |
| Fe–N–C/PdNC | 0.97 | 0.87 | ~4 | −51.1 | [61] |
| Sr/FeNC-2 | 0.90 | 0.85 | 3.91 | −27 | [62] |
| Fe5-PANI/C-MCS | 1.09 | 0.85 | ~4 | −85.1 | [63] |
| Co,Nb-MoS2/TiO2 HSs | 0.96 | 0.86 | 3.96 | −56.1 | [64] |
| Co3O4-C3N4/rGO | 0.97 | 0.81 | 3.95 | −87.2 | [65] |
| Fe–N–C 900 | 0.982 | 0.871 | 3.96 | −71 | [66] |
| Fe3C@N-CNTs/800 | 0.98 | 0.85 | 4 | −75 | [67] |
| Co5-N-C-900 | 0.99 | 0.86 | 4 | −75 | [68] |
| ACTP5@Co,N-800 | 1.0 | 0.891 | 3.95 | −74 | [69] |
| Co2P/CoP@NPC-1 | 0.986 | 0.93 | 3.76 | −69 | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobele, G.; Volperts, A.; Plavniece, A.; Zhurinsh, A.; Upskuviene, D.; Balciunaite, A.; Niaura, G.; Colmenares-Rausseo, L.C.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Thermochemical Activation of Wood with NaOH, KOH and H3PO4 for the Synthesis of Nitrogen-Doped Nanoporous Carbon for Oxygen Reduction Reaction. Molecules 2024, 29, 2238. https://doi.org/10.3390/molecules29102238
Dobele G, Volperts A, Plavniece A, Zhurinsh A, Upskuviene D, Balciunaite A, Niaura G, Colmenares-Rausseo LC, Tamasauskaite-Tamasiunaite L, Norkus E. Thermochemical Activation of Wood with NaOH, KOH and H3PO4 for the Synthesis of Nitrogen-Doped Nanoporous Carbon for Oxygen Reduction Reaction. Molecules. 2024; 29(10):2238. https://doi.org/10.3390/molecules29102238
Chicago/Turabian StyleDobele, Galina, Aleksandrs Volperts, Ance Plavniece, Aivars Zhurinsh, Daina Upskuviene, Aldona Balciunaite, Gediminas Niaura, Luis César Colmenares-Rausseo, Loreta Tamasauskaite-Tamasiunaite, and Eugenijus Norkus. 2024. "Thermochemical Activation of Wood with NaOH, KOH and H3PO4 for the Synthesis of Nitrogen-Doped Nanoporous Carbon for Oxygen Reduction Reaction" Molecules 29, no. 10: 2238. https://doi.org/10.3390/molecules29102238
APA StyleDobele, G., Volperts, A., Plavniece, A., Zhurinsh, A., Upskuviene, D., Balciunaite, A., Niaura, G., Colmenares-Rausseo, L. C., Tamasauskaite-Tamasiunaite, L., & Norkus, E. (2024). Thermochemical Activation of Wood with NaOH, KOH and H3PO4 for the Synthesis of Nitrogen-Doped Nanoporous Carbon for Oxygen Reduction Reaction. Molecules, 29(10), 2238. https://doi.org/10.3390/molecules29102238













